Abstract
Pathophysiological anomalies in autosomal dominant and recessive forms of polycystic kidney disease (PKD) may derive from impaired function/formation of the apical central monocilium of ductal epithelia such as that seen in the Oak Ridge polycystic kidney or orpk (Ift88Tg737Rpw) mouse and its immortalized cell models for the renal collecting duct. According to a previous study, Na/H exchanger (NHE) activity may contribute to hyperabsorptive Na+ movement in cilium-deficient (“mutant”) cortical collecting duct principal cell monolayers derived from the orpk mice compared with cilium-competent (“rescued”) monolayers. To examine NHE activity, we measured intracellular pH (pHi) by fluorescence imaging with the pH-sensitive dye BCECF, and used a custom-designed perfusion chamber to control the apical and basolateral solutions independently. Both mutant and rescued monolayers exhibited basolateral Na+-dependent acid-base transporter activity in the nominal absence of CO2/HCO3−. However, only the mutant cells displayed appreciable apical Na+-induced pHi recoveries from NH4+ prepulse-induced acid loads. Similar results were obtained with isolated, perfused collecting ducts from orpk vs. wild-type mice. The pHi dependence of basolateral cariporide/HOE-694-sensitive NHE activity under our experimental conditions was similar in both mutant and rescued cells, and 3.5- to 4.5-fold greater than apical HOE-sensitive NHE activity in the mutant cells (pHi 6.23–6.68). Increased apical NHE activity correlated with increased apical NHE1 expression in the mutant cells, and increased apical localization in collecting ducts of kidney sections from orpk vs. control mice. A kidney-specific conditional cilium-knockout mouse produced a more acidic urine compared with wild-type littermates and became alkalotic by 28 days of age. This study provides the first description of altered NHE activity, and an associated acid-base anomaly in any form of PKD.
Keywords: intracellular pH, cilia, acid-base, NHE, epithelial cell, cystic kidney disease
polycystic kidney disease (PKD) causes a remodeling of the kidney and other tissues, leading to the dilatation of tubules and ducts, and/or their full encapsulation into fluid-filled cysts (14, 35, 43). The latter structures become isolated from the rest of the nephron and parenchyma, while dilated tubules remain contiguous with the tissue (35). In humans, the autosomal recessive form of PKD (ARPKD) causes dilatation of tubules and ducts in the collecting duct (CD) system of the kidney and the bile duct of the liver early in life (23). Many infants survive an initial, poorly defined postnatal phase of respiratory insufficiency to later develop early-onset, severe, and debilitating hypertension that can cause multiple deleterious effects leading to renal failure and vascular problems (14). Autosomal dominant PKD (ADPKD) is more common than ARPKD, but progresses more slowly in general (35). ADPKD involves the formation of encapsulated cysts throughout the kidney, and ductal systems of the liver and pancreas in some cases (23). Vascular aneurysms are also prevalent in ADPKD, but difficult to study in animal models (36). Similar to that seen in ARPKD, hypertension precedes significant kidney remodeling and renal decline in ADPKD (30). Our laboratories are interested in the mechanisms of Na+ hyperabsorption that may underlie this debilitating hypertensive phenotype found in both forms of PKD, as well as other chronic kidney diseases.1
Our collaborative PKD research group studies cilium-deficient cell and tissue models of PKD in mice to understand the human disease. Moyer et al. (25) identified and characterized the Oak Ridge polycystic kidney or orpk (Ift88Tg737Rpw) mouse that exhibits a PKD phenotype seen in human patients with ARPKD. These mice have an insertional mutation in the Ift88 gene that encodes the protein IFT88, which is required for proper development of primary monocilia in epithelia, including the cortical collecting duct (CCD) of kidney. We previously found that epithelial sodium channel (ENaC)-driven Na+ absorption was upregulated fourfold in monolayers of cilium-deficient principal cells (PCs) cultured from CCD of orpk mice vs. cilium-competent cells rescued by IFT88 cDNA transfection (27). Such Na+ hyperabsorption may be linked to Ca2+ and ATP signaling pathways. For example, cilium-deficient cells exhibit increased apical Ca2+ entry, but impaired flow-induced Ca2+ signaling (18, 34). In addition, the cilium-driven Ca2+ signal may require mechanically induced ATP secretion into the apical medium that is impaired in cilium-deficient cell monolayers vs. cilium-competent controls (18). The cilium-driven Ca2+ signal originates from endoplasmic reticulum (ER) stores, and perhaps specialized ER cisternae beneath the primary cilium (18).
During the course of our initial ENaC study performed on well-polarized cell monolayers, we found that the amiloride analogs ethylisopropyl amiloride (EIPA) and dimethyl amiloride (DMA) inhibited Na+ hyperabsorption at concentrations more specific to Na/H exchangers (NHEs) than to ENaC (27). These analogs may inhibit mouse ENaC at low micromolar concentrations in a manner similar to amiloride, phenamil, and benzamil. However, an alternative hypothesis is that the analogs inhibit one or more NHEs, which contribute to Na+ hyperabsorption in cilium-deficient cell monolayers.
To assess the function and localization of NHEs in cilium-deficient mutant monolayers and cilium-competent rescued monolayers of CCD PCs, we used ratiometric fluorescence imaging with the pH-sensitive dye BCECF and a custom-designed flow chamber to characterize NHE activity on the apical and basolateral membranes selectively. The mutant monolayers compared with the rescued monolayers displayed pronounced apical NHE activity, which correlated with increased apical NHE1 expression. Apical NHE1 expression was also greater in collecting ducts from kidney sections of orpk vs. control mice. In agreement with the monolayer data, the luminal Na+-elicited mean intracellular pH (pHi) recovery rate from an acid load was greater in principal and intercalated cells in microperfused CDs from orpk vs. control mice. Furthermore, kidney-specific conditional cilium-knockout mice compared with littermate controls produced more acidic urine and became alkalotic. We hypothesize that an increase in apical NHE activity, as well as the associated pH-induced stimulation of ENaC activity will promote Na+ hyperabsorption and contribute to hypertension in either or both forms of PKD.
MATERIALS AND METHODS
Generating the Hoxb7 cre-lox kidney-specific conditional cilium-knockout mouse model.
Generating the conditional Ift88tm1Bky (hereinafter called Ift88flox) mutant allele was described previously (16). Kidney-specific mutant animals were established by first crossing heterozygous null Ift88 (Ift88tm1.1Bky/+, hereinafter called Ift88WT/Δ) mutants with a transgenic line expressing Cre recombinase under the Hoxb7 promoter (Hoxb7-cre) (47). The Ift88WT/Δ; Hoxb7-cre males were then crossed with the homozygous flox mice (Ift88flox/flox). The resulting Ift88flox/Δ; Hoxb7-cre were used as experimental animals while the Ift88WT/flox; Hoxb7-cre mice were used as littermate controls. Mice were genotyped by PCR using primers designed to amplify a region of genomic DNA flanking one of the loxP sites (wild-type and flox alleles) or spanning the region deleted with Cre-mediated recombination (null allele; Ift88Δ). Primer sequences are available on request. All mice were maintained in Association for Assessment and Accreditation of Laboratory Animal Care International-certified mouse facilities at the University of Alabama at Birmingham (UAB) with protocols approved by the UAB Institutional Animal Care and Use Committee (IACUC).
Isolating kidneys and performing immunofluorescence.
Orpk and wild-type kidneys were isolated from postnatal day 21 (P21) mice. Kidneys were cut equally along the longer axis, fixed in PBS containing 4% paraformaldehyde (PFA) overnight (O/N) at 4°C, rinsed in PBS, and infiltrated in PBS containing 30% sucrose O/N at 4°C. Tissue was then immersed in OCT, frozen, and stored at −80°C. Cryosections (8–10 μm; 4 sections per slide) were rinsed in PBS, refixed with PBS containing 4% PFA for 10 min at room temperature (RT), rinsed in PBS, and permeabilized with PBS containing 0.1% Triton X-100 for 30 min at RT. Sections were rinsed and incubated in blocking buffer [PBS containing 1% bovine serum albumin (BSA), 0.1% Triton X-100, 0.05% Tween-20, and 1% normal goat serum] for 1 h at RT in a humid chamber. Sections were then incubated in blocking buffering containing 1:1,000 rabbit affinity-purified polyclonal anti-NHE1 (Millipore, Chemicon, Billerica, MA) O/N at 4°C. Sections were washed 3 × 10 min in warm PBS and then incubated in blocking buffer containing 1:1,000 FITC-conjugated goat anti-rabbit IgG (Invitrogen, Molecular Probes, Grand Island, NY) and 1:300 rhodamine-labeled Dolichosbiflorus or DBA (Vector Labs, Burlingame, CA) for 1 h at RT. After sections were washed 3 × 10 min in PBS, they were then counterstained with Hoechst (1:1,000 in PBS) to label nuclei, and mounted in Immu-Mount (ThermoFisher Scientific, Waltham, MA). All images were captured on a Perkin-Elmer (Waltham, MA) confocal spinning disk microscope, and images were processed and analyzed using Volocity software (Perkin-Elmer).
Isolating tubules and performing immunofluorescence.
Kidneys were dissected in cold PBS and cut into small pieces. Tubules were isolated by collagenase digestion in DMEM without serum at 37°C as previously described (32). Tubules in solution were fixed in 4% PFA with 0.2% Triton X-100, washed several time in PBS, blocked in 2% BSA in PBS, and probed with 1:1,000 dilution of anti-acetylated-α-tubulin (Sigma, St Louis, MO) in 1% BSA. DNA was labeled with Hoechst (1:1,000, Sigma). Images were captured with a Nikon TE2000 microscope equipped with a CoolSnap HQ CCD camera.
Culturing cells.
The collecting duct principal cells derived from an orpk mouse model of ARPKD and the genetically rescued cells with wild-type Ift88 cDNA (BAP-Tg737), as well as our approach for generating and culturing additional mutant and rescued cell clones have been described previously (27).
Forming monolayers.
Cells were seeded onto translucent polyester 12-mm diameter Snapwell permeable filter supports (Corning, Corning, NY) with membranes containing 0.4 μm pores. Cell media was replaced every 2 days with a minimum apical volume of 300 μl and basolateral volume of 2 ml. Experiments were conducted on both young (5–9 days old) and old (35–40 days old) monolayers with a high transepithelial resistance >5,000 Ω·cm2. In some experiments, results were pooled from young and old monolayers, as well as from each clone of the mutant and genetically rescued monolayers.
Performing immunohistochemistry on polarized monolayers.
