Abstract
Glucocorticoids (GCs) are important regulators of skeletal muscle mass, and prolonged exposure will induce significant muscle atrophy. To better understand the mechanism of skeletal muscle atrophy induced by elevated GC levels, we examined three different models: exogenous synthetic GC treatment [dexamethasone (DEX)], nutritional deprivation, and denervation. Specifically, we tested the direct contribution of the glucocorticoid receptor (GR) in skeletal muscle atrophy by creating a muscle-specific GR-knockout mouse line (MGRe3KO) using Cre-lox technology. In MGRe3KO mice, we found that the GR is essential for muscle atrophy in response to high-dose DEX treatment. In addition, DEX regulation of multiple genes, including two important atrophy markers, MuRF1 and MAFbx, is eliminated completely in the MGRe3KO mice. In a condition where endogenous GCs are elevated, such as nutritional deprivation, induction of MuRF1 and MAFbx was inhibited, but not completely blocked, in MGRe3KO mice. In response to sciatic nerve lesion and hindlimb muscle denervation, muscle atrophy and upregulation of MuRF1 and MAFbx occurred to the same extent in both wild-type and MGRe3KO mice, indicating that a functional GR is not required to induce atrophy under these conditions. Therefore, we demonstrate conclusively that the GR is an important mediator of skeletal muscle atrophy and associated gene expression in response to exogenous synthetic GCs in vivo and that the MGRe3KO mouse is a useful model for studying the role of the GR and its target genes in multiple skeletal muscle atrophy models.
Keywords: muscle RING finger 1, muscle atrophy F-box, dexamethasone, nutritional deprivation, denervation
glucocorticoids (GCs) are widely used clinically for their anti-inflammatory properties; however, prolonged exposure to synthetic GCs can lead to detrimental side effects, including insulin resistance (51), osteoporosis (39), and muscle atrophy (29, 46). Furthermore, elevated endogenous levels of GCs associated with catabolic conditions and diseases such as diabetes mellitus (21, 22), sepsis (19, 59), starvation (63), and metabolic acidosis (30) can contribute to muscle atrophy. Endogenous GCs, like cortisol in humans and corticosterone in rodents, are released naturally from the adrenal cortex via stress-mediated or circadian activation of the hypothalamic-pituitary-adrenal axis. Adrenalectomy inhibits protein degradation in rats with diabetes mellitus, and proteolysis is restored with GC replacement (32). In addition, treatment of animals with the glucocorticoid receptor (GR) antagonist RU-486, an antagonist which competitively binds to the GR, inhibits muscle loss in response to sepsis and burn injury (18). Thus, although general inhibition of GC signaling inhibits muscle atrophy in these cases, the relative contribution of the skeletal muscle GR in induction of atrophy by exogenous and endogenous GCs is still poorly understood.
Skeletal muscle size is dependent on many factors, including innervation, loading, growth factors, and hormones, all of which contribute to regulating the relative balance between protein synthesis and degradation. The synthetic GC dexamethasone (DEX) induces a decrease in muscle mass in rodents (42) as well as upregulation of two E3 ubiquitin ligase genes, muscle RING finger 1 (MuRF1) and muscle atrophy F-box (MAFbx) (4). Although the precise mechanism of GC-induced atrophy is still largely unknown, previous studies have shown alterations in both protein degradation (10, 20, 52, 64) and synthesis (12, 13, 47, 63) pathways. GC-induced muscle atrophy likely involves activation of the GR, a member of the nuclear hormone receptor family that acts as a transcription factor, altering the expression of target genes such as MuRF1 (60). Investigation into the in vivo role of the GR in various developmental and homeostatic processes was originally hampered by the fact that mice with homozygous null alleles for the GR die perinatally due to respiratory failure (11). Therefore, mouse lines were created with loxP sites flanking exons 2 or 3 of the GR gene to allow for tissue-specific deletion upon regulated expression of Cre recombinase. This approach has been employed successfully in the liver (24, 36, 56), pancreas (16), T cells (6), macrophages (57), osteoblasts (39), and nervous system (5, 23, 55) to determine tissue-specific phenotypes due to reduced GR expression. Therefore, by utilizing the Cre-lox system, the mechanism of GC action in atrophy selectively mediated by the GR expressed in skeletal myofibers can be examined in vivo. Recently, this strategy has been used to demonstrate the critical role of the GR in supporting muscle atrophy in streptozotocin-induced diabetes mellitus (22).
The role of the GR in DEX-induced muscle atrophy may be questioned given that high doses are generally required to induce muscle loss when administered alone, although DEX has a higher affinity for the GR than natural GCs like cortisol and corticosterone. Furthermore, non-GR-mediated genomic responses to synthetic GC treatment have been reported recently (15, 37, 54). In addition, GCs may induce muscle atrophy secondary to their effects on other cell types or tissues; i.e., the effects on skeletal muscle myofibers may not be completely cell autonomous. GCs negatively affect the extracellular matrix by suppressing collagen production by fibroblasts (31, 40, 50), decrease vascularity and inhibit angiogenesis (28, 62), and decrease food intake in some rodent models (14, 57a), all of which may indirectly affect muscle atrophy independently from the myofiber-expressed GR. Therefore, to address the role of the GR in muscle atrophy, we generated a mouse line with a muscle-specific deletion of the GR and subjected those mice to three commonly used in vivo atrophy models: DEX treatment as an example of high-dose anti-inflammatory GC therapy, nutritional deprivation, and denervation. The line was also used to examine the role of the GR in the induction of specific atrophy-associated genes under these conditions.
EXPERIMENTAL PROCEDURES
Animal care and handling.
Muscle-specific GR-knockout mice were generated by crossing MCKcre (muscle creatine kinase promoter driving Cre recombinase) mice [B6.FVB(129S4)-Tg(Ckmm-cre)5Khn/J; Jackson Laboratories, Bar Harbor, ME] on an FVB background, with mice bearing GR alleles with loxP sites flanking exon 3 on a Balb/c background (GRloxp) (55). Genotyping of all mice was performed using genomic DNA from tail tips prepared using a DNeasy Blood and Tissue kit (Qiagen, Germantown, MD), primers that flank the 5′ most loxP site (GRflox forward 5′-GGCATGCACATTACTGGCCTTCT-3′, GRflox reverse 5′-CCTTCTCATTCCATGTCAGCATGT-3′), and primers within the MCKcre transgene 5′-ACAAAAGGTTTTGCCCTCCT-3′ and 5′-GTGAAACAGCATTGCTGTCACTT-3′. Breeding pairs of homozygous GRloxP mice and heterozygous MCKcre/heterozygous GRloxP mice were used to produce heterozygous MCKcre/homozygous GRloxP muscle-specific GR-knockout mice (designated as MGRe3KO). Recombination in various tissues was determined by PCR of genomic DNA using the GRflox forward primer used above and 5′-GTGTAGCAGCCAGCTTACAGGA-3′ (downstream of the 3′ most loxP site); PCR of nonrecombined alleles yields an ∼2,400-bp band, whereas successful recombination missing exon 3 yields a 400-bp band. Homozygous GRloxP littermates of MGRe3KO mice were used as the controls in all experiments. Animals were housed in ventilated cages with ad libitum access to food and water and a 12:12-h dark-light cycle. For terminal experiments, mice were anesthetized with 2–4% inhaled isoflurane for tissue removal and then euthanized by exsanguinations. All animal experiments were approved by the University of California-Davis Institutional Animal Care and Use Committee.
Voluntary exercise.
Female 3-mo-old mice were housed individually in cages equipped with voluntary running wheels for 30 days (n = 5–6). Activity was measured continuously and binned in 15-min increments and saved every 24 h (Lafayette Instruments, Lafayette, IN). Distance run as well as average speed were collected with each bin. To normalize for the variation in activity between individual animals, we scored the onset of activity when the first bin recorded an average of 20% of maximum activity, which was then sustained over the course of the night.
