Abstract
Although oxidative stress is a hallmark of important vascular disorders such as diabetic retinopathy, it remains unclear why the retinal microvasculature is particularly vulnerable to this pathophysiological condition. We postulated that redox-sensitive ion channels may play a role. Using H2O2 to cause oxidative stress in microvascular complexes freshly isolated from the adult rat retina, we assessed ionic currents, cell viability, intracellular oxidants, and cell calcium by using perforated-patch recordings, trypan blue dye exclusion, and fura-2 fluorescence, respectively. Supporting a role for the oxidant-sensitive ATP-sensitive K (KATP) channels, we found that these channels are activated during exposure of retinal microvessels to H2O2. Furthermore, their inhibition by glibenclamide significantly lessened H2O2-induced microvascular cell death. Additional experiments established that by increasing the influx of calcium into microvascular cells, the KATP channel-mediated hyperpolarization boosted the vulnerability of these cells to oxidative stress. In addition to the KATP channel-dependent mechanism for increasing the lethality of oxidative stress, we also found that the vulnerability of cells in the capillaries, but not in the arterioles, was further boosted by a KATP channel-independent mechanism, which our experiments indicated involves the oxidant-induced activation of calcium-permeable nonspecific cation channels. Taken together, our findings support a working model in which both KATP channel-independent and KATP channel-dependent mechanisms render the capillaries of the retina particularly vulnerable to oxidative stress. Identification of these previously unappreciated mechanisms for boosting the lethality of oxidants may provide new targets for pharmacologically limiting damage to the retinal microvasculature during periods of oxidative stress.
Keywords: capillaries, KATP channels, retina, microvessels
oxidative stress is thought to play an important role in a number of pathological disorders including diabetes (1). In the case of sight-threatening diabetic retinopathy, it is well established that oxidative stress is increased in the retina in general and in microvascular cells in particular (3, 13). Consistent with the importance of oxidants in the pathogenesis of diabetic retinopathy, antioxidant treatment of experimental models of diabetes can diminish microvascular cell death (11, 13), which is a hallmark of this disorder (2, 17). However, despite the likely significance of oxidative stress in retinal vascular disease, much remains to be elucidated about how the microvasculature of the retina responds to this pathophysiological condition. In addition, it is unclear why the capillary portion of the retinal vasculature is particularly vulnerable to disorders, such as diabetes, in which oxidative stress is increased (1, 13). To help address these gaps in knowledge, this study focused on redox-sensitive ion channels of the retinal microvasculature. These channels were of interest since it seemed likely that their activity would be affected by oxidative stress. Furthermore, oxidative stress-induced changes in ion channel function were of interest because ion channel activity has been causally linked with death pathways in a variety of nonretinal cells (16, 20, 27).
An initial focus of this study was on redox-sensitive ATP-sensitive K (KATP) channels. While these channels are known to be important in microvascular physiology due to their role in generating a vasomotor response to extracellular signals such as adenosine (7, 12), we postulated that oxidant-activated KATP channels may also play a pathophysiological role during oxidative stress by causing a hyperpolarization-induced rise in intracellular calcium, which has been implicated in the oxidant-induced death of nonretinal cells (4). It seemed likely that a KATP channel-induced hyperpolarization would increase microvascular cell calcium since nonspecific cation (NSC) channels, rather than voltage-dependent calcium channels (VDCCs), are the predominant calcium-permeable ion channels in retinal capillaries (14, 21). To assess this putative pathophysiological scenario, an aim of this study was to determine whether a mechanism dependent on KATP channels, calcium influx, and increased cell calcium boosts the vulnerability of retinal microvessels to oxidative stress.
We report that consistent with the capillaries being particularly vulnerable to oxidative stress, exposure of freshly isolated retinal microvascular complexes to H2O2 resulted in substantially more cell death in the capillaries than in the precapillary tertiary arterioles. Experiments also indicated that a KATP channel/Ca2+ influx pathway increased the lethality of oxidative stress in both the capillaries and tertiary arterioles. In addition to this KATP channel-dependent pathway, we found that the lethality of oxidative stress in the capillaries was further boosted by a KATP channel-independent mechanism, which is likely to involve the oxidant-induced activation of NSC channels. Based on these experimental results, we propose that the activation of both KATP channel-dependent and KATP channel-independent mechanisms accounts for the particularly great vulnerability of retinal capillaries to oxidative stress.
MATERIALS AND METHODS
Animal use conformed to the guidelines of the American Physiological Society's Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training and was approved by the University of Michigan Committee on the Use and Care of Animals. This study used Long-Evans rats (Charles River, Cambridge, MA), which were maintained on a 12 h alternating light/dark cycle.
Microvessel isolation.
A modified tissue print technique was used to isolate microvascular complexes from the retinas of rats (7, 19) that were killed with a rising concentration of carbon dioxide. As detailed previously (7), the procedure for microvessel isolation included the rapid removal of retinas, excision of adherent vitreous, and incubation for ∼24 min at 30°C in Earle's balanced salt solution supplemented with 0.5 mM EDTA, 6 U papain (Worthington Biochemicals, Freehold, NJ), and 2 mM cysteine. Subsequently, retinas were placed in solution A, which consisted of 140 mM NaCl, 3 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, 10 mM Na-Hepes, 15 mM mannitol, and 5 mM glucose at pH 7.4 with osmolarity adjusted to 310 mosmol/l. After each retina was quadrisected, a retinal quadrant was positioned vitreal surface up onto the glass bottom of a chamber containing solution A and then very gently compressed by a 15 mm diameter glass coverslip (Warner Instrument, Hamden, CT) onto which microvascular complexes adhered. This process was repeated several times with new coverslips. Typically, four to six coverslips containing microvascular complexes were obtained from a pair of retinas. Experiments were performed at room temperature, i.e., 22–23°C. Prior to the onset of exposure to H2O2, cell viability in the isolated retinal microvascular complexes was 96.3 ± 0.4% (n = 94 microvessel-containing coverslips) and 94.2 ± 0.4% (n = 119) for tertiary arterioles and capillaries, respectively.