Polarized cells grown on permeable supports were washed with PBS and fixed with 4% PFA in PBS for 10 min at RT. Cells were then permeabilized with 0.5% Triton X-100 in PBS for 10 or 30 min, and subsequently washed with PBS. Cells were blocked in 1% BSA in PBS for 30 min, and then incubated O/N in PBS containing primary antibodies [1:80 monoclonal mouse anti-NHE1 (Millipore, Chemicon) or 1:1,500 rabbit affinity-purified polyclonal mouse anti-NHE1 (Millipore, Chemicon); 1:200 anti-detyrosinated α-tubulin or 1:2,000 monoclonal mouse anti-acetylated tubulin (Sigma)] at 4°C in a moist, dark chamber. The cells were subsequently washed with 0.2% Tween-20 in PBS, then incubated in PBS containing conjugated secondary antibodies [1:5,000 or 1:4,000 Alexa Fluor 488 and Alexa Fluor 594 (Invitrogen, Molecular Probes)] for 1 h, and finally washed with PBS. The membrane was cut out and mounted with ProLong Gold antifade reagent with DAPI (Invitrogen, Molecular Probes) on slides for en face imaging of the monolayer, as well as in a folded orientation allowing side-view (xz plane) imaging of cells at the apex of the fold. Fluorescent-labeled cells were analyzed on a Leica scanning laser confocal microscope configured with both a 488-nm Argon Ion laser (5 mW) and a 568-nm Krypton Ion (10 mW) laser in the High Resolution Imaging Facility at UAB.
Performing cell-surface biotinylation.
Cell-surface biotinylation was performed as previously described (11, 21) on polarized monolayers (7 day) grown in Transwell filters. EZ-Link-Sulfo-NHS-SS-Biotin (200 μM; ThermoFisher Scientific, Pierce) in Ca2+/Mg2+-PBS was added to either the apical or basolateral side for 1 h at 4°C, and then subsequently removed with Ca2+/Mg2+-PBS washes and application of 20 mM glycine as a quencher in Ca2+/Mg2+-PBS for 10 min at 4°C. Cells were next lysed by first applying cold RIPA buffer (150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris at pH 7.4) containing the Protease Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN), and then sonicating the cell suspension. The cell lysates were precleared by centrifugation at 13,523 g for 20 min at 4°C. The BCA protein assay kit (ThermoFisher Scientific, Pierce) was used to measure and equalize the protein concentration of the cell lysates, which were then incubated O/N at 4°C in lysis buffer containing streptavidin-agarose beads (ThermoFisher Scientific, Pierce). The beads were centrifuged at 13,523 g for 1 min, and then subjected to the following washes (with subsequent centrifugations): 2× lysis buffer, 2× Tris-buffered saline (TBS), and 1× TE buffer. Biotinylated proteins were eluted by incubating the beads in Laemmli sample buffer for 5 min at 95°C. Eluted proteins were separated on 4–12% Tris-glycine gradient gels (Invitrogen), and then transferred to Immobilin-P polyvinylidene difluoride membranes (Millipore). Membranes were incubated in TBST (TBS + 0.1% Tween-20 + 2.5% dry milk powder) first for 2 h containing a 1:500 dilution of monoclonal mouse anti-NHE1 antibody (Millipore, Chemicon), and then for 1 h containing a 1:10K dilution of the secondary antibody, goat anti-mouse coupled to horseradish peroxidase (HRP) (ThermoFisher Scientific, Pierce). Bound HRP was detected by SuperSignal chemiluminescence (ThermoFisher Scientific, Pierce) as revealed by exposure to HXR Film (Hawkins X-Ray Supply).
Measuring pHi in polarized cell monolayers.
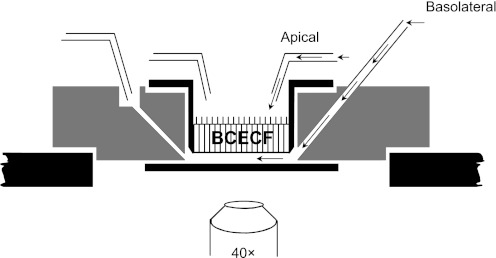
To study the relative activity of Na+-stimulated pHi regulation across the apical versus basolateral membranes of the cell monolayers, we used a flow chamber to perfuse the apical and basolateral surfaces selectively. A schematic of the “sided” flow chamber is shown in Fig. 1.
Fig. 1.
Perfusion chamber with independent control of apical and basolateral solutions. The custom-designed perfusion chamber was designed by Drs. Peter Komlosi and P. Darwin Bell (34). [Figure modified from Fig. 1 of Siroky et al. (34) with permission.]
Before each experiment, the monolayer was washed twice with Ringer solution and incubated for 20 min in a Ringer solution containing 5 μM BCECF-AM, the cell-permeant form of the pH-sensitive dye BCECF. The monolayer was subsequently washed twice with Ringer solution to remove nonhydrolyzed dye, and then placed in Ringer solution for another 5–10 min. A filter was snapped out its support, placed in the double-sided perfusion flow chamber (Fig. 1), and imaged using a ×40 PlanFl objective of an IX-70 inverted Olympus microscope equipped for fluorescence imaging. The monolayer was continuously perfused with solutions heated to 37° ± 0.5°C by an automatic temperature controller (TC-344B, Warner Instruments, Hamden, CT). BCECF was alternately excited with 495-nm and 440-nm light using a high-speed wavelength changer (Lambda DG-4, Sutter Instruments, Novato, CA), and emitted light at 530 nm was captured by a digital CCD camera (Orca II, Hamamatsu, Bridgewater, NJ). Because fluorescence from 495-nm excitation (I495) is pH sensitive and that from 440-nm excitation (I440) is relatively pH insensitive, the ratio (I495/I440) is predominantly a function of pHi and independent of changes in dye concentration, light path, etc. during experiments. I495 and I440 values were corrected for background fluorescence from dye-free monolayers and the baseline settings of the camera. I495/I440 values were converted to pHi values using the high-K+/nigericin technique (Supplemental Fig. S1).2 MetaFluor software (Universal Imaging, Westchester, PA) was used for image and data acquisition. Each “n” represents 8–12 cells in one of 9 regions designated by a 3 × 3 grid imposed over the entire field of cells.
As described by Bevensee and Boron (5), H+ pseudoflux (φ) in moles per unit cell volume per unit time (e.g., μM s−1) is the product of the rate of pHi change (i.e., dpHi/dt) and the total H+ buffering power (βT). For cells bathed in the nominal absence of an open buffering system such as CO2/HCO3−, βT is equal to the intrinsic H+ buffering power (βi). In experiments where stepwise decreases in the extracellular NH3/NH4+ concentration elicited corresponding stepwise decreases in pHi, βi was computed as Δ[NH4+]i/ΔpHi (Supplemental Fig. S2). The pHi dependence of βi was similar for both mutant and rescued cell monolayers.
Generating solutions for cell monolayer studies.
The standard Na+-containing Ringer solution contained (in mM): 140 NaCl, 5 KCl, 1.5 CaCl2, 1.5 MgCl2, 10 HEPES, and NaOH necessary to titrate the solution to pH 7.4 at 37° C. In Na+-free solutions, the Na+ substitute was N-methyl-d-glucammonium (NMDG+), and pH was titrated to 7.4 at 37°C with HCl. In Na+-free, 20 mM NH3/NH4+-containing solutions, 20 mM NMDG-Cl was replaced with equimolar NH4Cl. The high-K+ solutions used for BCECF calibration contained the following (in mM): 130 KCl, 15 NMDG-Cl, 1.5 CaCl2, 1.5 MgCl2, 10 HEPES, and either KOH or HCl necessary to titrate each solution to the desired pH at 37°C. BCECF-AM from Invitrogen, Molecular Probes was prepared as a 5-mM stock solution in dimethyl sulfoxide (DMSO). HOE-694 and HOE-642 (cariporide) were generous gifts from Aventis (Frankfurt, Germany), and were prepared as 50-mM stock solutions in DMSO. Nigericin (Sigma) was prepared as a 10-mM stock solution in 100% ethanol. All other chemicals were obtained from either Sigma or Fisher.
Generating animals used in microperfusion studies.
The generation and characterization of orpk mice on the FVB/N genetic background have previously been described (25). Mice for the in-vitro microperfusion experiments were housed and bred in the Mount Sinai School of Medicine animal care facility. Newborns were allowed to remain with their mothers until weaning. Animals were fed standard mouse chow and given free access to food and water.
Newborn animals homozygous for the orpk mutation were easily identified by their hindlimb polydactyly (48). Orpk mutants rarely survived beyond P21 on the FVB/N background. Genotyping of all animals was performed by PCR analysis of DNA isolated from tail biopsies, performed at the time of death, using previously described conditions (46). Animals studied between 6 and 12 days of age were euthanized in accordance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). Animal protocols were reviewed and approved by the Mount Sinai IACUC.
Dissecting and perfusing single collecting ducts.
Kidneys were removed from a midline incision and then sliced into 2-mm coronal sections. Single tubules were dissected freehand in cold (4°C) Na-Ringer solution. A single tubule was studied from each animal. Each isolated collecting duct (CD) was immediately transferred to a temperature-controlled specimen chamber that was assembled with a no. 1 coverslip (Corning) painted with a 1-μl drop of Cell-Tak (Collaborative Biomedical Products, Bedford, MA). The chamber was mounted on the stage of a Nikon Eclipse TE300 inverted epifluorescence microscope linked to a Cascade 512F camera (Photometrics, Tucson, AZ), which was interfaced with a digital imaging system (MetaFluor). The CD was mounted on concentric glass pipettes, cannulated, and then positioned directly on the Cell-Tak to immobilize the segment for the duration of the experiment as previously described (22). Tubules were initially perfused and bathed at 37°C with Na-Ringer solution for a 30-min equilibration period before each experiment. The bathing solution was continuously exchanged throughout the experiment at a rate of 10 ml/h using a syringe pump (Razel, Stamford, CT) and maintained at 37°C.
Measuring pHi in microperfused CDs.
Each CD was incubated for 20 min in 20 μM BCECF-AM (Invitrogen, Molecular Probes) added to the bathing medium. BCECF-loaded principal cells or PCs (dull in appearance) and intercalated cells or ICs (bright) were visualized using a Nikon S Fluor ×40 objective (numeric aperture 0.9, working distance 0.3) as previously described (22). After being rinsed, BCECF-loaded cells were alternately excited at 490-nm and 440-nm light using a DG-4 high-speed excitation wavelength switcher (Sutter Instruments). Images of the fluorescence emission at 530 nm from the 490-nm excitation (I490) and the 440-nm excitation (I440) were acquired at intervals ranging from 2 to 15 s using MetaFluor image acquisition software (Universal Imaging), and were stored on a Digital Instruments computer. I490/I440 ratios overlying at least two randomly identified PCs and ICs residing in the wall of each tubule were subsequently calculated using our commercially available digital image analysis system (MetaFluor).
