Abstract
The peptide hormone ghrelin is released from a distinct group of gastrointestinal cells in response to caloric restriction, whereas its levels fall after eating. The mechanisms by which ghrelin secretion is regulated remain largely unknown. Here, we have used primary cultures of mouse gastric mucosal cells to investigate ghrelin secretion, with an emphasis on the role of glucose. Ghrelin secretion from these cells upon exposure to different d-glucose concentrations, the glucose antimetabolite 2-deoxy-d-glucose, and other potential secretagogues was assessed. The expression profile of proteins involved in glucose transport, metabolism, and utilization within highly enriched pools of mouse ghrelin cells and within cultured ghrelinoma cells was also determined. Ghrelin release negatively correlated with d-glucose concentration. Insulin blocked ghrelin release, but only in a low d-glucose environment. 2-Deoxy-d-glucose prevented the inhibitory effect of high d-glucose exposure on ghrelin release. mRNAs encoding several facilitative glucose transporters, hexokinases, the ATP-sensitive potassium channel subunit Kir6.2, and sulfonylurea type 1 receptor were expressed highly within ghrelin cells, although neither tolbutamide nor diazoxide exerted direct effects on ghrelin secretion. These findings suggest that direct exposure of ghrelin cells to low ambient d-glucose stimulates ghrelin release, whereas high d-glucose and glucose metabolism within ghrelin cells block ghrelin release. Also, low d-glucose sensitizes ghrelin cells to insulin. Various glucose transporters, channels, and enzymes that mediate glucose responsiveness in other cell types may contribute to the ghrelin cell machinery involved in regulating ghrelin secretion under these different glucose environments, although their exact roles in ghrelin release remain uncertain.
Keywords: secretion
the peptide hormone ghrelin is the endogenous ligand of the growth hormone secretagogue receptor (GHSR) and is named for its ability to stimulate growth hormone release (32, 59). Ghrelin also regulates gastrointestinal motility, chronic stress-induced mood-related behaviors, and alcohol-seeking behaviors, among many other actions (1, 13, 15, 18, 31, 32, 34, 37). Perhaps best studied are ghrelin's actions in signaling and responding to states of energy insufficiency. Regarding its role in signaling energy-insufficient states, ghrelin levels are known to rise prior to set meals, following food deprivation, and after weight loss linked to exercise, cachexia, and anorexia nervosa (9, 10, 33, 39, 42, 45, 55, 57, 60). Several lines of evidence suggest that the rise in plasma ghrelin upon caloric restriction is likely related, at least in part, to binding of norepinephrine released from the sympathetic nervous system to β1-adrenergic receptors embedded in the plasma membranes of ghrelin cells (14, 28, 41, 63). Regarding ghrelin's role in responding to energy-insufficient states, infusions of ghrelin or GHSR agonists increase body weight via proorexigenic actions and/or decreases in energy expenditure (1, 43, 53, 59, 61). Ghrelin shifts fuel preference away from metabolic utilization of fat as an energy source and increases the expression of fat storage-promoting enzymes (51, 56, 59). Also, ghrelin plays an obligatory role in mediating various hedonic components of eating (4). Of note, ghrelin's orexigenic actions seemingly help reverse the rises in ghrelin induced originally by energy insufficiency, as evidenced by a lowering of its circulating levels following a meal (10, 11). In lean humans, studies with isocaloric drinks have demonstrated that ingested proteins are most effective in lowering ghrelin, whereas ingested lipids are least effective; carbohydrates result in the largest initial drop and also a subsequent rebound above preprandial levels (20). It can be postulated that ghrelin secretion might be regulated by nutrients acting directly at the level of the ghrelin cell.
Here, we have focused on the mediation of ghrelin secretion by the interaction of d-glucose directly with the ghrelin cell. This was achieved by establishing a model in which pools of dispersed mouse gastric mucosal cells, containing both ghrelin cells and nonghrelin cells, were grown in primary culture. The isolation and culture protocol offered by this in vitro system disrupts the usual gastric mucosal cell-cell interactions and thus facilitates the identification of direct ghrelin secretagogues and conditions linked to modulation of ghrelin secretion.
MATERIALS AND METHODS
Animals.
Male C57Bl6/J mice aged 8–12 wk fed a standard chow diet (Teklad Global 16% Protein Rodent Diet no. 2016; Harland Laboratory, Madison, WI) were used for harvesting cells. Animal studies were approved by the Institutional Animal Care and Use Committees at University of Texas Southwestern Medical Center and Saitama University.
Isolation of gastric mucosal cells.
The following isolation protocol was reported previously, albeit in less detail (48, 50). Following terminal anesthetization of mice using chloral hydrate (700 mg/kg body wt ip), the stomachs were exposed, and surgical suture was used to tie off the lumen of the stomach proximally from the esophagus and distally from the duodenum. The stomachs were quickly excised such that the sutures remained attached to the stomachs and were placed in cold PBS (Gibco, Gaithersburg, MD). A small, ∼5-mm-long incision was placed in the nonglandular forestomach, and the stomach was emptied of the bulk of any ingested food matter by gently pushing the food out through the incision using a blunt forceps. The stomach was turned inside out by pushing the distal part of the stomach through the incision in the forestomach. Next, a blunt 20-gauge metal needle attached to a syringe was advanced into the inside-out stomach through the forestomach incision, and a piece of suture was used to tighten the incisional opening against the needle shaft. Cold DMEM with no glucose (Gibco) was injected into the stomach to inflate it, after which the needle was removed and the incisional opening completely tied off using the previously placed suture. The procedure from time of anesthetization to inflation of the stomach took 15–20 min, and each animal was done sequentially. The inflated, inside-out stomachs were placed temporarily in DMEM with no glucose on ice while awaiting the isolation of all the specimens. In our hands, the remaining steps of the isolation protocol described below were delayed another 20 min while these specimens were transported to the next location.
Next, the stomachs were removed from the cold DMEM and gently and rapidly brushed with a Kimwipe to remove any residual food particles. Our preparations for the ghrelin secretion studies (see below) were done using three stomachs, and all of them were placed into a single 50-ml conical tube containing 5 ml of digestion solution [2.5 mg of Dispase II (Roche Diagnostics, Indianapolis, IN) per 1 ml of PBS] per stomach [our preparations for fluorescent-activated cell sorting (FACS) separation were done using 3–5 stomachs, with the 4th or 4th and 5th stomachs being placed in a second 50-ml conical tube containing another 5 ml of digestion solution per stomach]. The tube was then incubated in a 37°C water bath for 90 min. The mucosal layer of each stomach was isolated by securing the forestomach with a forceps such that the stomach was held against the inside lip of a 100-ml glass beaker containing 10 ml of room-temperature DMEM-F-12 (which happened to contain 17.49 mM d-glucose; Mediatech, Manassas, VA) supplemented with 10% (vol/vol) FBS (Atlanta Biologicals, Lawrenceville, GA) and 100 U/ml penicillin plus 100 μg/ml streptomycin sulfate (Gibco), and then a polyethylene transfer pipet (13-711-7M; Fisher Scientific, Pittsburgh, PA) was used to squirt some of the media over the sides of the stomach; while squirting, the transfer pipet was also scraped against the stomach as a further means to mechanically release the mucosal cells into the media. The cell suspension for each stomach was transferred to a 15-ml conical tube (1 tube for the cell suspension from each stomach), after which the tubes were centrifuged at 1,200 rpm for 3 min. For each tube, the supernatant was removed with a polyethylene transfer pipet, and the remaining cellular pellet was resuspended with another transfer pipet in 2.5 ml of 0.25% trypsin-EDTA (Gibco), followed by incubation in a 37°C water bath for 5 min. To inactivate the trypsin, 10 ml of DMEM-F-12 supplemented with 10% FBS was added, after which the cells were again resuspended and then collected by filtering through a 100-μm nylon mesh (Cell Strainer, 352360; BD Falcon, Bedford, MA). The filtered cells were centrifuged at 1,200 rpm for 3 min, the supernatants were removed, and then the cellular pellets from all three stomachs were resuspended in a total of 5 ml of DMEM-F-12 supplemented with 10% FBS, 100 U/ml penicillin plus 100 μg/ml streptomycin, and 50 μM octanoate-BSA [note that the octanoate-BSA conjugate was prepared as a 10-mM stock solution by dissolving 16.62 mg of sodium octanoate (Sigma, St. Louis, MO) in 6.67 ml of 0.9% NaCl (Sigma) and adding 3.33 ml of fatty acid-free BSA (Sigma)]. The number and concentration of isolated cells were determined using a hemocytometer. On average, we were able to isolate 2 × 106 cells from each stomach. The same medium was used to dilute the cells to a final concentration of 1 × 105 cells/ml.
Ghrelin secretion studies.
