Abstract
Medical records and liver histology of 68 English springer spaniels (ESS) with a histological diagnosis of CH were reviewed retrospectively. PCR was performed on liver tissue for canine adenovirus-1 (CAV-1), canine parvovirus, canine herpesvirus and pathogenic Leptospira species. Follow-up information was obtained to calculate survival times. Median age at presentation was three years seven months (range, seven months to eight years five months) and there were 48 female and 20 male dogs. Clinical signs were non-specific and five dogs were asymptomatic. All dogs had an increase in serum activity of one or more hepatobiliary enzymes. Histopathology demonstrated hepatocyte necrosis and apoptosis with varying amounts of fibrosis. A predominantly lymphoplasmacytic infiltrate throughout the hepatic parenchyma was found in all 68 dogs, but 45 of these dogs also had a neutrophilic component to the inflammatory infiltrate. There was no significant copper accumulation and no aetiological agent was identified by PCR. The median survival time was 189 days (range, 1 to 1211 days), 38 dogs died within three months and 12 dogs survived more than a year following diagnosis.
CANINE chronic hepatitis (CH) is characterised histologically by the presence of hepatocellular apoptosis or necrosis, a variable mononuclear or mixed inflammatory infiltrate, regeneration and fibrosis (Van den Ingh and others 2006). Several breeds of dog have been shown to be predisposed to the development of CH including the American and English cocker spaniel, West Highland white terrier, Scottish terrier, labrador retriever and dobermann (Andersson and Sevelius 1991). The disease is seen more commonly in middle-aged and older animals and a gender predisposition has been observed in several breeds.
Known causes of canine CH include the viruses CAV-1 (Chouinard and others 1998) and herpesvirus (Schulze and Baumgärtner 1998), bacteria including leptospires (Bishop and others 1979) and Helicobacter species (Fox and others 1996) and several toxins and drugs (Bunch 1993). Breed-associated defects in copper metabolism have been described. Other metabolic defects such as α1-antitrypsin deficiency have been linked to CH in the cocker spaniel (Sevelius and others 1994). Primary immune-mediated mechanisms have also been suggested as a cause of canine CH but have never been conclusively demonstrated (Andersson and Sevelius 1992, Weiss and others 1995). The aetiology of CH in most dogs remains idiopathic (Poldervaart and others 2009).
We have recently reported on an increased prevalence of CH in the English springer spaniels (ESS) in the UK (Buxton and others 2009) although a detailed description of the disease has not been published. The aim of the current study was therefore to describe the history, clinical signs, clinicopathologic abnormalities, diagnostic imaging findings, histological appearance and outcome of ESSs with CH. The authors also aimed to investigate the role of potential aetiological factors including copper and infectious agents.
Materials and methods
The study period was between June 2006 and September 2010. The study population consisted of ESSs presenting to the Queen's Veterinary School Hospital (QVSH), Department of Veterinary Medicine, University of Cambridge, and additional cases. Details of these additional cases were obtained via contact with primary and referral practices within the UK. Cases were included when results of hepatic histology were consistent with a diagnosis of CH according to criteria developed by the World Small Animal Veterinary Association (WSAVA) Liver Standardization Group (Van den Ingh and others 2006), when complete case records were available and if the outcome could be assessed either from the case records or by contact with the owner or veterinary surgeon. Data obtained included signalment, clinical signs, physical examination findings, results of laboratory tests and imaging. Laboratory data examined included serum biochemistry, complete blood count and coagulation times. Serum biochemistry was performed at the time of diagnosis and included measurement of alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl aminotransferase (GGT), total serum bilirubin (TBIL), albumin, globulin, cholesterol, urea and bile acids. Complete blood count (CBC) included a total white blood cell count, differential white cell counts and haematocrit. Because not all dogs had platelet numbers confirmed by examination of a blood film, this parameter was not assessed. Coagulation times included prothrombin time and partial thromboplastin time. Data are presented as median (range). Additional information on long-term outcome was obtained from the clinical records or via telephone conversation with the referring veterinary surgeon or owners. Follow-up times were based on a censor dated September 30, 2010.
Liver samples were obtained percutaneously with ultrasound guidance and using a Tru-cut biopsy needle (n=35), laparoscopically (n=8), via wedge biopsy at exploratory surgery (n=22) or at postmortem examination within 30 minutes of death (n=3). Liver tissue was fixed in 10 per cent neutral-buffered formalin and routinely processed for histopathological examination. Paraffin-embedded tissue was obtained for review by the authors from all dogs presenting at other centres. All sections were stained with haematoxylin and eosin, reticulin, periodic acid Schiff and rhodanine. Copper accumulation was scored on sections with rhodanine stain as zero (absence or rare copper-positive cells), one (few random copper-positive cells), two (moderate numbers of copper-positive cells) and three (many copper-positive cells in all zones) using a previously established semiquantitative scoring system (Shih and others 2007).
