Abstract
The human placenta is key to pregnancy outcome, and the elevated oxidative stress present in many complicated pregnancies contributes to placental dysfunction and suboptimal pregnancy outcomes. We tested the hypothesis that pomegranate juice, which is rich in polyphenolic antioxidants, limits placental trophoblast injury in vivo and in vitro. Pregnant women with singleton pregnancies were randomized at 35∼38 wk gestation to 8 oz/day of pomegranate juice or apple juice (placebo) until the time of delivery. Placental tissues from 12 patients (4 in the pomegranate group and 8 in the control group) were collected for analysis of oxidative stress. The preliminary in vivo results were extended to oxidative stress and cell death assays in vitro. Placental explants and cultured primary human trophoblasts were exposed to pomegranate juice or glucose (control) under defined oxygen tensions and chemical stimuli. We found decreased oxidative stress in term human placentas from women who labored after prenatal ingestion of pomegranate juice compared with apple juice as control. Moreover, pomegranate juice reduced in vitro oxidative stress, apoptosis, and global cell death in term villous explants and primary trophoblast cultures exposed to hypoxia, the hypoxia mimetic cobalt chloride, and the kinase inhibitor staurosporine. Punicalagin, but not ellagic acid, both prominent polyphenols in pomegranate juice, reduced oxidative stress and stimulus-induced apoptosis in cultured syncytiotrophoblasts. We conclude that pomegranate juice reduces placental oxidative stress in vivo and in vitro while limiting stimulus-induced death of human trophoblasts in culture. The polyphenol punicalagin mimics this protective effect. We speculate that antenatal intake of pomegranate may limit placental injury and thereby may confer protection to the exposed fetus.
Keywords: placenta, hypoxia, intrauterine growth restriction, nutrition, supplementation
the human placenta is a transient organ that is pivotal for a successful pregnancy and has lifelong influences on the mother and child (18, 25, 33, 43). Abnormal placental development, placental injury, or both, associate with preterm birth, hypertensive disorders, central nervous system injury, and intrauterine growth restriction (22, 44). Among all the pregnancies worldwide, ∼5–10% result in suboptimal outcomes due to placental dysfunction. Such dysfunction derives from etiologic heterogeneity that ranges from maldevelopment of the placenta in the first half of pregnancy to maladaptation of the placenta exposed to stimuli in the maternal environment during the second half of pregnancy. Importantly, the most common pathways to placental dysfunction show a histopathology indicating injury to placental villi, with notable effects on the trophoblast bilayer.
Bathed in maternal blood, the outer layer of placental villi is the syncytiotrophoblast, a unique epithelium and a true syncytium with multiple nuclei in a common cytoplasm (32). This structure is pivotal to the function of syncytiotrophoblast, which mediates the bulk of maternal-to-fetal exchange of gases, nutrients, and wastes. The layer also directionally secretes pregnancy-specific hormones, provides selective transport functions, and conducts metabolic activities important to the protection of the fetus from oxidative stress and toxic agents in the maternal blood. Underlying cytotrophoblasts can fuse with the syncytiotrophoblast, allowing its growth and repair during pregnancy (32). Placental dysfunction, irrespective of pregnancy malady, associates with nitrative and oxidative stress and generation of reactive oxygen species (6). The combined effects of these stimuli yield suboptimal function of villous trophoblasts through both molecular and morphological mechanisms. A therapeutic intervention with a low-risk profile, high availability to populations around the world, and the potential to positively modulate villous development and responses to stimuli would be an advance in translational medicine.
Pomegranate is ingested around the world and is attributed health benefits. In addition to its antioxidants, pomegranate juice contains molecules that enhance the release of nitric oxide (29). The effects of pomegranate on nitric oxide may be advantageous in pregnancy, as suggested by a recent randomized trial that showed improved pregnancy outcomes in women supplemented with l-arginine, likely because of its effects on nitric oxide production (45). Burgeoning data in nonpregnant populations, animal models, and in vitro studies show pomegranate juice has beneficial effects on cardiovascular function, especially on endothelial cells (16, 20). Notably, pomegranate juice is neuroprotective of oxidative stress and central nervous system injury in the perinatal period of mouse and rat with maternal ingestion during pregnancy (26, 47). Because it is bathed in maternal blood, the placental syncytiotrophoblast is positioned to respond to the antioxidants, nitric oxide-modulating agents, and other compounds in pomegranate juice. However, the effects of pomegranate juice on human pregnancy are unstudied, despite use of pomegranate by pregnant women for centuries. We herein test the hypothesis that pomegranate juice reduces oxidative stress in human placentas in vivo and stimulus-induced injury to human trophoblasts in explants and primary cultures in vitro.
MATERIALS AND METHODS
Patients.
This study was approved by the Institutional Review Board of Washington University School of Medicine in St. Louis, MO. Twenty pregnant women with singleton pregnancies and no evidence of pregnancy complications were randomized at 35∼38 wk gestation to a treatment or placebo group. Patients were instructed to drink 8 oz of pomegranate juice (POM Wonderful, Los Angeles, CA) or apple juice (Tree Top Apple Juice; Tree, Selah, WA), which is known to have low levels of polyphenols, daily until delivery. Each participant was asked to keep a dietary diary to document compliance. Only 12 placenta (pomegranate group, n = 4; control group, n = 8) samples were obtained, due to the timing of the delivery, from the participant within 30 min of delivery. Avoiding areas with visible infarcts or calcifications, samples were taken midway between the chorionic and basal plates of all lobules of each placenta except at the periphery and rinsed in 4°C PBS. Samples were then either snap-frozen in liquid nitrogen for protein analysis or fixed in 4% formalin for 24 h and embedded in paraffin for immunohistochemical staining.
For in vitro experiments, villous explants were obtained from four patients who were not enrolled in the in vivo study, with normal singleton pregnancies at 39 wk gestation without labor after a scheduled repeat C-section. The explants were cultured at 37°C under 20% or <1% oxygen with 5% CO2 in DMEM, as described previously (11), for 5 days followed by 2 days culture in phenol red-free DMEM with pomegranate juice (1%, vol/vol) or glucose (7.5 mM; Sigma), which is equivalent to the glucose content of pomegranate juice (38). At day 7, explants were cultured an additional 24 h under 20% oxygen or <1% oxygen or for 4 h in 20% oxygen in the presence or absence of the hypoxia mimetic cobalt chloride (200 nM; Sigma) or the protein kinase inhibitor staurosporine (0.5 μM; Tocris, Ellisville, MO).
