Abstract
Calmodulin (CaM) binding sites were recently identified on the cytoplasmic loop (CL) of at least three α-subfamily connexins (Cx43, Cx44, Cx50), while Cx40 does not have this putative CaM binding domain. The purpose of this study was to examine the functional relevance of the putative Cx43 CaM binding site on the Ca2+-dependent regulation of gap junction proteins formed by Cx43 and Cx40. Dual whole cell patch-clamp experiments were performed on stable murine Neuro-2a cells expressing Cx43 or Cx40. Addition of ionomycin to increase external Ca2+ influx reduced Cx43 gap junction conductance (Gj) by 95%, while increasing cytosolic Ca2+ concentration threefold. By contrast, Cx40 Gj declined by <20%. The Ca2+-induced decline in Cx43 Gj was prevented by pretreatment with calmidazolium or reversed by the addition of 10 mM EGTA to Ca2+-free extracellular solution, if Ca2+ chelation was commenced before complete uncoupling, after which gj was only 60% recoverable. The Cx43 CL136–158 mimetic peptide, but not the scrambled control peptide, or Ca2+/CaM-dependent kinase II 290–309 inhibitory peptide also prevented the Ca2+/CaM-dependent decline of Cx43 Gj. Cx43 gap junction channel open probability decreased to zero without reductions in the current amplitudes during external Ca2+/ionomycin perfusion. We conclude that Cx43 gap junctions are gated closed by a Ca2+/CaM-dependent mechanism involving the carboxyl-terminal quarter of the connexin CL domain. This study provides the first evidence of intrinsic differences in the Ca2+ regulatory properties of Cx43 and Cx40.
Keywords: connexin 40, intercellular communication, ionomycin, calmidazolium
gap junctions, formed by docking of two gap junction hemichannels (or connexons) between neighboring cells, allow the transfer of ions and small molecules between coupled cells (15, 20, 58). The connexin gap junction proteins have four transmembrane domains, linked by one intracellular and two extracellular loops, with the NH2- and COOH-termini both located in the cytosol (15, 20). Gap junctions play an important role in maintaining cell and tissue function and homeostasis. For example, the low intercellular resistance pathway formed by connexin 43 (Cx43) and connexin 40 (Cx40) gap junctions in the working myocardium is critical for propagating electrical signals to allow rapid, synchronized contraction. Loss of function connexin mutations has been linked to several serious human diseases, including oculodentodigital dysplasia for Cx43, the most abundantly expressed human connexin (17, 22, 24, 25, 37, 47, 49, 51). Identification of factors that regulate gap junction communication and their underlying mechanisms are physiologically relevant and significant to understanding the causes of gap junction-related diseases. Multiple factors, including transjunctional voltage (Vj), intracellular pH (pHi), intracellular Ca2+ concentration ([Ca2+]i), and protein phosphorylation, modulate gap junction intercellular communication (20, 41).
Increases in [Ca2+]i were proposed to downregulate gap junction-mediated electrical coupling since the early observations of the “healing-over” of cardiac muscle and were later confirmed by direct observation of electrical coupling and [Ca2+]i in cardiac and nonelectrically excitable cells (9–13, 50). Electrical uncoupling of cells paired by gap junctions with increased [Ca2+]i has since been observed in several cell types, including cardiac myocytes, astrocytes, Henson cells, Novikoff hepatoma cells, cultured lens cells, rat liver cells, and crayfish septate axons (1, 4, 5, 18, 26, 41, 61). Gap junction uncoupling is also mediated by intracellular acidification, although the independent role of protons (H+), or Ca2+, is questioned by numerous studies (6, 26, 40, 52, 55, 63). Calmodulin (CaM), a cytosolic Ca2+ binding protein, was proposed to have a role in the Ca2+-induced uncoupling of gap junctions, since CaM inhibitors were observed to prevent the response (38, 39, 42, 44). CaM colocalizes to the junctional plaques of Cx32 and Cx50 in transfected cells (44, 65). Török and coworkers (54) reported that the first 21 amino acids located on the NH2 terminus of Cx32 and amino acids 216–230 located on the COOH terminus bound CaM in a Ca2+-dependent manner with dissociation constants (Kd) of 27 nM and 1.2 μM. Recently, a CaM binding site was assigned to the second half of the cytoplasmic loop (CL) domain of Cx43, Cx44, and Cx50 and confirmed using surface plasmon resonance, circular dichroism, fluorescence spectroscopy, and nuclear magnetic resonance (7, 66, 67). Zhou et al. (67) indicated that the 136–158 amino acids of Cx43 bind with CaM with 1:1 stoichiometry in a Ca2+-dependent manner. However, the functional consequences of the connexin-CaM protein-protein interactions, other than Cx32 and Cx50, are unknown.
To examine the functional relevance of the predicted Cx43 CaM binding domain (CaMBD), we utilized the dual whole cell patch-clamp technique to directly evaluate the functional regulation of Cx43 and Cx40 gap junctions by Ca2+ and CaM. In contrast to Cx43, there is no putative CaM binding site in the CL region of Cx40, which led us to hypothesize a differential Ca2+-dependent regulation of Cx43 and Cx40. We demonstrate that elevation of intracellular Ca2+ of Neuro-2a (N2a)-Cx43 cells by ionomycin in the presence of normal extracellular Ca2+ is sufficient to uncouple Cx43, but not Cx40, gap junctions. This process is dependent on CaM and the predicted Cx43 CL CaM binding site, since acute treatment with the CaM inhibitor calmidazolium (CDZ), intracellular application of Ca2+/CaM-dependent kinase II (CaMKII) 290–309 inhibitory or Ac-136KYGIEEHGKVKMRGGLLRTYIIS158-NH2 Cx43–3 peptides inhibited the Ca2+-mediated uncoupling response. Our findings suggest that the CaM binding site of Cx43136–158 is critical for the Ca2+-induced closure of Cx43 gap junctions.
MATERIALS AND METHODS
Prediction of CaMBDs.
The topology and orientation of the transmembrane regions of the human Cx43 (Fig. 1A) were drawn based on predictions using four different programs, including SOSUI (34), TMHMM (23), MEMSAT (21), and HMMTOP (56). Sequence alignments of the gap junction proteins Cx43 (accession ID: NP_000156; human), Cx40 (accession ID: NP_005257; human), and CaMKII (accession ID: AAH40457; rat) were carried out by using the ClustalW2 algorithm (53). The probability of CaM binding regions in these proteins was predicted by CaM Target Database (64).
Fig. 1.