Studies were run on isolated gastric mucosal cells from a total of three C57BL6/J mice at a time since greater variation was observed when cells were isolated from more than three mice at a time, suggesting that extended isolation time impairs cell recovery and function. The cells were placed into the wells of poly-l-lysine-coated 24-well plates (1 × 105 cells/well in 1 ml of medium) and incubated in humidified 95% air and 5% CO2 at 37°C. Upon initial plating, most of the mixed population of cells was not in contact with one another, with the exception of the occasional doublets. For the Fig. 1 studies, after a 16- to 18-h incubation, the medium was aspirated from the cells and replaced with serum-free DMEM containing 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, 50 μM octanoate-BSA, and 5 mM glucose with or without one of the following reagents: epinephrine (10 μM; Sigma), norepinephrine (10 μM; Sigma), insulin (100 nM; Sigma), somatostatin (100 nM; Phoenix Pharmaceuticals, Belmont, CA), secretin (100 nM; Phoenix Pharmaceuticals), endothelin-1 (100 nM; Peptides International, Louisville, KY), or PMA (20 μM; Tocris Bioscience, Minneapolis, MN). The cells were exposed to these potential secretagogues for 6 h in humidified 95% air and 5% CO2 at 37°C. For the Fig. 2 and Fig. 3 studies, which examined the effects of d-glucose, the plated cells were treated similarly after the 16- to 18-h incubation for 6 h in serum-free DMEM containing 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, 50 μM octanoate-BSA, and the stated concentrations of d-glucose, l-glucose (Sigma), norepinephrine, insulin, 2-deoxy-d-glucose (Sigma), tolbutamide (Sigma), and diazoxide (Sigma). Stock solutions of tolbutamide and diazoxide were prepared in DMSO, resulting in DMSO concentrations within the media of 0.05 (vol/vol) and 0.025% (vol/vol), respectively; 0.05% (vol/vol) DMSO was also included in the appropriate control wells for Fig. 3, D and E. Following the 6-h incubations, the medium was immediately collected and centrifuged at 3,000 rpm for 5 min. To stabilize acyl ghrelin, the supernatants had 1 N HCl added to achieve a final HCl concentration of 0.1 N and were stored at −80°C.
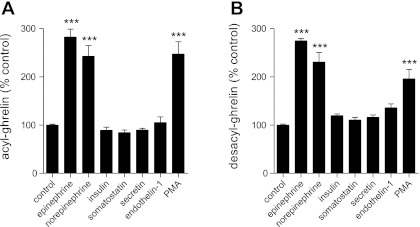
Fig. 1.
Secretion of ghrelin upon exposure of primary cultures of dispersed mouse gastric mucosal cells to peptide hormones and other compounds. Levels of acyl ghrelin (A) and desacyl ghrelin (B) released into the culture medium (DMEM; 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, 50 μM octanoate-BSA, and 5 mM glucose) upon 6-h incubation of dispersed mouse gastric mucosal cells in medium alone (control) or medium containing epinephrine (10 μM), norepinephrine (10 μM), insulin (100 nM), somatostatin (100 nM), secretin (100 nM), endothelin-1 (100 nM), and PMA (20 μM). Each bar represents the ghrelin level in the medium relative to that observed under the control condition (A: 462.6 ± 9.2 pg/ml; B: 1,995.4 ± 45.7 pg/ml) and is shown as the mean ± SE for 3 wells containing 1 × 105 cells/well in 500 μl of medium. ***P < 0.001, level of statistical significance.
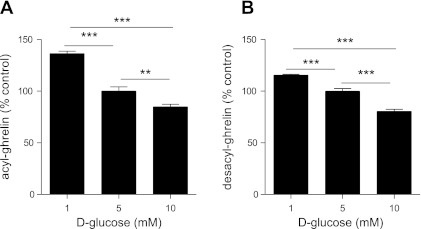
Fig. 2.
Effect of glucose on secretion of ghrelin from primary cultures of dispersed mouse gastric mucosal cells. Levels of acyl ghrelin (A) and desacyl ghrelin (B) released into the culture medium upon 6-h incubation of dispersed mouse gastric mucosal cells in medium (DMEM; 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, and 50 μM octanoate-BSA) containing 1, 5, and 10 mM d-glucose. Each bar represents the ghrelin level in the medium relative to that observed upon incubation in 5 mM d-glucose (A: 789.1 ± 48.1 pg/ml; B: 1,919.8 ± 83.3 pg/ml) and is shown as the mean ± SE for 4 wells containing 1 × 105 cells/well in 500 μl of medium. **P < 0.01 and ***P < 0.001, level of statistical significance.
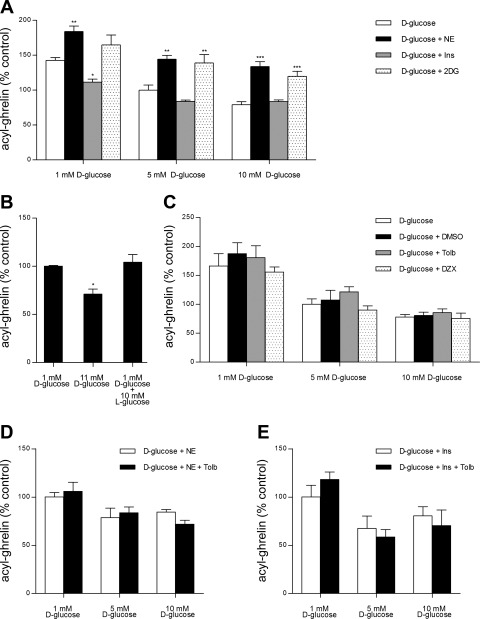
Fig. 3.
Impact of ambient d-glucose concentration on the ghrelin secretagogic effects of various reagents. Levels of acyl ghrelin released into the culture medium upon 6-h incubation of dispersed mouse gastric mucosal cells in medium (DMEM; 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, and 50 μM octanoate-BSA) containing various concentrations of d-glucose ± 10 μM norepinephrine (NE), 100 nM insulin (Ins), or 10 mM 2-deoxy-d-glucose (2DG) (A), 10 mM l-glucose (B), 0.05% (vol/vol) DMSO, 500 μM tolbutamide (Tolb), or 100 μM diazoxide (DZX) (C), 10 μM NE ± 500 μM Tolb (D), and 100 nM Ins ± 500 μM Tolb (E). In A, the patterned bars in each set represent the ghrelin levels in the medium relative to that observed upon incubation in 1 (636.5 ± 37.0 pg/ml), 5 (444.7 ± 34.8 pg/ml), or 10 mM d-glucose (348.9 ± 10.1 pg/ml) alone (open bars) and are shown as means ± SE for 2 different cell preparations each containing 3 wells. In B, each bar represents the ghrelin level in the medium relative to that observed upon incubation in 1 mM d-glucose (853.8 ± 6.9 pg/ml) and is shown as the mean ± SE for 4 wells. In C, the patterned bars in each set represent the ghrelin levels in the medium relative to that observed upon incubation in 1 (624.2 ± 95.7 pg/ml), 5 (376.3 ± 51.7 pg/ml), or 10 mM d-glucose (313.7 ± 35.2 pg/ml) alone (open bars) and are shown as means ± SE for 2 different cell preparations each containing 2–3 wells. In D and E, each bar represents the ghrelin level in the medium relative to that observed upon incubation in 1 mM d-glucose + 10 μM NE (884.4 ± 63.7 pg/ml) and 1 mM d-glucose + 100 nM Ins (378.0 ± 86.0 pg/ml), respectively, and is shown as the mean ± SE for 2 different cell preparations each containing 2–3 wells. Wells for all these studies contained 1 × 105 cells/well in 500 μl of medium. Asterisks in A denote the level of statistical significance relative to the open bar of the set, and asterisks in B denote the significant effect of the condition noted. *P < 0.05, **P < 0.01, and ***P < 0.001.
Of note, it is customary when preparing plasma for subsequent assay of acyl ghrelin to add a protease inhibitor such as PHMB (p-hydroxymercuribenzoate) prior to the HCl stabilization step as another step to stabilize the acyl group (26, 35, 58). To test the requirement for the protease inhibitor with the current cell culture-based system, pilot secretion studies were performed as described above in serum-free DMEM medium (containing penicillin, streptomycin, and octanoate-BSA) plus 1, 5, or 10 mM d-glucose (as described above) with or without 0.05% DMSO. Each condition was run four times in each of two separate trials. After the usual 6-h incubation, a stock solution of PHMB was added to the supernatants from one-half of the wells (to achieve a final concentration of 1 mM) prior to the HCl stabilization step. PHMB had no statistically significant effect on assayed acyl ghrelin levels, and thus for all subsequent trials, PHMB was not used.
Measurement of ghrelin levels.
Ghrelin levels were determined using rat acylated ghrelin (no. 10006307) and rat unacylated ghrelin (no. 10008953) ELISA kits (Cayman Chemical, Ann Arbor, MI). Absorbance data were collected via a BioTek spectrophotometer, using the KC Junior program (BioTek Instruments, Winooski, VT).
Isolation of ghrelin cell-enriched and non-ghrelin cell-enriched pools of gastric mucosal cells using ghrelin-humanized Renilla reniformis green fluorescent protein transgenic mice.
Gastric mucosal cells were isolated from ghrelin-humanized Renilla reniformis green fluorescent protein (hrGFP) reporter mice (hrGFP10) (48), as described above. After being washed in PBS, cells were resuspended in FACS buffer (Hanks' balanced salt solution containing 3% fetal bovine serum, 0.5 mM EDTA, 0.1% BSA, 10 U/ml DNase I, and 20 mg/ml glucose). The cells were sorted with a DakoCytomation MoFlo (Dako, Carpinteria, CA) at the University of Texas Southwestern Flow Cytometry Multi-User Core Facility on the basis of size, complexity, and intensity of GFP fluorescence (at 530 nm) and fluorescence at 585 nm. Three independent preparations (3–5 mice were used for each independent preparation) were included in the subsequent analyses.