Immunohistochemistry and immunofluorescence.
Five-micrometer sections were stained for heat shock protein (Hsp) 90 (Cell Signaling Technology, Danvers, MA), E-cadherin, caspase-cleaved cytokeratin 18 intermediate filaments (cl-Cyt 18), and DNA, as described (24).
For the data in Figs. 1C and 2, 12 randomly captured images were quantified by three examiners blinded to conditions who each assigned scores of 1 (worst) to 5 (best) for the integrity of the trophoblast layer and the overall morphology of villi based on the intensity of staining of E-cadherin, which identifies the apical and basal plasma membranes of syncytiotrophoblast and the plasma membrane of cytotrophoblasts. For quantification of Hsp 90, Z-stack images were collapsed into mean signal intensity and quantified using ImageJ software. For cl-Cyt 18 quantification, two blinded examiners evaluated slides and recorded the average number of sites with cl-Cyt 18 per each field of view (212 × 212 μm).
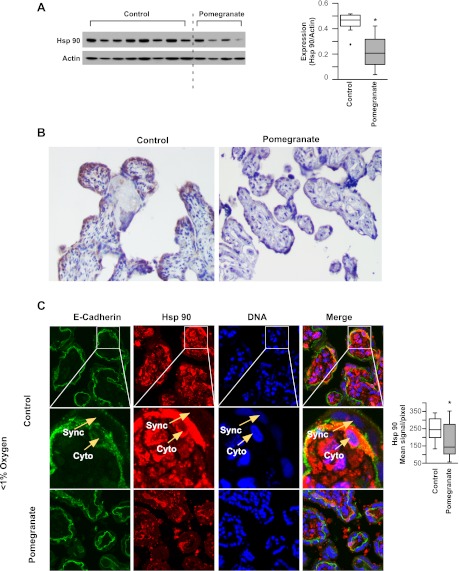
Fig. 1.
Pomegranate juice reduces labor-induced oxidative stress in term human placenta in vivo and in placental explants. A: left, lysates from placentas delivered following labor by women administered pomegranate juice (n = 4) or apple juice (n = 8), as control, were immunoblotted with heat shock protein (Hsp) 90 antibody. Right, a summary graph of densitometry of the Western blot shown on the left. Student's t-test, *P < 0.05. B: representative images of immunohistochemistry for Hsp 90 of placentas from women administered pomegranate juice or placebo. C: immunofluorescence staining of Hsp 90 and E-cadherin, for trophoblast surface membranes, in placental explants from patients that were not enrolled in the in vivo study, exposed to pomegranate juice or glucose, as control, in hypoxia (<1% O2). Images in the middle show the enlarged area outlined in the row on top. Cytotrophoblasts (Cyto) or syncytiotrophoblasts (Sync) were distinguished by E-cadherin staining (green). Hsp 90 and DNA were shown in red or blue separately. The Hsp 90 signal quantification, shown on the right, was presented as mean signal per pixel (n = 12, Student's t-test, *P < 0.05).
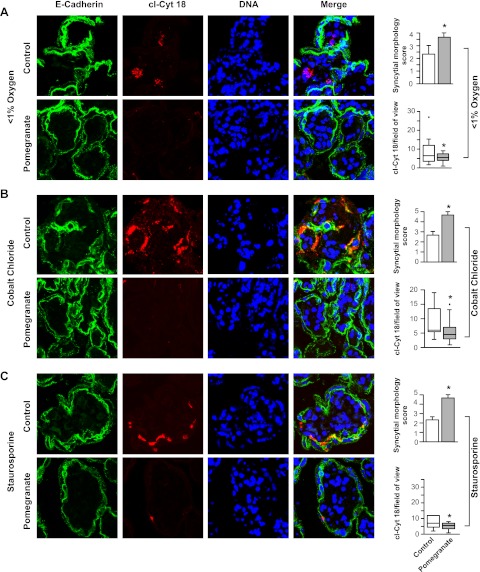
Fig. 2.
Immunofluorescence staining of cleaved cytokeratin 18 (cl-Cyt 18) in placental explants exposed to hypoxia (A), cobalt chloride (B), or staurosporine (C), cultured with pomegranate juice compared with glucose, as control. Graphs on the far right summarize the syncytial morphology score (n = 4, *P < 0.05, Mann-Whitney rank test) or cl-Cyt 18 levels in pomegranate juice compared with glucose control (n = 12, *P < 0.05, Student's t-test).
Isolation and culture of primary human trophoblasts.