A: the connexins (Cx) are composed of four transmembrane (TM) segments, two extracellular loops, a short NH2-terminus (region 1), one cytoplasmic loop linking TM2 and TM3 (region 2 and region 3), and a longer COOH-terminal tail (region 4). The numerical score (1–9) represents the prediction probability of each amino acid in a potential calmodulin (CaM) binding site. PCBS is the overall probability for the entire potential CaM binding domain (CaMBD) predicted by the CaM Target Database. The predicted CaMBD of Cx43 is located in the distal portion of the Cx43 intracellular loop (region 3). Similar to the CaMBD of Ca2+/CaM-dependent kinase II (CaMKII), the Cx43 CaMBD conforms to the 1–5-10 CaM-binding mode subclass with hydrophobic residues at positions 1, 5, and 10. In addition, they have conserved positive residues (underlined). On the other hand, there are major amino acid differences between Cx40 and Cx43/CaMKII (highlighted in gray). The corresponding Cx40 sequence does not have conserved positive residues such as K298 and K300 bracketing the hydrophobic L299 residue in CaMKII. PCBS for the corresponding region of Cx40 is 0 instead of 13 for Cx43 and 9 for CaMKII. Potential CaM binding regions corresponding to the four connexin intracellular domains are listed in the table: h, human; m, mouse; B, basic residues; Δ, hydrophobic residues; CaMBD, CaM binding domain; (Max), maximum score of CaM binding prediction for whole peptide fragment; PCBS, probability of CaM binding predicted by the CaM Target Database; * for score 1∼3; ** for score 4∼6; *** for score 7∼9, N.B., no prediction for CaM binding. B: the decline in normalized Cx43 gap junction conductance (Gj) was temporally correlated with a threefold increase in cytosolic Ca2+ concentration ([Ca2+]i) induced by 1 μM ionomycin + 1.8 mM CaCl2 saline superfusion. The Cx43-N2a cell [Ca2+]i measurements were obtained by ratiometric fura 2 imaging in independent experiments from the dual whole cell patch Gj measurements. [Ca2+]o, extracellular Ca2+ concentration. Values are means ± SE.
Cell cultures.
Mouse N2a neuroblastoma cells stably expressing rat Cx43 or rat Cx40 have been described previously (27, 29). N2a cells were cultured in minimum essential media, supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 g/l G418 sulfate (Invitrogen).
Cardiomyocyte cultures.
Neonatal mouse atrial and ventricular myocyte primary cultures were prepared according to previously published methods (28, 30). Newborn C57Bl/6 mice were anesthetized with isoflurane, and the hearts excised in accordance with procedures approved by State University of New York Upstate Medical University Committee for the Humane Use of Animals. The atria and ventricles were dissociated separately in a Ca2+- and Mg2+-free collagenase type II (≅1 mg/ml, Worthington Biochemicals) dissociation solution containing the following (in mM): 116 NaCl, 5.4 KCl, 1.0 NaH2PO4, and 5.5 dextrose. The supernatant was collected after each of four 10-min dissociation cycles, passed through a 70-μm cell strainer (Falcon), and centrifuged at low speed, and the cell pellet was resuspended in M199 media supplemented with 10% fetal bovine serum. The primary cell cultures were enriched for cardiomyocytes by differential cell adhesion for 30 min and plated onto 35-mm culture dishes.
Solutions.
The standard external solution (SES) contained the following (in mM): 142 NaCl, 1.3 KCl, 4 CsCl, 2 tetraethylammonium chloride, 0.8 MgSO4, 0.9 NaH2PO4, 1.8 CaCl2, 5.5 dextrose, 10 HEPES-NaOH (pH 7.4). A nominally Ca2+-free SES was prepared in the same manner without 1.8 mM CaCl2. To remove trace amounts of Ca2+, 10 mM EGTA were added. Ionomycin (calcium salt, Sigma) and CDZ chloride (Calbiochem) were added to SES. Perfusion of the 35-mm cell culture dish was gravity fed at a rate of 1 ml/min (3 ml bath volume) via an inflow line positioned <500 μm from the recording cell pair. Patch pipettes were filled with a KCl internal pipette solution (IPS KCl, in mM): 140 KCl, 1.0 MgCl2, 3.0 CaCl2, 5.0 BAPTA, 25 HEPES; pH titrated to 7.4 (using 1 N KOH). BAPTA (5 mM) titrated with 3 mM CaCl2 sets the solution [Ca2+] to ≈250 nM (MAXCHELATOR software). The [Ca2+] of IPS KCl was verified by calibrated in vitro fluorescence measurements using conventional fura 2 fluorescence measurement methods (19). The calibrated Kd of fura 2 was 78 ± 8 nM, and the measured free [Ca2+] of the pipette solution was 137 ± 2 nM, based on the in vitro measurement. In select experiments, the Cx43–3, Cx43-scrambled (-scr), or the calmodulin-dependent protein kinase II 290–309 (Axxora) peptides were added to both patch pipette solutions (67). To test for the effects of whole cell dialysis and Ca2+-BAPTA buffering during conventional ruptured patch recordings, some experiments were performed using perforated patch methods by adding 50 μM β-escin to the IPS KCl (16). All external and internal solutions were adjusted to a final osmolarity of 310 mosM.
Dual whole cell patch-clamp recording.
Gap junction currents (Ij) were recorded in the dual whole cell configuration, according to previously published methods (60). Both cells in the dual whole cell patch mode were held at −40 mV. Ij was measured in response to a +20 mV Vj pulse applied to one cell for 5 s every 15 s (4 pulses/min). To better resolve unitary Cx43 gap junction channel currents for the purpose of calculating the single-channel conductance (γj), the command Vj pulse was increased to +45 mV. The Ij values recorded during these Vj steps were recorded, and the macroscopic junctional conductance (gj) was calculated by the following equation: gj = −ΔI2/[V1 − (I1·Rel 1) − V2 + (I2·Rel 2)] (60), where I1 and I2 are whole cell currents, V1 and V2 are command voltages, and Rel 1 and Rel 2 are patch electrode resistances. In all experiments, Cx43 gj was normalized to the initial baseline control gj value before ionomycin perfusion (Gj = gj/gj,control, Table 1). All whole cell current recordings were low-pass filtered at 500 Hz and digitized at 2 kHz using pClamp8.2, and graphical analysis was performed using Origin 7.5 software. The Student's paired t-test was performed to test for statistically significant (P value < 0.05) alterations in Gj during ionomycin perfusion for each experimental group. One-way ANOVA analysis and the Bonferroni means comparison test was performed to test for statistical significance between the means of different experimental groups.
Table 1.
