Abstract
Microglia constitute the main immune defense in the central nervous system. In response to neuronal injury, microglia become activated, acquire phagocytic properties, and release a wide range of pro-inflammatory mediators that are essential for the annihilation of the neuronal insult. Although the role of microglial activation in acute neuronal damage is well defined, the pathophysiological processes underlying destructive or protective role to neurons following chronic exposure to microglial activation is still a subject of debate. It is likely that chronic exposure induces detrimental effects by promoting neuronal death through the release of neurotoxic factors. Positron emission tomography (PET) imaging with the use of translocator protein (TSPO) radioligands provides an in vivo tool for tracking the progression and severity of neuroinflammation in neurodegenerative disease. TSPO expression is correlated to the extent of microglial activation and the measurement of TSPO uptake in vivo with PET is a useful indicator of active disease. Although understanding of the interaction between radioligands and TSPO is not completely clear, there is a wide interest in application of TSPO imaging in neurodegenerative disease. In this article, we aim to review the applications of in vivo microglia imaging in neurodegenerative disorders such as Parkinson’s disease, Huntington’s disease, Dementias, and Multiple Sclerosis.
Keywords: dementia, Huntington, microglia, multiple sclerosis, Parkinson, PET, PK11195
Introduction
Microglia account for approximately 10% of the adult brain cell population and represent the first and main form of immune defense in the central nervous system (CNS; Lawson et al., 1990; Kreutzberg, 1996). Upon CNS injury and disease, microglia become activated and they can be identified and distinguished from their resting phenotype based on a combination of morphological and immunophenotypic changes (Dheen et al., 2007; Ransohoff and Perry, 2009). Microglia initiate immune responses by enhancing the expression of toll-like receptors (TLR) and a wide range of pro-inflammatory mediators such as tumor necrosis factor-alpha (TNFα), interleukin (IL)-1 and IL-6 for the removal of the CNS threat (Suzumura et al., 1996; Hartlage-Rübsamen et al., 1999; Bsibsi et al., 2002; Floden et al., 2005). Microglia may also fulfill a neuroprotective role via the release of neurotrophic factors and promotion of neurogenesis for the restoration of normal physiology (Stadelmann et al., 2002). Hence, the acute inflammatory response is generally beneficial, as it tends to minimize injury and promotes tissue repair. However, chronic neuroinflammation is closely related to various neurodegenerative disorders such as Parkinson’s disease (PD), Huntington’s disease (HD), Dementias, and Multiple sclerosis (MS), although the consequences of sustained microglial activation in these diseases is unclear.
Activated microglia upregulate expression of the 18-kDa translocator protein (TSPO; Chen and Guilarte, 2008; Cosenza-Nashat et al., 2009; Scarf et al., 2009). TSPO are found in abundance throughout the body in peripheral organs (i.e., liver and adrenals), and hematogenous cells, but are present at very low levels in the normal healthy CNS (Banati, 2002). Functionally, TSPO has several biological functions including the control of cholesterol transport and neurosteroid synthesis (Papadopoulos et al., 2006), and may also be involved in the release of pro-inflammatory cytokines during inflammation (Choi et al., 2002; Wilms et al., 2003).
Enhanced TSPO expression can be detected in vivo by using positron emission tomography (PET) imaging with the selective TSPO radioligand 11C-PK11195 (Benavides et al., 1988; Pike et al., 1993; Banati et al., 1999), with evidence that increases in 11C-PK11195 binding potential (BPND) correspond to activation of microglia (Stephenson et al., 1995; Conway et al., 1998; Banati et al., 2000). Although TSPO is also expressed by reactive astrocytes, a 11C-PK11195 PET study of patients with hippocampal sclerosis, a condition histopathologically characterized by marked astrogliosis, did not yield results that were significantly different to healthy normal controls (Banati et al., 1999). This is consistent with the view that reactive astrocytes, in vivo, do not significantly contribute to the 11C-PK11195 signal. Therefore, in the absence of invading blood borne cells or severe focal leakage of blood-brain barrier, the increased PK11195 binding is likely to indicate the transition of microglia from a resting to an activated state, and is due to an increase in the number, rather than the affinity, of TSPO (Banati et al., 2000). Hence, the measurement of TSPO uptake using PET provides an in vivo tool to monitor progression and severity of neuroinflammation and is a useful indicator of active CNS disease. This article aims to review the use of PET imaging to promote the understanding of activated microglia in neurodegenerative disease.
Parkinson’s Disease and Related Disorders
Parkinson’s disease is the second most common neurodegenerative disorder of the elderly and is associated with the motor symptoms of tremor, bradykinesia, and rigidity. It is characterized by the extended loss of dopaminergic neurons in the substantia nigra pars compacta, resulting in a deficiency of dopamine in the striatum (Braak et al., 2006), and the presence of alpha-synuclein (α-synuclein)-containing lewy bodies. PD is the most common of a group of parkinsonian movement disorders that also includes Multiple system atrophy (MSA), Corticobasal degeneration (CBD), and Progressive supranuclear palsy (PSP).
The presence of activated microglia close to dopaminergic neurons in post-mortem PD patient brains (McGeer et al., 1988a; Mogi et al., 1994; Langston et al., 1999; Imamura et al., 2003), and PD animal models (Czlonkowska et al., 1996; Kim et al., 2009) suggests a close relationship between neurodegeneration and neuroinflammation in PD. Numerous investigations have proposed a deleterious role of microglial activation in PD based on the vulnerability of dopaminergic neurons to various microglia-derived pro-inflammatory cytokines (Ferrari et al., 2006; Stone et al., 2009; De Lella Ezcurra et al., 2010), while α-synuclein can directly induce activation of microglia (Zhang et al., 2005). However, it seems that the plasticity of microglia must be considered with regards to their contribution in PD, and their role; whether beneficial or detrimental, it may depend on the stimuli present and the stage of disease (Li et al., 2007; Michelucci et al., 2009; Sanchez-Guajardo et al., 2010).
Further clues regarding the role of activated microglia has also come from in vivo PET imaging studies (Table 1). Significant microglial activation, as reflected by an increase in 11C-PK11195 BPND was reported in the midbrain and putamen of PD patients when compared to controls, and was found to correlate positively with the motor severity of Parkinsonism (Ouchi et al., 2005; Bartels et al., 2010). These findings suggest that activated microglia has a pathogenic importance in the disease and indicate that the early introduction of a neuroprotective drug to suppress microglial activation could be favorable in PD. Additionally, PD patients exhibited significantly increased 11C-PK11195 BPND in the basal ganglia, pons, and frontal and temporal cortical regions (Gerhard et al., 2006a). In this study, the increased microglial activation remained unchanged for 2 years, while the patients deteriorated clinically during this period. Hence, it is likely that microglia are activated early in PD, where they remain activated for longer periods and possibly drive progression of the disease (Gerhard et al., 2006a).
Table 1.
Positron emission tomography imaging studies assessing microglia in Parkinsonian disorders.
| Study | Disorder | Subjects | PET technique | Main findings |
|---|---|---|---|---|
| Ouchi et al. (2005) | PD | 10 Early PD patients, 10 NC | 11C-PK11195 | 11C-PK11195 BPND in patients significantly higher than controls Midbrain 11C-PK11195 BPND values correlated positively with motor disability |
| Gerhard et al. (2006a) | PD | 18 PD patients, 11 NC | 11C-PK11195 | Significantly increased 11C-PK11195 BPND in pons, basal ganglia, and frontal and temporal cortical regions |
| 11C-PK11195 signal remained stable for 2 years in subset of patients | ||||
| Bartels et al. (2010) | PD | 14 PD patients, 8 NC | 11C-PK11195 | Higher contralateral putamen and midbrain 11C-PK11195 BPND in patients than controls |
| Gerhard et al. (2003) | MSA | 5 MSA patients, 6 NC | 11C-PK11195 | MSA patients showed significantly increased 11C-PK11195 BPND in regions reflecting the known distribution of pathologic changes in MSA |
| Gerhard et al. (2006b) | PSP | 4 PSP patients, 7 NC | 11C-PK11195 | Significantly increased 11C-PK11195 BPND in basal ganglia, midbrain, frontal lobe, and cerebellum of patients compared to controls |
| Microglial activation remained stable as demonstrated in follow-up scans of two patients | ||||
| Gerhard et al. (2004) | CBD | 4 CBD patients, 5 NC | 11C-PK11195 | CBD patients had significantly increased 11C-PK11195 BPND in the cortical regions and basal ganglia that correspond to known distribution of pathological changes in CBD |
| Henkel et al. (2004) | CBD | 1 CBD patient | 11C-PK11195 | Marked asymmetric microglial activation in corresponding areas of basal ganglia and temporal and parietal cortices |
BPND, binding potential; CBD, corticobasal degeneration; MSA, multiple system atrophy; NC, normal control; PD, Parkinson’s disease; PSP, progressive supranuclear palsy.
