Abstract
Oculocerebrorenal syndrome of Lowe (OCRL) gene product is a phosphatidyl inositol 4,5-bisphosphate [PI(4,5)P2] 5-phosphatase, and mutations of OCRL cause Lowe syndrome and Dent disease, both of which are frequently associated with hypercalciuria. Transient receptor potential, vanilloid subfamily, subtype 6 (TRPV6) is an intestinal epithelial Ca2+ channel mediating active Ca2+ absorption. Hyperabsorption of Ca2+ was found in patients of Dent disease with increased Ca2+ excretion. In this study, we tested whether TRPV6 is regulated by OCRL and, if so, to what extent it is altered by Dent-causing OCRL mutations using Xenopus laevis oocyte expression system. Exogenous OCRL decreased TRPV6-mediated Ca2+ uptake by regulating the function and trafficking of TRPV6 through different domains of OCRL. The PI(4,5)P2 5-phosphatase domain suppressed the TRPV6-mediated Ca2+ transport likely through regulating the PI(4,5)P2 level needed for TRPV6 function without affecting TRPV6 protein abundance of TRPV6 at the cell surface. The forward trafficking of TRPV6 was decreased by OCRL. The Rab binding domain in OCRL was involved in regulating the trafficking of TRPV6. Knocking down endogenous X. laevis OCRL by antisense approach increased TRPV6-mediated Ca2+ transport and TRPV6 forward trafficking. All seven Dent-causing OCRL mutations examined exhibited alleviation of the inhibitory effect on TRPV6-mediated Ca2+ transport together with decreased overall PI(4,5)P2 5-phosphatase activity. In conclusion, OCRL suppresses TRPV6 via two separate mechanisms. The disruption of PI(4,5)P2 5-phosphatase activity by Dent-causing mutations of OCRL may lead to increased intestinal Ca2+ absorption and, in turn, hypercalciuria.
Keywords: transient receptor potential, vanilloid subfamily, subtype 6; oculocerebrorenal syndrome of Lowe
hypercalciuria is the most common metabolic abnormality in patients with kidney stones; however, the detailed molecular mechanisms underlying hypercalciuria are not well understood (9, 14, 40). Increased intestinal Ca2+ absorption, decreased renal Ca2+ reabsorption, or loss of Ca2+ from the bone may all contribute to the pathogenesis of hypercalciuria (14). The common form of hypercalciuria, idiopathic hypercalciuria, appears to be a disorder of polygenic trait, which provides limited evidence to the pathogenesis of hypercalciuria (40). On the other hand, some monogenic disorders are also associated with hypercalciuria. One of these disorders is Dent disease, an X-linked disorder featuring low-molecular-weight proteinuria, hypercalciuria, nephrocalcinosis, and kidney stone (10). Understanding the mechanism of hypercalciuria in Dent's disease may provide insights into the pathogenesis of idiopathic hypercalciuria.
Mutations in either the CLCN5 (24) or OCRL gene (15) may result in Dent's disease. CLCN5 gene encodes CLC-5, a H+/Cl− antiporter in the endosome (39). Removal of Clcn5 gene in mice results in a decreased level of endocytic receptor megalin due to the recycling defect in the proximal tubule and in turn causes proteinuria (35, 55). OCRL is a protein associated with oculocerebrorenal syndrome of Lowe, an X-linked disorder featuring congenital cataract, mental retardation, and renal proximal tubulopathy (1). OCRL is a phosphatidyl inositol 4,5-bisphosphate [PI(4,5)P2] 5-phosphatase (58) localized to the Golgi complex (11, 31) and endosome (5). OCRL regulates PI(4,5)P2 balance in these cellular compartments and in turn recruits different proteins for membrane trafficking. Mice lacking Ocrl gene or its homolog Inpp5b gene appear to be normal; however, mice lacking both Ocrl and Inpp5b exhibit embryonic lethality (18). Interestingly, when the Ocrl null mice express human INPP5B but not the mouse Inpp5b, they survive and develop syndrome similar to Dent disease (3). Thus the two lipid phosphatases could compensate for each other, and they are indispensable for some important cellular processes.
The pathogenesis of hypercalciuria in Dent disease is not well understood; however, it may be attributable to the elevated 1,25-dihydroxyvitmain D3 [1,25(OH)2D3] level (42), increased intestinal absorption (27), and renal Ca2+ leak and increased bone resorption (42) in Clcn5 knockdown or knockout animal models. About one-half of Dent disease patients have fasting hypercalciuria, and all patients exhibit exaggerated increase in Ca2+ excretion in response to oral Ca2+ loading (37). The elevated response to oral Ca2+ load and the elevated 1,25(OH)2D3 level in Dent patients are consistent with the involvement of hyperabsorption of Ca2+ under Dent disease conditions. Little is known about the mechanism underlying hypercalciuria caused by OCRL mutations.
TRPV6 (previously known as CaT1) is a Ca2+ channel expressed in the apical membrane of intestinal epithelial cells, where it mediates the first step of active Ca2+ absorption (33, 59). Trpv6 KO mice exhibit a 60% reduction in intestinal Ca2+ absorption (2). TRPV6 is highly regulated by 1,25(OH)2D3 at transcriptional level due to the presence of multiple vitamin D responsive elements in its promoter region (29). A 90% reduction of duodenal TRPV6 mRNA was observed in vitamin D receptor knockout mice (52). An ancestral haplotype of TRPV6 is associated with hyperabsorption of Ca2+ in a kidney stone patient (47). Intestinal overexpression of TRPV6 in mice resulted in elevated plasma Ca2+ level, soft tissue calcification, hypercalciuria, and bladder stone (7). This suggests that elevated TRPV6 activity could result in hypercalciuria.
In a Clcn5 knockout model of Dent disease, TRPV6 mRNA level is significantly elevated, likely due to increased 1,25(OH)2D3 in this mouse model (42). We observed that CLC-5 decreased protein abundance of TRPV6 when coexpressed in Xenopus laevis oocytes (30). We hypothesize that TRPV6 is also regulated by OCRL based on the evidence that OCRL is involved in protein trafficking. In this study, we examined this hypothesis and found OCRL inhibits TRPV6 via its two mechanisms by different domains. This regulation is impaired by Dent-causing mutations, arguing for a role of this regulation in the pathogenesis of hypercalciuria in Dent disease.
MATERIALS AND METHODS
cDNA constructs.
The human TRPV6 cDNA was described previously (32). The human OCRL cDNAs were purchased from Open Biosystems (Huntsville, AL) and were subcloned into the X. laevis oocytes expression vector pIN (20, 21) with Sal I and BamH I. To construct HA (hemagglutinin epitope)-tagged OCRL, OCRL cDNA was subcloned into pIN-HA vector with Mlu I and Sal I, and HA cDNA was linked to the 5′-terminus of OCRL cDNA with Mlu I site. Different fragments of OCRL were amplified from pIN-OCRL by PCR with AccPrime Pfx kit (Invitrogen, Carlsbad, CA) and cloned into pIN-HA or pIN-FLAG vector with Mlu I and Sal I. Mutants were generated using the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA) following the manufacturer's instruction and confirmed by sequencing.
Cloning of partial X. laevis OCRL cDNA.
Because no sequence information on X. laevis OCRL (xOCRL) was available for designing antisense oligodeoxynucleotides, we decided to clone partial xOCRL cDNA. Based on the predicted Xenopus tropicalis OCRL cDNA sequence (GenBank accession no. XM_002938379), we selected two primers in the conserved domain to amplify xOCRL cDNA fragment from X. laevis oocyte total RNA using RT-PCR approach. The two primers were 5′-agctacgcgtACCAAACACCCAGTCTG-3′ (1,458–1,474 of XM_002938379, sense) and 5′-agctacgcgtGTCACTGGTTTTGAGTTCC-3′ (2,394–2,376 of XM_002938379, antisense). The Mlu I restriction endonuclease cutting sites (underlined in the primers) were used to clone the amplified products to pIN vector for sequencing. The resultant 864-bp xOCRL cDNA fragment encodes 288 amino-acids mostly in the 5-phosphatase domain of xOCRL. The sequence information of xOCRL has been deposited to GenBank (accession no. JQ613219).
Ca2+ uptake in X. laevis oocytes.
In vitro transcription, injection of the resultant capped synthetic complementary RNAs (cRNAs) into oocytes, and 45Ca2+ uptake assay in oocytes were conducted as described previously (20, 21). The animal protocol used in this study was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. The cRNAs were injected at 12.5 ng/oocytes for each cRNA unless stated otherwise. In experiments involving knockdown endogenous OCRL, antisense oligodeoxynucleotide against X. laevis OCRL (anti-xOCRL, 5′-CATTCCAACAAGGCGG-3′) or control oligodeoxynucleotide (control, 5-CTAAAATACCGCAGCC-3′) were injected 1 day before injection of TRPV6 cRNA. Ca2+ uptake data are presented as mean values from at least two experiments with 7 oocytes per group, using the means ± SE as the index of dispersion.
Western blot analysis and biotinylation.
