Abstract
AS160 and its closely related protein TBC1D1 have emerged as key mediators for both insulin- and contraction-stimulated muscle glucose uptake through regulating GLUT4 trafficking. Insulin increases AS160 phosphorylation at multiple Akt/PKB consensus sites, including Thr649, and promotes its binding to 14-3-3 proteins through phospho-Thr649. We recently provided genetic evidence that AS160-Thr649 phosphorylation/14-3-3 binding plays a key role in mediating insulin-stimulated glucose uptake in muscle. Contraction has also been proposed to increase phosphorylation of AS160 and TBC1D1 via AMPK, which could be detected by a generic phospho-Akt substrate (PAS) antibody. Here, analysis of AS160 immunoprecipitates from muscle extracts with site-specific phospho-antibodies revealed that contraction and AICAR caused no increase but rather a slight decrease in phosphorylation of the major PAS recognition site AS160-Thr649. In line with this, contraction failed to enhance 14-3-3 binding to AS160. Consistent with previous reports, we also observed that in situ contraction stimulated the signal intensity of PAS antibody immunoreactive protein of ∼150–160 kDa in muscle extracts. Using a TBC1D1 deletion mutant mouse, we showed that TBC1D1 protein accounted for the majority of the PAS antibody immunoreactive signals of ∼150–160 kDa in extracts of contracted muscles. Consistent with the proposed role of AS160-Thr649 phosphorylation/14-3-3 binding in mediating glucose uptake, AS160-Thr649Ala knock-in mice displayed normal glucose uptake upon contraction and AICAR in isolated muscles. We conclude that the previously reported PAS antibody immunoreactive band ∼150–160 kDa, which were increased upon contraction, does not represent AS160 but TBC1D1, and that AS160-Thr649Ala substitution impairs insulin- but neither contraction- nor AICAR-stimulated glucose uptake in mouse skeletal muscle.
Keywords: Akt substrate of 160 kDa, phosphorylation, 14-3-3, contraction, 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside, glucose transport
the uptake of glucose into skeletal muscles for storage as glycogen or generation of energy represents a major regulatory event in whole body glucose homeostasis. This process is highly responsive to insulin and physical exercise. Insulin-stimulated glucose uptake into skeletal muscles operates via the PI 3-kinase-PKB (also known as Akt)-stimulated translocation of glucose transporter 4 (GLUT4) from intracellular storage vesicles to the plasma membrane (reviewed in Refs. 8, 14, 16, 27). One mediator of these events is Akt substrate of 160 kDa (AS160, also known as TBC1D4), a Rab GTPase-activating protein (RabGAP), which is phosphorylated at multiple sites including Ser325, Ser348, Ser577, Ser595, Thr649, Ser673, and Ser758 (numbering according to mouse AS160; the corresponding residues on human AS160 are Ser318, Ser341, Ser570, Ser588, Thr642, Ser666, and Ser751) (11, 29, 31). Insulin-induced phosphorylation of Akt substrate of 160 kDa (AS160) increases its binding to 14-3-3 proteins mainly via Thr649 phosphorylation, which has been proposed to inhibit its RabGAP function toward downstream Rab(s) through yet unknown mechanisms, consequently promoting GTP loading of Rab(s) and, hence, GLUT4 translocation (11, 24). Besides insulin, contractions also promote glucose uptake through GLUT4 translocation independently of the proximal insulin receptor signaling pathway, induced at least in part through AMP-activated protein kinase (AMPK) (3, 12, 19, 26). Several studies have shown that muscle contractions in rodents induce phosphorylation signals for a protein of ∼160 kDa, as detected by a phospho-Akt substrate (PAS) antibody (20, 21, 34) that can detect Akt substrates containing the generic Akt phosphomotif (Arg-Xaa-Arg-Xaa-Xaa pSer/Thr, Xaa, any amino acids). This signal was ascribed to AS160 because the PAS antibody recognizes phosphorylated AS160 (predominantly at Thr649) (11, 24, 31). These data led to the hypothesis that AS160 would regulate both insulin- and contraction-stimulated glucose uptake by functioning as a critical signaling node for independent stimulators of skeletal muscle glucose uptake. Subsequently, Kramer et al. (21) overexpressed AS160–4P mutant (in which Ser325, Ser595, Thr649, and Ser758 had been mutated to alanine) in skeletal muscles through electroporation and found that both insulin- and contraction-mediated glucose uptake were significantly impaired in vivo.
While others have suggested that AMPK phosphorylates the main PAS site (Thr649), in our previous studies, AMPK failed to significantly phosphorylate AS160 Thr649 or promote 14-3-3 binding to AS160 either in cell-free assays or in cell lines including L6 muscle and 3T3-L1 adipose cells (4, 5, 11). Instead, we found that AMPK robustly phosphorylates Ser231 on the related RabGAP TBC1D1 (numbering according to mouse protein), thereby promoting its binding to 14-3-3 proteins in muscle cells (5, 23). Overexpression of a TBC1D1–4P mutant (in which Ser231, Thr499, Thr590, and Ser621 were replaced by alanine) led to a decrease in contraction-induced glucose uptake in mouse skeletal muscles (1). On the basis of our findings (5, 11) and others' (1, 24, 31), we hypothesize that AS160/14-3-3 interaction mainly regulates insulin-induced glucose uptake, while TBC1D1/14-3-3 interaction plays a role in AMPK-mediated glucose uptake into skeletal muscles. Toward addressing this hypothesis, we recently generated a knock-in mouse model in which AS160 Thr649 was mutated to a nonphosphorylatable alanine to prevent insulin-induced 14-3-3 binding to AS160 (6). AS160 knock-in mice exhibited impaired insulin sensitivity with lower insulin-induced glucose uptake into skeletal muscles, suggesting that AS160 Thr649 phosphorylation and/or its binding to 14-3-3 plays an important role in regulating insulin-induced glucose uptake into muscles (6).