Multiple system atrophy is a sporadic neurodegenerative disorder involving a progressive akinetic-rigid syndrome, autonomic failure, and cerebellar dysfunction. It is associated by the appearance of abnormal glial cytoplasmic inclusions (GCI) containing (α-synuclein aggregates and neuronal loss within the nigrostriatal and olivopontocerebellar regions (Lantos and Papp, 1994). The presence of activated microglia is also a prominent feature of MSA (Schwarz et al., 1998). In an in vivo PET study of MSA patients, significant 11C-PK11195 BPND was observed in the putamen, pallidum, pons, substantia nigra pars compacta, and dorsolateral prefrontal cortex, reflecting the known distribution of neuropathological changes in MSA (Gerhard et al., 2003). Although the role of microglia in MSA is inconclusive, microglial activation localization correlated significantly with the locations of GCIs in specific neuroanatomical systems affected in MSA (Ishizawa et al., 2004). A correlation between extent of microglial activation and dopaminergic neurodegeneration has also been reported (Stefanova et al., 2007).
Progressive supranuclear palsy is an adult-onset progressive neurodegenerative disease of unknown cause, characterized by PD-like symptoms such as postural instability and bradykinesia. The pathological hallmark of the disease is neurofibrillary tangles consisting of hyperphosphorylated tau, accompanied by neuronal loss in the thalamus, basal ganglia, and specific brainstem regions (Hauw et al., 1994). Several early studies including immunohistochemical investigations have confirmed the possible involvement of activated microglia in PSP (Kida et al., 1992; Komori et al., 1998; Ishizawa et al., 2000; Ishizawa and Dickson, 2001). 11C-PK11195 PET have also reported significant levels of activated microglia in brain regions known to be affected by the disease process such as the midbrain, cerebellum, pons, frontal lobe, and basal ganglia (Gerhard et al., 2006b). Although these results were unable to support a direct causal contribution to neurodegeneration in PSP, they are at least suggestive of a role of microglia in the disease.
Corticobasal degeneration is a neurodegenerative disorder that affects both cortical and basal ganglial regions, with considerable clinical heterogeneity between patients. Typically, CBD features an asymmetric hypokinetic-rigid syndrome, coupled with alien limb phenomenon and cortical sensory impairment that is unresponsive to dopaminergic therapy (Rebeiz et al., 1968; Gibb et al., 1989). Information on the association of activated microglia in CBD is limited, and mainly coming from immunohistochemical-based assessments (Armstrong et al., 2000; Ishizawa and Dickson, 2001). However, more recent in vivo PET investigations have attempted to quantify microglial activation in CBD patients. Increased 11C-PK11195 BPND was observed in regions such as the caudate nucleus, putamen, substantia nigra pars compacta, pons, and pre- and post central gyrus (Gerhard et al., 2004; Henkel et al., 2004) that correspond to the expected neuropathological changes seen in CBD (Ishizawa and Dickson, 2001; Dickson et al., 2002). These results indicate an involvement of activated microglia in pathogenesis of CBD.
Huntington’s Disease
Huntington’s disease is an autosomal, dominant inherited progressive neurodegenerative disorder associated with motor, cognitive, and psychiatric symptoms. It is caused by an abnormal polyglutamine-repeat expansion on the IT15 gene that codes huntingtin, and involves the progressive loss of medium spiny dopaminergic receptor-bearing striatal GABA-ergic neurons (Vonsattel and DiFiglia, 1998). Although the role of chronic neuroinflammation in the HD pathogenesis is not fully understood, post-mortem assessments have reported high levels of activated microglia close to degenerating neurons (McGeer et al., 1988b; Messmer and Reynolds, 1998; Singhrao et al., 1999; Sapp et al., 2001). Upregulated inflammatory cytokines have also been detected in the striatum and plasma, indicative of an inflammatory component in HD (Dalrymple et al., 2007; Björkqvist et al., 2008).
In vivo imaging studies using 11C-PK11195 PET have found increased microglial activation in both premanifest HD gene carriers and manifest HD patients when compared to healthy controls (Table 2; Pavese et al., 2006; Tai et al., 2007; Politis et al., 2008, 2011). In premanifest HD patients, significant increases in 11C-PK11195 BPND in the striatum and hypothalamus was reported, which correlated inversely with neuronal dysfunction as measured by 11C-Raclopride; a marker of dopaminergic D2/D3 receptor availability (Tai et al., 2007; Politis et al., 2008). Interestingly, microglial activation in the striatum, and regions related to cognitive function has been shown to predict the 5-year disease clinical onset in premanifest HD patients (Tai et al., 2007; Politis et al., 2011). These results imply that microglial activation is an early event in the HD disease course, with a possible pathogenic involvement that is associated with a subclinical progression of the disease.
Table 2.
Positron emission tomography imaging studies assessing microglia in Huntington’s disease.
| Study | Subjects | PET technique | Main findings |
|---|---|---|---|
| Pavese et al. (2006) | 11 manifest HD patients, 10 NC | 11C-PK11195 | Significantly increased 11C-PK11195 BPND in patients than controls Increased 11C-PK11195 uptake correlated positively with disease severity |
| Tai et al. (2007) | 11 premanifest HD subjects, 10 NC | 11C-PK11195 | Significantly higher striatal 11C-PK11195 BPND that correlated inversely with D2 receptor availability |
| Higher striatal uptake correlated with 5 year probability of clinical disease onset | |||
| Politis et al. (2008) | 10 premanifest HD subjects, 9 manifest HD patients, 10 NC | 11C-PK11195 | Significantly increased hypothalamic 11C-PK11195 BPND in both premanifest and manifest subjects compared to controls |
| Inverse correlation between increased hypothalamic 11C-PK11195 BPND and D2 receptor availability | |||
| Politis et al. (2011) | 8 premanifest HD subjects, 8 manifest HD patients, 16 NC | 11C-PK11195 | In premanifest subjects, increased microglial activation in cognitive regions correlated with 5 year probability of clinical disease onset. |
| In manifest HD patients, significantly increased 11C-PK11195 BPND in globus pallidus, anterior prefrontal cortex, and limbic striatum |
BPND, binding potential; HD, Huntington’s disease; NC, normal control.
In manifest HD patients, significant 11C-PK11195 BPND in the striatum, hypothalamus, and various cortical regions was found, that correlated with greater disease burden and higher motor disability (Pavese et al., 2006; Politis et al., 2008, 2011). The cortical microglial activation is likely to indicate the involvement of cortical neurons in HD, a well-recognized phenomenon as the disease progresses. Collectively, these findings are consistent with the post-mortem studies (Messmer and Reynolds, 1998; Sapp et al., 2001) and suggest a detrimental microglial contribution to the ongoing neuronal degeneration in HD.
Dementia
Dementias are a group of disorders that are expected to affect more than 100 million people by 2050 raising remarkable financial costs for healthcare (Wimo et al., 2003). AD is the most common cause of dementia and is the most common neurological disorder of the elderly. AD is characterized by the presence of amyloid plaques, neurofibrillary tangles, and activated microglia (for review, see Hardy and Selkoe, 2002). There is a plethora of evidence from post-mortem human AD studies (McGeer et al., 1988a; Venneti et al., 2009) and animal models (Frautschy et al., 1998; Stalder et al., 1999; Leung et al., 2011) reporting a high accumulation of activated microglia in close proximity with the amyloid plaques, and upregulated levels of pro-inflammatory cytokines (Akiyama et al., 2000; Eikelenboom et al., 2002).
Positron emission tomography enables a broad range of functional processes to assess the AD brain in vivo (Table 3). 11C-PK11195 has been used to demonstrate increased levels of activated microglia in both AD animal models (Venneti et al., 2009) and AD patients (Cagnin et al., 2001; Edison et al., 2008; Yokokura et al., 2011). In AD patients, significant 11C-PK11195 BPND was consistently observed in the temporal, parietal, and occipital cortices, regions known to be affected by AD pathology (Cagnin et al., 2001; Edison et al., 2008; Yokokura et al., 2011). The increased activated microglia also inversely correlated with the patient Mini-Mental State Examination (MMSE) scores, which is compatible with a role of microglia in neuronal damage (Edison et al., 2008). Interestingly, elevated levels of activated microglia were also detected in patients with amnestic mild cognitive impairment (MCI; Okello et al., 2009a), although this was not observed in another study assessing MCI patients (Wiley et al., 2009). MCI could represent an early precursor stage of AD, since it was found that MCI patients with increased amyloid load were significantly more likely to clinically convert to AD within 3 years (Okello et al., 2009b). Therefore, microglial activation could be an early event in the AD pathogenesis that begins at the MCI stage.
Table 3.