At the end of each experiment, an intracellular dye calibration was performed using the high-K+/nigericin technique as previously described (10). Linear regression analysis was used to generate a calibration curve that was then used for conversion of calculated ratios to pHi. Steady-state pHi values of a given cell were calculated as the arithmetic average of at least six readings. The mean pHi of PCs or ICs in a tubule was calculated as the mean pHi of at least two cells. All pHi results are reported as the mean of n tubules.
Generating solutions for microperfusion studies.
The compositions of the solutions used have been previously described (10), and are presented in Supplemental Table S1. All experiments were performed in the nominal absence of CO2/HCO3− (to minimize the contribution of HCO3−-dependent transporters), and HEPES-buffered solutions were adjusted to pH 7.4 and 290–300 mosmol/kgH2O. A calibration solution was titrated to the appropriate pH using either HCl or KOH.
Measuring plasma and urine pH in mice.
A pH electrode for small samples (9810BN from Thermo Orion, Beverly, MA) connected to a portable pH and pH/ISE meter (model 290Aplus from Thermo Orion) was used to measure both blood and urine pH. After mice were weighed and urine samples were collected, the mice were intraperitoneally anesthetized with a cocktail of ketamine (80–100 μg/g mouse) and xylazine (8–10 μg/g mouse) in PBS. Mice older than 7 days sometimes needed additional ketamine + xylazine to be fully anesthetized. The abdominal and thoracic cavities were then opened and fresh blood was extracted from the cavities of the beating heart with a syringe. Both blood and urinary pH were measured immediately after the samples were collected/voided. Samples were obtained from mice at the following postnatal days: 7, 14, 21 (range: 19 to 23), and 28.
Performing statistical analyses.
Data are expressed as means ± SD or SE. Means of data sets were compared using the Student's t-test, and P < 0.05 was considered significant. For pHi recoveries from acid loads, pHi vs. time traces were first fit with single exponentials. The pHi dependencies of dpHi/dt values were then fit with third-order polynomials using Prism 5, and mean values in increments of 0.025 pH units were determined using a custom-written Excel macro by Dr. Christof J. Schwiening (currently in the Dept. of Physiology, Univ. of Cambridge, UK). Slopes and y-intercepts of linear fits to data were obtained using the Regression function of Excel. For microperfusion data, the initial rate of change in pHi (ΔpHi/Δt) observed in response to a change in luminal or bath solution was calculated by linear regression analysis of the rate of recovery over ∼30 s as previously described (10).
Some of this work has been published in preliminary form (28).
RESULTS
Increased Na+-dependent pHi regulation across the apical membrane of cilium-deficient vs. cilium-competent CCD PC monolayers.
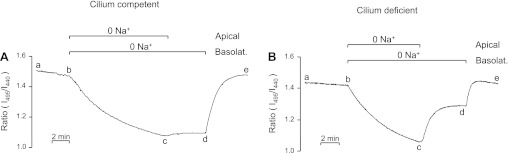
In our pilot experiments, we examined noncalibrated BCECF fluorescence ratios in both a cilium-competent (“rescued”) monolayer (Fig. 2A) and a cilium-deficient (“mutant”) monolayer (Fig. 2B). After the ratio stabilized (ab), removing external Na+ on both sides of the monolayer induced a decrease in the ratio (bc) due primarily to reverse NHE activity and unmasked background acid loading. Na+ was subsequently reintroduced first to the apical side of the monolayer, and then to the basolateral side to assess Na+-stimulated ratio recoveries typically due primarily to NHE activity. In cilium-competent monolayers (Fig. 2A), returning apical Na+ had little effect on the ratio (cd), although returning basolateral Na+ elicited a full recovery (de). In cilium-deficient monolayers (Fig. 2B) however, returning apical Na+ caused a pronounced increase in the ratio (cd), which increased further to the initial steady-state ratio after returning basolateral Na+ (de). The greater apical Na+-induced ratio recovery in the cilium-deficient vs. -competent monolayers is unlikely to be due to altered paracellular permeability because the monolayers both have high transepithelial resistances (>5,000 Ω·cm2). The greater ratio recovery is consistent with more apical NHE activity in the cilium-deficient monolayers than cilium-competent monolayers.
Fig. 2.
Increased Na+-dependent intracellular pH (pHi) regulation across the apical membrane of cilium-deficient vs. cilium-competent cortical collecting duct (CCD) principal cell monolayers. Initial pHi experiments were performed without a BCECF calibration, and results are presented as the ratio of 495-nm to 440-nm excitation (I495/I440). A: cilium-competent monolayer. Removing external Na+ on both sides of the monolayer elicited an I495/I440 decrease (bc), which was unaffected by first returning apical Na+ (cd) but was reversed by then returning basolateral Na+ (de). n = 17. B: cilium-deficient monolayer. The experimental protocol was identical to that described in A. Returning apical Na+ in the continued absence of basolateral Na+ caused the ratio to increase more than halfway back to the initial value (cd). Subsequently returning basolateral Na+ elicited a further increase in the ratio (de) to the initial value. n = 24.
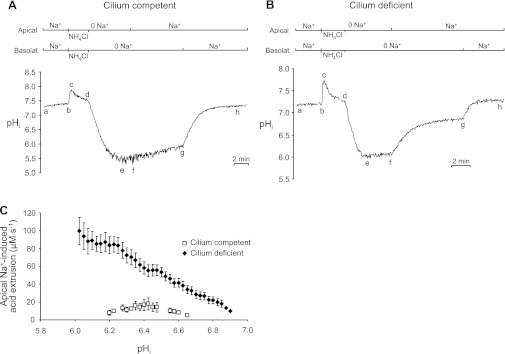
To characterize and compare pHi regulation in the monolayers further, we acid loaded cells using the NH4+-prepulse technique (6), and then assessed the rate of pHi recovery when external Na+ was returned to the apical or basolateral membrane. In these BCECF-calibrated experiments, the cilium-competent cells (n = 45) and cilium-deficient cells (n = 59) had the same mean resting pHi of 7.21 ± 0.02. As shown in Fig. 3A on cilium-competent cells, after the initial steady-state pHi was obtained (ab), the cells from both sides were exposed to a Na+-free solution containing 20 mM NH3/NH4+ for 2 min (bcd). pHi initially increased (bc) due to the rapid cellular influx of NH3, which combined with intracellular H+ to form NH4+. This phase was followed by a slower acidification (cd) due to the stimulation of acid loading and/or influx of NH4+, which dissociated into H+ and NH3. Removing the NH3/NH4+ solution caused pHi to decrease below the initial steady-state (de) as accumulated NH4+ dissociated into both NH3, which diffused out of the cells, and H+, which remained trapped in the cells.
Fig. 3.
Increased Na+-stimulated pHi regulation across the apical membrane of cilium-deficient vs. cilium-competent CCD principal cell monolayers. A: cilium-competent monolayer. Following an NH4+ prepulse-induced acid load (bcde), there was little pHi recovery in the continued absence of external Na+ (ef), or after returning apical Na+ alone (fg). Subsequently returning basolateral Na+ elicited a pronounced increase in pHi (gh). B: cilium-deficient monolayer. The experimental protocol was identical to that described for A. Returning apical Na+ in the continued absence of basolateral Na+ elicited a large increase in pHi (fg), which was further stimulated by also returning basolateral Na+ (gh). C: pHi dependence of apical Na+-induced acid extrusion. Total acid extrusion was plotted for cilium-competent and -deficient monolayers from segment-fg pHi recoveries in panel A/B-type experiments; n ≥ 3 for each symbol.
Following the acid load there was little pHi recovery (ef) in the absence of both apical and basolateral Na+. Returning apical Na+ had little effect on the slow pHi recovery rate (fg) in the cilia-competent cells. However, returning basolateral Na+ elicited a pronounced increase in pHi (gh). Similar results were obtained from two other CCD PC monolayer clones from the mutant mice. These data are in agreement with previous observations (8, 12, 40, 41) that CCD PCs with well-formed apical monocilia exhibit mainly, if not exclusively, basolateral NHE activity.
The same experiment performed on cilium-deficient monolayers yielded different results (Fig. 3B). After pHi stabilized (ab) and the cells were acid loaded by applying and removing 20 mM NH3/NH4+ (bcde), there was little subsequent pHi recovery in the absence of apical and basolateral Na+ (ef). However, returning apical Na+ elicited a marked increase in pHi (fg) that accounted for most of the total pHi recovery, which was completed by subsequently returning basolateral Na+ (gh). Similar results were obtained from two other CCD PC monolayer clones from the mutant mice that were subsequently “rescued” with Ift88 cDNA. Thus, there appears to be a Na+-dependent acid-extrusion mechanism on the apical membrane of the cilium-deficient cells that is absent or considerably less active in the cilium-competent cells.
From Fig. 3, A and B, experiments, we used the segment-fg pHi recoveries to calculate the pHi dependencies of apical Na+-induced total acid extrusion. Cilium-deficient cell monolayers displayed 3- to 10-fold greater acid extrusion (pHi range: 6.2–6.65) upon returning apical Na+ than cilium-competent cell monolayers (Fig. 3C).
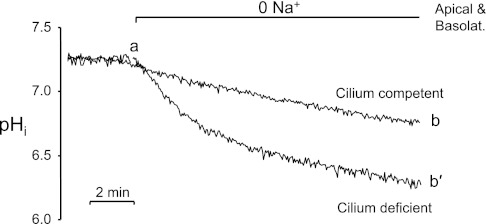
Enhanced apical Na+-dependent acid extrusion in the cilium-deficient cells was further supported by the observation that removing external Na+ on both sides of the monolayers simultaneously caused the resting pHi to decrease faster and to a greater extent in the cilium-deficient cells (Fig. 4, ab′) than the cilium-competent cells (ab). The faster pHi decrease is consistent with reversal of a more active NHE or the unmasking of more pronounced background acid loading in the mutant cells. Quantitating and comparing NHE activity in the mutant and rescued cells requires using NHE inhibitors as described in the next section.
Fig. 4.
Faster 0 Na+-induced acidification in cilium-deficient vs. cilium-competent cell monolayers. Removing both apical and basolateral Na+ elicited a decrease in pHi that was larger and faster in the cilium-deficient monolayers (ab′) than the cilium-competent cell monolayers (ab).
Pharmacologic analysis of apical NHE activity in mutant cell monolayers.
To quantify the contribution of NHE activity to pHi recoveries from acid loads, we performed experiments similar those in Fig. 3 and assessed the effect of applying amiloride analogs and more specific NHE inhibitors. In these experiments, we added the inhibitor after the acid load, but 30 s before returning the external Na+. In some of these experiments, cells were acid loaded twice, and the inhibitor was added immediately before returning external Na+ after the second acid load. We found that amiloride, as well as amiloride analogs such as EIPA and DMA, inhibited the apical Na+-induced pHi recovery (data not shown). However, because such inhibitors are not diagnostic for specific NHE subtypes, we examined the effect of HOE-694 and HOE-642 (cariporide)—two inhibitors that are more selective for NHE1 than NHE2, NHE3, and NHE4 (24). One caveat in our work and interpretation below is that HOE compound IC50 values for NHE1 through -4 have been determined mainly in rat and rabbit preparations (24), whereas our studies were performed on cells derived from transgenic mice. For comparative purposes, we examined the effect of NHE inhibitors on both apical and basolateral acid extrusion following an acid load.
