Abstract
Although body fat distribution strongly predicts metabolic health outcomes related to excess weight, little is known about the factors an individual might exhibit that predict a particular fat distribution pattern. We utilized the meal fatty acid tracer-adipose biopsy technique to assess upper and lower body subcutaneous (UBSQ and LBSQ, respectively) meal fat storage in lean volunteers who then were overfed to gain weight. Meal fatty acid storage in UBSQ and LBSQ adipose tissue, as well as daytime substrate oxidation (indirect calorimetry), was measured in 28 nonobese volunteers [n = 15 men, body mass index = 22.1 ± 2.5 (SD)] before and after an ∼8-wk period of supervised overfeeding (weight gain = 4.6 ± 2.2 kg, fat gain = 3.8 ± 1.7 kg). Meal fat storage (mg/g adipose tissue lipid) in UBSQ (visit 1: 0.78 ± 0.34 and 1.04 ± 0.71 for women and men, respectively, P = 0.22; visit 2: 0.71 ± 0.24 and 0.90 ± 0.37 for women and men, respectively, P = 0.08) and LBSQ (visit 1: 0.60 ± 0.23 and 0.48 ± 0.29 for women and men, respectively, P = 0.25; visit 2: 0.62 ± 0.24 and 0.65 ± 0.23 for women and men, respectively, P = 0.67) adipose tissue did not differ between men and women at either visit. Fractional meal fatty acid storage in UBSQ (0.31 ± 0.15) or LBSQ (0.19 ± 0.13) adipose tissue at visit 1 did not predict the percent change in regional body fat in response to overfeeding. These data indicate that meal fat uptake trafficking in the short term (24 h) is not predictive of body fat distribution patterns. In general, UBSQ adipose tissue appears to be a favored depot for meal fat deposition in both sexes, and redistribution of meal fatty acids likely takes place at later time periods.
Keywords: adipose tissue, body fat distribution, [3H]triolein, fat biopsy
obesity is largely a result of positive energy balance leading to tissue gain over time. Much of the tissue gained during a period of overeating or decreased energy expenditure is body fat. The distribution of body fat has been repeatedly shown to be of importance regarding health consequences of excess weight. In particular, it is established that an upper body fat distribution is associated with an increased risk of type 2 diabetes, dyslipidemia, and cardiovascular disease, among other conditions (5, 7, 8).
Several predictors of weight gain over time, in specific populations, have emerged. For example, a low fasting respiratory quotient (RQ; indicative of a low whole body fat oxidation) (24, 39), altered carbohydrate balance/oxidation (6, 21), low resting metabolic rate (36, 37), and reduced spontaneous physical activity (15) have been associated with excess weight gain. However, very little is known about the factors that might predict differences in regional fat gain, which could be almost as important as total weight gain in determining the metabolic effects of excess fat.
The use of meals that contain fatty acid tracers, as developed by Marin et al. (16–18), allows for direct analysis of the trafficking of dietary fat into various body compartments. In particular, it is possible to trace meal fat into regional body fat stores to determine if storage occurs differentially in the upper vs. lower body fat depots. This technique has been used extensively (26, 31, 33) to assess regional fat storage in various conditions over the short term (24 h). In this study, we assessed whether interindividual differences in regional meal fat storage and mediators of meal fat storage [lipoprotein lipase (LPL)] are predictive of regional fat gain. To accomplish this, we used the meal fatty acid tracer-adipose biopsy protocol to determine short-term subcutaneous fat storage and then asked nonobese men and women to overeat for ∼8 wk to gain ∼3 kg of fat. We hypothesized that those individuals who more readily store meal fatty acids in the upper body region at baseline would, in turn, gain more upper body fat in response to overeating. To gain a fuller understanding of lipid metabolism, we also measured substrate oxidation (indirect calorimetry), as well as 24-h profiles of free fatty acids (FFA) and chylomicron and nonchylomicron triglycerides (TG). The insulin responses to overfeeding for this group of volunteers have been published in a study designed to assess whether baseline insulin sensitivity predicted upper body subcutaneous or visceral fat gain with overfeeding (32).
METHODS
Subjects.
The study was approved by the Mayo Clinic Institutional Review Board, and informed, written consent was obtained from 28 volunteers (15 men). Subjects were recruited if their body mass index was <26 kg/m2 and they were taking no medications, with the exception of oral contraceptives. Three subjects reported a family history of type 2 diabetes. Prior to participation in the study, a complete blood count and a chemistry panel were documented to be within normal limits. We previously reported the adipose tissue cellular characteristics from these studies (29) and the relationship between indexes of insulin resistance and regional fat gain (32).