Positron emission tomography imaging studies assessing microglia in dementias.
| Study | Disorder | Subjects | PET technique | Main findings |
|---|---|---|---|---|
| Cagnin et al. (2001) | AD | 8 AD patients, 15 NC | 11C-PK11195 | AD patients showed significantly increased regional 11C-PK11195 BPND in the entorhinal, temporoparietal, and cingulate cortex |
| Edison et al. (2008) | AD | 13 AD patients, 10 NC | 11C-PK11195 | Significant increased 11C-PK11195 BPND in the cortical regions |
| Inverse correlation between increased cortical microglial activation and MMSE scores | ||||
| Yokokura et al. (2011) | AD | 11 AD patients, 10 NC | 11C-PK11195 | Significantly increased 11C-PK11195 uptake in the parietotemporal regions of patients than controls |
| Inverse correlation between dementia scores and 11C-PK11195 BPND values | ||||
| Wiley et al. (2009) | AD, MCI | 6 mild-moderate AD patients, 6 MCI patients, 5 NC | 11C-PK11195 | No significant differences in brain 11C-PK11195 BPND between subject groups |
| Okello et al. (2009a) | MCI | 14 MCI patients, 10 NC | 11C-PK11195 | 5 of 13 MCI subjects had increased cortical 11C-PK11195 BPND compared to controls |
| Cagnin et al. (2004) | FTLD | 5 FTLD patients, 8 NC | 11C-PK11195 | Significantly increased 11C-PK11195 BPND in the frontotemporal regions |
AD, Alzheimer’s disease; BPND, binding potential; FTLD, frontotemporal lobar degeneration; MCI, mild cognitive impairment; MMSE, mini-mental state examination; NC, normal controls.
Despite the evidence suggestive of a pathogenic role of activated microglia in AD, it is hypothesized that the accumulation of amyloid plaques is actually due to a failure in microglial clearance mechanisms that would normally remove the protein (Bornemann et al., 2001; DiCarlo et al., 2001; Napoli and Neumann, 2009). This indicates a beneficial, rather than detrimental role of microglia in AD. Notwithstanding the abundance of activated microglia close to senile plaques, they maybe inefficient in the clearance of amyloid, hence, resulting in aggregate formation (Bolmont et al., 2008). It has been shown that in the presence of pro-inflammatory cytokines, phagocytic functions of microglia are compromised (Koenigsknecht-Talboo and Landreth, 2005). Therefore, microglia may confer a dichotomous role in AD, where early microglial activation is possibly neuroprotective involving the removal of amyloid. However, chronic neuroinflammation may downregulate amyloid clearance mechanisms, thus, promoting aggregation and progression of disease.
Frontotemporal lobar degeneration (FTLD) which includes frontotemporal dementia is the name given to a group of pathologically, clinically, and genetically heterogeneous disorders involving focal atrophy of the frontal and temporal lobes, while unlike AD, with sparing of the parietal and occipital regions (Neary et al., 1998). Another important dissimilarity between AD and FTLD pathology is the absence of amyloid plaque formation (Paulus et al., 1993; Mirra and Hyman, 2002). Rather, the key histopathological features of FTLD, depending on subtype, includes tau deposition (including Pick bodies) and ubiquitin-positive, tau-negative inclusions (Munoz et al., 2003; Uchihara et al., 2003). In vivo PET imaging of FTLD patients detected enhanced microglial activation in the expected frontotemporal regions (Cagnin et al., 2004). In the same study, significant 11C-PK11195 BPND in the bilateral putamen is also consistent with previous neuropathological data showing the involvement of the basal ganglia in FTLD (Mirra and Hyman, 2002). These observations indicate the presence of an active microglial response that reflects progressive neuronal degeneration. Importantly, the detection of increased microglial activation in affected regions in FTLD suggests that microglial responses occur independently of amyloid deposition, and that neuronal loss alone is enough to induce activation (Cagnin et al., 2004). However, whether this applies to AD pathogenesis requires further investigation.
Multiple Sclerosis
Multiple sclerosis is a disease characterized pathologically by inflammatory demyelination and axonal transection, and is the most common cause of non-traumatic disability in young adults (Compston and Coles, 2008).
The involvement of activated microglia has long been proposed in MS (Benveniste, 1997). Post-mortem investigations have detected activated microglia in the cortical GM of MS patients (De Groot et al., 2001; Peterson et al., 2001; Petzold et al., 2002), while histopathological studies have implicated microglia in lesion pathogenesis (for review, see Lassmann, 2008). An observed correlation between neuronal loss and microglial activation was reported in animal experimental MS (Rasmussen et al., 2007). Significant levels of activated microglia was also found in MS patients, especially in the progressive forms of disease that are associated with neurodegeneration (Kutzelnigg et al., 2005; Magliozzi et al., 2010), and selective ablation of parenchymal microglia was able to prevent demyelination and axonal damage (Heppner et al., 2005).
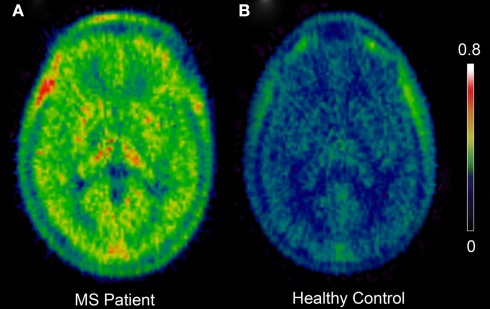
Pathological aspects of MS such as neuroinflammation, demyelination, and neurodegeneration may be explored in vivo with PET (for review, see Kiferle et al., 2011). PET with 11C-PK11195 and other tracers has demonstrated inflammatory processes with microglial involvement in MS (Figure 1; Table 4). In animal experimental MS and human post-mortem brains it has been shown that 11C-PK11195 uptake corresponds to the distribution pattern of activated microglia (Banati et al., 2000). It has also been demonstrated that there is increased 11C-PK11195 BPND in areas of focal pathology identified by T1- and T2-weighted MRI (Vowinckel et al., 1997; Banati et al., 2000) and in gadolinium-enhanced T1-weighted MRI (Debruyne et al., 2003). Increased 11C-PK11195 BPND was observed in normal-appearing gray and white anatomical structures (Banati et al., 2000; Debruyne et al., 2003; Versijpt et al., 2005). This is in line with the hypothesis that inflammatory processes initiated by microglia early in MS may constitute the real burden of disease, associated with invisible microglia-mediated damage that occur independently of relapses (Kesselring, 1990; Confavreux et al., 2000). A positive correlation has been suggested between ligand uptake and disease duration, disability, and brain atrophy (Banati et al., 2000; Debruyne et al., 2003; Versijpt et al., 2005), although the correlations were not consistently replicated across the different studies. However, recent data from our group found a significant association between high 11C-PK11195 BPND in the cortical gray matter and disability in patients with secondary progressive MS, and with higher 11C-PK11195 BPND in the secondary progressive group than the relapse-remitting MS group (Politis et al., in press). These findings are consistent with a detrimental role of microglia in MS. Enhanced microglial activation in MS has also been detected using the more recently developed TSPO tracers 11C-vinpocetine and 11C-PBR28 (Vas et al., 2008; Oh et al., 2011).
Figure 1.
Positron emission tomography images showing increased 11C-PK11195 BPND in a Multiple Sclerosis patient (A) when compared to a healthy normal control (B). Color bar represents intensity of 11C-PK11195 tracer binding (BPND). BPND, binding potential; MS, multiple sclerosis.
Table 4.
Positron emission tomography imaging studies assessing microglia in multiple sclerosis.
| Study | Subjects | PET technique | Main Findings |
|---|---|---|---|
| Vowinckel et al. (1997) | 2 MS patients | 11C-PK11195 | High 11C-PK11195 BPND in MRI-defined active MS lesions |
| Banati et al. (2000) | 12 MS patients (RR, SP, PP), 8 NC | 11C-PK11195 | Increased global and focal (in active MS lesions) 11C-PK11195 BPND in MS patients |
| Debruyne et al. (2003) | 22 MS patients (RR, SP, PP), 7 NC | 11C-PK11195 | Increased 11C-PK11195 BPND in MRI-Gadolinium lesions. Higher uptake in T2 lesions during relapse |
| Positive correlation between 11C-PK11195 BPND and disease duration | |||
| Versijpt et al. (2005) | 22 MS patients (RR, SP, PP), 8 NC | 11C-PK11195 | Significant correlation between brain atrophy and both disease duration and severity For NAWM, 11C-PK11195 BPND increased with amount of atrophy |
| T2-lesional 11C-PK11195 BPND values decreased according to increasing atrophy | |||
| Vas et al. (2008) | 4 MS patients | 11C-PK11195, 11C-vinpocetine | Regional uptake values increased in regions of brain damage for both tracers, but markedly higher for 11C-vinpocetine than 11C-PK11195 |
| Oh et al. (2011) | 11 MS patients, 7 NC | 11C-PBR28 | High 11C-PBR28 in MRI-gadolinium lesions in patients |
| Increase in tracer uptake preceded appearance of gadolinium enhancement | |||
| No difference in global 11C-PBR28 uptake between patients and healthy controls | |||
| Politis et al. (in press) | 16 MS patients (RR, SP), 8 NC | 11C-PK11195 | Significant correlation between cortical GM 11C-PK11195 BPND and disease severity Higher 11C-PK11195 in SP than RR patients |
BPND, binding potential; GM, gray matter; MRI, magnetic resonance image; MS, multiple sclerosis; NAWM, normal-appearing white matter; NC, normal control; PP, primary progressive multiple sclerosis; RR, relapse-remitting multiple sclerosis; SP, secondary progressive multiple sclerosis.