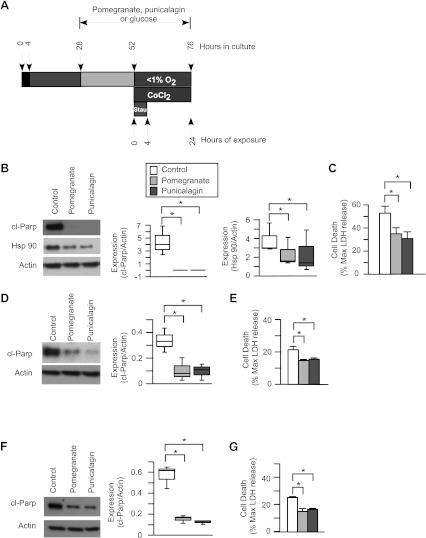
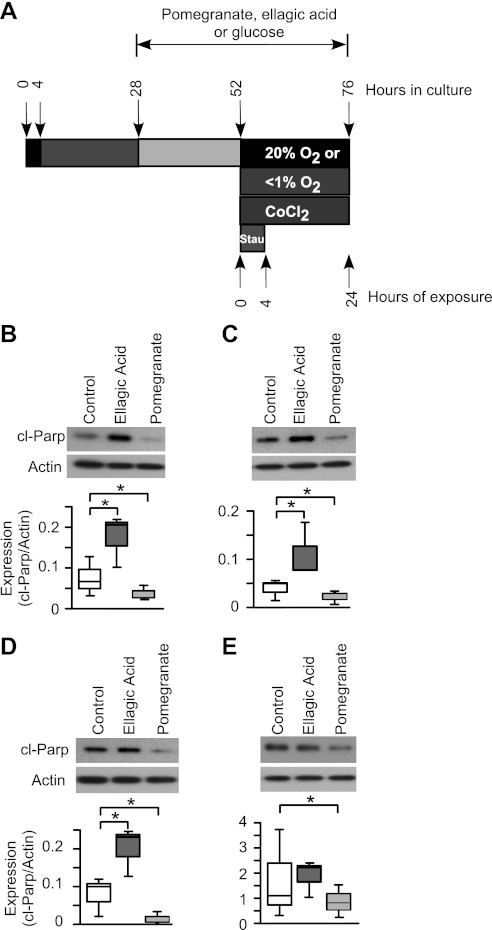
Primary trophoblasts were isolated from term human placental tissue that is not enrolled in the in vivo study. The trophoblasts were cultured, and the percentage of nuclei fused into syncytia (typically >80%) after 52 h was examined using E-cadherin staining as previously described (11). Except for the cultures used in Figs. 3B, 5B, and 5E, cultures were continued at 28 h in culture in phenol red-free DMEM with 10% charcoal-stripped FBS, with medium supplemented with glucose (7.5 mM), pomegranate juice (1% vol/vol), ellagic acid (40 μM; Sigma), or punicalagin (33.8 μM; Chengdu Purity Phytochemicals) at 37°C in 20% oxygen for another 24 h. The doses selected for ellagic acid and punicalagin were equivalent to the amount contained in the pomegranate juice (1% vol/vol) that we added to the cell culture (38). There was no significant change in the rate of trophoblasts fusion after pomegranate juice treatment compared with control (data not show). The cells were then exposed to cobalt chloride (200 nM), staurosporine (0.5 μM), or <1% oxygen with or without pomegranate juice, ellagic acid, or punicalagin for the listed time in the Figs. 1–7 and the corresponding legends.
Fig. 3.
The effect of hypoxia on the apoptosis in syncytiotrophoblasts. A: diagram of primary human trophoblast culture and hypoxia exposure. Primary human trophoblasts (PHTs) were derived from placentas of uncomplicated, unlabored pregnancies that were not enrolled in the in vivo study, delivered at 39 wk by cesarean section. PHTs were cultured for 4 h to allow the attachment, followed by 48 h continuous culture to let the trophoblasts differentiate, before exposure to hypoxia (<1% O2) for up to 24 h. B: Western blots of cleaved poly(ADP)-ribose polymerase (cl-Parp) and cl-Cyt 18 in syncytiotrophoblasts. The syncytiotrophoblasts were subjected to hypoxia for up to 24 h. Top, Western blots of cl-Parp and cl-Cyt 18. Middle and bottom, summary graphs of densitometry of Western blots. cl-Parp and cl-Cyt 18 were normalized to actin. In all graphs, n = 3. *P < 0.05, 2-way ANOVA, with Bonferroni post hoc test.
Fig. 5.
The effect of pomegranate juice on the syncytiotrophoblasts exposed to cobalt chloride or staurosporine. A: diagram of pomegranate juice, cobalt chloride, or staurosporine exposure for the primary human trophoblasts. The trophoblasts were pretreated with pomegranate juice (1%, vol/vol) or glucose (7.5 mM) for 24 h before exposure to cobalt chloride for up to 24 h, or staurosporine for up to 4 h. B: top, Western blot of cl-Cyt 18 in syncytiotrophoblasts cultured in regular DMEM for 52 h were exposed to cobalt chloride (CoCl2, 200 nM) for up to 24 h. Bottom, summary graphs of Western blots of cl-Cyt 18 at time (t) = 24 h, n = 3. *P < 0.05, t-test. C: top, Western blots of cl-Parp and cl-Cyt 18 in syncytiotrophoblasts exposed to CoCl2 with pomegranate juice or glucose, as control, for up to 24 h. Middle and bottom, summary graphs of densitometry of cl-Parp and cl-Cyt 18 expression. D: LDH analysis of syncytiotrophoblasts exposed to CoCl2 with pomegranate compared with control. E: top, Western blots of cl-Parp and cl-Cyt 18 in staurosporine (0.5 μM)-exposed syncytiotrophoblasts cultured in regular DMEM for up to 4 h. Middle and bottom, summary graphs of Western blots of cl-Parp and cl-Cyt 18, n = 3. *P < 0.05, 1-way ANOVA, with Bonferroni post hoc test. F: top, Western blots of cl-Parp and cl-Cyt 18 in syncytiotrophoblasts exposed to staurosporine compared with control for up to 4 h with pomegranate juice compared with control. Middle and bottom, summary graphs of densitometry of cl-Parp and cl-Cyt 18. G: LDH analysis of syncytiotrophoblasts exposed to staurosporine with pomegranate juice compared with control. In C, D, F, and G, n = 3. *P < 0.05, 2-way ANOVA, with Bonferroni post hoc test.
Fig. 7.
The effect of punicalagin on the syncytiotrophoblasts exposed to hypoxia, cobalt chloride, or staurosporine. A: diagram of pomegranate juice or punicalagin exposure for the primary human trophoblasts under hypoxia or exposed to CoCl2 or staurosporine. The trophoblasts were pretreated with pomegranate juice (1%, vol/vol), punicalagin (33.8 μM), or glucose (7.5 mM) for 24 h before exposure to hypoxia, or CoCl2 (200 nM) for 24 h, or staurosporine (0.5 μM) for 4 h. B: left, Western blots of cl-Parp and Hsp 90 in hypoxic trophoblasts treated with pomegranate juice or punicalagin compared with control. Second to the third panel: summary graphs of Western blots of cl-Parp and Hsp 90 shown on the left. C: LDH analysis of syncytiotrophoblasts under hypoxia with pomegranate juice or punicalagin compared with control for 24 h. D: left, Western blot of cl-Parp in trophoblasts exposed to CoCl2 with pomegranate juice or punicalagin compared with control. Right: summary graph of Western blot of cl-Parp shown on the left. E: LDH analysis of syncytiotrophoblasts exposed to CoCl2 with pomegranate juice or punicalagin compared with control for 24 h. F: left, Western blot of cl-Parp in trophoblasts exposed to staurosporine with pomegranate juice or punicalagin compared with control. Right, summary graph of Western blot of cl-Parp shown on the left. G: LDH analysis of syncytiotrophoblasts exposed to staurosporine with pomegranate juice or punicalagin compared with control for 4 h. In all graphs, n = 3. *P < 0.05, 1-way ANOVA, with Bonferroni post hoc test.
