Abstract
Because hepatic cysteine dioxygenase (CDO) appears to play the major role in controlling cysteine catabolism in the intact rat, we characterized the effect of a lack of hepatic CDO on the regulation of cysteine and its metabolites at the whole body level. In mice with liver-specific deletion of CDO expression, hepatic and plasma cysteine levels increased. In addition, in mice with liver-specific deletion of CDO expression, the abundance of CDO and the proportion of CDO existing as the mature, more active isoform increased in extrahepatic tissues that express CDO (kidney, brown fat, and gonadal fat). CDO abundance was also increased in the pancreas, where most of the enzyme in both control and liver CDO-knockout mice was in the more active isoform. This upregulation of CDO concentration and active-site cofactor formation were not associated with an increase in CDO mRNA and thus presumably were due to a decrease in CDO degradation and an increase in CDO cofactor formation in association with increased exposure of extrahepatic tissues to cysteine in mice lacking hepatic CDO. Extrahepatic tissues of liver CDO-knockout mice also had higher levels of hypotaurine, consistent with increased metabolism of cysteine by the CDO/cysteinesulfinate decarboxylase pathway. The hepatic CDO-knockout mice were able to maintain normal levels of glutathione, taurine, and sulfate. The maintenance of taurine concentrations in liver as well as in extrahepatic tissues is particularly notable, since mice were fed a taurine-free diet and liver is normally considered the major site of taurine biosynthesis. This redundant capacity for regulation of cysteine concentrations and production of hypotaurine/taurine is additional support for the body's robust mechanisms for control of body cysteine levels and indicates that extrahepatic tissues are able to compensate for a lack of hepatic capacity for cysteine catabolism.
Keywords: sulfur amino acids, sulfate, glutathione
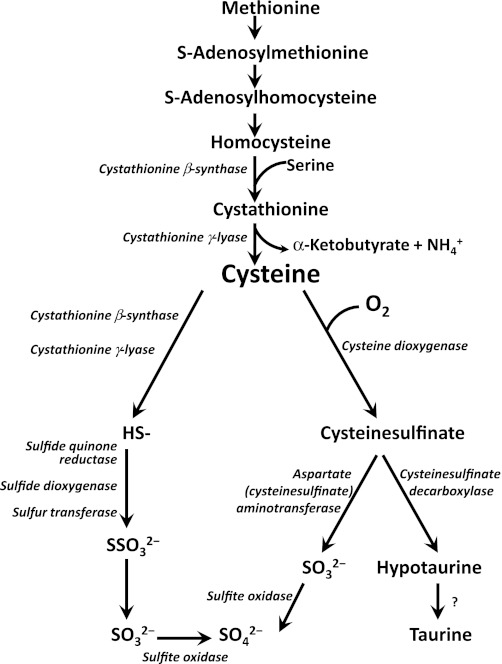
a major pathway of cysteine metabolism, and the major pathway for taurine synthesis, is the oxidative pathway in which cysteine is first oxidized to cysteinesulfinate in a reaction catalyzed by cysteine dioxygenase (CDO). Cysteinesulfinic acid is further metabolized by either 1) decarboxylation to form hypotaurine, which can be further oxidized to taurine, or 2) transamination to the unstable product 3-sulfinylpyruvate, which gives rise to pyruvate and sulfite, with sulfite being further oxidized to sulfate (Fig. 1). Alternative routes of cysteine catabolism in vivo include the desulfhydration reactions catalyzed by cystathionine γ-lyase and cystathionine β-synthase, which can give rise to sulfate, but not taurine, as an end product of cysteine metabolism.
Fig. 1.
Simplified scheme of methionine and cysteine metabolism to taurine and inorganic sulfur. Transsulfuration of methionine transfers methionine sulfur to serine to form cysteine. Cysteine is converted to taurine and sulfate by oxidative metabolism initiated by cysteine dioxygenase. Cysteine can also be catabolized by desulfhydration reactions. Whether the oxidation of hypotaurine to taurine is enzymatic or nonenzymatic in mammalian tissues is uncertain.
Hepatic CDO is highly regulated in response to changes in dietary protein or sulfur amino acid intake, undergoing up to 30-fold changes in concentration and up to 10-fold changes in catalytic efficiency (9, 10, 18, 24, 26). Studies with rodents and cultured cells (hepatocytes, adipocytes) have shown that changes in CDO concentration and activity are highly responsive to cysteine availability in the diet or culture medium (9, 11, 26). The regulation of CDO concentration has been shown to be posttranscriptional and posttranslational, and changes in CDO concentration are the consequence of changes in the rate of its ubiquitination and proteasomal degradation (9, 26). In addition to regulation at the level of enzyme concentration, the activity state of CDO is influenced by the posttranslational formation of a thioether cross-link at the active site; the protein cofactor that is produced by the cysteinyltyrosine cross-link increases the catalytic efficiency of CDO (10). Formation of this cofactor is dependent upon substrate (cysteine) turnover by CDO, making formation of the mature and more active CDO as well as an increase in CDO concentration dependent on cellular cysteine uptake. The mature form of CDO can be detected as a band that runs at a slightly lower apparent molecular mass than the immature form upon polyacrylamide gel electrophoresis. That both the response to CDO turnover and cofactor formation depend on cysteine rather than any precursor or metabolite of cysteine has been demonstrated both in vivo and in vitro (8–10).
In rodents, the concentration and activity of CDO is highest in liver, with lower levels present in adipose tissue, pancreas, kidney, and lung (24, 27). Despite the expression of CDO in several tissues, studies in intact rats fed different levels of dietary protein or sulfur amino acids indicated that only hepatic CDO changed in response to the changes in sulfur amino acid intake (27). In rats fed a diet containing 400 g/kg casein, hepatic CDO activity was 34 times and CDO protein abundance was 12.5 times the levels observed in rats fed a diet containing 100 g/kg casein. These changes in CDO were associated with hepatic cysteine, glutathione, and taurine levels that were 3.5, 2.2, and 21.2 times, respectively, those in rats fed the low-protein diet. In the same study, extrahepatic tissues showed no changes in CDO abundance or activity and no changes in the tissue concentrations of cysteine, glutathione, or taurine. The lack of response of extrahepatic tissues may be a reflection of the efficiency of liver in removing sulfur amino acids from the portal circulation, converting them to glutathione, taurine, and sulfate (13).
Dominy et al. (11) demonstrated that changes in CDO levels contribute to the control of intracellular cysteine concentrations. The robust regulation of hepatic CDO activity suggests that cysteine homeostasis is very important to the living organism. To gain further understanding of the physiological importance of CDO, we recently explored the ability of germ line CDO-knockout mice to maintain cysteine homeostasis (30). Lack of CDO resulted in extremely low plasma and tissue taurine levels (i.e., 2–7% of wild-type control levels), consistent with the major role of the CDO/cysteinesulfinate decarboxylase pathway in taurine production in mammals. Cysteine levels in CDO-knockout mice were somewhat higher than those in wild-type control mice (e.g., 2 times the wild-type level in plasma and 1.5 times the wild-type level in liver). Furthermore, evidence for excess catabolism of cysteine through desulfhydration pathways, giving rise to hydrogen sulfide, was obtained (30).