Microglial activation may contribute to the mechanism of axonal injury via the release of soluble factors that may either directly or indirectly cause neuronal dysfunction (Peterson et al., 2001; Barnett and Prineas, 2004; Dutta and Trapp, 2006; Zipp et al., 2006; Dal Bianco et al., 2008; Lassmann, 2008; Magliozzi et al., 2010), and consequently result in progressive increase in impairment and disability. However, activated microglia may also exert protective functions with MS through the release of neurotrophic factors (Stadelmann et al., 2002; Napoli and Neumann, 2009), and triggering of remyelination mechanisms (Li et al., 2005; Setzu et al., 2006). This suggests a possible dichromatic role of microglia in MS.
New TSPO Ligands
11C-PK11195 was the first tracer to be consistently used for the study of activated microglia and neuroinflammation in vivo. However, limitations associated with the application of 11C-PK11195 include a high level of non-specific binding (Petit-Taboué et al., 1991), and a poor signal to noise ratio, which complicates its quantification (Boutin et al., 2007). This has prompted the search for novel PET tracers (termed, second generation radioligands) with improved capacities to quantify TSPO expression.
Radioligands such as 11C-PBR28, 11C-DAA1106, 18F-FEDAA1106, and 18F-PBR111 have recently been developed to image TSPO in vivo (Gulyás et al., 2002; Ikoma et al., 2007; Fujita et al., 2008; Vas et al., 2008; Yasuno et al., 2008; Oh et al., 2011; for a review, see Chauveau et al., 2008). Published data using the second generation ligands 11C-DAA1106 (Ikoma et al., 2007) and 18F-FEDAA1106 (Fujimura et al., 2006) in humans were promising, with both tracers showing significantly higher cerebral uptake than 11C-PK11195. Furthermore, increased 11C-DAA1106 binding was reported in AD patients (Yasuno et al., 2008) that were similar to the previous studies that used 11C-PK11195 (Cagnin et al., 2001).
The only published study using the second generation radioligand 11C-PBR28 found areas of focal increases in radiotracer binding in the brain of MS patients (Oh et al., 2011). Interestingly, the increased focal 11C-PBR28 binding preceded the development of some gadolinium-enhancing lesions. Brain parenchymal 11C-PBR28 binding in MS patients was positively correlated with the duration of the disease, however it was not significantly higher than that of healthy volunteers. Interpretation of these results is limited by the lack of characterization of the binding affinity pattern, which might have significantly affected the comparison between subjects.
It has been recently demonstrated that there are three different affinity patterns for second generation TSPO ligands in healthy volunteers as well as patients with MS, which was evident with all the ligands tested (11C-PBR28; 11C-PBR06; 18F-PBR111; Owen et al., 2010). This presents a methodological problem, as differences in PET signal across subjects cannot be safely interpreted as differences in target density, but may reflect differences in the affinity pattern. A possible approach to solve this problem is based on the use of peripheral binding affinity, which can be characterized to classify subjects into one of the groups, as differences in affinity status between individuals have been shown to be present on peripheral cells as well (Owen et al., 2010).
Interestingly, the difference in binding patterns observed with second generation radioligands was not observed with 11C-PK11195. Also, in vitro autoradiography data using 11C-PK11195 suggest a receptor density (BMAX) significantly higher than that found using second generation ligands. It could be speculated that 11C-PK11195 and newer ligands bind to distinct sites within the TSPO molecule.
Although, data obtained from first generation studies have been promising and suggested that 11C-PK11195 could be useful to image acute inflammatory lesions and microglial activation in MS, a conclusive demonstration of the potential of TSPO imaging for the application as disease biomarker, indicative of microglial activation in MS, is still lacking. Furthermore, despite second generation ligands constituting a potential improvement relative to 11C-PK11195 at least from a methodological point of view, a clear advantage in their clinical application as disease biomarkers has not been demonstrated yet.
For these reasons, we aim to characterize a second generation TSPO PET radioligand in vivo in humans, and to evaluate its application as a disease biomarker in MS.
Among second generation TSPO tracers, 18F-PBR111 presents different advantages, as there is low difference in its affinity for TSPO between high, medium, low affinity binders. Also, it could be potentially used in clinical applications as it is labeled with fluorine-18. Promising preclinical data, and ongoing studies in neurological patients, suggest it could be a good choice amongst second generation TSPO ligands to progress into studies in MS patients.
Conclusion
Inflammation coupled with the presence of activated microglia seems to be a common feature of a wide range of CNS diseases. However, despite a large number of research studies, the exact role of microglia in chronic neurodegenerative diseases remains uncertain. In line with the high plasticity of microglia that allows them to perform numerous CNS functions, microglia are likely to play a dichromatic role in disease, depending on signals present in their microenvironment and the duration of activation. While early microglial activation could represents a beneficial response (i.e., removal of CNS threat, promoting tissue repair and removal of misfolded protein), chronic exposure could induces detrimental effects by promoting neuronal death (i.e., through the sustained release of neurotoxic factors), thus, contributing to progression of disease. PET imaging with the use of TSPO radioligands provides a valuable tool that allows us to track the progression and severity of neuroinflammation in the living brain, and is a useful indicator of active CNS disease. Therefore, the early detection of microglia using PET could offer opportunities for pharmacological interventions to limit the potential disruptive effects of chronic microglial activation. Furthermore, with the development of newer TSPO tracers, the potential for PET imaging research to promote our understanding of activated microglia in CNS disease can only increase.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Our own research is supported by the Michael J Fox Foundation for Parkinson’s Research USA, the Parkinson’s UK, and the Cure Huntington’s Disease Initiative Foundation USA.
References
- Akiyama H., Arai T., Kondo H., Tanno E., Haga C., Ikeda K. (2000). Cell mediators of inflammation in the Alzheimer disease brain. Alzheimer Dis. Assoc. Disord. 14(Suppl. 1), 47–53 [DOI] [PubMed] [Google Scholar]
- Armstrong R. A., Cairns N. J., Lantos P. L. (2000). A quantitative study of the pathological lesions in the neocortex and hippocampus of twelve patients with corticobasal degeneration. Exp. Neurol. 163, 348–356 10.1006/exnr.2000.7392 [DOI] [PubMed] [Google Scholar]
- Banati R. B., Goerres G. W., Myers R., Gunn R. N., Turkheimer F. E., Kreutzberg G. W., Brooks D. J., Jones T., Duncan J. S. (1999). [11C](R)-PK11195 positron emission tomography imaging of activated microglia in vivo in Rasmussen’s encephalitis. Neurology 53, 2199–2203 10.1212/WNL.53.9.2199 [DOI] [PubMed] [Google Scholar]
- Banati R. B., Newcombe J., Gunn R. N., Cagnin A., Turkheimer F. E., Heppner F., Price G., Wegner F., Giovannoni G., Miller D. H., Perkin G. D., Smith T., Hewson A. K., Bydder G., Kreutzberg G. W., Jones T., Cuzner M. L., Myers R. (2000). The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain 123(Pt 11), 2321–2337 10.1093/brain/123.11.2321 [DOI] [PubMed] [Google Scholar]
- Banati R. B. (2002). Visualizing microglial activation in vivo. Glia 40, 206–217 10.1002/glia.10144 [DOI] [PubMed] [Google Scholar]