To resolve the apparent inconsistencies in the literature, here we initially performed an extensive side-by-side comparison of the effect of contraction on the multisite phosphorylation of AS160 and TBC1D1 in mouse skeletal muscles. We then further investigated the potential role of phosphorylation of AS160 Thr649 and its binding to 14-3-3 in regulating AMPK-mediated glucose uptake into skeletal muscles in AS160 Thr649Ala knock-in mice and in animals deficient in TBC1D1.
MATERIALS AND METHODS
Materials.
Recombinant human insulin was from Novo Nordisk (Denmark), and AICAR from Toronto Research Chemicals (Canada). Microcystin-LR was from Linda Lawton (Robert Gordon's University, Aberdeen, UK), protease inhibitor cocktail tablets (no. 1697498) from Roche Diagnostics (Mannheim, Germany), and precast SDS-polyacrylamide gels from Invitrogen (Paisley, UK). Protein G-Sepharose was from GE Healthcare (Buckinghamshire, UK). 2-Deoxy-d-[1-3H]glucose and d-[1-14C]mannitol were from PerkinElmer (Seer Green, UK). All other chemicals were from BDH Chemicals or Sigma-Aldrich (Poole, UK).
Antibodies.
Sheep antibodies against total and phosphorylated AS160 and TBC1D1 were as previously described (5, 11). Sheep antibody against AMPKα2 (28) was provided by D. Grahame Hardie (University of Dundee). The antibody that recognizes phosphorylated Thr649 on AS160 (441071G) was from Invitrogen. The pan-14-3-3 antibody was K-19 from Santa Cruz Biotechnology. Antibodies that recognize phosphorylated Thr172 on AMPK (cat. no. 2535), phosphorylated Ser212 on ACC2 (acetyl-CoA carboxylase-2; cat. no. 3661), anti-AMPKα (cat. no. 2532), and anti-ACC (cat. no. 3676), and PAS antibody (cat. no. 9611) were from Cell Signaling Technology.
Generation of TBC1D1 deletion mutant mice.
A conditional TBC1D1-deficient mouse was generated by the transgenic service facility at University of Dundee, in which the fourth exon of tbc1d1 was flanked by loxP-Cre excision sites. The fourth exon of tbc1d1 was excised in the in TBC1D1 deletion mutant mouse by mating with the Bal1 mouse line, in which the Cre recombinase is expressed in all tissues resulting in an in-frame deletion of tbc1d1. TBC1D1 deletion mutant mice are viable and display no apparent adverse phenotype or growth defect.
Mouse breeding and genotype analysis.
All animal studies and breeding were approved by the University of Dundee Ethics Committee and performed under a United Kingdom Home Office project license. All the mice were housed with a 12:12-h light-dark cycle and free access to food and water unless stated otherwise. Genotyping of Thr649Ala-AS160 knock-in mice and wild-type littermate controls was performed by PCR using genomic DNA isolated from ear biopsy as previously described (6). Genotyping of the TBC1D1 deletion mutant allele was carried out by PCR using the following primers: 5′-TGGTTTACTGTGGCAGGAGGCAT-3′ and 5′-TATCAGGTTATTTAAAAAAATCCAGTTACCTGG-3′, and the wild-type allele was genotyped using the primers: 5′-TGGTTTACTGTGGCAGGAGGCAT-3′ and 5′-CACTGGGCTTTGTCTCTGATACTG-3′.
Tissue lysis and immunoprecipitations.
Unless otherwise stated, mouse tissues were homogenized with a Polytron tissue processor (Kinematica) in ice-cold lysis buffer [50 mM Tris·HCl, pH 7.4, 1 mM EDTA, 1 mM EGTA, 1% (wt/vol) Triton X-100, 1 mM sodium o-vanadate, 10 mM sodium glycerophosphate, 50 mM NaF, 5 mM sodium pyrophosphate, 0.27 M sucrose, 2 μM microcystin-LR, 1 mM benzamidine, 0.1% (vol/vol) 2-mercaptoethanol, and Complete proteinase inhibitor mixture (Roche)]. Lysates were clarified by centrifugation, snap-frozen in liquid N2, and stored at −80°C until analysis. Protein concentrations were determined with Bradford reagent (Thermo Scientific).
AS160 and TBC1D1 were immunoprecipitated with anti-AS160 (1 μg antibody/mg tissue lysate protein) and anti-TBC1D1 (1 μg antibody/mg tissue lysate protein), respectively. Briefly, lysates were precleared by incubation with protein G-Sepharose for 1 h at 4°C. The supernatants were then incubated with the antibody-coupled protein G-Sepharose overnight at 4°C. The immunoprecipitates were washed and eluted in SDS sample buffer for subsequent Western blot analysis as previously described (4, 5).
Western blots and quantitation.
After SDS-PAGE, proteins were transferred onto nitrocellulose membranes, blocked with 5% milk in TBS-T, and incubated at 4°C for 16 h using the indicated antibodies at 1 μg/ml in 5% milk in TBS-T. Detection was performed using horseradish peroxidase-conjugated secondary antibodies (Promega) and ECL (enhanced chemiluminescence reagent; GE Healthcare). The images were scanned, imported into an Odyssey imaging system (LI-COR Biosciences), and quantified.
