Abstract
The endocannabinoid system is highly implicated in the development of insulin resistance associated with obesity. It has been shown that antagonism of the CB1 receptor improves insulin sensitivity (SI). However, it is unknown whether this improvement is due to the direct effect of CB1 blockade on peripheral tissues or secondary to decreased fat mass. Here, we examine in the canine dog model the longitudinal changes in SI and fat deposition when obesity was induced with a high-fat diet (HFD) and animals were treated with the CB1 antagonist rimonabant. SI was assessed (n = 20) in animals fed a HFD for 6 wk to establish obesity. Thereafter, while HFD was continued for 16 additional weeks, animals were divided into two groups: rimonabant (1.25 mg·kg−1·day−1 RIM; n = 11) and placebo (n = 9). Euglycemic hyperinsulinemic clamps were performed to evaluate changes in insulin resistance and glucose turnover before HFD (week −6) after HFD but before treatment (week 0) and at weeks 2, 6, 12, and 16 of treatment (or placebo) + HFD. Magnetic resonance imaging was performed to determine adiposity- related changes in SI. Animals developed significant insulin resistance and increased visceral and subcutaneous adiposity after 6 wk of HFD. Treatment with RIM resulted in a modest decrease in total trunk fat with relatively little change in peripheral glucose uptake. However, there was significant improvement in hepatic insulin resistance after only 2 wk of RIM treatment with a concomitant increase in plasma adiponectin levels; both were maintained for the duration of the RIM treatment. CB1 receptor antagonism appears to have a direct effect on hepatic insulin sensitivity that may be mediated by adiponectin and independent of pronounced reductions in body fat. However, the relatively modest effect on peripheral insulin sensitivity suggests that significant improvements may be secondary to reduced fat mass.
Keywords: cannabinoid 1
obesity is highly correlated with insulin resistance and the development of type 2 diabetes. This relationship is reversed when weight loss occurs, suggesting that obesity promotes insulin resistance. However, the mechanisms underlying the tight relationship between adiposity and insulin resistance remain unclear.
The endocannabinoid system may be an important component in insulin resistance with obesity (22, 23). Overactivity of the endocannabinoid system is closely related to abdominal obesity and type 2 diabetes. In addition, cannabinoid 1 (CB1) receptors have been found not only in the brain but also in the liver, skeletal muscle, and gut, as well as the pancreas, all of which play a role in glucose metabolism and type 2 diabetes (15). It is now well understood that in addition to the central effects of the endocannabinoid system there is also a peripheral effect on several tissues such as the liver, adipose tissue, and skeletal muscle that influences insulin sensitivity (21). It has also been demonstrated that insulin has an inverse relationship with plasma endocannabinoids (7) and that chronic treatment of adipocytes with insulin results in a permanent elevation of endocannabinoid signaling (13), suggesting that dysregulation of the peripheral endocannabinoid system may be intimately linked with changes in insulin sensitivity.
Although it is apparent that chronic CB1 receptor antagonism in obese animals and humans has beneficial effects on the development of insulin resistance (6, 19), it is still unknown whether those beneficial actions are a consequence of decreased fat mass or the direct result of CB1 receptor blockade on peripheral tissues. Therefore, in this study, we sought to look at the effects of the CB1 receptor antagonist rimonabant when insulin resistance and obesity are induced by a hypercaloric high-fat diet (HHFD).
METHODS
Animals.
Male mongrel dogs (n = 20, 30.0 ± 0.8 kg) used in a corresponding publication (20) were housed in the Keck School of Medicine at the University of Southern California (USC) Vivarium under controlled kennel conditions (12:12-h light-dark cycle). Animals were accepted into this study following physical examination and a comprehensive blood panel. A chronic catheter attached to vascular access ports (Instech Solomon, Plymouth Meeting, PA) was surgically implanted 2 wk prior to the beginning of the study and secured subcutaneously to the underlying musculature at the back of the animal's neck. The catheter was inserted in the jugular vein and advanced to the right atrium for sampling of central venous blood. Access points for the ports were shaved and swabbed with providone-iodide before each sampling needle was inserted. Catheters were flushed with heparinized saline (10 U/ml) at least once/wk. Dogs were accustomed to laboratory procedures and were used for experiments only if judged to be in good health, as determined by visual observation, body temperature, and hematocrit. On the morning of each experiment, 19-gauge angiocatheters (Allegiance Healthcare, Ontario, CA) were inserted percutaneously into the saphenous vein for glucose infusion. The experimental protocol was approved by the USC Institutional Animal Care and Use Committee.
Diet.