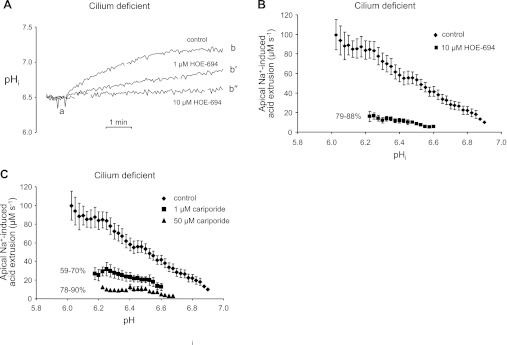
Figure 5A contains three representative traces of apical Na+-induced pHi recoveries from NH4+ prepulse-induced acid loads either in the absence or presence of two different concentrations of apical HOE-694. For clarity, we only show pHi vs. time traces after the acid loads; HOE-694 was added immediately prior to the traces shown. Compared with the full pHi recovery observed under control conditions in the absence of the inhibitor (ab), the pHi recovery was a little more than 50% inhibited by 1 μM HOE-694 (ab′), and almost completely blocked by 10 μM HOE-694 (ab″). From the pHi recoveries, we computed the pHi dependencies of apical Na+-induced acid extrusion in the presence and absence of 10 μM HOE-694. Both apical acid-extrusion plots were approximately linear over the reported pHi ranges, and 10 μM HOE-694 inhibited the apical acid extrusion by 79–88% in the pHi range of 6.23–6.6 (Fig. 5B). Thus, the apical NHE in mutant cell monolayers was quite sensitive to low doses of HOE-694.
Fig. 5.
Pharmacologic assessment of apical Na+-stimulated acid extrusion in cilium-deficient cell monolayers. A: HOE-694-sensitive pHi recovery from an acid load. In Fig. 3B-type experiments, HOE-694 was added apically immediately before returning apical Na+ (point a) in the partial traces shown. Returning apical Na+ elicited the expected pHi increase (ab), which was reduced by 1 μM HOE-694 (ab′) and eliminated by 10 μM HOE-694 (ab″). B: HOE-694 sensitivity of apical Na+-induced acid extrusion. Using data from panel A-type experiments, the pHi dependencies of total acid extrusion (♦) and HOE-694-insensitive acid extrusion (■) were calculated from the pHi recoveries denoted by segments ab and ab″, respectively. Total acid extrusion data (♦) are replotted from Fig. 3C; n ≥ 3 for each symbol. C: cariporide sensitivity of apical Na+-induced acid extrusion. Panel A-type experiments and panel B-type analyses were performed, except with the Na/H exchanger (NHE) inhibitor cariporide at 1 μM (■) and 50 μM (▴). Total acid extrusion data (♦) are replotted from Fig. 3C; n ≥ 3 for each symbol.
We performed similar experiments and analyses on the cilium-deficient monolayers with cariporide. All three apical acid-extrusion plots shown in Fig. 5C were approximately linear over the reported pHi ranges. Compared with apical total acid extrusion in the absence of inhibitor, 1 μM cariporide [which completely inhibits NHE1 but not NHE2 in mice (2)] reduced apical acid extrusion by 59–70% in the pHi range 6.18–6.60. Thus, an apical NHE sensitive to low doses of cariporide is responsible for the majority of acid extrusion during the Na+-stimulated recovery of pHi from an acid load. Fifty micromolar cariporide [which inhibits NHE2 activity in mice (2)] inhibited the remaining apical acid extrusion an additional ∼20% in a similar pHi range of 6.23–6.68. The low apical acid extrusion remaining in the presence of 50 μM cariporide is very similar to the low apical total acid extrusion seen in the cilium-competent monolayers (Fig. 3C). In summary, the HOE-694 and cariporide data are consistent with increased activity and/or expression of NHE1, and possibly NHE2 on the apical cell surface of cilium-deficient monolayers.
Pharmacologic analysis of basolateral NHE function in mutant and rescued cell monolayers.
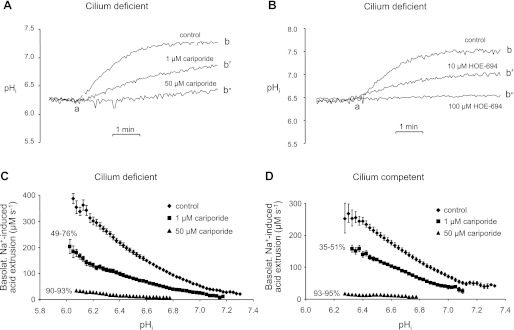
We examined the effect of cariporide and HOE-694 on the basolateral Na+-induced pHi recoveries from acid loads using the approach described for Fig. 5. In Fig. 6A, we show three representative traces of basolateral Na+-induced pHi recoveries from NH4+ prepulse-induced acid loads in cilium-deficient cells either in the absence or presence of two concentrations of basolateral cariporide. Compared with the full pHi recovery observed in the absence of inhibitor (ab), the pHi recovery was inhibited ∼50% by 1 μM cariporide (ab′), and nearly blocked by 50 μM cariporide (ab″). Similar results on the cilium-deficient cells were obtained with higher concentrations (e.g., 10 and 100 μM) of HOE-694 (Fig. 6B). Performing similar experiments on the cilium-competent cells, we found that both cariporide and HOE-694 also inhibited a large fraction of the pHi recovery elicited by returning basolateral Na+ (not shown).
Fig. 6.
Pharmacologic assessment of basolateral Na+-stimulated acid extrusion in cilium-deficient and -competent cell monolayers. A: cariporide-sensitive pHi recovery from an acid load in cilium-deficient monolayers. The experimental protocol and data presentation are similar to that shown in Fig. 5A. Returning basolateral Na+ elicited the expected pHi increase (ab), which was reduced by 1 μM cariporide (ab′), and almost eliminated by 50 μM cariporide (ab″). B: HOE-694-sensitive pHi recovery from an acid load in cilium-deficient monolayers. The experimental protocol was similar to that shown in A, except with the NHE inhibitor HOE-694. Returning basolateral Na+ elicited the expected pHi increase (ab), which was reduced by 10 μM HOE-694 (ab′) and eliminated by 100 μM HOE-694 (ab″). C: cariporide sensitivity of basolateral Na+-induced acid extrusion in cilium-deficient monolayers. Data from panel A-type experiments with Fig. 5B-type analyses were used to calculate the pHi dependencies of total acid extrusion (♦), 1 μM cariporide-insensitive acid extrusion (■), and 50 μM cariporide-insensitive acid extrusion (▴); n ≥ 3 for each symbol. D: cariporide sensitivity of basolateral Na+-induced acid extrusion in cilium-competent monolayers; n ≥ 3 for each symbol.
From the pHi recoveries, we computed the pHi dependencies of basolateral acid extrusion for both cilium-deficient (Fig. 6C) and cilium-competent cells (Fig. 6D). We observed two general differences between the total acid-extrusion plots from the basolateral vs. apical membranes. First, basolateral acid extrusion was considerably greater than apical acid extrusion. For example, at pHi ∼6.0, total acid extrusion was approximately fourfold greater on the basolateral membrane (Fig. 6C) than apical membrane (Fig. 5B) of cilium-deficient cells. Second, for the cilium-deficient cells, the basolateral acid-extrusion plot was more hyperbolic (Fig. 6C) compared with the apical plot, which was more linear (Fig. 3C).
For cilium-deficient cells, 1 μM cariporide moderately reduced basolateral total acid extrusion, whereas 50 μM cariporide severely reduced the acid extrusion (Fig. 6C). Similar results were obtained with the other related NHE inhibitor, HOE-694. For instance, 10 μM HOE-694 reduced basolateral acid extrusion by 36–78% in the pHi range 6.20–7.23, whereas 100 μM HOE-694 reduced the acid extrusion by 82–92% in the pHi range 6.14–6.98 (data not shown). Similar basolateral acid-extrusion plots with these two HOE compounds were obtained with cilium-competent cells. For rescued cell monolayers, 1 μM cariporide moderately reduced basolateral total acid extrusion, whereas 50 μM cariporide severely reduced the acid extrusion (Fig. 6D). Furthermore, 10 μM HOE-694 reduced the acid extrusion by 76–93% (pHi range 6.45–6.98), and 100 μM HOE-694 reduced the acid extrusion by 95–97% (pHi range 6.45–6.63, data not shown). According to these data, the pharmacologic profile of basolateral NHE activity is similar in mutant and rescued cell monolayers, and similar to apical NHE activity in the mutant cell monolayers.
Comparing the pHi dependencies of apical and basolateral NHE activity in cell monolayers.
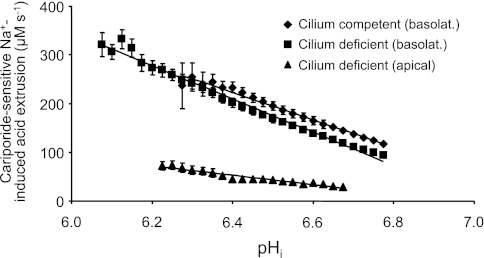
To compare basolateral and apical NHE activities under our experimental condition of returning external Na+ to one side of the monolayer, we plotted in Fig. 7 the 50 μM cariporide-sensitive pHi dependences of 1) apical acid extrusion in the cilium-deficient monolayers (from Fig. 5C data), 2) basolateral acid extrusion in the cilium-deficient monolayers (from Fig. 6C data), and 3) basolateral acid extrusion in the cilium-competent monolayers (from Fig. 6D data). These cariporide-sensitive plots were generated by subtracting the plots of cariporide-insensitive acid extrusion from the corresponding plots of total acid extrusion. Cariporide-sensitive acid extrusion on the basolateral sides of the cilium-deficient and -competent monolayers were similar. Furthermore, cariporide-sensitive acid extrusion on the basolateral side of either monolayer was 3.5- to 4.5-fold greater than that on the apical side of the mutant monolayer in the pHi range 6.23–6.68. In conclusion, apical NHE activity induced in the mutant monolayers is considerably less than basolateral NHE activity in both mutant and rescued monolayers.
Fig. 7.
pHi dependencies of apical and basolateral cariporide-sensitive Na+-induced acid extrusion in cilium-deficient and -competent cell monolayers. Cariporide-sensitive acid extrusion on the basolateral side was best fit with the line y = −280× + 2,017 for the cilium-competent cell monolayers (♦), and y = −341× + 2,389 for the cilium-deficient cell monolayers (■). Cariporide-sensitive acid extrusion on the apical side of cilium-deficient cell monolayers was best fit with the line y = −97× + 673 (▴).