AICAR tolerance test.
Mice were deprived of food for 4 h (9:00 AM to 1:00 PM) and weighed, and basal blood glucose was measured using a Breeze 2 glucometer (Bayer) following tail incision. The mice were administered 250 mg/kg AICAR (dissolved in PBS) by intraperitoneal injection, and blood glucose was measured at the indicated times.
In situ muscle contraction.
After mice were anesthetized with pentobarbital sodium (90 mg/kg body wt ip), the sciatic nerves to both hindlimbs were surgically exposed and electrodes attached. In situ contraction of hindlimb muscles through electrical stimulation of sciatic nerves was performed on one leg for either 5 min (for glucose uptake) or 10 min (for biochemistry/Western blotting), while the other leg served as sham-operated (noncontracted) control, as previously described, (30). After contraction, mice were killed by cervical dislocation extensor digitorum longus (EDL) muscles from both legs were isolated, and 2-deoxyglucose uptake was performed as described below. For study of signaling proteins, various hindlimb muscles were rapidly removed and snap-frozen in liquid nitrogen.
Muscle incubation and glucose uptake ex vivo.
Tendons from both ends of either soleus or EDL muscles, as indicated, were tied with suture (silk 4-0) and mounted on an incubation apparatus. The muscles were incubated with or without 2 mM AICAR in KRB buffer for 50 min (for study of signaling proteins, the muscles were snap-frozen in liquid nitrogen after incubation) (17), and glucose uptake was monitored for 10 min as previously described (30). For contraction-induced glucose uptake, EDL muscles were removed after in situ contraction and preincubated for 15 min in KRB buffer, and glucose uptake was performed for 10 min as previously described (30). After glucose uptake, the muscles were snap-frozen in liquid nitrogen. The muscles were weighed, hydrolyzed in 1 M NaOH at 80°C for 10 min, and neutralized in 1 M HCl, and radioisotopes in the muscles were counted using a Tri-Carb 2800TR scintillation counter (PerkinElmer).
Statistical analysis.
Unless stated otherwise, data analysis was performed via ANOVA (SigmaStat) with the Holm-Sidak method for posttest, and differences were considered statistically significant at P < 0.05.
RESULTS
In situ contraction induces changes in AS160 phosphorylation in wild-type and Thr649Ala knock-in mice.
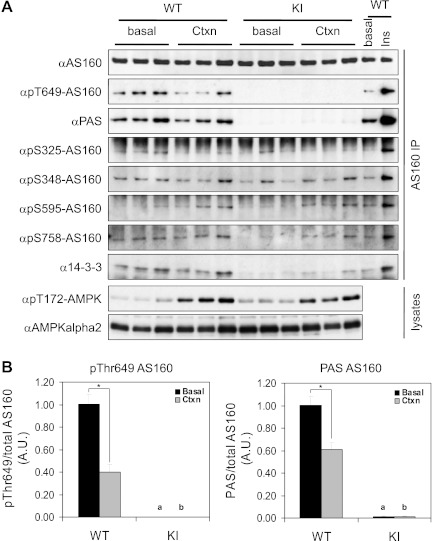
In situ contraction of hindlimb muscles was stimulated via sciatic nerve for 10 min in wild-type and Thr649Ala-AS160 knock-in mice, after which AMPKα T-loop phosphorylation (Thr172) was markedly elevated in both genotypes to a similar extent (Figs. 1A and 2A). While others have reported that contraction (both ex vivo and in situ) promoted AS160 Thr649 phosphorylation (20, 34), we observed that in situ contraction resulted in a small but significant (P < 0.05) decrease in Thr649 phosphorylation paralleled by a decreased PAS phosphorylation signal for AS160 immunoprecipitated from extracts of both wild-type gastrocnemius (GAS) and tibialis anterior (TA) muscles (Figs. 1B and 2B, respectively). As expected, these signals were absent from Thr649Ala-AS160 protein from muscle extracts of Thr649Ala-AS160 knock-in mice (Figs. 1, A and B and 2, A and B), which confirms the specificity of the phospho-Thr649 antibody. Consistent with the key role of Thr649 phosphorylation in mediating 14-3-3 protein binding to AS160, we observed that contraction did not increase the amount of 14-3-3 proteins coimmunoprecipitating with AS160 from muscle extracts of wild-type mice, whereas insulin increased 14-3-3 binding to AS160 (positive control), and the 14-3-3/AS160 complex was barely detectable in muscle extracts from Thr649Ala knock-in mice (negative control) (Fig. 1A).
Fig. 1.
Phosphorylation of Akt substrate of 160 kDa (AS160) and TBC1D1 in gastrocnemius muscles from wild-type (WT) and AS160-Thr649Ala knock-in (KI) mice upon in situ contraction. In situ contraction of hindlimb muscles via sciatic nerve was performed for 10 min on one leg (Ctxn) from anesthetized KI mice and WT littermates (13–14 wk old), and the other leg served as sham-operated control (basal). For insulin stimulation, KI mice and WT littermates (12 wk old) were anesthetized, injected with 150 mU/g ip insulin (Ins) or saline (basal), and gastrocnemius (GAS) muscle was removed and snap-frozen 20 min after injection. AS160 protein was immunoprecipitated from 3 mg of extracts of GAS muscles and their phosphorylation determined using indicated site-specific phospho-antibodies. 14-3-3 Proteins were detected using 14-3-3 K19 antibody in AS160 immunoprecipitates. Total and phosphorylated AMPK were determined in 40 μg of muscle extracts using anti-AMPKα2 and anti-pT172AMPK antibodies, respectively. A: representative blots showing total and phosphorylated AS160, AMPK, and 14-3-3. B: quantitation of AS160 Thr649/PAS phosphorylation; n = 5. *P < 0.05; aP < 0.05 vs. WT (basal); bP < 0.05 vs. WT (Ctxn).
