Abstract
Skeletal muscle atrophy during bed rest is attributed, at least in part, to slower basal muscle protein synthesis (MPS). Essential amino acids (EAA) stimulate mammalian target of rapamycin (mTORC1) signaling, amino acid transporter expression, and MPS and are necessary for muscle mass maintenance, but there are no data on the effect of inactivity on this anabolic mechanism. We hypothesized that bed rest decreases muscle mass in older adults by blunting the EAA stimulation of MPS through reduced mTORC1 signaling and amino acid transporter expression in older adults. Six healthy older adults (67 ± 2 yr) participated in a 7-day bed rest study. We used stable isotope tracers, Western blotting, and real-time qPCR to determine the effect of bed rest on MPS, muscle mTORC1 signaling, and amino acid transporter expression and content in the postabsorptive state and after acute EAA ingestion. Bed rest decreased leg lean mass by ∼4% (P < 0.05) and increased postabsorptive mTOR protein (P < 0.05) levels while postabsorptive MPS was unchanged (P > 0.05). Before bed rest acute EAA ingestion increased MPS, mTOR (Ser2448), S6 kinase 1 (Thr389, Thr421/Ser424), and ribosomal protein S6 (Ser240/244) phosphorylation, activating transcription factor 4, L-type amino acid transporter 1 and sodium-coupled amino acid transporter 2 protein content (P < 0.05). However, bed rest blunted the EAA-induced increase in MPS, mTORC1 signaling, and amino acid transporter protein content. We conclude that bed rest in older adults significantly attenuated the EAA-induced increase in MPS with a mechanism involving reduced mTORC1 signaling and amino acid transporter protein content. Together, our data suggest that a blunted EAA stimulation of MPS may contribute to muscle loss with inactivity in older persons.
Keywords: physical inactivity, mammalian target of rapamycin, L-type amino acid transporter
short-term bed rest typically occurs during hospitalization for acute disease or injury and is a major contributor to functional decline in older adults (18). Significant loss of lean tissue is a consequence of bed rest in both young and older adults even in the absence of disease (14, 30). Ageing accelerates the inactivity-induced loss of muscle mass with bed rest (30), whereas it does not seem to worsen cast-immobilization muscle loss (24, 41). Previous studies have indicated that reductions in muscle tissue following bed rest or immobilization may be driven by a slower muscle protein synthesis (MPS) rate at rest (7, 14, 17, 19). However, no studies have explored the effect of immobilization on the muscle protein anabolic response to nutrients or the cellular mechanisms that underlie the reduction in muscle size in older adults during bed rest or hospitalization.
Anabolic stimulation by nutrients, particularly amino acids, is a powerful modulator of skeletal muscle anabolism and is a fundamental process for maintenance of skeletal muscle mass. Previous studies have shown that acute stimulation of MPS by feeding is attenuated in healthy, community-dwelling older adults (22, 27, 46) but can be normalized by adequate intake of amino acids, particularly leucine (28, 42).
Amino acid stimulation of MPS is tightly regulated by the mammalian target of rapamycin (mTORC1) signaling pathway (8, 16). Activated mTORC1 upregulates mRNA translation by targeting downstream substrates such as ribosomal S6 kinase 1 (S6K1) and 4E binding protein 1 (4EBP1) (10, 29). However, in order for exogenous amino acids to stimulate mTORC1 signaling they have to first enter the muscle cell via active transport. Mounting evidence suggests that a unique class of amino acid transporters, L-type amino acid transporter 1 (LAT1) and sodium-coupled neutral amino acid transporter 2 (SNAT2), are sensitive to changes in nutrient status and are associated with mTORC1 activation and MPS (25, 33, 36). A recent study (2) reported that SNAT2 and LAT1 are strategically arranged in the plasma membrane to aid the influx of amino acids (i.e., branched-chain amino acids). Additionally, SNAT2 may activate mTORC1 through a signaling mechanism independent of increases in intracellular amino acid concentrations (37). We have recently shown that amino acids and resistance exercise increase amino acid transporter mRNA expression and protein content (12, 13). However, there are currently no data regarding the impact of inactivity on nutrient-induced changes in amino acid transporter expression.
Therefore, the primary purpose of this study was to determine the effects of 7 days of bed rest on essential amino acid (EAA)-stimulated MPS, skeletal muscle mTORC1 signaling, and amino acid transporter expression and protein content in healthy older adults. We hypothesized that the inactivity-induced decrease in muscle mass would be associated with a blunted EAA-induced stimulation of MPS with a mechanism involving decreased mTORC1 signaling and amino acid transporter expression.
METHODS
Screening of participants.
We studied six healthy, older subjects (5 male, 1 female; age range: 60–73 yr). EAA-induced change in MPS was the primary outcome variable used to calculate sample size and power. Subject characteristics and body composition are shown in Table 1. The subjects were recruited through the Pepper Older American Independent Center Volunteer Registry. The subjects were habitually active, independent older adults but were not engaged in any regular exercise training program at the time of enrollment, defined as one or more sessions of moderate- to high-intensity aerobic or resistance exercise per week. Exclusion criteria included, but were not limited to: heart, lung, blood, vascular, liver, kidney, infectious, oncologic, neurologic disease, dementia or other significant psychiatric disease, current weight loss or dieting, obesity, and diabetes. All subjects gave written informed consent before participating in the study, which was approved by the Institutional Review Board of the University of Texas Medical Branch (which is in compliance with the Declaration of Helsinki).
Table 1.