Protocol.
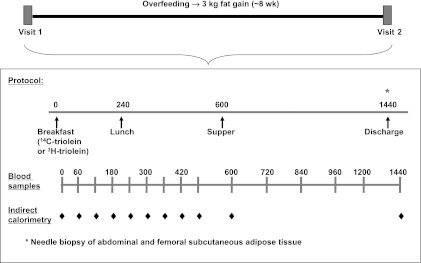
A schematic of the general protocol is depicted in Fig. 1. A baseline, weight maintenance diet was provided to the subjects by the Mayo Clinic General Clinical Research Center (GCRC) metabolic kitchen for 10 days prior to the test meal to ensure constant macronutrient composition (50% carbohydrate, 35% fat, and 15% protein), as previously described (25). If weight changed by ≥1 kg, the amount of food provided to the subjects was adjusted accordingly. Body composition was assessed by dual-energy X-ray absorptiometry (DXA) and computed tomography (see below).
Fig. 1.
Schematic of the study protocol. Patients were admitted for 2 inpatient stays of 2 nights each, separated by an overfeeding period of ∼8 wk. Inpatient studies were identical in nature, except for the radiolabeled meal fat tracer given: [14C]triolein during visit 1 and [3H]triolein during visit 2.
After the baseline diet control run-in period, the subjects were admitted to the GCRC at 1700 for their first inpatient study (visit 1). They consumed their metabolic kitchen-prepared evening meal at 1800 and fasted, except for water, until the next morning. Energy intake during this 2-day inpatient admission was based on the weight maintenance energy intake established during the previous 10 days. On the next morning, the subjects were given a liquid meal at 0800 (Ensure Plus, Ross Laboratories) to which 19.4 ± 0.7 μCi of [1-14C]triolein (Perkin Elmer, Boston, MA) had been added. The breakfast meal was followed by solid food meals at 1300 and 1800 that were prepared in the metabolic kitchen specifically to meet each subject's energy requirements. Meal fatty acid oxidation was estimated using 14CO2 production rates, as previously described (26, 31, 33).
Indirect calorimetry was performed as shown in Fig. 1, and blood samples were taken hourly until 1600 and then less frequently until 0800 on the following morning (day 2). Abdominal and femoral adipose tissue biopsies were performed under local anesthesia under sterile conditions 24 h after consumption of the fatty acid tracer-containing breakfast.
Immediately after visit 1, the subjects began the overfeeding phase of the study, with a goal weight gain of 8–10 pounds (∼3 kg of fat) over ∼8 wk. Supplemental food from the GCRC metabolic kitchen was provided to increase the daily energy intake of the subjects, as previously described (25, 29, 32). The rate of fat gain was determined by a DXA scan ∼4 wk into the overfeeding period.
After each subject had gained ∼3 kg of body fat, the subjects again received 10 days of meals from the GCRC metabolic kitchen. The macronutrient content of these meals was the same as that of the meals prior to visit 1 but was in excess of weight maintenance intake. The increase in energy intake compared with the feeding period before visit 1 was determined by the calorie amount of the supplements consumed by the subjects to that point. Daily weights were measured to ensure that subjects were maintaining or still slightly increasing body weight.
The second inpatient stay at the GCRC (visit 2) was identical to visit 1, except the tracer used to follow meal fatty acid metabolism was [3H]triolein (Perkin Elmer) (83.2 ± 5.2 μCi). Meal fatty acid oxidation was estimated using 3H2O production, as previously described (26, 31, 33).
The level of energy intake during visit 2 was the same as that during visit 1 to allow for comparison of meal fat response between visits. Three subjects were mistakenly given additional calories during visit 2. This did not impact the results, and these data are included in the analysis.
Assays and methods.
DXA (DPX-IQ, Lunar Radiation, Madison, WI) was used to assess total body fat, leg fat, and total fat-free mass. Duplicate scans were performed at visits 1 and 2, and in the middle of the overfeeding period a single scan was obtained to determine the proportion of weight gain that was fat gain. Visceral fat was determined at visits 1 and 2 using the three-slice computed tomographic images at the levels of L2–3, L3–4, and L4–5 in combination with DXA abdominal fat analysis using the region of interest software program (22).
O2 uptake and CO2 output were measured hourly with indirect calorimetry using a metabolic cart (Delta Trac, Sensor Medics, Yorba Linda, CA) starting immediately before the test meal, as depicted in Fig. 1. Energy expenditure and substrate utilization were calculated according to the equations of Wier et al. (38). Urine was quantitatively collected for 24 h to assess 3H2O losses and urinary nitrogen excretion (33). The oxidation of meal fatty acids traced with [1-14C]- and [3H]triolein was determined as previously described (26). Breath samples for 14CO2 specific activity were collected at the same time as blood samples (Fig. 1). Heparin releasable LPL activity (20) was measured using established methods, and plasma oleate concentration was measured using HPLC (10).
