Abstract
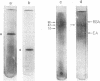
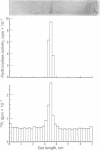
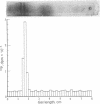
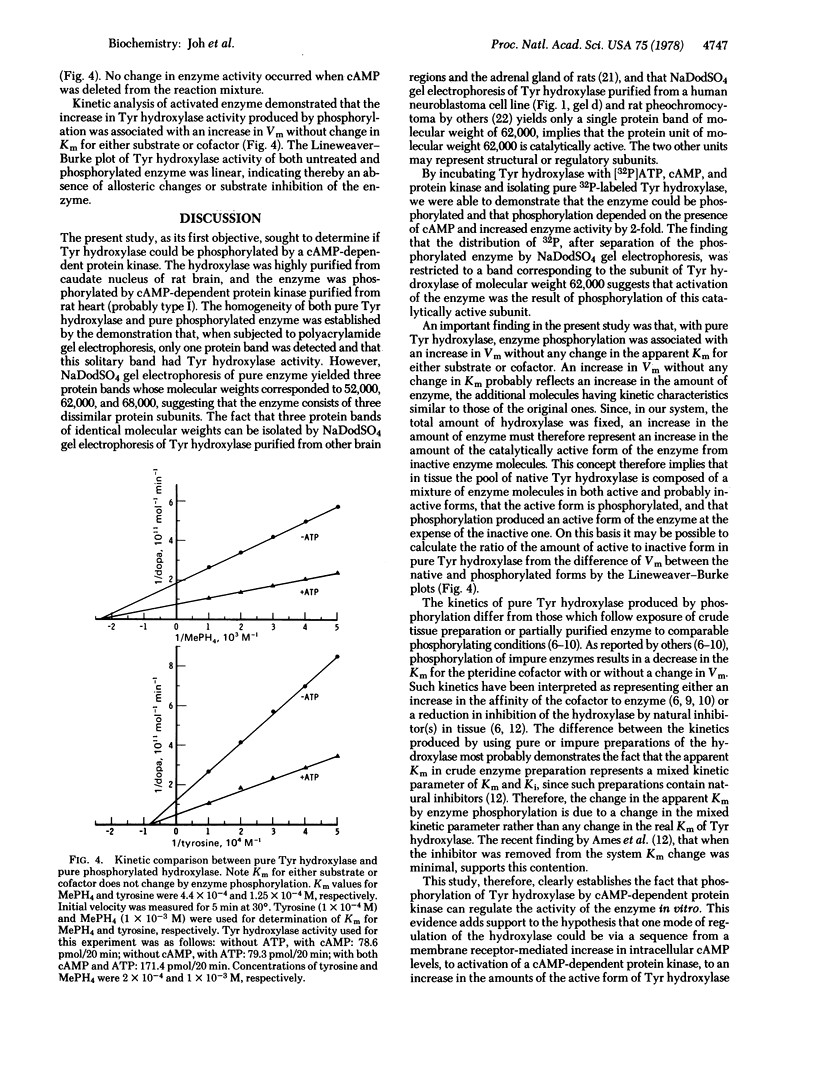
Tyrosine hydroxylase [tyrosine monooxygenase, L-tyrosine, tetrahydropteridine:oxygen oxidoreductase (3-hydroxylating), EC 1.14.16.2] was highly purified from rat caudate nuclei. When the pure hydroxylase was phosphorylated by incubation with cyclic AMP-dependent protein kinase and [32P]ATP, 32P and tyrosine hydroxylase activity were detected after polyacrylamide gel electrophoresis in a single protein band. After sodium dodecyl sulfate gel electrophoresis, 32P was detected only in a probably active subunit of tyrosine hydroxylase of molecular weight 62,000. Phosphorylation of the hydroxylase increased its activity by 2-fold, and was associated with an increase in Vm without any change in Km for either substrate or cofactor. We propose that the pool of native tyrosine hydroxylase is composed of a mixture of enzyme molecules in both active and probably inactive forms, that the active form is phosphorylated, and that phosphorylation produces an active form of the enzyme at the expense of an inactive one.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames M. M., Lerner P., Lovenberg W. Tyrosine hydroxylase. Activation by protein phosphorylation and end product inhibition. J Biol Chem. 1978 Jan 10;253(1):27–31. [PubMed] [Google Scholar]
- Coyle J. T. Tyrosine hydroxylase in rat brain--cofactor requirements, regional and subcellular distribution. Biochem Pharmacol. 1972 Jul 15;21(14):1935–1944. doi: 10.1016/0006-2952(72)90006-8. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Harris J. E., Morgenroth V. H., 3rd, Roth R. H., Baldessarini R. J. Regulation of catecholamine synthesis in the rat brain in vitro by cyclic AMP. Nature. 1974 Nov 8;252(5479):156–158. doi: 10.1038/252156a0. [DOI] [PubMed] [Google Scholar]
- Hoeldtke R., Kaufman S. Bovine adrenal tyrosine hydroxylase: purification and properties. J Biol Chem. 1977 May 25;252(10):3160–3169. [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVITT M., SPECTOR S., SJOERDSMA A., UDENFRIEND S. ELUCIDATION OF THE RATE-LIMITING STEP IN NOREPINEPHRINE BIOSYNTHESIS IN THE PERFUSED GUINEA-PIG HEART. J Pharmacol Exp Ther. 1965 Apr;148:1–8. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewander T., Joh T. H., Reis D. J. Tyrosine hydroxylase: delayed activation in central noradrenergic neurons and induction in adrenal medulla elicited by stimulation of central cholinergic receptors. J Pharmacol Exp Ther. 1977 Mar;200(3):523–534. [PubMed] [Google Scholar]
- Lloyd T., Kaufman S. Evidence for the lack of direct phosphorylation of bovine caudate tyrosine hydroxylase following activation by exposure to enzymatic phosphorylating conditions. Biochem Biophys Res Commun. 1975 Oct 6;66(3):907–917. doi: 10.1016/0006-291x(75)90726-3. [DOI] [PubMed] [Google Scholar]
- Lovenberg W., Bruckwick E. A., Hanbauer I. ATP, cyclic AMP, and magnesium increase the affinity of rat striatal tyrosine hydroxylase for its cofactor. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2955–2958. doi: 10.1073/pnas.72.8.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenroth V. H., 3rd, Hegstrand L. R., Roth R. H., Greengard P. Evidence for involvement of protein kinase in the activation by adenosine 3':5'-monophosphate of brain tyrosine 3-monooxygenase. J Biol Chem. 1975 Mar 10;250(5):1946–1948. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Reis D. J., Joh T. H., Ross R. A. Effects of reserpine on activities and amounts of tyrosine hydroxylase and dopamine-beta-hydroxylase in catecholamine neuronal systems in rat brain. J Pharmacol Exp Ther. 1975 Jun;193(3):775–784. [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Cyclic AMP-dependent protein kinase from bovine heart muscle. Methods Enzymol. 1974;38:308–315. doi: 10.1016/0076-6879(74)38047-0. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]