PCR for CAV-1, canine parvovirus, canine herpesvirus and pathogenic Leptospira species was performed on liver tissue from all dogs. Liver tissue was fresh frozen, stored in a commercially available nucleic acid preservative (RNAlater; Invitrogen), formalin-fixed or formalin-fixed, paraffin-embedded. DNA was extracted using a commercially available kit (DNeasy blood and tissue kit; Qiagen), according to the manufacturer's instructions with minor adjustments. To confirm successful DNA extraction, a control PCR was performed using primers specific for the canine cardiac actin gene (Brouillette and others 2000). PCR primers for the detection of CAV-1, parvovirus and herpesvirus were designed on conserved regions within genes of a candidate virus family. When multiple sequences were available for the same pathogen, the conserved parts of the genome were found by aligning the different genes of the different family members using commercially available software (ClustalW; EMBL-EBI). Gene sequences were retrieved from the GenBank sequence database, National Centre for Biotechnology Information (NCBI). Primers for the detection of pathogenic Leptospira species were as previously published (Fearnley and others 2008). Primers were checked for secondary structure and primer-dimers. All primer sequences are given in Table 1. Positive controls for CAV-1, canine parvovirus and Leptospira PCR consisted of DNA extracted from fresh frozen liver tissue from infected canine cases. Positive control for canine herpesvirus PCR consisted of DNA extracted from archived canine herpesvirus grown in cell culture. As a negative control, water was used as a template for each PCR reaction. Amplification was performed using 2 µl of extracted DNA, 0.2 µM of each primer, 1.25 units DNA polymerase (GoTaq DNA polymerase; Promega), 50 mM Tris-HCl (pH 9.0), 50 mM NaCl, 3.0 mM MgCl2, 0.2 mM of each dNTP and distilled water to a final volume of 50 µl. After a single denaturation step at 95°C for two minutes, 35 cycles of amplification were performed in a thermal cycler (Bio-Rad iCycler; Bio-Rad Laboratories). Each cycle consisted of 30 seconds of denaturation at 95°C, 30 seconds of annealing at a temperature specific for each primer (Table 1) and 45 seconds of elongation at 72°C. Amplified PCR products were resolved by electrophoresis in a 1.5 per cent agarose gel, stained with ethidium bromide and viewed under UV transillumination.
Table 1.
Primer sequences
| Target | Forward primer sequence (5′-3′) | Reverse primer sequence (5′-3′) | Annealing temperature (°C) |
|---|---|---|---|
| Canine cardiac actin gene | AGCACTGTTAGAGACACCTG | CGGATAGCACGTTGTTGGCA | 57 |
| Canine adenovirus-1 | TGCTGCCACAATGGTCTTAC | CCACAGTGGGGTTTCTGAAC | 60 |
| Canine parvovirus | GACTGGGAATCGGAAGTTGA | CAATGCCAGCCTTGATCTTT | 59 |
| Canine herpesvirus | CTCTGGGAGATGCGATTGAT | AAACTTCAGGAGCGCTTTCA | 59 |
| Pathogenic Leptospira species | TGAGTAACACGTGGGTAATCTTCC | CAGGTACCATCATCACATYGCTGC | 62 |
A molecular mimic was used to determine the sensitivity of the CAV-1, parvovirus and herpesvirus PCRs. Briefly, this was achieved by individually cloning the amplified fragment of interest from each of these pathogens into the cloning vector pJET1.2 (Fermentas) according to the manufacturer's instructions. Chemically competent Escherichia coli cells (One Shot Top10 E coli; Invitrogen) were transformed with this construct and plasmid DNA was extracted from E coli grown overnight in liquid culture (Plasmid mini kit; Qiagen). Plasmid DNA was quantified using a spectrofluorescent method (Quant-iT PicoGreen dsDNA reagent; Invitrogen). A 10-fold dilution series was made by diluting the plasmid DNA in polyinosinic-polycytidylic acid (Poly I:C) from 1 × 108 to 1 × 100 copies. Following linearisation of plasmid DNA, PCR was performed on each of the standards (methodology as previously described) and products evaluated on an agarose gel to ascertain the sensitivity of the PCR. The lowest standard plasmid dilution in which a visible DNA band of the expected size was observed was used to determine the sensitivity of each PCR.