Because hepatic CDO appears to play the major role in controlling cysteine catabolism in the intact rat, we pursued further characterization of the role of hepatic CDO in regulating levels of cysteine and its metabolites. We crossed conditional CDO-knockout mice with mice expressing Cre recombinase driven by the albumin promoter to generate mice with liver-specific knockout of CDO. Because other cells that express CDO respond to changes in cysteine concentration in vitro (28), we hypothesized that plasma cysteine levels would increase in mice without hepatic CDO and that this would then result in an increase in CDO abundance and activity in other tissues.
MATERIALS AND METHODS
Generation of floxed CDO targeting construct and CDO+/− mice.
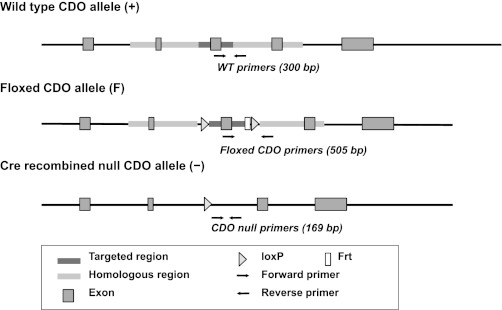
C57BL/6 mice were generated using a Flox/Cre protocol. The floxed mice contained a targeted construct with loxP sites on either side of exon 3 of the CDO1 gene (Fig. 2). Recombination of these loxP sites in liver of mice expressing Cre recombinase driven by the albumin promoter yields hepatocyte-specific knockout of CDO.
Fig. 2.
Generation and analysis of the cysteine dioxygenase (CDO)-null mutation in hepatocytes. Schematic representation of the wild-type (WT) allele (+), the floxed CDO gene (F) showing the targeted region containing exon 3 flanked by loxP sites (and with an Frt site remaining from removal of the Neo cassette), and the null CDO allele (−) after Cre-mediated deletion of the targeted region. Genotyping of tail DNA was performed using primer sets indicated by the arrows; the sequences for these primers have been reported (30).
Conditional CDO-knockout (CDOF/F) mice generated in our laboratory (30) were crossed with C57BL/6-Tg(Alb-cre)21Mgn/J mice (Jackson Laboratories) that express a transgene for Cre recombinase driven by the albumin gene promoter (AlbCre). Offspring with the CDOF/+AlbCre+ genotype were further crossed to obtain CDOF/FAlbCre+ mice. Finally, CDOF/FAlbCre+ and CDOF/F mice were interbred to obtain the CDOF/FAlbCre+ and CDOF/F littermates used in this study. Also included in this study as a control for the insertion of the AlbCre gene into the genome were CDO+/+AlbCre+ mice. Because albumin expression begins around the time of birth in mice, the disruption of the floxed CDO gene is expected to occur postnatally. Design of targeting vectors and genotyping were done as described previously (30). In this article, the experimental mice are referred to as follows: LKO (liver knockout; CDOF/FAlbCre+), FC (floxed control; CDOF/F), and CC (Cre control; CDO+/+AlbCre+).
Mice were housed in a pathogen-free barrier facility maintained at 23°C and 45–50% humidity, with a 14-h dark period (lights off from 0600 to 2000). All animals in the breeding colony had access to an irradiated semipurified rodent diet (7012 Teklad LM-485; Harlan Laboratories), and access to water was ad libitum. All experimental procedures involving live animals were conducted with the approval of the Cornell University Institutional Animal Care and Use Committee.
Dietary treament.
Mice were weaned at 28 days and placed on a semipurified diet that provided 8.0 g/kg diet of methionine equivalents [1 g of cyst(e)ine = 1.24 g of methionine equivalents]; this level of sulfur amino acids is similar to that supplied by the semipurified rodent diet. Pups had unrestricted access to this diet up until postnatal day 49. At the beginning of week 8, mice were switched to a diet that provided a slightly higher level of sulfur amino acids (i.e., 12.3 g/kg diet of methionine equivalents). Mice consumed this diet for 1 wk before the experiment was terminated at postnatal day 56, when tissue samples were collected. The composition of the experimental diets is shown in Table 1; diets were prepared in pelleted form by Dyets (Bethlehem, PA).
Table 1.
Composition of mouse diets
| 20% Soy Protein Isolate + 0.31% l-Methionine (Basal) | 20% Casein + 0.42% l-Methionine + 0.15% l-Cystine (High SAA) | |
|---|---|---|
| Soy protein isolate | 200 | 0 |
| Vitamin-free casein | 0 | 200 |
| l-Methionine | 3.1 | 1.5 |
| l-Cystine | 0 | 4.2 |
| Cornstarch | 397 | 395 |
| Dextrinized cornstarch | 132 | 132 |
| Sucrose | 100 | 100 |
| Fiber (Solka-Floc) | 50 | 50 |
| Soybean oil | 70 | 70 |
| AIN-93G-MX mineral mix | 35 | 35 |
| AIN-93G-VX vitamin mix | 10 | 10 |
| Choline bitartrate | 2.5 | 2.5 |
| tert-Butylhydroquinone | 0.014 | 0.014 |
| Total methionine equivalents | 8.0 | 12.3 |
Values are in g/kg diet; 1 g of cyst(e)ine = 1.24-g methionine equivalents.
SAA, sulfur amino acids.
Sample collection.
At the end of the 7-day period on the sulfur amino acid-enriched diet (i.e., between 1000 and 1400 on day 8), mice were euthanized with CO2, and blood and tissues were collected. Blood was drawn via cardiac puncture into EDTA-coated tubes. Then the liver, kidneys, pancreas, brown fat (interscapular) pads, and gonadal fat (epididymal or periuterine plus periovarian) pads were removed and immediately frozen in liquid nitrogen. Blood was centrifuged at 18,000 g at 4°C, and plasma was removed and frozen in liquid nitrogen. Tissues and plasma were stored at −80°C until analysis; the stability of CDO protein, aminothiols, and other measured metabolites in samples stored for ≤6 mo under these conditions was verified in preliminary studies.
Measurement of CDO relative abundance.