Control macroscopic junctional conductance values
| Initial gj, nS |
||||
|---|---|---|---|---|
| Experiment | Means | SE | N | |
| Connexin | ||||
| Cx43 | 1.8 mM CaCl2 | 22.3 | 5.2 | 6 |
| 0 mM CaCl2 | 9.2 | 3.3 | 6 | |
| 10 mM EGTA, partial uncoupling | 19.2 | 7.7 | 5 | |
| 10 mM EGTA, complete uncoupling | 19.1 | 5.9 | 5 | |
| 2 μM calmidazolium | 30.5 | 5.6 | 6 | |
| 1 μM Cx43-3 peptide | 14.2 | 4.5 | 6 | |
| 1 μM Cx43-scr peptide | 10.9 | 3.4 | 6 | |
| 100 nM CaMKII peptide | 18.5 | 8.8 | 6 | |
| Cx40 | 1.8 mM CaCl2 | 6.3 | 1.9 | 6 |
| 0 mM CaCl2 | 5.0 | 1.4 | 6 | |
| Cardiomyocytes | ||||
| Atrial | 1.8 mM CaCl2 | 42.9 | 5.6 | 7 |
| Ventricular | 1.8 mM CaCl2 | 47.1 | 6.4 | 6 |
N, no. of experiments. All experiments included bath saline perfusion with 1 μM ionomycin. gj, junctional conductance; Cx, connexin; scr, scrambled; CaMKII, Ca2+/calmodulin-dependent kinase II.
[Ca2+]i measurements.
N2a-Cx43 cells were cultured on 25-mm-diameter poly-l-lysine-coated glass coverslips for 48 h, rinsed with PBS, and loaded with 1 μM fura 2-AM (Invitrogen) for 15 min at 37°C. The coverslips were transferred to a microperifusion chamber mounted on a Nikon TE2000U inverted microscope equipped with ×40 SuperFluor oil immersion objective (48). Images of fura 2-loaded cells were obtained with a Roper Scientific Cascade 650 charge-coupled device digital camera using Metamorph software. The cells were perfused (5 ml/min, 1-ml bath volume within the microperifusion chamber) with SES until equilibrated at 22°C, after which 1 μM ionomycin perfusion commenced. [Ca2+]i was calculated as described (19, 48).
RESULTS
Bioinformatic analysis of CaM binding to the cytosolic loop of Cx40 and Cx43.
The Ca2+ regulatory properties of Cx40, the second most abundant myocardial gap junction protein, have not been specifically investigated, so we performed a CaM binding site analysis for Cx40 analogous to that used for Cx43 (67). The bioinformatic analysis did not reveal a connexin CL CaM binding site for Cx40. This is contrary to the presence of predicted CaM binding sites in Cx43, Cx44 (the sheep ortholog of human Cx46), or Cx50. Alternate CaM sites of lower probability were predicted for both Cx43 and Cx40 (Fig. 1A) (7, 66, 67). When aligned with the reported 1–5-10 subclass CaMKIIα CaM binding site (33), the conserved positively charged residues bordering the hydrophobic residue at position 1, such as K298/K300 in CaMKIIα (pdb code 1CDM) and K146/R148 in Cx43, are replace by A144/Q146 in Cx40. In the structure of CaM-CaMKIIα, the K298/K300 residues directly interact with the CaMKII target peptide. Intrinsic sequence differences between Cx40 139–156, Cx43 141–158, and CaMKII 293–310 sequences suggest that Cx40 does not bind CaM in the cytosolic loop region and is less susceptible to regulation by Ca2+.
Differential Ca2+ dependence of Cx43 and Cx40 gap junction uncoupling.
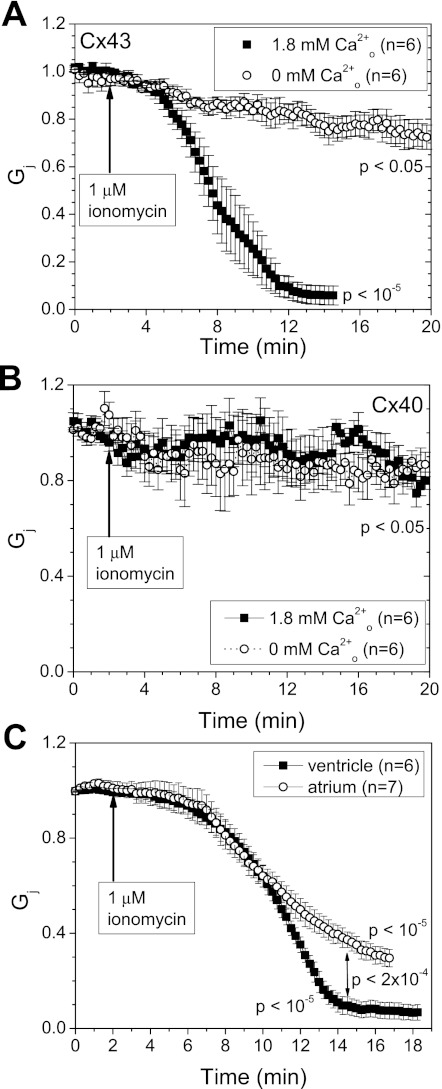
To test whether extracellular Ca2+ influx can induce uncoupling between N2a cells stably expressing Cx43, we perfused N2a-Cx43 cell pairs with SES or Ca2+-free SES containing 1 μM ionomycin. After a 2-min control recording period, Cx43 Gj declined by 95 ± 4% within 15 min following application of ionomycin in Ca2+-containing SES (Fig. 1B, top). We also performed dual whole cell perforated patch experiments with 50 μM β-escin to determine whether whole cell Ca2+ buffering with BAPTA modifies the response (16). Two β-escin perforated patch experiments proceeded to uncouple completely with a slightly delayed onset and slower time course of uncoupling, the opposite of what might be expected if excessive Ca2+ buffering and dilution of endogenous CaM were limiting factors in the response (data not shown). In separate imaging experiments, N2a-Cx43 cell [Ca2+]i was observed to gradually increase threefold from a resting level of 80 ± 3 to 250 ± 10 nM, parallel to the change in gj (Fig. 1B, bottom). Both panels in Fig. 1B present a similar temporal response to 1 μM ionomycin perfusion, although the N2a-Cx43 cell Gj and [Ca2+]i responses are not directly correlated. Assuming the same percentage increase in [Ca2+]i in whole cell patch-clamped N2a-Cx43 cell pairs, the [Ca2+]i levels are estimated to increase from 140 to 360 nM during the ionomycin perfusion, Gj measurement experiments.