DEX treatments.
Three-month-old female mice were treated with the synthetic GC DEX (water-soluble DEX; Sigma, St. Louis, MO) for periods of 6 h and 3, 7, and 14 days. For the 6-h DEX treatment, a single dose of 10 mg/kg body wt was given by intraperitoneal injection. For the 3-, 7-, and 14-day DEX treatments, 0.1 mg/ml water-soluble DEX was provided in the drinking water, which was replenished every 2nd day. Mice were given ad libitum access to food, and water consumption and body weight were measured every other day. Based on daily water consumption, mice were given a dose of ∼10 mg·kg−1·day−1 of DEX. At the designated time points a final body weight was taken, and under isoflurane (Abbott Laboratories, Abbott Park, IL) anesthesia the spleen, tibialis anterior (TA), triceps surae (TS; soleus, plantaris, and medial and lateral gastrocnemius), and heart were dissected, weighed, and frozen in liquid nitrogen. Those muscles used for histology were pinned on cork at a fixed length and frozen in liquid nitrogen-cooled isopentane.
Nutritional deprivation.
Four-month-old female mice were subjected to 3 days of 80% food restriction and not allowed to lose >20% of their initial body weight, according to the approved animal protocol. For these experiments mice were housed individually, and daily ad libitum food intake was monitored for 1 wk. At the start of week 2, food was removed from the cage at 0900, and then at 1700 each day 20% of each animal's ad libitum food intake was added to the cage. Body weight was recorded each day, and on the morning of day 4 body weight was measured, and under general anesthesia the hindlimb muscles were removed for RNA processing and quantitative PCR analysis.
Denervation studies.
Four-month-old female mice were denervated under isoflurane anesthesia, and the sciatic nerve on the right leg was exposed by a small (5 mm) incision in the midthigh and transected. The incision was closed with suture, and the mice were given an analgesia (0.5–1.0 mg/kg sc buprenorphine) and allowed to recover. Right and left hindlimb muscles were collected at 1 and 14 days following denervation, as described above.
Real-time PCR analysis of mRNA expression.
Total RNA was extracted from whole tricep surae using TRIzol reagent (Invitrogen, Camarillo, CA) according to the manufacturer's specifications. Tissues were homogenized in TRIzol with a Tekmar Tissuemizer and sheared with a 21-guage needle three times. cDNA synthesis was performed using Quantitech reverse transcription kit (Qiagen, Germantown, MD) according to the manufacturer's specifications. Quantitiative PCR (qPCR) was performed using a laser 7900 HTA FAST platform, SYBR green (Applied Biosystems, Carlsbad, CA), and primers designed by Primer Express (Applied Biosystems) and ordered from Invitrogen. Primer sequences were as follows: GR 5′-TCGCAGGCCGCTCAGT-3′ and 5′-GGAGGTGGTCCCGTTGCT-3′, MuRF1 5′-GCTGGTGGAAAACATCATTGACAT-3′ and 5′-CATCGGGTGGCTGCCTTT-3′, MAFbx 5′-CTTTCAACAGACTGGACTTCTCGA-3′ and 5′-CAGCTCCAACAGCCTTACTACGT-3′, forkhead box (Fox) O1 5′-AAGAGCGTGCCCTACTTCAA-3′ and 5′-TGCTGTGAAGGGACAGATTG-3′, forkhead transcription factor O3 (FoxO3) 5′-CAGGCTCCTCACTGTATTCAGCTA-3′ and 5′-CATTGAACATGTCCAGGTCCAA-3′, metallothionein 2 (MT2) 5′-ATAGACCATGTAGAAGCCTAGCCTTT-3′ and 5′-GGCTTTTATTGTCAGTTACATGCTTTATAG-3′, eukaryotic initiation factor 4E-binding protein (4E-BP1) 5′-GGCGGCACGCTCTTCA-3′ and 5′-TCCGACACTCCATCAGAAATTTC-3′, and collagen 1A1 (Col1A1) 5′-AGAGCCTGAGTCAGCAGATTGAG-3′ and 5′-CCAGTACTCTCCGCTCTTCCA-3′. All samples were normalized to GAPDH 5′-CCAGCCTCGTCCCGTAGAC-3′ and 5′-ATGGCAACAATCTCCACTTTGC-3′ with the exception of samples used in denervation experiments, which were normalized to Rpl39 5′-CAAAATCGCCCTATTCCTCA-3′ and 5′-AGACCCAGCTTCGTTCTCCT-3′.
Northern blot analysis.
For Northern blot analysis of gene expression after denervation, C57BL/6 mice were unilaterally denervated as described above for a given time (between 1 and 14 days), and both contralateral and denervated muscles were collected from each mouse for processing. Total RNA was isolated using the protocol as above for qPCR. Northern analysis was conducted using a random hexamer-labeled MuRF1 cDNA probe essentially as described previously (16) and was normalized to a methylene blue stain of the transferred membrane.
Histological analysis.
The TS complex was sectioned on a Leica (Buffalo Grove, IL) CM3050S cryostat at −21°C. Serial sections (10 μm) were stained with laminin (Sigma) for determination of fiber cross-sectional area. Serial cross sections were stained with myosin heavy chain fast antibody (Vector Laboratories, Burlingame, CA) at a 1:300 dilution. Digital images of the stained sections were taken under ×200 total magnification using an Axio Imager M1 light microscope (Zeiss). Fiber cross-sectional area was measured in the gastrocnemius (GA; lateral and medial heads) muscles. For the GA, five nonoverlapping regions were identified, and a total of 100–400 fibers were analyzed within each region using AxioVision software (Zeiss). The same five regions were analyzed across all mice.
Western blot analysis.
Muscle was powdered on dry ice within cryovial tubes, of which ∼30 mg of powdered tissue was placed in a clean tube and homogenized using a polytron in 400 μl of cold sucrose lysis buffer [50 mM Tris, pH 7.5, 250 mM sucrose, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 50 mM NaF, 5 mM Na2(PO4)2 with fresh 0.5 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM NaVO4, and 0.1% DTT]. This was briefly vortexed and centrifuged at 4°C for 10 min at 11,000 rpm to remove insoluble material. Protein concentrations were determined in triplicate using a Bradford Assay (Bio-Rad, Richmond, CA). Ten to 20 micrograms of protein was subjected to SDS-PAGE on acrylamide gels ranging from 10 to 15% and transferred to polyvinylidene diflouride membranes. Membranes were blocked in 4% nonfat dairy milk in Tris-buffered saline with 0.1% Tween-20 added for 1 h and then probed with primary antibody overnight at 4°C. Antibodies used included GR (M-20), p-Foxo1 (forkhead class O; Ser256), Foxo1 (Santa Cruz Biotechnology, Santa Cruz, CA), p-Akt (Ser473), Akt, p-GSK3β (Ser9), GSK-3β, p-4E-BP1 (Ser65), and 4E-BP1 (Cell Signaling Technology, Danvers, MA), all of which were used at a concentration of 1:1,000. The next day, membranes were washed and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies at 1:10,000 for 1 h at room temperature. Immobilon Western Chemiluminescent HRP substrate (Millipore) was then added to the membranes and exposed in a Chemigenius Bioimaging System (Syngene).
Statistical analysis.
Results are presented as means ± SD unless otherwise indicated. All data were analyzed by two-way ANOVA or Student's t-test. Tukey's post hoc analysis was used to determine differences when interactions existed. Statistical significance was set at P < 0.05.
RESULTS
Development of muscle-specific GR-knockout mice.