As illustrated photographically and schematically in published reports (14, 15, 28), the isolated microvascular complexes included, from distal to proximal, a network of capillaries with abluminally located pericytes that appear as “bumps on a log” with a density of ≤4 per 100 μm, a precapillary tertiary arteriole with ≥5 “dome-shaped” mural cell somas per 100 μm, and a secondary arteriole encircled by a layer of “doughnut-shaped” smooth muscle cells. By visual inspection with the aid of phase-contrast microscopy, it is straightforward to identify the pericytes of the capillaries, the abluminal mural cells of the tertiary arterioles, and the smooth muscle cells encircling the secondary arterioles. Determination of the abluminal cell density is a convenient and consistent method for the identification of capillaries, tertiary arterioles and secondary arterioles.
Cell viability assay.
As we have done previously (9, 10, 22), cells in isolated retinal microvascular complexes that failed to exclude trypan blue dye were classified as dead. The trypan blue assay was performed by exposing microvessel-containing coverslips to 0.04% trypan blue in solution A for 15 min. After being washed in solution A, microvessel-containing coverslips were examined at ×100 magnification with an inverted microscope equipped with bright-field optics. Since it was straightforward to distinguish precapillary tertiary arterioles and capillaries based on their differences in abluminal cell density, we tallied cell viability separately for these two portions of the distal microvasculature of the retina. However, because trypan blue-containing cells typically were swollen, identification of these cells as being endothelial or abluminal was uncertain, and thus, subclassification of microvascular cells into these two types was not done in the cell death assays. After cell viability was assessed prior to the onset of exposure to H2O2, the location on each coverslip of the assayed microvascular complex was carefully documented so that cell viability in the identical portion of the microvascular complex could be reassessed after H2O2 exposure. As with the initial assessment of cell death, the second quantification of cell death was done after a 15-min exposure of the microvessels to 0.04% trypan blue in solution A.
Perforated-patch recordings.
The perforated-patch configuration of the patch clamp technique was used to detect ionic currents in microvascular complexes located on coverslips positioned in a recording chamber (volume = 1 ml), which was perfused (∼1.5 ml/min) with solutions from a gravity-fed system using multiple reservoirs. The recording chamber and the reservoirs for the perfusates were open to the air; no additional oxygenation was administrated. Recording pipettes were filled with a solution consisting of 50 mM KCl, 65 mM K2SO4, 6 mM MgCl2, 10 mM K-Hepes, 60 μg/ml amphotericin B, and 60 μg/ml nystatin at pH 7.4 with the osmolarity adjusted to 280 mosmol/l; the perfusates consisted of solution A without or with 15 μM H2O2 plus or minus 0.5 μM glibenclamide.
Recording pipettes, which had resistances of 5–10 MΩ, were mounted in the holder of a patch-clamp amplifier (Axopatch 200B; MDS Analytical Technologies, Union City, CA) and were positioned onto the surface of capillary pericytes or tertiary arteriolar mural cells. Earlier publications show images of single pericytes loaded with a dye administered via a patch pipette [Fig. 1, B and C, in Oku et al. (18) and Fig. 6B of Kawamura et al. (8)]; indicative of the ease of identifying pericytes, we found in these dye-loading experiments that when the goal was to patch onto a pericyte, an endothelial cell was never (0 of 36 recordings) directly filled with dye (18). It is also not challenging to identify tertiary arteriolar mural cells, which have characteristic dome-shaped somas at a density of ≥5 per 100 μm (15).
Fig. 1.
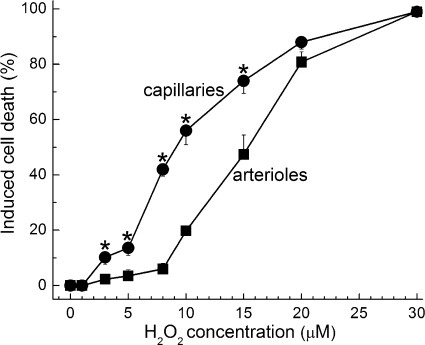
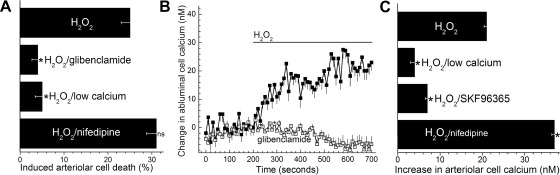
Induced cell death in capillaries and the precapillary tertiary arterioles in retinal microvascular complexes exposed for 20 h to various concentrations of H2O2. For each point, 10.9 ± 0.5 microvessel-containing coverslips were assayed. *H2O2-induced cell death was significantly greater in capillaries than in tertiary arterioles; the P values comparing capillary and arteriolar cell death induced by 3, 5, 8, 10, and 15 μM H2O2 were 0.014, 0.007, <0.001, <0.001, and 0.003, respectively.