Calculations.
The approach to measuring the quantity of [14C]- and [3H]triolein in the liquid test meal and the methods used to measure adipose tissue lipid surface area and fractional meal fat storage have been previously reported (26).
The regional fat gain between visit 1 and visit 2 needed to be adjusted for individual differences in absolute amount of fat gain between subjects. We therefore calculated regional fat gain as a percentage of the total change in fat mass. We also assessed all relationships that are reported as the absolute changes in regional fat mass, and results were similar.
Statistics.
Values are means ± SD, unless stated otherwise. Data were analyzed using SAS version 8.02 and SAS Enterprise Guide version 4.1. P < 0.05 was taken as significant. t-Tests with unequal variances were used to compare variables between men and women, where appropriate, since body fat distribution often tracks by sex. Because differences between upper and lower body subcutaneous (UBSQ and LBSQ, respectively) adipose tissue were expected, within-subject comparisons (i.e., UBSQ vs. LBSQ) were analyzed using a paired t-test. When testing for pre- vs. post-weight gain changes in plasma FFA (oleate) and chylomicron and nonchylomicron TG concentrations over the 24-h interval, we used a paired t-test with P < 0.01 considered significant to partially account for multiple testing issues.
RESULTS
The anthropometric characteristics and body composition of the subjects are reported elsewhere (29, 32). Briefly, men were heavier and had more visceral fat and a higher body mass index. Women had greater percent body fat and greater absolute amounts of UBSQ and LBSQ fat. Women and men had significant increases in these three fat depots from visit 1 to visit 2 (P ≤ 0.05). The increases in visceral, UBSQ, and LBSQ fat were 0.4 ± 0.3, 1.9 ± 1.0, and 1.6 ± 0.8 kg, respectively. For the entire group of men and women, fasting plasma insulin concentrations on day 1 of visits 1 and 2 averaged 4.4 ± 1.8 and 5.6 ± 2.9 μU/ml (P = 0.06).
Energy expenditure and substrate utilization were assessed by indirect calorimetry during the morning and afternoon of day 1 of the inpatient study visits. The results are provided in Table 1. Fasting RQ did not differ between men and women at either visit. Fasting RQ increased to a similar extent from visit 1 to visit 2, but the increase was statistically significant only in men (P < 0.005). Energy expenditure, estimated over 10 h, was significantly greater in men than women at both visits (P < 0.0001) and increased significantly from visit 1 to visit 2 only in men (P < 0.05). The 10-h oxidation of carbohydrate, fat, and protein, in grams or as a percentage of the 10-h energy expenditure, did not differ significantly by sex during visit 1. During visit 2, however, whole body carbohydrate and fat oxidation was greater in men than women (P < 0.05). In women, there was a significant alteration in whole body substrate oxidation from visit 1 to visit 2, with an increase in carbohydrate oxidation and a concomitant decrease in fat oxidation (P < 0.05). In men, carbohydrate oxidation increased from visit 1 to visit 2 (P < 0.05), but the change in fat oxidation did not reach statistical significance (P = 0.08).
Table 1.
Energy expenditure and substrate utilization from indirect calorimetry
| Women | Men | P Value | |
|---|---|---|---|
| Visit 1 | |||
| RQ | 0.80 ± 0.07 | 0.79 ± 0.05 | 0.8938 |
| EE, kcal/10 h | 662 ± 65 | 837 ± 81 | <0.0001 |
| Visit 2 | |||
| RQ | 0.83 ± 0.06 | 0.83 ± 0.05 | 0.8473 |
| EE, kcal/10 h | 646 ± 71 | 870 ± 98 | <0.0001 |
| 10-h substrate oxidation | |||
| Visit 1 | |||
| Carbohydrate, g | 77 ± 29 | 100 ± 43 | 0.1024 |
| Fat, g | 29 ± 12 | 37 ± 15 | 0.1378 |
| Protein, g | 24 ± 9 | 28 ± 16 | 0.4754 |
| Visit 2 | |||
| Carbohydrate, g | 96 ± 29 | 130 ± 47 | 0.0253 |
| Fat, g | 19 ± 11 | 31 ± 15 | 0.0317 |
| Protein, g | 25 ± 11 | 22 ± 16 | 0.4857 |
| Substrate as percentage of 10-h EE | |||
| Visit 1 | |||
| %Carbohydrate | 47 ± 17 | 47 ± 16 | 0.9818 |
| %Fat | 39 ± 16 | 40 ± 17 | 0.8526 |
| %Protein | 14 ± 5 | 13 ± 8 | 0.6697 |
| Visit 2 | |||
| %Carbohydrate | 58 ± 16 | 58 ± 18 | 0.9740 |
| %Fat | 26 ± 5 | 32 ± 17 | 0.3435 |
| %Protein | 16 ± 7 | 10 ± 8 | 0.0497 |
Values are means ± SD. EE, energy expenditure; RQ, respiratory quotient. P values reflect differences between men and women.