Western blot and biotinylation were performed as described previously (20, 21). Monoclonal anti-HA antibody (product no. H9658, 1:5,000 dilution) was purchased from Sigma-Aldrich (St. Louis, MO). The rabbit customer-made anti-TRPV6 antiserum (1:3,000 dilution) was described previously (57). Mouse anti-human OCRL (sc-100386, 1:1,000) and anti-β-actin antibody (sc-47778, 1:5,000 dilution) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-FLAG antibody (F7425, 1:2,000 dilution) was purchased from Sigma-Aldrich. Appropriate horseradish peroxidase-conjugated secondary antibodies (1:5,000 dilution) were purchased from Santa Cruz Biotechnology. Chemiluminescence was detected using a SuperSignal West Femto maximum sensitivity substrate or SuperSignal West Pico chemiluminescent substrate kit (Pierce, Rockford, IL) in accordance with the manufacturer's protocol.
RNA isolation and RT-PCR.
RNA was isolated from rat intestinal tissues or X. laevis oocytes as described previously (57). One microgram of total RNA was reverse-transcribed with random primer and SuperScript III first-strand kit (Invitrogen, Carlsbad, CA). GAPDH, which is a housekeeping gene, was served as a control gene to verify the consistency of reverse transcription. Rat TRPV6 fragment was amplified by PCR using the following primers: 5′-CATCTTCCAGACAGAGGACCC-3′ (1,527–1,547 of open reading frame, sense) and 5′-TTAGATCTGGTACTCCCAGCCCT-3′ (2,184–2,162, antisense). For rat OCRL, the primers used were 5′- GAATGTGAAGTTTCGGCAGC-3′ (1,719–1,738, sense) and 5′- AGGGATTGTCTCAGGAATGCT-3′ (2,337–2,317, antisense). For X. laevis OCRL (xOCRL), the primers used were 5′-AGCTACGCGTGCACATCCTTGCAAGAAAGA-3′ (sense) and 5′-AGCTGTCGACTTACATGTGGCTGCGGTATTTTAG-3′ (antisense), and the Mlu I and Sal I endonuclease cutting sites were included in sense and antisense primers, respectively, to clone the amplified cDNA in to pIN-HA vector. For X. laevis GAPDH, primers used were 5′-CGGATTTGGCTGTATTGG-3′ (65–82, sense) and 5′ CAGGATTCCCTTCATTGG-3′ (860–843, antisense). Amplification reactions were performed with Premix rTaq (New England Biolabs, Ipswich, MA) for 35 cycles for TRPV6, 30 cycles for OCRL, and 18 cycles for GAPDH using Mastercycler (Eppendorf, Westbury, NY).
Trafficking of TRPV6.
For assessing the effect of exogenous OCRL on the trafficking of TRPV6, oocytes were injected with TRPV6 cRNA alone or with HA-OCRL at 12.5 ng/oocyte and for assessing endogenous OCRL on TRPV6 trafficking, 1 day before injection of TRPV6 cRNA, oocytes were injected with an antisense oligodeoxynucleotide against xOCRL (anti-XOCRL) or nonspecific oligodeoxynucleotide (control). After being cultured at 18°C for 36 h postinjection, oocytes were subjected to injection with cRNA of Rab11a S25N, which inhibit recycling (38, 53), or incubation with brefeldin A (BFA, Tocris Bioscience, Ellisville, MO) at 20 μg/ml, or both. The Ca2+ uptake assay was carried out at 0, 12, and 24 h later. For controlling the effects not specific to TRPV6, separate oocyte groups were injected with the same combinations of cRNAs or water except for the TRPV6 cRNA. The control groups were treated the same way as the experimental groups. Ca2+ uptake values in the experimental groups were subtracted by the control groups to obtain TRPV6-mediated Ca2+ uptake. Data are expressed as percentage of the TRPV6-mediated Ca2+ uptake value at 0 h.
Pulse and chase experiments.
Oocytes were injected with cRNAs of glutathione S-transferase (as control), HA-OCRL or its mutants and at 12.5 ng/oocyte each. Oocytes were incubated at 18°C in 0.5× L-15 medium. Twelve hours later, FLAG-TRPV6 cRNA (25 ng/oocyte) was injected into the preinjected oocytes. After a 5-h incubation period, part of the oocytes in each group was isolated and the total proteins were extracted, and the remaining oocytes were incubated with cycloheximide (100 μg/ml), MG-132 (20 μM), and chloroquine (100 μM) to block protein synthesis, proteasomal, and lysosomal degradation, respectively. Oocyte proteins were extracted at 5, 10, or 24 h from application of the inhibitors. TRPV6 protein levels were analyzed by Western blot using anti-FLAG antibody.
Assessment of 5-phosphatase activity.
Oocytes were injected with water or cRNA of HA-OCRL or its mutants, and 36 h later the total proteins were extracted with lysis buffer (100 mM NaCl, 20 mM Tris·Cl, and 1% Triton X-100 plus protease inhibitor mixture, pH 7.6). For each assay, 10 oocytes were extracted with 200 μl lysis buffer and the amount corresponding to 0.1 μl of the lysate was mixed into 25 μl phosphatase buffer (20 mM Tris·Cl pH 7.2, 150 mM NaCl, 3 mM MgCl2, and 200 μg/ml BSA) that contained 2,000 pmol PI(4,5)P2 diC8 (Echelon Biosciences, Salt Lake City, UT), and the reaction mixture was incubated at 37°C for 30 min, followed by incubation at 75°C for 3 min to inactive the phosphatase. Background free phosphate in water-injected oocytes was determined by the same procedure without addition of PI(4,5)P2 diC8. The phosphatase activity was evaluated by the production of free phosphate using malachite green phosphatase assay kit (Echelon Biosciences, Salt Lake City, UT) following the manufacturer's instructions and described briefly as follows. Firstly, free phosphate standard (provided in the kit) was diluted to a series of concentrations ranging from 0 to 1,600 pmol at 200-pmol intervals in 25-μl solutions. Then, the standards or reaction mixtures were mixed with 100 μl malachite green solution (provided in the kit) and incubated at room temperature for 20 min. The absorbance of each reaction mixture at 620 nm was determined with a spectrometer. Standard curves were plotted using the regression program of SPSS software 13.0 (IBM, New York, NY) to fit the data. The values of free phosphate in the reaction mixtures were determined based on their absorbance at 620 nm using standard curves.
Immunofluorescent staining.
Rat intestinal tissue section and immunostaining were performed as described previously (57). Rabbit anti-TRPV6 antibody was described previously (57). Mouse anti-OCRL antibody (sc-100386, 1:100), bovine anti-mouse IgG-TR (sc-2788, 1:1,000), and goat anti-rabbit IgG-FITC (sc-2012, 1:500) were all purchased from Santa Cruz Biotechnology, (Santa Cruz, CA).
RESULTS
Expression of OCRL in rat intestine.
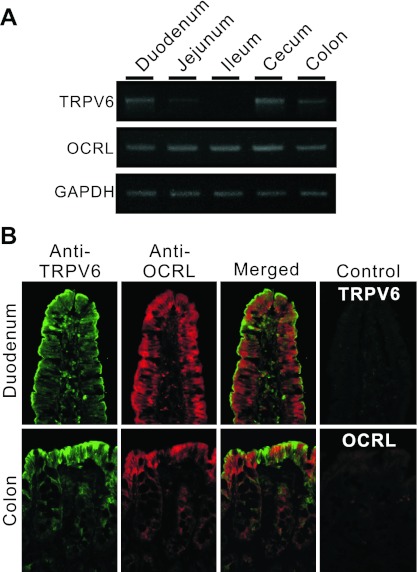
OCRL has been shown to be present in all human tissues and cell lines examined, including intestinal tissues (22). We confirmed the distribution of OCRL mRNA in rat intestinal tract by RT-PCR (Fig. 1A). For comparison, we also measured the TRPV6 mRNA level in the intestinal tract. Unlike TRPV6, which exhibited a decreasing mRNA gradient from proximal to distal small intestine as observed previously (33, 57), OCRL mRNA level did not vary significantly throughout the rat intestine (Fig. 1A) as observed previously in humans (22). OCRL antibody exhibited significant immunostaining in epithelia of rat duodenum and colon where TRPV6 was strongly expressed (Fig. 1B). Although TRPV6 and OCRL signals were identified in the same cells, TRPV6 proteins were mostly distributed to the apical membrane whereas OCRL staining was mostly observed in intracellular compartment (Fig. 1B). No specific staining was observed using preimmune serum for TRPV6 antibody or without the primary antibody of OCRL (Fig. 1B).
Fig. 1.
Distribution of oculocerebrorenal syndrome of Lowe (OCRL) and transient receptor potential, vanilloid subfamily, subtype 6 (TRPV6) in rat intestinal tract. A: RT-PCR results showing the expression of OCRL and TRPV6 in rat intestine at mRNA level. B: double immunofluorescent staining of rat duodenal and colon tissues with OCRL and TRPV6 antibodies. TRPV6 antiserum labeled strongly in apical membrane in rat duodenum and colon. OCRL antibody exhibited intracellular staining, especially in the subapical region of epithelial cells in duodenum and colon. Preimmune serum (for control of TRPV6 staining) and secondary antibody alone (for control of OCRL staining) exhibited no specific staining. Magnification = ×250.
OCRL decreases TRPV6-mediated Ca2+ uptake in X. laevis oocytes.