The present study also confirmed the ability of the PCR to detect CAV-1, parvovirus and Leptospira species in formalin-fixed, paraffin-embedded liver tissue by performing PCR on DNA extracted from such tissues in dogs with natural infections. Specificity was assessed in silico for primer pairs for CAV-1, parvovirus and herpesvirus by searching the GenBank sequence database, NCBI and, apart from the target genes, other sequences of significance were not identified.
Results
Signalment, history, clinical signs and physical examination
Sixty-eight cases were identified, 10 of which presented to the QVSH. The median age at presentation was three years seven months (range, seven months to eight years five months). There were 48 female and 20 male dogs (female:male ratio 2.6:1). Seven of 20 entire female dogs were in oestrus or had been in oestrus in the two months before presentation. Four dogs had a prior diagnosis of hypothyroidism and were on thyroid replacement medication. Two dogs had been diagnosed with osteoarthritis and were currently being treated with non-steroidal anti-inflammatory drugs (NSAIDs). One dog had been diagnosed with bacterial pyoderma and was currently being treated with cephalexin. Two dogs had received oral corticosteroids within the three months before presentation and one dog had received a topical corticosteroid preparation. Clinical signs at presentation included lethargy (n=63), decreased appetite (n=58), vomiting (n=34), weight loss (n=31), diarrhoea (n=21) and polydipsia (n=21). Five dogs were asymptomatic at presentation and were investigated only for increases in serum liver enzymes. Abnormalities on physical examination included icterus (n=37), hyperthermia (n=24), poor body condition (n=23), ascites (n=17) and abdominal pain (n=9).
Clinicopathologic findings
All cases had serum biochemistry and a CBC performed and a concurrent smear examination was performed in 57 cases. The clinicopathologic findings in all cases are given in Table 2. ALT and ALP were elevated in all dogs with median values of 690 and 821 iu/l, respectively. AST and GGT were elevated in 75 and 63 per cent of cases with median values of 361 and 26 iu/l, respectively. Bilirubin was increased in 69 per cent of cases with a median value of 77µmol/l. Albumin and urea, were reduced in 38 and 22 per cent of cases, respectively. Resting bile acids were increased in 76 per cent of cases with a median value of 87 µmol/l. Prothrombin time and partial thromboplastin time were measured in 49 cases and were increased in 24 and 21 per cent of cases, respectively. The median white blood cell count was 16.1 × 109/l and 77 per cent of cases had an elevated white blood cell count.
Table 2.
Results of clinical pathology
| Parameter (units) | Median value | Range of values | Reference interval | Percentage of cases with a value above the reference interval | Percentage of cases with a value below the reference interval |
|---|---|---|---|---|---|
| Alkaline phosphatase (iu/l) | 821 | 85-4902 | 26-107 | 100 | 0 |
| Alanine aminotransferase (iu/l) | 690 | 127-2018 | 14-67 | 100 | 0 |
| Aspartate aminotransferase (iu/l) | 361 | 10-1043 | 12-49 | 75 | 3 |
| γ-Glutamyl aminotransferase (iu/l) | 26 | 0-129 | 0-10 | 63 | N/A |
| Total bilirubin (µmol/l) | 77 | 9-203 | 0-12 | 69 | N/A |
| Albumin (g/l) | 22 | 18-45 | 25-41 | 7 | 38 |
| Globulin (g/l) | 38 | 21-53 | 24-47 | 12 | 7 |
| Cholesterol (mmol/l) | 4.4 | 1.2-12.1 | 3.3-6.5 | 35 | 22 |
| Urea (mmol/l) | 6.2 | 1.8-10.2 | 2.5-7.4 | 6 | 26 |
| Resting bile acids (µmol/l) | 87 | 8-310 | 0-10 | 76 | N/A |
| Prothrombin time (seconds) | 12.2 | 7.1-62.8 | 7.6-11.6 | 24 | 2 |
| Partial thromboplastin time (seconds) | 31.0 | 15.0-96.5 | 12.5-25.5 | 21 | 0 |
| Haematocrit (l/l) | 0.41 | 0.27-0.59 | 0.37-0.55 | 12 | 12 |
| White blood cell count (×109/l) | 16.1 | 5.5-36.4 | 6.0-17.0 | 77 | 2 |
| Neutrophil count (×109/l) | 12.1 | 6.3-33.9 | 3.0-11.5 | 74 | 0 |
N/A Not applicable
Diagnostic imaging
Thirty-three dogs had lateral or ventrodorsal abdominal radiographs taken and of those 27 had reduced liver size and 16 had splenomegaly. Sixteen dogs had both a reduced liver size and splenomegaly. Ultrasonography was performed in all dogs. The liver appeared small in 49 dogs and normal in size in the remainder. Changes in hepatic parenchymal echogenicity were present in 62 dogs and included hypoechogenicity (n=12), hyperechogenicity (n=10) or a combination of hypo- and hyperechogenicity (n=40) when compared with the echogenicity of the spleen. Six dogs had a normal appearance to the liver on ultrasound. An abdominal effusion was present in 27 dogs.