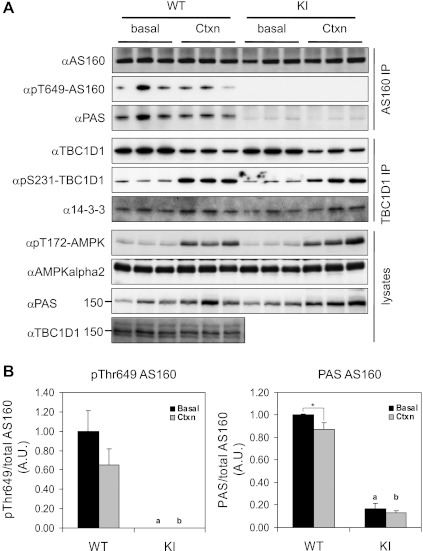
Fig. 2.
Phosphorylation of AS160 and TBC1D1 in tibialis anterior (TA) muscles from WT and KI mice upon in situ contraction. Hindlimb muscles were contracted in situ via sciatic nerve stimulation for 10 min on one leg (Ctxn) from anesthetized KI mice and WT littermates (13–14 wk old), and the other leg served as sham-operated control (basal). AS160 and TBC1D1 proteins were sequentially immunoprecipitated from 2 mg of extracts of TA muscles and their phosphorylation determined using indicated site-specific phospho-antibodies. Total and phosphorylated AMPK were determined in 40 μg of muscle extracts using anti-AMPKα2 and anti-pT172AMPK antibodies, respectively. phospho-Akt substrate (PAS) signal ∼160 kDa was detected in muscle extract using PAS antibody. A: representative blots showing total and phosphorylated AS160, TBC1D1, and AMPK. B: quantitation of AS160 Thr649/PAS phosphorylation; n = 5. *P < 0.05; aP < 0.05 vs. WT (basal); bP < 0.05 vs. WT (Ctxn).
Phosphorylation of AS160 on four other known sites, namely Ser325, Ser348, Ser595, and Ser758, was assessed using the respective phosphospecific antibodies on AS160 proteins immunoprecipitated from the extracts of GAS muscle (Fig. 1A). In contrast to insulin, in situ contraction did not increase the phosphorylation of Ser325, Ser348, and Ser758 in GAS muscle. However, in situ contraction increased the phosphorylation of Ser595 of AS160 to a similar extent (∼1.9-fold) in muscle extracts from both wild-type and AS160-Thr649Ala knock-in mice, although the magnitude was much weaker than with insulin (Fig. 1A).
In situ contraction induces changes in TBC1D1 phosphorylation in wild-type and AS160 knock-in mice.
Since PAS phosphorylation of AS160 has been mostly inferred in previous studies from signals detected in tissue lysates rather than immunopurified protein (20, 34), we next investigated PAS phosphorylation in muscle extracts. A PAS phosphorylation signal at ∼150–160 kDa was indeed increased in response to in situ contraction in TA muscle extracts from both wild-type and AS160 knock-in mice (Fig. 2A, bottom).
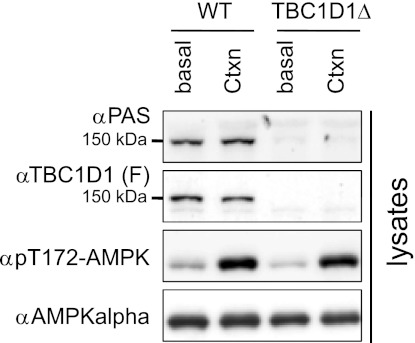
The related RabGAP, TBC1D1, also contains a phosphorylation site detected by PAS antibody at Thr590 (5, 25), which could be phosphorylated upon muscle contraction (10, 23). However, the PAS/Thr590 phosphorylation of immunoprecipitated TBC1D1 was largely unaltered, in parallel with a moderate decrease of immunoprecipitatable TBC1D1 in contracted TA muscle from both wild-type and AS160-Thr649Ala knock-in mice (data not shown). In contrast, Ser231 phosphorylation of TBC1D1 and coimmunoprecipitated 14-3-3 protein were robustly induced following in situ contraction in TA muscle extracts from both genotypes (Fig. 2A). We noticed that the amount of immunoprecipitatable TBC1D1 was consistently less, in parallel with a slight decrease of total TBC1D1 in the lysates (Figs. 2A and 3), in the contracted muscle under our experimental conditions (possibly owing to changes in the recognition of the antibody due to a posttranslation modification, although we do not know the accurate reason for this), which could potentially underestimate the PAS/Thr590 phosphorylation. Therefore, we further addressed whether the PAS signal at ∼150–160 kDa in extracts from contracted muscle was from TBC1D1 by using a TBC1D1 deletion mutant mouse in which the fourth exon of tbc1d1 was excised, resulting in an in-frame deletion of tbc1d1. The full-length TBC1D1 protein was not detectable in muscle lysates from the TBC1D1 deletion mutant mouse as expected (Fig. 3), whereas total AS160 expression was slightly increased (data not shown). Importantly, the PAS signal at ∼150–160 kDa was diminished in the extracts from the contracted TA muscle of the TBC1D1 deletion mutant mice (Fig. 3), showing that the PAS signal at ∼150–160 kDa in contracted TA muscle is most likely derived from TBC1D1.