Frozen tissues samples were homogenized in nine volumes of ice-cold lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.5% NP-40) containing 1× Complete Protease Inhibitor Cocktail (Roche; except 4× protease inhibitor for pancreas homogenates) to prepare 10% (wt/vol) tissue homogenates of liver, kidney, brown fat, gonadal fat, and pancreas. Homogenates were centrifuged at 20,000 g for 20 min at 4°C, and the supernatants were stored at −80°C until Western blotting of the soluble proteins was performed. Protein concentration of the supernatant was determined using the BCA Protein Assay kit (Thermo Scientific Pierce Protein Research Products). Proteins were separated by SDS-PAGE, transferred to a membrane, and immunoblotted for CDO, as described previously (25, 30). Immunoreactive protein bands were visualized and quantified using a Li-COR Odyssey infrared imaging system and software scanner (Li-COR Biosciences). CDO values were normalized using actin (liver, kidney, gonadal fat) or GAPDH (brown fat) as a control for loading and transfer.
Measurement of CDO mRNA relative abundance.
RNA was isolated using the RNeasy mini kit or the RNeasy Lipid tissue mini kit (for fat) according to the manufacturer's directions (Qiagen). Complementary DNA was reverse transcribed using Applied Biosystems High Capacity cDNA kit (Applied Biosystems) and quantified using Power Sybr Green (Applied Biosystems) in conjunction with a Roche 480 Lightcycler (Roche Diagnostics). Primers for CDO were forward (5′-3′) GATTCTGTGCTGGGGTGAA and reverse (5′-3′) CAGTGGGAGTCCGTGTGAT. Values for CDO mRNA were normalized to values for actin mRNA.
Measurement of total cystine, total homocysteine, and total glutathione.
Aminothiols (thiols plus disulfides, after reduction) were measured by an HPLC method based on components of the methods described by Nolin et al. (19), Frick et al. (12), and Kuhn et al. (16). For liver and fat, an aliquot of the ice-cold homogenate prepared for CDO Western blotting, as described above, was immediately mixed with an equal volume of 10% (wt/vol) trichloroacetic acid (TCA) solution to denature and precipitate protein. To prevent hydrolysis of glutathione by γ-glutamyltranspeptidase, kidney and pancreas were homogenized directly in 5% (wt/vol) TCA. The acid supernatant was obtained by centrifuging for 20 min at 20,000 g at 4°C. The supernatant was then frozen at −80°C until the HPLC analysis was performed. Standards ranged from 0.1 to 10 μM homocysteine, 2 to 200 μM cysteine, and 20 to 2,000 μM glutathione. Standards were diluted 1:1 in 10% (wt/vol) TCA. Recoveries for internal standards added to tissue samples were complete for all tissue samples as prepared and analyzed.
On the day the tissue samples were to be analyzed, supernatants were thawed on ice. To reduce any disulfides present, samples were treated with tris(2-carboxyethyl)phosphine (TCEP). To a 50-μl aliquot of tissue supernatant or standard, 125 μl of 125 mM borate buffer (pH 9.0) with 1 mM EDTA was added, followed by 4 μl of 200 mM TCEP in 125 mM borate buffer (final pH 7–8) and 11 μl of 1.55 M NaOH. After the diluted sample was incubated at room temperature for 15 min, thiols were derivatized by adding 50 μl of a solution of 1.5 mg/ml borate buffer of ammonium 7-fluorobenzo-2-oxa-I,3-diazole-4-sulphonate, pH 9.0, and incubating the sample mixture at 60°C for 1 h. Then 20 μl of the final sample solution was injected onto a C18 reversed-phase column, and aminothiol derivatives were separated using 100 mM potassium phosphate buffer, pH 2.1, with 5% (vol/vol) acetonitrile as the mobile phase and a flow rate of 1.5 ml/min. Fluorescence of derivatives in the eluate was detected using an excitation wavelength of 385 nm and an emission wavelength of 515 nm. Following chromatography of each sample, the HPLC column was washed with 70% acetonitrile and then reequilibrated with mobile phase.
Plasma samples were similarly analyzed for total aminothiol levels except for their initial preparation. For plasma analysis, plasma was thawed on ice on the day of analysis. A 30-μl aliquot of the plasma was mixed with 3 μl of a 75-mM solution of TCEP in 125 mM borate buffer (final pH 7–8), and the mixture was incubated for 30 min at 4°C. Then 30 μl of 10% (wt/vol) TCA-1 mM EDTA was added, and the mixture was centrifuged to obtain the acid supernatant. To 20 μl of the acid supernatant, 4 μl of 1.55 M NaOH, 50 μl of 125 mM borate buffer (pH 9.5) containing 5 mM EDTA, and 20 μl of ammonium 7-fluorobenzo-2-oxa-I,3-diazole-4-sulphonate solution (1.5 mg/ml in borate buffer, pH 9.5) were added. This mixture was allowed to incubate for 1 h at 60°C. HPLC analysis was then carried out as described for tissue samples.
Measurement of hypotaurine and taurine.
For the measurement of taurine and hypotaurine, aliquots of plasma and of the tissue homogenates prepared as described for CDO Western blotting were treated by addition of three volumes of 5% (wt/vol) sulfosalicylic acid and mixing. Acidified samples were centrifuged at 20,000 g for 20 min at 4°C. The supernatants were then frozen at −80°C for later analysis. On the day of analysis, the acid supernatants or standards (1–1,000 μM) were diluted 1:50 in 200 mM borate buffer, pH 10.4, and then analyzed by an HPLC method using precolumn derivatization with o-phthaldialdehyde (OPA). Chromatography of derivatized samples was conducted on a 4.6 × 150 mm Nova-Pak C18 column equipped with a C18 guard cartridge. Under conditions of no flow, 75 μl of OPA-2-mercaptoethanol-derivatizing reagent and then a 50-μl volume of the diluted sample were injected into the precolumn tubing by an automatic sample injector (WISP Model 712; Waters). The derivatizing reagent was prepared fresh daily in a small brown glass container by mixing 7 mg of OPA with 300 μl of 95% ethanol, 10 ml of 100 mM borate buffer (pH 10.4), and 20 μl of 2-mercaptoethanol. A 1-min delay was programmed prior to flow of mobile phase being initiated to allow reaction of amines with OPA. Amino acids were separated by gradient elution using two buffers. Buffer A was 50 mM potassium phosphate buffer (pH 7.0)−3.5% (vol/vol) tetrahydrofuran, and buffer B was 50 mM potassium phosphate buffer (pH 7.0)−3.5% (vol/vol) tetrahydrofuran-40% (vol/vol) acetonitrile. Buffers were filtered through 0.45-μm filters before use. Flow rate was 1.0 ml/min, and column temperature was maintained at room temperature. Mobile phase was started at 80% A-20% B and then changed to 30% A-70% B over 20 min. In between samples, the column was washed with 100% B and then reequilibrated to 80% A-20% B. Detection of OPA-derivatized compounds was performed using excitation and emission peaks at 360 and 455 nm, respectively.
Measurement of plasma sulfate.