To examine the Ca2+ dependence of the uncoupling response, N2a-Cx43 cell pairs were also perfused with 1 μM ionomycin in Ca2+-free SES (Fig. 2A). Cx43 Gj declined by 20 ± 4% within 10 min and achieved a minimum Gj of 72 ± 5% after 20 min in the absence of external CaCl2. The Cx43 Gj response in the presence of 1.8 mM Ca2+-SES (Fig. 1B) is contrasted with the Ca2+-free SES results in this panel. The response of N2a-Cx40 gap junctions was also examined. There was no difference in Cx40 Gj following application of ionomycin in SES or Ca2+-free SES; the maximum decrease in Cx40 Gj under these conditions was ≤20%. Patch electrode series resistance errors, which increase with higher gj values (60), did not influence these measurements, since our stable N2a-Cx40 cell clones exhibited lower gj values, on average, than our N2a-Cx43 cells (Table 1). These observations provide the first evidence for an intrinsic difference in the Ca2+ regulatory properties of Cx43 and Cx40 and are consistent with the bioinformatic CaM binding site predictions. To determine whether the differential sensitivity of Cx40 and Cx43 gap junctions to Ca2+-induced uncoupling translated into functional differences between atrial and ventricular myocardial gap junctions, neonatal mouse atrial and ventricular myocyte cell pairs were superfused with SES containing 1 μM ionomycin (Fig. 2C). The decline in ventricular myocyte Gj resembled the ionomycin-induced changes in N2a-Cx43 Gj. In contrast, atrial Gj declined 20% less than ventricular Gj (P < 0.0002, one-way ANOVA). This is consistent with a partial contribution of less Ca2+-sensitive Cx40 gap junctions to the incomplete atrial uncoupling response.
Fig. 2.
A and B: Neuro-2a (N2a)-Cx43 cell pairs (A) or N2a-Cx40 cell pairs (B) were perfused with 1 μM ionomycin in the presence or absence of added 1.8 mM CaCl2 to the bath saline solution. In nominally zero CaCl2 saline, Cx43 Gj declined by <30% over 20 min (P < 0.05) compared with a 95% decline within 15 min in the presence of 1.8 mM external CaCl2 (P < 10−5). In contrast, Cx40 Gj declined by <20%, whether in nominally zero or normal external CaCl2 conditions (P < 0.05). C: neonatal mouse atrial or ventricular cardiomyocyte Gj decreased by 73 ± 11 or 93 ± 8%, respectively, when perfused with 1 μM ionomycin + 1.8 mM CaCl2 (P < 10−5). Values are means ± SE.
Reversibility of Ca2+-dependent Cx43 gap junction uncoupling.
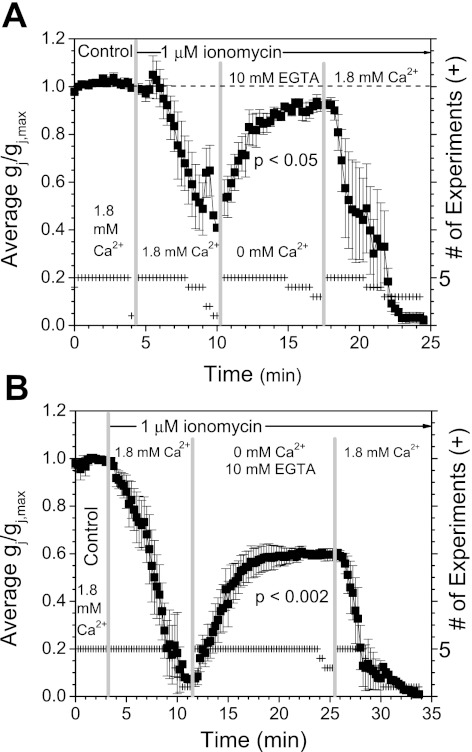
The above data suggest that Ca2+ influx is responsible for the uncoupling of Cx43 gap junctions. Therefore, we hypothesize that this process should be reversible if the external Ca2+ is removed during ionomycin perfusion. To test this hypothesis, the 1 μM ionomycin perfusion solution was switched from 1.8 mM CaCl2 to nominally 0 CaCl2 + 10 mM EGTA during the uncoupling phase. When the external bath perfusion was switched to 0 Ca2+/EGTA saline when the decline in Cx43 Gj reached ∼50% (≤5 min), Gj increased to ≈90% of its initial value (Fig. 3A). Application of 1.8 mM CaCl2 saline demonstrated that the recoverable Cx43 Gj remained responsive to Ca2+-induced uncoupling since reexposure of cells to SES containing Ca2+ decreased Gj by 98%. The level of recovery induced by removal of extracellular Ca2+ was dependent on the magnitude of the Ca2+-induced reduction in Cx43 Gj. If the decline in Gj was >90%, then only 60% of the initial Cx43 Gj was restored by applying 0 Ca2+/EGTA Ca2+ SES (Fig. 3B). This suggests that nearly one-half of the Cx43 gap junctions become irreversibly closed after Ca2+-dependent uncoupling attains maximum levels.
Fig. 3.
A: the reversibility of the calcium-induced uncoupling of Cx43 gap junctions was examined by alternating 1 μM ionomycin perfusion with 1.8 mM CaCl2 or 0 mM added calcium plus 10 mM EGTA to chelate the trace amounts of calcium in the deionized water used to prepare the saline solutions. If the perfusion solution was switched to the chelated calcium saline when Gj had declined by ∼50%, Cx43 Gj recovered to 92 ± 3% of its initial value (P < 0.05). This Cx43 Gj was responsive to external calcium, since reperfusion with 1.8 mM CaCl2 saline produced a 98 ± 2% decline in Gj. B: if the decline in Cx43 Gj was allowed to achieve maximum uncoupling before perfusion with the 0 mM CaCl2 + 10 mM EGTA solution, Gj was recoverable only to ∼60% of initial values (P < 0.002). Again, this reversible Cx43 Gj was responsive to calcium, since reexposure to 1.8 mM CaCl2 saline produced complete uncoupling. Since the perfusion times for the 5 experiments in each data set were not identical, the time base was shifted to align with the onset of the 1.8 mM CaCl2 and 0 mM + 10 mM EGTA chelated calcium saline perfusions (+ = no. of experiments at each time point). Values are means ± SE.
The Ca2+ regulation of Cx43 gap junctions is blocked by CaM inhibitors.