Fig. 3.
Phosphorylation of TBC1D1 in TA muscles from WT and TBC1D1 deletion mutant mice upon in situ contraction. Hindlimb muscles were contracted in situ via sciatic nerve stimulation for 10 min on one leg (Ctxn) from anesthetized TBC1D1 deletion mutant mice and WT littermates (8 wk old), and the other leg served as sham-operated control (basal). Total and phosphorylated TBC1D1 and AMPK were determined in 40 μg of muscle extracts using anti-TBC1D1, anti-AMPKα2, and anti-pT172AMPK antibodies, respectively. PAS signal at ∼150–160 kDa was detected in muscle extract using PAS antibody.
AS160-Thr649Ala knock-in mutation does not impair contraction-induced glucose uptake into muscle.
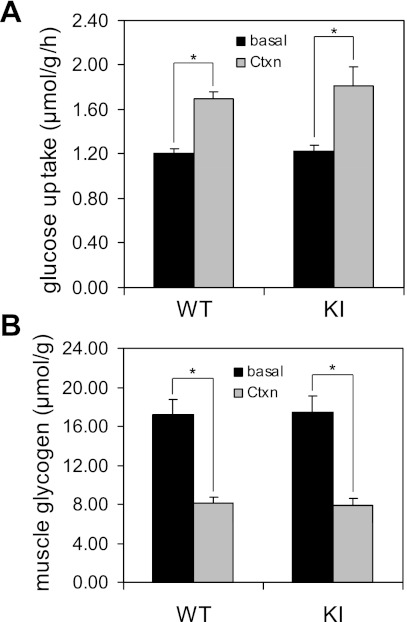
We next performed glucose uptake assays in isolated EDL and soleus muscles from both wild-type and AS160-Thr649Ala knock-in mice after in situ contraction. As expected, and consistent with our previous study (30), in situ contraction significantly increased (>40% over basal, P < 0.05) glucose uptake rates in wild-type EDL muscles (Fig. 4A). The increase in contraction-stimulated glucose uptake was nearly identical comparing the EDL muscles from AS160-Thr649Ala knock-in and wild-type littermates (Fig. 4A). Similarly, the AS160-Thr649Ala mutation did not inhibit the glucose uptake in the isolated soleus muscle from the knock-in mice upon in situ contraction (data not shown). High intensity in situ contraction is known to be energy-demanding stress that robustly depletes muscle glycogen storage (35). In situ contraction caused a significant decrease (∼50%) in glycogen content in TA muscle from the same wild-type animals used for glucose uptake assays (Fig. 4B). No difference was observed in the muscle glycogen content between wild-type and AS160-Thr649Ala knock-in mice in either sham-operated or contracted TA muscle (Fig. 4B), indicating that the intensity of contraction was comparable in the two genotypes. Since muscle glycogen content is inversely related to muscle glucose uptake (13), these data also suggest that the lack of inhibition on contraction-stimulated glucose uptake in muscles from AS160-Thr649Ala mice was not due to alterations in muscle glycogen levels.
Fig. 4.
Glucose uptake in EDL muscle and glycogen content in TA muscle from WT and KI mice upon in situ contraction. In situ contraction of hindlimb muscles via sciatic nerve was performed for 5 min on one leg (Ctxn) from anesthetized KI mice and WT littermates (8 wk old), and the other leg served as sham-operated control (basal). After contraction, EDL and TA muscles were removed from both legs, and glucose uptake (A) determined in EDL muscle and muscle glycogen content (B) determined in TA muscle as described in materials and methods; n = 5. *P < 0.05.
Pharmacological activation of AMPK by AICAR does not promote AS160 Thr649 phosphorylation in isolated skeletal muscles ex vivo.
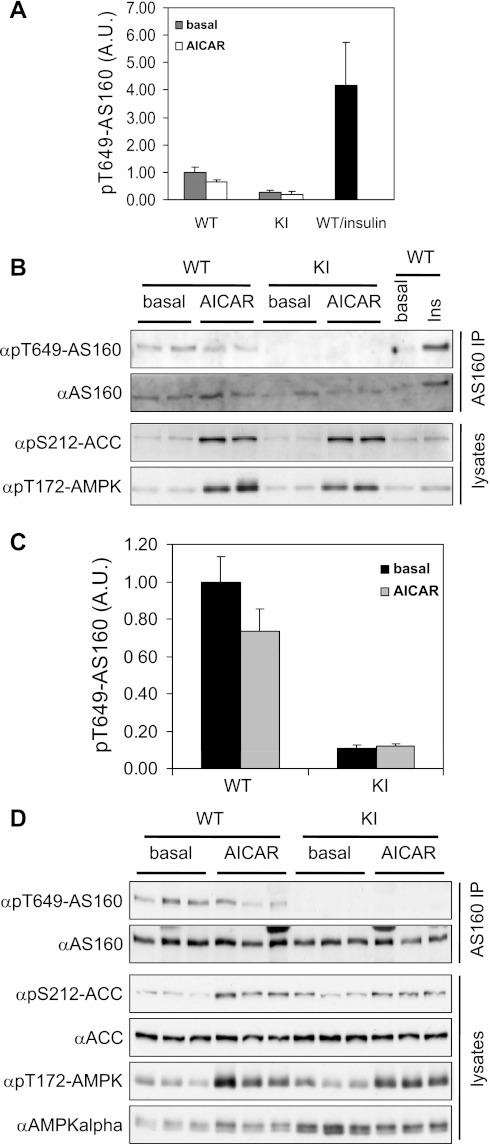
Next, we assessed the effect of AMPK activation on phosphorylation of AS160 Thr649 in isolated muscles ex vivo. We employed the well-established pharmacological AMPK activator AICAR, which elicited similar increases in phosphorylation of AMPK and its bona fide substrate ACC in isolated soleus and EDL muscles of both AS160-Thr649Ala and wild-type mice (Fig. 5, B and D, respectively). We observed that Thr649 phosphorylation of AS160 was slightly decreased when immunoprecipitated protein was analyzed from extracts of wild-type muscles (Fig. 5, A and C). Phospho-Thr649-AS160 was undetectable in Thr649Ala knock-in muscles (Fig. 5, B and D) as shown previously (Figs. 1 and 2).