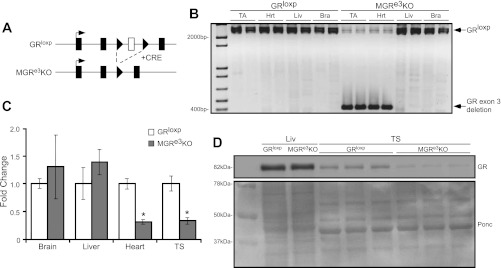
To reduce GR expression specifically in muscle tissue, we crossed mice bearing a transgene with the muscle creatine kinase (MCK) promoter driving Cre recombinase (7) with the GRloxP mouse line that has loxP sites flanking exon 3 of the GR (Fig. 1A) (55). These mice, designated MGRe3KO, had normal birth and growth rates, with no significant difference in body size of either sex compared with age- and sex-matched GRloxP littermates upon reaching sexual maturity. The only observed phenotype to date in unchallenged animals was an increase (P < 0.05) in hindlimb muscle wet weights in MGRe3KO mice compared with GRloxP littermates (see Fig. 2C). However, the mean myofiber cross-sectional areas (CSAs) were not different between the two groups (see Fig. 2F). Recombination of the GR gene occurred selectively in skeletal muscle and heart (Fig. 1B). Detectable recombination did not occur in the liver or brain. GR gene expression was analyzed using qPCR and was decreased significantly in the heart and TS muscle but unchanged in the brain and liver (Fig. 1C) of MGRe3KO mice. Western blot analysis was performed on liver and TS samples, and a substantial decrease in GR protein levels was seen in skeletal muscle but not in the liver (Fig. 1D). Taken together, GR expression is strongly reduced in skeletal muscle, and any residual expression is likely due to lack of recombination in nonmuscle cells such as fibroblasts present in whole muscle samples. For the rest of the studies in this article we used female mice only, since previous studies have shown that female mice are more sensitive to exogenous glucocorticoids than males (1, 10).
Fig. 1.
Generation of muscle-specific glucocorticoid receptor (GR) knockout mice (MGRe3KO). A: schematic depicting alleles of GRloxP and recombined GR in MGRe3KO mouse lines. Exon 3 is flanked by 2 loxP sites (arrowheads), and intervening DNA is removed to create the MGRe3KO when Cre recombinase is expressed under the control of the muscle-specific muscle creatine kinase promoter. B: PCR performed on genomic DNA extracted from 1-mo-old GRloxP and MGRe3KO mouse tissues: tibialis anterior (TA), heart (Hrt), liver (Liv), and brain (Bra). Specific recombination of GR gene is seen with the generation of a smaller band predicted at 400 bp (GR exon 3 deletion), as opposed to the nonrecombined band at ∼2,400 bp (GRloxP). C: GR mRNA expression from cDNA from Bra, Liv, Hrt, and skeletal muscle [triceps surae (TS)]. mRNA expression was normalized to GAPDH expression. D: Western blot showing GR protein expression from GRloxP and MGRe3KO liver and TS tissues; Ponceau (Ponc) stain demonstrating equal loading between GRloxP and MGRe3KO samples is shown below. Error bars represent means ± SD; n = 3–4 animals/group. *P < 0.01 (vs. control).
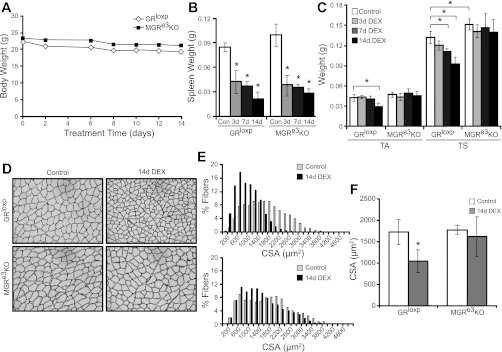
Fig. 2.
Dexamethasone (DEX) treatment induces skeletal muscle atrophy in GRloxP but not in MGRe3KO mice. A: body weight changes throughout the 14-day treatment regimen of 10 mg·kg−1·day−1 of water-soluble DEX. B: spleen weight changes between controls and 3-, 7-, and 14-day DEX-treated GRloxP and MGRe3KO mice. C: TA and TS wet weights were obtained from control, 3-, 7-, and 14-day DEX-treated GRloxP, or MGRe3KO mice as indicated. D: representative cross-sectional areas (CSA) from the gastrocnemius of control or 14-day DEX-treated GRloxP (top) or MGRe3KO mice (bottom) imaged by laminin immunohistochemistry. E: corresponding values representing 3 animals/treatment group, with 5 pictures/gastrocnemius section of each animal taken at ×20 magnification and 100–400 myofibers/picture counted, are shown. Gray bars represent fibers from control animals, and black bars represent fibers from 14-day DEX-treated animals. F: average of cross-sectional area measurements shown in E. Open bars, control animals; gray bars, 14-day DEX-treated animals. Error bars represent means ± SD of n = 3–4 animals/group. *P < 0.01 (vs. control).
Since glucocorticoids have been linked to circadian changes in peripheral clock activity and metabolism (2, 49), we monitored voluntary wheel running activity in GRloxP and MGRe3KO for 30 days. Although there was considerable variation in the total amount of wheel running during the night in both lines, neither GRloxP nor MGRe3KO mice showed any difference in circadian patterns of activity, with average onset of activity 18:06 ± 0.01 and 18:06 ± 0.004, respectively (lights were turned off from 1800 to 0600 daily). The difference in nightly activity in both lines of mice varied equally in that neither line showed a significant trend toward more or less activity. Subtle differences that may become important over long experimental protocols or over the lifetime of the animal may require much larger cohorts to be definitively revealed. Overall, the presence or absence of the GR in skeletal muscle does not appear to affect growth rate or daily activity patterns, and the only obvious phenotype in unchallenged animals observed to date is a slightly larger adult skeletal muscle mass without increased myofiber CSA.
DEX-induced atrophy is strongly attenuated in MGRe3KO mice.
To determine whether the GR in skeletal muscle mediates DEX-induced atrophy, we treated MGRe3KO and GRloxP mice with or without 10 mg·kg−1·day−1 DEX for 3, 7, and 14 days in 3-mo-old female mice. Pilot studies in our laboratory had shown that lower doses of DEX did not induce atrophy to a significant extent in the mixed FVB/Balb/c background. Additional pilot studies also showed that there was no significant difference in the amount of food or water consumed between control and DEX-treated wild-type mice over the course of 10 days when there was significant muscle atrophy. Both GRloxP and MGRe3KO mice steadily lost modest but similar amounts of total body weight over the 14-day treatment period (total weight loss ∼10%; Fig. 2A), and body weights at all time points were not significantly different between GRloxP and MGRe3KO mice. Therefore, any muscle sparing observed in MGRe3KO mice would not likely be due to changes in food or water intake compared with GRloxP mice. Spleen weights were taken as a measurement of equivalent DEX dosing since it has been shown that glucocorticoids induce splenocyte apoptosis (41). Spleen weight significantly and similarly decreased in both GRloxP and MGRe3KO mice, suggesting that both groups received equivalent DEX dosing (Fig. 2B). Heart weights were not significantly different from each other at baseline or from controls after following DEX treatment. In response to 14 days of DEX treatment, a significant decrease in the wet weights of the TS (32.1%) and TA (31.8%) was found in DEX-treated GRloxP mice compared with untreated GRloxP mice (Fig. 2C). In the TS, significant atrophy (19%) was also apparent after 7 days of DEX treatment (Fig. 2C). However, in the MGRe3KO mice, no loss of muscle mass was observed in the TA over the course of the 14 days of DEX treatment, and only a modest but not statistically significant loss in the TS following 7 or 14 days of DEX treatment was observed. Measurement of cross-sectional area of type II fibers in the medial and lateral heads of the GA muscle, verified by fast myosin heavy chain isoform staining of serial sections, confirmed the changes in the wet weight measurements. After 14 days of DEX treatment, a significant decrease in mean cross-sectional area (39.6%) was apparent in the GA of GRloxP mice, whereas no significant decrease was observed in the MGRe3KO mice (Fig. 2, D–F).