Subject characteristics and body composition of healthy, older adults before (pre-bed rest) and after (post-bed rest) bed rest
| Pre-Bed Rest | Post-Bed Rest | Δ, % | P Value | |
|---|---|---|---|---|
| Age, yr | 67.2 ± 1.7 | |||
| Height, cm | 173.7 ± 1.7 | |||
| Weight, kg | 74.8 ± 3.7 | 73.5 ± 3.9 | −1.8 ± 1.4 | 0.26 |
| Lean mass, kg | 50.4 ± 2.9 | 48.8 ± 2.5 | −3.0 ± 1.0 | 0.03 |
| Leg lean mass, kg | 18.3 ± 1.1 | 17.5 ± 1.0 | −4.1 ± 0.9 | 0.01 |
| Trunk lean mass, kg | 23.3 ± 1.4 | 22.5 ± 1.2 | −3.3 ± 1.5 | 0.08 |
| Fat mass, kg | 21.4 ± 3.4 | 21.8 ± 3.4 | 1.9 ± 0.6 | 0.01 |
| Body fat, % | 28.8 ± 3.5 | 30.1 ± 3.3 | 5.1 ± 1.5 | 0.02 |
| Body mass index, kg/m2 | 24.7 ± 0.9 | 24.3 ± 1.1 | −1.8 ± 1.4 | 0.29 |
Values are means ± SE; n = 6 subjects in each group. P value determined from a paired t-test (pre vs. post-bed rest).
Diet and activity level stabilization.
Enrolled subjects were admitted in the Institute for Translational Sciences Clinical Research Center 3 days before beginning strict bed rest. During these 3 days, diet and activity level (Step Activity Monitor; Cyma, Seattle, WA) were closely monitored to maintain body weight. Total caloric intake was predetermined based on the Harris-Benedict equation adjusted for activity level (pre-bed rest: 1.6; during bed rest: 1.3). Daily caloric intake was evenly distributed over three meals (0900, 1300, 1800) and composed of a macronutrient distribution of 15% protein, 55% carbohydrate, and 30% fat as previously reported (14, 34). Subjects were provided water ad libitum. The day before initiation of bed rest, a dual-energy X-ray absorptiometry (DXA) scan (iDXA; General Electric) was performed to determine body composition. Subjects refrained from eating for 3 h and remained supine for ∼60 min before completion of DXA testing. DXA testing was repeated on day 6 of bed rest at the same time of day and conditions as the initial DXA scan. We chose to conduct the DXA scan on day 6 of bed rest (rather than day 7) to minimize variability in DXA measurement due to potential fluid changes from the post-bed rest infusion experiment (3).
Seven-day bed rest.
The experimental treatment consisted of 7 days of bed rest in a hospital bed with established safety and comfort provisions consistent with previous long-term bed rest studies conducted at our institution (14, 30). During bed rest, a slight head and shoulder elevation with a maximum of two pillows was allowed. Adherence to bed rest was monitored via video surveillance by nursing staff 24 h a day. To reduce discomfort and risk of deep venous thrombosis during bed rest, subjects were 1) encouraged to change horizontal position periodically (i.e., roll to side), 2) provided with serial compression devices and compression stockings on their lower limbs, and 3) given daily passive range of motion to lower-extremity major joints to reflect standard of care for bedridden individuals. D-dimer was measured daily before and during bed rest (days 1–7) to monitor for deep venous thrombosis. D-dimer values remained within the normal range in all subjects throughout the study. Bathing and hygiene activities were performed during bed rest while toilet privileges were limited to a bedside commode.
Infusion protocol.
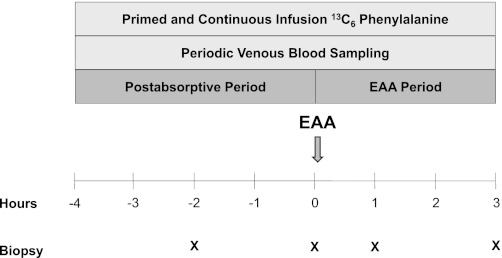
A stable isotope infusion experiment was performed on each of the enrolled subjects during the morning of day 1 and repeated at the same time on the morning of day 7 of bed rest. Each infusion experiment lasted 7 h, was divided up into a postabsorptive and EAA period, and included a stable isotope tracer infusion, venous blood draws, and four muscle biopsies (Fig. 1). Specifically, after an overnight fast, a polyethylene catheter was inserted in an antecubital vein for the infusion of stable isotope tracers. Another polyethylene catheter was inserted retrogradely in a vein of the contralateral hand, which was heated, for arterialized blood sampling. After background blood sampling, a primed, infusion of l-[ring-13C6]phenylalanine was started (priming dose: 2 μmol/kg, infusion rate: 0.05 μmol·kg−1·min−1) and continued until the end of the experiment. Approximately 2 h after starting the tracer infusion, a muscle biopsy was taken from the vastus lateralis. Later (2 h), another muscle biopsy was taken, marking the end of the postabsorptive period. Immediately after the second biopsy, subjects ingested ∼12 g of crystalline EAA (Sigma Aldrich, St. Louis, MO). Additionally, muscle biopsies were taken 1 and 3 h after EAA ingestion, and blood was periodically sampled during the 3 h following EAA ingestion. The EAA mixture was enriched (7.3%) with l-[ring-13C6]phenylalanine to maintain the isotopic steady state. The composition of the EAA mixture was the following: histidine (1.2 g), isoleucine (1.0 g), leucine (2.5 g), lysine (2.5 g), methionine (0.8 g), phenylalanine (1.1 g), threonine (1.2 g), and valine (1.5 g). The proportion of EAA in the mixture was comparable to that found in beef and has been shown to effectively stimulate MPS in older individuals (42, 44).
Fig. 1.
Schematic of infusion protocol. Protocol was conducted before and after 7 days of bed rest. EAA, essential amino acids.
Muscle biopsy procedure.
During the pre-bed rest infusion experiment, muscle biopsies were taken from the vastus lateralis muscle of one leg using aseptic technique, local anesthesia (1% lidocaine), and a 5-mm Bergström biopsy needle. The first and second muscle biopsies (postabsorptive period) were taken from a single incision with the needle inserted at an angle to separate the two sampling sites by at least 5 cm. The third and fourth biopsies (EAA period) were taken from a separate incision, ∼7 cm apart from the first and angling the needle as described above. During the post-bed rest infusion experiment, biopsies were taken from the opposite leg as described above. All muscle tissue was immediately blotted, flash-frozen in liquid nitrogen, and stored at −80°C for later analysis.