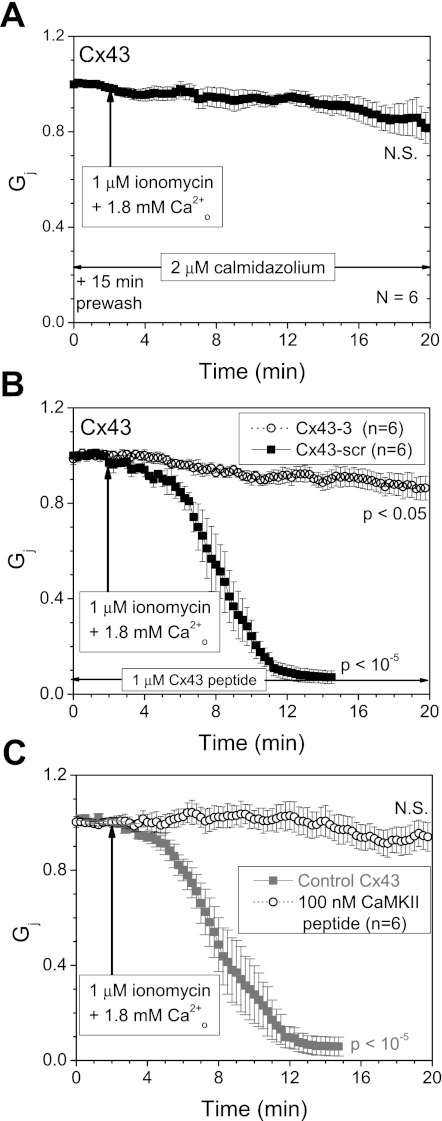
To determine whether CaM was involved in Ca2+-induced uncoupling of Cx43 gap junctions, N2a-Cx43 cells were pretreated with 2 μM CDZ for 15 min before patch-clamp recording. In the presence of CDZ, the steady-state Cx43 Gj of 83 ± 7% was not significantly different from initial values after 20-min exposure to Ca2+-containing SES (Fig. 4A). To address whether the Cx43 CL136–158 CaMBD was involved in the Ca2+/CaM-dependent uncoupling of Cx43, we added amino acid sequence-specific (Cx43–3) or scrambled (Cx43-scr) peptides to both whole cell patch pipettes. Since the apparent Kd of the Cx43–3 peptide for CaM in the presence of 100 mM KCl and 1 mM CaCl2 is 860 nM (67), 1 μM of the Cx43 CL peptides was added to each of the whole cell patch pipettes for these experiments. The Cx43–3 peptide, which mimics the Cx43 CL CaMBD sequence, limited the Ca2+-induced reduction of Cx43 Gj to 14 ± 5% of initial values, although this Gj decline was statistically significant at the P < 0.05 value. Cx43-scr was without significant effect: Cx43 Gj decreased by 93 ± 3% following the application of ionomycin (Fig. 4B). The canonical CaMKII CaMBD 290–309 inhibitory peptide (Kd = 50 nM, see Fig. 1A) prevented any significant Ca2+-induced Cx43 Gj decline. We also determined the effects of K146E+R148E and M147Q+L151E+I156E mutations to the Cx43 CaMBD sequence that eliminate CaM binding to this CL domain (67). These combinatorial hydrophilic or hydrophobic site mutations failed to induce any functional N2a cell coupling and inefficiently formed gap junction plaques in HeLa cells (data not shown).
Fig. 4.
A: pretreatment with 2 μM calmidazolium (CDZ) prevented the calcium-induced decrease in Cx43 Gj, limiting the decline to <20% after 20 min of 1 μM ionomycin, 1.8 mM CaCl2 exposure (P > 0.05). All Gj values were normalized to the initial junctional conductance (gj) value recorded over a 2-min period before ionomycin bath perfusion (mean ± SE, N = 6). B: the involvement of the Cx43 CL136–158 CaMBD in the Ca2+/CaM-dependent uncoupling response of Cx43 was examined by including 1 μM of the Cx43 mimetic (Cx43–3; Ac-KYGIEEHGKVKMRGGLLRTYIIS-NH2) or scrambled control (Cx43-scr; Ac-LGGEYLVTMESKIHIKGKRIGYR-NH2) in both whole cell pipettes during ionomycin/CaCl2 perfusion. The scrambled peptide (■) failed to prevent the calcium-induced decline in Cx43 Gj (P < 10−5), whereas the Cx43–3 mimetic peptide (○) limited the decrease in Gj to <15% (P < 0.05). C: inclusion of 100 nM CaMKII 290–309 peptide in both patch pipettes also preserved Cx43 Gj relative to control experiments (from Fig. 1B; P > 0.05), further demonstrating the involvement of CaMBD in the Ca2+-induced rundown of Cx43 Gj. Values are means ± SE.
Mechanism for Cx43 gap junction closure by Ca2+.
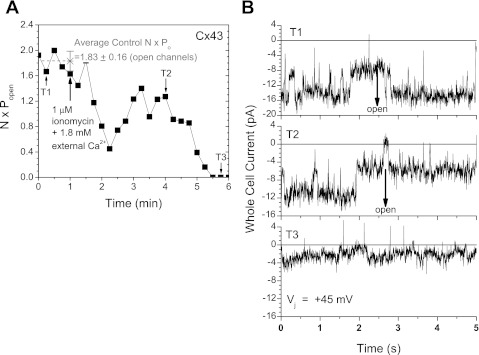
Channel blockade can occur by two basic mechanisms: pore block, where a poorly permeable or impermeable ion (e.g., Ca2+) inhibits the ion conduction pathway, or by gating between the open and closed configurations of the channel. A gating mechanism of channel closure is characterized by reductions in the cumulative product of the number of observed open channels (N) and the open probability (Po), whereas ionic block typically results in rapid flickering channel currents and an overall reduction in single-channel conductance (γ). In an attempt to distinguish between these two mechanisms, Cx43 gap junction channel currents were resolved in one low Gj N2a-Cx43 cell pair during perfusion with ionomycin in SES. The average value of N·Po for the observed channels was determined for each Vj pulse during this 1 μM ionomycin SES perfusion experiment. On average, 1.83 channels were open during four baseline control Vj pulses (Fig. 5A). This N·Po value declined to zero following a stochastic but gradual trend downward during the 6-min recording period. The unitary Cx43 gap junction channel current fluctuations from three time points (T1–T3; baseline control, declining, and final uncoupled phases) illustrate the decline in the product of N·Po without any decrease in the single-channel current (Fig. 5B). During the 5-min recording period, Cx43 γj averaged 114 ± 1 pS (SE).
Fig. 5.
A: a plot of the cumulative number of open channels (N) and open time probability (Po) from an N2a-Cx43 cell pair that exhibited a maximum of 3 open gap junction channels. On average, 1.83 Cx43 channels were open during the 1-min control period before 1 μM ionomycin + 1.8 mM CaCl2 perfusion. N·Po declined to zero in <5 min of ionomycin/CaCl2 perfusion. B: Cx43 gap junction channel currents recorded during the +45 mV, 5-s baseline control (T1), declining Gj (T2), and the final phase (T3) transjunctional voltage (Vj) pulses. The cumulative N·Po values were 1.67, 1.27, and 0 open channels per unit time, respectively. Value is mean ± SD.