Expression of NHE1 on the apical membrane of mutant cell monolayers.
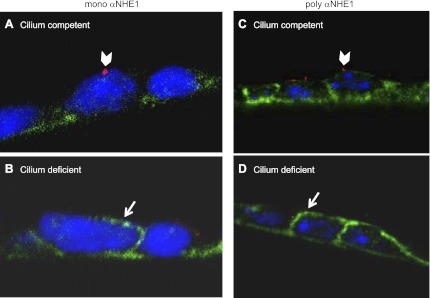
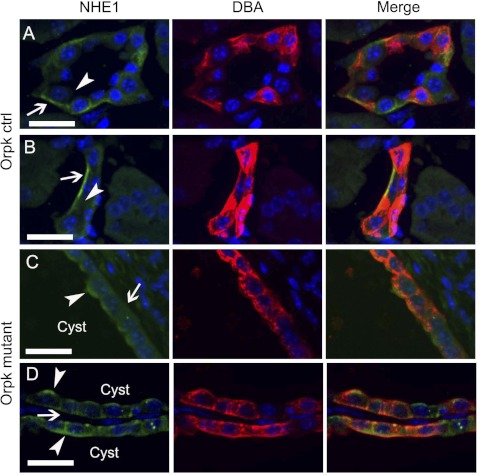
The aforementioned pharmacologic data are consistent with increased expression of an HOE-sensitive Na/H exchanger on the apical membrane of mutant cell monolayers. We therefore used immunohistochemistry to examine and compare cellular localization of the two most HOE-sensitive Na/H exchangers, NHE1 and NHE2, in mutant and rescued cell monolayers. Images were captured in the xz plane of cell monolayers grown on filter membranes and folded so that both the apical and basolateral membranes could be visualized at the crease of the fold. An antibody to NHE2 did not appreciably label mutant or rescued cells (data not shown). However, as shown in Fig. 8A, a monoclonal antibody to NHE1 (mono αNHE1) primarily labeled the basolateral membrane and perhaps some intracellular targets of cilium-competent cells, which were evident from apical antibody labeling to the cilia-protein, detyrosinated α-tubulin (arrowhead). In contrast, as shown in Fig. 8B, mono αNHE1 labeled not only the basolateral membrane, but also the apical membrane in the majority of cilium-deficient cells (arrow). As expected, these mutant cells showed no or minimal antibody labeling to detyrosinated α-tubulin. The arrow in Fig. 8B points to the apical surface based on the orientation of our filter fold, and the fact that other cells outside of the field shown label with α-tubulin on that side of the monolayer. Similar results were obtained with a polyclonal antibody to NHE1 or poly αNHE1 (Fig. 8, C and D). In conclusion, increased activity of an apical Na/H exchanger correlates with stimulated expression of apical NHE1 in cilium-deficient cell monolayers.
Fig. 8.
Localization of NHE1 in cilium-competent and -deficient cells. Monolayers grown on permeable supports were colabeled with either a monoclonal or a polyclonal antibody to NHE1 (mono αNHE1 or poly αNHE1, respectively) (in green), and antibodies to tubulin (in red). Folded membranes allowed for side-view imaging of the apical and basolateral membranes. A: a cilium-competent cell with a detyrosinated α-tubulin-labeled cilium (arrowhead) displayed predominantly basolateral expression of NHE1. B: a cilium-deficient cell displayed both apical (arrow) and basolateral expression of NHE1. Labeling was repeated in another experimental set. C: a cilium-competent cell with an acetylated tubulin-labeled cilium (arrowhead) displayed predominantly basolateral expression of NHE1. D: a cilium-deficient cell displayed both apical (arrow) and basolateral expression of NHE1. In all panels, DNA was stained with DAPI (in blue).
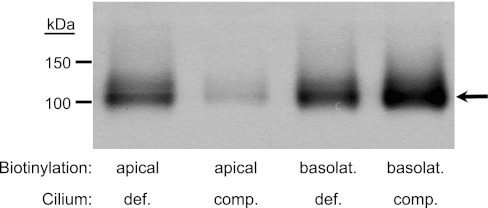
We also performed cell-surface biotinylation to isolate apical or basolateral membranes and assess membrane-targeted NHE1 expression in the mutant vs. rescued monolayers. We labeled either apical or basolateral membranes with EZ-Link-Sulfo-NHS-SS-Biotin, and then isolated membrane-specific protein by pull-down with streptavidin. We loaded an equal amount of total protein per lane, separated the proteins by SDS-PAGE (gradient), and then immunoblotted with the monoclonal αNHE1 (1:500) as shown in Fig. 9. NHE1 expression (arrow) in the apical membrane of the cilium-deficient, mutant cells (lane 1) was considerably greater than in the cilium-competent, rescued cells (lane 2), a finding in agreement with the immunohistochemistry data (Fig. 8). Consistent with our functional data, NHE1 expression was higher in the basolateral vs. apical membranes of both the rescued and mutant cells as evident by stronger labeling in lanes 3 and 4 that contained samples diluted fourfold. Interestingly, basolateral expression was somewhat greater in the rescued vs. mutant cells in two of the three Fig. 9-type experiments performed, although this finding did not lead to a difference in NHE activity in our functional assay (Fig. 7).
Fig. 9.
NHE1 in biotinylated protein from apical and basolateral membranes of cilium-competent and -deficient monolayers. The apical membrane (lanes 1 and 2) or the basolateral membrane (lanes 3 and 4) from either cilium-deficient cells (lanes 1 and 3) or cilium-competent cells (lanes 2 and 4) were selectively biotinylated before being subjected to pull-down by streptavidin. Basolateral membrane protein samples were diluted 4-fold. According to immunoblot analysis, NHE1 expression is higher in the basolateral vs. apical membrane. Furthermore, cilium deficiency is associated with an increase in apical and a decrease in basolateral NHE1 expression. n = 3, although in one experiment, NHE1 expression in the basolateral membranes of the two monolayers was similar.
We extended our immunochemical analysis of mislocalized NHE1 expression in the cell monolayers to kidney sections from the orpk and wild-type mice (Fig. 10). As shown in Fig. 10, A and B, on kidney sections from wild-type mice, the polyclonal antibody to NHE1 predominantly labeled the basolateral membrane (arrows) compared with the apical membrane (arrowheads) of collecting ducts identified by rhodamine-labeled DBA staining. However, in kidney sections with cysts from orpk mice (panel sets in Fig. 10, C and D), the NHE1 antibody labeled both the apical membrane (arrowheads) as well as the basolateral membrane (arrows) of the ducts. The monoclonal NHE1 antibody labeled both apical and basolateral membranes in mutant and control kidney sections, although there appeared to be more nonspecific labeling. In summary, the polyclonal antibody data on kidney sections are consistent with the increased NHE1 expression on the apical membrane of the mutant monolayers shown by immunocytochemistry (Fig. 8) and surface biotinylation-streptavidin pull-down (Fig. 9).
Fig. 10.
Localization of NHE1 in wild-type (WT) and Oak Ridge polycystic kidney (orpk) cystic kidneys. A and B: in 2 sets of representative confocal images from postnatal day 21 (P21) WT kidney sections, NHE1 (green) localized to the basolateral membrane (arrow) but not the apical membrane (arrowhead) of collecting ducts identified by DBA labeling (red). ctrl, Control. C and D: in two sets of representative confocal images from P21 Orpk cystic kidneys, NHE1 (green) localized to both the basolateral membrane (arrow) and the apical membrane (arrowhead) of collecting ducts identified by DBA labeling (red). In all panels, nuclei were counterstained with Hoechst (blue). “Cyst” refers to the lumen of a cyst. Scale bars, 25 μm.
Na+-dependent pHi regulation in microperfused isolated collecting ducts from orpk mutant mice.
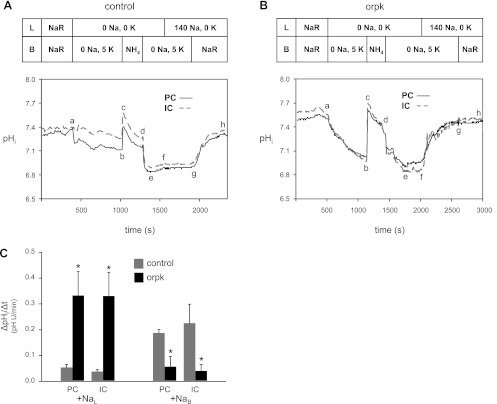
Both our functional and localization data are consistent with altered pHi physiology of cilium-deficient vs. cilium-competent CCD PC monolayers. To explore such altered pHi physiology in a more intact preparation, we examined luminal and basolateral Na+-dependent pHi regulation in microperfused isolated CDs from age-matched orpk mutant and control mice. Representative traces of the patterns of pHi recovery from NH4+ prepulse-induced acid loads observed in age-matched control and orpk CDs are shown in Fig. 11. After the CDs were equilibrated in the Na-Ringer solution, the resting pHi was 7.45 ± 0.04 in dull PCs and 7.43 ± 0.03 in bright ICs [P = not significant (NS)] of control mice (n = 3). Removing luminal and basolateral Na+ simultaneously elicited a mild decrease in pHi (ab, Fig. 11A), likely due to either reverse NHE activity and/or unmasked background acid loading. In the continued absence of external Na+, the cells were then acid loaded by transiently applying 30 mM basolateral NH4Cl (bcde). After pHi decreased to 6.91 ± 0.03 in PCs and 6.90 ± 0.05 in ICs (point e), there was little pHi recovery in the absence of both luminal and basolateral Na+ (ef). Similarly, returning luminal Na+ failed to stimulate the pHi recovery (fg), a finding consistent with minimal apical NHE activity in control CDs. In contrast, returning basolateral Na+ elicited an abrupt increase in pHi to the resting pHi (gh). The data from control CDs are consistent with predominant Na+-dependent acid extrusion on the basolateral membrane with minimal contribution from the apical membrane.
Fig. 11.