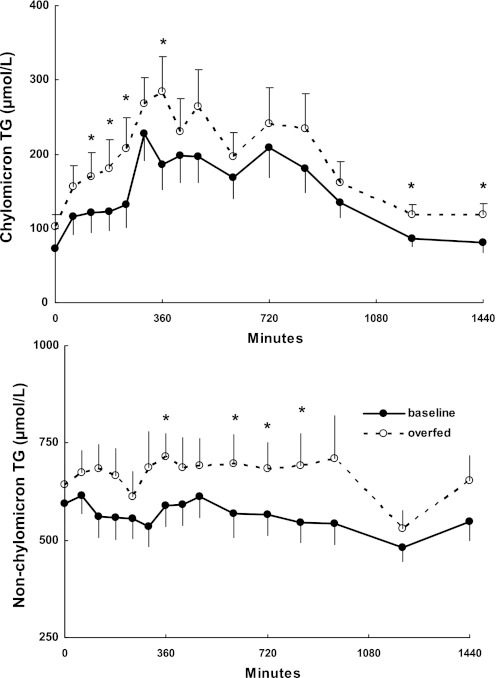
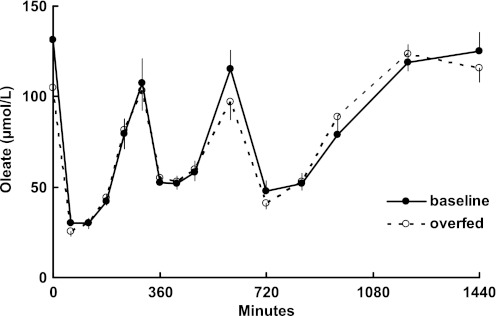
Plasma concentrations of chylomicron and nonchylomicron TG throughout the visit 1 and 2 study days are depicted in Fig. 2. For the combined group of men and women, both TG fractions were significantly (P < 0.01) greater at several time points during visit 2 than visit 1. Study day plasma oleate concentrations, a reflection of adipose tissue lipolysis, are depicted in Fig. 3. At no time were plasma oleate concentrations significantly different between the pre- and post-weight gain study days.
Fig. 2.
Daytime chylomicron and nonchylomicron triglyceride (TG) concentrations during the baseline study and following overfeeding. *P < 0.01 vs. baseline at the same time point.
Fig. 3.
Daytime plasma free fatty acid (oleate) concentrations during the baseline study and following overfeeding. At no time were concentrations significantly different between visits 1 and 2.
Fasting femoral adipose tissue LPL activity (mmol FFA released·h−1·g lipid−1) was significantly greater in women than men (Table 2) at both visits, whereas fasting abdominal subcutaneous adipose tissue LPL activity was not different. In women, femoral LPL activity was significantly greater than abdominal LPL activity (P ≤ 0.05), but this was not the case in men. There was no significant change in regional, fasting LPL activity in women after overfeeding. In men, overfeeding induced a significant increase in fasting femoral (P < 0.05), but not abdominal, LPL activity.
Table 2.
LPL activity and meal fat storage
| Women | Men | P Value | |
|---|---|---|---|
| Fasted LPL activity, mmol FFA released·h−1·g lipid−1 | |||
| Visit 1 | |||
| UBSQ | 0.34 ± 0.15 | 0.25 ± 0.18 | 0.22 |
| LBSQ | 0.84 ± 0.44 | 0.22 ± 0.09 | 0.0008 |
| Visit 2 | |||
| UBSQ | 0.45 ± 0.29 | 0.30 ± 0.17 | 0.21 |
| LBSQ | 0.90 ± 0.52 | 0.44 ± 0.28 | 0.01 |
| Storage, mg meal fat/g lipid | |||
| Visit 1 | |||
| UBSQ | 0.78 ± 0.34 | 1.04 ± 0.71 | 0.22 |
| LBSQ | 0.60 ± 0.23 | 0.48 ± 0.29 | 0.25 |
| Visit 2 | |||
| UBSQ | 0.71 ± 0.24 | 0.90 ± 0.37 | 0.077 |
| LBSQ | 0.62 ± 0.24 | 0.65 ± 0.23 | 0.67 |
Values are means ± SD. LPL, lipoprotein lipase; FFA, free fatty acid; UBSQ, upper body subcutaneous region; LBSQ, lower body subcutaneous region.