Histopathology
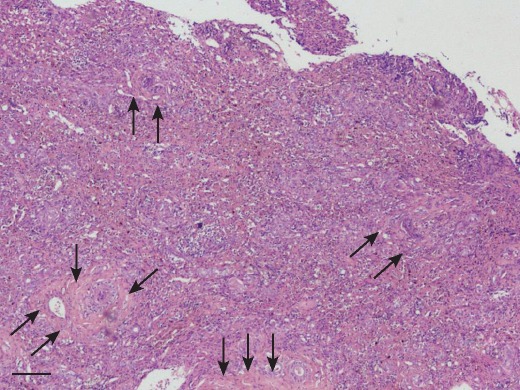
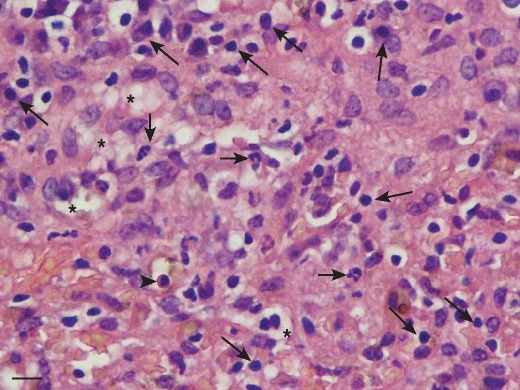
CH was diagnosed in all dogs and the predominant inflammatory cell type was the lymphocyte, with all dogs having lesser number of plasma cells (Fig 1 and Fig 2). Forty-five dogs also had a neutrophilic inflammatory cell infiltrate, but in number, neutrophils were less than the lymphocytes. The inflammatory cell infiltrate was present both in the portal area and throughout the hepatic parenchyma. Hepatocyte necrosis and apoptosis predominantly located in areas of inflammatory cell infiltrate were prominent in all dogs. Hepatocyte vacuolation was present in 49 dogs. All dogs had evidence of increased fibrous connective tissue on reticulin staining and 38 dogs had bridging fibrosis and of these 22 had cirrhosis. Cases with bridging fibrosis had combinations of portal-portal (n=30), portal-central (n=24) and central-central (n=18). Rhodanine staining for copper was positive in 21 dogs and this was scored as one in 19 dogs and two in two dogs. The distribution of copper was multifocal and excess copper was located primarily in the periportal region in all dogs.
Fig 1.
Low-power photomicrograph of liver tissue demonstrating disruption to the normal architecture by a marked and generalised infiltrate of inflammatory cells and also bundles of fibrous tissue (arrows). Haematoxylin and eosin. Original magnification ×40
Fig 2.
High-power photomicrograph of liver tissue demonstrating the presence of mononuclear cells including lymphocytes and plasma cells (long arrows), an occasional neutrophil (short arrows) and apoptotic hepatocyte (arrow head). Hepatocyte vacuolation is also present (asterisks). Haematoxylin and eosin. Original magnification ×400
PCR for aetiological agents
The sensitivity of each PCR in genome copies per reaction was 1 × 101 (adenovirus-1), 1 × 101 (parvovirus) and 1 × 102 (herpesvirus). PCR amplification of DNA extracted from formalin-fixed, paraffin-embedded liver tissue was positive for CAV-1, parvovirus and Leptospira species.
Liver tissue used for DNA extraction consisted of fresh frozen (n=15), stored in a commercially available nucleic acid preservative (RNAlater; Invitrogen) (n=48), formalin-fixed (n=3) or formalin-fixed, paraffin-embedded (n=2). After DNA extraction and PCR amplification, all liver tissues were positive for the canine cardiac actin gene. All positive controls for each PCR reaction were positive, while the negative controls were negative. PCR for the identification of CAV-1, parvovirus, herpesvirus and pathogenic Leptospira species was negative in all cases.
Treatment
Dogs were treated with a variety of medications according to clinician preference including ursodeoxycholic acid, prednisolone, antibiotics, S-adenosylmethionine, H2 receptor antagonists, antiemetics, lactulose, spironolactone and furosemide.