MPS and blood and muscle phenylalanine concentrations.
Muscle tissue samples (biopsies 1–4) were ground, and intracellular free amino acids and muscle proteins were extracted as previously described (9). Blood and intracellular free concentrations and l-[ring-13C6]phenylalanine enrichments were determined by gas chromatography-mass spectrometry (GCMS, 6890 Plus CG, 5973N MSD, 7683 autosampler; Agilent Technologies, Palo Alto, CA) after addition of an appropriate internal standard (l-[15N]phenylalanine) (47). Mixed-muscle protein-bound phenylalanine enrichment was analyzed by GCMS after protein hydrolysis and amino acid extraction (9), using the external standard curve approach (5). We calculated MPS by measuring the incorporation rate of the phenylalanine tracer into the proteins and using the precursor-product model to calculate the synthesis rate:
where ΔEp is the increment in protein-bound phenylalanine enrichment between two sequential biopsies, t is the time between the two sequential biopsies, and EM(1) + EM(2) are the phenylalanine enrichments in the free intracellular pool in the two sequential biopsies. Data are expressed as percent per hour (%/h).
Whole body protein breakdown.
Postabsorptive whole body endogenous phenylalanine rate of appearance was calculated using the single-pool model as the ratio between the tracer infusion rate and the venous phenylalanine enrichment (47).
Western blotting.
Specific details to the Western blotting procedure can be found elsewhere (9). Whole muscle homogenates (50 μg) were loaded on a 7.5 and 15% polyacrylamide gel (Criterion; Bio-Rad, Hercules, CA), depending on the molecular weight of the protein, and subjected to electrophoresis (150 V) for 1 h. Each gel contained six samples from a single subject (pre- and post-bed rest: basal, 1 and 3 h EAA) loaded in duplicate and a molecular weight ladder. An internal control (rodent muscle homogenate) was loaded in duplicate on each gel for band normalization and comparisons across blots. Protein was transferred (50 V; 1 h) to a polyvinylidene diflouride membrane and then blocked for 1 h at room temperature with 5% nonfat dry milk (NFDM) in Tris-buffered saline in 0.1% Tween 20 (TBST). Membranes were incubated overnight in primary antibody (diluted in 5% NFDM or bovine serum albumin). The next morning, blots were rocked in secondary antibody for 1 h at room temperature and then serially washed in TBST. Chemiluminescence reagent (ECL Plus; GE Healthcare) was applied to each blot for 5 min. Optical density measurements were obtained with a phosphoimager (ChemiDoc; Bio-Rad). Membranes containing phospho-detected proteins were stripped (25 mM glycine, pH 2.0, and 1% SDS) of primary and secondary antibodies and then reprobed for total protein. Densitometric analysis was performed using Quantity One 4.5.2 software (Bio-Rad). After subtracting out background, all Western blot data were normalized to the internal control, and replicate samples were averaged. Western blot data in Figs. 1–6 were reported as fold change in phosphorylation from postabsorptive values (basal). Reporting Western blot data as phosphorylation alone reduces variability, improves power, and is consistent with previous publications from our laboratory (13, 21).
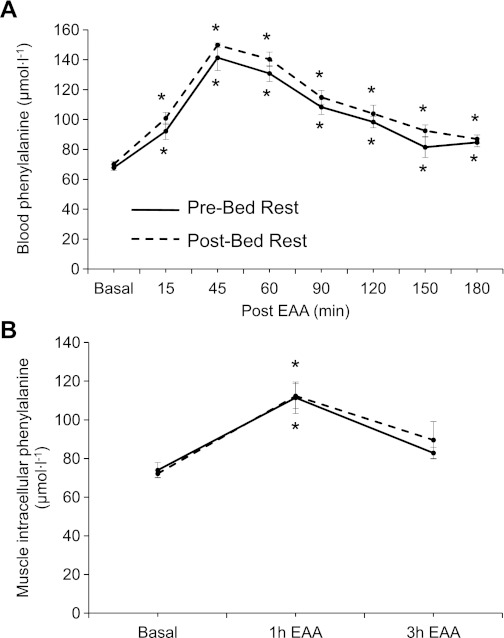
Fig. 2.
Data represent blood (A) and muscle phenylalanine (B) concentrations in the postabsorptive state (basal) and after ingestion of EAA in older adults (n = 6). Solid line, before bed rest (pre-bed rest); broken line, after 7 days of bed rest (post-bed rest). *Different from basal (P < 0.05). Values are presented as means ± SE.
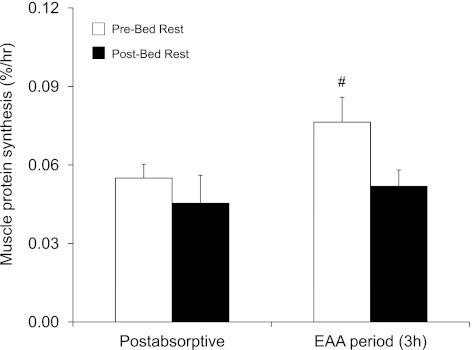
Fig. 3.
Mixed-muscle protein synthesis in skeletal muscle of older adults (n = 6) in the postabsorptive state and during the EAA ingestion period (3 h; 0–3 h post-EAA). #Different from post-bed rest during the 3-h EAA period (P = 0.05). Values are presented as means ± SE.
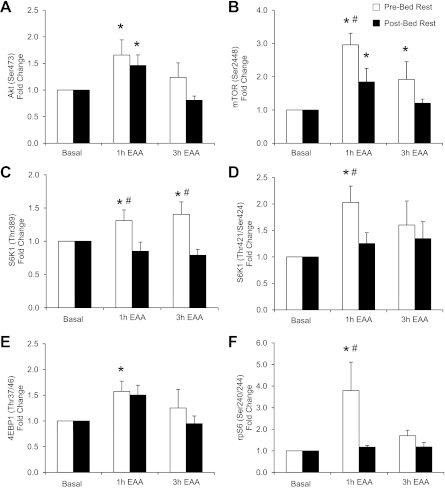
Fig. 4.