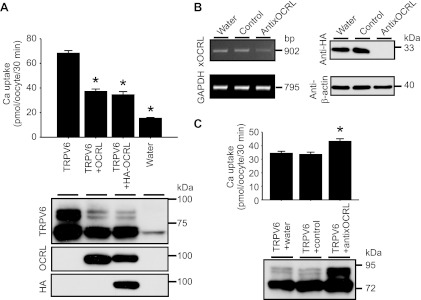
To evaluate the potential effect of OCRL on TRPV6, we injected cRNAs for OCRL and TRPV6 in X. laevis oocytes and TRPV6-mediated Ca2+ uptake was assessed 2 days later (Fig. 2A). TRPV6-mediated Ca2+ uptake value was decreased by 58.7 ± 4.1% in the presence of OCRL (Fig. 2A, top). TRPV6 protein abundance, especially the complexly N-glycosylated forms with higher molecular weight (20), was also significantly reduced as assesses by Western blot analysis (Fig. 2A, bottom). NH2-terminal tagging OCRL with HA epitope (HA-OCRL) did not significantly affect the ability of OCRL to inhibit TRPV6 (Fig. 2A).
Fig. 2.
OCRL decreased TRPV6-meidated Ca2+ uptake. A: Ca2+ uptake values of control oocytes injected with water or oocytes injected with complementary RNAs (cRNAs) for TRPV6, TRPV6 and OCRL, and TRPV6 and hemagglutinin (HA)-OCRL (12.5 ng/oocyte for each cRNA) 2 days earlier. Data are presented as means ± SE of 3 independent experiments with 21 oocytes in each group. *P < 0.01 vs. TRPV6 alone group. Representative Western blot analyses for proteins extracted from different groups using antibodies against TRPV6, OCRL, and HA epitope are shown (bottom). B: effectiveness of anti-xOCRL oligodeoxynucleotide in knocking down xOCLR mRNA and protein in Xenopus laevis oocytes. Left: RT-PCR results for xOCRL from oocytes injected with anti-xOCRL, control oligodeoxynucleotide, and water for 1 day. Right: Western blot analysis with HA antibody showing exogenous expressed HA-tagged xOCRL fragment knocked down by anti-xOCRL but not by control oligodeoxynucleotide. C: Ca2+ uptake of oocytes injected with antisense against endogenous xOCRL (anti-x-OCRL), control oligodeoxynucleotide or water 1 day before injection of TRPV6 cRNA. Data are presented as means ± SE of 4–5 independent experiments with 28–35 oocytes in each group. *P < 0.01 vs. water or control group.
To evaluate the effect of endogenous X. laevis OCRL (xOCRL) on TRPV6, we cloned a part of xOCRL cDNA (GenBank accession no. JQ613219) from oocytes. Based on the sequence of xOCRL, we designed antisense oligodeoxynucleotide against xOCRL (anti-xOCRL). RT-PCR results indicated anti-xOCRL successfully decreased endogenous xOCRL mRNA in oocytes relative to control oligodeoxynucleotide and water (Fig. 2B, left). In the absence of an antibody against xOCRL, to confirm the effectiveness of anti-xOCRL antisense oligodeoxynucleotide in knocking down xOCRL protein, we cloned a xOCRL fragment (encoding 288 amino acids that are mainly located in the 5-phosphatase domain) into pIN-HA vector for expression in oocytes. The cRNA for the HA-tagged xOCRL fragment was injected into oocytes, and 12 h later, anti-xOCRL, control oligodeoxynucleotide, or water was injected. Two days later, xOCRL abundance was analyzed by Western blot. In contrast to oocytes injected with control oligodeoxynucleotide or water, the HA-tagged xOCRL fragment was not detectable in oocytes injected with anti-xOCRL oligodeoxynucleotide, whereas β-actin was unaffected (Fig. 2B, right). These results confirmed the capability of anti-xOCRL to effectively knockdown xOCRL, even though the exact levels of endogenous xOCRL in different groups were not known. To evaluate the effect of anti-xOCRL on TRPV6-mediated Ca2+ uptake, TRPV6 cRNA was injected 1 day after injection of anti-xOCRL, control oligodeoxynucleotide, or water. Compared with control and water, anti-xOCRL oligodeoxynucleotide significantly increased TRPV6-mediated Ca2+ uptake and the abundance of complexly glycosylated bands of TRPV6 (Fig. 2C). These observations indicate that endogenous xOCRL inhibits TRPV6, similar to exogenous OCRL.
The 5-phosphatase domain of OCRL regulates TRPV6 Ca2+ uptake activity.
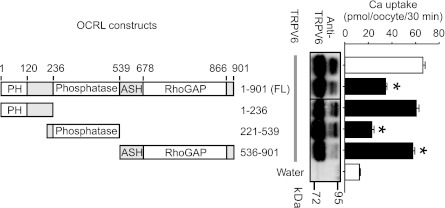
To identify key domains in OCRL that are important in regulating TRPV6, we constructed various HA-tagged OCRL fragments and evaluated their effects on TRPV6 (Fig. 3). The NH2-terminal (amino-acids 1–236) or COOH-terminal region (amino-acids 536–901) alone had minimal effects on TRPV6. In contrast, the middle section (construct 221–539) that contains the 5-phosphatase domain (amino-acids 237–539) exhibited robust inhibiting effect on TRPV6-mediated Ca2+ uptake (65.4 ± 3.0% inhibition). It has been shown that TRPV6 channel is inactivated when PI(4,5)P2 is depleted and application of PI(4,5)P2 prevents TRPV6 inactivation (48). Therefore, the 5-phosphatase activity of OCRL may suppress TRPV6 by decreasing the level of PI(4,5)P2. The Ca2+ uptake values were not always correlated to the abundance of the complexly glycosylated bands of TRPV6 in Fig. 3, because complexly glycosylated TRPV6 proteins only contributed to a small fraction of functional TRPV6 at the plasma membrane and the majority of TRPV6 detected at the plasma membrane was in core-glycosylated form (20). In addition, PI(4,5)P2 may only affect the function of TRPV6 without a significant effect on the membrane abundance of TRPV6. The 536–901 construct appeared to reduce the abundance of complexly glycosylated TRPV6, likely due to the Rab binding domain in the construct that affects the forward trafficking of TRPV6 (see below and Fig. 6E).
Fig. 3.
Effects of OCRL fragments on TRPV6. Ca2+ uptake values in oocytes expressing TRPV6 alone or together with different HA-tagged OCRL fragments (left) are shown. Each group includes 14–21 oocytes from 2–3 independent experiments. Representative Western blot analysis (jointed from the same blot) with TRPV6 antibody is also shown. *P < 0.01 vs. TRPV6 alone group.
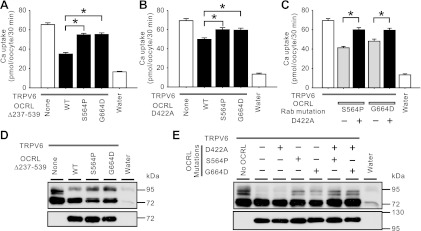
Fig. 6.
5-Phosphatase domain and Rab binding domain could act independently in regulating TRPV6. A: Ca2+ uptake in oocytes expressing TRPV6 alone or TRPV6 and HA-OCRL construct lacking the 5-phosphatase domain (Δ237–539) or HA-OCRL construct with either S564P or G664D mutation. B: Ca2+ uptake in oocytes expressing TRPV6 alone or TRPV6 and the D422A mutant of HA-OCRL in the absence or presence of either S564P or G664D mutation. C: Ca2+ uptake in oocytes expressing TRPV6 alone or TRPV6 and HA-OCRL S564P or G664D mutant in the presence or absence of the D422A mutation. D and E: representative Western blot analyses with TRPV6 and HA antibodies, respectively, for groups in A-C. cRNAs of HA-OCRL constructs (wild-type or mutant) were injected at 25 ng/oocyte and TRPV6 cRNA was injected at 12.5 ng/oocyte. Each group included 18–21 oocytes from 3 independent experiments. *P < 0.01.
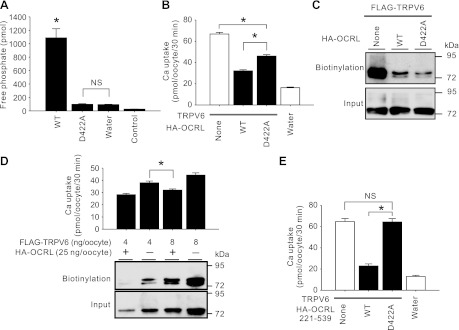
Mutations of conserved amino acids in the inositol polyphosphate phosphatase catalytic domain homologues abolish the 5-phosphatase activity of the platelet 75-kDa inositol polyphosphate 5-phosphatase II (19). As a member of the inositol polyphosphate phosphatase family, OCRL also possesses these conserved amino acid residues in its catalytic phosphatase domain. When a conserved aspartate residue in OCRL was mutated to alanine (D422A), the phosphatase activity was abolished (Fig. 4A). The D422A mutation also alleviated the inhibitory effect of OCRL on TRPV6 (Fig. 4B). Coexpression of OCRL decreased the surface abundance of TRPV6 protein (Fig. 4C). Unlike TRPV6 Ca2+ uptake activity, TRPV6 protein abundance at the cell surface was not restored by the D422A mutation (Fig. 4C). This suggests the effect of the 5-phosphatase of OCRL is on the Ca2+ uptake activity of TRPV6. By manipulating the level of TRPV6 cRNA injected, we observed a condition that the surface abundance of TRPV6 protein was higher in a group of oocyte coexpressing TRPV6 and OCRL but the Ca2+ uptake activity was actually lower than the group expressing TRPV6 alone with a lower level of TRPV6 protein at the cell surface (Fig. 4D). This suggested that TRPV6 Ca2+ uptake activity is inhibited by OCRL likely through its PI(4,5)P2 phosphatase activity. To confirm the role of the catalytic activity of the 5-phosphatase domain in regulating TRPV6, we introduced the D422A mutation in the 5-phosphatase alone construct (amino-acids 221–539). In this background, the D422A mutation completely abolished the inhibitory effect on TRPV6 (Fig. 4E). Comparing Fig. 4, B and E, it became clear that 1) the 5-phosphatase activity is capable of regulating TRPV6 on its own, and 2) other mechanisms in OCRL also play a significant role in regulating TRPV6.