Western blotting.
Placental tissue was homogenized, primary human trophoblasts were lysed in radioimmunoprecipitation buffer (1% Nonidet P-40, 0.5% deoxycholate, and 0.1% SDS in PBS), and Western blotting was done using antibodies against cl-Cyt 18, cleaved poly(ADP)-ribose (cl-Parp), Hsp 90, or actin, followed by horseradish peroxidase and chemiluminescent detection, as described (11, 12). Protein levels were determined by densitometry of bands on films after normalization to actin levels.
Lactate dehydrogenase assay.
Levels of lactate dehydrogenase (LDH) released from cells into the culture medium were assessed using a cytotoxicity detection kit (Roche, Indianapolis, IN) according to the manufacturer's instructions, and data are shown as a percentage of the maximum LDH obtained by complete cell lysis.
Statistical analysis.
All the experiments were conducted using at least three different placentas from which multiple explants were cultured in each paradigm, or primary cell isolates, from at least three different placentas. Student's t-test, one-way ANOVA, two-way ANOVA with Bonferroni post hoc test, or the Mann-Whitney rank test were performed as listed in the legends for Figs. 1–7, using KaleidaGraph software, version 4.1.0 for Macintosh. A P < 0.05 was significant. Data are presented as means ± SD.
RESULTS
Twenty pregnant women were randomized to receive pomegranate (n = 10) or apple juice (n = 10). Twelve placentas were analyzed with eight women in the apple juice group and four women in the pomegranate group (Table 1). There was no significant difference in the timing of commencement or length of juice exposure. The women in the apple juice group had shorter time in labor (7.4 ± 5.5 h) compared with the women in the pomegranate juice (14.2 ± 3.8 h, P = 0.02). There were no adverse infant outcomes with no medical concerns in the neonatal period for any of the infants.
Table 1.
Pregnancy and delivery characteristics of women randomized to pomegranate or apple juice
| Gestational Age, wk |
||||||
|---|---|---|---|---|---|---|
| Patient No. | Weight, kg | Group | At enrollment | At delivery | Duration of Consumption, days | Duration of Labor, h |
| 1 | 79 | Apple | 37 | 39 | 12 | 5 |
| 2 | 101 | Apple | 35 | 39 | 22 | 2.5 |
| 3 | 92 | Apple | 35 | 39 | 27 | 2 |
| 4 | 67 | Apple | 37 | 40 | 23 | 3 |
| 5 | 82 | Apple | 37 | 39 | 12 | 8 |
| 6 | 81 | Apple | 38 | 38 | 6 | 7.5 |
| 7 | 61 | Apple | 36 | 39 | 23 | 17 |
| 8 | 86 | Apple | 35 | 38 | 27 | 14 |
| 9 | 67 | Pomegranate | 37 | 39 | 15 | 10 |
| 10 | 97 | Pomegranate | 38 | 40 | 18 | 12.5 |
| 11 | 63 | Pomegranate | 36 | 39 | 22 | 19 |
| 12 | 89 | Pomegranate | 35 | 40 | 34 | 15 |
We tested the hypothesis that pomegranate juice reduces labor-induced oxidative stress in term human placenta in vivo. Hsp 90, a marker of oxidative stress, was significantly lower in the placentas of women who received pomegranate juice compared with women who received apple juice (Fig. 1A). Immunohistochemical staining showed that the expression of Hsp 90 was most marked in the villous trophoblast bilayer compared with the villous core, and the overall expression of Hsp 90 was significantly lower in placental villi from women who received pomegranate juice compared with control (Fig. 1, A and B). We next exposed villous explants from placentas of uncomplicated term pregnancies to <1% oxygen to model a common clinical malady of placental underperfusion with hypoxia in pregnancy. We found that Hsp 90 expression was lower in villi exposed to pomegranate juice compared with the glucose control (Fig. 1C).
We hypothesized that pomegranate juice decreased apoptosis induced by low oxygen exposure of villous explants because hypoxia and oxidative stress enhance apoptosis in trophoblasts (11). Explants cultured in low oxygen tension exposed to pomegranate juice exhibited a more robust morphology compared with control villi cultured in hypoxic conditions, as reflected by E-cadherin immunostaining showing a more intact trophoblast bilayer (Fig. 2A). Importantly, villous explants exposed to pomegranate juice showed lower expression of the cytoplasmic marker of apoptosis cl-Cyt 18, a caspase-cleaved form of an intermediate filament protein (Fig. 2A).
Cobalt chloride is a hypoxia mimetic that induces apoptosis in many cell types, including placental trophoblasts (42, 50), largely by stabilizing hypoxia-inducible factor-1α (46), and staurosporine is a kinase inhibitor that also induces apoptosis in many cell types (36, 49), including placental trophoblasts (11, 12). Remarkably, in villous explants, pomegranate juice improved the morphology of the syncytiotrophoblast and decreased the level of apoptosis induced by both cobalt chloride and staurosporine (Fig. 2, B and C). Collectively, these results indicate that villous explants cultured in pomegranate juice show decreased oxidative stress and reduced levels of cell death induced by each of three potent inducers of apoptosis.