Fig. 5.
Phosphorylation of AS160 Thr649 in isolated soleus and EDL muscles from WT and KI mice upon AICAR stimulation ex vivo. Soleus and EDL muscles from KI mice and WT littermates (10–14 wk old) were incubated with or without 2 mM AICAR ex vivo. AS160 was immunoprecipitated from 200 μg of muscle extracts, and Thr649 phosphorylation was determined using a site-specific phospho-antibody. Phosphorylated ACC and AMPK were determined in 40 μg of muscle extracts using antibodies that recognize phosphorylated ACC-Ser212 and AMPK-Thr172. Total ACC and AMPKα were determined in 40 μg of muscle extracts. A: quantitation of AS160 Thr649 phosphorylation in soleus muscles; n = 5. B: representative blots showing total and phosphorylated AS160 on immunoprecipitated AS160 and phosphorylated ACC and AMPK in muscle extracts. C: quantitation of AS160 Thr649 phosphorylation in EDL muscles; n = 5. D: representative blots showing total and phosphorylated AS160 on immunoprecipitated AS160 and total and phosphorylated ACC and AMPK.
AS160-Thr649Ala knock-in mutation does not impair AICAR-induced glucose uptake in isolated muscles.
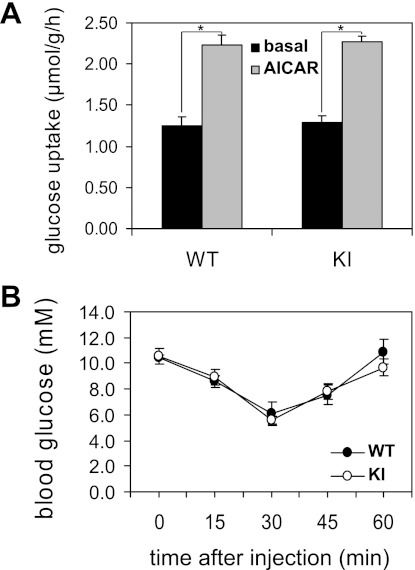
We then performed glucose uptake assays in isolated muscles upon AICAR stimulation. Incubation of isolated EDL muscle ex vivo with AICAR increased uptake of glucose into wild-type muscles nearly twofold (Fig. 6A). The AS160-Thr649Ala mutation did not inhibit AICAR-stimulated glucose uptake into EDL muscle from knock-in mice compared with wild-type littermates (Fig. 6A). Similarly, AICAR significantly stimulated glucose uptake in isolated soleus muscle from both AS160 knock-in mice and wild-type littermates (data not shown).
Fig. 6.
Glucose uptake in isolated EDL muscle upon AICAR stimulation, and AICAR tolerance test in WT and KI mice. A: EDL muscle from AS160-Thr649Ala knock-in mice and WT littermates (10–12 wk old) was incubated with or without 2 mM AICAR ex vivo. After incubation, muscle glucose uptake was determined as described in materials and methods; n = 6–7. *P < 0.05. B: KI mice and WT littermates (8 wk old) were partially fasted for 4 h before injection with AICAR (250 mg/kg body wt ip). Blood glucose levels were monitored before and after AICAR injection at indicated time points; n = 4–5.
AS160-Thr649Ala knock-in mutation does not alter whole body AICAR sensitivity.
A bolus injection of AICAR causes substantial hypoglycemia through the suppression of hepatic glucose production and stimulation of muscle glucose uptake, and this effect was significantly blunted in transgenic mice that overexpress dominant negative AMPK in muscle (9, 22). We injected a bolus of AICAR (250 mg/kg body wt ip) into both wild-type and AS160-Thr649Ala knock-in mice and monitored blood glucose clearance kinetics. AICAR decreased blood glucose levels at similar rates and extents in the two genotypes (Fig. 6B), indicating that whole body AICAR sensitivity in causing hypoglycemia was not altered in AS160-Thr649Ala knock-in mice.