Fig. 4.
5-Phosphatase domain played a role in downregulating TRPV6. A: free phosphate produced from phosphatidyl inositol 4,5-bisphosphate [PI(4,5)P2] diC8 by lysate of oocytes injected with cRNA for wild-type HA-OCRL (WT), D422A mutant, or water. Control group shows the free phosphate in from lysates of water injected oocytes without addition of PI(4,5)P2 diC8. Data derived from 3 independent experiments are expressed as means ± SE. *P < 0.01 vs. D422A or water group; NS, not significant (P > 0.05). B. Ca2+ uptake in oocytes expressing TRPV6 alone or TRPV6 plus HA-tagged wild-type or D422A mutant of OCRL. cRNAs of OCRL constructs (wild-type or mutant) were injected at 25 ng/oocyte and TRPV6 cRNA was injected at 12.5 ng/oocyte. Data of 39–42 oocytes/group from 6 independent experiments are shown. C: biotinylated FLAG-TRPV6 in oocytes expressing FLAG-TRPV6 alone or together with HA-OCRL wild type or its D422A mutant detected by FLAG antibody. D: surface abundance of FLAG-TRPV6 detected by biotinylation vs. Ca2+ uptake value among groups of oocytes injected with 4 or 8 ng FLAG-TRPV6 cRNA/oocyte, with or without 25 ng of HA-OCRL cRNA/oocyte. Data are derived from 14–56 oocytes in 2–8 independent experiments. E: Ca2+ uptake of oocytes expressing TRPV6 alone or TRPV6 plus HA-tagged OCRL 5-phosphatase domain (amino acids 221–539) or the domain containing D422A mutation. Each group included 21 oocytes from 3 independent experiments. *P < 0.01; NS, not significant (P > 0.05).
Rab protein binding domain of OCRL regulates TRPV6 protein abundance.
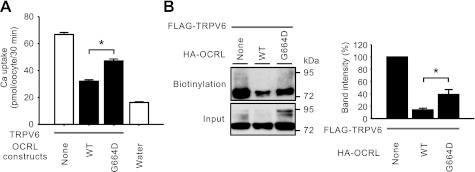
Rab GTPases are monomeric G proteins regulating many steps of membrane trafficking (44). OCRL has been shown to be a binding partner for Rab proteins in Golgi apparatus and early endosome (17). The minimal sequence of OCRL required for Rab binding (amino-acids 555–678) is mainly localized to the ASH domain (16). Mutations such as G664D in OCRL disrupt its binding to Rab proteins (17). To evaluate the role of Rab binding in the regulation of TRPV6 by OCRL, we assessed the effect of G664D mutant on TRPV6 (Fig. 5). The G664D mutant exhibited weakened ability to inhibit TRPV6-mediated uptake (Fig. 5A). In contrast to the 5-phosphatase disrupting D442A mutant, the G664D mutant also partially restored the TRPV6 protein abundance at the cell surface (Fig. 5B).
Fig. 5.
Role of Rab binding domain of OCRL in regulating TRPV6. A: Ca2+ uptake in oocytes expressing TRPV6 alone or TRPV6 and HA-tagged wild-type OCRL or G664D mutants, which is defective in Rab binding. cRNAs of HA-OCRL constructs were injected at 25 ng/oocyte. Data of 39–42 oocytes/group from 6 independent experiments are shown. *P < 0.01. B: representative Western blot analysis of biotinylated FLAG-TRPV6 proteins detected with FLAG antibody. Summary of the band intensity of 3 independent blots are shown at right. *P < 0.01.
Because Rab interaction stimulates the 5-phosphatase activity of OCRL (17), we wondered whether the relieving effect of Rab binding mutations on TRPV6 is related to a potential alteration of 5-phosphatase activity. To test this, we introduced G664D and S564P, another Rab binding mutation (17) in the 5-phosphatase domain-lacking and D422A mutants, respectively (Fig. 6, A and B). The two Rab binding-defective mutations were able to relieve the inhibitory effect of the 5-phosphatase-defective OCRL proteins on TRPV6-mediated Ca2+ uptake, suggesting that the OCRL Rab binding function may regulate TRPV6 independently. On the other hand, in the background of Rab binding-defective S564P or G664D mutation, the 5-phosphatase activity still played a role in inhibiting TRPV6 because the D422A mutation significantly relieved the inhibitory effects of the Rab binding-defective OCRL on TRPV6 (Fig. 6C). However, the D422A mutation had little effect on the abundance of the complexly N-glycosylated TRPV6 (Fig. 6E). In contrast, the Rab binding mutations (S564P and G664D) significantly relieved the inhibitory effect of OCRL on the abundance of the complexly N-glycosylated bands of TRPV6 (Fig. 6E). This effect of S564P and G664D mutations was preserved in the 5-phosphatase-lacking construct (Fig. 6D) and 5-phosphatase-defective construct (Fig. 6E). These results indicate Rab binding and 5-phosphatase activity of OCRL could act independently in inhibiting TRPV6.
OCRL regulates TRPV6 trafficking.
To further understand the mechanisms by which TRPV6 is regulated by OCRL, we evaluated the effect of biosynthesis and degradation of TRPV6. The total amount of TRPV6 protein synthesized in 5-h time period was not altered by OCRL expression, and the degradation of TRPV6 protein was only slightly enhanced in the presence of OCRL (data not shown). Thus TRPV6 protein synthesis and degradation appeared not to be the key steps regulated by OCRL. We then evaluated whether TRPV6 trafficking is affected by OCRL. After being synthesized in the endoplasmic reticulum, TRPV6 proteins can be delivered through the secretory pathway or an alternative pathway to the plasma membrane (20). The majority of TRPV6 protein reaches the plasma membrane through the alternative pathway where it bypasses the trans-Golgi network and therefore is not complexly glycosylated (20). There is also a TRPV6 protein-recycling pool under the plasma membrane, where TRPV6 can go back and forth between the recycling pool and plasma membrane (53). It is well established that Rab11 is involved in the recycling process (38). The Rab11a S25N mutation, which disrupts GTP binding, interacts directly with TRPV6 and inhibits its recycling (53). Rab11a S25N mutant is thus a tool to block TRPV6 recycling.
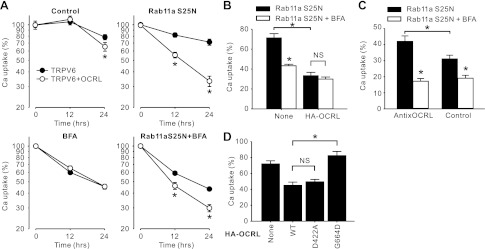
A very moderately more rapid decrease in TRPV6-mediated Ca2+ uptake was observed in the presence of HA-OCRL compared with that in the absence of HA-OCRL (TRPV6-mediated Ca2+ uptake was 65.5 ± 5.5% and 79.0 ± 4.1% of those at time 0, respectively, in presence and absence of HA-OCRL 24-h later; Fig. 7A, control). By expression of Rab11a S25N to inhibit the recycling of TRPV6 back to cell surface, a much more dramatic decline in TRPV6-mediated Ca2+ uptake was revealed in the group coinjected with HA-OCRL than the TRPV6 alone group (Fig. 7A, Rab11a S25N). One day postinjection of Rab11a S25N cRNA, the Ca2+ uptake value decreased to 33.4 ± 3.3% in presence of HA-OCRL compared with 71.3 ± 4.3% in the absence of HA-OCRL. Rab11a S25N proteins were rapidly synthesized in oocytes and high levels of Rab11a S25N protein were maintained at least from 3 to 24 h after injection (data not shown). It has been shown that unlike some Rab proteins, Rab11 does not bind to OCRL (17); thus it is unlikely Rab11a influences the function of OCRL. This suggested that the recycling of TRPV6 from the recycling pool to the plasma membrane may be enhanced in the presence of HA-OCRL. Since the recycling was blocked, an alteration in either the forward or the reverse trafficking or both should account for the observed effects of OCRL on TRPV6 in the presence of Rab11a S25N.
Fig. 7.
OCRL regulates TRPV6 trafficking. A: trafficking of TRPV6 in the presence and absence of HA-OCRL. Thirty-six hours after injection of cRNA(s) for TRPV6 or TRPV6 plus HA-OCRL each at 12.5 ng/oocyte, oocytes were injected with cRNA of Rab11a S25N at 12.5 ng/oocyte, treated with brefeldin A (BFA, 20 μg/ml), or both. TRPV6-mediated Ca2+ uptake was assessed at 0, 12, and 24 h after treatment. Data from 30–35 oocytes of 5 independent experiments are represented as percent of the value at 0 h in each group. *P < 0.01 vs. TRPV6 alone group. B: effect of BFA on TRPV6-mediated Ca2+ uptake was diminished in the presence of HA-OCRL. Data are derived from the Rab11aS25N group and Rab11a S25N + BFA group at 24 h in A. C: effect of BFA on TRPV6-mediated Ca2+ uptake was increased when endogenous xOCRL was knocked down by the anti-xOCRL oligodeoxynucleotide. The process of this experiment was similar to that described in B except that oligodeoxynucleotides were injected 1 day before injection of TRPV6 cRNA. Data are derived from 3 independent experiments. D: Rab binding disrupting G664D mutation, but not the phosphatase-dead D422A mutation, abolished the effect of HA-OCRL on TRPV6 in the presence of Rab11a S25N as described in A. Data from 23–28 oocytes of 4 independent experiments are expressed as percentage of the Ca2+ uptake value at 0 h. *P < 0.01; NS, not significant (P > 0.05).