Plasma sulfate levels were measured by HPLC separation and conductivity detection, as described by Hoffman et al. (14). Plasma was centrifuged using a 3,000-MW centrifugal filter (Amicon Ultracel; Millipore) to remove proteins prior to HPLC on a Hamilton PRP X-100 anion exchange column (Hamilton, Reno, NV), using a 2.5-mM phthalate buffer as the mobile phase. A conductivity detector was used to measure levels of sulfate in the eluate.
Statistical analysis.
Results were analyzed by ANOVA for effects of genotype and sex. Tukey's test was used for comparison of individual means. Differences were considered significant at P ≤ 0.05.
RESULTS
As shown in Table 2, disruption of the CDO gene in liver tissue had no effect on the body weight of the LKO mice, which was the same as that of the FC and CC mice. Additionally, the body weights of both LKO and control mice were stable during the experimental feeding of the sulfur amino acid-enriched diet. Male mice weighed significantly more (P ≤ 0.01) than female mice, as expected. No clinical phenotype was observed in the LKO mice; they appeared to be identical to the FC and CC control mice.
Table 2.
Body weight of LKO and control mice
| Body Weight at 7 wk (When Placed on SAA-Enriched Diet) |
Body Weight at 8 wk (When Experiment was Terminated) |
Change in Weight While on SAA-Enriched Diet |
||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| LKO | 23.9 ± 0.4 | 18.0 ± 0.7 | 24.0 ± 0.6 | 18.7 ± 0.5 | 0.07 ± 0.24 | −0.90 ± 0.37 |
| FC | 25.4 ± 0.6 | 18.1 ± 0.5 | 26.3 ± 0.8 | 18.1 ± 0.4 | 0.90 ± 0.51 | −0.06 ± 0.20 |
| CC | 26.0 ± 0.8 | 20.7 ± 0.7 | 25.3 ± 0.6 | 21.0 ± 0.5 | −0.70 ± 0.62 | 0.35 ± 0.15 |
Values are means ± SE for 4–8 mice and in g.
LKO, liver knockout; FC, floxed control; CC, Cre control.
Significant effect of sex difference on body weight, P < 0.01. Change in body weight over the week on SAA-enriched diet was not significantly different from zero for any of the groups.
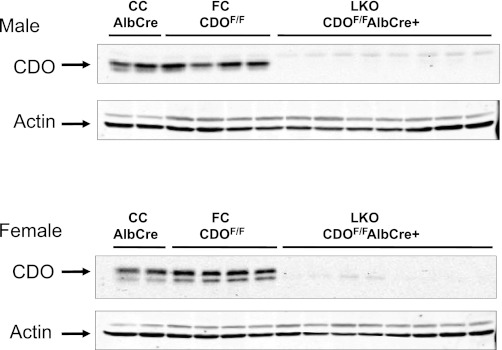
Relative CDO abundance was measured in the livers of LKO, FC, and CC mice, as shown in Fig. 3. The absent or negligible CDO in the liver of the LKO mice demonstrates that expression of albumin-Cre recombinase had disrupted the CDO1 gene in hepatocytes before the mice were 8 wk of age. As expected, liver of the FC and CC mice expressed CDO, which migrated as two bands.
Fig. 3.
Western blots of liver of liver knockout (LKO), floxed control (FC), and Cre control (CC) mice for CDO expression. Each lane was loaded with 50 μg of total soluble liver protein. Two bands are typically observed when samples containing CDO are separated by SDS-PAGE. These 2 bands correspond to an immature less active isoform (upper band) and a mature more active isoform (lower band). The upper and lower bands migrate with apparent molecular masses of 23 and 22.5 kDa, respectively. Actin is also shown as a loading control. Because we used CDO antibody raised in rabbits, we used β-actin antibody raised in mice; the β-actin antibody raised in mice reacts with another protein in liver samples that is seen here as a lighter band above the β-actin band. This 2nd band is not observed if we use β-actin antibody raised in rabbits.
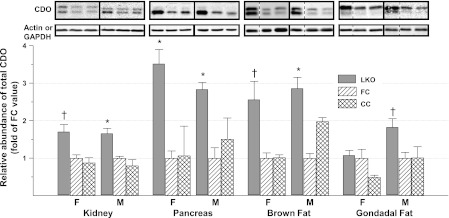
CDO abundance was measured by western blotting in kidney, pancreas, brown fat, and gonadal fat, as shown in Fig. 4. The relative abundance of CDO increased significantly (P ≤ 0.05) in the pancreas of both female and male LKO mice and in kidney and brown fat of male mice relative to either FC or CC control mice. CDO abundance in kidney and brown fat of female mice and in the epididymal fat of male LKO mice tended to be greater than that in control mice (P ≤ 0.10). CDO abundance in gonadal fat of female mice did not differ from that of control mice (P > 0.10). Analysis of tissue CDO mRNA by real-time PCR confirmed that the mechanism for upregulation of extrahepatic CDO concentration was not transcriptional, as reported previously for liver CDO. The abundance of CDO mRNA in the extrahepatic tissues was the same in LKO, FC, and CC mice for male mice and also for female mice.
Fig. 4.
Relative abundance of CDO in nonhepatic tissues of LKO, FC, and CC mice. Representative blots are shown, with each lane loaded with 50 μg of total soluble protein. Boxes surrounded by solid lines were from different gels. Dotted lines indicate where a lane was deleted so that the order of blots would be the same as in the bar graph. Values in bar graph are means ± SE for 4–7 mice, with the mean for the FC mice shown as 1.0. *Value for LKO mice is greater than those for control mice, P < 0.05. †Value for LKO mice tends to be greater than those for control mice, P < 0.10. F, female; M, male.
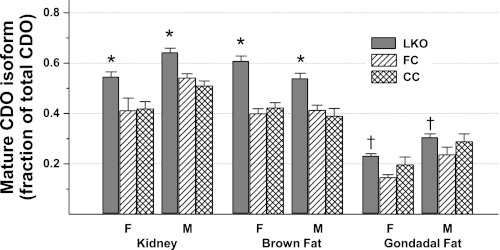
In addition to the increase in total CDO abundance, the proportion of CDO existing as the mature, more active isoform also increased in the kidney, brown fat, and gonadal fat of LKO mice. As shown in Fig. 5, the fraction of total CDO present as the mature isoform was 18–58% higher in extrahepatic tissues of LKO mice than in control mice. CDO in pancreas (not shown in Fig. 5) ran almost entirely as the lower band, or mature isoform, in both LKO and control mice. The presence of most CDO as the mature isoform in pancreas suggests either that CDO degradation occurs more slowly or that the catalytic turnover of cysteine by CDO is more rapid in the pancreas than in other tissues.
Fig. 5.