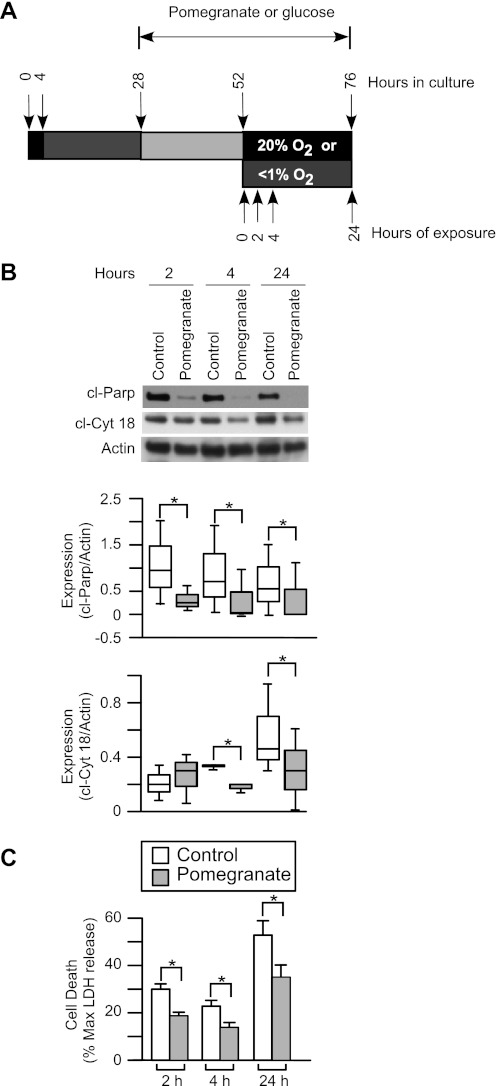
Using a primary human trophoblast system, illustrated in Fig. 3A, we confirmed that culture of syncytiotrophoblasts in <1% oxygen compared with standard culture conditions of 20% oxygen yielded a time-dependent increase in apoptosis, as reflected by increased levels of caspase-specific cleavage products of poly(ADP)-ribose polymerase (cl-Parp), a marker of apoptosis in the nucleus, and of cl-Cyt 18, a marker of apoptosis in the cytoplasm (Fig. 3B). Compared with cultures with added glucose, cultures with pomegranate juice exhibited significantly lower hypoxia-induced apoptosis, as measured by levels of both cl-Parp and cl-Cyt 18 (P < 0.05, Fig. 4, A and B). Finally, medium levels of LDH, an indicator of global cell death, were significantly reduced by pomegranate juice compared with glucose (P < 0.05, Fig. 4C). Collectively, these results indicate that pomegranate juice diminishes hypoxia-induced death of primary cultures of syncytiotrophoblasts.
Fig. 4.
The effect of pomegranate juice on the syncytiotrophoblasts under hypoxic conditions. A: diagram of pomegranate juice treatment and hypoxia exposure for the primary human trophoblasts. The trophoblasts were pretreated with pomegranate juice (1%, vol/vol) or glucose (7.5 mM) for 24 h before exposure to hypoxia for up to 24 h. B: top, Western blots of cl-Parp and cl-Cyt 18 in syncytiotrophoblasts under hypoxic conditions for up to 24 h with pomegranate juice compared with glucose. Middle and bottom, summary graphs of densitometry of cl-Parp and cl-Cyt 18 expression, n = 3. *P < 0.05, 2-way ANOVA, with Bonferroni post hoc test. C: lactate dehydrogenase (LDH) analysis of syncytiotrophoblasts with pomegranate juice compared with control under hypoxia. Media from these cultures were collected and assayed for leakage of LDH, a marker of cell death, n = 3. *P < 0.05, 2-way ANOVA, with Bonferroni post hoc test.
Cultured syncytiotrophoblasts exposed to the hypoxia mimetic cobalt chloride diagramed in Fig. 5A also showed increased levels of apoptosis in a time-dependent manner (Fig. 5B). Compared with control, pomegranate juice significantly reduced the levels of cl-Parp and cl-Cyt 18 (P < 0.05, Fig. 5C) and LDH in the culture medium (P < 0.05, Fig. 5D). We had examined cultures by phase microscopy during exposure to staurosporine and found that, after 24 h exposure, many cells were detached from the culture surface (data not shown). We thus targeted our analysis to syncytiotrophoblasts exposed to vehicle control or staurosporine for 4 h. Consistent with our previous results (11), staurosporine significantly induced apoptosis in a time-dependent manner, as measured by the increased levels of cl-Parp and cl-Cyt 18 (Fig. 5E). Remarkably, pomegranate juice attenuated staurosporine-induced cell death, as shown by reduced expression of cl-Parp, cl-Cyt 18, and of LDH in the medium (Fig. 5, F and G). Collectively, these results indicate that pomegranate juice has anti-apoptotic effects and limits injury from very potent chemical inducers of cell death.
Two prevalent polyphenols in pomegranate juice are ellagic acid and punicalagin (38). We tested the hypothesis that these agents were involved in the protection of syncytiotrophoblast apoptosis by pomegranate juice. As diagramed in Fig. 6A, pomegranate juice decreased spontaneous apoptosis in syncytiotrophoblasts cultured in standard conditions compared with control (Fig. 6B) and under conditions of stress induced by hypoxia, cobalt chloride, or staurosporine (Fig. 6, CE). Interestingly, ellagic acid showed increased apoptosis in the cultured syncytiotrophoblasts compared with control (Fig. 6B), indicating that this polyphenol was not protective. In contrast, punicalagin significantly decreased trophoblast death, as shown by reduced levels of cl-Parp (Fig. 7B) and medium LDH (Fig. 7C). We verified that both pomegranate juice and punicalagin had antioxidant effects, reflected by reduced Hsp 90 levels under conditions of hypoxia (Fig. 7B). In all cases where punicalagin and pomegranate treatment of primary cultures were directly compared (Fig. 7, B and C), punicalagin was equally effective at reducing cell death or oxidative stress. Moreover, punicalagin reduced death of syncytiotrophoblasts exposed to cobalt chloride or staurosporine, as shown by reduced levels of cl-Parp (Fig. 7, D and F) and medium LDH (Fig. 7, E and G) compared with control.
Fig. 6.