Protein kinase B (Akt), mammalian target of rapamycin (mTOR), and downstream signaling. Data represent phosphorylation of Akt at Ser473 (A), mTOR at Ser2448 (B), S6 kinase 1 (S6K1) at Thr389 (C), S6K1 at Thr421/Ser424 (D), 4E binding protein 1 (4EBP1) at Thr37/46 (E), and ribosomal protein S6 (rpS6) at Ser240/244 (F) in the postabsorptive state (basal) and 1 and 3 h after ingestion of EAA in skeletal muscle of older adults (n = 6). *Different from basal (P < 0.05). #Different from postbed rest at corresponding time point (P < 0.05). Values are presented as means ± SE.
Fig. 5.
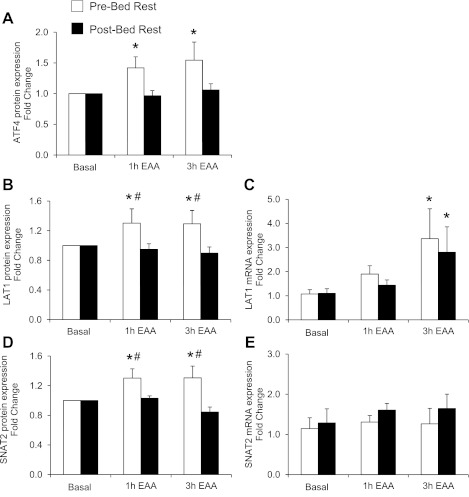
Activating transcription factor 4 (ATF4) and amino acid transporters. Data represent ATF4 protein content (A), L-type amino acid transporter 1 (LAT1) protein content (B), LAT1 mRNA expression (C), sodium-coupled neutral amino acid transporter 2 (SNAT2) protein content (D), and SNAT2 mRNA expression (E) in the postabsorptive state (basal) and 1 and 3 h after ingestion of EAA in skeletal muscle of older adults (n = 6). *Different from basal (P < 0.05). #Different from post-bed rest at corresponding time point (P < 0.05). Values are presented as means ± SE.
Fig. 6.
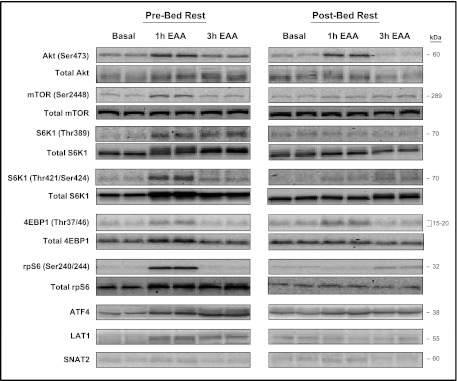
Representative phospho and total protein Western blot images in the postabsorptive state (basal) and after (1 and 3 h) the ingestion of EAA before (pre-bed rest) and after (post-bed rest) 7 days of bed rest.
Antibodies.
Rabbit polyclonal antibodies were purchased from Cell Signaling (Beverley, MA) unless otherwise indicated: total and phospho-Akt (Ser473), total and phospho-mTOR (Ser2448), total and phospho-S6K1 (Thr389; Santa Cruz, Biotechnology, Santa Cruz, CA), phospho-S6K1 (Thr421/Ser424), total and phospho-4EBP1 (Thr37/46), total and phospho-ribosomal protein S6 (rpS6) (Ser240/244), activating transcription factor 4 (ATF4) (Santa Cruz Biotechnology), LAT1 (Abcam, Cambridge, MA), and SNAT2 (Santa Cruz Biotechnology). Anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody was purchased from GE Healthcare.
RNA extraction, cDNA synthesis, and semiquantitative real-time PCR.
Total RNA, cDNA synthesis, and real-time qPCR were conducted as previously reported (13). Total RNA was isolated by homogenizing 15–20 mg tissue with a hand-held homogenizing dispenser (T10 Basic Ultra Turrax; IKA, Wilmington, NC) in a solution containing 1 ml of Tri reagent. The RNA was separated into an aqueous phase using 0.2 ml of chloroform and precipitated from the aqueous phase using 0.5 ml of isopropanol. Extracted RNA was washed with 1 ml of 75% ethanol, dried, and then suspended in a known amount of nuclease-free water. RNA integrity was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). The RNA integrity number was 8.8 ± 0.1 (1–10 scale; 10 = highest integrity). RNA concentration was determined using the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). RNA was DNase-treated using a commercially available kit (DNA-free; Ambion, Austin, TX). Afterward, 1 μg of total RNA was reverse transcribed into cDNA according to the manufacturers' directions (iScript; Bio-Rad). Real-time qPCR was carried out with an iQ5 Multicolor Real Time PCR cycler (Bio-Rad). cDNA was analyzed with SYBR green fluorescence (iQ SYBR green supermix; Bio-Rad). All isolated RNA and cDNA samples were stored at −80°C until analyzed. Primer sequences used in this experiment have been published previously (13). β2-Microglobulin was used as a housekeeping gene, since it remained stable after bed rest and in response to EAA ingestion. Relative fold changes were determined from the cycle threshold (CT) values using the 2−ΔΔCT method (31).
Statistical analysis.
A two-way repeated-measures ANOVA was used to analyze differences across time (basal and 1 and 3 h after EAA ingestion) and between treatments (before vs. after bed rest). Post hoc tests (Fisher's least-significant difference) were conducted to assess specific interactions. Analysis of subject characteristics, body composition, and baseline protein content and mRNA expression between treatments was conducted using a paired t-test. Significance was set at P < 0.05. All values are presented as means ± SE. All analyses were performed with SigmaPlot (version 12.0).
RESULTS
Body composition before and after bed rest.