DISCUSSION
The proposal that AS160 phosphorylation represents a convergence point mediating both insulin- and contraction-induced glucose transport in skeletal muscles (21) originally arose from the observation that a protein running at ∼160 kDa on SDS-PAGE gave a contraction-induced signal with the PAS antibody in mouse and human muscle extracts, which was ascribed to AS160 phosphorylation (7, 20, 34). The PAS antibody does recognize phosphorylated Thr649 on AS160 (11, 24). However, in contrast to previous reports (20, 34), we observed in this study that both PAS and Thr649 phosphorylation of immunoprecipitated AS160 were slightly decreased in various mouse skeletal muscle types upon in situ contraction or AICAR stimulation (Figs. 1, 2, and 5). In agreement with previous reports (20, 34), we did detect a PAS signal of a protein of ∼150–160 kDa in muscle extracts, which was increased upon contraction in muscle extracts from wild-type mice (Fig. 2A). However, this signal cannot be from Thr649 of AS160, as it was also present in muscle extracts from AS160-Thr649Ala knock-in mice (Fig. 2A). In addition, a contraction-induced PAS signal was absent from immunoprecipitated muscle AS160. Rather, the opposite was observed, as PAS/Thr649 phosphorylation of AS160 decreased slightly when muscles were contracted or stimulated with AICAR. In contrast, Bruss et al. (2) reported a PAS signal from AS160 immunoprecipitated from isolated rat muscle extracts, which was stimulated by contraction and AICAR ex vivo, and that the contraction response was inhibited by the PI 3-kinase inhibitor wortmannin. One possible reason for this discrepancy is that Bruss et al. reported that, under their experimental conditions, ex vivo muscle contractions robustly stimulated Akt phosphorylation, and wortmannin inhibited both Akt and AS160 phosphorylation; consequently, PAS/AS160 phosphorylation under their experimental conditions could be Akt dependent. However, this does not explain AICAR-mediated AS160 phosphorylation, as there was no detectable increase in Akt phosphorylation whereas AMPK was robustly activated. Another likelihood is that the polyclonal PAS antibody is produced by immunizing animals with a proprietary pool of phospho-Akt substrate peptides. Although PAS antibody reproducibly recognized phosphorylated Thr649 on AS160 (11, 24), whether there was any batch variation in the ability for PAS antibody to recognize additional phosphorylation sites on AS160 cannot be ruled out (e.g., phospho-Ser595). A third consideration is the variable experimental conditions employed in different laboratories for muscle contraction/exercise in rodents and humans. In line with this consideration, PAS phosphorylation of AS160 was found either increased (7, 15, 33) or unaltered (7, 34) in human muscle in response to different types of exercises. Although exercise did not increase PAS/Thr590 phosphorylation of TBC1D1 in human muscle (18), muscle contraction did enhance PAS phosphorylation of TBC1D1 in rodents (10, 23, 32). Our findings using the TBC1D1 deletion mutant mice (Fig. 3) strongly support a fourth possibility, that the contraction-induced PAS signal at ∼150–160 kDa derives from TBC1D1 in mouse (23, 32) whose PAS recognition site is Thr590 (5, 25). The importance of this PAS phosphorylation of TBC1D1 in mediating contraction-stimulated glucose uptake in muscle still needs to be established.
Consistent with contraction and AICAR slightly decreasing PAS/Thr649 phosphorylation of AS160, glucose uptake in response to these stimuli was not altered in skeletal muscles from the AS160-Thr649Ala knock-in mice compared with wild-type littermates. Thus, there is no apparent role for AS160 Thr649 phosphorylation in contraction- or AICAR-induced glucose uptake under the conditions/protocols tested here. In contrast, overexpression of the AS160–4P mutant in skeletal muscles inhibited contraction-stimulated glucose uptake in vivo (21). There are at least two possible explanations to account for this discrepancy. First, the other three sites (Ser325, Ser595, and Ser758) mutated in the AS160–4P mutant might be more important in mediating contraction- or AICAR-induced muscle glucose uptake. Note that Ser595 has been shown as a potential AMPK site (11), and its phosphorylation was increased upon in situ contraction in muscle extracts from both wild-type and AS160-Thr649Ala knock-in mice (Fig. 1). However, any role of AS160 Ser595 phosphorylation in mediating contraction-induced muscle glucose uptake remains to be investigated. Second is the likelihood that overexpressing the mutant protein disrupts the normal hierarchy of regulation. For example, AS160 can interact with the related RabGAP TBC1D1 (5, 25), and it is currently not clear whether overexpression of AS160–4P mutant can interfere with TBC1D1 protein in skeletal muscles, which is robustly phosphorylated on Ser231 upon contraction and AICAR stimulation. Overexpression of a TBC1D1–4P mutant (in which Ser231, Thr499, Thr590, and Ser621 were replaced by alanine) also decreased contraction-induced glucose uptake in skeletal muscles (1). Again, it is not clear whether overexpression of TBC1D1–4P mutant interferes with AS160 in muscles. Better tools to study the role of TBC1D1 phosphorylation in mediating contraction- or AICAR-induced glucose uptake into muscles are therefore needed. In our working hypothesis, TBC1D1 Ser231 phosphorylation and its resultant 14-3-3 interaction mediates AMPK-regulated muscle glucose uptake. This hypothesis will be addressed by generation of knock-in mice in which TBC1D1 Ser231 is mutated to nonphosphorylatable alanine.
In summary, in contrast to the proposed role of AS160 Thr649 phosphorylation as a convergence point mediating both insulin- and contraction-induced muscle glucose transport (20, 34), we find that both contractions and AICAR do not promote (but, rather modestly inhibit) AS160 Thr649 phosphorylation in mouse skeletal muscles. Consistent with these findings, the AS160-Thr649Ala knock-in mutation did not impair glucose uptake in skeletal muscles in response to either stimuli.