Outcome
The median survival time of all dogs was 189 days (range, 1 to 1211 days). The median survival time of symptomatic dogs was 171 days (range, 1 to 1211 days) and that of asymptomatic dogs was 203 days (median 66 to 413 days). Thirty-eight dogs died within three months and 12 dogs survived more than a year following diagnosis. Fifteen dogs are alive to date. Of the dogs that died, all died or were euthanased due to liver-related complications. There was no clear association between treatments and survival time.
Discussion
This study shows that CH occurs in young to middle-aged ESSs and there is a female predisposition. A female predisposition to CH has also been reported in the dobermann (Crawford and others 1985) and labrador retriever (Andersson and Sevelius 1991). We do not know the gender distribution of the ESS population in the UK however, and it is possible that a female overrepresentation may exist. The majority of ESSs presented with non-specific clinical signs, typical of canine CH in other breeds (Sevelius 1995, Poldervaart and others 2009). Five dogs were asymptomatic, and were investigated for an increase in liver enzymes found on routine blood testing for other reasons.
Clinicopathologic findings were consistent with hepatic disease and in all dogs included an increase in one or more of the serum enzymes ALT, AST, ALP or GGT. Total bilirubin was increased in nearly 70 per cent of dogs concomitant with the physical examination finding of jaundice and appears to be a particular feature of CH in the ESSs. The percentage of cases showing elevation in total bilirubin is higher than that reported in the labrador retriever (Shih and others 2007), dobermann (Johnson and others 1982) and a variety of other breeds with CH (Sevelius 1995). Non-specific indicators of a reduction in hepatic function present in some dogs included hypoalbuminaemia and decreased urea. The most common ultrasonographic abnormality was a change in hepatic echotexture to a combination of hypo- and hyperechogenicity, followed by a reduction in hepatic size. The finding of normal hepatic echotexture on ultrasound in some dogs despite a histological diagnosis of CH highlights the insensitivity of this test for the detection of changes suggestive of CH. Ultrasonographic changes were not correlated with histopathological findings but are likely non-specific and compatible with several different pathological processes (Feeney and others 2008). It therefore appears that the best initial screening test for the presence of CH in the ESS is the finding of increased serum liver enzymes in a young to middle-aged dog.
Confirmation of the diagnosis of CH requires hepatic histopathology. The histological appearance is consistent with standardised criteria for CH developed by the WSAVA Liver Standardization Group (Van den Ingh and others 2006). The inflammatory cell infiltrate in ESSs was predominantly lymphocytic with lesser number of plasma cells, although some cases also had a neutrophilic component to their disease. Despite the acute presentation of clinical signs in some dogs, all dogs had evidence of hepatic fibrosis on histology. It was also noteworthy that despite the absence of clinical signs in some dogs, hepatic histopathology revealed significant liver disease in all dogs. The presence of a number of necrotic and apoptotic hepatocytes in areas of inflammation appears to be a particular feature of CH in the ESSs. Vacuolation of hepatocytes was an additional feature although this change is likely non-specific and representative of a variety of hepatic insults (Ishak 2000).
The present study failed to identify any aetiological factors for CH in the ESSs. The aetiology of CH in the majority of cases of canine CH is also unknown, although a proportion of cases are due to disorders of copper metabolism (Poldervaart and others 2009). Disorders of copper metabolism have been reported in the Bedlington, Skye and West Highland white terriers, dalmatian, dobermann and labrador retriever (Watson 2004). This diagnosis is made by documenting increased copper by quantitative analysis, the histological finding of copper accumulation in hepatocytes around the central vein and response to treatment with copper-chelating drugs (Hultgren and others 1986). There was no evidence of significant copper accumulation based on semiquantitative histological staining in the present study. When present, all but two dogs had only a few copper-laden hepatocytes and these were located primarily in the periportal region. The two remaining dogs had moderate numbers of copper-laden hepatocytes located in a similar region. This pattern of copper accumulation is likely secondary to cholestasis as seen in human cholestatic liver disease (Owen and others 1977). Quantitative copper analysis was not deemed necessary in the present study as there is good correlation between the amount of copper visualised by histological staining and quantitative measurement (Schultheiss and others 2002, Hoffmann and others 2006).