We next used BFA, which disrupts Golgi apparatus and in turn prohibits insertion of newly synthesized proteins to the plasma membrane (23), to block the forward trafficking of TRPV6 after 36-h expression of TRPV6 or TRPV6 and HA-OCRL (Fig. 7A, BFA). The decrease in TRPV6-mediated Ca2+ uptake was not different in the presence and absence of HA-OCRL with 12-h or 24-h BFA treatment (20 μM). This suggests the forward trafficking of newly synthesized proteins may account for the difference caused by HA-OCRL observed in the control group of Fig. 7A.
We next blocked both the recycling and forward trafficking with both Rab11a S25N and BFA. Under this condition, TRPV6-mediated Ca2+ uptake decreased significantly more rapidly in the presence of HA-OCRL than in the absence of HA-OCRL (Fig. 7A, Rab11a S25N + BFA). Since both forward trafficking and recycling back to plasma membrane were blocked, the difference should be attributed to the reverse trafficking of TRPV6. Thus this result indicates the reverse trafficking of TRPV6 was accelerated by OCRL.
Comparing the difference between the effect of Rab11a S25N alone (Fig. 7A, Rab11a S25N) and that together with BFA (Fig. 7A, Rab11a S25N + BFA), it became apparent that the forward trafficking of TRPV6 was also decreased significantly by OCRL. In the absence of HA-OCRL, Ca2+ uptake was decreased by 39.3% (from 71.3 ± 4.3 to 43.3 ± 1.5%) with 24-h BFA treatment. By contrast, only 10.5% (from 33.4 ± 3.3 to 29.9 ± 2.1%) was observed in the presence of HA-OCRL (Fig. 7B). This indicated that the process blocked by BFA was largely diminished in the presence of OCRL when the recycling of TRPV6 was blocked (Fig. 7B). To evaluate endogenous X. laevis xOCRL on TRPV6 trafficking, we injected anti-xOCRL or control oligodeoxynucleotide into oocytes 1 day before injection of TRPV6 cRNA. The effectiveness of this anti-xOCRL oligodeoxynucleotide in knocking down of xOCRL was evaluated earlier (Fig. 2, B and C). In the presence of Rab11a S25N, Ca2+ uptake was decreased by 59.1% (from 42.0 ± 3.4 to 17.2 ± 1.7%) within 24-h BFA treatment in the presence of anti-xOCRL. In contrast, only 38.7% (from 31.4 ± 2.3 to 19.1 ± 1.8%) was observed in the presence of control oligodeoxynucleotide (Fig. 7C). This suggests that endogenous xOCRL acts similarly to BFA (Fig. 7C). In addition, knocking down xOCRL by anti-xOCRL did not prevent the decrease of TRPV6-mediated Ca2+ uptake in the presence of Rab11a S25N and BFA, and 24 h later the remaining Ca2+ uptake value was 17.2 ± 1.7% for anti-xOCRL group and 19.1 ± 1.8% for control oligodeoxynucleotide (Fig. 7C), indicating that the endogenous OCRL may not accelerate the reverse trafficking of TRPV6. Taken together the results, both exogenous and endogenous OCRL blocked the forward trafficking of newly synthesized TRPV6 proteins.
To further understand whether the phosphatase activity or the Rab protein binding domain plays a critical role in regulating TRPV6 trafficking, we evaluated the effects of D422A and G664D mutant, respectively, in the presence of Rab11a S25N. Twenty-four hours after injection of Rab11a S25N cRNA, the inhibitory effect of HA-OCRL on TRPV6-mediated Ca2+ uptake was completely abolished by the G664D mutation. In contrast, the D422A mutation had no significant effect (Fig. 7D). This indicated that it was the Rab protein binding domain, not the phosphatase activity, played an essential role in regulating TRPV6 trafficking.
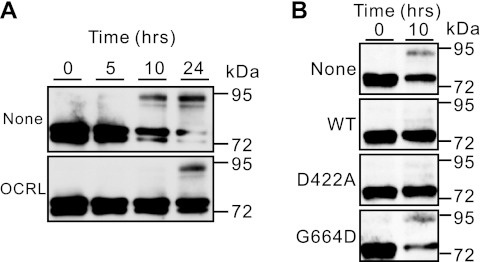
To confirm the effect of OCRL on TRPV6 forward trafficking, we conducted pulse and chase experiments. In this experiment, TRPV6 was expressed for 5 h in the presence or absence of preexpressed HA-OCRL and then protein synthesis was blocked by cycloheximide (100 μg/ml). Because the TRPV6 proteins made in the 5-h period could not be degraded through the proteasomal and lysosomal pathways in the presence of MG-132 (20 μM) and chloroquine (100 μM), part of these proteins was expected to go through the secretory pathway. Along the way, the proteins will be core glycosylated in the endoplasmic reticulum and then be complexly glycosylated in the Golgi apparatus. Thus the forms of glycosylation in TRPV6 reflect its forward trafficking process, even though part of TRPV6 goes to the plasma membrane through an alternative pathway (20). As shown in Fig. 8A, it is evident that significant amount of complexly glycosylated TRPV6 appeared in the absence of OCRL 10 h after the administration of the drugs. In contrast, roughly the same level of complexly glycosylated TRPV6 protein appeared at 24 h in the presence of HA-OCRL (Fig. 8A). In addition, there was a significant decrease in the abundance of the nonglycosylated and core-glycosylated TRPV6 as the complexly glycosylated forms increased in the absence of HA-OCRL; the same was not obvious in the presence of HA-OCRL due to the lower level of complexly glycosylated TRPV6 (Fig. 8A). Thus HA-OCRL significantly slowed down the forward trafficking process. This is likely the reason that the complexly glycosylated forms of TRPV6 were decreased by OCRL (Figs. 2 and 6E).
Fig. 8.
HA-OCRL slowed down the transition of nonglycosylated and core-glycosylated forms of TRPV6 (∼72 kDa) to complexly glycosylated forms (∼95 kDa; A) and Rab binding disrupting G664D mutation, but not the phosphatase-dead D422A mutation, abolished this effect (B). See materials and methods for details.
The involvement of phosphatase activity and Rab binding domain in the forward trafficking of TRPV6 was further evaluated at the 10-h time point when the complexly glycosylated forms of TRPV6 became apparent in the absence of OCRL (Fig. 8B). The G664D mutation abolished the blocking effect of TRPV6 forward trafficking, whereas the D422A mutant behaved similar to the wild-type OCRL. This is consistent with the different effects of the two mutations on TRPV6 trafficking (Fig. 7D), supporting the role of the Rab binding domain in regulating TRPV6 trafficking. Thus the different effects of the Rab binding mutations (S564P and G664D) and the 5-phosphatase mutation (D422A) on the complexly glycosylated forms of TRPV6 (Fig. 6E) likely reflected their differential effects on the ability of OCRL to control TRPV6 trafficking.
OCRL Dent-causing mutations relieve the suppressing effect on TRPV6.
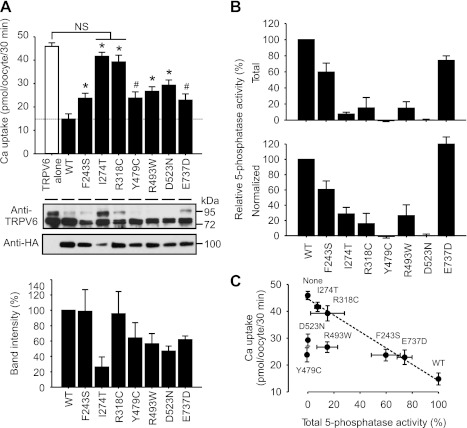
To examine whether the regulation of TRPV6 by OCRL is relevant to the pathogenesis of hypercalciuria in Dent disease, we next determined to what extent the Dent-causing mutations of OCRL affect the regulation of TRPV6. All mutations examined, including R318C and Y479C (15), F243S (4), I274T and R493W (51), and D523N and E737D (49), are associated with hypercalciuria. Compared with the wild-type OCRL, all these mutations significantly relieved the suppression of TRPV6-mediated Ca2+ uptake by OCRL, ranging from 25.9 ± 8.7% for E737D to 86.3 ± 5.6% for I274T (Fig. 9A, top), and the TRPV6 protein abundance varied with different mutations (Fig. 9A, middle). Some OCRL mutants had lower steady-state protein level than the wild-type OCRL (Fig. 9A, middle and bottom), and this might be one of the reasons that they were not as effective in suppressing TRPV6. The PI(4,5)P2 5-phosphatase activity was decreased in all the mutants tested (Fig. 9B, bottom). Six out of the seven mutations are localized in the 5-phosphatase domain. When normalized to the protein level, their phosphatase activities were all decreased (Fig. 9B, bottom). The E737D mutation, which is outside the 5-phosphatase domain, did not show a decrease in the 5-phosphatase activity after normalization (Fig. 9B, bottom).