Glucocorticoid-induced gene expression in skeletal muscle is inhibited in MGRe3KO mice.
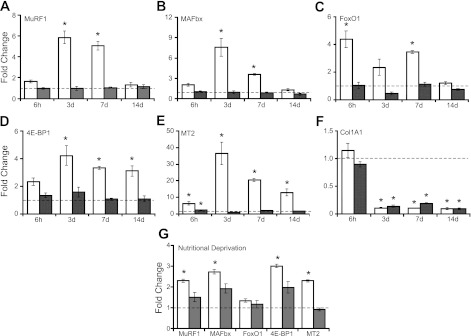
A number of GC-responsive genes have been identified in skeletal muscle, but the direct role of muscle GR in their regulation has not been determined in vivo in response to exogenous high-dose GCs. Therefore, we examined the regulation of muscle gene expression using quantitative PCR on RNA from TS muscle after 6 h and 3, 7, and 14 days of DEX treatment. Upregulation of the atrophy-associated genes MuRF1 and MAFbx was blocked completely in MGRe3KO mice at all time points examined (Fig. 3, A and B). The upregulation of several additional DEX-responsive genes was also blocked in MGRe3KO mice, including the FoxO1 gene implicated in regulation of downstream atrophy-associated genes that include MuRF1, the 4E-BP1 gene linked to inhibition of cap dependent translation, and the more broadly expressed GC response element (GRE)-containing MT2 gene (Fig. 3, C–E). FoxO3a expression was generally much lower than FoxO1 in TS muscle samples but basically showed the same overall pattern: 2.2-fold ± 0.4 and 1.5-fold ± 0.15 upregulated in GRloxP mice but not upregulated and even slightly downregulated 1.5-fold ± 0.17 and 1.2-fold ± 0.06 in the MGRe3KO mice at 3 and 14 days, respectively. Interestingly, Col1A1 expression was repressed to the same degree by DEX in both GRloxP and MGRe3KO mice, which was expected since Col1A1 is expressed normally in nontargeted fibroblast cells in the muscle extracellular matrix (Fig. 3F) and is known to be repressed by GCs (45). Therefore, the equal degree of Col1A1 repression in both MGRe3KO and GRloxP mice provides supporting evidence that the MGRe3KO animals lack GR-mediated signaling specifically in myofibers.
Fig. 3.
Glucocorticoid-induced gene expression changes in TS muscles of GRloxP and MGRe3KO mice. Expression of select glucocorticoid-responsive genes muscle RING finger 1 (MuRF1; A), muscle atrophy F-box (MAFbx; B), metallothionein 2 (MT2; C), eukaryotic translation intitiation factor 4E-binding protein 1 (4E-BP1; D), forkhead transcription factor O1 (FoxO1; E), and collagen 1A1 (Col1A1; F) expressed as fold changes in DEX-treated animals relative to untreated control animals, as determined by quantitative PCR. G: gene expression changes in TS muscles in nutritionally deprived vs. ad libitum-fed controls. Open bars represent expression changes in GRloxP mice; gray bars represent expression changes in MGRe3KO mice. Dashed line represents the level of 1-fold change, i.e., no change in DEX-treated or nutritionally deprived expression relative to untreated controls. Error bars represent means ± SD of n = 3 animals/group. *P < 0.05 (vs. control).
We also examined gene expression in response to elevated endogenous GCs. Extended periods of starvation or nutritional deprivation generally result in decreased muscle mass (63) and are accompanied by increased endogenous GCs and decreased insulin and IGF-I secretion (35). The role of the GR in mediating gene expression changes during nutritional deprivation has not been examined extensively to date, except for MAFbx (atrogin-1), whose upregulation was blunted under starvation conditions in a different strain of muscle-specific GRKO mice (22). GRloxP and MGRe3KO mice were subjected to 3 days of nutritional deprivation to 20% of their normal food intake. Body weight decreased by nearly 15% over the 3 days in both GRloxP and MGRe3KO mice. Unexpectedly, we did not observe statistically significant atrophy under these conditions in either TS or TA muscles of either group of mice. Spleen weight decreased >30% in both groups, indicating that endogenous glucocorticoids were indeed elevated. Upon examination of gene expression changes in GRloxP and MGRe3KO mice, there was strong attenuation in the induction of MuRF1 and MAFbx, 4E-BP1, and MT2 expression in MGRe3KO mice (Fig. 3G). In contrast, FoxO1 (Fig. 3G) was not induced significantly by nutritional deprivation in either GRloxp or MGRe3KO mice. Likewise, FoxO3a expression was only modestly (but significantly) upregulated equally in both GRloxP and MGRe3KO mice (1.7-fold ± 0.05 and 1.7-fold ± 0.25, respectively).
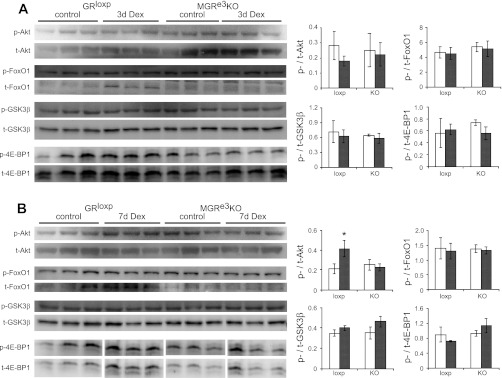
DEX induction of FoxO1 and 4E-BP1 protein levels is inhibited in MGRe3KO mice.
Altering the balance between protein synthesis and degradation can lead to changes in muscle mass. Our previous studies implicated suppression of protein synthesis, rather than activation of various proteolytic activities, as the predominant means by which exogenous GCs induce atrophy in mice (1); thus we examined protein expression and phosphorylation of certain key proteins in the phosphatidylinositol 3 (PI3)-kinase/Akt pathway. Expression levels and phosphorylation state of Akt, GSK-3β, FoxO1, and 4E-BP1 were examined at two time points: 3 days after DEX treatment prior to significant atrophy and 7 days after DEX treatment, when there is significant atrophy in GRloxP, which is prevented in MGRe3KO mice. Overall, no obvious differences in phosphorylated/total ratios were found (Fig. 4, A and B). However, total FoxO1 protein expression was elevated with 3 and 7 days of DEX treatment in GRloxP but not MGRe3KO mice. Total 4E-BP1 induction was only readily apparent at 7 days in GRloxP mice; modest induction was seen in one DEX-treated sample from MGRe3KO mice, but overall expression was clearly blunted in these animals without concurrent decreases in phosphorylated levels.
Fig. 4.
Protein expression from TS in GRloxP and MGRe3KO mice after DEX treatment. Western blots of phosphorylated (p)-Akt, total (t)-Akt, p-FoxO1, t-FoxO1, p-GSK-3β, t-GSK-3β, p-4E-BP1, and t-4E-BP1 from 3- (A) and 7-day (B) DEX-treated GRloxP and MGRe3KO mice. Phosphorylated to total protein ratios from control (open bars) vs. DEX-treated (gray bars) GRloxP (loxp) and MGRe3KO (KO) mice are shown at right. Error bars represent means ± SD of n = 3 animals/group. *P < 0.05 (vs. control).
Denervation induces skeletal muscle atrophy independent of the GR.