CH in the ESSs shares some of the features of human viral hepatitis including a predominantly lymphocytic inflammation, necrosis and apoptosis in areas of inflammation and mild to moderate hepatocyte vacuolation (Ishak 2000), suggesting the possibility of the involvement of a previously undocumented viral agent. Although studies using a candidate virus approach to identify canine hepatitis viruses have been unsuccessful (Rakich and others 1986, Chouinard and others 1998, Boomkens and others 2005), a transmissible agent capable of causing acute CH has been described previously in dogs in the UK (Jarrett and O'Neil 1985, Jarrett and others 1987). No further work to identify the transmissible hepatitis-causing agent was performed (W. F. Jarrett, personal communication). The investigation for potential infectious aetiological agents in the present cases consisted of PCR for known hepatotrophic viruses and pathogenic Leptospira species. PCR was negative in all dogs, which is in agreement with previous studies of CH in a variety of dog breeds (Boomkens and others 2005). Although Helicobacter species have been proposed to cause CH in some dogs (Fox and others 1996), testing for the presence of Helicobacter species was not performed. Helicobacter organisms were not visible on histopathological sections viewed under light microscopy, although this can be insensitive for diagnosis. More specific histological stains for Helicobacter organisms, such as Warthin-Starry, were not used.
Primary immune-mediated mechanisms have also been suggested to cause canine CH (Andersson and Sevelius 1992, Weiss and others 1995, Poitout and others 1997, Dyggve and others 2011) but have never been conclusively demonstrated. The ESS is predisposed to the development of other autoimmune diseases, such as immune-mediated haemolytic anaemia (Reimer and others 1999). The presence of concurrent hypothyroidism at the time of diagnosis in four dogs raises the possibility of an autoimmune aetiology to CH in this breed, although this may be a coincidental finding. The occurrence of oestrus or recent oestrus at the time of diagnosis in seven dogs also suggests that immunological dysregulation predisposing to the development of disease or worsening current disease may be present. In human beings, the worsening of autoimmune liver disease occurs in pregnancy due to changes in the concentration of oestrogen and progesterone (Candia and others 2005), although studies to document the effects of these hormones on canine CH have not been performed. Histological features such as a predominance of foci of plasma cells and multinucleated hepatocytes characteristic of human autoimmune hepatitis (Ishak 2000), are however, not typical features of CH in the ESS.
The potential for a toxic aetiology to CH in the ESS exists. Three dogs were receiving NSAIDs at the time of diagnosis and these drugs have been associated with the development of a hepatopathy in a number of dog breeds particularly the labrador retriever (MacPhail and others 1998). It is unknown whether the ESS is similarly affected. No dog was being treated with other known hepatotoxic drugs, although environmental or dietary exposure to potentially hepatotoxic substances could not be ruled out.
Survival time ranged from 1 to 1211 days, with a median survival of 189 days. This compares to a median survival time of 374 days (range 1 to 2645 days) in labrador retrievers with CH (Shih and others 2007). Previous studies examining survival in different breeds of dogs with CH report a mean survival time ranging from 4.1 months (Poldervaart and others 2009) to 19 months (Strombeck and others 1988). Interestingly, the survival time of the asymptomatic dogs in this study was similar to that of the symptomatic dogs. No standardised treatment regimen was used in the present study, so it was not possible to assess the effects of treatment on survival. In a separate study, however, the authors have recently demonstrated that treatment with corticosteroids has no effect on survival in ESSs with CH (Shawcroft and others 2010). This is contrary to a previous study that reported improved survival in a variety of dog breeds treated with corticosteroids (Strombeck and others 1988) and might suggest that the aetiology of CH in the ESS is not predominantly autoimmune, but more studies are necessary to confirm this.
In conclusion, this study documents a breed-related CH that occurs in ESSs. The disease should be considered in young to middle-aged ESSs, which present with increases in liver enzymes, and some dogs may be asymptomatic. The diagnosis is confirmed by hepatic histopathology in which widespread predominantly lymphocytic inflammatory cell infiltrate is seen. Varying degrees of fibrosis and other criteria for a diagnosis of CH are also present. The aetiology is unknown but the disease does not appear to be related to currently known canine hepatotrophic viruses, pathogenic Leptospira species or copper accumulation. No specific treatments exist and the disease carries a relatively poor prognosis.
Acknowledgments
The authors thank the owners and veterinary surgeons who contributed data to this study. The authors are also grateful to histopathology laboratories for providing tissues for analysis. NHB gratefully acknowledges the Wellcome Trust for supporting his Fellowship.