Fig. 9.
Dent-causing mutations impaired the suppression of TRPV6 by OCRL. A: Assessment of the effects of Dent-causing OCRL mutations in HA-OCRL construct on TRPV6-mediated Ca2+ uptake. Representative Western blot analyses with TRPV6 and HA antibodies, respectively, are shown at middle. Band intensities of disease-causing mutants relative to wild-type HA-OCRL from 5 independent experiments are shown at bottom. Amount of cRNAs for HA-OCRL constructs was injected at 25 ng/oocyte. Ca2+ uptake value of each group was obtained by subtracting that of oocytes injected with water. Each group includes 21 oocytes from 3 independent experiments. *P < 0.01, #P < 0.05; NS, not significant (P > 0.05). B: relative PI(4,5)P2 5-phosphatase activities of disease-causing mutants. Total activity (top) and activity normalized (bottom) by the protein level of the mutants as reflected by their band intensities are shown. Data from 3 independent experiments are presented. Background value from the group injected with water was subtracted from each group. C: plot of TRPV6-mediated Ca2+ uptake value as the function of PI(4,5)P2 5-phosphatase activity of disease-causing mutants and WT HA-OCRL. Value of 5-phosphatase activity in oocytes expressing TRPV6 alone without exogenous HA-OCRL (None) was set to 0. Dashed line represents a curve fitting by linear regression without the 3 outlier (Y479C, R493W, and D523N).
In general, the value of TRPV6-mediated Ca2+ uptake under the regulation of OCRL mutants was inversely related to the level of total 5-phosphatase activity of these mutants (Fig. 9C). Four of the mutants, including I274T, F243S, R318C, and E737D, together with the wild-type and none OCRL, fit well with a linear relationship between Ca2+ uptake activity and 5-phosphatase activity (R2 = 0.986). The other three mutants, including Y479C, R493W, and D523N, differed from the relationship. Interestingly, the three mutants blocked the complexly glycosylated bands of TRPV6 more completely than the wild-type (Fig. 9A, middle). It is likely that the remaining inhibitory ability of the three mutants on TRPV6 was related to TRPV6 forward trafficking and the ability was somehow enhanced by the disease-causing mutations. The intensity of the complexly glycosylated bands was restored by the I274T mutation, this is likely due to that the low protein level of this mutant diminished its ability to regulate TRPV6 trafficking via Rab binding ability. Thus, even though the impaired 5-phosphatase activity apparently is a major factor contributing to the impaired suppression of TRPV6 by the disease-causing mutations, other factors, such as protein stability and the ability to regulate TRPV6 trafficking, are also likely involved.
DISCUSSION
Hypercalciuria is a major manifestation of Dent disease; however, its pathogenesis is not well understood. In this study, we showed that, similar to CLC-5 (30), OCRL is capable of inhibiting the intestinal Ca2+ channel TRPV6, which plays a critical role in active Ca2+ absorption. The PI(4,5)P2 5-phosphatase activity and the Rab binding domain each plays a role in this regulation. The suppression effect of the abundance of the complexly N-glycosylated TRPV6 is caused by the blockade of forward trafficking. All Dent-causing mutations of OCRL tested relieved the suppression of TRPV6 to various extents. This is largely due to the reduction of 5-phosphatase activity of these mutants increased the level of PI(4,5)P2 required for normal TRPV6 Ca2+ uptake function. To our knowledge, this is the first report on the regulation of OCRL on a channel/transporter. Our observations may provide clues for the pathogenesis of hypercalciuria in Dent disease and hypercalciuria in general.
OCRL represents an uncommon phenomenon that dysfunction of one gene cause two different disorders: Lowe's syndrome (25) and Dent disease (8). Lowe's syndrome is a multisystem disorder characterized by anomalies affecting the eye, the nervous system, and the kidney. Dent disease, on the other hand, is a disorder of the kidney. It is unclear how different mutations in one gene result in two different disorders. One possibility is that as a multifunctional protein, OCRL, regulates different processes in different organs. A specific mutation of OCRL may affect the regulation of OCRL on one process more than others. OCRL is comprised of several domains. The catalytic PI(4,5)P2 5-phosphatase domain of OCRL is flanked by a PH domain at the NH2-terminal region, an ASH domain (36) that also contains the Rab binding domain (16), and a catalytically inactive RhoGAP-like (28) domain at the COOH-terminal region. These domains may coordinate in regulating different processes. They may also have independent roles in regulating different proteins. Because the regulation of TRPV6 by OCRL involves multiple domains of OCRL, it could serve as a model system in evaluating disease-causing mutations of OCRL. In the current study, we focused on the PI(4,5)P2 5-phosphatase domain and the Rab binding domain.
The PI(4,5)P2 5-phosphatase domain is the catalytic domain in OCRL. This activity decreases the level of PI(4,5)P2. As TRPV6 is positively regulated by PI(4,5)P2 (48), we anticipated that the 5-phosphatase domain could play an essential role in regulating TRPV6. Indeed, the 5-phosphatase domain alone was capable of inhibiting TRPV6 more robustly than the full-length OCRL (Fig. 3); however, the D422A mutant, which abolished the inhibitory effect of the phosphatase domain on TRPV6 (Fig. 4E), only relieved approximately one-third of the inhibitory effect of OCRL (Fig. 4B). This is supported by the fact that the 5-phosphatase domain lacking OCRL construct still robustly inhibits TRPV6 (Fig. 6A). Thus the catalytic domain is important but only plays its limited role in inhibiting TRPV6. The increased inhibitory effect of 5-phosphatase domain alone construct on TRPV6 may be due to the mislocalization of the construct because of the absence of potential localization signals in other domains. In contrast to the compartmented localization of OCRL, the 5-phosphatase domain alone construct may localize ubiquitously in the cell and breakdown of PI(4,5)P2 in the plasma membrane and, in turn, reduce TRPV6 activity.
OCRL interacts with small GTPases such as Rac and Rab (13, 17), which are involved in membrane trafficking in organelles such as endosome and trans-Golgi network. The interaction of OCRL with Rab proteins may target OCRL in relevant organelles such as endosome and trans-Golgi network and regulate the PI(4,5)P2 and PI(4)P balance in these organelles and in turn membrane trafficking (17, 26). In this study, we found mutations disrupting Rab binding also impair the inhibitory effects of OCRL. It has been proposed that the 5-phosphatase activity and the Rab binding may coordinate in regulating the PI(4,5)P2 and PI(4)P abundance in cellular organelles (17). Our results indicate that 5-phosphatase activity and Rab binding may function independently in regulating TRPV6 (Fig. 6). This does not exclude the coordination between them, but each domain appears to take part in separated processes in regulating TRPV6. The full-length OCRL decreases the level of complexly N-glycosylated bands and surface abundance of TRPV6 (Figs. 2, 4, and 6) and disruption of the 5-phosphatase activity by D422A mutation does not change this effect. In contrast, disruption of Rab binding by G664D mutation largely restored the abundance of surface protein and the complexly glycosylated bands of TRPV6 (Figs. 5 and 6E) because of the restoration of TRPV6 forward trafficking (Figs. 7 and 8). This is another line of evidence supporting the independent actions of the two domains in inhibiting TRPV6.
Our results suggest that two mechanisms for the effects of OCRL on TRPV6. The first one is that OCRL controls the PI(4,5)P2 level in the cell membrane and, in turn, controls the activity and Ca2+-dependent inactivation of TRPV6 as revealed by previous studies (48). This mechanism is likely responsible for the alleviated suppression of TRPV6 by most of the Dent disease-causing mutants, as six out of seven mutants tested exhibit decreased 5-phosphatase activity (Fig. 9B). In fact, the majority of the Dent-causing missense mutants are in the phosphatase domain (4, 15, 41, 49, 51). There is an inverse relationship between the 5-phosphatas activity of OCRL mutants and the TRPV6-mediated Ca2+ uptake under the regulation of these OCRL mutants (Fig. 9C). Thus this mechanism is likely a main mechanism relevant to the regulation of TRPV6 activity. This mechanism should not be limited to the system tested, as it has been shown in the other cells that TRPV6 is regulated by PI(4,5)P2 (48).
The second mechanism is that OCRL also regulates TRPV6 trafficking. This regulation may involve multiple domains of TRPV6; however, we focused on the Rab binding domain as it is critical in this regulation. OCRL decreases the complexly glycosylated forms and surface abundance of TRPV6. This effect was largely independent of the 5-phosphatase activity, as the D422A mutation did not restore either of the effects (Fig. 4, C and E). This suggests that even though the PI(4,5)P2 5-phosphatase activity is capable of inhibiting TRPV6 activity, its contribution to TRPV6 trafficking is limited. On the other hand, OCRL could inhibit TRPV6 in the absence of its phosphatase activity, such as in the construct without the phosphatase domain or in the construct carrying the D422A mutation. The Rab binding-disrupting G664D mutation significantly reduced the inhibitory effect of phosphatase-deficient OCRL (Fig. 6, A and B). It also significantly restored the abundance of surface protein and the complexly glycosylated forms of TRPV6 (Figs. 5B and 6E) and the trafficking of TRPV6 (Figs. 7D and 8B). It is worth noting that even though the phosphatase-disrupting D422A mutation does not restore the band intensity, the restoration of these bands by the S564P and G664D mutations was more robust in the presence of the D422A mutation (Fig. 6E). This suggests that the 5-phosphatase activity also plays a minor role in the complex glycosylation of TRPV6, although it may not be the determinant step. In fact, three of the Dent-causing mutations that differ from the linear relationship between the Ca2+ uptake and the 5-phosphatase activities all show a significant decrease in the complexly glycosylated forms of TRPV6 (Fig. 9, A and C), indicating an effect of TRPV6 trafficking is involved. How these 5-phosphatase-disrupting mutations are involved in regulating TRPV6 trafficking requires further investigation.