RNA extraction and quantitative real-time polymerase chain reaction.
The hrGFP-positive pools and the hrGFP-negative pools were adjusted to contain a matched number of cells (6,500, 10,000, and 12,500 cells for preparations 1, 2, and 3, respectively). The cells in each pool were collected by centrifugation at 4°C at 3,000 rpm for 10 min. Total RNA was extracted from these cellular pellets using a standard guanidium thiocyanate-phenol-chloroform extraction protocol after the addition of RNA STAT-60 (Tel-Test, Friendswood, TX). Total RNA was also extracted from two different mouse ghrelinoma cell lines, SG-1 and PG-1 (63), using RNA STAT-60. Concentration and relative purity of the RNA were determined using a Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA), and it was stored at −80°C until use. Complementary DNA was synthesized for each individual sample using Superscript III reverse transcriptase (Life Technologies, Grand Island, NY). Quantitative PCR was performed using the iTaq SYBR Supermix (Bio-Rad Laboratories, Hercules, CA) and an ABI 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA). Initial template denaturation (3 min at 95°C) was performed, followed by 40 cycles of denaturation (15 s at 95°C), annealing, and extension (45 s at 60°C). Reactions were evaluated by the comparative threshold cycle (CT) method, using cyclophilin as the invariant control gene. Such was done separately for each of the three different hrGFP-positive pools, each of the three different hrGFP-negative pools, and each of the preparations of SG-1 and PG-1 cells. Previously, we have reported comparing the CT values of several genes amplified from FACS-separated gastric mucosal cells with the CT values of a separate housekeeping gene, 36B4, and observed results similar to those determined with cyclophilin (50). Sequences of primers are listed in Table 1. Primers used (Table 1) were as published previously [ghrelin, cyclophilin, and ghrelin O-acyltransferase (48, 50)], newly designed [somatostatin receptor (SSTR)2, SSTR5, secretin receptor, GLUT2, hexokinase 1, hexokinase 2, and hexokinase 3], or gifts from Joyce Repa (University of Texas Southwestern Medical Center). The newly designed primers were designed using Primer Express Software (Life Technologies). These primer pairs were further tested for specificity to the gene of interest by analysis using the Basic Local Alignment Search Tool and Primer-BLAST (National Center for Biotechnology Information). Only those primer pairs for which neither of the two primers bound with 100% specificity to the gene of interest, did not bind to another gene of interest, and/or did not amplify another random DNA fragment <1 kb in size were used. The efficiencies of the primers were validated by verifying a slope of approximately −3.3 when the logs of the cDNA concentration at different serial dilutions were compared with the CT (cDNA dilutions ranged from 50 to 0.016 ng of cDNA). The primers were designed to amplify regions of cDNA that in the corresponding genomic DNA span introns to further ensure the amplification of cDNA derived from mRNA rather than residual genomic DNA.
Table 1.
Quantitative real-time PCR primer sequences
| Mouse Gene | Sequences of Primer Sets |
|---|---|
| Ghrelin | 5′-GTCCTCACCACCAAGACCAT-3′ |
| 5′-TGGCTTCTTGGATTCCTTTC-3′ | |
| GOAT | 5′-TCCACAGCCTGGCTCTTTAAAC-3′ |
| 5′-GCCGCGTGGAGGAGAGA-3′ | |
| Insulin receptor | 5′-CGAGTGCCCGTCTGGCTATA-3′ |
| 5′-GGCAGGGTCCCAGACATG-3′ | |
| SSTR1 | 5′-GCTACGCCAAGATGAAGACCG-3′ |
| 5′-GCTCATCAGCAATAGCCAGGTTT-3′ | |
| SSTR2 | 5′-ACCCCAGCCCTGAAAGG-3′ |
| 5′-CGCAGCTGTTGGCATAGGT-3′ | |
| SSTR3 | 5′-CTACGGCTTCCTCTCCTACCG-3′ |
| 5′-CGACGTGATGGTCTTAGCAGGA-3′ | |
| SSTR4 | 5′-ACTGGAGGTGCTGAGGAAGA-3′ |
| 5′-TCTTGGTGAAAGGGACTTGC-3′ | |
| SSTR5 | 5′-TGGCTGACGTGTTGTTTATGTTG-3′ |
| 5′-ACCAGGCGGCACAAGAAG-3′ | |
| Secretin receptor | 5′-TACTCTTCCCCGCAGATGAC-3′ |
| 5′-AGATAGAGGCCCTCCACCAG-3′ | |
| Endothelin receptor A | 5′-GCGGATCGCCCTTAGTGA-3′ |
| 5′-GATGACAACCAAGCAGAAGACAGT-3′ | |
| Endothelin receptor B | 5′-CTGCGAAATGCTCAGGAAGAA-3′ |
| 5′-CGAGGACCAGGCAGAAGACT-3′ | |
| PKCα | 5′-GAAACTCACAGACTTCAACTT-3′ |
| 5′-ATCTTGATGGCGTACAGTTC-3′ | |
| PKCβ | 5′-AATGGCAACAGGGACCGGAT-3′ |
| 5′-TACCCTTCCGCTCTGAGAGC-3′ | |
| PKCγ | 5′-TGGCCGATGCTGACAACTGC-3′ |
| 5′-TGGGGGATGGAGAAGGGATG-3′ | |
| GLUT1 | 5′-CGTCGTTGGCATCCTTATTG-3′ |
| 5′-GAGGCCACAAGTCTGCATTG-3′ | |
| GLUT2 | 5′-TCCAACCACACTCAGGGGTG-3′ |
| 5′-AAGCTGAGGCCAGCAATCTG-3′ | |
| GLUT3 | 5′-TGTTGGACTCTTTGTCAACCG-3′ |
| 5′-GGCCAGCAAGTTGACTAGAAGC-3′ | |
| GLUT4 | 5′-CCTTTCTCATTGGCATCATTTC-3′ |
| 5′-CACGGCCAAGACATTGTTG-3′ | |
| GLUT5 | 5′-GGGCCGTCAATGTGTTCAT-3′ |
| 5′-CCGACGAGGAGTAGGAATCG-3′ | |
| Glucokinase | 5′-CCGTGATCCGGGAAGAGAA-3′ |
| 5′-GGGAAACCTGACAGGGATGAG-3′ | |
| Hexokinase 1 | 5′-CGAGCCTAGATTGCGGAATC-3′ |
| 5′-AAATTCCTCTCTCCTTTTTACAGCAT-3′ | |
| Hexokinase 2 | 5′-GTCTCGGATATTGAAGACGATAAGGA-3′ |
| 5′-GCGCGTGGACACAATCTG-3′ | |
| Hexokinase 3 | 5′-CTGACAGTGTCTGTGGGAGTAGATG-3′ |
| 5′-GGCTAGCTTCCGGACTGTTG-3′ | |
| Kir6.2 | 5′-GCAGAGCCCAGGTACCGTACT-3′ |
| 5′-CGTTGCAGTTGCCTTTCTTG-3′ | |
| SUR1 | 5′-TCTCGCCTTTTTCCGAATG-3′ |
| 5′-TGTCGATGCCATCAATGATA-3′ | |
| Cyclophilin | 5′-TGGAGAGCACCAAGACAGACA-3′ |
| 5′-TGCCGGAGTCGACAATGAT-3′ |
GOAT, ghehlin O-acyltransferase; SSTR, somatostatin receptor; GLUT, glucose transporter; SUR1, sulfonylurea type 1 receptor.
Statistical analysis.
Data are expressed as means ± SE. GraphPad Prism 5 software (GraphPad Software, San Diego, CA) was used to perform all statistical analyses. One-way anaylsis of variance (ANOVA) followed by Dunnett's post hoc test was used to assess the effects on desacyl ghrelin and/or acyl ghrelin secretion of various reagents (all in a single d-glucose environment; Fig. 1), d-glucose concentration (Fig. 2), norepinephrine, insulin, and 2-deoxy-d-glucose for each of three different d-glucose concentrations (Fig. 3A), and d-glucose concentration ± l-glucose (Fig. 3B) or DMSO, tolbutamide, and diazoxide for each of three different d-glucose concentrations (Fig. 3C). Two-way ANOVA was used to assess the effects of d-glucose concentration and tolbutamide (and the interaction between them) on norepinephrine-stimulated acyl ghrelin levels (Fig. 3D) and on insulin-influenced acyl ghrelin levels (Fig. 3E). For the quantitative real-time polymerase chain reaction (qPCR) studies, we determined the mean ± SE for each of the tissue types and then used a one-way ANOVA followed by Dunnett's post hoc test to determine statistical significance. P < 0.05 was considered statistically significant.
RESULTS
Validation of dispersed mouse gastric mucosal cell primary culture model.