P value reflects differences between men and women.
Meal fat storage (mg meal fat/g adipose tissue lipid) is shown in Table 2. Assessed in this manner, the regional (UBSQ and LBSQ) meal fat storage did not differ between men and women at either visit. Women had a significantly greater total meal fatty acid storage in UBSQ than LBSQ fat at both study visits (P ≤ 0.05), similar to men (P ≤ 0.001). Meal fat storage (mg meal fat/g adipose tissue lipid) remained similar in both adipose depots at visit 2 in women, but storage into LBSQ adipose tissue increased significantly (P < 0.05) in men.
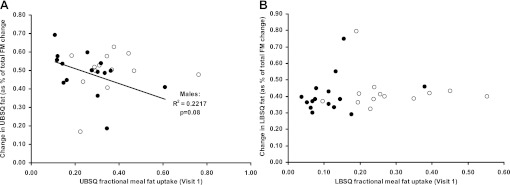
Fractional meal fat storage in the UBSQ fat (Fig. 4A) and LBSQ fat (Fig. 4B) at visit 1 did not predict the change in regional body fat (as percentage of total fat mass change) in response to overfeeding in either sex. Similarly, neither meal fat storage in subcutaneous fat nor meal fatty acid oxidation at baseline predicted visceral fat gain (data not shown). Because some of the data were not normally distributed, we also analyzed these relationships using logarithmically transformed values; there remained no significant associations using this approach.
Fig. 4.
Change in upper and lower body subcutaneous (UBSQ and LBSQ, respectively) adipose tissue as a percentage of total body fat mass (FM) change from visit 1 to visit 2 vs. UBSQ and LBSQ adipose tissue fractional meal fat storage (percentage of meal fat tracer given) at visit 1. No significant correlations were found.
Similar to previous findings (18), we found no relationship between fasting LPL and regional meal fat storage at 24 h (data not shown). The following relationships were tested and found to be insignificant; thus the data are not shown: 1) there was no relationship between fasting regional LPL at the start of the study and change in regional fat mass stores as a percentage of total fat mass change; and 2) changes in meal fat storage from before to after weight gain were unrelated to regional fat gain.
DISCUSSION
We hypothesized that regional meal fat storage over the short term (24 h) would predict regional fat gain during the deposition of ∼3 kg of fat in response to 2–3 mo of overeating. Our data suggest that we can reject this hypothesis. Although we found a slight, nonsignificant, increase in overall meal fat storage from visit 1 to visit 2, this is likely a result of the increase in fat mass, not vice versa. Additionally, we found that neither baseline, fasting LPL activity nor the change in fasting LPL activity with overfeeding predicted regional fat gain over time. We admit that fasting LPL activity generally is not a good predictor of short-term meal fat storage (33, 34). However, we wished to understand whether baseline LPL and/or the changes in regional LPL activity might be an indicator of adipose tendencies to respond to excess fat intake with greater regional fat gain; postabsorptive LPL activity did not relate to regional fat gain with overfeeding.
The metabolic milieu of these nonobese subjects in response to fat gain deserves comment. Our subjects gained upper body fat via adipocyte hypertrophy and lower body fat by adipocyte hyperplasia (29) and developed mild insulin resistance with regard to glucose metabolism as measured by integrated daytime plasma insulin concentrations (32). We report that overfeeding/fat gain resulted in higher chylomicron TG concentrations in the context of the same dietary challenge (suggesting reduced clearance) and higher nonchylomicron TG concentrations. Plasma FFA (oleate) concentrations following fat gain were not greater than baseline (Fig. 3), which, combined with the minor increases in insulin in these subjects, suggests that insulin resistance with regard to adipose tissue lipolysis did not develop. However, without a more specific assessment of adipose tissue lipolysis insulin sensitivity, we cannot exclude the possibility of a minor change in this parameter. In general, the adipose tissue adaptations to modest fat gain in nonobese adults are more complex than we anticipated.
Meal fat storage, as a percentage of total meal fat or in milligrams of meal fat per gram of adipose tissue, was greater in the UBSQ than the LBSQ fat in men and women. This is consistent with previous findings (25, 35) and indicates that, in the short term, meal fat is not bound by sex-specific adipose distribution. This is interesting also in light of the similar increase in UBSQ and LBSQ fat with weight gain in the men and women in our study. That is, men did not gain more than women in the UBSQ region, as might be expected. This suggests that some redistribution of fat from one fat depot to another, or to other adipose compartments in the body, is likely, even in the face of a positive energy balance.