Dogs were fed a weight-maintaining standard diet of one can of Hill's Prescription Diet (10% carbohydrate, 9% protein, 8% fat, 0.3% fiber, and 73% moisture; Hill's Pet Nutrition, Topeka, KS) and 825 g of dry chow (40% carbohydrate, 26.2% protein, 14% fat, and 2.9% fiber; LabDiet, Richmond, IN) for a period of 2–3 wk to ensure weight stabilization before any experiments were conducted. This standard diet consisted of 3,885 kcal/day: 38.3% from carbohydrates, 26.1% from protein, and 34.5% from fat. Following weight stabilization (week −6), dogs were maintained on a HHFD in which the standard diet was supplemented with 6 g/kg of prediet body weight of cooked bacon grease. This HHFD consisted of a total of 5,236 kcal/day: 28.4% carbohydrates, 19.4% protein, and 51.4% fat. Throughout the study, dogs were presented with their meals at 9 AM and given 3 h to eat, after which the meal was removed.
Rimonabant treatment.
Following 6 wk of high-fat diet (HFD; week 0), the animals were divided into two groups, rimonabant (RIM; n = 11) or placebo (PBO; n = 9). Animals were matched for body weight (RIM = 31.7 ± 1.3 kg, PBO = 31.8 ± 1.5 kg). Rimonabant (Sanofi-Aventis, Paris, France) was encapsulated (AMC pharmacy, Burbank, CA) and administered orally at 1.25 mg·kg−1·day−1, whereas the PBO group received gelatin capsules. The dose of rimonabant was chosen on the basis of a study carried out in a small group of dogs (n = 5) testing different doses ranging from 1.25 to 5 mg·kg−1·day−1. The dose of 1.25 mg·kg−1·day−1 was chosen because it did not produce any adverse clinical effects. Animals were maintained on the HHFD throughout the 16 wk of treatment.
Magnetic resonance imaging.
During weeks −6, 0, 2, 6, 12, and 16 of the study, magnetic resonance imaging (MRI) scans were performed on the dogs, as described previously (11). Thirty 1-cm axial abdominal images (T1 slices; TR 500 TE:14) were obtained using a General Electric 1.5 Tesla Horizon (software version 5.7) magnet. Of the 30 images obtained, ∼20 of these images were used for analysis of total trunk body fat, depending on the relative torso length of the animal. Images were analyzed using Scion Image (Alpha 4.0.3.2; Scion, Frederick, MD), which quantifies fat tissue (pixel value 121–254) and other tissue (20–120) in each slice. Fat volume was calculated by dividing the number of pixels counted as fat by the ratio of the total number of pixels (256 × 256) and known area (34.9 × 34.9 cm) for a 1-cm image. Total trunk fat and tissue were estimated as the integrated fat or tissue across all 20 slices. Percent fat was calculated as the total trunk fat divided by the total trunk tissue. Omental fat was defined as fat within the peritoneal cavity in an 11-cm region of the thorax, using the slice at the level where the left renal artery branches from the abdominal aorta as a midpoint landmark. Percent omental fat was calculated as the omental fat divided by the total tissue area in these same slices.
Euglycemic hyperinsulinemic clamps.
The euglycemic hyperinsulinemic clamps were performed as described previously (11) during weeks −6, 0, 2, 6, 12, and 16 of the study. Animals were familiarized with the Pavlov sling ≥1 wk before the first experiment. At ∼6:30 AM on the day of the clamp, animals were brought to the laboratory and placed in the Pavlov sling. A 19-gauge angiocatheter was placed in a saphenous vein and secured. Approximately 30 min later (t = −120 min), a primed continuous infusion of high-performance liquid chromatography-purified [3-3H]glucose (25 μCi + 0.25 μCi/min infusion; PerkinElmer, Boston, MA) was started. After tracer equilibration, basal samples were taken at −30, −20, −10, and −1 min. At time t = 0 min, a somatostatin infusion (1.0 μg·min−1·kg−1; Bachem California, Torrance, CA) was started to suppress endogenous insulin and glucagon secretion and was continued for the duration of the experiment. Porcine insulin was infused (0.75 mU·kg−1·min−1; Eli Lilly, Indianapolis, IN) into the saphenous vein to attain hyperinsulinemia. Glucose was clamped at basal by a variable glucose infusion labeled with d-[3-3H]glucose (2.0 μCi/g) to minimize fluctuations in plasma-specific activity. Blood samples were drawn from the jugular catheter every 10 min from −30 to 60 min, every 15 min from 60 to 120 min, and then every 10 min from 120 to 180 min.
Samples for assay of insulin and d-[3-3H]glucose were taken in tubes precoated with lithium fluoride and heparin (Brinkmann Instruments, Westbury, NY). The tubes for insulin and glucose also contained 50 μl of EDTA. Samples for free fatty acid and glycerol assay were taken in tubes with EDTA and paraoxon to inhibit lipase activity. All samples were immediately centrifuged, and plasma was separated and stored at −80°C for further analysis.
Assays.
Glucose was measured with a YSI 2300 autoanalyzer (Yellow Springs Instruments, Yellow Springs, OH). Free fatty acids were measured utilizing a colorimetric assay based on the acylation of coenzyme A from Wako (NEFA C; Wako Pure Chemical Industries, Richmond, VA). Insulin was measured by an ELISA developed originally for human serum or plasma (Linco Research, St. Charles, MO) and adapted for dog plasma using a dog standard kindly provided by Novo Nordisk. The method is based on two murine monoclonal antibodies that bind to different epitopes of insulin but do not bind to proinsulin.