Fig. 6.
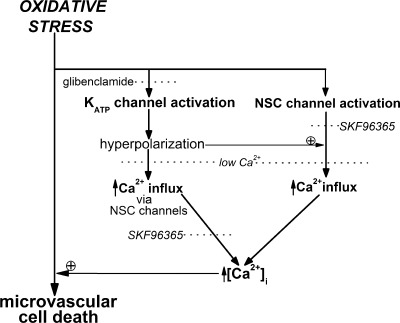
A working model based on the results of this study illustrating mechanisms by which the vulnerability of retinal microvessels to oxidative stress is boosted. During a period of oxidative stress, KATP channels are activated, and the resulting hyperpolarization boosts the electrochemical gradient for the influx of calcium via NSC channels located in the retinal microvasculature. As a consequence of increased calcium influx, capillary cell calcium increases, and this, we propose, exacerbates the lethality of oxidative stress, as has been established for nonretinal cells (4). In addition, our experiments indicate that calcium influx is further boosted in the capillaries, but not in the tertiary arterioles, by a KATP channel-independent mechanism that is likely to involve an oxidative stress-induced activation of capillary NSC channels.
Recordings with a seal resistance of ≥10 GΩ seal and an access resistance of <25 MΩ were used. Currents were filtered with a four-pole Bessel filter, digitally sampled, and stored on a computer equipped with pClamp (MDS Analytical Technologies) and Origin (OriginLab, Northampton, MA) software. Due to the presence of gap junction pathways in retinal microvessels (18, 25), the currents detected by a perforated-patch pipette sealed onto an abluminal cell includes not only the current generated by the sampled pericyte, but also currents transmitted electrotonically from neighboring vascular cells. Based on recordings made in isolated retinal microvessels in the absence and presence of gap junction uncouplers, we found that ∼95% of the current monitored via a perforated-patch pipette is generated by the microvascular neighbors of the sampled cell (8, 25).
For the generation of current-voltage (I-V) plots, currents were evoked by voltage step protocols controlled by pCLAMP. Voltage steps were made from a holding potential of −58 mV. Calculated liquid junction potentials were corrected after data collection. The paired Student's t-test was used to compare the mean conductances measured in the absence and presence of H2O2 at 10 mV intervals, from −48 mV to −18 mV (Fig. 2B) or +2 mV to +32 mV (Fig. 4). The zero-current potential was defined as the membrane potential.
Fig. 2.
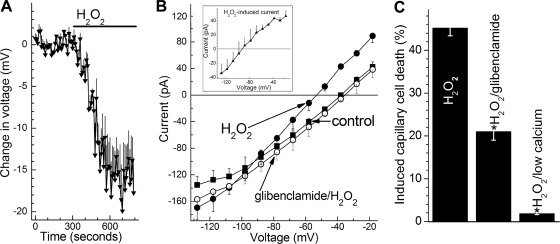
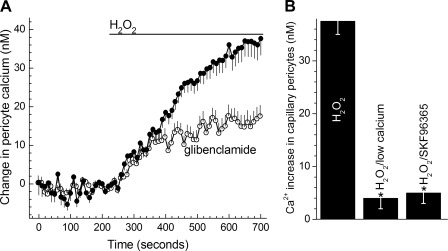
KATP channel activation in retinal microvessels during exposure to H2O2. A: time course for the hyperpolarizing effect of 15 μM H2O2. Values are the means of 4 experiments. During H2O2 exposure, the membrane potential increased significantly (P < 0.001). B: during continuous recordings (n = 4), current-voltage (I-V) plots were generated during exposure of the retinal microvascular complex to solution A (control, ■), 9 ± 2 min after the onset of exposure to solution A supplemented with 15 μM H2O2 (●), and finally 11 ± 2 min after the addition of 0.5 μM glibenclamide to the H2O2-containing solution (○). Exposure to H2O2 significantly (P = 0.001, paired t-test) increased the ionic conductance; glibenclamide significantly (P = 0.001, paired t-test) decreased the H2O2-induced conductance. Inset: I-V relations of the H2O2-induced conductance, which is the difference between the control and H2O2 I-V plots shown in this panel. C: effect of glibenclamide and low extracellular calcium on H2O2-induced capillary cell death. Microvessels were exposed for 6 h to 15 μM H2O2 in solution A without additives (H2O2 group), in solution A with 0.5 μM glibenclamide, or in solution A lacking added calcium. For the H2O2, H2O2/glibenclamide, and H2O2/low calcium groups, 14, 5, and 6 microvessel-containing coverslips were assayed, respectively. H2O2-induced capillary cell death was significantly (*P < 0.001) less in the glibenclamide and low calcium groups.
Fig. 4.
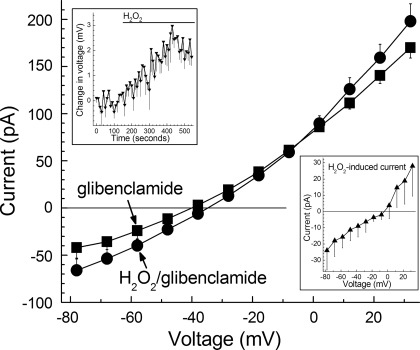
Effect of H2O2 on I-V relations recorded in retinal microvascular complexes whose KATP channels were blocked by glibenclamide. I-V relations were determined in solution A supplemented with 0.5 μM glibenclamide (■) and then again 6 ± 1 min after the addition of 15 μM H2O2 (●). I-V plots are the means of 4 experiments. Inset, top: time course for the H2O2-induced depolarization (P < 0.001) of the glibenclamide-treated microvascular complexes whose I-V relations are shown in the main panel. Inset, bottom: I-V relationship of the H2O2-induced conductance determined by calculating the difference in the plots shown in the main panel.