Redistribution of meal fat over time has been previously shown in animals (2–4) and humans (18), probably through a variety of routes. Ravikumar et al. (23) showed that a significant portion of dietary fat is taken up by the liver; some of this dietary fat is eventually exported in VLDL particles (9) and potentially stored in adipose tissue (19, 28). Redistribution from rapidly to slowly turning over fatty acid adipose tissue depots may also be possible. Dietary fatty acid storage into visceral fat is very efficient in humans with small visceral fat depots (11), which implies high rates of release in these individuals. The delivery of these fatty acids to the liver via the portal vein may create a second source of recent dietary fat for VLDL synthesis. Combined, these observations likely account for the findings of Marin et al. (18), who also performed repeated adipose tissue biopsies and found that tracer accumulates slowly in subcutaneous adipose tissue (abdominal and femoral) over 1 mo. In addition, plasma FFA can be restored in body fat via a direct uptake pathway independent of VLDL (1, 12–14, 27) in a manner that may serve to redistribute fatty acids between depots in a sex-specific manner. An additional confounding factor is the unexpected appearance of new LBSQ adipocytes (29) during weight gain. By definition, we cannot know the fat storage tendencies of these as yet unformed adipocytes prior to the overfeeding intervention, which may also help explain why our baseline measures of meal fat storage were completely unable to predict regional fat gain.
We also observed that, in women, the change in LBSQ fat, as a percentage of total fat mass change, seems to be capped at ∼40% of total fat mass (Fig. 4B). This is somewhat surprising, because women tend to carry more of their body fat in the lower body stores. There are not many longer-term overfeeding studies in women with which to compare our data. Yet it begs the possibility that there is a limit to how much fat can be stored in the LBSQ. Perhaps this is a different value for all women, but perhaps, at some threshold level, women start to accumulate more fat, as a percentage of total, in the UBSQ.
It is of interest to note that expression of the data as milligrams of meal fat per gram of adipose tissue, or as a percentage of meal fat, was not a factor in the ability of regional meal fat tracer storage to predict regional weight gain. The lack of a relationship between short-term meal fat storage and regional fat gain, therefore, is quite robust. We recently showed that the microenvironment differs remarkably in regional fat depots (30). In fact, it is possible that meal fat storage may only predict fat gain if the main mechanism of expansion is hypertrophy, given that we observed adipocyte hyperplasia in the LBSQ adipose tissue (29).
There are several limitations to this study. Meal fat storage was determined only in subcutaneous adipose tissue, and it is possible that the storage in visceral fat may predict visceral fat gain. However, visceral fat accounts for only ∼5% of meal fat storage (1) and, thus, is of lesser importance to overall dietary fatty acid trafficking than subcutaneous fat. We studied only nonobese adults, which begs the question of whether meal fat storage in obese adults might predict whether additional, excess regional fat gain is also independent of meal fat storage. Testing that hypothesis is likely to be even more challenging, however, given the even greater reluctance of already obese adults to participate in weight gain studies.
In conclusion, meal fat uptake over 24 h is not a predictor of regional body fat gain over ∼8 wk in lean, healthy subjects. Similarly, regional fasting adipose tissue LPL did not predict regional body fat gain over the study period. In general, meal fat uptake appears to be greater in the UBSQ fat region in men and women. Therefore, it may be that other mechanisms that favor fat trafficking and redistribution, such as concurrent lipolysis and redistribution via VLDL and FFA, are potentially more likely to favor differences in upper body vs. lower body fat distribution.
GRANTS
This research was supported by National Institutes of Health Grants DK-45343 (M. D. Jensen), DK-50456 (M. D. Jensen), and RR-00585 (M. D. Jensen) and the Mayo Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.B.V. and M.D.J. are responsible for conception and design of the research; S.B.V. and M.D.J. performed the experiments; S.B.V. and M.D.J. analyzed the data; S.B.V. and M.D.J. interpreted the results of the experiments; S.B.V. and M.D.J. prepared the figures; S.B.V. and M.D.J. drafted the manuscript; S.B.V. and M.D.J. edited and revised the manuscript; S.B.V. and M.D.J. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank the volunteers who participated in the study, as well as the staff of the Mayo Clinic GCRC. We also thank Carol Siverling, Jessica Eastman, Darlene Lucas, Debra Harteneck, and Rita Nelson for their work on the study.
Present affiliation of S. B. Votruba: Obesity and Diabetes Clinical Research Section, NIH/NIDDK, Phoenix, AZ.