Samples for [3H]glucose tracer assay were deproteinized using barium hydroxide and zinc sulfate. The supernatants were then evaporated in a vacuum, reconstituted in water, and counted in Ready Safe scintillation fluid (Beckman liquid scintillation fluid; Beckman Instruments, Fullerton, CA). Tracer infusates were processed identically to plasma samples.
Calculations.
The time course of endogenous glucose production (EGP) and glucose disappearance (Rd) during the euglycemic hyperinsulinemic clamp were calculated using Steele's model with a labeled glucose infusion, as described previously (9). Derivatives of all time course data were calculated with OOPSEG (5). Basal was defined as the average of four samples taken every 10 min from t = −30 to 0 min, and steady state was defined as the average of four samples taken from t = 150 to 180 min. Whole body insulin sensitivity was calculated using the equation
where ΔGinf is the difference in glucose infusion rate at steady state from basal, ΔI is the difference in plasma insulin at steady state from basal, and G is the steady-state plasma glucose concentration. Peripheral and hepatic insulin sensitivity were calculated using the same equation, in which ΔGinf was substituted by either ΔRd or ΔEGP, respectively.
Statistical analyses.
All experimental data are expressed as means ± SE. Repeated-measures ANOVA with Bonferroni posttest was used to compare all time course data within treatment groups. Paired Student's t-tests were used to identify the significantly different time point pairs and to compare all metabolic parameters at fasting and steady state within groups. Nonpaired t-tests were used to compare means between groups. All analyses were done using GraphPad InStat (GraphPad Software, La Jolla, CA).
RESULTS
Body composition.
Six weeks of the HHFD resulted in a substantial increase in both total trunk body fat and body weight (Fig. 1A). Total trunk fat increased from 733 ± 59 cm3 at week −6 to 996 ± 61 cm3 at week 0 (P < 0.05), and body weight was increased from 30.0 ± 0.8 to 32.0 ± 0.9 kg (P < 0.05).
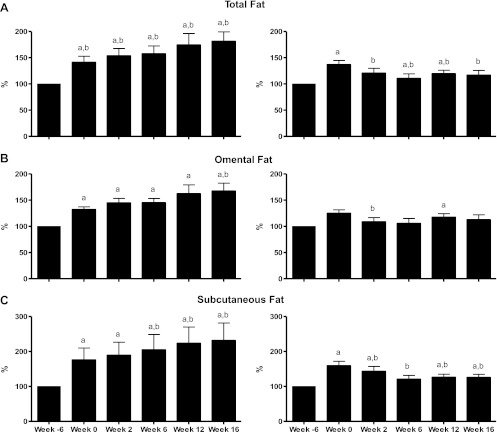
Fig. 1.
Changes in total body fat, omental fat, and subcutaneous fat depots in the placebo- (left) vs. the rimonabant-treated animals (right). aP < 0.05 vs. week −6; bP < 0.05 vs. week 0.
In the PBO group, this increase in total trunk adiposity was maintained up to week 16 of the study, at which time total trunk fat volume was 1,122 ± 195 cm3 (P < 0.05 vs. week −6). The extensive increase in total trunk fat was due to an accumulation of fat tissue in the omental and subcutaneous depots (Fig. 1, B and C), which increased by 39 ± 9 and 92 ± 39%, respectively, from prediet levels (P < 0.05 vs. week −6 for both).
In contrast, RIM treatment resulted in a 12 ± 4% decrease in total body fat after only 2 wk of treatment. This decrease was due to a reduction in both subcutaneous and omental fat (−10.6 ± 3.8 and −12.9 ± 4.9%, respectively, from week 0, P < 0.05, for both), which continued for the remainder of the study, such that subcutaneous fat had decreased by 19.5 ± 5.0% (P < 0.05 vs. week 0) and omental fat had decreased by 9.8 ± 6.4% [P = not significant (NS)]. Likewise, total body fat continued to decrease for the remainder of the study such that total body fat volume was 936 ± 108 cm3 by week 16 of treatment, accounting for a 14 ± 3% decrease in total body fat despite continued high-fat hypercaloric feeding. These changes observed in body fat are discussed in detail in a previous publication from our laboratory (20).
Glucose.
Despite a 50 ± 14% increase in total trunk fat (see above) over the course of the study, fasting glucose did not increase with fat feeding (Table 1) or at any time point during the treatment period (Table 2). This is consistent with previous results (11). In addition, glucose was well clamped during each experiment throughout the study (P = NS for all experiments; basal vs. steady-state, paired t-test), and the time course for plasma glucose during the clamp did not differ between weeks (P = NS, paired t-test; data not shown).
Table 1.