Calcium imaging.
As detailed previously, freshly isolated retinal microvascular complexes were incubated in solution A supplemented with 5 μM fura-2AM (Molecular Probes, Eugene, OR) at 37°C for 90 min. After allowing the AM ester to be cleaved, we positioned a coverslip containing fura-loaded microvessels in a 200 μl recording chamber that was perfused (∼1.5 ml/min) continuously. Digital imaging was performed using a Nikon Eclipse E600 FN microscope, an optical sensor (Sensicam, Cooke, Auburn Hills, MI), and a high-intensity mercury lamp coupled to an Optoscan Monochromator (Cairn Research, Faversham, UK); imaging equipment and data collection were controlled using MetaFluor software (Molecular Devices, Sunnyvale, CA). As previously noted, abluminal cells in the isolated retinal microvascular complexes were well loaded with fura-2 while little of this fluorescent dye entered the endothelial cells (26); isolated microvascular complexes lacked detectable autofluorescence.
Fluorescent intensities were measured every 10 s at 340 nm and 380 nm within region of interests (ROIs), each of which encircled a single abluminal cell soma. Visualization of the microvessels with the aid of differential interference contrast microscopy was used to help confirm that ROIs were of abluminal cell somas. As detailed earlier, Mn2+ quenching not routinely performed because only a minimal amount of fluorescence in isolated retinal microvascular is derived from fura-2 that is not sensitive to free Ca2+ (7). Background fluorescence of the optical system was measured in each experiment by placing some ROIs in cell-free areas of the coverslip. After subtracting this background, we calculated the F340/F380 ratio and converted it to the intracellular calcium concentration by use of a standard equation (5) in which Rmin and Rmax were determined as detailed previously (26). For each ROI, the baseline value was defined as the mean cell calcium concentration during the minute (six samples) preceding the onset of exposure to H2O2. At each sampling time, the change in calcium concentration from the baseline level was calculated for each ROI. For the data shown in Figs. 3 and 5, the increase in calcium concentration was the difference between the baseline value and the mean concentration during the final six sampling periods in the last minute of a 400-s exposure to 15 μM H2O2.
Chemicals.
Unless otherwise noted, chemicals were from Sigma-Aldrich.
Statistics.
Data are given as means ± SE. For the comparison of the means of two groups, probability was evaluated by the two-tailed Student's t-test with the Bonferroni correction used to adjust the P value for significance. For multiple comparisons, an analysis of variance was performed using commercially available software (Origin version 8) with the subsequent application of the Bonferroni correction. P ≥ 0.05 indicated lack of a significant difference.
RESULTS
Capillary vulnerability to oxidative stress.
In initial experiments using retinal microvascular complexes freshly isolated from the rat retina, we assessed the effect of various concentrations of H2O2 on the viability of cells in the capillaries and precapillary tertiary arterioles (Fig. 1). Indicative that the capillary portion of the retinal microvasculature is particularly vulnerable to oxidative stress, we found that over the range of 3–15 μM H2O2, significantly (P ≤ 0.014) more cell death was induced in the capillaries; the half-maximally effective concentration for H2O2-induced cell death in capillaries and tertiary arterioles was 9 and 15 μM, respectively.
KATP channel-dependent mechanism.
What accounts for the relatively high vulnerability of capillaries to oxidative stress? Based on the idea that certain physiological features of the capillaries increase their vulnerability to pathophysiological conditions such as oxidative stress, we evaluated the role of redox-sensitive KATP channels, which are predominantly located in the capillary portion of the retinal microvasculature (7). In an initial series of experiments, we asked whether a KATP conductance is activated during oxidative stress caused by exposure to H2O2. As shown in Fig. 2A and B, perforated-patch recordings showed that exposure to 15 μM H2O2 resulted in the rapid activation of a hyperpolarizing current that was sensitive to the KATP channel blocker glibenclamide; of note, this hyperpolarization reversed by 81 ± 4% (n = 4) during a 7 min washout of H2O2. We concluded from these electrophysiological experiments that KATP channels are activated in the retinal microvasculature during a period of oxidative stress.
To further establish a role for KATP channels in establishing the vulnerability of capillaries to oxidative stress, we assessed the effect of glibenclamide on H2O2-induced capillary cell death. Figure 2C shows that treatment of retinal microvessels with this KATP channel blocker significantly (P < 0.001) lessened that amount of capillary cell death induced by 15 μM H2O2. This finding supported the idea that the lethality of H2O2-induced oxidative stress was increased by a KATP channel-dependent mechanism.