REFERENCES
- 1. Ali AH, Koutsari C, Mundi M, Stegall MD, Heimbach JK, Taler SJ, Nygren J, Thorell A, Bogachus LD, Turcotte LP, Bernlohr D, Jensen MD. Free fatty acid storage in human visceral and subcutaneous adipose tissue: role of adipocyte proteins. Diabetes 60: 2300–2307, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bessesen DH, Rupp CL, Eckel RH. Dietary fat is shunted away from oxidation, toward storage in obese Zucker rats. Obes Res 3: 179–189, 1995. [DOI] [PubMed] [Google Scholar]
- 3. Bessesen DH, Rupp CL, Eckel RH. Trafficking of dietary fat in lean rats. Obes Res 3: 191–203, 1995. [DOI] [PubMed] [Google Scholar]
- 4. Bessesen DH, Vensor SH, Jackman MR. Trafficking of dietary oleic, linolenic, and stearic acids in fasted or fed lean rats. Am J Physiol Endocrinol Metab 278: E1124–E1132, 2000. [DOI] [PubMed] [Google Scholar]
- 5. Björntorp P. Metabolic implications of body fat distribution. Diabetes Care 14: 1132–1143, 1991. [DOI] [PubMed] [Google Scholar]
- 6. Eckel RH, Hernandez TL, Bell ML, Weil KM, Shepard TY, Grunwald GK, Sharp TA, Francis CC, Hill JO. Carbohydrate balance predicts weight and fat gain in adults. Am J Clin Nutr 83: 803–808, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Gastaldelli A, Miyazaki Y, Pattiti M, Matsuda M, Mahankali S, Santini E, DeFronzo RA, Ferrannini E. Metabolic effects of visceral fat accumulation in type 2 diabetes. J Clin Endocrinol Metab 87: 5098–5103, 2002. [DOI] [PubMed] [Google Scholar]
- 8. Gillum RF. The association of body fat distribution with hypertension, hypertensive heart disease, coronary heart disease, diabetes and cardiovascular risk factors in men and women aged 18–79 years. J Chron Dis 40: 421–428, 1987. [DOI] [PubMed] [Google Scholar]
- 9. Heath RB, Karpe F, Milne RW, Burdge GC, Wootton S, Frayn KN. Dietary fatty acids make a rapid and substantial contribution to VLDL-triacylglycerol in the fed state. Am J Physiol Endocrinol Metab 292: E732–E739, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Jensen MD. Gender differences in regional fatty acid metabolism before and after meal ingestion. J Clin Invest 96: 2297–2303, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jensen MD, Sarr MG, Dumesic DA, Southorn PA, Levine JA. Regional uptake of meal fatty acids in humans. Am J Physiol Endocrinol Metab 285: E1282–E1288, 2003. [DOI] [PubMed] [Google Scholar]
- 12. Koutsari C, Ali AH, Mundi MS, Jensen MD. Storage of circulating FFA in adipose tissue of postabsorptive humans: quantitative measures and implications for body fat distribution. Diabetes 60: 2032–2040, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koutsari C, Dumesic DA, Patterson BW, Votruba SB, Jensen MD. Plasma free fatty acid storage in subcutaneous and visceral adipose tissue in postabsorptive women. Diabetes 57: 1186–1194, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Koutsari C, Snozek CL, Jensen MD. Plasma NEFA storage in adipose tissue in the postprandial state: sex-related and regional differences. Diabetologia 51: 2041–2048, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levine JA, McCrady SK, Lanningham-Foster LM, Kane PH, Foster RC, Manohar CU. The role of free-living daily walking in human weight gain and obesity. Diabetes 57: 548–554, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Marin P, Andersson B, Ottosson M, Olbe L, Chowdhury B, Kvist H, Holm G, Sjostrom L, Bjorntorp P. The morphology and metabolism of intra-abdominal adipose tissue in men. Metabolism 41: 1242–1248, 1992. [DOI] [PubMed] [Google Scholar]
- 17. Marin P, Oden B, Olbe L, Bengtsson BA, Bjorntorp P. Assimilation of triglycerides in subcutaneous and intra-abdominal adipose tissues in vivo in men: effects of testosterone. J Clin Endocrinol Metab 81: 1018–1022, 1996. [DOI] [PubMed] [Google Scholar]
- 18. Marin P, Rebuffe-Scrive M, Bjorntorp P. Uptake of triglyceride fatty acids in adipose tissue in vivo in man. Eur J Clin Invest 20: 158–165, 1990. [DOI] [PubMed] [Google Scholar]