Fasting metabolic parameters before and after fat feeding
| Week −6 | Week 0 | |
|---|---|---|
| Gllucose, mg/dl | 94 ± 2 | 95 ± 1 |
| Insulin, pM | 50 ± 4 | 66 ± 6* |
| MCR, ml·kg−1·min−1 | 20.4 ± 0.9 | 17.9 ± 1.1* |
| FFA, mM | 0.60 ± 0.04 | 0.69 ± 0.04 |
Values are means ± SE. MCR, metabolic clearance rate; FFA, free fatty acids.
P < 0.05 vs. week −6.
Table 2.
Fasting parameters during the treatment period in placebo and rimonabant groups
| Week 0 | Week 2 | Week 6 | Week 12 | Week 16 | |
|---|---|---|---|---|---|
| Placebo | |||||
| Glucose, mg/dl | 95 ± 2.1 | 90 ± 1 | 92 ± 1 | 94 ± 2 | 91 ± 2 |
| Insulin, pM | 72 ± 13 | 67 ± 8 | 67 ± 11 | 69 ± 15 | 72 ± 15 |
| FFA, mM | 0.67 ± 0.05 | 0.65 ± 0.04 | 0.78 ± 0.07 | 0.79 ± 0.05 | 0.76 ± 0.05 |
| Rimonabant | |||||
| Glucose, mg/dl | 96 ± 2 | 95 ± 2 | 93 ± 2 | 97 ± 2 | 93 ± 3 |
| Insulin, pM | 62 ± 3 | 68 ± 9 | 64 ± 8 | 77 ± 10 | 63 ± 10 |
| FFA, mM | 0.70 ± 0.06 | 0.81 ± 0.0 | 0.76 ± 0.06 | 0.68 ± 0.04 | 0.71 ± 0.06 |
Values are means ± SE.
Free fatty acids.
Similarly, there was no change in the fasting levels of free fatty acids with either HFD (Table 1) or during treatment (Table 2). In addition, there was no change in the ability of insulin to suppress free fatty acids since steady-state levels during the clamp did not differ between weeks or treatment groups (data not shown).
Insulin.
In sharp contrast to glucose, hypercaloric high-fat feeding caused an increase in fasting insulin (Table 1). At week −6, fasting insulin was 50 ± 4 pM. By week 0, fasting insulin had increased by 46 ± 17% (66 ± 6 pM, P < 0.05 vs. week −6) in response to the HFD.
Fasting insulin remained increased throughout the study in the PBO group, and by week 16 of the study, fasting insulin was 72 ± 15 pM. Surprisingly, there was no change in fasting insulin levels in response to RIM treatment. Following the increase in fasting levels due to HFD, plasma insulin did not change in response to RIM treatment (week 0: 62 ± 3 vs. week 2: 68 ± 9 pM, P = NS), and this remained for the duration of the study (Table 2).
Examining the time course data (not shown) and calculating the steady-state values of plasma insulin during the clamp, we found that compared with week −6, there was a tendency for the steady-state concentrations of plasma insulin to be increased during the clamps at week 0 (227 ± 9 vs. 287 ± 33 pM, P = 0.065), indicating a reduction in the metabolic clearance rate (MCR) of insulin during fat. MCR was reduced from 20.4 ± 0.9 ml·kg−1·min−1 at week −6 to 17.9 ± 1.1 ml·kg−1·min−1 at week 0 (P < 0.05). This reduction in insulin clearance remained for the duration of the study in both the PBO and RIM treatment groups (Table 3).
Table 3.
Steady-state metabolic parameters in placebo and rimonabant groups
| Week 0 | Week 2 | Week 6 | Week 12 | Week 16 | |
|---|---|---|---|---|---|
| Placebo | |||||
| Insulin, pM | 307 ± 59 | 262 ± 17 | 286 ± 23 | 299 ± 19 | 324 ± 19 |
| MCR, ml·kg−1·min−1 | 17.1 ± 1.7 | 17.7 ± 1.0 | 16.5 ± 1.3 | 15.5 ± 0.9 | 14.3 ± 0.8 |
| Ginf, mg·kg−1·min−1 | 7.9 ± 1.1 | 7.6 ± 1.4 | 8.1 ± 1.1 | 10.2 ± 1.4 | 9.2 ± 0.9 |
| Rd, mg·kg·−1min−1 | 8.3 ± 1.5 | 7.2 ± 1.2 | 7.5 ± 1.0 | 8.9 ± 1.2 | 8.4 ± 0.9 |
| EGP, mg·kg−1·min−1 | −0.3 ± 0.3 | −0.3 ± 0.4 | −0.7 ± 0.6 | −0.8 ± 0.6 | −0.8 ± 0.5 |
| Rimonabant | |||||
| Insulin, pM | 271 ± 36 | 238 ± 13 | 255 ± 12 | 286 ± 25 | 279 ± 13 |
| MCR, ml·kg−1·min−1 | 18.5 ± 1.5 | 19.4 ± 0.9 | 18.0 ± 0.9 | 16.2 ± 0.8 | 16.6 ± 1.0 |
| Ginf, mg·kg−1·min−1 | 5.8 ± 0.8 | 7.9 ± 0.7* | 8.1 ± 0.7* | 9.2 ± 0.9* | 8.8 ± 0.9* |
| Rd, mg·kg−1·min−1 | 7.1 ± 0.7 | 7.3 ± 0.6 | 7.7 ± 0.6 | 8.2 ± 0.9 | 8.6 ± 0.9 |
| EGP, mg·kg−1·min−1 | 1.0 ± 0.6 | −0.4 ± 0.4 | −0.5 ± 0.6 | −0.8 ± 0.7 | −0.5 ± 0.4* |
Values are means ± SE. EGP, endogenous glucose production; Ginf, glucose infusion rate; Rd, glucose disappearance.