In the current study, we examined ghrelin secretion from preparations of dispersed mouse gastric mucosal cells grown for a brief time in primary culture. Here, cells comprising the gastric mucosa of adult mice were enzymatically and mechanically separated from the stomach, dispersed such that they were no longer in contact with one another, and then studied in primary culture. Approximately 0.3 to 1% of this mixed population of gastric mucosal cells is thought to be ghrelin cells (48). A similar isolation protocol and primary cell culture system was described previously for rat stomach cells, although in that system, the isolated cells were first exposed to Percoll centrifugation to enrich for ghrelin cells prior to plating (49). Previously, we also used an identical isolation protocol to prepare gastric mucosal cells from ghrelin-hrGFP (humanized Renilla reniformis green fluorescent protein) transgenic reporter mice for FACS to generate highly enriched pools of ghrelin cells (see more below) (48). Of note, despite several manipulations to the protocol, the survivability of the hrGFP-positive gastric mucosal cells was shown to drop significantly following their enrichment by FACS analysis, thus precluding studies of these enriched pools in primary culture.
To confirm the survivability and functionality of the ghrelin cells within the nonsorted, dispersed gastric mucosal cell primary culture model used here, we first treated the cells with two adrenergic agonists, epinephrine and norepinephrine. Previous studies have consistently demonstrated a ghrelin secretory action for epinephrine and norepinephrine. For instance, in vivo studies in rats and mice have shown induction of ghrelin secretion upon stimulation of the sympathetic nervous system (41, 63). Both of these compounds also potently stimulate ghrelin release when infused via microdialysis probes into the gastric mucosa of rats (14) and when added to the culture media of immortalized ghrelinoma cell lines (28, 63). These data, along with the finding of high levels of β1-adrenergic receptors on ghrelin cells isolated from ghrelin-hrGFP reporter mice, suggest that these catecholaminergic agents may act directly on ghrelin cells to stimulate ghrelin release (63). Here, both epinephrine and norepinephrine (10 μM) potently stimulated ghrelin release from the dispersed gastric mucosal cell primary culture system (Fig. 1), thus confirming this system as a valid model with which to investigate ghrelin secretion.
Regulation of ghrelin secretion by other peptide hormones and compounds.
We also investigated the effects of the peptide hormones insulin, somatostatin, secretin, and endothelin-1 on ghrelin release using the dispersed gastric mucosal cell primary culture model. These hormones were studied given previous work suggesting that they play a role in ghrelin secretion (see below). When cells were incubated in the presence of 5 mM d-glucose, none of the mean acyl ghrelin levels that accumulated in the culture media in response to 100 nM insulin (413.1 ± 29.3 pg/ml), 100 nM somatostatin (390.3 ± 24.7 pg/ml), 100 nM secretin (414.8 ± 17.3 pg/ml), or 100 nM endothelin-1 (487.4 ± 53.8 pg/ml) was statistically different from that released under basal conditions (462.6 ± 9.2 pg/ml) when assessed using 1-way ANOVA (Fig. 1A). Nor did any of these hormones affect levels of desacyl ghrelin that accumulated in the culture media (Fig. 1B).
Effects of the phorbol ester PMA were also examined. This compound directly alters the activity of key modulators downstream of G protein-coupled and other plasma membrane-bound receptor signaling cascades, which in other systems affect secretory activity. PMA and other phorbol esters bind to the C1b regulatory domain of members of the conventional protein kinase C family, including PKCα, PKCβ, and PKCγ, and activate those PKC isozymes, thus bypassing their normal activation by binding of diacylglycerol generated by phospholipase C (7). Phorbol esters also bind members of the novel PKC and RasGRP families. Here, 20 μM PMA potently increased both acyl ghrelin and desacyl ghrelin in the culture medium (Fig. 1).
mRNA expression profiles of ghrelin cells.
Since the dispersed gastric mucosal cell primary culture model consists primarily of nonghrelin cells in addition to ghrelin cells, we next sought to determine whether highly purified populations of ghrelin cells express the receptors and molecular targets for the peptide hormones and compound tested. We prepared highly enriched pools of gastric ghrelin cells by taking advantage of the hrGFP reporter present in a previously described line of ghrelin hrGFP transgenic mice (hrGFP10 line) (48). qPCR was performed on mRNAs isolated from both the hrGFP-positive (ghrelin cell-enriched) and the hrGFP-negative (non-ghrelin cell-enriched) pools, and the average levels of the mRNA species of interest relative to that of the housekeeping gene cyclophilin were determined. Previously, this method has been used to confirm expression within ghrelin cells of prohormone convertases 1/3 and 2 (48), ghrelin O-acyltransferase (50), and β1-adrenergic receptor (63). Similar analyses were done using mouse ghrelinoma cell lines SG-1 and PG-1 established previously from mice bearing ghrelinomas induced by a tissue-specific SV40 T-antigen transgene (63).
To confirm adequacy of the FACS separation, we first assessed levels of ghrelin and ghrelin O-acyltransferase within each pool. Messenger RNAs for ghrelin and ghrelin O-acyltransferase were significantly higher within the hrGFP-positive pools, as expected (Table 2).
Table 2.
Relative levels of mRNAs in FACS-separated gastric mucosal cells from hrGFP mice and in 2 ghrelinoma cell lines
| FACS-Separated Gastric Mucosal Cells |
Ghrelinoma Cell Lines |
|||
|---|---|---|---|---|
| mRNA | Non-ghrelin cell-enriched (hrGFP-negative) pools | Ghrelin cell-enriched (hrGFP-positive) pools | SG-1 | PG-1 |
| Ghrelin | 1.0 (28.9 ± 0.6)# | 45,529 ± 2,635* | 533 ± 34.6 | 1,086 ± 180 |
| GOAT | 1.0 (33.7. ±0.2) | 691 ± 216* | 140 ± 16.6 | 99.4 ± 6.0 |
| Insulin receptor | 1.0 (29.5 ± 0.3) | 4.50 ± 1.71 | 4.1 ± 0.7 | 8.5 ± 2.2* |
| SSTR1 | 1.0 (33.1 ± 0.2) | 32.1 ± 11.8* | 4.0 ± 0.5 | 2.2 ± 1.3 |
| SSTR2 | 1.0 (30.7 ± 0.5) | 2.8 ± 0.9 | 1.3 ± 0.2 | 2.9 ± 1.2 |
| SSTR3 | 1.0 (34.0 ± 0.9) | 155.5 ± 31.8* | 3.8 ± 0.9 | 26.1 ± 0.5 |
| SSTR4 | Not detected† | Not detected† | (29.42 ± 0.34)‡ | (29.42 ± 0.17)‡ |
| SSTR5 | 1.0 (33.4 ± 0.4) | 3.5 ± 0.7* | 0.8 ± 0.4 | 0.2 ± 0.0 |
| Secretin receptor | 1.0 (29.0 ± 0.6) | 1.4 ± 0.6* | 0.004 ± 0.001* | 0.007 ± 0.003* |
| Endothelin receptor A | 1.0 (33.5 ± 0.4) | 2.9 ± 0.3* | 0.4 ± 0.1 | 0.2 ± 0.07 |
| Endothelin receptor B | Not detected† | Not detected† | Not detected† | (33.5 ± 0.2)‡ |
| PKCα | 1.0 (33.7 ± 0.2) | 1.4 ± 0.4 | 2.2 ± 0.2 | 4.3 ± 1.8* |
| PKCβ | 1.0 (26.3 ± 0.7) | 1.0 ± 0.3 | Not detected† | 0.070 ± 0.003 |
| PKCγ | Not detected† | Not detected† | Not detected† | Not detected† |
| GLUT1 | 1.0 (26.6 ± 0.5) | 1.6 ± 0.3 | 1.4 ± 0.1 | 3.1 ± 0.3* |
| GLUT2 | Not detected† | Not detected† | Not detected† | Not detected† |
| GLUT3 | 1.0 (33.2 ± 0.4) | Not detected† | 59.6 ± 6.9* | 221 ± 11* |
| GLUT4 | 1.0 (32.8 ± 0.3) | 13.2 ± 1.2 | 87.8 ± 17.6* | 246 ± 13* |
| GLUT5 | 1.0 (31.0 ± 0.6) | 74.3 ± 11.0* | 120 ± 22.0* | 199 ± 17* |
| Glucokinase | 1.0 (31.2 ± 0.4) | 75.6 ± 15.5* | 8.4 ± 2.0 | 32.2 ± 2.7 |
| Hexokinase 1 | 1.0 (27.3 ± 0.5) | 3.5 ± 0.0 | 13.9 ± 1.4* | 8.5 ± 0.8* |
| Hexokinase 2 | 1.0 (28.5 ± 0.7) | 1.2 ± 0.1 | 3.1 ± 0.3* | 1.5 ± 0.6 |
| Hexokinase 3 | Not detected† | Not detected† | (29.2 ± 0.6)‡ | (32.9 ± 0.3)‡ |
| Kir6.2 | 1.0 (31.6 ± 0.7) | 106 ± 4* | 129 ± 25* | 144 ± 10* |
| SUR1 | 1.0 (31.9 ± 0.5) | 104 ± 24 | 187 ± 33* | 326 ± 31* |
Values are means ± SE.
FACS, fluorescent-activated cell sorting; hrGFP, humanized Renilla reniformis green fluorescemt protein.