We next asked how the activation of KATP channels increases the vulnerability of the retinal microvasculature to oxidative stress. To address this question, we postulated that by increasing the electrochemical gradient for calcium influx, the hyperpolarization caused by the opening of KATP channels would increase the intracellular concentration of calcium, which is known to influence the response of a variety of non-retinal cells to oxidative stress (4). We surmised that hyperpolarization would increase calcium influx since the predominant calcium-permeable ion channels in retinal capillaries are NSC channels (21), and the activity of VDCCs is minimal in retinal capillaries (14). In agreement with this scenario, Fig. 3A shows that soon after the onset of H2O2 exposure, capillary cell calcium increased significantly (P < 0.0001); of note, this calcium increase reversed over the course of a 400-s washout of H2O2. Consistent with the importance of KATP channels in causing the H2O2-induced rise in capillary cell calcium, inhibition of these channels with glibenclamide significantly (P < 0.001) lessened the H2O2-induced increase in capillary cell calcium (Fig. 3A). Furthermore, consistent with an influx of calcium accounting for this increase in capillary cell calcium, the H2O2-induced rise in capillary cell calcium was significantly (P < 0.0001) less when the extracellular solution lacked added calcium (Fig. 3B). Suggestive that this influx occurs via NSC channels, Fig. 3B also shows that the H2O2-induced rise in capillary cell calcium was significantly (P < 0.0001) less in the presence of SKF-96365, which inhibits the NSC conductance in retinal microvessels (21). Thus, the experimental results shown in Fig. 3 are consistent with the proposal that during a period of oxidative stress, the activation of hyperpolarizing KATP channels causes capillary cell calcium to increase by a mechanism dependent upon the influx of calcium via NSC channels.
Fig. 3.
Effect of glibenclamide, low extracellular calcium, and SKF-96365 on the H2O2-induced increase in calcium in capillary pericytes. A: time course for the change in the intracellular concentration of capillary pericytes during exposure of retinal microvascular complexes to solution A supplemented with 15 μM H2O2 in the absence (n = 59) or presence of 0.5 μM glibenclamide (n = 46). B: effect of low calcium and the nonspecific cation (NSC) channel inhibitor SKF-96365 on the H2O2-induced increase in capillary pericyte calcium. For the H2O2 group, microvessels were initially monitored in solution A without additives and then in solution A plus 15 μM H2O2 for 400 s. For the H2O2/low calcium group, microvessels were initially monitored in solution A without added calcium prior to the addition of 15 μM H2O2. For the H2O2/SKF-96365 group, microvessels were exposed to solution A supplemented with 50 μM SKF-96365 before the addition of 15 μM H2O2. For each group, 41 ± 5 regions of interest (ROIs) were monitored. The increase in pericyte calcium was determined for each group by subtracting the average calcium concentration of the monitored ROIs during the final 6 10-s samples of a 400-s exposure to H2O2 from the mean intracellular calcium concentration during the minute preceding H2O2 exposure. Low calcium and SKF-96365 significantly (*P < 0.001) lessened the H2O2-induced increase in pericyte calcium.
Indicative that the influx of calcium plays a key role in increasing the lethality of oxidative stress, we found that the data in Fig. 2C show that omission of added calcium from the extracellular solution decreased (P < 0.0001) H2O2-induced capillary cell death from 45.2 ± 1.8% (n = 15) to only 1.8 ± 0.2% (n = 6). Unfortunately the effect of SKF-96365 on H2O2-induced capillary cell death could not be assessed because of its toxicity with exposure times of >1 h, although capillary cell viability was not significantly affected by the 15-min exposures to SKF-96365 used in the calcium-imaging experiments.
Taken together, the experimental findings presented in Figs. 2 and 3 supported a working model in which capillary cell death during a period of oxidative stress is boosted, at least in part, by a mechanism involving KATP channels whose activation causes a hyperpolarization-driven calcium influx via NSC channels and, thereby, results in an increase in intracellular calcium that is proposed to exacerbate the lethality of oxidative stress.
KATP channel-independent mechanism.
In addition to supporting the importance of KATP channel activation in boosting oxidative stress-induced capillary cell death, experiments also indicated that a mechanism independent of KATP channels was involved. In support of a KATP channel-independent mechanism playing a role, we observed that although treatment of retinal microvessels with 0.5 μM glibenclamide is known to near-totally inhibit microvascular KATP channels (7, 12, 24), blockade of these ion channels caused only a ∼50% diminution in the amount of capillary cell death induced by 15 μM H2O2 (Fig. 2C). Thus, it appeared that a mechanism independent of KATP channels accounts for ∼50% of the H2O2-induced capillary cell death.
The question then arose as to the identity of the KATP channel-independent mechanism. Based on our finding that nearly all of the increases in capillary cell calcium (Fig. 3B) and capillary cell death (Fig. 2C) induced by H2O2 were dependent on extracellular calcium, we surmised that the KATP channel-independent mechanism for boosting the vulnerability of capillaries to oxidative stress involves an increase in the influx of calcium. Furthermore, since most of the H2O2-induced increase in capillary cell calcium was prevented by the NSC channel blocker, SKF-96365 (Fig. 3B), it seemed likely that the KATP channel-independent pathway involved calcium influx via NSC channels. These deductions led us to postulate that the oxidative stress-induced activation of NSC channels is a putative KATP channel-independent mechanism for increasing calcium influx into capillary cells during oxidative stress. This seemed to be a reasonable possibility since NSC channels in vascular cells of various nonretinal tissues are known to be activated by oxidative stress (6, 23).
To test the idea that NSC channels are activated during oxidative stress, perforated-patch recordings were obtained from glibenclamide-treated microvessels before and during exposure to 15 μM H2O2. To enhance our ability to detect the activation of a depolarizing NSC conductance during H2O2 exposure, the KATP channel-mediated hyperpolarization was prevented by treating the microvessels with glibenclamide. Consistent with NSC channels being activated during oxidative stress, Fig. 4 shows that 15 μM H2O2 rapidly induced a depolarizing conductance that had a reversal potential close to Ecations, i.e., −5 mV; this conductance reversed by 83 ± 6% (n = 3) during a 5-min washout of H2O2. Taken together, our findings support a mechanism in which the activation of NSC channels, as well as KATP channel activation, plays a role in the calcium influx-dependent rise in capillary cell calcium, which we propose boosts the vulnerability of retinal microvascular cells to oxidative stress.