DISCUSSION
The ability of Ca2+ to induce closure of gap junctions has been proposed since the early observations that cut cardiac muscle “heals-over” in the presence, but not absence, of external Ca2+ (10, 12). The ability of elevated intracellular Ca2+ to close gap junctions is considered to be a protective mechanism by isolating healthy cells from their injured neighbors. The direct modulation of intercellular gap junction communication by intracellular Ca2+ or CaM has been controversial since the publication of contradictory reports claiming that pHi or [Ca2+]i directly modulate junctional communication (26, 40, 41, 50, 52, 55). Using a bioinformatics approach, we identified putative CaM binding sites on the carboxyl-terminal portion of the connexin CL of three α-subfamily connexins: Cx43, sheep Cx44 (Cx46), and Cx50 (7, 66, 67). While CaM has previously been demonstrated to bind to at least two connexins, Cx32 and Cx50 (54, 65), the mechanisms by which Ca2+ or CaM modulate connexin gap junctions remain poorly defined.
In this study, we predicted that Cx40 does not contain a CaM binding site in the CL domain. In addition, we directly tested the involvement of Ca2+, CaM, and the CL CaMBD in the regulation of Cx43 and Cx40 gap junctions (Figs. 1–4). We have shown that Ca2+ influx uncoupled N2a-Cx43 cell pairs, while Cx40-N2a cell pairs were not uncoupled by Ca2+. Atrial gap junctions, which contain equal amounts of Cx40 and Cx43, uncoupled by 20% less than ventricular gap junctions comprised only of Cx43 (28). The relative insensitivity of Cx40 gap junctions to the Ca2+/CaM gating mechanism suggests that atrial gap junctions may not close as completely in response to Ca2+ overload conditions, which may be relevant to atrial fibrillation. The apparent insensitivity of Cx40 gap junctions to uncoupling by hundreds of nanomolar cytosolic [Ca2+] in this study does not preclude blockade by submillimolar or millimolar [Ca2+], as evidenced by the closing of Cx40 hemichannels in the presence of 3.6 mM Ca2+ (3). The decline in Cx43 Gj was inhibited by the omission of external CaCl2 or pretreatment with the CaM inhibitor CDZ (Figs. 2 and 4A). The addition of 1 μM ionomycin in the presence of normal extracellular [Ca2+] produced a threefold increase in N2a-Cx43 cytosolic [Ca2+] of ≈200 nM, within the physiological range of changes in systolic [Ca2+]i. Whole cell dialysis by the ruptured patch electrode recordings may alter the time course of the response due to internal Ca2+ buffering and dilution of endogenous CaM, but complete uncoupling was still observed (e.g., β-escin perforated patch experiment, data not shown). These results are consistent with previous findings in HeLa cells that the extracellular addition of Ca2+ in the presence of ionomycin could inhibit Cx43 cell-to-cell dye transfer (31). In those experiments, HeLa-Cx43 cell intracellular Ca2+ was elevated by 200–500 nM, with the addition of 1.8 or 21.8 mM CaCl2 in the presence of ionomycin, and the decrease in intercellular dye transfer was inhibited by CDZ, but not by chemical inhibitors of CaMKII or protein kinase C. By our measurements of the differential initial [Ca2+]i levels in fura 2 loaded or BAPTA/CaCl2-buffered patch clamped N2a-Cx43 cells before 1 μM ionomycin perfusion with 1.8 mM CaCl2 SES, the [Ca2+]i level should increase within the estimated range of 400–800 nM [Ca2+]i in N2a-Cx43 cell pairs. This estimated range for Cx43 Gj uncoupling is consistent with previous estimations of a threshold [Ca2+]i for ventricular Gj uncoupling of 685 nM (9). Directly correlated [Ca2+]i and Gj measurements have not yet been performed in paired cardiomyocytes; these experiments are planned for the next phase of this project using wild-type and available cardiac connexin-modified (e.g., Cx40 knockout) mice (28).
Chelation of all external Ca2+ with 10 mM EGTA inhibited Ca2+/ionomycin-induced uncoupling in our N2a-Cx43 cells. Interestingly, the magnitude of Gj recovery was dependent on the extent of uncoupling at the time the Ca2+-free SES was administered. Gj recovery achieved >90%, if Ca2+ chelation began before complete uncoupling, but was only partially reversible if complete uncoupling was allowed to develop (Fig. 3). Previous Ca2+ ionophore experiments have indicated that the induced elevation in [Ca2+]i is paralleled by a crystallization and decrease in particle spacing in gap junction plaques (43). Our data are consistent with the interpretation that prolonged exposure to elevated [Ca2+]i induces an irreversible gap junction uncoupling that may be correlated with plaque crystallization.
We directly tested for the involvement of the Cx43 CaMBD in Ca2+/CaM-dependent regulation of Cx43 gap junctions using Cx43 CL136–158 mimetic or scrambled peptides. Zhou et al. (67) described a Cx43–3 peptide corresponding to the 136–158 amino acid CL domain of Cx43 that bound CaM in the presence of Ca2+ with a Kd around 860 ± 20 nM. Intracellular addition of this peptide prevented the decline in Cx43 Gj, presumably by binding Ca2+/CaM and preventing its association with functioning Cx43 gap junctions (Fig. 4B). The Cx43-scr control peptide does not bind CaM and had no effect on the Ca2+-induced uncoupling. These results also suggest that the other regions of Cx43 are not likely to be involved in the Ca2+/CaM regulation (Fig. 1A).
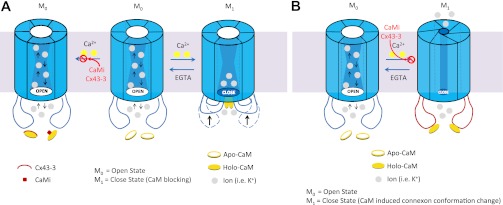
Based on our results, we hypothesize that Ca2+/CaM binding to the Cx43 CL136–158 domain induces a gating response that closes the Cx43 gap junctions. The gating mechanism of closure is favored over the alternative direct divalent cation block of Cx43 gap junction channels, since a decline in N·Po was observed without significant alteration of single-channel current amplitudes upon exposure to ionomycin and extracellular Ca2+ (Fig. 5). We have similarly repeatedly observed an all-or-none channel closure response with Cx50 gap junctions (7), thus substantiating the gap junction channel-gating mechanism by Ca2+/CaM. Our data, however, cannot distinguish between alternative connexin domain gating conformations for the Ca2+/CaM hypothesis, only that individual Cx43 gap junction channels close in an all-or-none manner in the presence of elevated cytosolic Ca2+. For instance, liver gap junctions, composed predominantly of Cx32, may possess connexin CaM binding amino and carboxyl terminal domains instead of the Cx43 CL site (14, 54). Holo CaM may act directly as a “cork” or induce a conformational change in the CL domain that occludes the cytoplasmic vestibule (mouth) of the gap junction channel, thereby restricting the passage of current-carrying ions (14, 45). There are several possible Ca2+/CaM gating mechanisms for connexin gap junctions that could be considered (Fig. 6). An alternative “iris” gating hypothesis based on the Unwin and Ennis (57) model suggests that the Ca2+-induced closure of liver gap junctions occurs by a decrease in the tilt angle of the six connexins comprising one connexon of a gap junction channel.