Ghrelin cell-enriched (hrGFP-positive) and non-ghrelin cell-enriched (hrGFP-negative) gastric mucosal cells were FACS separated, and mRNAs from these pools and from SG-1 and PG-1 ghrelinoma cell lines were extracted and quantified by quantitative real-time PCR, as described in materials and methods. Cyclophilin mRNA was used as an invariant control. Each value represents the amount of mRNA relative to that in the hrGFP-negative pool, which is arbitrarily defined at 1.0 and is shown as the means ± SE of 3 different preparations. Each determination was done in duplicate. #Values in parentheses denote the mean ± SE of threshold cycle values.
Level significantly different from non-ghrelin cell-enriched pools (P < 0.05), as determined by 1-way ANOVA followed by Dunnett's post hoc analysis.
Threshold cycle value ≥35.
In instances when mRNA was not detected in the hrGFP-negative pools but was detected in 1 or more of the other cell types (thereby precluding a relative comparison with the levels observed in the hrGFP-negative pools), the threshold cycle values for the other cell types are listed in parentheses.
Insulin receptor mRNA was observed in both the hrGFP-negative pools and the ghrelin cell-enriched pools (with higher levels observed in the latter) as well as in both ghrelinoma cell lines. Expression of five different SSTRs was also examined. Marked elevations in SSTR1 and SSTR3 mRNA were observed in the hrGFP-positive pools compared with the hrGFP-negative pools. SSTR2 and SSTR5 were increased slightly within the hrGFP-positive pools, whereas SSTR4 was not detected in either FACS-separated pool. All of these SSTRs were observed in the ghrelinoma cell lines. Secretin receptor mRNA was observed in both FACS-separated pools but was barely detectable in the ghrelinoma cell lines. mRNAs for entothelin receptors A and B were barely detected or not detected in the FACS-separated cells and the ghrelinoma cells. mRNAs encoding PKCα and PKCβ were found in both FACS-separated pools and both ghrelinoma cell lines, although PKCβ levels in the ghrelinoma cell lines were much lower than in the hrGFP-positive pools; PKCγ mRNA was not detected in any sample.
Effects of glucose on ghrelin release.
We next investigated the effects of exposing the primary cultures to different concentrations of d-glucose. Compared with levels of acyl ghrelin released into the media in an environment of 5 mM d-glucose, which is equivalent to a normal blood glucose level (90 mg/dl), increased d-glucose (10 mM, which is equivalent to 180 mg/dl) lowered the amount of acyl ghrelin released by 16% (Fig. 2A). On the other hand, when ambient d-glucose levels were lowered to 1 mM (the equivalent of 18 mg/dl), acyl ghrelin release was enhanced, resulting in a 31% increase in acyl ghrelin levels in the media (Fig. 2A). Similar effects of raising and lowering d-glucose were noted for desacyl ghrelin (Fig. 2B). These changes were statistically significant.
The effect of glucose on ghrelin release was explored further by incubating the cells with different concentrations of d-glucose together with norepinephrine, insulin, 2-deoxy-d-glucose, or l-glucose (Fig. 3, A and B). Norepinephrine (10 μM) was effective at stimulating ghrelin secretion not only in the presence of 5 mM d-glucose, as had been observed in Fig. 1, but also in the presence of lower (1 mM) and higher (10 mM) d-glucose (Fig. 3A). The d-glucose-dependent pattern of ghrelin release induced by norepinephrine seemed to mirror that observed in the absence of norepinephrine only at a higher mean level (Fig. 3A). Also, as had been observed in Fig. 1, insulin (100 nM) had no statistically significant effect on ghrelin release in the condition of 5 mM d-glucose; insulin was similarly ineffective at altering ghrelin release in the presence of 10 mM d-glucose (Fig. 3A). However, when the cells were exposed to 1 mM d-glucose, insulin did restrict ghrelin release in a statistically significant manner (Fig. 3A). The glucoprivic d-glucose analog 2-deoxy-d-glucose, which is taken up into cells where it subsequently blocks d-glucose metabolism, was also tested. The addition of 2-deoxy-d-glucose (10 mM) to culture medium containing 10 mM d-glucose blocked the expected decrease in ghrelin release otherwise observed upon ambient d-glucose concentration being raised (Fig. 3A). Compared with incubations without 2-deoxy-d-glucose, the addition of 2-deoxy-d-glucose raised ghrelin secretion observed under conditions of 10 mM d-glucose and 5 mM d-glucose, whereas no statistically significant difference in ghrelin secretion was observed upon the addition of 2-deoxy-d-glucose to culture medium with 1 mM d-glucose (Fig. 3A).
The importance of d-glucose metabolism within the cells as a mechanism for d-glucose-induced inhibition of ghrelin release was suggested further upon incubation of the primary cultures with l-glucose, which is the diastereoisomer of d-glucose and does not serve as a substrate for glycolysis (Fig. 3B). In contrast to the reduction in ghrelin release observed upon incubation of the cells in 11 mM d-glucose compared with 1 mM d-glucose, the addition of 10 mM l-glucose had no effect on ghrelin release (Fig. 3B).
Mechanistic aspects of glucose-mediated changes to ghrelin release.
The enhanced ghrelin release observed in the primary culture system upon 2-deoxy-d-glucose-induced glucoprivation, which impersonates a state of low ambient d-glucose, supports the assertions that ghrelin secretion is inversely proportional to the ambient d-glucose concentrations to which ghrelin cells are directly exposed and that metabolism of d-glucose is essential to decrease ghrelin release. Thus, we next investigated the expression of various glucose metabolism-related enzymes and channels that help other cell types sense and respond to changes in d-glucose concentration, using a qPCR-based strategy.
Messenger RNAs encoding several members of the glucose transporter (GLUT) family of facilitative glucose transporters were localized to ghrelin cell-enriched (hrGFP-positive) gastric mucosal cell pools (Table 2). These included GLUT1, GLUT4, and GLUT5. GLUT1 expression was nearly as high in the hrGFP-negative pools as in the hrGFP-positive pools. GLUT2 was not detected within either pool, whereas GLUT3 seemed restricted to the non-ghrelin cell-enriched pools. Nonetheless, GLUT3 was observed at fairly high levels in both ghrelinoma cell lines. GLUT4 was higher in both ghrelinoma cell lines, and GLUT5 was higher within the hrGFP-positive pools and the ghrelinoma cell lines.
Messenger RNA encoding glucokinase, which is known to catalyze the rate-limiting step in glucose metabolism in pancreatic β-cells, was also highly enriched in the hrGFP-positive pool, as were mRNAs encoding the inwardly rectifying potassium channel Kir6.2 and the high-affinity sulfonylurea type 1 receptor (SUR1; Table 2). Expression of hexokinase 1 and hexokinase 2, which, similar to glucokinase, also phosphorylate glucose, was modest within both FACS-separated pools, whereas hexokinase 3 was not detected. Glucokinase, hexokinase 1, hexokinase 2, Kir6.2, and SUR1 also were expressed in ghrelinoma cells, as was a low level of hexokinase 3.
A complex of Kir6.2 and SUR1 comprises the well-characterized pancreatic β-cell ATP-sensitive potassium (KATP) channel (27). Within the pancreatic β-cell, this Kir6.2-SUR1 complex plays a key role in insulin secretion, whereby closure of KATP channels and the ensuing depolarization of β-cells is the major pathway by which d-glucose-induced insulin secretion, as well as sulfonyurea-induced insulin secretion, occurs (8). The opening of KATP channels is the mechanism by which the drug diazoxide works to inhibit insulin secretion in cases of congenital hyperinsulinism (25, 30). Because of the presence of both Kir6.2 and SUR1 at such high levels within the ghrelin cell-enriched pools and the ghrelinoma cell lines, we examined the effects of both the sulfonylurea tolbutamide and diazoxide on ghrelin release from the primary cell cultures (Fig. 3C). Once again, acyl ghrelin release was inversely proportional to the amount of d-glucose in the media (1 vs. 5 vs. 10 mM d-glucose). However, concentrations of tolbutamide (500 μM) and diazoxide (100 μM) shown in other in vitro studies to influence insulin release from pancreatic β-cells and/or islets (25, 40) neither enhanced nor inhibited ghrelin secretion at d-glucose concentrations simulating hypoglycemic, normoglycemic, or hyperglycemic conditions (Fig. 3C). Nor did tolbutamide significantly alter the effects of norepinephrine or insulin on ghrelin release (Fig. 3, D and E).