Arteriolar vulnerability to oxidative stress.
Because viability assays revealed that H2O2 caused cell death not only in the capillaries but also in the precapillary tertiary arterioles (Fig. 1), we asked whether KATP channels play a role in increasing oxidative stress-induced cell death in the arterioles, as well as in the capillaries. Supporting the importance of KATP channels, Fig. 5A shows that H2O2-induced arteriolar cell death was markedly (P = 0.001) less in glibenclamide-treated microvessels. Based on these findings, we concluded that the activation of KATP channels increases the vulnerability to oxidative stress of arterioles, as well as capillaries.
Fig. 5.
Effects of oxidative stress on the tertiary arterioles of the retinal microvasculature. A: arteriolar cell death induced by 15 μM H2O2 under various experimental conditions. Microvessels were exposed for 6 h to 15 μM H2O2 in solution A without additives (H2O2 group), in solution A with 0.5 μM glibenclamide, in solution A lacking added calcium, or in solution A plus 10 μM nifedipine. For each group, 8 ± 2 microvessel-containing coverslips were assayed. H2O2-induced arteriolar cell death was significantly (*P < 0.001) less in the H2O2/glibenclamide and H2O2/low calcium groups. Nifedipine did not significantly affect H2O2-induced arteriolar cell death. B: time course for the change in the intracellular calcium concentration of tertiary arteriolar cells during exposure to solution A supplemented with 15 μM H2O2 in the absence (n = 24) or presence of 0.5 μM glibenclamide (n = 25). C: effect of extracellular calcium, SKF-96365, and nifedipine on the H2O2-induced increase in the intracellular calcium concentration of arteriolar mural cells. For the H2O2 group, microvessels were exposed to solution A without additives followed by exposure to solution A plus 15 μM H2O2 for 400 s. For the H2O2/low calcium group, microvessels were initially monitored in solution A without added calcium prior to the addition of 15 μM H2O2. For the H2O2/SKF-96365 group, microvessels were perfused with solution A supplemented with 50 μM SKF-96365 before the addition of 15 μM H2O2. For the H2O2/nifedipine group, microvessels were perfused with solution A supplemented with 10 μM nifedipine prior to the addition of 15 μM H2O2. For each group, 63 ± 21 ROIs were monitored. The increase in calcium was determined as noted in the legend of Fig. 3 and detailed in materials and methods. The H2O2-induced increase in mural cell calcium was significantly (*P < 0.001) less in the H2O2/low calcium and H2O2/SKF-96365 groups; nifedipine significantly (*P < 0.001) increased the H2O2-induced increase in mural cell calcium.
To help determine the mechanism by which KATP channel activation increases the lethality of oxidative stress in the tertiary arterioles, we asked whether arteriolar cell calcium is increased during a period of oxidative stress. As shown in Fig. 5B, arteriolar cell calcium increased significantly (P < 0.001) during exposure of retinal microvascular complexes to 15 μM H2O2. Supporting the importance of KATP channels in mediating this H2O2-induced rise in arteriolar cell calcium, glibenclamide eliminated (P < 0.001) this effect of H2O2 (Fig. 5B).
Consistent with the H2O2-induced rise in arteriolar cell calcium being dependent on an influx of extracellular calcium, this calcium increase during H2O2 exposure was ∼80% less (P < 0.001) when the bathing solution lacked added calcium (Fig. 5C). Suggestive that the H2O2-induced influx of calcium was via NSC channels, the blocker of these channels, SKF-96365, significantly (P < 0.001) decreased the H2O2-induced increase in arteriolar calcium (Fig. 5C). Furthermore, indicative of the importance of calcium influx in establishing the lethality of oxidative stress in the arterioles, arteriolar cell death induced by 15 μM H2O2 was reduced from 25.1 ± 1.8 to 5.0 ± 1.6% (P < 0.001) when added calcium was eliminated from the extracellular solution (Fig. 5A). Thus, these results led us to conclude that similar to capillary cells, a mechanism involving polyamines, KATP channels, and calcium influx increases the vulnerability of arteriolar cells to oxidative stress.
We also considered the possibility that a mechanism independent of KATP channels may play a role in establishing the vulnerability of tertiary arterioles to oxidative stress. However, inconsistent with this, a vast majority of the H2O2-induced arteriolar cell death was prevented by treatment with the KATP channel blocker, glibenclamide (Fig. 5A). This contrasted significantly (P < 0.001) with the capillaries where only ∼50% of the H2O2-induced cell death was insensitive to glibenclamide-treatment (Fig. 2C). Furthermore, in contrast to glibenclamide's partial inhibition of the H2O2-induced increase in capillary cell calcium (Fig. 3A), glibenclamide treatment eliminated the rise in arteriolar cell calcium during H2O2 exposure (Fig. 5B). Also, in contrast to the observation made in patch-clamp recordings from the capillaries, exposure to 15 μM H2O2 did not elicit a significant effect on the I-V relations (n = 4) of glibenclamide-treated arterioles. Taken together, these findings led us to conclude that the H2O2-induced arteriolar cell death is almost exclusively mediated by a KATP channel-dependent mechanism and a mechanism independent of KATP channels is of relatively little importance in this portion of the retinal microvasculature.