After 7 days of bed rest, total lean mass and leg lean mass decreased by 3.0 ± 1.0 and 4.1 ± 0.9%, respectively. Trunk lean mass tended to decrease by 3.3 ± 1.5% after bed rest (P = 0.08). Fat mass increased by 1.9 ± 0.6% and relative body fat percentage increased by 5.1 ± 1.5% (P < 0.05). There were no changes in total body weight or body mass index after bed rest (P > 0.05) (Table 1).
Blood and muscle phenylalanine concentrations in the postabsorptive period and in response to acute EAA.
Blood phenylalanine concentrations (Fig. 2A) increased 15, 45, 60, 90, 120, 150, and 180 min after EAA intake both before and after 7 days of bed rest (P < 0.05). Muscle phenylalanine (Fig. 2B) concentrations increased 1 h (P < 0.05) after EAA ingestion before and after bed rest. Blood and muscle phenylalanine concentrations were not affected by bed rest in the postabsorptive period or in response to acute EAA ingestion (P > 0.05).
Endogenous whole body phenylalanine rate of appearance (relative to lean muscle mass), a marker of whole body breakdown, was not affected by bed rest either in the postabsorptive period or in response to acute EAA ingestion (P > 0.05): postabsorptive period (pre: 0.94 ± 0.04; post: 0.89 ± 0.04 μmol·kg−1·min−1); 1 h after EAA (pre: 0.98 ± 0.04; post: 0.94 ± 0.03 μmol·kg−1·min−1), 2 h after EAA (pre: 0.90 ± 0.03; post: 0.89 ± 0.02 μmol·kg−1·min−1), and 3 h after EAA (pre: 0.89 ± 0.05; post: 0.84 ± 0.02 μmol·kg−1·min−1).
MPS in the postabsorptive state and in response to acute EAA ingestion.
Following acute EAA ingestion, MPS (0–3 h post-EAA) increased by ∼40% before but not after bed rest (P = 0.05, pre- vs. post-bed rest). Postabsorptive MPS rate was slightly, but not statistically, different before (0.055 ± 0.005%/h) compared with after (0.045 ± 0.011%/h) bed rest (P > 0.05) (Fig. 3).
Protein kinase B (Akt), mTOR, ATF4, and amino acid transporter protein content and amino acid transporter mRNA expression in the postabsorptive state.
After bed rest, postabsorptive total mTOR protein content was increased (P < 0.05), whereas LAT1 mRNA expression tended to be higher (P = 0.06). There were no changes in total protein content for protein kinase B (Akt), S6K1, rpS6, 4EBP1, ATF4, and SNAT2 with bed rest (P > 0.05) (Table 2).
Table 2.
Total protein and mRNA expression levels for select mTORC1 signaling and amino acid transporters (mRNA and protein) in the postabsorptive state before and after bed rest in healthy, older adults
| Pre-Bed Rest | Post-Bed Rest | P Value | |
|---|---|---|---|
| Total protein | |||
| Akt | 0.60 ± 0.12 | 0.65 ± 0.12 | 0.84 |
| mTOR | 1.00 ± 0.28 | 1.44 ± 0.42 | 0.03 |
| S6K1 | 1.22 ± 0.14 | 1.55 ± 0.25 | 0.10 |
| 4EBP1 | 0.42 ± 0.05 | 0.52 ± 0.09 | 0.13 |
| rpS6 | 0.68 ± 0.20 | 0.97 ± 0.33 | 0.15 |
| ATF4 | 1.00 ± 0.26 | 1.12 ± 0.14 | 0.47 |
| LAT1 | 0.73 ± 0.23 | 1.02 ± 0.31 | 0.10 |
| SNAT2 | 1.08 ± 0.49 | 1.41 ± 0.64 | 0.10 |
| mRNA | |||
| LAT1 | 1.07 ± 0.18 | 1.77 ± 0.31 | 0.06 |
| SNAT2 | 1.15 ± 0.27 | 1.11 ± 0.30 | 0.86 |
Values are means ± SE; n = 6 subjects in each group.
Akt, protein kinase B; mTOR, mammalian target of rapamycin; 4EBP1, 4E binding protein 1; rpS6, ribosomal protein S6; ATF4, activating transcription factor 4; LAT1, L-type amino acid transporter 1; SNAT2, sodium-coupled neutral amino acid transporter 2.
P values were determined from a paired t-test (pre- vs. post-bed rest). Total protein data were calculated by normalizing arbitrary density units to an internal loading control. Postabsorptive mRNA data were determined from the 2−ΔΔCT method relative to pre-bed rest.
Akt and mTOR signaling in response to acute EAA ingestion.
Akt phosphorylation (Ser473; Fig. 4A) increased 1 h after EAA (P < 0.05 vs. postabsorptive state) both before (1.7 ± 0.3-fold) and after (1.5 ± 0.2-fold) bed rest. However, there was a trend for Akt (Ser473) to be higher before (1.2 ± 0.3-fold) bed rest 3 h following acute EAA ingestion compared with after (0.8 ± 0.1-fold) bed rest (P = 0.08). Before bed rest, mTOR phosphorylation (Ser2448; Fig. 4B) increased 1 h (3.0 ± 0.4-fold) and 3 h (1.9 ± 0.5-fold) following EAA ingestion (P < 0.05 vs. postabsorptive state) while after bed rest mTOR (Ser2448) increased only 1 h after EAA (1.9 ± 0.4-fold) (P < 0.05 vs. postabsorptive state). The phosphorylation of mTOR (Ser2448) was significantly greater 1 h following EAA ingestion before bed rest compared with after bed rest (P < 0.05). Before bed rest, S6K1 phosphorylation (Thr389; Fig. 4C) increased 1 h (1.3 ± 0.2-fold) and 3 h (1.4 ± 0.2-fold) after EAA ingestion (P < 0.05 vs. postabsorptive state) and was significantly greater compared with the post-bed rest values (P < 0.05). There were no changes in S6K1 phosphorylation (Thr389) following EAA ingestion after bed rest (P > 0.05). Before bed rest, S6K1 phosphorylation (Thr424/Ser421; Fig. 4D) increased 1 h after EAA ingestion (2.0 ± 0.3-fold, P < 0.05 vs. postabsorptive state) and was significantly greater compared with the post-bed rest value (P < 0.05). In fact, there were no changes in S6K1 phosphorylation following EAA ingestion after bed rest (P > 0.05). Before bed rest, 4EBP1 phosphorylation (Thr37/46; Fig. 4E) increased 1 h following EAA ingestion (1.6 ± 0.2-fold, P < 0.05 vs. postabsorptive state). After bed rest, there was a trend for 4EBP1 (Thr37/46) to increase 1 h following EAA ingestion (1.5 ± 0.2-fold, P = 0.06 vs. postabsorptive state). Before bed rest, rpS6 phosphorylation (Ser240/244; Fig. 4F) increased 1 h after EAA ingestion (3.8 ± 1.3-fold, P < 0.05 vs. postabsorptive state). The phosphorylation of rpS6 (Ser240/244) 1 h following EAA ingestion was significantly greater before compared with post-bed rest (P < 0.05). Ribosomal protein S6 phosphorylation did not change with EAA ingestion after bed rest (P > 0.05).