DISCUSSION
Here, we used primary cultures of dispersed mouse gastric mucosal cells to investigate ghrelin secretion. This model was validated by the demonstration of ghrelin secretion by epinephrine and norepinephrine, which were shown previously to have the same effect in vitro, using ghrelinoma cell lines, as well as in vivo (14, 28, 41, 63). Neither insulin, somatostatin, secretin, nor endothelin-1 had a statistically significant effect on ghrelin release from the dispersed cells upon incubation in the presence of 5 mM d-glucose. Similarly to the catecholamines used, the phorbol ester PMA, which is best characterized as an activator of several members of the protein kinase C family, markedly enhanced ghrelin secretion. Messenger RNAs encoding the receptors and direct molecular targets of these peptide hormones and compounds were found at varying levels within highly enriched pools of ghrelin cells and within two different mouse ghrelinoma cell lines at levels ranging from very high (relative to non-ghrelin cell-enriched pools of gastric mucosal cells) to barely detectable. Compared with that observed in an environment of 5 mM d-glucose, which simulates a normoglycemic state, ghrelin release from the cultured cells was inhibited by high d-glucose (10 mM) and stimulated by low d-glucose (1 mM). Metabolism of d-glucose within the cells seemed to be essential for its inhibitory effect on ghrelin release since the reductions in ghrelin levels achieved by incubation in media with both 5 and 10 mM d-glucose (compared with the higher ghrelin release observed in 1 mM d-glucose) were blocked by the glucoprivic agent 2-deoxy-d-glucose. Corroborating this latter point, l-glucose had no effect on ghrelin release. However, an inhibitory effect of insulin on ghrelin release was observed, such as that which occurred only in conditions of low d-glucose (1 mM). Messenger RNAs encoding several channels and enzymes responsible for mediating the effects of glucose on secretion in other cell types, including hexokinase 1, hexokinase 2, glucose transporters GLUT1, GLUT4, and GLUT5, glucokinase, and both components of the pancreatic β-cell KATP channel (Kir6.2 and SUR1), were all expressed highly within ghrelin cells, with the latter five genes also being highly enriched within ghrelin cells compared with other gastric mucosal cell types. Despite the presence of the KATP channel within ghrelin cells, neither the sulfonylurea agent tolbutamide (a KATP channel blocker) nor diazoxide (a KATP channel activator) exerted any apparent direct effect on ghrelin secretion.
There are several noteworthy discussion points and implications for these results. The first relates to the use of the dispersed gastric mucosal cell system to study ghrelin secretion. This model provides for gastric mucosal cells that have been physically separated from their neighbors and helps to isolate any observed effects on ghrelin secretion to direct effects on ghrelin cells. Caveats of the system include possible indirect paracrine effects of substances released into the culture medium by the other nonghrelin cells that comprise the majority of cells in culture. Possible untoward effects of the enzymatic and mechanical dispersion protocols might also influence the observed results. It is assumed that disruptive mechanical changes to the cells are magnified with FACS analysis, precluding FACS-separated ghrelin cells from ghrelin-hrGFP mice from being studied in primary culture, although mRNA expression data from highly enriched, FACS-separated pools of ghrelin cells does help provide perspective into the secretion data obtained using the primary culture system. As used here, the dispersed gastric mucosal cell system was able to confirm findings of catecholamine-induced ghrelin secretion observed in immortalized mouse ghrelinoma cell lines and using various other modalities (28, 63). Although the mouse ghrelinoma cell lines are predicted to be key tools to studying ghrelin secretion in the future, the variations in mRNA expression described here (for instance, undetectable GLUT3 in ghrelin cell-enriched pools vs. markedly elevated GLUT3 within SG-1 and PG-1 cells) expose some potential limitations of the ghrelinoma cell lines as models of normal ghrelin cell function. Similar types of differences result in altered glucose sensitivity within immortalized pancreatic β-cell lines, thereby somewhat limiting their utility as an exact replica of wild-type β-cells (12).
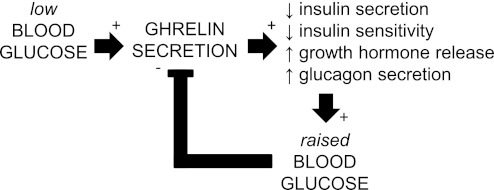
The ability of d-glucose to regulate ghrelin secretion at the level of the ghrelin cell was not necessarily unexpected, especially when viewed from the perspective of known prandial-related changes in ghrelin levels (10, 11). Perhaps more tantalizing would be to speculate on the relevance of this finding to ghrelin's important role as a regulator of blood glucose. Several studies have now demonstrated clearly that ghrelin increases blood glucose levels, likely via multiple mechanisms. Ghrelin administration to rodents dose-dependently increases fasting blood glucose, lowers insulin levels, and attenuates insulin responses during glucose tolerance testing (15, 16). Similar ghrelin-mediated effects on blood glucose and/or insulin release have been demonstrated in isolated rodent islets and pancreata, in ghrelin-overexpressing mice, and in humans (3, 6, 15, 16, 47). Ghrelin also directly stimulates glucagon secretion from pancreatic α-cells (5). Conversely, GHSR deletion lowers blood glucose, enhances insulin sensitivity, and lowers plasma glucagon (5, 36, 46, 64). Ghrelin deletion also improves glucose tolerance, whereas simultaneous deletion of both leptin and ghrelin improves the insulin-resistant phenotype characteristic of leptin deficiency (16, 17, 54). Strikingly, mice lacking ghrelin O-acyltransferase show a progressive decline in fasting blood glucose to the point of near death after only 1 wk of 60% calorie restriction (62). Administration of either acyl ghrelin or growth hormone to ghrelin O-acyltransferase-deficient mice normalizes blood glucose and prevents death under these conditions, thus suggesting an essential role for ghrelin in maintaining a minimum blood glucose level to allow the survival of severely calorie-restricted mice (62). The findings here demonstrating enhanced ghrelin secretion upon exposure of ghrelin cells to low ambient d-glucose (or simulation of a hypoglycemic state by exposure of ghrelin cells to 2-deoxy-d-glucose) and decreased ghrelin secretion upon exposure of ghrelin cells to high ambient d-glucose, together with published work describing ghrelin-induced elevations in blood glucose, suggest that ghrelin participates in blood glucose homeostatic pathways in an analogous manner to its role in body weight homeostasis. As mentioned previously, ghrelin levels rise in energy-insufficient states to stimulate food intake and fat storage, after which levels again fall. The findings here support a model by which ghrelin also both signals hypoglycemic states (its plasma levels rise) and responds to hypoglycemic states (by reducing insulin release and insulin sensitivity and by enhancing growth hormone and glucagon secretion) and then also responds appropriately upon reversal of the hypoglycemia (its plasma levels fall) (Fig. 4).
Fig. 4.
Ghrelin's roles in blood glucose control. The findings here support a model by which ghrelin both signals and responds to hypoglycemic states. Ghrelin secretion is stimulated when low blood glucose levels are sensed by ghrelin cells. Following ghrelin secretion, blood glucose is raised, at least in part, as a result of ghrelin's ability to reduce insulin release and insulin sensitivity and enhance growth hormone secretion and glucagon secretion. The raised blood glucose then feeds back upon the ghrelin cells, leading to a reduction in ghrelin secretion.
We also investigated the ability of insulin to directly interact with ghrelin cells to regulate ghrelin secretion. Such an action would be relevant not only to ghrelin's actions in signaling and responding to hypoglycemia but also its actions on eating and body weight. Previously, it has been proposed that meal-related changes in insulin might contribute to meal-related fluxes in circulating ghrelin (20). However, the results of previous studies examining the ability of insulin to directly interact with ghrelin cells to affect ghrelin release have been inconsistent. Our own group did not observe any statistically significant decrease in ghrelin release from SG-1 or PG-1 mouse ghrelinoma cell lines (63), whereas another stomach ghrelinoma cell line (MGN3-1) generated using a similar transgenic strategy did reduce its release of ghrelin in a statistically significant manner and also reduced ghrelin and ghrelin O-acyltransferase mRNA levels upon exposure to insulin (28, 29). Here, reduction in ghrelin release by insulin appeared dependent on the ambient d-glucose concentration. As such, whereas insulin was ineffective at reducing ghrelin release in a statistically significant manner in the presence of either 5 or 10 mM d-glucose, it did reduce ghrelin release from the dispersed gastric mucosal cell primary cultures in conditions of 1 mM d-glucose. Insulin's dependency on glucose concentration may explain our previous findings in the SG-1 and PG-1 cell lines, which were performed in culture medium with 5.56 mM d-glucose (63), although it wouldn't explain the findings in MGN3-1 cells, which were performed at 25 mM d-glucose (28, 29). The presence of insulin receptor mRNAs within highly enriched populations of ghrelin cells as well as within the SG-1, PG-1, and MGN3-1 ghrelinoma cells (28, 29) does support the idea that insulin can act directly on ghrelin cells to modulate ghrelin cell physiology.
Indeed, previous in vivo studies in humans also support the notion that both insulin and d-glucose can regulate ghrelin release (19, 20, 38). In one of these studies, infusion of insulin with purposeful maintenance of normoglycemia (90 mg/dl), achieved by infusion of dextrose, led to a rapid fall in acyl ghrelin levels, suggesting that insulin suppresses ghrelin release independently of the degree of glycemia (19). Subsequent hypoglycemia (50 mg/dl), achieved by maintaining the insulin infusion rate but lowering the dextrose infusion rate, had no significant effect on ghrelin levels, suggesting that hypoglycemia does not reverse the fall in ghrelin induced by hyperinsulinemia (19). Induction of hyperglycemia (160 mg/dl), achieved by maintaining the insulin infusion rate but raising the dextrose infusion rate, resulted in a further decline in plasma ghrelin, suggesting that high blood glucose also suppresses ghrelin release independently of insulin (19). Obviously, there are some differences in d-glucose-related sensitivity to insulin in this human study (19) and the current in vitro culture system that will need to be explored further.