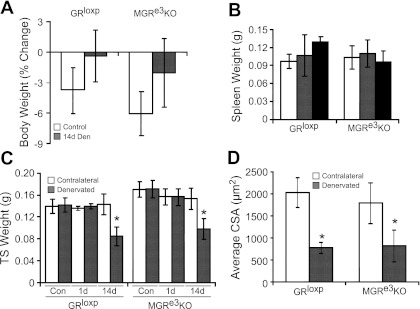
Denervation is a well-known inducer of muscle atrophy; however, the role of the GR in denervation-induced atrophy and associated gene expression changes has not been determined unequivocally. Denervation resulted in similar changes in TS wet weights (Fig. 5C) in GRloxP and MGRe3KO mice. The significant loss of mass in the TS of both strains was consistent with the significant decrease in fiber cross-sectional area observed in both GRloxP (61.9%) and MGRe3KO (53.8%) mice following 14 days of denervation (Fig. 5D). Thus, the presence of the GR in skeletal muscle is not essential for muscle atrophy caused by denervation, as opposed to induction of muscle atrophy by exogenous and endogenous GCs.
Fig. 5.
Denervation induces a similar degree of atrophy in both GRloxP and MGRe3KO littermates. A: body weights of GRloxP and MGRe3KO mice before and after 14-day denervation (14d Den). B: spleen weight from control animals (open bars) and 1 (gray bars) and 14 days (black bars) after denervation in GRloxP and MGRe3KO mice. C: wet weight of both contralateral (open bars) and denervated TS muscles (gray bars) at 1 (1d) and 14 days (14d) as well as from control (Con) nondenervated animals. D: average CSA. Values represent 3 animals/treatment group; 5 pictures/gastrocnemius section of each animal were taken at ×20 magnification, and 100–400 myofibers were counted in each picture. Open bars represent contralateral gastrocnemius, and gray bars are 14d denervated gastrocnemius. Error bars represent means ± SD; n = 3 for control groups, and n = 6 for Den groups. *P < 0.05 (vs. control).
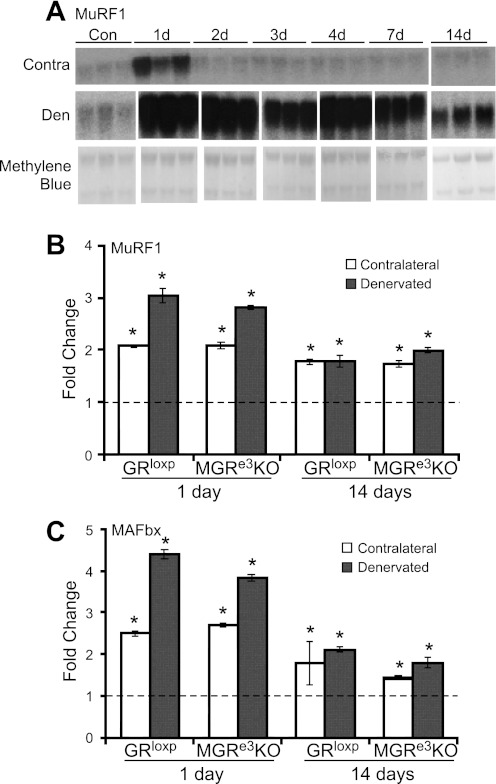
Gene expression in muscle from the contralateral, unoperated limb was also examined since the contralateral limb is often used as an internal control for the denervated muscle. Initially, a time course of MuRF1 expression was examined in contralateral and denervated TS muscles over 14 days. An upregulation of MuRF1 RNA occurred in the contralateral side after 1 day of denervation but returned to baseline by 2 days of denervation. In contrast, MuRF1 expression in the denervated muscle was elevated at 1 day and remained elevated for all time points but declined back toward baseline at 14 days (Fig. 6A). To determine whether this transient increase in MuRF1 expression was related to activation of the GR immediately following surgery, we measured MuRF1 and MAFbx expression in the contralateral limb of denervated MGRe3KO mice. Interestingly, a similar increase in MuRF1 and MAFbx expression was observed on the contralateral side relative to untreated control mice in both GRloxP mice and MGRe3KO mice 1 day after denervation (Fig. 6, B and C). This observation suggests that the transient elevation in MuRF1 expression in the contralateral limb was not related to elevated GCs following the surgery. Furthermore, these data show that a transient increase in MuRF1 and MAFbx expression does not lead to muscle atrophy.
Fig. 6.
MuRF1 and MAFbx expression patterns after denervation are similar between GRloxP and MGRe3KO mice. A: Northern blot of MuRF1 expression in TS muscle of C57BL/6 mice from either contralateral (Contra) or denervated (Den) limbs (n = 3). A methylene blue stain of the membrane was used to demonstrate equal RNA loading. Gene expression changes for MuRF1 (B) and MAFbx (C) in 1- and 14-day Den and Contra (nondenervated) TS muscles. Dashed lines represent the level of 1-fold change, i.e., no change in expression relative to unoperated controls. Error bars represent means ± SD of n = 3 animals/group. *P < 0.05 (vs. control).
DISCUSSION
Elevated endogenous levels of GCs are associated with muscle atrophy in many different pathophysiological conditions. Furthermore, steroid myopathy is a serious side effect of synthetic GC treatment for conditions ranging from asthmas, certain leukemias or other cancers, and a variety of autoimmune diseases. Although the requirement for intact GC signaling in skeletal muscle under these conditions has been inferred from adrenalectomy or treatment with RU-486, a GR (and progesterone receptor) antagonist, we chose a genetic approach to determine the role of the GR by creating a mouse with a muscle-specific deletion of the GR, which we designated MGRe3KO. These mice subsequently allowed us to examine the conditions under which skeletal muscle atrophy is mediated by the GR specifically expressed in muscle tissue. No obvious morphological or behavioral differences were observed in MGRe3KO mice, except for modest but significantly larger TS muscle wet weights. The mechanism responsible for the larger TS muscle weights in the MGRe3KO mice was not determined here since it is beyond the scope of the current study, but interestingly, myofiber cross-sectional areas were not larger in MGRe3KO mice than in their control littermates. This suggests that the increased muscle mass in MGRe3KO mice could be due to increased vascularity, as one possibility, since GCs have been shown to repress VEGF expression in skeletal muscle (3).
The role of skeletal muscle GR in mediating atrophy due to excess synthetic GC treatment was examined first in the MGRe3KO mice following an acute injection of DEX and following chronic daily DEX treatment for 3–14 days. The MGRe3KO mice were remarkably resistant to the atrophy-inducing effects of DEX observed in GRloxP mice over the entire 14-day treatment period. Since food and water intake are not affected by DEX in our mice, and overall activity did not show obvious differences between strains, we conclude that muscle sparing is the result of the absence of a DEX-induced genetic program leading to atrophy in MGRe3KO mice. Consistent with muscle sparing, DEX induction of MuRF1 and MAFbx, two genes linked to the induction of atrophy in multiple models, was completely blocked in MGRe3KO mice. Recently, we showed that deletion of MuRF but not MAFbx strongly inhibited muscle atrophy in response to DEX treatment (1) to an extent very similar to what we observed in MGRe3KO mice. Although other GR target genes in skeletal muscle may also contribute to a lesser degree, the remarkably similar DEX-induced responses in our MuRF1-KO and MGRe3KO models support the conclusion that MuRF1 is a major downstream target of the GR in the muscle atrophy pathway induced by exogenous GCs.