The effect of ellagic acid on the syncytiotrophoblasts under standard conditions or exposure to multiple stimuli. A: diagram of pomegranate juice or ellagic acid exposure for the primary human trophoblasts under standard conditions, hypoxia, or exposed to CoCl2 or staurosporine. The trophoblasts were pretreated with pomegranate juice (1%, vol/vol), ellagic acid (40 μM), or glucose (7.5 mM) for 24 h before exposure to hypoxia, or CoCl2 (200 nM) for 24 h, or staurosporine (0.5 μM) for 4 h. BE: Western blots of cl-Parp (top) and summary graphs of densitometry of cl-Parp (bottom). B: 20% O2, standard conditions. C: <1% O2, hypoxic conditions. D: cultures with CoCl2. E: cultures with staurosporine. In all graphs, n = 3. *P < 0.05, 1-way ANOVA, with Bonferroni post hoc test.
DISCUSSION
The data show that pomegranate juice reduces oxidative stress in human placental villi in vivo and in villous explants and primary trophoblast cultures in vitro. Pomegranate juice limits both apoptotic and global cell death in cultures of syncytiotrophoblasts exposed to harsh insults. The polyphenol punicalagin mimics the ability of pomegranate to protect villous trophoblast from oxidative and chemical stress. We speculate that antenatal ingestion of pomegranate juice by mothers may limit placental injury in susceptible pregnancies, while realizing the preliminary nature of our in vivo data.
Biological oxidative stress is defined by an imbalance between antioxidants and free radicals, and this phenomenon associates with pregnancy maladies (6, 7). Labor is an in vivo model of oxidative stress for the human placenta (14, 19). The human placenta in the third trimester is perfused by modified uterine spiral arterioles that carry oxygenated maternal blood to the intervillous space where exchange of gases and nutrients occurs over the trophoblast layer of villi. Transient contractions of the myometrium during human labor compress the myometrial blood vessels and yield intermittent reductions of maternal uteroplacental blood flow into the chorioallantoic placenta (5). This creates intermittent hypoxia and reoxygenation due to decreased then increased blood flow over the placental villi, resulting in oxidative stress of the trophoblasts (14). Using Hsp 90 as a marker of oxidative stress, as previously documented in placental villi (14), we exploited this human model to test our hypothesis that pomegranate juice can protect trophoblasts from oxidative stress. We initially randomized equal numbers of patients to either apple or pomegranate juice, but unscheduled timing of deliveries in this otherwise low-risk population allowed us to sample only 12 of the 20 total placentas originally randomized for the study. Levels of pomegranate polyphenols in maternal blood and cord blood were measured and were increased in both maternal and cord blood of subjects who took pomegranate juice (data not shown). Importantly, we found significant differences in oxidative stress in the placentas of women consuming pomegranate compared with apple juice, encouraging us to proceed with in vitro studies. Although we had small numbers of placenta (n = 4) from the pomegranate-exposed mothers, these mothers also experienced longer labors (Table 1) that would usually increase oxidative stress or markers. Our limited sample size in vivo led to expansion of the hypotheses and in vitro testing. Moreover, a pilot randomized study of pomegranate juice in 40 pregnancies complicated by intrauterine growth restriction is in progress, and these placentas will yield a larger number of in vivo samples to further evaluate the impact of pomegranate juice. The clinical significance of this intervention on mothers and infants remains to be fully evaluated.
Oxidative stress triggers inflammation, endothelial dysfunction, and a reduction in nitric oxide with an increased production of peroxynitrite, a highly reactive free radical (4). The realization that placental maladaptation and increased oxidative stress contributes to the pathophysiology of preeclampsia stimulated enthusiasm for prenatal ingestion of pharmacologic dosages of vitamins C and E, as antioxidants to limit oxidative stress in pregnancies at risk. Although small clinical trials suggested benefits (10), larger trials showed no benefit from these vitamins (15, 28, 31, 34, 35) and a debated small risk for harm (48). We speculate that the scavenging of ROS by high doses of vitamins C and E is too extensive and prevents the low levels of intracellular ROS that are important as mediators of key signaling pathways (6). Notably, signaling by ROS is important in the formation of placental syncytiotrophoblast (2, 27). One reactive oxygen molecule is nitric oxide, and pomegranate juice enhances the release of nitric oxide from endothelium in nonpregnant sources (37). Increased nitric oxide production may be beneficial to pregnancy, since a recent randomized trial showed improved pregnancy outcomes in pregnant women supplemented with l-arginine to enhance nitric oxide production (45). Further studies are needed to determine if pomegranate juice treatment of human trophoblast also enhances nitric oxide levels and if this is important in the ability of pomegranate juice to protect trophoblasts from injury.
Pomegranate juice limited trophoblast demise from exogenous stress induced by rather potent insults, including low oxygen tension, the hypoxia mimetic cobalt chloride, and the protein kinase inhibitor staurosporine. Pomegranate juice contains high levels of polyphenols, including anthocyanins and ellagitannins (29), with >90% of the antioxidant activity from the latter. The major ellagitannin is punicalagin. Ellagitannins do not appear to be absorbed directly (9) but are first hydrolyzed to ellagic acid, which can circulate in the blood for several hours. Further metabolism of ellagic acid, in part by gut bacteria, results in demethyellagic acid glucuronide, urolithin derivatives, and other metabolites that can be found in the circulation for up to 48 h after ingestion (38). These metabolites in vivo likely provide some of the biological effects reported for pomegranate juice consumption (48). Our finding that punicalagin, at a concentration equivalent to the concentration reported for this polyphenol in 8 oz of pomegranate juice (41), mimics the protection of trophoblasts afforded by pomegranate supports the concept that the components in the juice likely work together to protect cells. The punicalagin is converted to ellagic acid by some cells (39), yet we found no protective effect from the latter polyphenol. Importantly, we instead found that ellagic acid added in pure form to medium resulted in more, not less, trophoblast cell death. Nutrient supplementation trials evaluating cardiovascular effects of vegetables and fruits relate the benefits observed to the combination of photochemical, fiber, and other nutrients in whole foods, instead of selected individual components (17, 21, 30, 40). In our in vitro systems, like others (1, 8, 13, 23), we directly applied pomegranate juice, punicalagin, or ellagic acid to the cell culture. We clearly recognize that compounds present in pomegranate juice are unlikely metabolized in vitro the same way as they are in vivo. What remains to be determined is if native punicalagin in pomegranate juice activates signaling pathways that protect trophoblast or if it is converted to compounds that work together with other chemicals in pomegranate for the protective effect.