- Barnett M. H., Prineas J. W. (2004). Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann. Neurol. 55, 458–468 10.1002/ana.20016 [DOI] [PubMed] [Google Scholar]
- Bartels A. L., Willemsen A. T., Doorduin J., de Vries E. F., Dierckx R. A., Leenders K. L. (2010). [11C]-PK11195 PET: quantification of neuroinflammation and a monitor of anti-inflammatory treatment in Parkinson’s disease? Parkinsonism Relat. Disord. 16, 57–59 10.1016/S1353-8020(10)70200-5 [DOI] [PubMed] [Google Scholar]
- Benavides J., Cornu P., Dennis T., Dubois A., Hauw J. J., MacKenzie E. T., Sazdovitch V., Scatton B. (1988). Imaging of human brain lesions with an omega 3 site radioligand. Ann. Neurol. 24, 708–712 10.1002/ana.410240603 [DOI] [PubMed] [Google Scholar]
- Benveniste E. N. (1997). Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J. Mol. Med. (Berl.) 75, 165–173 10.1007/s001090050101 [DOI] [PubMed] [Google Scholar]
- Björkqvist M., Wild E. J., Thiele J., Silvestroni A., Andre R., Lahiri N., Raibon E., Lee R. V., Benn C. L., Soulet D., Magnusson A., Woodman B., Landles C., Pouladi M. A., Hayden M. R., Khalili-Shirazi A., Lowdell M. W., Brundin P., Bates G. P., Leavitt B. R., Möller T., Tabrizi S. J. (2008). A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington’s disease. J. Exp. Med. 205, 1869–1877 10.1084/jem.20080178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolmont T., Haiss F., Eicke D., Radde R., Mathis C. A., Klunk W. E., Kohsaka S., Jucker M., Calhoun M. E. (2008). Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J. Neurosci. 28, 4283–4292 10.1523/JNEUROSCI.4814-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornemann K. D., Wiederhold K. H., Pauli C., Ermini F., Stalder M., Schnell L., Sommer B., Jucker M., Staufenbiel M. (2001). Abeta-induced inflammatory processes in microglia cells of APP23 transgenic mice. Am. J. Pathol. 158, 63–73 10.1016/S0002-9440(10)63945-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin H., Chauveau F., Thominiaux C., Kuhnast B., Grégoire M. C., Jan S., Trebossen R., Dollé F., Tavitian B., Mattner F., Katsifis A. (2007). In vivo imaging of brain lesions with [(11)C]CLINME, a new PET radioligand of peripheral benzodiazepine receptors. Glia 55, 1459–1468 10.1002/glia.20562 [DOI] [PubMed] [Google Scholar]
- Braak H., Rub U., Del Tredici K. (2006). Cognitive decline correlates with neuropathological stage in Parkinson’s disease. J. Neurol. Sci. 248, 255–258 10.1016/j.jns.2006.05.011 [DOI] [PubMed] [Google Scholar]
- Bsibsi M., Ravid R., Gveric D., van Noort J. M. (2002). Broad expression of Toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 61, 1013–1021 [DOI] [PubMed] [Google Scholar]
- Cagnin A., Brooks D. J., Kennedy A. M., Gunn R. N., Myers R., Turkheimer F. E., Jones T., Banati R. B. (2001). In-vivo measurement of activated microglia in dementia. Lancet 358, 461–467 10.1016/S0140-6736(01)05625-2 [DOI] [PubMed] [Google Scholar]
- Cagnin A., Rossor M., Sampson E. L., Mackinnon T., Banati R. B. (2004). In vivo detection of microglial activation in frontotemporal dementia. Ann. Neurol. 56, 894–897 10.1002/ana.20332 [DOI] [PubMed] [Google Scholar]
- Chauveau F., Boutin H., Van Camp N., Dollé F., Tavitian B. (2008). Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur. J. Nucl. Med. Mol. Imaging 35, 2304–2319 10.1007/s00259-008-0908-9 [DOI] [PubMed] [Google Scholar]
- Chen M. K., Guilarte T. R. (2008). Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol. Ther. 118, 1–17 10.1016/j.pharmthera.2007.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. B., Khoo C., Ryu J. K., van Breemen E., Kim S. U., McLarnon J. G. (2002). Inhibition of lipopolysaccharide-induced cyclooxygenase-2, tumor necrosis factor-alpha and [Ca2+]i responses in human microglia by the peripheral benzodiazepine receptor ligand PK11195. J. Neurochem. 83, 546–555 10.1046/j.1471-4159.2002.01122.x [DOI] [PubMed] [Google Scholar]
- Compston A., Coles A. (2008). Multiple sclerosis. Lancet 372, 1502–1517 10.1016/S0140-6736(08)61620-7 [DOI] [PubMed] [Google Scholar]
- Confavreux C., Vukusic S., Moreau T., Adeleine P. (2000). Relapses and progression of disability in multiple sclerosis. N. Engl. J. Med. 343, 1430–1438 10.1056/NEJM200011163432001 [DOI] [PubMed] [Google Scholar]
- Conway E. L., Gundlach A. L., Craven J. A. (1998). Temporal changes in glial fibrillary acidic protein messenger RNA and [3H]PK11195 binding in relation to imidazoline-I2-receptor and alpha 2-adrenoceptor binding in the hippocampus following transient global forebrain ischaemia in the rat. Neuroscience 82, 805–817 10.1016/S0306-4522(97)00321-7 [DOI] [PubMed] [Google Scholar]
- Cosenza-Nashat M., Zhao M. L., Suh H. S., Morgan J., Natividad R., Morgello S., Lee S. C. (2009). Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol. Appl. Neurobiol. 35, 306–328 10.1111/j.1365-2990.2008.01006.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czlonkowska A., Kohutnicka M., Kurkowska-Jastrzebska I., Czlonkowski A. (1996). Microglial reaction in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced Parkinson’s disease mice model. Neurodegeneration 5, 137–143 10.1006/neur.1996.0020 [DOI] [PubMed] [Google Scholar]
- Dal Bianco A., Bradl M., Frischer J., Kutzelnigg A., Jellinger K., Lassmann H. (2008). Multiple sclerosis and Alzheimer’s disease. Ann. Neurol. 63, 174–183 10.1002/ana.21240 [DOI] [PubMed] [Google Scholar]
- Dalrymple A., Wild E. J., Joubert R., Sathasivam K., Björkqvist M., Petersén A., Jackson G. S., Isaacs J. D., Kristiansen M., Bates G. P., Leavitt B. R., Keir G., Ward M., Tabrizi S. J. (2007). Proteomic profiling of plasma in Huntington’s disease reveals neuroinflammatory activation and biomarker candidates. J. Proteome Res. 6, 2833–2840 10.1021/pr0700753 [DOI] [PubMed] [Google Scholar]
- De Groot C. J., Bergers E., Kamphorst W., Ravid R., Polman C. H., Barkhof F., van der Valk P. (2001). Post-mortem MRI-guided sampling of multiple sclerosis brain lesions: increased yield of active demyelinating and (p) reactive lesions. Brain 124, 1635–1645 10.1093/brain/124.8.1635 [DOI] [PubMed] [Google Scholar]
- De Lella Ezcurra A. L., Chertoff M., Ferrari C., Graciarena M., Pitossi F. (2010). Chronic expression of low levels of tumor necrosis factor-alpha in the substantia nigra elicits progressive neurodegeneration, delayed motor symptoms and microglia/macrophage activation. Neurobiol. Dis. 37, 630–640 10.1016/j.nbd.2009.11.018 [DOI] [PubMed] [Google Scholar]
- Debruyne J. C., Versijpt J., Van Laere K. J., De Vos F., Keppens J., Strijckmans K., Achten E., Slegers G., Dierckx R. A., Korf J., De Reuck J. L. (2003). PET visualization of microglia in multiple sclerosis patients using [11C]PK11195. Eur. J. Neurol. 10, 257–264 10.1046/j.1468-1331.2003.00571.x [DOI] [PubMed] [Google Scholar]
- Dheen S. T., Kaur C., Ling E. A. (2007). Microglial activation, and its implications in the brain diseases. Curr. Med. Chem. 14, 1189–1197 10.2174/092986707780597961 [DOI] [PubMed] [Google Scholar]
- DiCarlo G., Wilcock D., Henderson D., Gordon M., Morgan D. (2001). Intrahippocampal LPS injections reduce Abeta load in APP+PS1 transgenic mice. Neurobiol. Aging 22, 1007–1012 10.1016/S0197-4580(01)00292-5 [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Bergeron C., Chin S. S., Duyckaerts C., Horoupian D., Ikeda K., Jellinger K., Lantos P. L., Lippa C. F., Mirra S. S., Tabaton M., Vonsattel J. P., Wakabayashi K., Litvan I. (2002). Office of rare diseases neuropathologic criteria for corticobasal degeneration. J. Neuropathol. Exp. Neurol. 61, 935–946 [DOI] [PubMed] [Google Scholar]
- Dutta R., Trapp B. D. (2006). [Pathology and definition of multiple sclerosis]. Rev. Prat. 56, 1293–1298 [PubMed] [Google Scholar]
- Edison P., Archer H. A., Gerhard A., Hinz R., Pavese N., Turkheimer F. E., Hammers A., Tai Y. F., Fox N., Kennedy A., Rossor M., Brooks D. J. (2008). Microglia, amyloid, and cognition in Alzheimer’s disease: an [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol. Dis. 32, 412–419 10.1016/j.nbd.2008.08.001 [DOI] [PubMed] [Google Scholar]