The MuRF1 gene has a functional GRE in its promoter and is thus a direct target of the GR (60); hence, lack of DEX induction in MGRe3KO mice was not completely unexpected. On the other hand, no GRE has been demonstrated conclusively in the vicinity of the MAFbx gene; rather, that gene's upregulation is thought to be mediated indirectly by DEX via prior induction of FoxO genes (43), which are well-known GC-inducible transcription factors (48). Since DEX-induced expression of both FoxO1 and FoxO3a is also blocked completely in MGRe3KO mice, the induction of multiple downstream genes involved in DEX-induced atrophy that are strongly dependent on prior FoxO upregulation would also be blocked in these mice, including MAFbx. Interestingly, the most robustly induced genes after an acute DEX injection were FoxO1 and MT2, and whereas MT2 is regulated via a clustered pair of GREs in the proximal promoter (25), the mechanism of DEX induction of FoxO1 by DEX is not currently known. However, FoxO1 cooperates with the GR at the more complex MuRF1 GRE (60), which predictably shows more delayed induction kinetics than FoxO1.
An additional, important consideration regarding transcriptional responses to DEX in skeletal muscle is that a previous study proposed an important nongenomic role for the GR in the induction of atrophy in the streptozotocin-induced diabetes model via direct interference with PI3 kinase activity (22). In those studies, strong GR-dependent suppression of phosphorylated Akt and FoxO1 was observed, with little change in total protein levels. The decreased ratio of phosphorylated/total FoxO1 levels was proposed as a potential indirect means of activation of downstream GC-inducible genes such as MAFbx/atrogin-1. Other studies, using primarily intact rats or cell culture models, have reported that high-dose GC inhibits Akt and downstream signaling events (8, 9, 64) even without prior inhibition of insulin signaling. Nevertheless, in the presence of chronic high-dose DEX in our mouse strains, phosphorylated Akt activity did not appear to be inhibited, as might be expected if there was a strong upstream inhibitory effect of liganded GR interacting with PI3 kinase. In fact, at 7 days, phosphorylated Akt levels (relative to total Akt) were elevated in wild-type animals undergoing atrophy, which was blunted in MGRe3KO mice that were not. We also examined FoxO1 and GSK-3β, two important downstream targets of Akt. GSK-3β, an inhibitor of protein synthesis and glycogen synthesis, was recently reported to play an important role in muscle atrophy resulting from impaired insulin/IGF-1 signaling or GC treatment (58). However, no changes in GSK-3β total or phosphorylated forms were observed with DEX treatment, consistent with a lack of effect on upstream Akt activity. However, as expected, total FoxO1 was induced only in GRloxP and not MGRe3KO mice at both time points. However, the phosphorylation level was not reduced, and phosphorylated/total FoxO1 ratios did not appear to change when quantified. Nonetheless, even if the ratio does not change, upregulation of more total FoxO1 should result in more available for activation of downstream targets, such as MuRF1, in collaboration with the GR. This end result appears to be accomplished via the GR in two different ways when the diabetes models vs. high-dose DEX treatment are compared. In the former, the primary effect may be on upstream signaling cascades, resulting in a decreased phosphorylation state of FoxO1 with little effect on total protein levels, and in the latter, the primary effect may be induction of FoxO1 protein with little effect on upstream signaling and overall phosphorylation state. Last, the effect of DEX on total and phosphorylated 4E-BP1 was examined, with a clear induction at 7 days in GRloxP, which is blunted in MGRe3KO mice. This protein is of interest since it is an inhibitor of cap-dependent translation and a target for inactivation by mTOR-mediated phosphorylation (26). Furthermore, GC-induced decreases in phosphorylated 4E-BP1 have been linked to induction of regulated in development and DNA damage-1 (REDD1), a potent inhibitor of mTOR activity. Despite upregulation of total 4E-BP1, we observed that DEX proportionally induced the phosphorylated forms as well, which is not consistent with upstream inhibition of mTOR. Studies on GC inhibition of mTOR and protein synthesis are generally performed at shorter time points than examined here (61); thus, earlier important effects mediated by DEX-induced REDD1 activity might have been missed in our chronic exposure studies. Furthermore along those lines, we also cannot rule out an earlier effect on of PI3-kinase inhibition, but our results are more consistent with a more direct effect of GR on atrophy-related gene expression under chronic high-dose DEX treatment. It is noteworthy that a subset of the genes we examined have well-characterized consensus GC response elements such as MuRF1 and MT2, and they were not induced at all in DEX-treated MGRe3KO mice.
Nutritional deprivation affects rodent physiology in multiple ways, including decreased insulin and IGF-I levels and signaling (35, 38) and the release of corticosterone from the adrenal gland (34). A previous study showed that the muscle-expressed GR was an important mediator of overnight starvation-induced atrophy and partially responsible for MAFbx induction under those conditions (22). With the milder nutritional deprivation regimen employed here (27), atrophy was not significant compared with controls in either strain despite significant body mass loss. However, when we examined an expanded set of atrophy-associated genes such as MuRF1, MAFbx, and 4E-BP1 gene expression, we found that upregulation of these genes during nutritional deprivation was strongly attenuated in the MGRe3KO mice but not completely blocked, as we observed with DEX treatment. Thus, the GR is only partially responsible for the induction of these genes during nutritional deprivation, and other pathways such as disinhibition by impaired insulin/IGF-I signaling or activation by fasting-induced cytokines must play an additional, important role for full activation. By contrast, upregulation of the MT2 gene was blocked completely in nutritionally deprived MGRe3KO mice, implying a simpler control of this gene via the presence or absence of elevated GC levels and the GR.
Finally, denervation of hindlimb muscles has been a well-studied model of muscle atrophy (17, 63), and atrophy is at least partially dependent on induction of functional MuRF1 and/or MAFbx genes. Recent evidence suggests that denervation induces atrophy via a signaling cascade involving induction and translocation of HDAC4 and -5, culminating in the induction of myogenin, as well as MuRF1 and MAFbx, which are downstream targets of myogenin in adult muscle (33). Thus, we did not necessarily expect this type of atrophy to be mediated through the GR, but it was formally possible since activation of the GR is permissive for several other signaling pathways (44), and the GR functionally interacts with FoxO1, which has been implicated in multiple atrophy models, including denervation. Furthermore, an important goal of this study was to examine the role of the GR in a broad range of muscle atrophy-inducing conditions whether or not they were linked previously to altered GC levels. Early (1 day) and late (14 days) time points were chosen to determine whether or not short-term gene expression changes were mediated through the GR as well as to look at the long-term atrophy of the TS on the denervated right side vs. the nonoperated (contralateral, or left) side. Use of the contralateral side is a common control in denervation studies, but no studies to our knowledge have examined whether there are gene expression changes relative to muscle from a control animal that has not undergone the procedure. First, we found that there was a virtually identical decrease in muscle mass in denervated TS compared with the contralateral control TS, and compared with muscle from control animals that were not denervated, in both GRloxP and MGRe3KO mice. This was confirmed by cross-sectional area analysis. Furthermore, we found that there were no differences in the pattern of MuRF1 or MAFbx gene expression between GRloxP and MGRe3KO mice at either 1 or 14 days postdenervation, consistent with the similar degree of atrophy observed. Thus, the GR is not essential for induction of denervation-induced atrophy. Interestingly, we observed modest but significant increases in MuRF1 and MAFbx gene expression on the contralateral sides of denervated mice, which were lower than in muscle from the denervated side but higher than in muscle from nonoperated control mice. Induction of contralateral MuRF1 is transient and returns to baseline levels by the 2nd day after surgery. Contralateral MuRF1 and MAFbx expression may be due to some systemic factor other than GCs that is induced by the surgical procedure itself, since expression changes were similar in both MGRe3KO and GRloxP mice. Regardless of the mechanism, this observation raises the issue that the contralateral muscle may not be a perfectly suitable control for gene expression studies following denervation, at least in the time period shortly after the procedure.