P < 0.05 vs. week 0.
Insulin sensitivity.
Associated with the increase in body fat and fasting hyperinsulinemia, there was a 27.1 ± 7.0% decrease in the glucose infusion rate (Fig. 2A) during steady state after a HHFD such that whole body insulin sensitivity (SI) decreased from 6.0 ± 0.5 dl·kg−1·min−1·pM−1 at week −6 to 3.6 ± 0.4 dl·kg−1·min−1·pM−1 at week 0 (P = 0.00003).
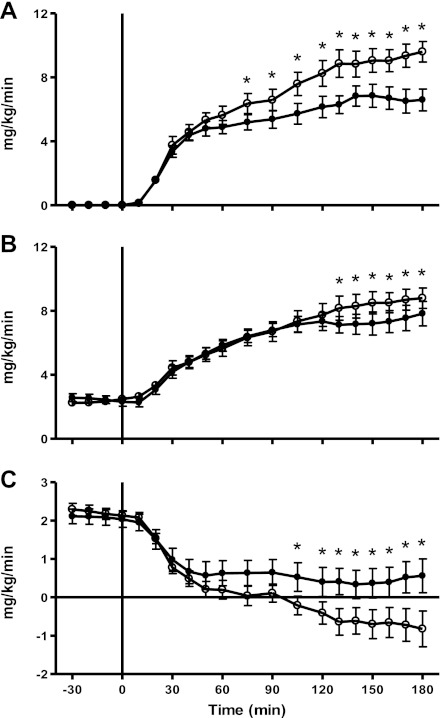
Fig. 2.
Time course data for glucose infusion rates (A), glucose uptake (B), and endogenous glucose production (C) during the clamp before (week −6; ○) and after fat diet (week 0; ●). *P < 0.05 vs. week −6.
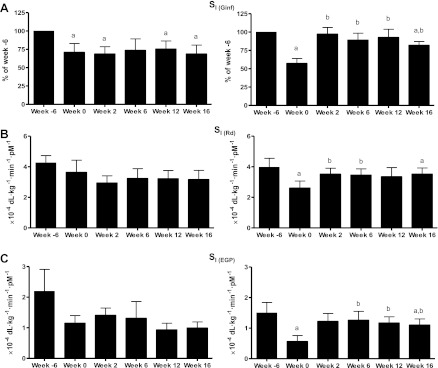
Whole body SI remained altered for the duration of the study in the placebo animals such that at week 16 SI was decreased by 31.3 ± 12.1% (P < 0.05; Fig. 3A) from week −6. In contrast, rimonabant animals showed complete restoration of whole body SI after only 2 wk of treatment. This improvement in whole body SI remained for almost the entire duration of the study, and by week 16 there was only a slight decrease in whole body SI (−18.1 ± 5.2%, P < 0.05).
Fig. 3.
Whole body insulin sensitivity (SI; presented as %week −6) (A), peripheral SI (B), and hepatic SI (C). Placebo group is shown at left, and rimonabant-treated animals are shown at right. aP < 0.05 vs. week −6; bP < 0.05 vs. week 0. Ginf, glucose infusion rate; Rd, glucose disappearance; EGP, endogenous glucose production.
Peripheral insulin action.
Associated with the increase in body fat and fasting hyperinsulinemia, there was a 26.5 ± 10.0% decrease in peripheral insulin action in response to the HHFD (Fig. 2B). Steady-state glucose uptake during a hyperinsulinemic euglycemic clamp decreased from 8.7 ± 0.5 mg·kg−1·min−1 at week −6 to 7.5 ± 0.5 mg·kg−1·min−1 at week 0 (P < 0.05). The time course data for glucose turnover and hepatic glucose production throughout the course of the study is shown in Fig. 4.
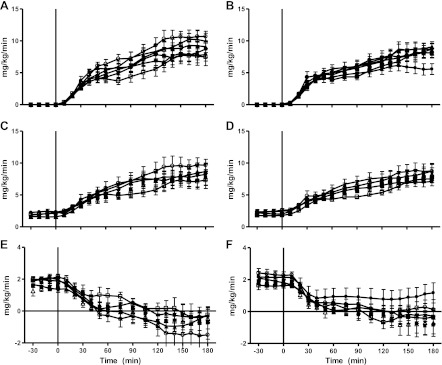
Fig. 4.