GRANTS
Thanks to Diabetes UK, the UK Medical Research Council, Dundee, district of Dundee Diabetes UK volunteer group, and pharmaceutical companies that sponsor the Division of Signal Transduction Therapy (AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck Serono, and Pfizer) for financial support.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.D., H.Y.W., K.S., and S.C. performed experiments; S.D., H.Y.W., and S.C. analyzed data; S.D., H.Y.W., K.S., C.M., and S.C. interpreted results of experiments; S.D., H.Y.W., and S.C. prepared figures; S.D., H.Y.W., K.S., C.M., and S.C. approved final version of manuscript; H.Y.W., K.S., C.M., and S.C. edited and revised manuscript; S.C. conception and design of research; S.C. drafted manuscript.
ACKNOWLEDGMENTS
We thank Gail Fraser for assistance with genotyping of mice, members of the resource unit for technical assistance, and Dr. Roger Hunter for critical reading of the manuscript. We thank Drs. Rachel Toth and Simon Arthur for helping in generation of TBC1D1 deletion mutant mouse. We thank Prof. D. Grahame Hardie for providing the AS160 phospho-Ser325, Ser348, Ser595, and Ser758 antibodies and AMPKα2 antibody.
Present address of S. Chen: Model Animal Research Center, Medical School of Nanjing University, Pukou District, Nanjing, 210061, China.
REFERENCES
- 1. An D, Toyoda T, Taylor EB, Yu H, Fujii N, Hirshman MF, Goodyear LJ. TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle. Diabetes 59: 1358–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54: 41–50, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab 32: 557–566, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Chen S, Mackintosh C. Differential regulation of NHE1 phosphorylation and glucose uptake by inhibitors of the ERK pathway and p90RSK in 3T3–L1 adipocytes. Cell Signal 21: 1984–1993, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, Mackintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J 409: 449–459, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Chen S, Wasserman DH, MacKintosh C, Sakamoto K. Mice with AS160/TBC1D4-Thr649Ala knock-in mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metab 13: 68–79, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deshmukh A, Coffey VG, Zhong Z, Chibalin AV, Hawley JA, Zierath JR. Exercise-induced phosphorylation of the novel Akt substrates AS160 and filamin A in human skeletal muscle. Diabetes 55: 1776–1782, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Foley K, Boguslavsky S, Klip A. Endocytosis, recycling, and regulated exocytosis of glucose transporter 4. Biochemistry 50: 3048–3061, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Fujii N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM, Goodyear LJ. AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem 280: 39033–39041, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Funai K, Cartee GD. Inhibition of contraction-stimulated AMP-activated protein kinase inhibits contraction-stimulated increases in PAS-TBC1D1 and glucose transport without altering PAS-AS160 in rat skeletal muscle. Diabetes 58: 1096–1104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geraghty KM, Chen S, Harthill JE, Ibrahim AF, Toth R, Morrice NA, Vandermoere F, Moorhead GB, Hardie DG, MacKintosh C. Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to IGF-1, EGF, PMA and AICAR. Biochem J 407: 231–241, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda) 21: 48–60, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Hargreaves M. Muscle glycogen and metabolic regulation. Proc Nutr Soc 63: 217–220, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Holman GD, Sandoval IV. Moving the insulin-regulated glucose transporter GLUT4 into and out of storage. Trends Cell Biol 11: 173–179, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Howlett KF, Mathews A, Garnham A, Sakamoto K. The effect of exercise and insulin on AS160 phosphorylation and 14-3-3 binding capacity in human skeletal muscle. Am J Physiol Endocrinol Metab 294: E401–E407, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab 5: 237–252, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Hunter RW, Treebak JT, Wojtaszewski JF, Sakamoto K. Molecular mechanism by which AMP-activated protein kinase activation promotes glycogen accumulation in muscle. Diabetes 60: 766–774, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jessen N, An D, Lihn AS, Nygren J, Hirshman MF, Thorell A, Goodyear LJ. Exercise increases TBC1D1 phosphorylation in human skeletal muscle. Am J Physiol Endocrinol Metab 301: E164–E171, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jessen N, Goodyear LJ. Contraction signaling to glucose transport in skeletal muscle. J Appl Physiol 99: 330–337, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 55: 2067–2076, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem 281: 31478–31485, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7: 1085–1094, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Pehmoller C, Treebak JT, Birk JB, Chen S, Mackintosh C, Hardie DG, Richter EA, Wojtaszewski JF. Genetic disruption of AMPK signaling abolishes both contraction- and insulin-stimulated TBC1D1 phosphorylation and 14-3-3 binding in mouse skeletal muscle. Am J Physiol Endocrinol Metab 297: E665–E675, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramm G, Larance M, Guilhaus M, James DE. A role for 14-3-3 in insulin-stimulated GLUT4 translocation through its interaction with the RabGAP AS160. J Biol Chem 281: 29174–29180, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Roach WG, Chavez JA, Miinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J 403: 353–358, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rose AJ, Richter EA. Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology (Bethesda) 20: 260–270, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Rowland AF, Fazakerley DJ, James DE. Mapping insulin/GLUT4 circuitry. Traffic 12: 672–681, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab 287: E310–E317, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab 295: E29–E37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J 24: 1810–1820, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, Goodyear LJ. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem 283: 9787–9796, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA, Wojtaszewski JF. AS160 phosphorylation is associated with activation of α- but not α2β2γ3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab 292: E715–E722, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 55: 2051–2058, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Witczak CA, Jessen N, Warro DM, Toyoda T, Fujii N, Anderson ME, Hirshman MF, Goodyear LJ. CaMKII regulates contraction- but not insulin-induced glucose uptake in mouse skeletal muscle. Am J Physiol Endocrinol Metab 298: E1150–E1160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]