In summary, our data strongly support the conclusion that the GR expressed in myofibers is the essential mediator of muscle atrophy and gene expression changes induced by systemic exposure to synthetic GCs such as DEX. Furthermore, the GR is at least partially responsible for muscle gene expression changes in response to elevated endogenous GCs during nutritional deprivation. By contrast, atrophy is equally induced in denervated hindlimb muscles in both GRloxP and MGRe3KO mice, confirming that the lack of the GR does not inhibit neurogenic-induced muscle atrophy. Preventing GR activation and downstream MuRF1 expression in skeletal muscle as a deleterious side effect of synthetic GC treatment provides an important clinical avenue for future research.
GRANTS
M. L. Watson received support from a National Institutes of Health training grant in Molecular and Cellular Biology (2-T32-GM-007377-31A1). J. D. Furlow and S. C. Bodine received support from the National Institute of Diabetes and Digestive and Kidney Diseases (1-RO1-DK-75801). J. P. Tuckerman received support from Deutsche Forschungsgemeinschaft (DFG; TU220/3 and TU220/6 within the priority program SPP1468, IMMUNOBONE) and the Boehringer Ingelheim Foundation. H. M. Reichardt was supported by DFG (RE1631/7-1) and Deutsche Krebshilfe (108713).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.L.W., H.M.R., J.P.T., S.C.B., and J.D.F. did the conception and design of the research; M.L.W., L.M.B., and S.C.B. performed the experiments; M.L.W., S.C.B., and J.D.F. interpreted the results of the experiments; M.L.W. prepared the figures; M.L.W. and J.D.F. drafted the manuscript; M.L.W., L.M.B., H.M.R., J.P.T., S.C.B., and J.D.F. edited and revised the manuscript; M.L.W., L.M.B., H.M.R., J.P.T., S.C.B., and J.D.F. approved the final version of the manuscript; M.L.W. and J.D.F. analyzed the data.
ACKNOWLEDGMENTS
We acknowledge Chris Craig-Veit for expert technical assistance as well as several Furlow Laboratory undergraduates who assisted with various aspects of the work: Elaine Garcia (genotyping/dissections), Lucia Dobrawa (genotyping/dissections), Rebecca Waters (CSA analysis), Matthew Barraza (running wheel experiments), and Brian Noh (genotyping/dexamethasone treatments). We also thank Andrew Philp for advice on Western blots and Darren Hwee for advice on activity measurements.
REFERENCES
- 1. Baehr LM, Furlow JD, Bodine SC. Muscle sparing in muscle RING finger 1 null mice: response to synthetic glucocorticoids. J Physiol 589: 4759–4776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289: 2344–2347, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Barel M, Perez OA, Giozzet VA, Rafacho A, Bosqueiro JR, do Amaral SL. Exercise training prevents hyperinsulinemia, muscular glycogen loss and muscle atrophy induced by dexamethasone treatment. Eur J Appl Physiol 108: 999–1007, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci USA 102: 473–478, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brewer JA, Khor B, Vogt SK, Muglia LM, Fujiwara H, Haegele KE, Sleckman BP, Muglia LJ. T-cell glucocorticoid receptor is required to suppress COX-2-mediated lethal immune activation. Nat Med 9: 1318–1322, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Brüning JC, Michael MD, Winnay JN, Hayashi T, Hörsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 2: 559–569, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Buren J, Lai YC, Lundgren M, Eriksson JW, Jensen J. Insulin action and signalling in fat and muscle from dexamethasone-treated rats. Arch Biochem Biophys 474: 91–101, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Cho JE, Fournier M, Da X, Lewis MI. Time course expression of Foxo transcription factors in skeletal muscle following corticosteroid administration. J Appl Physiol 108: 137–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E, Glass DJ. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab 6: 376–385, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schütz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev 9: 1608–1621, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Dardevet D, Sornet C, Grizard J. Glucocorticoid-induced insulin resistance of protein synthesis is independent of the rapamycin-sensitive pathways in rat skeletal muscle. J Endocrinol 162: 77–85, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Desler MM, Jones SJ, Smith CW, Woods TL. Effects of dexamethasone and anabolic agents on proliferation and protein synthesis and degradation in C2C12 myogenic cells. J Anim Sci 74: 1265–1273, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Dumas JF, Simard G, Roussel D, Douay O, Foussard F, Malthiery Y, Ritz P. Mitochondrial energy metabolism in a model of undernutrition induced by dexamethasone. Br J Nutr 90: 969–977, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology 151: 4811–4819, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gesina E, Tronche F, Herrera P, Duchene B, Tales W, Czernichow P, Breant B. Dissecting the role of glucocorticoids on pancreas development. Diabetes 53: 2322–2329, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Goldberg AL. Protein turnover in skeletal muscle. II. Effects of denervation and cortisone on protein catabolism in skeletal muscle. J Biol Chem 244: 3223–3229, 1969 [PubMed] [Google Scholar]
- 18. Hasselgren PO. Glucocorticoids and muscle catabolism. Curr Opin Clin Nutr Metab Care 2: 201–205, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Hobler SC, Tiao G, Fischer JE, Monaco J, Hasselgren PO. Sepsis-induced increase in muscle proteolysis is blocked by specific proteasome inhibitors. Am J Physiol Regul Integr Comp Physiol 274: R30–R37, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Hong DH, Forsberg NE. Effects of dexamethasone on protein degradation and protease gene expression in rat L8 myotube cultures. Mol Cell Endocrinol 108: 199–209, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Hu Z, Lee IH, Wang X, Sheng H, Zhang L, Du J, Mitch WE. PTEN expression contributes to the regulation of muscle protein degradation in diabetes. Diabetes 56: 2449–2456, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Hu Z, Wang H, Leed IH, Du J, Mitch WE. Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J Clin Invest 119: 3059–3069, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kellendonk C, Eiden S, Kretz O, Schutz G, Schmidt I, Tronche F, Simon E. Inactivation of the GR in the nervous system affects energy accumulation. Endocrinology 143: 2333–2340, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Kellendonk C, Opherk C, Anlag K, Schutz G, Tronche F. Hepatocyte-specific expression of Cre recombinase. Genesis 26: 151–153, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Kelly EJ, Sandgren EP, Brinster RL, Palmiter RD. A pair of adjacent glucocorticoid response elements regulate expression of two mouse metallothionein genes. Proc Natl Acad Sci USA 94: 10045–10050, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kimball SR, Jefferson LS. Control of translation initiation through integration of signals generated by hormones, nutrients, and exercise. J Biol Chem 285: 29027–29032, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewis MI, Bodine SC, Kamangar N, Xu X, Da X, Fournier M. Effect of severe short-term malnutrition on diaphragm muscle signal transduction pathways influencing protein turnover. J Appl Physiol 100: 1799–1806, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Logie JJ, Ali S, Marshall KM, Heck MM, Walker BR, Hadoke PW. Glucocorticoid-mediated inhibition of angiogenic changes in human endothelial cells is not caused by reductions in cell proliferation or migration. PLoS One 5: e14476, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ma K, Mallidis C, Bhasin S, Mahabadi V, Artaza J, Gonzalez-Cadavid N, Arias J, Salehian B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am J Physiol Endocrinol Metab 285: E363–E371, 2003 [DOI] [PubMed] [Google Scholar]