Safety concerns accompany the introduction of any therapeutic maneuver, especially in pregnancy. There are no controlled studies on the safety of pomegranate juice in pregnancy, but pomegranate polyphenol extracts up to 1,400 mg have been described as safe in nonpregnant human studies (3). Importantly, there are no reported serious adverse effects from ingestion of pomegranate, and data have accumulated suggesting benefits in prevention of cancer and arteriosclerosis from agents present in pomegranate. The literature describing cardiovascular benefits of pomegranate juice in humans and murine models was recently reviewed, and the limited studies available indicate that this product has antioxidant, antiatherogenic, anti-inflammatory, and antihypertensive effects (3).
In summary, we found that pomegranate juice, and specifically its most abundant polyphenol punicalagin, reduce oxidative stress in vivo and in vitro and attenuate spontaneous and induced apoptosis in villous explants and cultured human trophoblasts. We speculate that maternal dietary supplementation with pomegranate juice during pregnancy has the potential to decrease the oxidative stress and stimulus-induced apoptosis of placental trophoblasts and may decrease the occurrence of placental dysfunction and thereby the maladies caused in pregnancy.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grants R01 HD-29190 (D. M. Nelson) and P30HD-062171 [Washington University Intellectual and Developmental Disabilities Research Center (T. Inder)] and The Foundation for Barnes-Jewish Hospital (D. M. Nelson).
DISCLOSURES
All the coauthors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Author contributions: B.C., M.G.T., and D.M.N. conception and design of research; B.C. and M.G.T. performed experiments; B.C., M.G.T., and J.S.S. analyzed data; B.C. and D.M.N. interpreted results of experiments; B.C. prepared figures; B.C. drafted manuscript; B.C., M.G.T., M.S.L., J.S.S., R.L., and D.M.N. edited and revised manuscript; B.C., M.G.T., M.S.L., J.S.S., R.L., T.I., and D.M.N. approved final version of manuscript.
REFERENCES
- 1. Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem 54: 980– 985, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Banerjee S, Chambers AE, Campbell S. Is vitamin E a safe prophylaxis for preeclampsia? Am J Obstet Gynecol 194: 1228–1233, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Basu A, Penugonda K. Pomegranate juice: a heart-healthy fruit juice. Nutr Rev 67: 49–56, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Booth AD, Wallace S, McEniery CM, Yasmin Brown J, Jayne DR, Wilkinson IB. Inflammation and arterial stiffness in systemic vasculitis: a model of vascular inflammation. Arthritis Rheum 50: 581–588, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Brar HS, Platt LD, DeVore GR, Horenstein J, Medearis AL. Qualitative assessment of maternal uterine and fetal umbilical artery blood flow and resistance in laboring patients by Doppler velocimetry. Am J Obstet Gynecol 158: 952–956, 1988 [DOI] [PubMed] [Google Scholar]
- 6. Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol 25: 287–299, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30: 473–482, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campos-Esparza MR, Sanchez-Gomez MV, Matute C. Molecular mechanisms of neuroprotection by two natural antioxidant polyphenols. Cell Calcium 45: 358–368, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Cerda B, Espin JC, Parra S, Martinez P, Tomas-Barberan FA. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur J Nutr 43: 205–220, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Chappell LC, Seed PT, Briley AL, Kelly FJ, Lee R, Hunt BJ, Parmar K, Bewley SJ, Shennan AH, Steer PJ, Poston L. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet 354: 810– 816, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Chen B, Longtine MS, Sadovsky Y, Nelson DM. Hypoxia downregulates p53 but induces apoptosis and enhances expression of BAD in cultures of human syncytiotrophoblasts. Am J Physiol Cell Physiol 299: C968–C976, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen B, Nelson DM, Sadovsky Y. N-myc down-regulated gene 1 modulates the response of term human trophoblasts to hypoxic injury. J Biol Chem 281: 2764–2772, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Chen PS, Li JH. Chemopreventive effect of punicalagin, a novel tannin component isolated from Terminalia catappa, on H-ras-transformed NIH3T3 cells. Toxicol Lett 163: 44–53, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Cindrova-Davies T, Yung HW, Johns J, Spasic-Boskovic O, Korolchuk S, Jauniaux E, Burton GJ, Charnock-Jones DS. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am J Pathol 171: 1168–1179, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conde-Agudelo A, Romero R, Kusanovic JP, Hassan SS. Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol 204: 503, e501–e503, e512, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Nigris F, Williams-Ignarro S, Sica V, Lerman LO, D'Armiento FP, Byrns RE, Casamassimi A, Carpentiero D, Schiano C, Sumi D, Fiorito C, Ignarro LJ, Napoli C. Effects of a pomegranate fruit extract rich in punicalagin on oxidation-sensitive genes and eNOS activity at sites of perturbed shear stress and atherogenesis. Cardiovasc Res 73: 414–423, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Erlund I, Koli R, Alfthan G, Marniemi J, Puukka P, Mustonen P, Mattila P, Jula A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr 87: 323–331, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Fowden AL, Forhead AJ, Coan PM, Burton GJ. The placenta and intrauterine programming. J Neuroendocrinol 20: 439–450, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Hung TH, Burton GJ. Hypoxia and reoxygenation: a possible mechanism for placental oxidative stress in preeclampsia. Taiwan J Obstet Gynecol 45: 189–200, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Ignarro LJ, Byrns RE, Sumi D, de Nigris F, Napoli C. Pomegranate juice protects nitric oxide against oxidative destruction and enhances the biological actions of nitric oxide. Nitric Oxide 15: 93–102, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Iribarren C, Folsom AR, Jacobs DR, Jr, Gross MD, Belcher JD, Eckfeldt JH. Association of serum vitamin levels, LDL susceptibility to oxidation, and autoantibodies against MDA-LDL with carotid atherosclerosis A case-control study The ARIC Study Investigators Atherosclerosis Risk in