In our study of tertiary arterioles, we also considered the possible role of VDCCs. These channels were of potential importance since the tertiary arterioles, unlike the capillaries, have a significant number of functional VDCCs (14), whose activity is decreased by hyperpolarization (21). To determine whether these channels are involved in establishing the lethality of oxidative stress in arterioles, cell death induced by 15 μM H2O was quantified in microvessels treated with 10 μM nifedipine, which inhibits nearly all VDCC current in retinal microvessels (14, 21). As shown in Fig. 5A, we found that nifedipine treatment did not significantly affect the amount of H2O2-induced arteriolar cell death. Of note, this lack of effect occurred even though inhibiting VDCCs with nifedipine prior to the onset of H2O2 exposure resulted in a larger (P < 0.001) H2O2-induced increase in arteriolar cell calcium (Fig. 5C). On the other hand, we also found that nifedipine decreased the basal concentration of calcium in arteriolar cells by 14 ± 1 nM (n = 24, P < 0.0001), and as a consequence, the peak concentration of arteriolar cell calcium during exposure to 15 μM H2O2 was not significantly affected by nifedipine. Specifically, the maximum H2O2-induced calcium level was 135 ± 1 nM (n = 24 ROIs) in the absence of nifedipine and 137 ± 1 nM (n = 53 ROIs) in the presence of this VDCC blocker. These findings, along with our observation that nifedipine did not alter H2O2-induced arteriolar cell death, provided support for the idea that it is the level of intracellular calcium, and not the absolute change in calcium, that plays a major role in establishing the lethality of oxidative stress.
DISCUSSION
This study of microvascular complexes freshly isolated from the adult rat retina revealed previously unappreciated mechanisms by which the lethality of oxidative stress is boosted. As illustrated in Fig. 6, our results support a working model in which the oxidative stress-induced activation of hyperpolarizing KATP channels increases the electrochemical gradient for calcium influx via the microvasculature's NSC channels and, thereby, causes a rise in capillary cell calcium, which we propose increases the lethality of oxidative stress, as has been reported for nonretinal cells (4). In addition to the role of the KATP channel/calcium influx pathway, our experimental results also revealed a KATP channel-independent mechanism that provides an additional boost in the lethality of oxidative stress in the capillaries. This KATP channel-independent mechanism appears to involve an oxidative stress-induced activation of capillary NSC channels. However, in contrast to the capillaries, our experiments indicate that a KATP channel-independent mechanism for exacerbating the deleterious effects of oxidative stress is of minimal importance in the tertiary arterioles.
This analysis of the retinal microvasculature's response to oxidative stress provides the first evidence that oxidant-sensitive ion channels play a critical role in making cells of the capillaries particularly prone to oxidative stress-induced death. Taken together, our findings indicate that capillaries are more vulnerable than precapillary arterioles to oxidative stress because KATP channel-dependent, as well as KATP channel-independent, mechanisms for boosting oxidant lethality are operative in the capillaries. In contrast, only the KATP channel/calcium influx pathway affects arteriolar cell vulnerability. Of note, we are not excluding that additional mechanisms also play a role in making retinal capillaries particularly vulnerable in pathological disorders associated with increased oxidative stress. For example, although evidence is lacking at present, it may be that in some diseases, the amount of oxidative stress in the capillaries is greater than the arterioles.
Our conclusions concerning mechanisms boosting the vulnerability of the retinal microvasculature to oxidative stress are based on experiments performed on microvascular complexes freshly isolated from the rat retina. An advantage of this experimental preparation is the ease with which capillaries and tertiary arterioles can be distinguished. This allowed us to compare the vulnerability of these microvascular regions to H2O2-induced oxidative stress. Another advantage of studying microvessels in isolation is the absence of potentially confounding effects caused by interactions of H2O2 with nonvascular retinal cells. On the other hand, conclusions based on a study of microvessels isolated from the retina will require in vivo validation, although performing the types of patch-clamping and fluorescence-imaging studies done in this study on retinal capillaries and tertiary arterioles located in oculo appears to be technically unfeasible at present. In addition, future studies are needed to characterize the effect of reactive oxygen species other than those associated with H2O2 exposure. Furthermore, because this study focused on the mechanism by which relatively modest concentrations of H2O2, i.e., ≤30 μM, cause microvascular cell death, we do not exclude that other mechanisms may be operative at higher H2O2 concentrations. Also, additional analyses will be needed to further characterize the specific type of NSC channel activated in the retinal microvasculature during a period of oxidative stress. Finally, it is clear that caution is warranted when extrapolating findings obtained with rodent retinal microvessels to the microvasculature of the human retina. Yet, despite caveats, the experimental approach used in this study revealed previously unappreciated mechanisms that boost the vulnerability of the retinal microvasculature to oxidative stress.
In summary, our analysis of freshly isolated retinal microvascular complexes showed that the KATP channel/calcium influx pathway significantly increases the vulnerability of both capillaries and arterioles to oxidative stress. In addition to this KATP channel-dependent mechanism, our experiments indicate that NSC channel activation further augments calcium flux into capillary cells and, thereby, increases the intracellular concentration of calcium, which we propose boosts the lethality of oxidative stress. Thus, our study indicates that the particularly great vulnerability of the capillaries to oxidative stress is a result of their having both the KATP channel-dependent and KATP channel-independent mechanisms for increasing oxidant-induced cell death (Fig. 6). A hope is that the discovery of previously unrecognized mechanisms for boosting the vulnerability of the retinal microvasculature to oxidative stress will provide new potential targets for pharmacological interventions to limit oxidant-induced cell damage in sight-threatening disorders such as diabetes.