- 30. May RC, Kelly RA, Mitch WE. Metabolic acidosis stimulates protein degradation in rat mucle by glucocorticoid-dependent mechanism. J Clin Invest 77: 614–621, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meisler N, Shull S, Xie R, Long GL, Absher M, Connolly JP, Cutroneo KR. Glucocorticoids coordinately regulate type I collagen pro alpha 1 promoter activity through both the glucocorticoid and transforming growth factor beta response elements: a novel mechanism of glucocorticoid regulation of eukaryotic genes. J Cell Biochem 59: 376–388, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Mitch WE, Bailey JL, Wang X, Jurkovitz C, Newby D, Price SR. Evaluation of signals activating ubiquitin-proteasome proteolysis in a model of muscle wasting. Am J Physiol Cell Physiol 276: C1132–C1138, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Moresi V, Williams AH, Meadows E, Flynn JM, Potthoff MJ, McAnally J, Shelton JM, Backs J, Klein WH, Richardson JA, Bassel-Duby R, Olson EN. Myogenin and class II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell 143: 35–45, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muir JL, Pfister HP. Corticosterone and prolactin responses to predictable and unpredictable novelty stress in rats. Physiol Behav 37: 285–288, 1986 [DOI] [PubMed] [Google Scholar]
- 35. O'Sullivan U, Gluckman PD, Breier BH, Woodall S, Siddiqui RA, McCutcheon SN. Insulin-like growth factor-1 (IGF-1) in mice reduces weight loss during starvation. Endocrinology 125: 2793–2794, 1989 [DOI] [PubMed] [Google Scholar]
- 36. Opherk C, Tronche F, Kellendonk C, Kohlmüller D, Schulze A, Schmid W, Schütz G. Inactivation of the glucocorticoid receptor in hepatocytes leads to fasting hypoglycemia and ameliorates hyperglycemia in streptozotocin-induced diabetes mellitus. Mol Endocrinol 18: 1346–1353, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Park YS, Ha Choi Y, Park CH, Kim KT. Nongenomic glucocorticoid effects on activity-dependent potentiation of catecholamine release in chromaffin cells. Endocrinology 149: 4921–4927, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rao RH. Adaptations in glucose homeostasis during chronic nutritional deprivation in rats: hepatic resistance to both insulin and glucagon. Metabolism 44: 817–824, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, Kirilov M, Mandic V, Takacz A, Schmidt-Ullrich R, Ostermay S, Schinke T, Spanbroek R, Zaiss MM, Angel PE, Lerner UH, David JP, Reichardt HM, Amling M, Schütz G, Tuckermann JP. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab 11: 517–531, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Riso EM, Ahtikoski A, Alev K, Kaasik P, Pehme A, Seene T. Relationship between extracellular matrix, contractile apparatus, muscle mass and strength in case of glucocorticoid myopathy. J Steroid Biochem Mol Biol 108: 117–120, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Rooman R, Koster G, Bloemen R, Gresnigt R, van Buul-Offers SC. The effect of dexamethasone on body and organ growth of normal and IGF-II-transgenic mice. J Endocrinol 163: 543–552, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Ruff RL, Martyn D, Gordon AM. Glucocorticoid-induced atrophy is not due to impaired excitability of rat muscle. Am J Physiol Endocrinol Metab 243: E512–E521, 1982 [DOI] [PubMed] [Google Scholar]
- 43. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21: 55–89, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Schoepe S, Schäcke H, Bernd A, Zöller N, Asadullah K. Identification of novel in vitro test systems for the determination of glucocorticoid receptor ligand-induced skin atrophy. Skin Pharmacol Physiol 23: 139–151, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Seene T. Turnover of skeletal muscle contractile proteins in glucocorticoid myopathy. J Steroid Biochem Mol Biol 50: 1–4, 1994 [DOI] [PubMed] [Google Scholar]
- 47. Shah OJ, Kimball SR, Jefferson LS. Acute attenuation of translation initiation and protein synthesis by glucocorticoids in skeletal muscle. Am J Physiol Endocrinol Metab 278: E76–E82, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Smith IJ, Alamdari N, O'Neal P, Gonnella P, Aversa Z, Hasselgren PO. Sepsis increases the expression and activity of the transcription factor Forkhead Box O 1 (FOXO1) in skeletal muscle by a glucocorticoid-dependent mechanism. Int J Biochem Cell Biol 42: 701–711, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. So AY, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci USA 106: 17582–17587, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sterling KM, Jr, Harris MJ, Mitchell JJ, DiPetrillo TA, Delaney GL, Cutroneo KR. Dexamethasone decreases the amounts of type I procollagen mRNAs in vivo and in fibroblast cell cultures. J Biol Chem 258: 7644–7647, 1983 [PubMed] [Google Scholar]
- 51. Stojanovska L, Rosella G, Proietto J. Evolution of dexamethasone-induced insulin resistance in rats. Am J Physiol Endocrinol Metab 258: E748–E756, 1990 [DOI] [PubMed] [Google Scholar]
- 52. Sun L, Trausch-Azar JS, Muglia LJ, Schwartz AL. Glucocorticoids differentially regulate degradation of MyoD and Id1 by N-terminal ubiquitination to promote muscle protein catabolism. Proc Natl Acad Sci USA 3339–3344, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tillis CC, Huang HW, Bi W, Pan S, Bruce SR, Alcorn JL. Glucocorticoid regulation of human pulmonary surfactant protein-B (SP-B) mRNA stability is independent of activated glucocorticoid receptor. Am J Physiol Lung Cell Mol Physiol 300: L940–L950, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 23: 99–103, 1999 [DOI] [PubMed] [Google Scholar]
- 56. Tronche F, Opherk C, Moriggl R, Kellendonk C, Reimann A, Schwake L, Reichardt HM, Stangl K, Gau D, Hoeflich A, Beug H, Schmid W, Schütz G. Glucocorticoid receptor function in hepatocytes is essential to promote postnatal body growth. Genes Dev 18: 492–497, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tuckermann JP, Kleiman A, Moriggl R, Spanbroek R, Neumann A, Illing A, Clausen BE, Stride B, Forster I, Habenicht AJ, Reichardt HM, Tronche F, Schmid W, Schutz G. Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J Clin Invest 117: 1381–1390, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57a. Tulipano G, Taylor JE, Halem HA, Datta R, Dong JZ, Culler MD, Bianchi I, Cocchi D, Giustina A. Glucocorticoid inhibition of growth in rats: partial reversal with the full-length ghrelin analog BIM-28125. Pituitary 10: 267–274, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Verhees KJ, Schols AM, Kelders MC, Op den Kamp CM, van der Velden JL, Langen RC. Glycogen synthase kinase-3β is required for the induction of skeletal muscle atrophy. Am J Physiol Cell Physiol 301: C995–C1007, 2011 [DOI] [PubMed] [Google Scholar]
- 59. Voisin L, Breuillé D, Combaret L, Pouyet C, Taillandier D, Aurousseau E, Obled C, Attaix D. Muscle wasting in a rat model of long-lasting sepsis results from the activation of lysosomal, Ca2+ -activated, and ubiquitin-proteasome proteolytic pathways. J Clin Invest 97: 1610–1617, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Waddell D, Baehr L, Van Den Brandt J, Johnsen S, Reichardt H, Furlow J, Bodine S. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol Endocrinol Metab 295: E785–E797, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang H, Kubica N, Ellisen LW, Jefferson LS, Kimball SR. Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J Biol Chem 281: 39128–39134, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O'Brien CA, Thostenson J, Roberson PK, Boskey AL, Clemens TL, Manolagas SC. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell 9: 147–161, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wing SS, Haas AL, Goldberg AL. Increase in ubiquitin-protein conjugates concomitant with the increase in proteolysis in rat skeletal muscle during starvation and atrophy denervation. Biochem J 307: 639–645, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhao W, Qin W, Pan J, Wu Y, Bauman WA, Cardozo C. Dependence of dexamethasone-induced Akt/FOXO1 signaling, upregulation of MAFbx, and protein catabolism upon the glucocorticoid receptor. Biochem Biophys Res Commun 378: 668–672, 2009 [DOI] [PubMed] [Google Scholar]