- Eikelenboom P., Bate C., Van Gool W. A., Hoozemans J. J., Rozemuller J. M., Veerhuis R., Williams A. (2002). Neuroinflammation in Alzheimer’s disease and prion disease. Glia 40, 232–239 10.1002/glia.10146 [DOI] [PubMed] [Google Scholar]
- Ferrari C. C., Pott Godoy M. C., Tarelli R., Chertoff M., Depino A. M., Pitossi F. J. (2006). Progressive neurodegeneration and motor disabilities induced by chronic expression of IL-1beta in the substantia nigra. Neurobiol. Dis. 24, 183–193 10.1016/j.nbd.2006.06.013 [DOI] [PubMed] [Google Scholar]
- Floden A. M., Li S., Combs C. K. (2005). Beta-amyloid-stimulated microglia induce neuron death via synergistic stimulation of tumor necrosis factor alpha and NMDA receptors. J. Neurosci. 25, 2566–2575 10.1523/JNEUROSCI.4998-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frautschy S. A., Yang F., Irrizarry M., Hyman B., Saido T. C., Hsiao K., Cole G. M. (1998). Microglial response to amyloid plaques in APPsw transgenic mice. Am. J. Pathol. 152, 307–317 [PMC free article] [PubMed] [Google Scholar]
- Fujimura Y., Ikoma Y., Yasuno F., Suhara T., Ota M., Matsumoto R., Nozaki S., Takano A., Kosaka J., Zhang M. R., Nakao R., Suzuki K., Kato N., Ito H. (2006). Quantitative analyses of 18F-FEDAA1106 binding to peripheral benzodiazepine receptors in living human brain. J. Nucl. Med. 47, 43–50 [PubMed] [Google Scholar]
- Fujita M., Imaizumi M., Zoghbi S. S., Fujimura Y., Farris A. G., Suhara T., Hong J., Pike V. W., Innis R. B. (2008). Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage 40, 43–52 10.1016/j.neuroimage.2007.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard A., Banati R. B., Goerres G. B., Cagnin A., Myers R., Gunn R. N., Turkheimer F. E., Good C. D., Mathias C. J., Quinn N., Schwarz J., Brooks D. J. (2003). [11C](R)-PK11195 PET imaging of microglial activation in multiple system atrophy. Neurology 61, 686–689 10.1212/01.WNL.0000078192.95645.E6 [DOI] [PubMed] [Google Scholar]
- Gerhard A., Pavese N., Hotton G., Turkheimer F., Es M., Hammers A., Eggert K., Oertel W., Banati R. B., Brooks D. J. (2006a). In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 21, 404–412 10.1016/j.nbd.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Gerhard A., Trender-Gerhard I., Turkheimer F., Quinn N. P., Bhatia K. P., Brooks D. J. (2006b). In vivo imaging of microglial activation with [11C](R)-PK11195 PET in progressive supranuclear palsy. Mov. Disord. 21, 89–93 10.1002/mds.20668 [DOI] [PubMed] [Google Scholar]
- Gerhard A., Watts J., Trender-Gerhard I., Turkheimer F., Banati R. B., Bhatia K., Brooks D. J. (2004). In vivo imaging of microglial activation with [11C](R)-PK11195 PET in corticobasal degeneration. Mov. Disord. 19, 1221–1226 10.1002/mds.20162 [DOI] [PubMed] [Google Scholar]
- Gibb W. R., Luthert P. J., Marsden C. D. (1989). Corticobasal degeneration. Brain 112, 1171–1192 10.1093/brain/112.5.1171 [DOI] [PubMed] [Google Scholar]
- Gulyás B., Halldin C., Sandell J., Karlsson P., Sóvágó J., Kárpáti E., Kiss B., Vas A., Cselényi Z., Farde L. (2002). PET studies on the brain uptake and regional distribution of [11C]vinpocetine in human subjects. Acta Neurol. Scand. 106, 325–332 10.1034/j.1600-0404.2002.01302.x [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D. J. (2002). The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356 10.1126/science.1072994 [DOI] [PubMed] [Google Scholar]
- Hartlage-Rübsamen M., Lemke R., Schliebs R. (1999). Interleukin-1beta, inducible nitric oxide synthase, and nuclear factor-kappaB are induced in morphologically distinct microglia after rat hippocampal lipopolysaccharide/interferon-gamma injection. J. Neurosci. Res. 57, 388–398 [DOI] [PubMed] [Google Scholar]
- Hauw J. J., Daniel S. E., Dickson D., Horoupian D. S., Jellinger K., Lantos P. L., McKee A., Tabaton M., Litvan I. (1994). Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 44, 2015–2019 10.1212/WNL.44.11.2015 [DOI] [PubMed] [Google Scholar]
- Henkel K., Karitzky J., Schmid M., Mader I., Glatting G., Unger J. W., Neumaier B., Ludolph A. C., Reske S. N., Landwehrmeyer G. B. (2004). Imaging of activated microglia with PET and [11C]PK 11195 in corticobasal degeneration. Mov. Disord. 19, 817–821 10.1002/mds.20040 [DOI] [PubMed] [Google Scholar]
- Heppner F. L., Greter M., Marino D., Falsig J., Raivich G., Hövelmeyer N., Waisman A., Rülicke T., Prinz M., Priller J., Becher B., Aguzzi A. (2005). Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat. Med. 11, 146–152 10.1038/nm1177 [DOI] [PubMed] [Google Scholar]
- Ikoma Y., Yasuno F., Ito H., Suhara T., Ota M., Toyama H., Fujimura Y., Takano A., Maeda J., Zhang M. R., Nakao R., Suzuki K. (2007). Quantitative analysis for estimating binding potential of the peripheral benzodiazepine receptor with [(11)C]DAA1106. J. Cereb. Blood Flow Metab. 27, 173–184 10.1038/sj.jcbfm.9600325 [DOI] [PubMed] [Google Scholar]
- Imamura K., Hishikawa N., Sawada M., Nagatsu T., Yoshida M., Hashizume Y. (2003). Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 106, 518–526 10.1007/s00401-003-0766-2 [DOI] [PubMed] [Google Scholar]
- Ishizawa K., Dickson D. W. (2001). Microglial activation parallels system degeneration in progressive supranuclear palsy and corticobasal degeneration. J. Neuropathol. Exp. Neurol. 60, 647–657 [DOI] [PubMed] [Google Scholar]
- Ishizawa K., Komori T., Sasaki S., Arai N., Mizutani T., Hirose T. (2004). Microglial activation parallels system degeneration in multiple system atrophy. J. Neuropathol. Exp. Neurol. 63, 43–52 [DOI] [PubMed] [Google Scholar]
- Ishizawa K., Lin W. L., Tiseo P., Honer W. G., Davies P., Dickson D. W. (2000). A qualitative and quantitative study of grumose degeneration in progressive supranuclear palsy. J. Neuropathol. Exp. Neurol. 59, 513–524 [DOI] [PubMed] [Google Scholar]
- Kesselring J. (1990). The pathogenesis of multiple sclerosis. Schweiz. Med. Wochenschr. 120, 1083–1090 [PubMed] [Google Scholar]
- Kida E., Barcikowska M., Niemczewska M. (1992). Immunohistochemical study of a case with progressive supranuclear palsy without ophthalmoplegia. Acta Neuropathol. 83, 328–332 10.1007/BF00296797 [DOI] [PubMed] [Google Scholar]
- Kiferle L., Politis M., Muraro P. A., Piccini P. (2011). Positron emission tomography imaging in multiple sclerosis-current status and future applications. Eur. J. Neurol. 18, 226–231 10.1111/j.1468-1331.2010.03154.x [DOI] [PubMed] [Google Scholar]
- Kim S., Moon M., Park S. (2009). Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson’s disease. J. Endocrinol. 202, 431–439 10.1677/JOE-09-0132 [DOI] [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J., Landreth G. E. (2005). Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J. Neurosci. 25, 8240–8249 10.1523/JNEUROSCI.1808-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T., Shibata N., Kobayashi M., Sasaki S., Iwata M. (1998). Inducible nitric oxide synthase (iNOS)-like immunoreactivity in argyrophilic, tau-positive astrocytes in progressive supranuclear palsy. Acta Neuropathol. 95, 338–344 10.1007/s004010050808 [DOI] [PubMed] [Google Scholar]
- Kreutzberg G. W. (1996). Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318 10.1016/0166-2236(96)10049-7 [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A., Lucchinetti C. F., Stadelmann C., Brück W., Rauschka H., Bergmann M., Schmidbauer M., Parisi J. E., Lassmann H. (2005). Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128, 2705–2712 10.1093/brain/awh641 [DOI] [PubMed] [Google Scholar]
- Langston J. W., Forno L. S., Tetrud J., Reeves A. G., Kaplan J. A., Karluk D. (1999). Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann. Neurol. 46, 598–605 [DOI] [PubMed] [Google Scholar]