Fig. 6.
A: a hypothetical model for the (gating) occlusion of the Cx43 gap junction channel opening by the direct blockade of Ca2+-bound CaM (holo-CaM) (45), the induction of a conformational change in the cytoplasmic loop of Cx43 (67), or a combination of both steric hindrance mechanisms. Inhibition of the CaM-Cx43 cytoplasmic loop interaction by addition of the CaMKII or Cx43–3 peptides or a CaM inhibitor (e.g., CDZ) prevents the open (M0) to closed (M1) transition. B: an alternative gating hypothesis, based on the dual conformational model proposed by Unwin and Ennis (57), whereby a <10° tilting of the connexin subunits within a connexon translates into narrowing of the TM pore that effectively occludes the channel. The gating process is also reversible upon Ca2+ removal (by EGTA).
One longstanding criticism of the Ca2+-gating hypothesis of gap junctions is that it requires millimolar external Ca2+ and elevations in [Ca2+]i that cause permanent hypercontracture of cardiomyocytes to induce intercellular uncoupling (32, 59, 63). Whether or not physiological [Ca2+]i and levels were sufficient to modulate Cx43 gap junctions was not entirely understood (8, 36, 62). Our new data are consistent with previous observations in ventricular myocytes or Novikoff hepatoma cells that dynamic changes within the cardiac [Ca2+]i range of 200 to 500 nM are sufficient to produce effects on ventricular Gj, although the involvement of CaM was not tested in these prior studies (26, 36). Human disease causing connexin mutations may also lead to pathophysiological conditions by producing dysregulation of gap junction gating mechanisms like Ca2+/CaM or pHi. Although yet to be proven, several Cx43 oculodentodigital dysplasia mutations occur within these CL regulatory domains, including I130T, K134E/N, G138S/R, G143D/S, K144E, V145G, M147T, R148G/Q, and T154A/N (37), that could possibly disrupt Ca2+/H+ chemical gating mechanisms, in addition to their loss-of-function or nonfunctional consequences. We were unable to test the functional relevance of the Cx43 CaMBD site K146E+R148E and M147Q+L151E+I156E mutations because they failed to form detectable gap junctions or induce functional electrical coupling (67), perhaps because connexin interactions with CaM are required for gap junction formation or the mutations cause protein misfoldings that are incompatible with gap junction assembly (2, 24).
Steady-state measurements of cardiac gj have suggested a synergistic action between H+ and Ca2+ ions (6, 63), although time course experiments have indicated that Cx43 or cardiac gj is insensitive to pHi or declines only when pHi drops below 6.1–6.5, provided that [Ca2+]i remains < 500 nM (26, 36). As shown in Figs. 1B and 2A, parallel to the increase of [Ca2+]i from a resting level of 80 ± 3 nM to a maximum value of 250 ± 10 nM, Cx43 gj decreased to near zero after ∼12 min of ionomycin perfusion. These findings suggest that a threefold increase in [Ca2+]i uncouples Cx43. Although we did not measure pHi in conjunction with Ca2+ imaging, it is unlikely that pH is involved under the experimental conditions used in our experiments, since both SES and patch electrode solutions were buffered to pH 7.4 with 10 or 25 mM HEPES, respectively. Our data, however, do not exclude interplay between Ca2+ and pH in cells under more physiological settings. The CaMBD is located adjacent to a putative pH gating domain on the CL of Cx43 (35). Additional studies are required to determine whether causal interrelationships between these domains modify gap junction conductance. Experiments to further define the α-subfamily connexin Ca2+/CaM gating mechanism, including possible connexin trans-domain interactions with CaM, as proposed for Cx32 (14, 54), are also planned.
Our data strongly suggest that Ca2+/CaM-dependent regulation of Cx43 gap junction conductance requires the carboxyl-terminal portion of the Cx43 CL domain. Cx40 gap junctions lack this sub-micromolar Ca2+/CaM-dependent regulatory mechanism. Cx43 gap junctions are closed by a gating mechanism characterized by a reduction in channel Po without accompanying reductions in channel current amplitude. The Ca2+/CaM-dependent uncoupling is fully reversible, but only if the rise in [Ca2+]i is blocked before complete uncoupling; only one-half of the Cx43 gap junctional conductance can be recovered after complete uncoupling occurs, suggestive of irreversible gap junction closure induced by prolonged elevated cytosolic Ca2+. The intracellular Ca2+ affinity and Cx43/CaM conformational changes necessary to induce this chemical gating mechanism will require additional experimentation.
GRANTS
This work is supported in part by National Institutes of Health (NIH) Grants HL-042220 to R. D. Veenstra, DK-074966 to M. W. Roe, and GM-081749 and EY-05684 to J. J. Yang.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Q.X., Y.C., J.J.Y., M.W.R., and R.D.V. conception and design of research; Q.X., R.F.K., and M.W.R. performed experiments; Q.X. and M.W.R. analyzed data; Q.X., J.J.Y., M.W.R., and R.D.V. interpreted results of experiments; Q.X., Y.C., and R.D.V. prepared figures; Q.X. and R.D.V. drafted manuscript; Q.X., R.F.K., Y.C., J.J.Y., M.W.R., and R.D.V. edited and revised manuscript; Q.X., R.F.K., Y.C., J.J.Y., M.W.R., and R.D.V. approved final version of manuscript.