Time course data for glucose infusion rates (A and B), glucose uptake (C and D), and EGP (E and F) during the clamp before fat diet (week −6; ○), after fat diet (week 0; ●), and during the treatment period at weeks 2 (□), 6 (■), 12 (▵), and 16 (▴). Placebo group is shown at left, and rimonabant-treated animals are shown at right.
Peripheral glucose uptake showed no further decrease in the placebo animals and in fact appeared to increase at week 16 of the study (8.4 ± 0.9 mg·kg−1·min−1; Table 3). However, this increase in steady-state glucose uptake was most likely due to the concomitant increase in MCR, and the combination of similar steady-state glucose uptake in the face of a modest increase in steady-state insulinemia during the clamp suggests peripheral insulin resistance (Fig. 3B).
In the RIM treatment group, peripheral glucose uptake showed no change with the onset of RIM treatment. At week 2 of treatment, steady-state glucose uptake was 7.3 ± 0.6 vs. 7.1 ± 0.7 mg·kg−1·min−1 at week 0 (P = NS). Although glucose uptake had a tendency to increase as the study continued (week 16: 8.6 ± 0.9 mg·kg−1·min−1, P = 0.10, vs. week 0; Table 3), this was most likely due to the increase in plasma insulin levels during steady state (see above).
Hepatic insulin action.
In addition to the decrease in peripheral insulin action seen with the HFD, there was also impairment in insulin's ability to suppress glucose production during the clamp. At week −6 of the study, hepatic insulin production during steady state was −0.7 ± 0.4 mg·kg−1·min−1 (Fig. 2C). Following the HFD, EGP during the clamp was 0.6 ± 0.4 mg·kg−1·min−1 (P < 0.05), signifying a 63.9 ± 19.6% decrease in hepatic insulin sensitivity in response to increased fat. This impairment in hepatic insulin action remained for the duration of the study in the placebo-treated animals (Fig. 3C).
Rimonabant treatment completely reversed the effects of fat diet on hepatic insulin resistance after only 2 wk. By week 2 of treatment, steady-state glucose production showed complete suppression by insulin (−0.4 ± 0.4 mg·kg−1·min−1, P < 0.05, vs. week 0; Table 3), indicating that hepatic insulin sensitivity was completely restored (Fig. 3C). This restoration of insulin's ability to suppress glucose production remained throughout the 16-wk period (EGP during steady state at week 16: −0.5 ± 0.4 mg·kg−1·min−1).
Adiponectin.
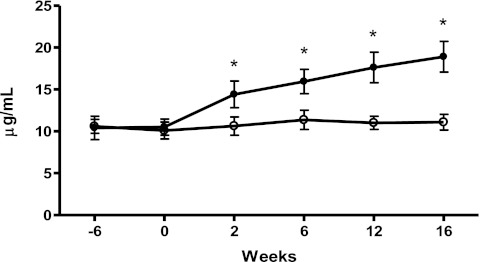
Fasting levels of plasma adiponectin did not change with the onset of HFD (week −6: 10.5 ± 0.8 μg/ml vs. week 0: 10.3 ± 0.7 μg/ml, P = NS). Plasma adiponectin remained unchanged for the duration of the study in the placebo group (Fig. 5). However, after only 2 wk of RIM treatment, there was a substantial increase in fasting adiponectin levels that continued for the remainder of the treatment period such that at week 16 plasma adiponectin had almost doubled from prefat levels to 18.9 ± 1.8 μg/ml (P = 0.0004 vs. week −6).
Fig. 5.
Basal adiponectin levels in the placebo group (○) and rimonabant-treated animals (●) over the course of the study. *P < 0.05 vs. placebo.
DISCUSSION
It is well known that obesity is associated with the impairment of insulin's ability to regulate glucose and that this impairment can be reversed when weight loss occurs. However, the mechanistic basis for this improvement is not yet understood. To examine the relationship between insulin sensitivity and body fat, we employed a CB1 receptor antagonist, rimonabant, which has been shown to have a profound effect in reducing body fat in both animal and human studies.
In this study, normal dogs were fed a hypercaloric high-fat diet for a period of 6 wk to render them obese and insulin resistant, after which they were placed randomly on either rimonabant treatment or placebo while still being maintained on high fat. There was a reduction in both body weight and body fat with rimonabant that became significant after only 2 wk of treatment and remained throughout the 16 wk despite a continued high-fat diet. As expected, administration of a diet high in fat also resulted in impaired insulin sensitivity both in the periphery and at the liver. Surprisingly, when obesity was improved by rimonabant treatment, there was only a moderate effect on peripheral insulin sensitivity. However, hepatic insulin resistance was improved significantly after only 2 wk of rimonabant treatment. This improvement in hepatic insulin sensitivity was associated with an increase in fasting adiponectin levels.