Fraction of total CDO present as the mature isoform (i.e., lower band) in nonhepatic tissues of LKO, FC, and CC mice. Values are means ± SE for 4–7 mice. *Value for LKO mice is greater than those for both control groups, P < 0.05. †Value for LKO mice is greater than that for F/F control mice but not different from that for the CC control group, P < 0.05.
To assess whether knockout of hepatic CDO influenced the plasma or tissue levels of cysteine and its metabolites or precursors, cysteine, glutathionine, homocysteine, hypotaurine, and taurine were measured in liver, kidney, pancreas, and plasma, and sulfate was measured in plasma. As shown in Table 3, 8-wk-old LKO mice had sufficient CDO to allow them to maintain relatively normal levels of cysteine and its metabolites. Plasma (P < 0.001) and liver (P < 0.01) cysteine levels were ∼15 and 45% higher, respectively, in LKO mice than in control mice, whereas kidney and pancreas cysteine levels were not different between LKO and control mice. The levels of cysteine metabolites (glutathione and taurine) were not different from control levels, even in liver of the LKO mice that were no longer expressing hepatic CDO.
Table 3.
Cysteine, glutathione, homocysteine, taurine, and sulfate levels in plasma and tissues of CDO LKO and control mice
| Females |
Males |
Statistics (P Value) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| LKO | FC | CC | LKO | FC | CC | G | S | G × S | |
| Plasma cysteine, μmol/l | 128 ± 10 | 106 ± 6 | 111 ± 5 | 101 ± 3 | 94 ± 2 | 86 ± 1 | <0.01 | <0.001 | |
| Liver cysteine, μmol/g tissue | 0.13 ± 0.01 | 0.09 ± 0.002 | 0.07 ± 0.003 | 0.10 ± 0.004 | 0.085 ± 0.01 | 0.07 ± 0.003 | <0.001 | <0.01 | |
| Kidney cysteine, μmol/g tissue | 0.30 ± 0.03 | 0.34 ± 0.03 | 0.35 ± 0.02 | 0.64 ± 0.03 | 0.63 ± 0.02 | 0.67 ± 0.01 | <0.001 | ||
| Pancreas cysteine, μmol/g tissue | 0.23 ± 0.02 | 0.23 ± 0.05 | 0.25 ± 0.02 | 0.25 ± 0.02 | 0.30 ± 0.03 | 0.25 ± 0.02 | |||
| Plasma glutathione, μmol/l | 68 ± 12 | 79 ± 23 | 102 ± 5 | 78 ± 11 | 86 ± 6 | 109 ± 29 | |||
| Liver glutathione, μmol/g tissue | 8.4 ± 0.5 | 7.0 ± 0.5 | 7.0 ± 0.5 | 10.8 ± 0.8 | 10.7 ± 0.3 | 9.2 ± 0.9 | <0.001 | ||
| Kidney glutathione, μmol/g tissue | 2.6 ± 0.1 | 2.5 ± 0.1 | 2.3 ± 0.2 | 3.0 ± 0.1 | 3.3 ± 0.3 | 3.2 ± 0.1 | <0.001 | ||
| Pancreas glutathione, μmol/g tissue | 2.0 ± 0.1 | 1.9 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.3 ± 0.1 | <0.05 | ||
| Plasma homocysteine, μmol/l | 5.33 ± 0.78 | 6.69 ± 1.36 | 5.42 ± 0.24 | 2.72 ± 0.27 | 6.86 ± 0.18 | 2.75 ± 0.09 | <0.001 | ||
| Liver homocysteine, nmol/g tissue | 1.65 ± 0.32 | 2.01 ± 0.29 | 1.64 ± 0.13 | 1.05 ± 0.10 | 1.22 ± 0.17 | 0.87 ± 0.19 | <0.01 | ||
| Kidney homocysteine, nmol/g tissue | 0.36 ± 0.02 | 0.47 ± 0.06 | 0.43 ± 0.07 | 1.00 ± 0.04 | 1.00 ± 0.04 | 1.03 ± 0.03 | <0.001 | ||
| Pancreas homocysteine, nmol/g tissue | 1.04 ± 0.18 | 0.73 ± 0.03 | 1.07 ± 0.40 | 0.99 ± 0.13 | 0.86 ± 0.04 | 1.03 ± 0.24 | |||
| Plasma sulfate, μmol/l | 1,104 ± 61 | 1,081 ± 149 | 1,070 ± 76 | 982 ± 57 | 1,081 ± 96 | 1,035 ± 83 | |||
| Plasma taurine, μmol/l | 545 ± 65 | 413 ± 52 | 450 ± 8 | 590 ± 41 | 637 ± 32 | 678 ± 54 | |||
| Liver taurine, μmol/g tissue | 15.4 ± 0.8 | 14.7 ± 0.9 | 15.0 ± 1.2 | 12.3 ± 0.8 | 12.7 ± 2.8 | 12.4 ± 2.7 | |||
| Kidney taurine, μmol/g tissue | 11.5 ± 0.4 | 11.1 ± 0.9 | 10.0 ± 1.0 | 9.6 ± 0.4 | 9.5 ± 0.4 | 10.8 ± 0.5 | <0.05 | ||
| Pancreas taurine, μmol/g tissue | 2.5 ± 0.1 | 3.4 ± 0.9 | 3.4 ± 0.5 | 3.2 ± 0.4 | 2.2 ± 0.1 | 4.3 ± 0.6 | |||
| Plasma hypotaurine, μmol/l | 14.5 ± 1.3 | 4.2 ± 0.3 | 5.8 ± 1.3 | 10.8 ± 1.1 | 5.1 ± 0.5 | 5.9 ± 0.1 | <0.01 | <0.01 | |
| Liver hypotaurine, μmol/g tissue | 0.06 ± 0.01 | 0.06 ± 0.003 | 0.08 ± 0.02 | 0.07 ± 0.01 | 0.10 ± 0.03 | 0.12 ± 0.02 | <0.05 | ||
| Kidney hypotaurine, μmol/g tissue | 0.34 ± 0.03 | 0.12 ± 0.01 | 0.12 ± 0.03 | 0.32 ± 0.02 | 0.13 ± 0.01 | 0.16 ± 0.02 | <0.001 | ||
| Pancreas hypotaurine, μmol/g tissue | 2.8 ± 0.1 | 1.3 ± 0.2 | 0.8 ± 0.1 | 4.4 ± 0.5 | 1.9 ± 0.2 | 1.9 ± 0.7 | <0.01 | <0.01 | |
Values are means ± SE for 4–8 mice; values for tissues are expressed as amount per gram wet weight of tissue.
CDO, cysteine dioxygenase.
Significant effects of genotype (G) or sex difference (S) and their interaction (G × S) were determined by ANOVA. Differences were accepted at P < 0.05. Levels of significance (<0.05) are reported in the last 3 columns.