Of note and as mentioned above, ingestion by healthy lean human volunteers of drinks composed predominantly of carbohydrates had been shown to have a biphasic effect on plasma ghrelin, first lowering its levels below baseline and subsequently raising its levels above baseline (20). The late overshoot of plasma ghrelin observed in that study coincided with blood glucose levels that were decreased below baseline and insulin levels that were only slightly elevated above baseline. This observation prompted the authors of that study to hypothesize that the late postcarbohydrate overshoot in plasma ghrelin may have resulted from lowered intracellular glucose metabolism associated with blood glucose levels that had decreased below baseline (20). Our findings here upon exposure of the primary cultures to low d-glucose or to the antimetabolite 2-deoxy-d-glucose support such a hypothesis. Also of interest as it relates to ghrelin's proposed roles in responding to states of both energy insufficiency and hypoglycemia, food intake in rats induced by 2-deoxy-d-glucose glucoprivation has been shown to be blunted by pretreatment with anti-ghrelin antibodies (52).
The mechanisms by which low d-glucose and high d-glucose states directly regulate ghrelin release have admittedly not been fully determined by the studies performed here. What does seem apparent, though, by the findings with 2-deoxy-d-glucose and l-glucose is that metabolism of d-glucose within ghrelin cells does contribute to an inhibition of ghrelin release. Entry of d-glucose into the ghrelin cell may occur via transport by one or more members of the facilitative glucose transporter family identified within ghrelin cells, including GLUT1, GLUT4, and GLUT5. Once within the ghrelin cell, metabolism might begin via interaction with glucokinase or one of the hexokinases, which were also identified within ghrelin cells. Glucokinase plays a key role in glucose metabolism within the pancreatic β-cell, where it serves as an important glucose sensor, and within other cell types. Further downstream, metabolites of d-glucose might change the activity of the Kir6.2/SUR1 KATP channel, the components of which also were identified within ghrelin cells, although exposure of the primary gastric mucosal cell pools to the sulfonylurea tolbutamide or to diazoxide, which are expected to close or open the KATP channel, respectively, had no effect on ghrelin release. However, although a mechanism by which glucose transport followed by glucose metabolism, KATP channel closure, cellular depolarization, and an ensuing stimulatory effect on secretion seems intuitive in a system such as the pancreatic β-cell (in which a high glucose state is associated with insulin secretion), such an equivalent pathway within ghrelin cells does not seem obvious (since ghrelin secretion is expected in low glucose states). In this regard, ghrelin cells seem more akin to pancreatic α-cells, in which glucagon secretion occurs in the setting of low glucose. Similarly to both pancreatic β-cells and ghrelin cells, pancreatic α-cells express Kir6.2/SUR1 complexes, and they also contain KATP conductance (2, 22, 44). Of note, whereas tolbutamide increases insulin release, its application to pancreatic α-cells has been shown in many (but not all) studies to suppress glucagon release (21–23). It has been proposed that the opposite effects of d-glucose and tolbutamide on insulin secretion by pancreatic β-cells and on glucagon secretion by pancreatic α-cells are the consequence of the two different cell types being equipped with different sets of voltage-dependent ion channels that lead to different electrophysiological characteristics (22). A similar phenomenon might occur within gastric ghrelin cells. Other similar features between ghrelin cells and pancreatic α-cells include lack of GLUT2 expression, whereas pancreatic β-cells contain high levels of GLUT2 (24). Differential expression of GLUT2, and more broadly, members of the facilitative glucose transport family, has been associated with differences in glucose transport kinetics (24) and may contribute to the differential cellular responses to d-glucose. More work will be required to more clearly define the mechanism by which d-glucose interacts with glucose transporters, glycolytic enzymes, and ion channels within the ghrelin cell to regulate ghrelin release.
We also investigated the effects of somatostatin, secretin, and endothelin-1 on ghrelin release based on published reports of such activity. For instance, gastric submucosal microinfusion of both secretin and endothelin-1 stimulated ghrelin secretion in rats, whereas somatostatin inhibited ghrelin release (14). Using that same technique, neither insulin nor d-glucose affected ghrelin release (14). MGN3-1 mouse ghrelinoma cells demonstrated somatostatin-induced suppression of ghrelin secretion and expression of SSTR2, SSTR3, SSTR5, and SSTR5 (but not SSTR1) but a lack of an effect of secretin on ghrelin release (28, 29). Here, we failed to demonstrate a statistically significant effect of somatostatin and secretin on ghrelin release. This is despite the finding of receptors for those peptide hormones in ghrelin cell-enriched pools of gastric mucosal cells, which would otherwise suggest the ability of somatostatin and secretin to affect ghrelin cell physiology in some way. It is possible that the sensitivity to ambient d-glucose concentration, which is observed here for norepinephrine and insulin, may also help to explain a lack of responsiveness to somatostatin and secretin. It also is possible that the effects of these agents on ghrelin secretion might be apparent only upon simultaneous exposure to other hormones. Endothelin-1 had no effect on ghrelin release here, which might coincide with the barely detectable or lack of detectable levels of its receptors within ghrelin cells.
In conclusion, we have used a dispersed gastric mucosal cell primary culture system and qPCR on highly enriched pools of ghrelin cells to investigate ghrelin secretion. In particular, the enhanced ghrelin release observed in low d-glucose conditions and the reduced ghrelin release observed in high d-glucose conditions, when viewed together with ghrelin's roles in the setting of severe caloric restriction to normalize and prevent life-threatening falls in blood glucose (62), help solidify an important role for ghrelin in blood glucose homeostasis.
GRANTS
These studies were made possible through the support of an International Research Alliance grant with the Novo Nordisk Foundation Center for Basic Metabolic Research (to J. M. Zigman), National Institutes of Health Grants 1-K08-DK-068069, 1-R01-DA-024680, and 1-R01-MH-085298 (to J. M. Zigman), and an Endocrine Fellows Foundation Development Grant in Diabetes, Obesity, and Fat Cell Biology (to P. K. Piper).
DISCLOSURES
The authors have no potential conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
I.S. and J.M.Z. did the conception and design of the research; I.S., W.-M.P., A.K.W., P.K.P., J.-C.C., S.O.-L., and J.M.Z. performed the experiments; I.S., W.-M.P., A.K.W., P.K.P., J.-C.C., and J.M.Z. analyzed the data; I.S., W.-M.P., A.K.W., P.K.P., J.-C.C., and J.M.Z. interpreted the results of the experiments; I.S., W.-M.P., A.K.W., and J.M.Z. prepared the figures; I.S., W.-M.P., and J.M.Z. drafted the manuscript; I.S., W.-M.P., A.K.W., and J.M.Z. edited and revised the manuscript; J.M.Z. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We acknowledge Chelsea Migura for maintenance of the animal colony and Joyce Repa (University of Texas Southwestern Medical Center) for the generous gift of qPCR primers and for the critical read-through of the manuscript.