ATF4 and amino acid transporter mRNA expression and protein content in response to acute EAA ingestion.
Before bed rest, ATF4 protein content (Fig. 5A) increased 1 h (1.4 ± 0.2-fold) and 3 h (1.5 ± 0.3-fold) following EAA ingestion (P < 0.05 vs. postabsorptive state). There was a trend for ATF4 protein content to be greater before bed rest than after bed rest following EAA (1 h, P = 0.08; 3 h, P = 0.06). After bed rest ATF4 protein content did not change from the postabsorptive value following EAA ingestion (P > 0.05). Before bed rest, protein content for LAT1 (Fig. 5B) increased 1 h (1.3 ± 0.2-fold) and 3 h (1.2 ± 0.2-fold) after EAA ingestion (P < 0.05). The increase in LAT1 protein content 1 and 3 h following EAA ingestion was greater before compared with after bed rest (P < 0.05). LAT1 protein content did not change significantly with EAA after bed rest (P > 0.05). However, LAT1 mRNA expression (Fig. 5C) increased both before (3.4 ± 1.2-fold) and after (2.8 ± 1.1-fold) bed rest 3 h after EAA ingestion (P < 0.05 vs. postabsorptive state). Before bed rest, SNAT2 protein content (Fig. 5D) increased 1 h (1.3 ± 0.1-fold) and 3 h (1.3 ± 0.2-fold) after EAA (P < 0.05 vs. postabsorptive state). The increase in SNAT2 protein content following EAA was greater before than after bed rest (P < 0.05). There were no changes in SNAT2 protein content in response to EAA after bed rest (P > 0.05). Conversely, SNAT2 mRNA expression (Fig. 5E) was not different after EAA ingestion before or after bed rest (P > 0.05). There were no changes in total protein content after EAA ingestion before or after bed rest (P > 0.05). Representative phosphorylated and total protein Western blot images for Akt and mTORC1 signaling and regulators of amino acid transport are reported in Fig. 6.
DISCUSSION
This study shows for the first time that 7 days of bed rest blunted MPS, mTORC1 signaling, and amino acid transporter protein content in response to acute EAA ingestion in healthy, older adults. While we observed a mild but nonsignificant decrease in postabsorptive MPS after bed rest, we found that the blunted MPS response to EAA ingestion was related to profound attenuations in mTOR (Ser2448), S6K1 (Thr389; Thr424/Ser421), and rpS6 (Ser240/244) phosphorylation and to a reduction in the response of LAT1 and SNAT2 protein content to EAA following 7 days of continuous bed rest. Together, our findings suggest that short-term bed rest mimicking acute hospitalization alters the normal protein anabolic response to feeding and is likely regulated by mTORC1 signaling and perhaps amino acid transporters. We suggest that alterations in the protein anabolic response to EAA ingestion may also contribute to accelerated muscle loss with inactivity in older adults.
A very exciting result of our study is that as little as 7 days of bed rest can desensitize healthy, older adult skeletal muscle to the anabolic influence of EAA intake. Previous studies had shown that 14 days of limb immobilization blunted the MPS response to a constant infusion of amino acids in healthy young adults (19). Furthermore, Kortebein et al. (30) showed that MPS measured over a 24-h period (including postabsorptive and three postprandial periods) was reduced after 10 days of bed rest in older adults. However, our data are unique, since we show for the first time that bed rest attenuated the protein anabolic response to feeding, specifically to an acute oral EAA bolus. At this time, we cannot further elaborate whether decrements in the synthesis rates of myofibrillar proteins following feeding are responsible for decreases in lean mass since our measurements are limited to the synthesis rate of mixed-muscle proteins. Additional studies are needed to address this question. Nonetheless, we propose that an impaired protein synthetic response to feeding is a contributor to inactivity-induced atrophy.
In our study, the increase in intracellular phenylalanine concentrations after acute EAA ingestion was not affected by 7 days of bed rest. On the contrary, we found that key regulators of mRNA translation within the mTORC1 signaling pathway were markedly nonresponsive following acute EAA ingestion after 7 days of bed rest. A cause-effect relationship between mTORC1 signaling and MPS can be inferred from our previous work showing that EAA stimulates MPS via a mechanism that can be blocked by the mTOR inhibitor rapamycin in human skeletal muscle (8).
We also found that the EAA-induced increase in LAT1 and SNAT2 protein was abolished by 7 days of bed rest. Several reports provide evidence that amino acid transporter (i.e., LAT1, SNAT2) mRNA expression can be mediated by an mTOR-dependent mechanism possibly regulated by the transcription factor ATF4 (1, 32, 35, 39, 40). We found that EAA increased ATF4 protein content before bed rest, an effect that disappeared after 7 days of bed rest. However, bed rest did not attenuate the normal EAA-induced increase in amino acid transporter mRNA (i.e., LAT1). These data suggest that other transcription factors independent of mTOR and ATF4 may be involved in the regulation of amino acid transporter transcription in inactive older adults. In a recent report from our laboratory, we suggested that signal transducer and activator of transcription 3 may be a transcriptional regulator of amino acid transporters during periods of cellular stress in older adults (e.g., postexercise recovery) (12). Taken together, these data indicate that bed rest reduced the normal EAA-stimulated increase in SNAT2 and LAT1 protein content with a mechanism that must still be elucidated.