Luminal and basolateral Na+-dependent pHi regulation of principal cells (PCs) and intercalated cells (IC) in microperfused isolated collecting ducts (CDs) from control and orpk mutant mice. A: control CD. Simultaneously removing luminal (L) and basolateral (B) Na+ caused a small decrease in pHi (ab). Following an NH4+ prepulse-induced acid load (bcde), there was little pHi recovery in the continued absence of external Na+ (ef), or after returning luminal Na+ alone (fg). Subsequently returning basolateral Na+ elicited a pronounced increase in pHi (gh). NaR, Na-Ringer solution. B: orpk CD. The experimental protocol was identical to that shown in A. Removing luminal and basolateral Na+ elicited a more pronounced decrease in pHi (ab). Following the subsequent acid load (bcde) with little pHi recovery in the absence of luminal and basolateral Na+ (ef), returning luminal Na+ in the continued absence of basolateral Na+ elicited a large increase in pHi (fg), which was unaffected by then returning basolateral Na+ (gh). C: luminal and basolateral Na+-stimulated pHi recovery rates (ΔpHi/Δt). Using data from panel A- and B-type experiments, the luminal Na+ (NaL)-stimulated ΔpHi/Δt was calculated from the pHi recovery at point f in PCs and ICs from control (gray bars) and orpk (black bars) CDs. The basolateral Na+ (NaB)-stimulated ΔpHi/Δt was calculated from the pHi recovery at point g in PCs and ICs from control (gray bars) and orpk (black bars) CDs. *P < 0.05 vs control.
Markedly different results were obtained with orpk mutant CDs (Fig. 11B). In CDs from age-matched orpk mice (n = 4), resting pHi was 7.50 ± 0.04 in PCs and 7.49 ± 0.04 in ICs (for both cells types, P = NS compared with control mice). As seen with control CDs, removing luminal and basolateral Na+ simultaneously elicited a decrease in pHi (ab, Fig. 11B). However, the rate and magnitude of the pHi decrease were markedly greater in the orpk CDs, a finding that parallels the Fig. 4 data on mutant and rescued cell monolayers. The NH4+-induced acid load (bcde) caused pHi to decrease to 7.07 ± 0.06 in PCs (P = NS vs. control PCs) and 7.01 ± 0.04 in ICs (P = NS vs. control ICs). Subsequently, little pHi recovery was observed in the absence of both luminal and basolateral Na+ (ef). However, in contrast to that observed with control CDs, returning luminal Na+ to the orpk CDs elicited a dramatic and almost complete pHi recovery to 7.46 ± 0.01 for PCs and 7.43 ± 0.03 for ICs (fg). The associated pHi recovery rate is considerably greater than the rate mediated by the H+- and H-K pumps in the rabbit CD (10). Subsequently returning basolateral Na+ had little further effect on pHi (gh).
From experiments similar to those shown in Fig. 11, A and B, we plotted the mean pHi recovery rate (ΔpHi/Δt) elicited by returning luminal Na+ (+NaL) and subsequently basolateral Na+ (+NaB) to PCs and ICs in control CDs (gray bars) or orpk CDs (black bars) (Fig. 11C). Note that the low mean ΔpHi/Δt values seen with basolateral Na+ in the orpk CD cells is expected because the preceding luminal Na+ addition induced almost complete pHi recovery. In summary, PCs and ICs from mutant CDs displayed greater luminal Na+-induced pHi recovery rates from acid loads than cells from control CDs. These data are consistent with the finding that cilium-deficient vs. cilium-competent monolayers display increased apical NHE activity.
Altered urine and blood pH in a kidney-specific conditional cilium-knockout mouse.
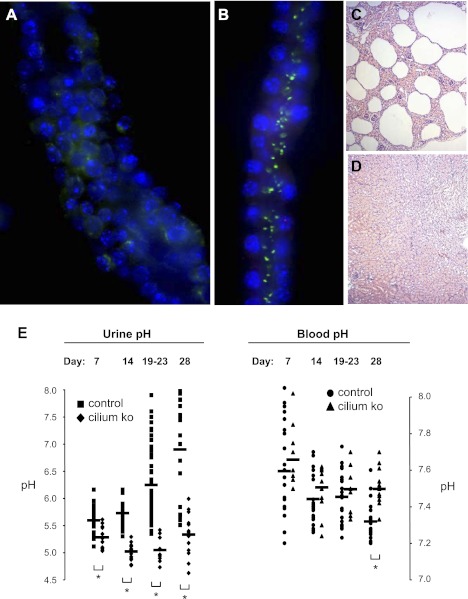
We next examined whether the heightened apical Na+-induced pHi recovery rates seen in both cell monolayers and microperfused CDs from the orpk mice correlates with altered acid-base handling in the intact animal. We measured the pH of freshly voided urine and blood samples from Hoxb7-cre; Ift88flox/Δ kidney-specific conditional cilium-knockout mice vs. littermate controls at postnatal days 7, 14, 21, and 28. Loss of cilia in collecting ducts from this renal cilium-deficient mouse model was evaluated by immunofluorescence analysis using antibodies against acetylated α-tubulin. Tubule identity was determined by positive DBA lectin staining (Vector Labs). A dilated collecting duct without cilia is shown in Fig. 12A, while a normal collecting duct with intact cilia is shown in Fig. 12B. Hoxb7-cre is expressed in the collecting duct epithelium, and conditional mutant mice rapidly develop cysts during the perinatal period. Histological analysis revealed cystic lesions in the kidneys from the conditional cilium-knockout mice (Fig. 12C) compared with wild-type littermates (Fig. 12D). Thus, this cilium-deficient mouse model recapitulates the renal cystic phenotype in other mouse models of both ARPKD and ADPKD. In summary, this conditional cilium-knockout mouse displays a renal cystic phenotype similar to the orpk mouse.
Fig. 12.
Hoxb7 cre-lox kidney-specific conditional cilium-knockout (KO) mice with cystic kidneys and acid-base anomalies. A: a collecting duct from a 7-day old Ift88flox/Δ; Hoxb7-cre mutant mouse displays a dilated tubule lumen and no cilium labeling with an antibody to acetylated α-tubulin (green). Nuclei are labeled with Hoechst (blue). B: a collecting duct from a 7-day old Ift88WT/flox; Hoxb7-cre control mouse displays cilium labeling with the acetylated α-tubulin (green) antibody. Nuclei are labeled with Hoechst (blue). C: cystic lesions are evident in cross sections of paraffin-embedded whole kidney from a 14-day-old mutant mouse. D: cystic lesions are absent in cross sections of paraffin-embedded whole kidney from a 14-day-old control mouse. E: fresh urine samples (left) and blood samples (right) were collected from 50 Hoxb7-cre; Ift88flox/Δ kidney-specific conditional cilium-knockout mice versus 116 littermate controls at postnatal days 7, 14, 19–23, and 28. Horizontal bars in the scatterplots represent mean pH values. *P < 0.01 (nonparametric Mann-Whitney t-test).
Compared with littermate controls, mutant mice at each postnatal age studied had significantly more acidic urine, which became more pronounced with age (Fig. 12E). Compared with littermate urine, mutant urine was more acidic by a mean of 0.3 pH units at day 7. The difference in mean pH between urine samples of mutant and control mice expanded to 0.7 pH units at day 14, 1.2 pH units at day 21, and 1.6 pH units at day 28. All differences were statistically significant. These data correlate with a progressively worsening cystic phenotype in the mutant animals. In fact, the mutant animals become severely debilitated with renal dysfunction in the weeks that follow postnatal day 28. The acidic urine of the mutant mice also influenced their blood pH. Compared with littermate blood, mutant blood was more alkaline by a mean of 0.18 pH units at day 28. Although not statistically significant, there was also a trend toward more alkaline blood of the mutant mice at the earlier postnatal ages of day 7, 14, and 21. Blood and urinary pH means ± SE for the knockout and control mice at the four ages are shown in Supplemental Table S2. This in vivo data in combination with the aforementioned in vitro data support the conclusion that enhanced apical NHE activity in this mouse model of cystic kidney disease leads to a decrease in urine pH and induces alkalosis.
DISCUSSION
This study provides the first description to our knowledge of a renal acid-base transport abnormality, as well as an acid-base disturbance associated with polycystic kidney disease. We found enhanced HOE-sensitive NHE activity on the apical surface of well-polarized renal cortical collecting duct principal cell monolayers derived from the orpk mouse deficient in primary cilia compared with “rescued” cells transfected with Ift88 cDNA. Furthermore, we observed faster luminal Na+-induced pHi recoveries from acid loads in isolated perfused collecting ducts (CDs) from the orpk mouse compared with CDs from littermate controls. The significance of these findings is highlighted by urine and blood pH measurements from mutant mice and their littermates in the first ∼4 wk of age showing early-onset, enhanced, and persistent urinary acidification, as well as the development of alkalosis in mutant animals. The data are most consistent with a renal tubular metabolic alkalosis, although a definitive determination requires arterial blood gas data to assess any respiratory response. Further characterization of the acid-base phenotype will require measurements of titratable acid and ammonium excretion.
On the basis of the aforementioned findings, the role of acid-base transporters in salt and pH homeostasis in PKD deserves further characterization. Although acid-base disturbances are not a diagnostic feature of PKD to our knowledge, we hypothesize that it would be informative to examine the acid-base status of at least some ARPKD and ADPKD patient populations, particularly those exhibiting hypertension. Urine pH may be a key biomarker for assessing PKD patient populations in the future. Therapeutically, NHE1 inhibitors targeted to the apical membrane of the tubule may be beneficial in treating hypertension and perhaps alkalosis in PKD patients.
Our approach to characterize the activity of an NHE selectively in one membrane of the polarized monolayers was to examine the HOE-sensitive, Na+-induced pHi recovery from an acid load from one side of the monolayer while the other side was continually bathed in a Na+-free solution to eliminate the contribution of any contaminating NHE activity from that side. We cannot exclude the possibility that Na+ influx across one membrane (e.g., due to the activities of NHE, ENaC, etc.) may raise intracellular Na+ and promote reverse NHE activity in the opposite membrane bathed in the absence of external Na+ and drive H+ into cells. Such reverse NHE activity would effectively “short circuit” the pHi recovery and lead to a calculated underestimate of the NHE activity in the opposite membrane. Surprisingly, we found that HOE-sensitive, Na+-induced pHi recoveries from an acid load were actually inhibited by HOE compounds present in the solution on the opposite membrane. For example, apical HOE compounds greatly inhibited basolateral Na+-induced acid extrusion in both the cilium-competent monolayer (Supplemental Fig. S3) and the cilium-deficient monolayer, and basolateral HOE compounds eliminated apical Na+-induced acid extrusion in the cilium-deficient monolayer.