REFERENCES
- 1. Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 120: 337–345, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Bokvist K, Olsen HL, Hoy M, Gotfredsen CF, Holmes WF, Buschard K, Rorsman P, Gromada J. Characterisation of sulphonylurea and ATP-regulated K+ channels in rat pancreatic A-cells. Pflugers Arch 438: 428–436, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deghenghi R, Ghigo E. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab 86: 5083–5086, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, Zigman JM. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest 121: 2684–2692, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chuang JC, Sakata I, Kohno D, Perello M, Osborne-Lawrence S, Repa JJ, Zigman JM. Ghrelin directly stimulates glucagon secretion from pancreatic alpha-cells. Mol Endocrinol 25: 1600–1611, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colombo M, Gregersen S, Xiao J, Hermansen K. Effects of ghrelin and other neuropeptides (CART, MCH, orexin A and B, and GLP-1) on the release of insulin from isolated rat islets. Pancreas 27: 161–166, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Colon-Gonzalez F, Kazanietz MG. C1 domains exposed: from diacylglycerol binding to protein-protein interactions. Biochim Biophys Acta 1761: 827–837, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Cook DL, Hales CN, Satin LS. Glucose suppresses ATP-inhibited K-channels in pancreatic beta-cells. Adv Exp Med Biol 211: 63–67, 1986 [DOI] [PubMed] [Google Scholar]
- 9. Cummings DE, Foster KE. Ghrelin-leptin tango in body-weight regulation. Gastroenterology 124: 1532–1535, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50: 1714–1719, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346: 1623–1630, 2002 [DOI] [PubMed] [Google Scholar]
- 12. D'Ambra R, Surana M, Efrat S, Starr RG, Fleischer N. Regulation of insulin secretion from beta-cell lines derived from transgenic mice insulinomas resembles that of normal beta-cells. Endocrinology 126: 2815–2822, 1990 [DOI] [PubMed] [Google Scholar]
- 13. Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun 280: 904–907, 2001 [DOI] [PubMed] [Google Scholar]
- 14. de la Cour CD, Norlen P, Hakanson R. Secretion of ghrelin from rat stomach ghrelin cells in response to local microinfusion of candidate messenger compounds: a microdialysis study. Regul Pept 143: 118–126, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Dezaki K, Hosoda H, Kakei M, Hashiguchi S, Watanabe M, Kangawa K, Yada T. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes 53: 3142–3151, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Dezaki K, Kakei M, Yada T. Ghrelin uses Galphai2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet beta-cells: novel signal transduction of ghrelin. Diabetes 56: 2319–2327, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Dezaki K, Sone H, Koizumi M, Nakata M, Kakei M, Nagai H, Hosoda H, Kangawa K, Yada T. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes 55: 3486–3493, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschop MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci 9: 381–388, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Flanagan DE, Evans ML, Monsod TP, Rife F, Heptulla RA, Tamborlane WV, Sherwin RS. The influence of insulin on circulating ghrelin. Am J Physiol Endocrinol Metab 284: E313–E316, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, Thorner MO, Cummings DE. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab 93: 1971–1979, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54: 1808–1815, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Göpel SO, Kanno T, Barg S, Weng XG, Gromada J, Rorsman P. Regulation of glucagon release in mouse -cells by KATP channels and inactivation of TTX-sensitive Na+ channels. J Physiol 528: 509–520, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gromada J, Ma X, Hoy M, Bokvist K, Salehi A, Berggren PO, Rorsman P. ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1−/− mouse alpha-cells. Diabetes 53, Suppl 3: S181–S189, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Heimberg H, De Vos A, Pipeleers D, Thorens B, Schuit F. Differences in glucose transporter gene expression between rat pancreatic alpha- and beta-cells are correlated to differences in glucose transport but not in glucose utilization. J Biol Chem 270: 8971–8975, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Henquin JC, Nenquin M, Sempoux C, Guiot Y, Bellanne-Chantelot C, Otonkoski T, de Lonlay P, Nihoul-Fekete C, Rahier J. In vitro insulin secretion by pancreatic tissue from infants with diazoxide-resistant congenital hyperinsulinism deviates from model predictions. J Clin Invest 121: 3932–3942, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hosoda H, Doi K, Nagaya N, Okumura H, Nakagawa E, Enomoto M, Ono F, Kangawa K. Optimum collection and storage conditions for ghrelin measurements: octanoyl modification of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood samples. Clin Chem 50: 1077–1080, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Inagaki N, Gonoi T, Clement JP, 4th, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science 270: 1166–1170, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Iwakura H, Ariyasu H, Hosoda H, Yamada G, Hosoda K, Nakao K, Kangawa K, Akamizu T. Oxytocin and dopamine stimulate ghrelin secretion by the ghrelin-producing cell line, MGN3–1 in vitro. Endocrinology 152: 2619–2625, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Iwakura H, Li Y, Ariyasu H, Hosoda H, Kanamoto N, Bando M, Yamada G, Hosoda K, Nakao K, Kangawa K, Akamizu T. Establishment of a novel ghrelin-producing cell line. Endocrinology 151: 2940–2945, 2010 [DOI] [PubMed] [Google Scholar]
- 30. James C, Kapoor RR, Ismail D, Hussain K. The genetic basis of congenital hyperinsulinism. J Med Genet 46: 289–299, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, Engel JA. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci USA 106: 11318–11323, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656–660, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Kojima S, Nakahara T, Nagai N, Muranaga T, Tanaka M, Yasuhara D, Masuda A, Date Y, Ueno H, Nakazato M, Naruo T. Altered ghrelin and peptide YY responses to meals in bulimia nervosa. Clin Endocrinol (Oxf) 62: 74–78, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Korbonits M, Goldstone AP, Gueorguiev M, Grossman AB. Ghrelin—a hormone with multiple functions. Front Neuroendocrinol 25: 27–68, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Liu J, Prudom CE, Nass R, Pezzoli SS, Oliveri MC, Johnson ML, Veldhuis P, Gordon DA, Howard AD, Witcher DR, Geysen HM, Gaylinn BD, Thorner MO. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab 93: 1980–1987, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Longo KA, Charoenthongtrakul S, Giuliana DJ, Govek EK, McDonagh T, Qi Y, DiStefano PS, Geddes BJ. Improved insulin sensitivity and metabolic flexibility in ghrelin receptor knockout mice. Regul Pept 150: 55–61, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci 11: 752–753, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCowen KC, Maykel JA, Bistrian BR, Ling PR. Circulating ghrelin concentrations are lowered by intravenous glucose or hyperinsulinemic euglycemic conditions in rodents. J Endocrinol 175: R7–R11, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, Herzog DB, Klibanski A. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab 289: E347–E356, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Mourad NI, Nenquin M, Henquin JC. Metabolic amplifying pathway increases both phases of insulin secretion independently of β-cell actin microfilaments. Am J Physiol Cell Physiol 299: C389–C398, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Mundinger TO, Cummings DE, Taborsky GJ., Jr Direct stimulation of ghrelin secretion by sympathetic nerves. Endocrinology 147: 2893–2901, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Nagaya N, Uematsu M, Kojima M, Date Y, Nakazato M, Okumura H, Hosoda H, Shimizu W, Yamagishi M, Oya H, Koh H, Yutani C, Kangawa K. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation 104: 2034–2038, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature 409: 194–198, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Nielsen LB, Ploug KB, Swift P, Ørskov C, Jansen-Olesen I, Chiarelli F, Holst JJ, Hougaard P, Pörksen S, Holl R, de Beaufort C, Gammeltoft S, Rorsman P, Mortensen HB, Hansen L; Hvidøre Study Group Co-localisation of the Kir6.2/SUR1 channel complex with glucagon-like peptide-1 and glucose-dependent insulinotrophic polypeptide expression in human ileal cells and implications for glycaemic control in new onset type 1 diabetes. Eur J Endocrinol 156: 663–671, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Riepl RL, Heiman ML, Lehnert P, Fichter M, Tschöp M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol 145: 669–673, 2001 [PubMed] [Google Scholar]
- 46. Qi Y, Longo KA, Giuliana DJ, Gagne S, McDonagh T, Govek E, Nolan A, Zou C, Morgan K, Hixon J, Saunders JO, Distefano PS, Geddes BJ. Characterization of the insulin sensitivity of ghrelin receptor KO mice using glycemic clamps. BMC Physiol 11: 1, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reed JA, Benoit SC, Pfluger PT, Tschöp MH, D'Alessio DA, Seeley RJ. Mice with chronically increased circulating ghrelin develop age-related glucose intolerance. Am J Physiol Endocrinol Metab 294: E752–E760, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Sakata I, Nakano Y, Osborne-Lawrence S, Rovinsky SA, Lee CE, Perello M, Anderson JG, Coppari R, Xiao G, Lowell BB, Elmquist JK, Zigman JM. Characterization of a novel ghrelin cell reporter mouse. Regul Pept 155: 91–98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sakata I, Tanaka T, Yamazaki M, Tanizaki T, Zheng Z, Sakai T. Gastric estrogen directly induces ghrelin expression and production in the rat stomach. J Endocrinol 190: 749–757, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Sakata I, Yang J, Lee CE, Osborne-Lawrence S, Rovinsky SA, Elmquist JK, Zigman JM. Colocalization of ghrelin O-acyltransferase and ghrelin in gastric mucosal cells. Am J Physiol Endocrinol Metab 297: E134–E141, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shimbara T, Mondal MS, Kawagoe T, Toshinai K, Koda S, Yamaguchi H, Date Y, Nakazato M. Central administration of ghrelin preferentially enhances fat ingestion. Neurosci Lett 369: 75–79, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Solomon A, De Fanti BA, Martinez JA. Peripheral ghrelin participates in the glucostatic signaling mediated by the ventromedial and lateral hypothalamus neurons. Peptides 27: 1607–1615, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Strassburg S, Anker SD, Castaneda TR, Burget L, Perez-Tilve D, Pfluger PT, Nogueiras R, Halem H, Dong JZ, Culler MD, Datta R, Tschöp MH. Long-term effects of ghrelin and ghrelin receptor agonists on energy balance in rats. Am J Physiol Endocrinol Metab 295: E78–E84, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab 3: 379–386, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Tanaka M, Naruo T, Muranaga T, Yasuhara D, Shiiya T, Nakazato M, Matsukura S, Nozoe S. Increased fasting plasma ghrelin levels in patients with bulimia nervosa. Eur J Endocrinol 146: R1–R3, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, Castaneda TR, Muzzin P, Schürmann A, Szanto I, Tschöp MH, Rohner-Jeanrenaud F. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest 116: 1983–1993, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tolle V, Kadem M, Bluet-Pajot MT, Frere D, Foulon C, Bossu C, Dardennes R, Mounier C, Zizzari P, Lang F, Epelbaum J, Estour B. Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J Clin Endocrinol Metab 88: 109–116, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Trivedi A, Babic S, Chanoine JP. Pitfalls in the determination of human acylated ghrelin plasma concentrations using a double antibody enzyme immunometric assay. Clin Biochem 45: 178–180, 2012 [DOI] [PubMed] [Google Scholar]
- 59. Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 407: 908–913, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Wisse BE, Frayo RS, Schwartz MW, Cummings DE. Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology 142: 3292–3301, 2001 [DOI] [PubMed] [Google Scholar]
- 61. Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR. Ghrelin causes hyperphagia and obesity in rats. Diabetes 50: 2540–2547, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA 107: 7467–7472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhao TJ, Sakata I, Li RL, Liang G, Richardson JA, Brown MS, Goldstein JL, Zigman JM. Ghrelin secretion stimulated by {beta}1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc Natl Acad Sci USA 107: 15868–15873, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 115: 3564–3572, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]