In contrast, hypotaurine levels were significantly (P < 0.01) greater in plasma, kidney, and pancreas of LKO mice than in CC or FC control mice, whereas liver hypotaurine levels were not different for LKO and control mice. The higher levels of hypotaurine in pancreas and kidney are consistent with the upregulation of CDO in these tissues. Although taurine and hypotaurine may be taken up from the plasma, most of the body hypotaurine/taurine pool is in the form of taurine, and the highest amounts of hypotaurine are found in tissues that actively synthesize it by the CDO/cysteinesulfinate decarboxylase pathway. It is notable that, for mice of all genotypes, hypotaurine levels in liver, kidney, and plasma were <3% of the taurine level in the same tissue. In contrast, hypotaurine levels in the pancreas were two to three orders of magnitude higher than those in other tissues, ranging from 24 to 137% of the pancreatic taurine level. It was surprising to us that hypotaurine was so high in pancreas, but the identity of hypotaurine was confirmed by the addition of hydrogen peroxide to samples to oxidize the hypotaurine to taurine, with the hypotaurine peak disappearing and the taurine peak increasing by an equivalent molar amount, as measured with the HPLC method used for this study. Further support for accuracy of these pancreatic hypotaurine values is the absence of hypotaurine in these same tissues from CDO−/− mice (30).
An overall effect of sex on cysteine and some cysteine metabolite levels was observed; plasma cysteine, liver cysteine, plasma homocysteine, and liver homocysteine were higher in female mice, whereas kidney cysteine, liver glutathione, kidney glutathione, pancreas glutathione, and kidney homocysteine were lower in female mice than in male mice. The metabolic basis of these differences was not explored. In previous work, we noted that hepatic CDO levels are significantly higher in female than in male mice, whereas hepatic cysteinesulfinate decarboxylase levels are significantly lower in female than in male mice (30).
DISCUSSION
In the intact mammal, the liver is normally the most active site for metabolism of dietary sulfur amino acids. Arteriovenous difference studies show that the liver removes methionine and cyst(e)ine from the portal blood and releases taurine, glutathione, and sulfate into the circulation (13). The dominant role of the liver in dietary sulfur amino acid metabolism is further illustrated by the robust upregulation of CDO in the liver, but not extrahepatic tissues, in response to an increased intake of dietary sulfur amino acids or protein (2, 27). Thus, hepatic CDO appears to play a somewhat unique role in regulating body cysteine homeostasis. Because CDO is also expressed in other tissues, including the adipose tissue, kidney, and pancreas, we questioned whether these tissues could also play an important role in cysteine metabolism, particularly in situations in which a first pass of amino acids through the liver does not occur (i.e., parenteral feeding) or in liver disease, in which hepatic sulfur amino acid metabolism is impaired. Our previous observations that CDO abundance and activity in adipocytes respond to changes in cysteine concentration in vitro suggested that CDO in extrahepatic tissues might respond in a similar manner should these tissues be exposed to an elevated cysteine concentration (28). Thus, we hypothesized that plasma cysteine levels would increase in mice with a liver-specific disruption of the CDO gene and that this would then result in an increase in CDO abundance and activity in extrahepatic tissues.
In this study, expression of CDO in hepatocytes was essentially eliminated by the time the mice were 7 wk old and used for this study. Previous studies have shown that the expression of genes driven by the albumin promoter occurs as a result of the process of hepatocyte differentiation, which begins before birth and is complete by about postnatal day 42 in mice (20, 32). In these LKO mice, the relative abundance of CDO in brown and white adipose tissues, kidney, and pancreas was significantly higher compared with FC and CC control mice, demonstrating that extrahepatic tissues of intact animals are able to upregulate CDO abundance in the absence of hepatic CDO or presumably in the face of an increase in circulating cyst(e)ine levels. Furthermore, greater increases were observed for the relative abundance of the more catalytically active isoform of CDO compared with the less active isoform, demonstrating that CDO activity in these tissues was increased by both an increase in CDO concentration and an increased formation of the mature isoform of CDO, just as is observed in livers of wild-type mice fed sulfur amino acid- or protein-enriched diets (9, 30).
In our previous work, we have shown that both the increase in CDO abundance, which is due mainly to decreased ubiquitination and degradation of CDO when cysteine levels are high, and the increase in catalytic efficiency, which is due to formation of the mature isoform of CDO, are due specifically to an increase in cellular cysteine supply or concentration (8, 9, 17, 21, 26). A greater abundance of total CDO and a higher proportion of CDO present as the mature isoform were observed in extrahepatic tissues of LKO mice. Thus, it is most likely that CDO in extrahepatic tissues in LKO mice was upregulated in response to higher circulating concentrations of cysteine, at least in the postprandial period. Indeed, plasma cysteine (P ≤ 0.01) and hepatic cysteine (P ≤ 0.001) levels were significantly higher in LKO mice than in control mice, consistent with the liver having an impaired ability to dispose of excess cysteine, resulting in higher circulating cysteine levels. Liver cysteine was 17–85% higher in LKO mice, with an average of 47% higher, than in control mice, which compares favorably with the 50% higher hepatic cysteine concentrations observed in the CDO−/− mouse and underscores the physiologically important role of liver in cysteine disposal (30). Plasma cysteine changes in the LKO mice were relatively small, ranging from 7% above control to 20% above control values (average of 15%). Nevertheless, a consistent and significant (P < 0.001) increase in plasma cysteine was observed in the LKO mice. Even in our earlier studies with the CDO−/− mouse (i.e., germ line CDO-knockout), plasma cysteine was only double that of wild-type mice (30). Kidney and pancreas cysteine levels were similar in LKO and control mice. Based on the current understanding of regulation of CDO in response to changes in cysteine, the observation of a sustained increase in extrahepatic CDO abundance and mature isoform abundance indicates that extrahepatic tissues were exposed to higher cysteine levels at some points during each 24-h period, perhaps mostly during the feeding periods, and this is consistent with the higher hepatic and plasma cysteine levels in LKO mice.
In the LKO mouse, the greater ability of extrahepatic tissues to catabolize cysteine probably accounts for the negligible effect of liver-specific CDO-knockout on plasma and extrahepatic tissue cysteine levels. Indeed, a rough estimate of the change in the capacity of pancreas for cysteine dioxygenation suggests that the observed changes in extrahepatic tissue CDO could compensate for the absent hepatic CDO. Based on relative measurements of CDO in various tissues (24), pancreas normally has about 25% as much CDO as the liver per gram of tissue, and this increases in the LKO mouse to about 75% as much CDO as in the wild-type liver. However, the mouse pancreas weighs about 17% as much as the liver, yielding an amount of CDO in the LKO mouse pancreas that is only about 13% of that in the liver of a wild-type mouse. However, essentially all of the pancreatic CDO is the the mature form compared with only about 25% of hepatic CDO in the wild-type mouse being in the mature form. Because the mature form has more than 10 times the catalytic efficiency of immature CDO, the pancreas of the LKO mouse would have about 100% of the capacity of the wild-type liver. In addition to this, the two- to fourfold higher cysteine concentration in pancreas than in liver should also increase flux through the cysteine dioxygenase reaction because the Km of CDO for cysteine is substantially higher (∼4 mM) than tissue cysteine concentrations (∼0.1 μmol/g for liver and 0.25 μmol/g for pancreas) (21).