- Lantos P. L., Papp M. I. (1994). Cellular pathology of multiple system atrophy: a review. J. Neurol. Neurosurg. Psychiatr. 57, 129–133 10.1136/jnnp.57.2.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H. (2008). Mechanisms of inflammation induced tissue injury in multiple sclerosis. J. Neurol. Sci. 274, 45–47 10.1016/j.jns.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Lawson L. J., Perry V. H., Dri P., Gordon S. (1990). Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39, 151–170 10.1016/0306-4522(90)90229-W [DOI] [PubMed] [Google Scholar]
- Leung E., Guo L., Bu J., Maloof M., El Khoury J., Geula C. (2011). Microglia activation mediates fibrillar amyloid-(β toxicity in the aged primate cortex. Neurobiol. Aging 32, 387–397 10.1016/j.neurobiolaging.2009.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Lu J., Tay S. S., Moochhala S. M., He B. P. (2007). The function of microglia, either neuroprotection or neurotoxicity, is determined by the equilibrium among factors released from activated microglia in vitro. Brain Res. 1159, 8–17 10.1016/j.brainres.2007.04.066 [DOI] [PubMed] [Google Scholar]
- Li W. W., Setzu A., Zhao C., Franklin R. J. (2005). Minocycline-mediated inhibition of microglia activation impairs oligodendrocyte progenitor cell responses and remyelination in a non-immune model of demyelination. J. Neuroimmunol. 158, 58–66 10.1016/j.jneuroim.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Magliozzi R., Howell O. W., Reeves C., Roncaroli F., Nicholas R., Serafini B., Aloisi F., Reynolds R. (2010). A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann. Neurol. 68, 477–493 10.1002/ana.22230 [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Itagaki S., Boyes B. E., McGeer E. G. (1988a). Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38, 1285–1291 10.1212/WNL.38.8.1285 [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Itagaki S., McGeer E. G. (1988b). Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neuropathol. 76, 550–557 10.1007/BF00689592 [DOI] [PubMed] [Google Scholar]
- Messmer K., Reynolds G. P. (1998). Increased peripheral benzodiazepine binding sites in the brain of patients with Huntington’s disease. Neurosci. Lett. 241, 53–56 10.1016/S0304-3940(97)00967-1 [DOI] [PubMed] [Google Scholar]
- Michelucci A., Heurtaux T., Grandbarbe L., Morga E., Heuschling P. (2009). Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-beta. J. Neuroimmunol. 210, 3–12 10.1016/j.jneuroim.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Mirra S. S., Hyman B. T. (2002). “Aging and dementia,” in Greenfield’s Neuropathology, Vol. 2, eds Graham D. I., Lantos P. L. (London: Arnold; ), 195–271 [Google Scholar]
- Mogi M., Harada M., Riederer P., Narabayashi H., Fujita K., Nagatsu T. (1994). Tumor necrosis factor-alpha (TNF- alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci. Lett. 165, 208–210 10.1016/0304-3940(94)90746-3 [DOI] [PubMed] [Google Scholar]
- Munoz D. G., Dickson D. W., Bergeron C., Mackenzie I. R., Delacourte A., Zhukareva V. (2003). The neuropathology and biochemistry of frontotemporal dementia. Ann. Neurol. 54(Suppl. 15), 24–28 10.1002/ana.10571 [DOI] [PubMed] [Google Scholar]
- Napoli I., Neumann H. (2009). Microglial clearance function in health and disease. Neuroscience 158, 1030–1038 10.1016/j.neuroscience.2008.06.046 [DOI] [PubMed] [Google Scholar]
- Neary D., Snowden J. S., Gustafson L., Passant U., Stuss D., Black S., Freedman M., Kertesz A., Robert P. H., Albert M., Boone K., Miller B. L., Cummings J., Benson D. F. (1998). Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51, 1546–1554 10.1212/WNL.51.6.1546 [DOI] [PubMed] [Google Scholar]
- Oh U., Fujita M., Ikonomidou V. N., Evangelou I. E., Matsuura E., Harberts E., Fujimura Y., Richert N. D., Ohayon J., Pike V. W., Zhang Y., Zoghbi S. S., Innis R. B., Jacobson S. (2011). Translocator protein PET imaging for glial activation in multiple sclerosis. J. Neuroimmune Pharmacol. 6, 354–361 10.1007/s11481-011-9273-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okello A., Edison P., Archer H. A., Turkheimer F. E., Kennedy J., Bullock R., Walker Z., Kennedy A., Fox N., Rossor M., Brooks D. J. (2009a). Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology 72, 56–62 10.1212/01.wnl.0000345004.84188.b9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okello A., Koivunen J., Edison P., Archer H. A., Turkheimer F. E., Någren K., Bullock R., Walker Z., Kennedy A., Fox N. C., Rossor M. N., Rinne J. O., Brooks D. J. (2009b). Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology 73, 754–760 10.1212/WNL.0b013e3181b23564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi Y., Yoshikawa E., Sekine Y., Futatsubashi M., Kanno T., Ogusu T., Torizuka T. (2005). Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann. Neurol. 57, 168–175 10.1002/ana.20338 [DOI] [PubMed] [Google Scholar]
- Owen D. R., Howell O. W., Tang S. P., Wells L. A., Bennacef I., Bergstrom M., Gunn R. N., Rabiner E. A., Wilkins M. R., Reynolds R., Matthews P. M., Parker C. A. (2010). Two binding sites for [3H]PBR28 in human brain: implications for TSPO PET imaging of neuroinflammation. J. Cereb. Blood Flow Metab. 30, 1608–1618 10.1038/jcbfm.2010.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V., Baraldi M., Guilarte T. R., Knudsen T. B., Lacapère J. J., Lindemann P., Norenberg M. D., Nutt D., Weizman A., Zhang M. R., Gavish M. (2006). Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 27, 402–409 10.1016/j.tips.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Paulus W., Bancher C., Jellinger K. (1993). Microglial reaction in Pick’s disease. Neurosci. Lett. 161, 89–92 10.1016/0304-3940(93)90147-D [DOI] [PubMed] [Google Scholar]
- Pavese N., Gerhard A., Tai Y. F., Ho A. K., Turkheimer F., Barker R. A., Brooks D. J., Piccini P. (2006). Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology 66, 1638–1643 10.1212/01.wnl.0000222734.56412.17 [DOI] [PubMed] [Google Scholar]
- Peterson J. W., Bö L., Mörk S., Chang A., Trapp B. D. (2001). Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann. Neurol. 50, 389–400 10.1002/ana.1123 [DOI] [PubMed] [Google Scholar]
- Petit-Taboué M. C., Baron J. C., Barré L., Travère J. M., Speckel D., Camsonne R., MacKenzie E. T. (1991). Brain kinetics and specific binding of [11C]PK 11195 to omega 3 sites in baboons: positron emission tomography study. Eur. J. Pharmacol. 200, 347–351 10.1016/0014-2999(91)90594-G [DOI] [PubMed] [Google Scholar]
- Petzold A., Eikelenboom M. J., Gveric D., Keir G., Chapman M., Lazeron R. H., Cuzner M. L., Polman C. H., Uitdehaag B. M., Thompson E. J., Giovannoni G. (2002). Markers for different glial cell responses in multiple sclerosis: clinical and pathological correlations. Brain 125, 1462–1473 10.1093/brain/awf165 [DOI] [PubMed] [Google Scholar]
- Pike V. W., Halldin C., Crouzel C., Barré L., Nutt D. J., Osman S., Shah F., Turton D. R., Waters S. L. (1993). Radioligands for PET studies of central benzodiazepine receptors and PK (peripheral benzodiazepine) binding sites – current status. Nucl. Med. Biol. 20, 503–525 10.1016/0969-8051(93)90082-6 [DOI] [PubMed] [Google Scholar]
- Politis M., Gianneti P., Su P., Turkheimer F., Keihaninejad S., Wu K., Waldman A., Malik O., Matthews P. M., Reynolds R., Nicholas R., Piccini P. (in press). Increased PK11195 PET binding in the cortex of MS patients correlates with disability. Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis M., Pavese N., Tai Y. F., Kiferle L., Mason S. L., Brooks D. J., Tabrizi S. J., Barker R. A., Piccini P. (2011). Microglial activation in regions related to cognitive function predicts disease onset in Huntington’s disease: a multimodal imaging study. Hum. Brain Mapp. 32, 258–270 10.1002/hbm.21008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis M., Pavese N., Tai Y. F., Tabrizi S. J., Barker R. A., Piccini P. (2008). Hypothalamic involvement in Huntington’s disease: an in vivo PET study. Brain 131(Pt 11), 2860–2869 10.1093/brain/awn244 [DOI] [PubMed] [Google Scholar]
- Ransohoff R. M., Perry V. H. (2009). Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 27, 119–145 10.1146/annurev.immunol.021908.132528 [DOI] [PubMed] [Google Scholar]