Interestingly, bed rest blunted the protein abundance of amino acid transporters but did not affect the normal EAA-induced increase in muscle and blood concentrations of phenylalanine. It is important to underscore that changes in intracellular concentrations do not necessarily reflect changes in amino acid trafficking across the cell membrane, since they can be due to changes in transport and/or changes in protein synthesis and breakdown. In other words, changes in transport rates may not be reflected by changes in the total intracellular amino acid pool. In this study, protein synthesis increased with acute EAA ingestion before but not after bed rest. Even if transport was reduced after bed rest as a consequence of a lower transporter protein content, amino acids may have accumulated in the cells due to lack of utilization for synthesis. It is also possible that transient increases of amino acid transporters following EAA ingestion may have no immediate effect on the acute stimulation of MPS. Amino acid transporters reside in vesicles and upon stimulation are recruited to the membrane for amino acid transport (26). Thus, the reservoir of amino acid transporters present in skeletal muscle during the postabsorptive state may be sufficient to deliver amino acids to the intracellular compartment upon EAA stimulation even after bed rest. There are also alternative hypotheses that may be considered. Phenylalanine (and presumably leucine) may have simply accumulated in muscle through alternate transporters independent of LAT1 (i.e., LAT2). Finally, the increase in amino acid transporter abundance may be a mechanism to sensitize the cell for an upcoming anabolic stimulus (i.e., contraction). This may be a cellular strategy to augment the MPS response upon a second stimulus (4, 11). Future studies are warranted to clarify the role of transient changes in amino acid transporter content following acute EAA ingestion.
A secondary aim of this experiment was to determine the effect of short-term bed rest on the postabsorptive rate of MPS in older adults. Postabsorptive MPS following bed rest in these older subjects was mildly (∼18%) but not significantly reduced. In addition, we did not observe changes in mTORC1 signaling that would be compatible with a reduced postabsorptive MPS after bed rest. Rather, total mTOR protein abundance increased, suggesting the existence of a compensatory mechanism to slow the rapid loss in muscle mass. Larger changes in postabsorptive MPS have been reported in younger subjects exposed to bed rest or immobilization (7, 14, 17, 19). Differences in pre-bed rest physical activity level between young and older individuals might have been responsible for this discrepancy (6). However, it is not possible to test this hypothesis because the previous studies did not report preimmobilization physical activity data. Alternatively, it is possible that we did not have enough power to detect significant changes in postabsorptive MPS with bed rest. A post hoc power calculation indicated that we would need 44 older subjects to detect a significant decrease in postabsorptive MPS. While it is possible that small changes in postabsorptive MPS may be physiologically relevant and can contribute to the losses in lean body mass with bed rest, our data suggest that the magnitude of such an effect is reduced with aging. Finally, our protein synthesis values are a composite measure of the synthesis of different protein subfractions (myofibrillar, sarcolemic, mitochondrial, etc.). Thus, some changes in the synthesis of specific proteins may have been masked by opposite changes of other protein subfractions.
Seven days of bed rest decreased total lean mass by 1.6 kg. The loss in total lean mass during bed rest was isolated to the legs (0.8 ± 0.2 kg) and likely the trunk (i.e., low back and hips; 0.8 ± 0.4 kg). Our results are comparable with those by Kortebein et al. (30) who found that 10 days of bed rest in healthy, older adults reduced total lean mass by 1.5 kg and leg lean mass by 0.95 kg. Thus, it appears that the majority of leg lean mass loss occurs within the first week of bed rest, and possibly earlier (45). This information is especially important for clinicians, since the average length of stay for hospitalized geriatric patients is ∼5–6 days (15). In addition to impaired stimulation of MPS, we cannot entirely exclude that muscle protein breakdown might have played a role in muscle loss during the initial days of bed rest (20, 23, 38, 45). Some reports suggest that protein breakdown may increase during immobilization (20, 38, 45). However, we did not observe any changes in whole body protein breakdown with 7 days of bed rest, which is consistent with previous reports (14, 43).
In conclusion, we found that 7 days of bed rest in healthy, older adults impaired the amino acid-stimulated MPS response, mTORC1 signaling, and amino acid transporter protein content. These novel findings suggest that the significant muscle loss induced by profound inactivity in older adults, such as bed rest and hospitalization, may be driven at least in part by a reduced sensitivity of skeletal muscle to anabolic stimulation of amino acids. Future studies are necessary to identify and test effective countermeasures to maintain an adequate muscle protein anabolic sensitivity to feeding in profoundly inactive and hospitalized older individuals.
GRANTS
This study was supported by NIH/NCRR UL1 RR-029876 and in part by National Institutes of Health Grants R01 AG-018311 (E. Volpi), P30 AG-024832 (E. Volpi), R01 AR-04987 (B. B. Rasmussen), K01 AG-038556 (M. J. Drummond), and NSBRI NNJ08ZSA002N (D. Paddon-Jones).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.J.D., J.M.D., C.S.F., D.K.W., D.M.G., P.T.R., K.L.T., M.M.M., D.P.-J., and E.V. performed experiments; M.J.D., J.M.D., C.S.F., D.K.W., D.M.G., P.T.R., K.L.T., M.M.M., and E.V. analyzed data; M.J.D., J.M.D., C.S.F., D.K.W., D.M.G., P.T.R., K.L.T., M.M.M., D.P.-J., B.B.R., and E.V. interpreted results of experiments; M.J.D. and E.V. prepared figures; M.J.D. and E.V. drafted manuscript; M.J.D., B.B.R., and E.V. edited and revised manuscript; M.J.D., J.M.D., C.S.F., D.K.W., D.M.G., P.T.R., K.L.T., M.M.M., D.P.-J., B.B.R., and E.V. approved final version of manuscript; M.J.D., D.P.-J., B.B.R., and E.V. conception and design of research.