It is unknown whether the beneficial effects of CB1 receptor blockade on glucose metabolism are a direct and independent effect or just a consequence of decreased fat mass. In particular, it has been implicated that increased visceral fat may play an integral role in the development of hepatic insulin resistance (4) due to the anatomic proximity of this fat bed to the liver. In this study, hepatic insulin sensitivity was virtually restored to prediet values after only 2 wk of rimonabant treatment. Although total trunk fat was decreased significantly after 2 wk of treatment, adiposity was not completely renormalized in this time frame. In particular, visceral fat content was not altered significantly by rimonabant treatment. Studies completed in our laboratory utilizing surgical omentectomy (12) to reduce body fat found that a similar reduction of total trunk fat resulted in an 18% increase in peripheral insulin sensitivity. These results suggest that the improvement in insulin sensitivity seen with rimonabant treatment must be due to other mechanisms.
It has been suggested that adiponectin is required to mediate rimonabant-induced improvement of insulin sensitivity (14). Adiponectin is well known to both enhance insulin's ability to suppress glucose production and decrease the storage of triglycerides in the liver and may, therefore, be directly involved in the development of hepatic insulin resistance associated with obesity (1, 3). It has also been demonstrated that chronic activation of CB1 receptors in adipocytes inhibits adiponectin expression, and this inhibition is reversed by treatment with rimonabant (13). Moreover, studies in adiponectin receptor knockout mice show rimonabant to be ineffective in improving insulin sensitivity despite significant improvement in body weight (14). In contrast, other studies, also in adiponectin receptor knockout mice, demonstrate that rimonabant can have a significant effect in partially restoring insulin sensitivity. (25). Hence, rimonabant has been shown to improve insulin sensitivity via both adiponectin-dependent and adiponectin-independent pathways. In the present study, although adiponectin levels did not change with the onset of a high-fat diet, we found that adiponectin levels were increased immediately with rimonabant treatment such that fasting adiponectin had increased by ∼50% after only 2 wk of rimonabant treatment. In addition to the striking increase seen in plasma adiponectin levels, we also found a marked increase in the expression of adiponectin receptors (ADR1 and ADR2) in the liver as well as an increase in the expression of carnitine palmitoyltransferase I and peroxisome proliferator-activated receptor-α (10). Although it is likely that there may also be adiponectin-independent effects of rimonabant, our results suggest that in the fat-fed dog model, the predominant effect of rimonabant may be to increase adiponectin, which will act on its own receptors in the liver and increase fat oxidation, thereby improving hepatic insulin action.
It has been demonstrated previously that there is upregulation of CB1 receptors in whole liver tissue of mice fed a high-fat diet for 3 wk (18). In addition, it has been found in mice that activation of CB1 receptors can lead to increased de novo fatty acid synthesis by inducing hepatic gene expression of the lipogenic transcription factor sterol regulatory element-binding protein-1c (SREBP-1c) and its downstream targets acetyl-CoA carboxylase-α and fatty acid synthase (17). SREBP-1c is a transcription factor that is known to be particularly instrumental in the liver in transducing the insulin signal for regulation of glucose metabolism (8). Interestingly, it has been found recently that adiponectin suppresses SREBP-1c expression in the liver (2). These data support the notion that the endocannabinoid system might affect insulin sensitivity through its action on hepatic metabolism via adiponectin.
The endocannabinoid system has been implicated in the development of insulin resistance and associated morbidities. Increased levels of the endocannabinoids anandamide and/or 2-arachidonoylglycerol have been found in diet-induced obesity (17), and it has been suggested that CB1 antagonists do not need to act centrally to be effective. Studies completed in rats have demonstrated that global blockade of CB1 results in increased insulin sensitivity compared with selective central blockade of CB1 (16), indicating that endcannabinoids may directly regulate metabolic processes by binding to CB1 receptors in peripheral tissues. Moreover, selective targeting of peripheral CB1 receptors has been shown to result in weight-independent improvements in glucose homeostasis (24). These studies, in combination with the results shown here, suggest that blockade of peripheral CB1 receptors may be responsible for the improvement in insulin sensitivity in the fat-fed dog model.
In conclusion, this is the first study to clearly delineate the effects of rimonabant on insulin sensitivity in a large animal model of obesity reminiscent to humans. Our results indicate that the CB1 receptor antagonist rimonabant has a profound effect on improving hepatic insulin sensitivity despite an only modest effect in reducing total body fat. Moreover, it appears that this improvement in hepatic insulin sensitivity occurs without a significant change in visceral fat, indicating that there may be a direct effect of rimonabant on the liver that may be mediated by adiponectin.
GRANTS
This work was supported by Sanofi-Aventis and a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-029867).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.P.K., R.N.B., and J.M.R. did the conception and design of the research; S.P.K., O.W., I.R.H., D.S., L.N.H., D.Z., M.L., C.M.K., K.J.C., J.D.C., M.K., V.I., and J.M.R. performed the experiments; S.P.K. and O.W. analyzed the data; S.P.K. interpreted the results of the experiments; S.P.K. prepared the figures; S.P.K. drafted the manuscript; S.P.K., R.N.B., and J.M.R. edited and revised the manuscript; R.N.B. and J.M.R. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We express our extreme gratitude to Dr. Erlinda Kirkman for excellent veterinarian expertise in facilitating these studies. We are very grateful to radiologist Dr. Linda Needham for help with the MRI scans and to Rita Thomas for excellent assistance with the MRI images. We also thank our lead laboratory animal technician Edward Zuñiga and laboratory assistant Edgardo Paredes.