- Rasmussen S., Wang Y., Kivisäkk P., Bronson R. T., Meyer M., Imitola J., Khoury S. J. (2007). Persistent activation of microglia is associated with neuronal dysfunction of callosal projecting pathways and multiple sclerosis-like lesions in relapsing–remitting experimental autoimmune encephalomyelitis. Brain 130(Pt 11), 2816–2829 10.1093/brain/awm219 [DOI] [PubMed] [Google Scholar]
- Rebeiz J. J., Kolodny E. H., Richardson E. P., Jr. (1968). Corticodentatonigral degeneration with neuronal achromasia. Arch. Neurol. 18, 20–33 10.1001/archneur.1968.00470310034003 [DOI] [PubMed] [Google Scholar]
- Sanchez-Guajardo V., Febbraro F., Kirik D., Romero-Ramos M. (2010). Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS ONE 5, e8784 10.1371/journal.pone.0008784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp E., Kegel K. B., Aronin N., Hashikawa T., Uchiyama Y., Tohyama K., Bhide P. G., Vonsattel J. P., DiFiglia M. (2001). Early and progressive accumulation of reactive microglia in the Huntington disease brain. J. Neuropathol. Exp. Neurol. 60, 161–172 [DOI] [PubMed] [Google Scholar]
- Scarf A. M., Ittner L. M., Kassiou M. (2009). The translocator protein (18 kDa): central nervous system disease and drug design. J. Med. Chem. 52, 581–592 10.1021/jm8011678 [DOI] [PubMed] [Google Scholar]
- Schwarz S. C., Seufferlein T., Liptay S., Schmid R. M., Kasischke K., Foster O. J., Daniel S., Schwarz J. (1998). Microglial activation in multiple system atrophy: a potential role for NF-kappaB/rel proteins. Neuroreport 9, 3029–3032 10.1097/00001756-199809140-00020 [DOI] [PubMed] [Google Scholar]
- Setzu A., Lathia J. D., Zhao C., Wells K., Rao M. S., Ffrench-Constant C., Franklin R. J. (2006). Inflammation stimulates myelination by transplanted oligodendrocyte precursor cells. Glia. 54, 297–303 10.1002/glia.20371 [DOI] [PubMed] [Google Scholar]
- Singhrao S. K., Neal J. W., Morgan B. P., Gasque P. (1999). Increased complement biosynthesis by microglia and complement activation on neurons in Huntington’s disease. Exp. Neurol. 159, 362–376 10.1006/exnr.1999.7170 [DOI] [PubMed] [Google Scholar]
- Stadelmann C., Kerschensteiner M., Misgeld T., Brück W., Hohlfeld R., Lassmann H. (2002). BDNF and gp145trkB in multiple sclerosis brain lesions: neuroprotective interactions between immune and neuronal cells? Brain 125(Pt 1), 75–85 10.1093/brain/awf015 [DOI] [PubMed] [Google Scholar]
- Stalder M., Phinney A., Probst A., Sommer B., Staufenbiel M., Jucker M. (1999). Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am. J. Pathol. 154, 1673–1684 10.1016/S0002-9440(10)65423-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N., Reindl M., Neumann M., Kahle P. J., Poewe W., Wenning G. K. (2007). Microglial activation mediates neurodegeneration related to oligodendroglial alpha-synucleinopathy: implications for multiple system atrophy. Mov. Disord. 22, 2196–2203 10.1002/mds.21671 [DOI] [PubMed] [Google Scholar]
- Stephenson D. T., Schober D. A., Smalstig E. B., Mincy R. E., Gehlert D. R., Clemens J. A. (1995). Peripheral benzodiazepine receptors are colocalized with activated microglia following transient global forebrain ischemia in the rat. J. Neurosci. 15(Pt 2), 5263–5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D. K., Reynolds A. D., Mosley R. L., Gendelman H. E. (2009). Innate and adaptive immunity for the pathobiology of Parkinson’s disease. Antioxid. Redox Signal. 11, 2151–2166 10.1089/ars.2009.2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A., Sawada M., Marunouchi T. (1996). Selective induction of interleukin-6 in mouse microglia by granulocyte-macrophage colony-stimulating factor. Brain Res. 713, 192–198 10.1016/0006-8993(95)01535-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai Y. F., Pavese N., Gerhard A., Tabrizi S. J., Barker R. A., Brooks D. J., Piccini P. (2007). Microglial activation in presymptomatic Huntington’s disease gene carriers. Brain 130(Pt 7), 1759–1766 10.1093/brain/awm044 [DOI] [PubMed] [Google Scholar]
- Uchihara T., Ikeda K., Tsuchiya K. (2003). Pick body disease and Pick syndrome. Neuropathology 23, 318–326 10.1046/j.1440-1789.2003.00523.x [DOI] [PubMed] [Google Scholar]
- Vas A., Shchukin Y., Karrenbauer V. D., Cselényi Z., Kostulas K., Hillert J., Savic I., Takano A., Halldin C., Gulyás B. (2008). Functional neuroimaging in multiple sclerosis with radiolabelled glia markers: preliminary comparative PET studies with [11C]vinpocetine and [11C]PK11195 in patients. J. Neurol. Sci. 264, 9–17 10.1016/j.jns.2007.07.018 [DOI] [PubMed] [Google Scholar]
- Venneti S., Lopresti B. J., Wang G., Hamilton R. L., Mathis C. A., Klunk W. E., Apte U. M., Wiley C. A. (2009). PK11195 labels activated microglia in Alzheimer’s disease and in vivo in a mouse model using PET. Neurobiol. Aging 30, 1217–1226 10.1016/j.neurobiolaging.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versijpt J., Debruyne J. C., Van Laere K. J., De Vos F., Keppens J., Strijckmans K., Achten E., Slegers G., Dierckx R. A., Korf J., De Reuck J. L. (2005). Microglial imaging with positron emission tomography and atrophy measurements with magnetic resonance imaging in multiple sclerosis: a correlative study. Mult. Scler. 11, 127–134 10.1191/1352458505ms1140oa [DOI] [PubMed] [Google Scholar]
- Vonsattel J. P., DiFiglia M. (1998). Huntington disease. J. Neuropathol. Exp. Neurol. 57, 369–384 10.1097/00005072-199805000-00001 [DOI] [PubMed] [Google Scholar]
- Vowinckel E., Reutens D., Becher B., Verge G., Evans A., Owens T., Antel J. P. (1997). PK11195 binding to the peripheral benzodiazepine receptor as a marker of microglia activation in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neurosci. Res. 50, 345–353 [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Lopresti B. J., Venneti S., Price J., Klunk W. E., DeKosky S.T., Mathis C.A. (2009). Carbon 11-labelled Pittsburgh Compound B and carbon 11-labelled ®-PK11195 positron emission tomographic imaging in Alzheimer disease. Arch. Neurol. 66, 60–67 10.1001/archneurol.2009.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms H., Claasen J., Röhl C., Sievers J., Deuschl G., Lucius R. (2003). Involvement of benzodiazepine receptors in neuroinflammatory and neurodegenerative diseases: evidence from activated microglial cells in vitro. Neurobiol. Dis. 14, 417–424 10.1016/j.nbd.2003.07.002 [DOI] [PubMed] [Google Scholar]
- Wimo A., Winblad B., Aguero-Torres H., von Strauss E. (2003). The magnitude of dementia occurrence in the world. Alzheimer Dis. Assoc. Disord. 17, 63–67 10.1097/00002093-200304002-00010 [DOI] [PubMed] [Google Scholar]
- Yasuno F., Ota M., Kosaka J., Ito H., Higuchi M., Doronbekov T. K., Nozaki S., Fujimura Y., Koeda M., Asada T., Suhara T. (2008). Increased binding of peripheral benzodiazepine receptor in Alzheimer’s disease measured by positron emission tomography with [11C]DAA1106. Biol. Psychiatry 64, 835–841 10.1016/j.biopsych.2008.04.021 [DOI] [PubMed] [Google Scholar]
- Yokokura M., Mori N., Yagi S., Yoshikawa E., Kikuchi M., Yoshihara Y., Wakuda T., Sugihara G., Takebayashi K., Suda S., Iwata Y., Ueki T., Tsuchiya K. J., Suzuki K., Nakamura K., Ouchi Y. (2011). In vivo changes in microglial activation and amyloid deposits in brain regions with hypometabolism in Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 38, 343–351 10.1007/s00259-010-1612-0 [DOI] [PubMed] [Google Scholar]
- Zhang W., Wang T., Pei Z., Miller D. S., Wu X., Block M. L., Wilson B., Zhang W., Zhou Y., Hong J. S., Zhang J. (2005). Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 19, 533–542 10.1096/fj.05-3851com [DOI] [PubMed] [Google Scholar]
- Zipp F., Hartung H. P., Hillert J., Schimrigk S., Trebst C., Stangel M., Infante-Duarte C., Jakobs P., Wolf C., Sandbrink R., Pohl C., Filippi M., CCR1 Antagonist Study Group (2006). Blockade of chemokine signaling in patients with multiple sclerosis. Neurology 67, 1880–1883 10.1212/01.wnl.0000244420.68037.86 [DOI] [PubMed] [Google Scholar]