Footnotes
Provenance not commissioned; externally peer reviewed
References
- Andersson M., Sevelius E. (1991) Breed, sex and age distribution in dogs with chronic liver-disease – a demographic-study. Journal of Small Animal Practice 32, 1–5 [Google Scholar]
- Andersson M., Sevelius E. (1992) Circulating autoantibodies in dogs with chronic liver disease. Journal of Small Animal Practice 33, 389–394 [Google Scholar]
- Bishop L., Strandberg J. D., Adams R. J., Brownstein D. G., Patterson R. (1979) Chronic active hepatitis in dogs associated with leptospires. American Journal of Veterinary Research 40, 839–844 [PubMed] [Google Scholar]
- Boomkens S. Y., Slump E., Egberink H. F., Rothuizen J., Penning L. C. (2005) PCR screening for candidate etiological agents of canine hepatitis. Veterinary Microbiology 108, 49–55 [DOI] [PubMed] [Google Scholar]
- Brouillette J. A., Andrew J. R., Venta P. J. (2000) Estimate of nucleotide diversity in dogs with a pool-and-sequence method. Mammalian Genome 11, 1079–1086 [DOI] [PubMed] [Google Scholar]
- Bunch S. E. (1993) Hepatotoxicity associated with pharmacologic agents in dogs and cats. Veterinary Clinics of North America: Small Animal Practice 23, 659–670 [DOI] [PubMed] [Google Scholar]
- Buxton R., Watson P. J., Vicek T., Scase T. J., Raffan E., Haugland S., Bailey S., Morrison L., Else R., Bexfield N. H. (2009) Breed, age and sex distribution in canine chronic hepatitis. In Proceedings of the British Small Animal Veterinary Association Congress. Birmingham, April 2 to 5, 2009 p 410 [Google Scholar]
- Candia L., Marquez J., Espinoza L. R. (2005) Autoimmune hepatitis and pregnancy: a rheumatologist's dilemma. Seminars in Arthritis and Rheumatism 35, 49–56 [DOI] [PubMed] [Google Scholar]
- Chouinard L., Martineau D., Forget C., Girard C. (1998) Use of polymerase chain reaction and immunohistochemistry for detection of canine adenovirus type 1 in formalin-fixed, paraffin-embedded liver of dogs with chronic hepatitis or cirrhosis. Journal of Veterinary Diagnostic Investigation 10, 320–325 [DOI] [PubMed] [Google Scholar]
- Crawford M. A., Schall W. D., Jensen R. K., Tasker J. B. (1985) Chronic active hepatitis in 26 Doberman pinschers. Journal of the American Veterinary Medical Association 187, 1343–1350 [PubMed] [Google Scholar]
- Dyggve H., Kennedy L. J., Meri S., Spillmann T., Lohi H., Speeti M. (2011) Association of Doberman hepatitis to canine major histocompatibility complex II. Tissue Antigens 77, 30–35 [DOI] [PubMed] [Google Scholar]
- Fearnley C., Wakeley P. R., Gallego-Beltran J., Dalley C., Williamson S., Gaudie C., Woodward M. J. (2008) The development of a real-time PCR to detect pathogenic Leptospira species in kidney tissue. Research in Veterinary Science 85, 8–16 [DOI] [PubMed] [Google Scholar]
- Feeney D. A., Anderson K. L., Ziegler L. E., Jessen C. R., Daubs B. M., Hardy R. M. (2008) Statistical relevance of ultrasonographic criteria in the assessment of diffuse liver disease in dogs and cats. American Journal of Veterinary Research 69, 212–221 [DOI] [PubMed] [Google Scholar]
- Fox J. G., Drolet R., Higgins R., Messier S., Yan L., Coleman B. E., Paster B. J., Dewhirst F. E. (1996) Helicobacter canis isolated from a dog liver with multifocal necrotizing hepatitis. Journal of Clinical Microbiology 34, 2479–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann G., Van Den Ingh T. S., Bode P., Rothuizen J. (2006) Copper-associated chronic hepatitis in labrador retrievers. Journal of Veterinary Internal Medicine 20, 856–861 [DOI] [PubMed] [Google Scholar]
- Hultgren B. D., Stevens J. B., Hardy R. M. (1986) Inherited, chronic, progressive hepatic degeneration in Bedlington terriers with increased liver copper concentrations: clinical and pathologic observations and comparison with other copper-associated liver diseases. American Journal of Veterinary Research 47, 365–377 [PubMed] [Google Scholar]
- Ishak K. G. (2000) Pathologic features of chronic hepatitis. A review and update. American Journal of Clinical Pathology 113, 40–55 [DOI] [PubMed] [Google Scholar]
- Jarrett W. F., O'Neil B. W. (1985) A new transmissible agent causing acute hepatitis, chronic hepatitis and cirrhosis in dogs. Veterinary Record 116, 629–635 [DOI] [PubMed] [Google Scholar]