GRANTS
This project was supported by National Institutes of Health Grants EY-12505, EY-07003, and DK-20572.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.F., A.N., T.Z., S.I.L., M.S., and D.G.P. conception and design of research; M.F., A.N., T.Z., S.I.L., and M.S. performed experiments; M.F., A.N., T.Z., S.I.L., M.S., and D.G.P. analyzed data; M.F., A.N., T.Z., S.I.L., M.S., and D.G.P. interpreted results of experiments; M.F., A.N., T.Z., S.I.L., M.S., and D.G.P. edited and revised manuscript; M.F., A.N., T.Z., S.I.L., M.S., and D.G.P. approved final version of manuscript; S.I.L. and D.G.P. prepared figures; D.G.P. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Bret Hughes for use of imaging equipment.
REFERENCES
- 1.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54: 1615–1625, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol 166: 366–378, 1961 [DOI] [PubMed] [Google Scholar]
- 3.Du Y, Miller CM, Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med 35: 1491–1499, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Ermak G, Davies KJA. Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol 38: 713–721, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 6.Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res 102: 347–355, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Ishizaki E, Fukumoto M, Puro DG. Functional KATP channels in the rat retinal microvasculature: topographical distribution, redox regulation, spermine modulation and diabetic alteration. J Physiol 587: 2233–2253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawamura H, Oku H, Li Q, Sakagami K, Puro DG. Endothelin-induced changes in the physiology of retinal pericytes. Invest Ophthalmol Vis Sci 43: 882–888, 2002 [PubMed] [Google Scholar]
- 9.Kobayashi T, Puro DG. Loss of insulin-mediated vasoprotection: early effect of diabetes on pericyte-containing microvessels of the retina. Invest Ophthalmol Vis Sci 48: 2350–2355, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Kodama T, Oku H, Kawamura H, Sakagami K, Puro DG. Platelet-derived growth factor-BB: a survival factor for the retinal microvasculature during periods of metabolic compromise. Curr Eye Res 23: 93–97, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Kowluru RA, Kern TS, Engerman RL, Armstrong D. Abnormalities of retinal metabolism in diabetes or experimental galactosemia. III. Effects of antioxidants. Diabetes 45: 1233–1237, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Li Q, Puro DG. Adenosine activates ATP-sensitive K+ currents in pericytes of rat retinal microvessels: role of A1 and A2a receptors. Brain Res 907: 93–99, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord 9: 315–327, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Matsushita K, Fukumoto M, Kobayashi T, Kobayashi M, Ishizaki E, Minami M, Katsumura K, Liao SD, Wu DM, Zhang T, Puro DG. Diabetes-induced inhibition of voltage-dependent calcium channels in the retinal microvasculature: role of spermine. Invest Ophthalmol Vis Sci 51: 5979–5990, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsushita K, Puro DG. Topographical heterogeneity of KIR currents in pericyte-containing microvessels of the rat retina: effect of diabetes. J Physiol 573: 483–495, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNulty S, Fonfria E. The role of TRPM channels in cell death. Pflügers Arch 451: 235–242, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest 97: 2883–2890, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oku H, Kodama T, Sakagami K, Puro DG. Diabetes-induced disruption of gap junction pathways within the retinal microvasculature. Invest Ophthalmol Vis Sci 42: 1915–1920, 2001 [PubMed] [Google Scholar]
- 19.Puro DG. Retinovascular physiology and pathophysiology: new experimental approach/new insights. Prog Retin Eye Res [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remillard CV, Yuan JX. Activation of K+ channels: an essential pathway in programmed cell death. Am J Physiol Lung Cell Mol Physiol 286: L49–L67, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Sakagami K, Wu DM, Puro DG. Physiology of rat retinal pericytes: modulation of ion channel activity by serum-derived molecules. J Physiol 521: 637–650, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugiyama T, Kawamura H, Yamanishi S, Kobayashi M, Katsumura K, Puro DG. Regulation of P2X7-induced pore formation and cell death in pericyte-containing retinal microvessels. Am J Physiol Cell Physiol 288: C568–C576, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Takahashi N, Mori Y. TRP channels as sensors and signal integrators of redox status changes. Front Pharmacol 2: 1–11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu DM, Kawamura H, Li Q, Puro DG. Dopamine activates ATP-sensitive K+ currents in rat retinal pericytes. Vis Neurosci 18: 935–940, 2001 [PubMed] [Google Scholar]
- 25.Wu DM, Miniami M, Kawamura H, Puro DG. Electrotonic transmission within pericyte-containing retinal microvessels. Microcirculation 13: 353–363, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Yamanishi S, Katsumura K, Kobayashi T, Puro DG. Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. Am J Physiol Heart Circ Physiol 290: H925–H934, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki D, Kito H, Yamamoto S, Ohya S, Yamamura H, Asai K, Imaizumi Y. Contribution of Kir2 potassium channels to ATP-induced cell death in brain capillary endothelial cells and reconstructed HEK293 cell model. Am J Physiol Cell Physiol 300: C75–C86, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Zhang T, Wu DM, Xu GZ, Puro DG. The electrotonic architecture of the retinal microvasculature: modulation by angiotensin II. J Physiol 589: 2383–2399, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]