In the wild-type animal, hepatic metabolism of cysteine is responsible for the majority of taurine synthesis (3, 4, 13). In the CDO−/− mouse, tissue and plasma taurine concentrations were reduced to less than 10% of wild-type levels (30). It is notable that the plasma and tissue levels of taurine were not different between the LKO and control mice in this study despite the animals being fed a diet that contained no preformed taurine. Clearly, the extrahepatic tissues that express CDO are capable of taurine synthesis in vivo, and the liver is able to actively take up taurine under these circumstances. This observation is consistent with the reported expression of CDO and cysteinesulfinate decarboxylase in white and brown adipose tissue, kidney, and pancreas and also with the ability of adipocytes to produce taurine (15, 24, 28, 29). This indicates that in vivo taurine synthesis by tissues that express CDO is determined largely by cysteine availability, which serves as both the substrate and the molecule that triggers an increase in CDO concentration and activity. Normally liver plays the major role, but if cysteine is administered intravenously or if hepatic metabolism of sulfur amino acids is impaired, other tissues should be able to supply sufficient taurine.
In contrast to taurine, greater levels of hypotaurine were present in plasma, kidney, and pancreas of the LKO mice compared with those in control mice. This observation is consistent with the increased CDO activity in extrahepatic tissues of LKO mice and with a role of these tissues in supplying hypotaurine and taurine to the circulation for uptake by other tissues, including liver in the LKO mice. Thus, although most of the body's hypotaurine/taurine pool is in the form of taurine, hypotaurine is a better indicator of active taurine biosynthesis. Previous work has shown that CDO activity plays the major role in determining the flux of cysteine to hypotaurine/taurine (1, 2, 5). Cysteinesulfinate decarboxyase, which catalyzes the decarboxylation of cysteinesulfinate to hypotaurine, is highly expressed in pancreas and kidney as well as in liver (24). Compared with other tissues, pancreas maintains a large proportion of its hypotaurine/taurine pool in the form of hypotaurine. It is possible that this reflects both active synthesis and high turnover of the hypotaurine/taurine pool in the pancreas.
Tissue and plasma glutathione, tissue and plasma homocysteine, and plasma sulfate levels were also sustained in the LKO mice, not being different than levels in control mice. Cysteine was clearly not limiting for glutathione synthesis, and liver-specific disruption of the CDO gene would not be expected to affect the ability of liver to synthesize glutathione. Similarly, the metabolism of methionine should be relatively normal in mice lacking hepatic CDO, and cysteine levels were minimally affected, consistent with our observation of similar homocysteine levels in LKO and control mice. Although disruption of the liver CDO gene would block sulfate production via the cysteinesulfinate transamination pathway, the observation that the plasma sulfate level was not different between wild-type and CDO−/− mice indicates that sulfate was produced by other pathways. This most likely was due largely to the ability of desulfhydration enzymes (i.e., cystathionine γ-lyase and cystathionine β-synthase) to release the reduced sulfur of cysteine, followed by the rapid conversion of sulfide to sulfate (6, 7, 22, 23, 24). Indeed, a block in hepatic taurine synthesis could actively promote inorganic sulfur production from cysteine because all catabolism of cysteine by desulfhydration enzymes eventually results in sulfate production, whereas cysteinesulfinate formed from cysteine by CDO is partitioned between conversion to taurine and catabolism to sulfate and pyruvate.
The LKO mice had a normal clinical phenotype, consistent with their ability to upregulate CDO in extrahepatic tissues to maintain cysteine catabolism by the oxidative pathway and hence, to maintain tissue taurine levels and minimize flux of cysteine through desulfhydration pathways. This is in contrast to the phenotype of germ line CDO-knockout mice (CDO−/−), which consists of a severe postnatal growth deficit, a high rate of postnatal mortality, joint hypermobility, wry nose, and microscopically visible abnormalities in the elastin fibers in the large arteries and in the parenchyma and small blood vessels of the lungs (30). CDO−/− mice have elevated levels of cysteine and glutathione, very low levels of taurine, and essentially no hypotaurine in plasma and tissues. Additionally, CDO−/− mice exhibit excess catabolism of cysteine by desulfhydration pathways as assessed by elevated acid-labile sulfur in tissues, elevated thiosulfate excretion in the urine, loss of cytochrome c oxidase activity, and slightly elevated plasma sulfate levels. Much of the pathology observed in CDO−/− mice is likely due to elevated cysteine levels and the consequential excess production of H2S because taurine supplementation had little effect on the phenotype of CDO−/− mice (30) and also because the pathologies observed in CDO−/− mice generally appear earlier and are different from those reported for mice lacking expression of the taurine transporter (31).
This study demonstrates that CDO in extrahepatic tissues is able to undergo upregulation of enzyme concentration and activity state in response to an increase in cysteine concentrations in the plasma and/or tissue. Generally, this is not necessary because the liver removes much of the cysteine during the first pass of the portal blood through the liver. The ability of extrahepatic CDO-expressing tissues to assume the roles of cysteine disposal and taurine biosynthesis when cysteine levels are elevated, perhaps because of a lack of liver function, can compensate largely for the loss of hepatic CDO activity. Except for increases in hypotaurine levels in plasma and CDO-expressing extrahepatic tissues, sulfur amino acid metabolites were maintained largely at normal levels in LKO mice at the expense of small increases in hepatic and plasma cysteine concentrations. This is yet another example of the body's robust mechanisms (and backup mechanisms) for control of body cysteine levels. When hepatic metabolism fails to control cysteine levels, CDO is induced in extrahepatic tissues, ensuring sustained production of hypotaurine and taurine and ensuring that cysteine levels in tissues do not rise markedly, which would give rise to the unregulated and excess production of H2S and other possibly other reduced sulfur compounds.
GRANTS
This project was supported by Grant DK-056649 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are reported by the authors.
AUTHOR CONTRIBUTIONS
I.U. and M.H.S. did the conception and design of the research; I.U., H.B.R., L.L.H., and C.C.J. performed the experiments; I.U., C.C.J., and M.H.S. analyzed the data; I.U., C.C.J., and M.H.S. interpreted the results of the experiments; I.U. and M.H.S. prepared the figures; I.U., H.B.R., L.L.H., C.C.J., and M.H.S. edited and revised the manuscript; I.U., H.B.R., L.L.H., C.C.J., and M.H.S. approved the final version of the manuscript; C.C.J. and M.H.S. drafted the manuscript.