Our data suggest that OCRL slows down the forward trafficking (Fig. 7). As BFA diminishes the difference between the presence and absence of OCRL in TRPV6 trafficking, the process disrupted by BFA, which is the delivery of newly synthesized proteins to the plasma membrane, is likely a process regulated by OCRL. This does not exclude the possibility that the recycling process is affected by OCRL as well as reported recently (54). The fact that blocking the recycling by Rab11a S25N revealed a significant difference in the decrease in Ca2+ uptake between the HA-OCRL expressing group and control group (Fig. 7A) is consistent with an accelerated recycling process by OCRL. Regardless, the inhibitory effects of OCRL on the TRPV6 trafficking process are disrupted by Rab binding-deficient G664D mutation, not by the phosphatase-deficient D422A mutation (Fig. 7D). The forward trafficking as revealed by the process of the N-glycosylation is inhibited by OCRL, and again, this inhibitory effect is abolished by the G664D mutation, not by the D422A mutation (Fig. 8B). Thus these lines of evidence indicate that TRPV6 trafficking is mainly regulated by the Rab binding activity of OCRL and this process could be separated from the 5-phosphatase activity of OCRL. OCRL can bind to Rab1 and Rab6 (17). Rab1 facilitates transport vesicle from ER to Golgi and Rab6 plays a role in retrograde trafficking from Golgi to ER and intra-Golgi transport (56). OCRL also binds to Rab8 (16), which plays critical role in vesicle trafficking from trans-Golgi network to plasma membrane (56). Thus the interaction of OCRL with Rab1, Rab6, or Rab8 might be involved in regulating the forward trafficking of TRPV6.
The effects of phosphatase activity, Rab binding domain could not explain all the inhibitory effect of OCRL on TRPV6, because TRPV6 is still inhibited in the presence of D422A/G664D double mutations (Fig. 6B). OCRL is a protein of multiple functional domains. A clathrin box in the PH domain helps to recruit OCRL to endocytic clathrin-coated pits (28). There is another clathrin box in the ASH-RhoGAP-like domain (5) and the clathrin adaptor protein AP-2 binding motif (50); these motifs are important for recruiting OCRL to clathrin-coated vesicles to facilitate endocytosis (6, 28) and vesicle trafficking between early endosome and trans-Golgi network (5). Our preliminary study indicates the clathrin binding motifs and AP-2 binding motif participate in the inhibition of TRPV6 (data not shown). The RhoGAP-like domain appears to limit the inhibitory effect of OCRL on TRPV6. The potential role of RhoGAP-like domain and other regulatory activities of OCRL on TRPV6, such as regulating actin polymerization (46), against-induced Ca2+ release (45), and interaction with Rab5 effector APPL1 (12), are yet to be assessed.
Although TRPV6 was originally identified from intestine (33), it is also expressed in other organs in humans including placenta, pancreas, prostates, kidney, and testis (34). Expressed sequence tags of TRPV6 were found in the brain, eye, blood, and several other human tissues. As OCRL is more ubiquitously expressed, it could certainly regulate TRPV6 in tissues other than the intestine. It is unclear what roles TRPV6 plays in organs such as the brain and the eye. Thus it is premature to speculate whether the regulation of TRPV6 by OCRL is involved in other abnormalities in Lowe syndrome. TRPV6 is unlikely the only channel protein regulated by OCRL, other channels, transporters, and receptors, such as some in the TRP super family, may also be targets of OCRL-mediated regulation.
In summary, OCRL suppresses TRPV6 via controlling the trafficking of TRPV6 protein via its Rab binding activity and TRPV6-mediated Ca2+ transport via its PI(4,5)P2 5-phosphatase activity. The latter regulation is impaired by Dent-causing OCRL mutations. Taken into consideration that CLC-5, the other protein associated with Dent disease also suppressed TRPV6 (30), TRPV6 activity is likely elevated due to the increased transcription of TRPV6 gene caused by increased 1,25(OH)2D3 (42) and/or relieved of suppression of TRPV6 under Dent conditions. Thus the dysregulation of TRPV6 by OCRL mutants likely contributes to the development of hypercalciuria in Dent disease and possibly Lowe syndrome, which is also frequently associated with hypercalciuria (43).
GRANTS
W. Z. is a recipient of postdoctoral fellowship award from the American Heart Association. H. J. is a recipient of a scholarship from China Scholarship Council. This work was supported by American Heart Association Greater Southeast Affiliate Grant 09GRNT2160024 and National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-072154 (to J.-B. Peng) and R01-DK-081463 (to H. Wu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.W. and J.-B.P. conception and design of research; G.W., W.Z., T.N., H.J., and J.-B.P. performed experiments; G.W., T.N., H.J., H.W., and J.-B.P. analyzed data; G.W., W.Z., T.N., H.W., and J.-B.P. interpreted results of experiments; G.W., W.Z., T.N., and J.-B.P. prepared figures; G.W. and J.-B.P. drafted manuscript; G.W., T.N., H.W., and J.-B.P. edited and revised manuscript; G.W., W.Z., T.N., H.J., H.W., and J.-B.P. approved final version of manuscript.
ACKNOWLEDGMENTS
Part of this work was presented at the 43rd Annual Meeting of the American Society of Nephrology, Denver, CO, on November 16-21, 2010.
REFERENCES
- 1. Attree O, Olivos IM, Okabe I, Bailey LC, Nelson DL, Lewis RA, McInnes RR, Nussbaum RL. The Lowe's oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature 358: 239–242, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Bianco SD, Peng JB, Takanaga H, Suzuki Y, Crescenzi A, Kos CH, Zhuang L, Freeman MR, Gouveia CH, Wu J, Luo H, Mauro T, Brown EM, Hediger MA. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Miner Res 22: 274–285, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bothwell SP, Chan E, Bernardini IM, Kuo YM, Gahl WA, Nussbaum RL. Mouse model for Lowe syndrome/Dent disease 2 renal tubulopathy. J Am Soc Nephrol 22: 443–448, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cho HY, Lee BH, Choi HJ, Ha IS, Choi Y, Cheong HI. Renal manifestations of Dent disease and Lowe syndrome. Pediatr Nephrol 23: 243–249, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Choudhury R, Diao A, Zhang F, Eisenberg E, Saint-Pol A, Williams C, Konstantakopoulos A, Lucocq J, Johannes L, Rabouille C, Greene LE, Lowe M. Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network. Mol Biol Cell 16: 3467–3479, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choudhury R, Noakes CJ, McKenzie E, Kox C, Lowe M. Differential clathrin binding and subcellular localization of OCRL1 splice isoforms. J Biol Chem 284: 9965–9973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cui M, Fleet J. Transgenic over-expression of human TRPV6 in intestine increases calcium absorption efficiency and improves bone mass in mice (Abstract). J Bone Miner Res 25, Suppl 1: 10–18-2010, 2010. [Available at http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=5cc85c32-51fa-465c-af36-e1db4338942d Accessed 16 Nov, 2010] [Google Scholar]
- 8. Dent CE, Friedman M. Hypercalcuric rickets associated with renal tubular damage. Arch Dis Child 39: 240–249, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Devuyst O, Pirson Y. Genetics of hypercalciuric stone forming diseases. Kidney Int 72: 1065–1072, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Devuyst O, Thakker RV. Dent's disease. Orphanet J Rare Dis 5: 28, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dressman MA, Olivos-Glander IM, Nussbaum RL, Suchy SF. Ocrl1, a PtdIns(4,5)P2 5-phosphatase, is localized to the trans-Golgi network of fibroblasts and epithelial cells. J Histochem Cytochem 48: 179–190, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Erdmann KS, Mao Y, McCrea HJ, Zoncu R, Lee S, Paradise S, Modregger J, Biemesderfer D, Toomre D, De CP. A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway. Dev Cell 13: 377–390, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faucherre A, Desbois P, Satre V, Lunardi J, Dorseuil O, Gacon G. Lowe syndrome protein OCRL1 interacts with Rac GTPase in the trans-Golgi network. Hum Mol Genet 12: 2449–2456, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Frick KK, Bushinsky DA. Molecular mechanisms of primary hypercalciuria. J Am Soc Nephrol 14: 1082–1095, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Hoopes RR, Jr, Shrimpton AE, Knohl SJ, Hueber P, Hoppe B, Matyus J, Simckes A, Tasic V, Toenshoff B, Suchy SF, Nussbaum RL, Scheinman SJ. Dent disease with mutations in OCRL1. Am J Hum Genet 76: 260–267, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hou X, Hagemann N, Schoebel S, Blankenfeldt W, Goody RS, Erdmann KS, Itzen A. A structural basis for Lowe syndrome caused by mutations in the Rab-binding domain of OCRL1. EMBO J 30: 1659–1670, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hyvola N, Diao A, McKenzie E, Skippen A, Cockcroft S, Lowe M. Membrane targeting and activation of the Lowe syndrome protein OCRL1 by rab GTPases. EMBO J 