These HOE data are consistent with the possibility that NHE activity in one membrane is being contaminated by a Na+ leak that stimulates NHE-mediated acid extrusion by the opposite membrane. We think this possibility is unlikely for the following three reasons. First, with both sides of the epithelium being continually perfused, it is unlikely that any small Na+ leak from one side will increase the Na+ concentration to any appreciable level of a continually perfused 0-Na+ solution on the other side. However, unstirred layers would contribute to any increase. Second, as shown in Fig. 3A, apical Na+ fails to stimulate any pHi recovery in a cilium-competent monolayer with basolateral membranes exposed to a Na+-free solution. Both the cilium-competent and cilium-deficient monolayers have transepithelial resistances >5 kΩ·cm2. Finally, from Fig. 3B-type experiments on cilium-deficient monolayers where apical and basolateral Na+ were added simultaneously (data not shown), the calculated pHi dependence of Na+-induced acid extrusion was approximately the sum of the apical and basolateral plots shown in Figs. 3C and 6C. In summary, HOE compounds applied to one membrane appear to inhibit NHE activity in the other membrane, perhaps due to intracellular accumulation or paracellular/transcellular movement to the opposite membrane. Alternatively, the apical and basolateral NHEs may be functionally coupled, similar to that observed in the isolated, perfused medullary thick ascending limb from rat (13) where inhibiting basolateral NHE1 induces cytoskeletal remodeling and F-actin depolymerization that inhibits apical NHE3 (42). In light of the complexities of using NHE inhibitors, we opted to assay for NHE activity in a given membrane by examining HOE-sensitive, Na+-induced pHi recoveries from acid loads with the other membrane continually exposed to a Na+-free solution. Nevertheless, observed HOE sensitivity of NHE activity in a membrane may be indirect if the HOE acts through inhibition of a functionally coupled NHE in the opposite membrane, even one that is nonfunctional or perhaps operating in reverse with 0 Na+.
On the basis of the finding that the mutant monolayers display increased apical NHE activity, the simplest explanation for the more acidic urine and alkalosis in the orpk mice is an increase in luminal NHE activity in the distal nephron. However, an apical NHE in the distal nephron with a low luminal pH is energetically unlikely to make a major contribution to H+ excretion, particularly if luminal Na+ is also low due to heightened Na+ reabsorption in PKD. There may be alternative or additional explanations for the acid-base disturbance in the orpk mice, including increased NHE activity promoting increased H+ excretion or HCO3− reabsorption in upstream nephron segments. HCO3− transporters may also play a role. Indeed, acid-base transport dysregulation has been documented in the choroid plexus (CP) of the orpk mouse where the mutant CP epithelium exhibits reduced Na+-coupled HCO3− transporter activity that could be resurrected by inhibiting PKA (4). Consistent with this finding in the CP, we have recently found that basolateral Na+-coupled HCO3− transporter activity is reduced in the cilium-deficient vs. -competent cell monolayers (29). In parallel with the NHE findings in the current study, reduced basolateral Na+ and HCO3− influx would contribute to an overall net increase in Na+ reabsorption and acid excretion. Thus, we propose that multiple acid-base transporters contribute to altered salt handling in this cell model of ARPKD.
Our work has implications regarding renal and whole body homeostasis in the pathophysiology of PKD. It would be valuable to determine how acid-base transport anomalies contribute to PKD pathogenesis in the kidney. Chronic hyperacidification of the final urine may affect collecting duct and urinary tract physiology in poorly understood ways. Furthermore, the development of metabolic alkalosis from increased H+ excretion will drive compensation by the respiratory system, as well as increase the proportion of B-type vs. A-type intercalated cells in the renal collecting duct system (26). B-type intercalated cell number needs to be examined in these cilium-deficient mouse models of PKD. Moreover, the reversion to an undifferentiated and hyper-proliferative phenotype for the cystic kidney epithelial cell in the collecting duct also prompted one of our laboratories to revisit the idea—as described by Aigner et al. (1)—of a “progenitor cell” in the collecting duct that may give rise to principal vs. intercalated cells. Perhaps, the cystic epithelial cell regains a “progenitor cell-like status” in the renal collecting duct that predisposes remodeling and abnormal solute and water transport.
Acid-base transport anomalies also contribute to PKD pathogenesis in other organs such as the choroid plexus as described above. Furthermore, increased apical NHE activity in the bile and pancreatic ducts would be expected to acidify bile and exocrine pancreas secretions that are normally alkaline. Such secretions would reduce the neutralization of stomach acid, as well as interfere with the breakdown of food and absorption of essential nutrients at points downstream in the small intestine that require a neutral to alkaline environment.
Our work draws attention to the following fundamental question in the cell physiology of PKD: How does disruption in the primary cilium alter membrane protein function and/or expression? Such disruption is likely to have structural and/or regulatory consequences. For example, loss of a functional primary cilium may reduce the function and expression of a membrane protein such as NHE by altering the apical membrane landscape and associated supporting cytoskeleton. Loss of the primary cilium may also promote dysfunctional autocrine and paracrine signaling associated with the apical cell surface. One of our laboratories has previously reported that the loss of a primary cilium causes an attenuation of autocrine purinergic signaling and flow-induced calcium signaling (18). Moreover, both the ATP/ADP-scavenger apyrase and the broad specificity calcium entry channel blocker gadolinium attenuate this flow-induced calcium signal. Jensen et al. (19) have made similar observations. Purinergic signaling modulates NHE activity through phospholipase C products and intracellular calcium (3, 38). We hypothesize that cilium-modulated change in the subapical cytoskeleton, autocrine-mediated extracellular signaling, and the associated intracellular signaling all contribute in altered function and expression of ENaC and NHE, and perhaps other channels and transporters responsible for salt and acid-base handling in the cortical collecting duct. We propose that changes in Na+-dependent channel and transporter activity/expression are an underlying primary cause of the profound and early-onset hypertension in pediatric patients affected by ARPKD (14) and may also contribute to the hypertension that precedes a decline in renal failure in ADPKD (30). The emergence of conditional cilium-knockout mice where the cilium is completely absent in tissues and cells will allow further testing of these hypotheses.
According to our localization data at the cell and tissue levels, increased apical NHE activity in the mutant cells can be at least partially explained by an increase in apical NHE expression—a finding not particularly surprising in light of studies documenting abnormal polarity of expression of epithelial membrane proteins in PKD cystic vs. noncystic epithelia. Na+-pump subunits and growth factor receptors are normally, if not exclusively, basolateral membrane proteins in renal collecting duct epithelia. However, individual Na+-pump subunits have been found in the apical membrane of cystic epithelial cells (31, 44), although it is not clear if they support Na+-pump activity (31, 32). There is more uniform agreement that growth factor receptors appear on both the apical surface, as well as the basolateral surface of cystic epithelia. In the current study, we present immunohistochemical evidence consistent with apical NHE expression of cilium-deficient principal cells derived from the renal collecting duct. In most studies (8, 12, 40, 41), except for a couple (15, 17), investigators find NHE expression exclusively on the basolateral surface of collecting duct cells. Indeed, we also found NHE1 predominantly localized to the basolateral membrane of collecting duct cells in kidney sections from control vs. orpk mice (Fig. 10).
Cariporide and HOE-694 at inhibitory doses for NHE1 inhibited the majority of the enhanced acid extrusion during apical Na+-induced pHi recoveries following an acid load in the mutant cilium-deficient cells. Nevertheless, higher concentrations that are expected to inhibit NHE2 eliminated the remaining enhanced acid extrusion. These functional data are consistent with NHE1, and with NHE2 to a lesser extent, being responsible for the enhanced apical NHE activity in the mutant cells. This interpretation, however, relies on the assumption that NHE subtype sensitivities to cariporide and HOE-694 in our mouse cells are similar to those reported in rat, rabbit, and human cells. Nevertheless, these functional data are supported by the localization data demonstrating increased expression of NHE1 in the apical membrane of the mutant vs. rescued cells. We were unable to detect any expression of NHE2 in either cell monolayer. However, we have confirmed the presence of NHE1 and 2 in our mutant and rescued cells by RT-PCR as well. In fact, using RT-PCR and an array of NHE primer pairs, we have detected NHE1, 2, and 4–8 in these principal cell models (not shown). These early data corroborate past work (15, 17). We could not PCR amplify NHE3—the predominant NHE in the apical membrane of the proximal tubule—despite the use of at least six different primer pairs. As such, there may be as many as seven NHE subtypes in our cilium-deficient and -competent cells. Thus, we must be cautious in interpreting our pharmacologic data. Further studies are required to characterize and compare the kinetic properties and pharmacologic sensitivities of the apical and basolateral NHEs in the mutant cells.
In summary, our results support the conclusion that cilium-deficient principal cells derived from the orpk mouse model of polycystic kidney disease have increased apical NHE activity at least partially due to mislocalized expression of NHE1. Increased apical NHE activity correlates with persistent urinary acidification in the kidney-specific conditional cilium-knockout mice, and the development of alkalosis as the kidneys become more cystic over time. Enhanced function and localization of apical NHE may also contribute to increased activity and expression of ENaC. The colocalization of NHE and ENaC proteins in the apical membrane is not a normal physiologic occurrence along the nephron (33, 39). Apical NHE activity will promote extracellular acidification, which can stimulate δENaC (45). Furthermore, Chalfant et al. (9) showed that intracellular alkalinization stimulates the activity of ENaC channels expressed in Xenopus oocytes. We hypothesize that coexpressed ENaC and NHE in the apical membrane of cilium-deficient cells promotes Na+ hyperabsorption in this cell and mouse model of ARPKD, and may represent the primary cause of hypertension in either or both ARPKD and ADPKD.
GRANTS
We gratefully acknowledge the support of National Institutes of Health (NIH) Grant R01 DK-67343 (to E. M. Schwiebert, then transferred to M. O. Bevensee), as well as the support of NIH Grant R01 DK-65655 (to B. K. Yoder). We acknowledge the support of the Recessive PKD Translational and Research Core Centers at the University of Alabama at Birmingham (P30 DK-074038), and the Pittsburgh Center for Kidney Research Physiology Core (P30 DK079307).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.O., W.L., N.S., B.K.Y., L.M.S., E.M.S., and M.O.B. conception and design of the research; D.O., X.L., W.L., V.C.R., and N.S. performed the experiments; D.O., X.L., W.L., N.S., B.K.Y., and M.O.B. analyzed the data; D.O., X.L., W.L., N.S., B.K.Y., L.M.S., E.M.S., and M.O.B. interpreted the results of the experiments; D.O., X.L., W.L., N.S., and M.O.B. prepared the figures; D.O., X.L., W.L., N.S., B.K.Y., L.M.S., E.M.S., and M.O.B. drafted the manuscript; D.O., X.L., W.L., N.S., B.K.Y., L.M.S., E.M.S., and M.O.B. edited and revised the manuscript; D.O., X.L., W.L., V.C.R., N.S., B.K.Y., L.M.S., E.M.S., and M.O.B. approved the final version of the manuscript.
Supplementary Material
ACKNOWLEDGMENTS
A special thanks to Dr. P. Darwin Bell (currently at the Medical University of South Carolina in Charleston, SC) for allowing us to perform some early experiments (Fig. 2) in his laboratory, and Dr. James A. Schafer for thoughtful comments and suggestions. We also thank Dr. Jürgen Pünter at Sanofi-Aventis Deutschland (Frankfurt, Germany) for providing the HOE compounds.
Present address of E. M. Schwiebert: DiscoveryBioMed, Inc., Innovation Depot, Unit 37, Suite L140, 1500 1st Avenue North, Birmingham, AL 35203 (e-mail: erik@discoverybiomed.com).