ACKNOWLEDGMENTS
We thank the research participants for their time and effort completing this study. Additionally, we are grateful to the nursing staff at the Institute for Translational Sciences Clinical Research Center, the Claude E. Pepper Older Americans Independence Center recruitment coordinators, and Dr. Shaheen Dhanani for assistance in screening, admitting, and assisting the subjects during data collection. We also thank Shelley Medina, Ming Zheng, and Junfang Hao for technical assistance.
REFERENCES
- 1.Adams CM. Role of the transcription factor ATF4 in the anabolic actions of insulin and the anti-anabolic actions of glucocorticoids. J Biol Chem 282: 16744–16753, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Baird FE, Bett KJ, MacLean C, Tee AR, Hundal HS, Taylor PM. Tertiary active transport of amino acids reconstituted by coexpression of System A and L transporters in Xenopus oocytes. Am J Physiol Endocrinol Metab 297: E822–E829, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Berg HE, Tedner B, Tesch PA. Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand 148: 379–385, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Phillips SM. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr 141: 568–573, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–009 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass spectrom 6: 421–424, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Cavanaugh JT, Coleman KL, Gaines JM, Laing L, Morey MC. Using step activity monitoring to characterize ambulatory activity in community-dwelling older adults. J Am Geriatr Soc 55: 120–124, 2007 [DOI] [PubMed] [Google Scholar]
- 7.de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol 585: 241–251, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr 141: 856–862, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576: 613–624, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol 106: 1374–1384, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol 104: 1452–1461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, Gundermann DM, Volpi E, Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol 111: 135–142, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 298: E1011–E1018, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab 270: E627–E633, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Fisher SR, Kuo YF, Graham JE, Ottenbacher KJ, Ostir GV. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med 170: 1942–1943, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol 582: 813–823, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson JN, Halliday D, Morrison WL, Stoward PJ, Hornsby GA, Watt PW, Murdoch G, Rennie MJ. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci (Lond) 72: 503–509, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Gill TM, Allore H, Guo Z. The deleterious effects of bed rest among community-living older persons. J Gerontol A Biol Sci Med Sci 59: 755–761, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol 586: 6049–6061, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glover EI, Yasuda N, Tarnopolsky MA, Abadi A, Phillips SM. Little change in markers of protein breakdown and oxidative stress in humans in immobilization-induced skeletal muscle atrophy. Appl Physiol Nutr Metab 35: 125–133, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr 140: 1970–1976, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guillet C, Prod'homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB j 18: 1586–1587, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Gustafsson T, Osterlund T, Flanagan JN, von Walden F, Trappe TA, Linnehan RM, Tesch PA. Effects of 3 days unloading on molecular regulators of muscle size in humans. J Appl Physiol 109: 721–727, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Hvid L, Aagaard P, Justesen L, Bayer ML, Andersen JL, Ortenblad N, Kjaer M, Suetta C. Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining. J Appl Physiol 109: 1628–1634, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J 19: 461–463, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Hyde R, Peyrollier K, Hundal HS. Insulin promotes the cell surface recruitment of the SAT2/ATA2 system A amino acid transporter from an endosomal compartment in skeletal muscle cells. J Biol Chem 277: 13628–13634, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 82: 1065–1073, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 291: E381–E387, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Kimball SR, Farrell PA, Jefferson LS. Invited Review: Role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol 93: 1168–1180, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 297: 1772–1774, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Malmberg SE, Adams CM. Insulin signaling and the general amino acid control response. Two distinct pathways to amino acid synthesis and uptake. J Biol Chem 283: 19229–19234, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136: 521–534, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, Ferrando AA. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab 89: 4351–4358, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Peng T, Golub TR, Sabatini DM. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol 22: 5575–5584, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peyrollier K, Hajduch E, Blair AS, Hyde R, Hundal HS. l-Leucine availability regulates phosphatidylinositol 3-kinase, p70 S6 kinase and glycogen synthase kinase-3 activity in L6 muscle cells: evidence for the involvement of the mammalian target of rapamycin (mTOR) pathway in the l-leucine-induced up-regulation of system A amino acid transport. Biochem J 350: 361–368, 2000 [PMC free article] [PubMed] [Google Scholar]
- 37.Pinilla J, Aledo JC, Cwiklinski E, Hyde R, Taylor PM, Hundal HS. SNAT2 transceptor signalling via mTOR: a role in cell growth and proliferation? Front Biosci 3: 1289–1299, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Reich KA, Chen YW, Thompson PD, Hoffman EP, Clarkson PM. Forty-eight hours of unloading and 24 h of reloading lead to changes in global gene expression patterns related to ubiquitination and oxidative stress in humans. J Appl Physiol 109: 1404–1415, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roos S, Kanai Y, Prasad PD, Powell TL, Jansson T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am J Physiol Cell Physiol 296: C142–C150, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Roos S, Lagerlof O, Wennergren M, Powell TL, Jansson T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. Am J Physiol Cell Physiol 297: C723–C731, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol 107: 1172–1180, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr 86: 451–456, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Symons TB, Sheffield-Moore M, Chinkes DL, Ferrando AA, Paddon-Jones D. Artificial gravity maintains skeletal muscle protein synthesis during 21 days of simulated microgravity. J Appl Physiol 107: 34–38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Dietetic Assoc 109: 1582–1586, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tesch PA, von Walden F, Gustafsson T, Linnehan RM, Trappe TA. Skeletal muscle proteolysis in response to short-term unloading in humans. J Appl Physiol 105: 902–906, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Cclin Endocrinol Metab 85: 4481–4490, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research Principles and Practice of Kinetic Analysis. Hobokon, NJ: Wiley-Liss, 2005 [Google Scholar]