25: 3750–3761, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janne PA, Suchy SF, Bernard D, MacDonald M, Crawley J, Grinberg A, Wynshaw-Boris A, Westphal H, Nussbaum RL. Functional overlap between murine Inpp5b and Ocrl1 may explain why deficiency of the murine ortholog for OCRL1 does not cause Lowe syndrome in mice. J Clin Invest 101: 2042–2053, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jefferson AB, Majerus PW. Mutation of the conserved domains of two inositol polyphosphate 5-phosphatases. Biochemistry 35: 7890–7894, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Jiang Y, Cong P, Williams SR, Zhang W, Na T, Ma HP, Peng JB. WNK4 regulates the secretory pathway via which TRPV5 is targeted to the plasma membrane. Biochem Biophys Res Commun 375: 225–229, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang Y, Ferguson WB, Peng JB. WNK4 enhances TRPV5-mediated calcium transport: potential role in hypercalciuria of familial hyperkalemic hypertension caused by gene mutation of WNK4. Am J Physiol Renal Physiol 292: F545–F554, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Johnson JM, Castle J, Garrett-Engele P, Kan Z, Loerch PM, Armour CD, Santos R, Schadt EE, Stoughton R, Shoemaker DD. Genome-wide survey of human alternative premRNA splicing with exon junction microarrays. Science 302: 2141–2144, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol 116: 1071–1080, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lloyd SE, Pearce SH, Fisher SE, Steinmeyer K, Schwappach B, Scheinman SJ, Harding B, Bolino A, Devoto M, Goodyer P, Rigden SP, Wrong O, Jentsch TJ, Craig IW, Thakker RV. A common molecular basis for three inherited kidney stone diseases. Nature 379: 445–449, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Lowe CU, Terrey M, MacLachlan EA. Organic-aciduria, decreased renal ammonia production, hydrophthalmos, and mental retardation; a clinical entity. AMA Am J Dis Child 83: 164–184, 1952 [DOI] [PubMed] [Google Scholar]
- 26. Lowe M. Structure and function of the Lowe syndrome protein OCRL1. Traffic 6: 711–719, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Luyckx VA, Leclercq B, Dowland LK, Yu AS. Diet-dependent hypercalciuria in transgenic mice with reduced CLC5 chloride channel expression. Proc Natl Acad Sci USA 96: 12174–12179, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mao Y, Balkin DM, Zoncu R, Erdmann KS, Tomasini L, Hu F, Jin MM, Hodsdon ME, De CP. A PH domain within OCRL bridges clathrin-mediated membrane trafficking to phosphoinositide metabolism. EMBO J 28: 1831–1842, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol 20: 1447–1461, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Na T, Zhang W, Peng JB. Chloride channel ClC-5 linked to Dent's disease decreases protein level of the intestinal calcium entry channel TRPV6 in Xenopus oocytes (Abstract). FASEB J 23: 998.15, 2009 [Google Scholar]
- 31. Olivos-Glander IM, Janne PA, Nussbaum RL. The oculocerebrorenal syndrome gene product is a 105-kD protein localized to the Golgi complex. Am J Hum Genet 57: 817–823, 1995 [PMC free article] [PubMed] [Google Scholar]
- 32. Peng JB, Brown EM, Hediger MA. Structural conservation of the genes encoding CaT1, CaT2, and related cation channels. Genomics 76: 99–109, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem 274: 22739–22746, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Peng JB, Chen XZ, Berger UV, Weremowicz S, Morton CC, Vassilev PM, Brown EM, Hediger MA. Human calcium transport protein CaT1. Biochem Biophys Res Commun 278: 326–332, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Piwon N, Gunther W, Schwake M, Bosl MR, Jentsch TJ. ClC-5 Cl−-channel disruption impairs endocytosis in a mouse model for Dent's disease. Nature 408: 369–373, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Ponting CP. A novel domain suggests a ciliary function for ASPM, a brain size determining gene. Bioinformatics 22: 1031–1035, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Reinhart SC, Norden AG, Lapsley M, Thakker RV, Pang J, Moses AM, Frymoyer PA, Favus MJ, Hoepner JA, Scheinman SJ. Characterization of carrier females and affected males with X-linked recessive nephrolithiasis. J Am Soc Nephrol 5: 1451–1461, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Ren M, Xu G, Zeng J, De Lemos-Chiarandini C, Adesnik M, Sabatini DD. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc Natl Acad Sci USA 95: 6187–6192, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scheel O, Zdebik AA, Lourdel S, Jentsch TJ. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 436: 424–427, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Scheinman SJ. Nephrolithiasis. Semin Nephrol 19: 381–388, 1999 [PubMed] [Google Scholar]
- 41. Shrimpton AE, Hoopes RR, Jr, Knohl SJ, Hueber P, Reed AA, Christie PT, Igarashi T, Lee P, Lehman A, White C, Milford DV, Sanchez MR, Unwin R, Wrong OM, Thakker RV, Scheinman SJ. OCRL1 mutations in Dent 2 patients suggest a mechanism for phenotypic variability. Nephron Physiol 112: 27–36, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Silva IV, Cebotaru V, Wang H, Wang XT, Wang SS, Guo G, Devuyst O, Thakker RV, Guggino WB, Guggino SE. The ClC-5 knockout mouse model of Dent's disease has renal hypercalciuria and increased bone turnover. J Bone Miner Res 18: 615–623, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Sliman GA, Winters WD, Shaw DW, Avner ED. Hypercalciuria and nephrocalcinosis in the oculocerebrorenal syndrome. J Urol 153: 1244–1246, 1995 [PubMed] [Google Scholar]
- 44. Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10: 513–525, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Suchy SF, Cronin JC, Nussbaum RL. Abnormal bradykinin signalling in fibroblasts deficient in the PIP2 5-phosphatase, ocrl1. J Inherit Metab Dis 32: 280–288, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Suchy SF, Nussbaum RL. The deficiency of PIP2 5-phosphatase in Lowe syndrome affects actin polymerization. Am J Hum Genet 71: 1420–1427, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suzuki Y, Pasch A, Bonny O, Mohaupt MG, Hediger MA, Frey FJ. Gain-of-function haplotype in the epithelial calcium channel TRPV6 is a risk factor for renal calcium stone formation. Hum Mol Genet 17: 1613–1618, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Thyagarajan B, Lukacs V, Rohacs T. Hydrolysis of phosphatidylinositol 4,5-bisphosphate mediates calcium-induced inactivation of TRPV6 channels. J Biol Chem 283: 14980–14987, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tosetto E, Addis M, Caridi G, Meloni C, Emma F, Vergine G, Stringini G, Papalia T, Barbano G, Ghiggeri GM, Ruggeri L, Miglietti N, Angelo D, Melis MA, Anglani F. Locus heterogeneity of Dent's disease: OCRL1 and TMEM27 genes in patients with no CLCN5 mutations. Pediatr Nephrol 24: 1967–1973, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Ungewickell A, Ward ME, Ungewickell E, Majerus PW. The inositol polyphosphate 5-phosphatase Ocrl associates with endosomes that are partially coated with clathrin. Proc Natl Acad Sci USA 101: 13501–13506, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Utsch B, Bokenkamp A, Benz MR, Besbas N, Dotsch J, Franke I, Frund S, Gok F, Hoppe B, Karle S, Kuwertz-Broking E, Laube G, Neb M, Nuutinen M, Ozaltin F, Rascher W, Ring T, Tasic V, van Wijk JA, Ludwig M. Novel OCRL1 mutations in patients with the phenotype of Dent disease. Am J Kidney Dis 48: 942–14, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA 98: 13324–13329, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van de Graaf SF, Chang Q, Mensenkamp AR, Hoenderop JG, Bindels RJ. Direct interaction with Rab11a targets the epithelial Ca2+ channels TRPV5 and TRPV6 to the plasma membrane. Mol Cell Biol 26: 303–312, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vicinanza M, Di CA, Polishchuk E, Santoro M, Di TG, Godi A, Levtchenko E, De Leo MG, Polishchuk R, Sandoval L, Marzolo MP, De Matteis MA. OCRL controls trafficking through early endosomes via PtdIns4,5P-dependent regulation of endosomal actin. EMBO J 30: 4970–4985, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang SS, Devuyst O, Courtoy PJ, Wang XT, Wang H, Wang Y, Thakker RV, Guggino S, Guggino WB. Mice lacking renal chloride channel, CLC-5, are a model for Dent's disease, a nephrolithiasis disorder associated with defective receptor-mediated endocytosis. Hum Mol Genet 9: 2937–2945, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Zhang W, Na T, Wu G, Jing H, Peng JB. Down-regulation of intestinal apical calcium entry channel TRPV6 by ubiquitin E3 ligase Nedd4–2. J Biol Chem 285: 36586–36596, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang X, Jefferson AB, Auethavekiat V, Majerus PW. The protein deficient in Lowe syndrome is a phosphatidylinositol-4,5-bisphosphate 5-phosphatase. Proc Natl Acad Sci USA 92: 4853–4856, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhuang L, Peng JB, Tou L, Takanaga H, Adam RM, Hediger MA, Freeman MR. Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Lab Invest 82: 1755–1764, 2002 [DOI] [PubMed] [Google Scholar]