Footnotes
This article is the topic of an Editorial Focus by Avner et al. (1a).
The online version of this article contains supplemental material.
REFERENCES
- 1. Aigner J, Kloth S, Jennings ML, Minuth WW. Transitional differentiation patterns of principal and intercalated cells during renal collecting duct development. Epithelial Cell Biol 4: 121– 140, 1995 [PubMed] [Google Scholar]
- 1a. Avner ED, McDonough AA, Sweeney WE., Jr Transport, cilia, and PKD: must we in(cyst) on interrelationships? Focus on “Increased Na+/H+ exchanger activity on the apical surface of a cilium-deficient cortical collecting duct principal cell model of polycystic kidney disease.” Am J Physiol Cell Physiol (March 7, 2012). doi:10.1152/ajpcell.00070.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bachmann O, Riederer B, Rossmann H, Groos S, Schultheis PJ, Shull GE, Gregor M, Manns MP, Seidler U. The Na+/H+ exchanger isoform 2 is the predominant NHE isoform in murine colonic crypts and its lack causes NHE3 upregulation. Am J Physiol Gastrointest Liver Physiol 287: G125– G133, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bagorda A, Guerra L, Di Sole F, Helmle-Kolb C, Favia M, Jacobson KA, Casavola V, Reshkin SJ. Extracellular adenine nucleotides regulate Na+/H+ exchanger NHE3 activity in A6-NHE3 transfectants by a cAMP/PKA-dependent mechanism. J Membr Biol 188: 249– 259, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banizs B, Komlosi P, Bevensee MO, Schwiebert EM, Bell PD, Yoder BK. Altered pHi regulation and Na+/HCO3− transporter activity in choroid plexus of cilia-defective Tg737orpk mutant mouse. Am J Physiol Cell Physiol 292: C1409– C1416, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Bevensee MO, Boron WF. Manipulation and regulation of cytosolic pH. In: Methods in Neurosciences, vol. 27. Measurement and Manipulation of Intracellular Ions, edited by Kracier J, Dixon SJ. New York, NY: Academic, 1995, p. 252– 273 [Google Scholar]
- 6. Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol 67: 91– 112, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyarsky G, Ganz MB, Sterzel RB, Boron WF. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3−. Am J Physiol Cell Physiol 255: C844– C856, 1988 [DOI] [PubMed] [Google Scholar]
- 8. Chaillet JR, Lopes AG, Boron WF. Basolateral Na-H exchange in the rabbit cortical collecting tubule. J Gen Physiol 86: 795– 812, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chalfant ML, Denton JS, Berdiev BK, Ismailov, Benos DJ, Stanton BA. Intracellular H+ regulates the α-subunit of ENaC, the epithelial Na+ channel. Am J Physiol Cell Physiol 276: C477– C486, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Constantinescu A, Silver RB, Satlin LM. H-K-ATPase activity in PNA-binding intercalated cells of newborn rabbit cortical collecting duct. Am J Physiol Renal Physiol 272: F167– F177, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Corbeil D, Röper K, Hannah MJ, Hellwig A, Huttner WB. Selective localization of the polytopic membrane protein prominin in microvilli of epithelial cells — a combination of apical sorting and retention in plasma membrane protrusions. J Cell Sci 112: 1023– 1033, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Ford P, Rivarola V, Kierbel A, Chara O, Blot-Chabaud M, Farman N, Parisi M, Capurro C. Differential role of Na+/H+ exchange isoforms NHE-1 and NHE-2 in a rat cortical collecting duct cell line. J Membr Biol 190: 117– 125, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Good DW, George T, Watts BA., III Basolateral membrane Na+/H+ exchange enhances HCO3− absorption in rat medullary thick ascending limb: evidence for functional coupling between basolateral and apical membrane Na+/H+ exchangers. Proc Natl Acad Sci USA 92: 12525– 12529, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guay-Woodford LM, Desmond RA. Autosomal recessive polycystic kidney disease: the clinical experience in North America. Pediatrics 111: 1072– 1080, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Guerra L, Sole FD, Volenti G, Ronco PM, Perlino E, Casavola V, Reshkin SJ. Polarized distribution of Na+/H+ exchanger isoforms in rabbit collecting duct cells. Kidney Int 53: 1269– 1277, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Intraflagellar transport is essential for endochondral bone formation. Development 134: 307– 316, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Hill C, Giesberts AN, White SJ. Expression of isoforms of the Na+/H+ exchanger in M-1 mouse cortical collecting duct cells. Am J Physiol Renal Physiol 282: F649– F654, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Hovater MB, Olteanu D, Hanson EL, Cheng NL, Siroky B, Fintha A, Komlosi P, Bell PD, Yoder BK, Schwiebert EM. Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal 4: 155– 170, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J. Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol 18: 2062– 2070, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Kizer NL, Lewis B, Stanton BA. Electrogenic sodium absorption and chloride secretion by an inner medullary collecting duct cell line (mIMCD-K2). Am J Physiol Renal Fluid Electrolyte Physiol 268: F347– F355, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Lawrence SP, Holman GD, Koumanov F. Translocation of the Na+/H+ exchanger 1 (NHE1) in cardiomyocyte responses to insulin and energy-status signaling. Biochem J 432: 515– 523, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol 289: F978– F988, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Martinez JR, Grantham JJ. Polycystic kidney disease: etiology, pathogenesis, and treatment. Dis Mon 41: 693– 765, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Masereel B, Pochet L, Laeckmann D. An overview of inhibitors of Na+/H+ exchanger. Eur J Med Chem 38: 547– 54, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Moyer JH, Lee-Tischler MJ, Kwon HY, Schrick JJ, Avner ED, Sweeney WE, Godfrey VL, Cacheiro NL, Wilkinson JE, Woychik RP. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science 264: 1329– 1333, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Narbaitz R, Kapal VK, Levine DZ. Induction of intercalated cell changes in rat pups from acid- and alkali-loaded mothers. Am J Physiol Renal Fluid Electrolyte Physiol 264: F415– F420, 1993 [DOI] [PubMed] [Google Scholar]
- 27. Olteanu D, Yoder BK, Liu W, Croyle MJ, Welty EA, Rosborough K, Wyss JM, Bell PD, Guay-Woodford LM, Bevensee MO, Satlin LM, Schwiebert EM. Heightened epithelial Na+ channel-mediated Na+ absorption in a murine polycystic kidney disease model epithelium lacking apical monocilia. Am J Physiol Cell Physiol 290: C952– C963, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Olteanu DS, Yoder BK, Bevensee MO, Schwiebert EM. NHE dysregulation in cilium deficient mouse renal principal cells from orpk mice (Abstract). FASEB J 21: 596.2, 2007. 17167067 [Google Scholar]
- 29. Liu X, Stephens LA, Olteanu D, Bevensee MO. Altered bicarbonate-dependent intracellular pH (pHi) regulation in a cortical collecting duct (CCD) principal cell line from a mouse model of autosomal recessive polycystic kidney disease (ARPKD) (Abstract). FASEB J 24: 1024.10, 2010 [Google Scholar]
- 30. Perrone RD, Miskulin DC. Hypertension in individuals at risk for autosomal dominant polycystic kidney disease: to screen or not to screen? Am J Kidney Dis 46: 557– 559, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Rohatgi R, Greenberg A, Burrow CR, Wilson PD, Satlin LM. Na transport in autosomal recessive polycystic kidney disease (ARPKD) cyst lining epithelial cells. J Am Soc Nephrol 14: 827– 836, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Schafer JA, Watkins ML, Li L, Herter P, Haxelmans S, Schlatter E. A simplified method for isolation of large numbers of defined nephron segments. Am J Physiol Renal Physiol 273: F650– F657, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Schild L. The epithelial sodium channel: from molecule to disease. Rev Physiol Biochem Pharmacol 151: 93– 107, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Siroky BJ, Ferguson WB, Fuson AL, Xie Y, Fintha A, Komlosi P, Yoder BK, Schwiebert EM, Guay-Woodford LM, Bell PD. Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am J Physiol Renal Physiol 290: F1320– F1328, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Sullivan LP, Wallace DP, Grantham JJ. Epithelial transport in polycystic kidney disease. Physiol Rev 78: 1165– 1191, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Takahashi H, Calvet JP, Dittemore-Hoover D, Yoshida K, Grantham JJ, Gattone VH., 2nd A hereditary model of slowly progressive polycystic kidney disease in the mouse. J Am Soc Nephrol 1: 980– 989, 1991 [DOI] [PubMed] [Google Scholar]
- 37. Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 18: 2210– 2218, 1979 [DOI] [PubMed] [Google Scholar]
- 38. Urbach V, Hélix N, Renaudon B, Harvey BJ. Cellular mechanisms for apical ATP effects on intracellular pH in human bronchial epithelium. J Physiol 543: 13– 21, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang T, Hropot M, Aronson PS, Giebisch G. Role of NHE isoforms in mediating bicarbonate reabsorption along the nephron. Am J Physiol Renal Physiol 281: F1117– F1122, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Wang X, Kurtz I. H+/base transport in principal cells characterized by confocal fluorescence imaging. Am J Physiol Cell Physiol 259: C365– C373, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Weiner ID, Hamm LL. Regulation of intracellular pH in the rabbit cortical collecting tubule. J Clin Invest 85: 274– 81, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Watts BA, III, George T, Good DW. The basolateral NHE1 Na+/H+ exchanger regulates transepithelial HCO3− absorption through actin cytoskeletal remodeling in renal thick ascending limb. J Biol Chem 280: 11439– 11447, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Wilson PD. Polycystic kidney disease. N Engl J Med 350: 151– 164, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Wilson PD, Sherwood AC, Palla K, Du J, Watson R, Norman JT. Reversed polarity of Na+-K+-ATPase: mislocation to apical plasma membranes in polycystic kidney disease epithelia. Am J Physiol Renal Fluid Electrolyte Physiol 260: F420– F430, 1991 [DOI] [PubMed] [Google Scholar]
- 45. Yamamura H, Ugawa S, Ueda T, Shimada S. Protons activate the δ-subunit of the epithelial Na+ channel in humans. J Biol Chem 279: 12529– 12534, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Yoder BK, Richards WG, Sommardahl C, Sweeney WE, Michaud EJ, Wilkinson JE, Avner ED, Woychik RP. Differential rescue of the renal and hepatic disease in an autosomal recessive polycystic kidney disease mouse mutant. A new model to study the liver lesion. Am J Pathol 150: 2231– 2241, 1997 [PMC free article] [PubMed] [Google Scholar]
- 47. Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129: 5301– 5312, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Zhang Q, Murcia NS, Chittenden LR, Richards WG, Michaud EJ, Woychik RP, Yoder BK. Loss of the Tg737 protein results in skeletal patterning defects. Dev Dyn 227: 78– 90, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.