- Jarrett W. F., O'Neil B. W., Lindholm I. (1987) Persistent hepatitis and chronic fibrosis induced by canine acidophil cell hepatitis virus. Veterinary Record 120, 234–235 [DOI] [PubMed] [Google Scholar]
- Johnson G. F., Zawie D. A., Gilbertson S. R., Sternlieb I. (1982) Chronic active hepatitis in Doberman pinschers. Journal of the American Veterinary Medical Association 180, 1438–1442 [PubMed] [Google Scholar]
- MacPhail C. M., Lappin M. R., Meyer D. J., Smith S. G., Webster C. R., Armstrong P. J. (1998) Hepatocellular toxicosis associated with administration of carprofen in 21 dogs. Journal of the American Veterinary Medical Association 212, 1895–1901 [PubMed] [Google Scholar]
- Owen C. A. Jr, Dickson E. R., Goldstein N. P., Baggenstoss A. H., McCall J. T. (1977) Hepatic subcellular distribution of copper in primary biliary cirrhosis. Comparison with other hyperhepatocupric states and review of the literature. Mayo Clinic Proceedings 52, 73–80 [PubMed] [Google Scholar]
- Poitout F., Weiss D. J., Armstrong P. J. (1997) Cell-mediated immune responses to liver membrane protein in canine chronic hepatitis. Veterinary Immunology and Immunopathology 57, 169–178 [DOI] [PubMed] [Google Scholar]
- Poldervaart J. H., Favier R. P., Penning L. C., Van Den Ingh T. S., Rothuizen J. (2009) Primary hepatitis in dogs: a retrospective review (2002-2006). Journal of Veterinary Internal Medicine 23, 72–80 [DOI] [PubMed] [Google Scholar]
- Rakich P. M., Prasse K. W., Lukert P. D., Cornelius L. M. (1986) Immunohistochemical detection of canine adenovirus in paraffin sections of liver. Veterinary Pathology 23, 478–484 [DOI] [PubMed] [Google Scholar]
- Reimer M. E., Troy G. C., Warnick L. D. (1999) Immune-mediated hemolytic anemia: 70 cases (1988-1996). Journal of the American Animal Hospital Association 35, 384–391 [DOI] [PubMed] [Google Scholar]
- Schultheiss P. C., Bedwell C. L., Hamar D. W., Fettman M. J. (2002) Canine liver iron, copper and zinc concentrations and association with histologic lesions. Journal of Veterinary Diagnostic Investigation 14, 396–402 [DOI] [PubMed] [Google Scholar]
- Schulze C., Baumgärtner W. (1998) Nested polymerase chain reaction and in situ hybridization for diagnosis of canine herpesvirus infection in puppies. Veterinary Pathology 35, 209–217 [DOI] [PubMed] [Google Scholar]
- Sevelius E. (1995) Diagnosis and prognosis of chronic hepatitis and cirrhosis in dogs. Journal of Small Animal Practice 36, 521–528 [DOI] [PubMed] [Google Scholar]
- Sevelius E., Andersson M., Jönsson L. (1994) Hepatic accumulation of α1-antitrypsin in chronic liver disease in the dog. Journal of Comparative Pathology 111, 401–412 [DOI] [PubMed] [Google Scholar]
- Shawcroft A., Watson P. J., Bexfield N. H., Collings A. J. (2010) Effect of prednisolone treatment on survival of dogs with chronic hepatitis. In Proceedings of the British Small Animal Veterinary Association Congress. Birmingham, April 8 to 11, 2010 p 458 [Google Scholar]
- Shih J. L., Keating J. H., Freeman L. M., Webster C. R. (2007) Chronic hepatitis in labrador retrievers: clinical presentation and prognostic factors. Journal of Veterinary Internal Medicine 21, 33–39 [DOI] [PubMed] [Google Scholar]
- Strombeck D. R., Miller L. M., Harrold D. (1988) Effects of corticosteroid treatment on survival time in dogs with chronic hepatitis: 151 cases (1977-1985). Journal of the American Veterinary Medical Association 193, 1109–1113 [PubMed] [Google Scholar]
- Van Den Ingh T., van Winkle T., Cullen J., Charles J., Desmet V. (2006) Morphological classification of parenchymal disorders of the canine and feline liver: 2 Hepatocellular death, hepatitis and cirrhosis. In Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Disease. 1st edn Ed WSAVA. Saunders Elsevier; pp 85–102 [Google Scholar]
- Watson P. J. (2004) Chronic hepatitis in dogs: a review of current understanding of the aetiology, progression and treatment. Veterinary Journal 167, 228–241 [DOI] [PubMed] [Google Scholar]
- Weiss D. J., Armstrong P. J., Mruthyunjaya A. (1995) Anti-liver membrane protein antibodies in dogs with chronic hepatitis. Journal of Veterinary Internal Medicine 9, 267–271 [DOI] [PubMed] [Google Scholar]