Communities. Arterioscler Thromb Vasc Biol 17: 1171–1177, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Kim MS, Hyo Kim J, Kry D, Ae Choi M, Ok Choi D, Gon Cho B, Jin YZ, Ho Lee S, Park BR. Effects of acute hypotension on expression of cFos-like protein in the vestibular nuclei of rats. Brain Res 962: 111–121, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Lee SI, Kim BS, Kim KS, Lee S, Shin KS, Lim JS. Immune-suppressive activity of punicalagin via inhibition of NFAT activation. Biochem Biophys Res Commun 371: 799–803, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Longtine MS, Chen B, Odibo AO, Zhong Y, Nelson DM. Caspase-mediated apoptosis of trophoblasts in term human placental villi is restricted to cytotrophoblasts and absent from the multinucleated syncytiotrophoblast. Reproduction 143: 107–121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Longtine MS, Nelson DM. Placental dysfunction and fetal programming: the importance of placental size, shape, histopathology, and molecular composition. Semin Reprod Med 29: 187–196, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loren DJ, Seeram NP, Schulman RN, Holtzman DM. Maternal dietary supplementation with pomegranate juice is neuroprotective in an animal model of neonatal hypoxic-ischemic brain injury. Pediatr Res 57: 858–864, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Martin KR, Barrett JC. Reactive oxygen species as double-edged swords in cellular processes: low-dose cell signaling versus high-dose toxicity. Hum Exp Toxicol 21: 71–75, 2002 [DOI] [PubMed] [Google Scholar]
- 28. McCance DR, Holmes VA, Maresh MJ, Patterson CC, Walker JD, Pearson DW, Young IS. Vitamins C and E for prevention of pre-eclampsia in women with type 1 diabetes (DAPIT): a randomised placebo-controlled trial. Lancet 376: 259–266, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCutcheon AUJ, Brown DJ. Scientific and clinical monograph for Pom Wonderful Pomegranate Juice. Am Botan Council 20: 1–19, 2008 [Google Scholar]
- 30. Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, Jacobs DR., Jr Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 83: 1369–1379, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet 367: 1145–1154, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Rampersad RCZM, Nelson DM. Development and anatomy of the human placenta. In: The Placenta: From development to Disease. Oxford, UK: Wiley-Blackwell, 2011, p. 19– 26 [Google Scholar]
- 33. Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet 366: 1797–1803, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, Pearson GD, Wapner RJ, Varner MW, Thorp JM, Jr, Mercer BM, Peaceman AM, Ramin SM, Carpenter MW, Samuels P, Sciscione A, Harper M, Smith WJ, Saade G, Sorokin Y, Anderson GB. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med 362: 1282–1291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS. Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med 354: 1796–1806, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Sarkar J, Singh N, Meena S, Sinha S. Staurosporine induces apoptosis in human papillomavirus positive oral cancer cells at G2/M phase by disrupting mitochondrial membrane potential and modulation of cell cytoskeleton. Oral Oncol 45: 974–979, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Schmitt CA, Dirsch VM. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide 21: 77–91, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr 136: 2481–2485, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Seeram NP, Lee R, Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin Chim Acta 348: 63–68, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Sesso HD, Buring JE, Norkus EP, Gaziano JM. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. Am J Clin Nutr 79: 47–53, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Siman CM, Sibley CP, Jones CJ, Turner MA, Greenwood SL. The functional regeneration of syncytiotrophoblast in cultured explants of term placenta. Am J Physiol Regul Integr Comp Physiol 280: R1116–R1122, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Stenger C, Naves T, Verdier M, Ratinaud MH. The cell death response to the ROS inducer, cobalt chloride, in neuroblastoma cell lines according to p53 status. Int J Oncol 39: 601–609, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Thornburg KL, O'Tierney PF, Louey S. Review: The placenta is a programming agent for cardiovascular disease. Placenta Suppl 31: S54–S59, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Toal M, Keating S, Machin G, Dodd J, Adamson SL, Windrim RC, Kingdom JC. Determinants of adverse perinatal outcome in high-risk women with abnormal uterine artery Doppler images. Am J Obstet Gynecol 198: 330 e331–e337, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Vadillo-Ortega F, Perichart-Perera O, Espino S, Avila-Vergara MA, Ibarra I, Ahued R, Godines M, Parry S, Macones G, Strauss JF. Effect of supplementation during pregnancy with l-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: randomised controlled trial. Br Med J 342: d2901, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vengellur A, LaPres JJ. The role of hypoxia inducible factor 1alpha in cobalt chloride induced cell death in mouse embryonic fibroblasts. Toxicol Sci 82: 638–646, 2004 [DOI] [PubMed] [Google Scholar]
- 47. West T, Atzeva M, Holtzman DM. Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury. Dev Neurosci 29: 363–372, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu H, Perez-Cuevas R, Xiong X, Reyes H, Roy C, Julien P, Smith G, von Dadelszen P, Leduc L, Audibert F, Moutquin JM, Piedboeuf B, Shatenstein B, Parra-Cabrera S, Choquette P, Winsor S, Wood S, Benjamin A, Walker M, Helewa M, Dube J, Tawagi G, Seaward G, Ohlsson A, Magee LA, Olatunbosun F, Gratton R, Shear R, Demianczuk N, Collet JP, Wei S, Fraser WD. An international trial of antioxidants in the prevention of preeclampsia (INTAPP). Am J Obstet Gynecol 202: 239, e231–e239, e210, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Yu AC, Liu RY, Zhang Y, Sun HR, Qin LY, Lau LT, Wu BY, Hui HK, Heung MY, Han JS. Glial cell line-derived neurotrophic factor protects astrocytes from staurosporine- and ischemia- induced apoptosis. J Neurosci Res 85: 3457–3464, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Zeno S, Zaaroor M, Leschiner S, Veenman L, Gavish M. CoCl(2) induces apoptosis via the 18 kDa translocator protein in U118MG human glioblastoma cells. Biochemistry 48: 4652–4661, 2009 [DOI] [PubMed] [Google Scholar]