REFERENCES
- 1. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79–83, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Awazawa M, Ueki K, Inabe K, Yamauchi T, Kaneko K, Okazaki Y, Bardeesy N, Ohnishi S, Nagai R, Kadowaki T. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun 382: 51–56, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7: 947–953, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Bjorntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 10: 493–496, 1990 [PubMed] [Google Scholar]
- 5. Bradley DC, Steil GM, Bergman RN. OOPSEG: a data smoothing program for quantitation and isolation of random measurement error. Comput Methods Programs Biomed 46: 67–77, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci 8: 585–589, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Di Marzo V, Verrijken A, Hakkarainen A, Petrosino S, Mertens I, Lundbom N, Piscitelli F, Westerbacka J, Soro-Paavonen A, Matias I, Van Gaal L, Taskinen MR. Role of insulin as a negative regulator of plasma endocannabinoid levels in obese and nonobese subjects. Eur J Endocrinol 161: 715–722, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Ferre P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res 68: 72–82, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Finegood DT, Bergman RN, Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes 36: 914–924, 1987 [DOI] [PubMed] [Google Scholar]
- 10. Kabir M, Stefanovski D, Hsu IR, Iyer M, Woolcott O, Zheng D, Catalano KJ, Chiu JD, Kim SP, Harrison LN, Ionut V, Lottati M, Bergman RN, Richey JM. Adiponectin Upregulation by CB-1 Antagonist Is Associated with a Reduction of Liver Fat Oxidation and Inflammatory Cytokine Gene Expression in the Fat-Fed Canine Model (Abstract). Diabetes 58: A381, 2009 [Google Scholar]
- 11. Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab 292: E1590–E1598, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Lottati M, Kolka CM, Dittman J, Yae S, Harrison LN, Mooradian V, Kirkman E, Bergman RN. Free Fatty Acids Modulate Insulin Sensitivity Following Reduction of Visceral Fat by Greater Omentectomy in the Obese Dog (Abstract). Obesity 16: S62, 2008 [Google Scholar]
- 13. Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, Petrosino S, Hoareau L, Festy F, Pasquali R, Roche R, Maj M, Pagotto U, Monteleone P, Di Marzo V. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab 91: 3171–3180, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Migrenne S, Lacombe A, Lefèvre AL, Pruniaux MP, Guillot E, Galzin AM, Magnan C. Adiponectin is required to mediate rimonabant-induced improvement of insulin sensitivity but not body weight loss in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 296: R929–R935, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Nogueiras R, Diaz-Arteaga A, Lockie SH, Velásquez DA, Tschop J, López M, Cadwell CC, Diéguez C, Tschöp MH. The endocannabinoid system: role in glucose and energy metabolism. Pharmacol Res 60: 93–98, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Nogueiras R, Veyrat-Durebex C, Suchanek PM, Klein M, Tschöp J, Caldwell C, Woods SC, Wittmann G, Watanabe M, Liposits Z, Fekete C, Reizes O, Rohner-Jeanrenaud F, Tschöp MH. Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes 57: 2977–2991, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 115: 1298–1305, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, Batkai S, Marsicano G, Lutz B, Buettner C, Kunos G. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest 118: 3160–3169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev 27: 73–100, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Richey JM, Woolcott OO, Stefanovski D, Harrison LN, Zheng D, Lottati M, Hsu IR, Kim SP, Kabir M, Catalano KJ, Chiu JD, Ionut V, Kolka C, Mooradian V, Bergman RN. Rimonabant prevents additional accumulation of visceral and subcutaneous fat during high-fat feeding in dogs. Am J Physiol Endocrinol Metab 296: E1311–E1318, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenson RS. Role of the endocannabinoid system in abdominal obesity and the implications for cardiovascular risk. Cardiology 114: 212–225, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Scheen AJ. The endocannabinoid system: a promising target for the management of type 2 diabetes. Curr Protein Pept Sci 10: 56–74, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Scheen AJ, Paquot N. Inhibitors of cannabinoid receptors and glucose metabolism. Curr Opin Clin Nutr Metab Care 11: 505–511, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, Makriyannis A, Kunos G. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest 120: 2953–2966, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Watanabe T, Kubota N, Ohsugi M, Kubota T, Takamoto I, Iwabu M, Awazawa M, Katsuyama H, Hasegawa C, Tokuyama K, Moroi M, Sugi K, Yamauchi T, Noda T, Nagai R, Terauchi Y, Tobe K, Ueki K, Kadowaki T. Rimonabant ameliorates insulin resistance via both adiponectin-dependent and adiponectin-independent pathways. J Biol Chem 284: 1803–1812, 2009 [DOI] [PubMed] [Google Scholar]