REFERENCES
- 1. Bagley PJ, Stipanuk MH. The activities of rat hepatic cysteine dioxygenase and cysteinesulfinate decarboxylase are regulated in a reciprocal manner in response to dietary casein level. J Nutr 124: 2410–2421, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Bagley PJ, Stipanuk MH. Rats fed a low protein diet supplemented with sulfur amino acids have increased cysteine dioxygenase activity and increased taurine production in hepatocytes. J Nutr 125: 933–940, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Bella DL, Stipanuk MH. Effects of protein, methionine, or chloride on acid-base balance and on cysteine catabolism. Am J Physiol Endocrinol Metab 269: E910–E917, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Bella, Hahn C, Stipanuk H. Effects of nonsulfur and sulfur amino acids on the regulation of hepatic enzymes of cysteine metabolism. Am J Physiol Endocrinol Metab 277: E144–E153, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Bella DL, Hirschberger LL, Hosokawa Y, Stipanuk MH. Mechanisms involved in the regulation of key enzymes of cysteine metabolism in rat liver in vivo. Am J Physiol Endocrinol Metab 276: E326–E335, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Chen X, Jhee KH, Kruger WD. Production of the neuromodulator H2S by cystathionine β-synthase via the condensation of cysteine and homocysteine. J Biol Chem 279: 52082–52086, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R. H2S biogenesis by human cystathionine γ-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem 284: 11601–11612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cresenzi CL, Lee JI, Stipanuk MH. Cysteine is the metabolic signal responsible for dietary regulation of hepatic cysteine dioxygenase and glutamate cysteine ligase in intact rats. J Nutr 133: 2697–2702, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Dominy JE, Hirschberger LL, Coloso RM, Stipanuk MH. Regulation of cysteine dioxygenase degradation is mediated by intracellular cysteine levels and the ubiquitin-26 S proteasome system in the living rat. Biochem J 394: 267–273, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dominy JE, Hwang J, Guo S, Hirschberger LL, Zhang S, Stipanuk MH. Synthesis of amino acid cofactor in cysteine dioxygenase is regulated by substrate and represents a novel post-translational regulation of activity. J Biol Chem 283: 12188–12201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dominy JE, Hwang J, Stipanuk MH. Overexpression of cysteine dioxygenase reduces intracellular cysteine and glutathione pools in HepG2/C3A cells. Am J Physiol Endocrinol Metab 293: E62–E69, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Frick B, Schröcksnadel K, Neurauter G, Wirleitner B, Artner-Dworzak E, Fuchs D. Rapid measurement of total plasma homocysteine by HPLC. Clin Chim Acta 331: 19–23, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Garcia RA, Stipanuk MH. The splanchnic organs, liver and kidney have unique roles in the metabolism of sulfur amino acids and their metabolites in rats. J Nutr 122: 1693–1701, 1992 [DOI] [PubMed] [Google Scholar]
- 14. Hoffman DA, Wallace SM, Verbeeck RK. Simple method for the determination of inorganic sulfate in human serum and urine using single-column ion chromatography. J Chromatogr B 565: 447–452, 1991 [DOI] [PubMed] [Google Scholar]
- 15. Ide T, Kushiro M, Takahashi Y, Shinohara K, Cha S. mRNA expression of enzymes involved in taurine biosynthesis in rat adipose tissues. Metabolism 51: 1191–1197, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Kuhn KS, Krasselt AI, Fürst P. Glutathione and glutathione metabolites in small tissue samples and mucosal biopsies. Clin Chem 46: 1003–1005, 2000 [PubMed] [Google Scholar]
- 17. Kwon YH, Stipanuk MH. Cysteine regulates expression of cysteine dioxygenase and γ-glutamylcysteine synthetase in cultured rat hepatocytes. Am J Physiol Endocrinol Metab 280: E804–E815, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Lee JI, Londono M, Hirschberger LL, Stipanuk MH. Regulation of cysteine dioxygenase and gamma-glutamylcysteine synthetase is associated with hepatic cysteine level. J Nutr Biochem 15: 112–122, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Nolin TD, McMenamin ME, Himmelfarb J. Simultaneous determination of total homocysteine, cysteine, cysteinylglycine, and glutathione in human plasma by high-performance liquid chromatography: application to studies of oxidative stress. J Chromatogr B Analyt Technol Biomed Life Sci 852: 554–561, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Postic C, Magnuson MA. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis 26: 149–150, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Siakkou E, Rutledge MT, Wilbanks SM, Jameson GN. Capturing crosslink formation with enzymatic activity in cysteine dioxygenase. Biochim Biophys Acta 1814: 2003–2009, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. Relative contributions of cystathionine β-synthase and γ-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem 284: 22457–22466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J 206: 267–277, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stipanuk MH, Ueki I. Dealing with methionine/homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. J Inherit Metab Dis 34: 17–32, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stipanuk MH, Dominy JE, Ueki I, Hirschberger LL. Measurement of Cysteine Dioxygenase Activity and Protein Abundance. Curr Protoc Toxicol 38: 6.15.1–6.15.25, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stipanuk MH, Hirschberger LL, Londono MP, Cresenzi CL, Yu AF. The ubiquitin-proteasome system is responsible for cysteine-responsive regulation of cysteine dioxygenase concentration in liver. Am J Physiol Endocrinol Metab 286: E439–E448, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Stipanuk MH, Londono M, Lee JI, Hu M, Yu AF. Enzymes and metabolites of cysteine metabolism in nonhepatic tissues of rats show little response to changes in dietary protein or sulfur amino acid levels. J Nutr 132: 3369–3378, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Ueki I, Stipanuk MH. 3T3-L1 adipocytes and rat adipose tissue have a high capacity for taurine synthesis by the cysteine dioxygenase/cysteinesulfinate decarboxylase and cysteamine dioxygenase pathways. J Nutr 139: 207–214, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ueki I, Stipanuk MH. Enzymes of the taurine biosynthetic pathway are expressed in rat mammary gland. J Nutr 137: 1887–1894, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ueki I, Roman HB, Valli A, Fieselmann K, Lam J, Peters R, Hirschberger LL, Stipanuk MH. Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am J Physiol Endocrinol Metab 301: E668–E684, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Warskulat U, Heller-Stilb B, Oermann E, Zilles K, Haas H, Lang F, Häussinger D. Phenotype of the taurine transporter knockout mouse. Methods Enzymol 428: 439–458, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Weisend CM, Kundert JA, Suvorova ES, Prigge JR, Schmidt EE. Cre activity in fetal albCre mouse hepatocytes: Utility for developmental studies. Genesis 47: 789–792, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]