- 19. Nellemann B, Gormsen LC, Christiansen JS, Jensen MD, Nielsen S. Postabsorptive VLDL-TG fatty acid storage in adipose tissue in lean and obese women. Obesity (Silver Spring) 18: 1304–1311, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nilsson-Ehle P, Schotz MC. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res 17: 536–541, 1976. [PubMed] [Google Scholar]
- 21. Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr 86: 625–632, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Potretzke AM, Schmitz KH, Jensen MD. Preventing overestimation of pixels in CT images of visceral fat. Obes Res 12: 1698–1701, 2004. [DOI] [PubMed] [Google Scholar]
- 23. Ravikumar B, Carey PE, Snaar JEM, Deelchand DK, Cook DB, Neely RDG, English PT, Firbank MJ, Morris PG, Taylor R. Real-time assessment of postprandial fat storage in liver and skeletal muscle in health and type 2 diabetes. Am J Physiol Endocrinol Metab 288: E789–E797, 2005. [DOI] [PubMed] [Google Scholar]
- 24. Ravussin E, Swinburn BA. Metabolic predictors of obesity: cross-sectional versus longitudinal data. Int J Obes 17 Suppl 3: S28–S31; discussion S41–S22, 1993. [PubMed] [Google Scholar]
- 25. Romanski SA, Nelson R, Jensen MD. Meal fatty acid uptake in adipose tissue: gender effects in non-obese humans. Am J Physiol Endocrinol Metab 279: E455–E462, 2000. [DOI] [PubMed] [Google Scholar]
- 26. Romanski SA, Nelson RM, Jensen MD. Meal fatty acid uptake in human adipose tissue: technical and experimental design issues. Am J Physiol Endocrinol Metab 279: E447–E454, 2000. [DOI] [PubMed] [Google Scholar]
- 27. Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes 56: 1369–1375, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Sondergaard E, Nellemann B, Sorensen LP, Gormsen LC, Christiansen JS, Ernst E, Dueholm M, Nielsen S. Similar VLDL-TG storage in visceral and subcutaneous fat in obese and lean women. Diabetes 60: 2787–2791, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tchoukalova Y, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci USA 107: 18226–18231, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tchoukalova YD, Koutsari C, Votruba SB, Tchkonia T, Giorgadze N, Thomou T, Kirkland JL, Jensen MD. Sex- and depot-dependent differences in adipogenesis in normal-weight humans. Obesity (Silver Spring) 18: 1875–1880, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Uranga AP, Levine J, Jensen M. Isotope tracer measures of meal fatty acid metabolism: reproducibility and effects of the menstrual cycle. Am J Physiol Endocrinol Metab 288: E547–E555, 2005. [DOI] [PubMed] [Google Scholar]
- 32. Votruba SB, Jensen MD. Insulin sensitivity and regional fat gain in response to overfeeding. Obesity (Silver Spring) 19: 269–275, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Votruba SB, Jensen MD. Sex-specific differences in leg fat uptake are revealed with a high-fat meal. Am J Physiol Endocrinol Metab 291: E1115–E1123, 2006. [DOI] [PubMed] [Google Scholar]
- 34. Votruba SB, Jensen MD. Sex differences in abdominal, gluteal, and thigh LPL activity. Am J Physiol Endocrinol Metab 292: E1823–E1828, 2007. [DOI] [PubMed] [Google Scholar]
- 35. Votruba SB, Mattison RS, Dumesic DA, Koutsari C, Jensen MD. Meal fatty acid uptake in visceral fat in women. Diabetes 56: 2589–2597, 2007. [DOI] [PubMed] [Google Scholar]
- 36. Weyer C, Pratley RE, Salbe AD, Bogardus C, Ravussin E, Tataranni PA. Energy expenditure, fat oxidation, and body weight regulation: a study of metabolic adaptation to long-term weight change. J Clin Endocrinol Metab 85: 1087–1094, 2000. [DOI] [PubMed] [Google Scholar]
- 37. Weyer C, Vozarova B, Ravussin E, Tataranni PA. Changes in energy metabolism in response to 48 h of overfeeding and fasting in Caucasians and Pima Indians. Int J Obes 25: 593–600, 2001. [DOI] [PubMed] [Google Scholar]
- 38. Wier LT, Ayers GW, Jackson AS, Rossum AC, Poston WS, Foreyt JP. Determining the amount of physical activity needed for long-term weight control. Int J Obes 25: 613–621, 2001. [DOI] [PubMed] [Google Scholar]
- 39. Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, Swinburn BA, Knowler WC, Bogardus C, Ravussin E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol Endocrinol Metab 259: E650–E657, 1990. [DOI] [PubMed] [Google Scholar]