REFERENCES
- 1. Adermark L, Lovinger DM. Electrophysiological properties and gap junction coupling of striatal astrocytes. Neurochem Int 52: 1365– 1372, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmad S, Martin PE, Evans WH. Assembly of gap junction channels: mechanism, effects of calmodulin antagonists and identification of connexin oligomerization determinants. Eur J Biochem 268: 4544– 4552, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Allen MJ, Gemel J, Beyer EC, Lal R. Atomic force microscopy of connexin40 gap junction hemichannels reveals calcium-dependent three-dimensional molecular topography and open-closed conformations of both the extracellular and cytoplasmic faces. J Biol Chem 286: 22139– 22146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernardini G, Peracchia C. Gap junction crystallization in lens fibers after an increase in cell calcium. Invest Ophthalmol Vis Sci 21: 291– 299, 1981 [PubMed] [Google Scholar]
- 5. Blodow A, Ngezahayo A, Ernst A, Kolb HA. Calmodulin antagonists suppress gap junction coupling in isolated Hensen cells of the guinea pig cochlea. Pflügers Arch 446: 36– 41, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Burt JM. Block of intercellular communication: interaction of intracellular H+ and Ca2+. Am J Physiol Cell Physiol 253: C607– C612, 1987 [DOI] [PubMed] [Google Scholar]
- 7. Chen Y, Zhou Y, Lin X, Wong HC, Xu Q, Jiang J, Wang S, Lurtz MM, Louis CF, Veenstra RD, Yang JJ. Molecular interaction and functional regulation of connexin50 gap junction by calmodulin. Biochem J 435: 711– 722, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dahl G, Isenberg G. Decoupling of heart muscle cells: correlation with increased cytoplasmic calcium activity and with changes of nexus ultrastructure. J Membr Biol 53: 63– 75, 1980 [DOI] [PubMed] [Google Scholar]
- 9. Dekker LRC, Fiolet JWT, VanBavel E, Coronel R, Opthof T, Spaan JAE, Janse MJ. Intracellular Ca2+, intercellular electrical coupling, and mechanical activity in ischemic rabbit papillary muscle. Effects of preconditioning and metabolic blockade. Circ Res 79: 237– 246, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Dèléze J. Calcium ions and the healing-over in heart fibers. In: Electrophysiology of the Heart, edited by Taccardi B, Marchetti C. Elmsford, NY: Pergamon, 1965, p. 147– 148 [Google Scholar]
- 11. Dèléze J, Loewenstein WR. Permeability of a cell junction during intracellular injection of divalent cations. J Membr Biol 28: 71– 86, 1976 [DOI] [PubMed] [Google Scholar]
- 12. De Mello WC, Motta GE, Chapeau M. A study on the healing-over of myocardial cell of toads. Circ Res 24: 475– 487, 1969 [DOI] [PubMed] [Google Scholar]
- 13. De Mello WC. Effect of intracellular injection of calcium and strontium on cell communication in the heart. J Physiol 250: 2331– 245, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dodd R, Peracchia C, Stolady D, Török K. Calmodulin association with connexin32-derived peptides suggests trans-domain interaction in chemical gating of gap junction channels. J Biol Chem 283: 26911– 26920, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Evans WH, Martin PE. Gap junctions: structure and function. Mol Membr Biol 19: 121– 136, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Fan JS, Palade P. Perforated patch recording with β-escin. Pflügers Arch 436: 1021– 1023, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Fenwick A, Richardson RJ, Butterworth J, Barron MJ, Dixon MJ. Novel mutations in GJA1 cause oculodentodigital syndrome. J Dent Res 87: 1021– 1026, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Firek L, Weingart R. Modification of gap junction conductance by divalent cations and protons in neonatal rat heart cells. J Mol Cell Cardiol 27: 1633– 1643, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440– 3450, 1985 [PubMed] [Google Scholar]
- 20. Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys 34: 325– 472, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Jones DT. Do transmembrane protein superfolds exist? FEBS Lett 423: 281– 285, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Kalcheva N, Qu J, Sandeep N, Garcia L, Zhang J, Wang Z, Lampe PD, Suadicani SO, Spray DC, Fishman GI. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proc Natl Acad Sci USA 104: 20512– 20516, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krogh A, Larsson B, von HG, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305: 567– 580, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Laird DW. The gap junction proteome and its relationship to disease. Trends Cell Biol 20: 92– 101, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Lamartine J, Munhoz EG, Kibar Z, Lanneluc I, Callouet E, Laoudj D, Lemaitre G, Hand C, Hayflick SJ, Zonana J, Antonarakis S, Radhakrishna U, Kelsell DP, Christianson AL, Pitaval A, Der KV, Fraser C, Blanchet-Bardon C, Rouleau GA, Waksman G. Mutations in GJB6 cause hidrotic ectodermal dysplasia. Nat Genet 26: 142– 144, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Lazrak A, Peracchia C. Gap junction gating sensitivity to physiological internal calcium regardless of pH in Novikoff hepatoma cells. Biophys J 65: 2002– 2012, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin X, Crye M, Veenstra RD. Regulation of connexin43 gap junctional conductance by ventricular action potentials. Circ Res 93: e63– e73, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Lin X, Gemel J, Glass A, Zemlin CW, Beyer EC, Veenstra RD. Connexin40 and connexin43 determine gating properties of atrial gap junction channels. J Mol Cell Cardiol 48: 238– 245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin X, Veenstra RD. Effect of transjunctional KCl gradients on the spermine inhibition of connexin40 gap junctions. Biophys J 93: 483– 495, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin X, Zemlin CW, Hennan J, Petersen JS, Veenstra RD. Enhancement of ventricular gap junction coupling by rotigaptide. Cardiovasc Res 79: 416– 426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lurtz MM, Louis CF. Intracellular calcium regulation of connexin43. Am J Physiol Cell Physiol 293: C1806– C1813, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Maurer P, Weingart R. Cell pairs isolated from adult guinea pig and rat hearts: effects of [Ca2+]i on nexal membrane resistance. Pflügers Arch 409: 394– 402, 1987 [DOI] [PubMed] [Google Scholar]
- 33. Meador WE, Means AR, Quiocho FA. Modulation of calmodulin plasticity in molecular recognition on the basis of x-ray structures. Science 262: 1718– 1721, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Mitaku S, Hirokawa T, Tsuji T. Amphiphilicity index of polar amino acids as an aid in the characterization of amino acid preference at membrane-water interfaces. Bioinformatics 18: 608– 616, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Morley GE, Taffet SM, Delmar M. Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys J 70: 1294– 1302, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Noma A, Tsuboi N. Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. J Physiol 382: 193– 211, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paznekas WA, Karczaski B, Vermeer S, Lowry RB, Delatycki M, Laurence F, Koivisto PA, Maldergem LV, Boyadjiev SA, Bodurtha JN, Jabs EW. GJA1 mutations, variants, and connexin43 dystfunction as it relates to the oculodentodigital dysplasia phenotype. Hum Mutat 30: 724– 733, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Peracchia C. Communicating junctions and calmodulin: inhibition of electrical uncoupling in Xenopus embryo by calmidazolium. J Membr Biol 81: 49– 58, 1984 [DOI] [PubMed] [Google Scholar]
- 39. Peracchia C. Calmodulin-like proteins and communicating junctions. Electrical uncoupling of crayfish axons in inhibited by the calmodulin inhibitor W7 and is not affected by cyclic nucleotides. Pflügers Arch 408: 379– 385, 1987 [DOI] [PubMed] [Google Scholar]
- 40. Peracchia C. Increase in gap junction resistance with acidification in crayfish septate axons is closely related to changes in intracellular calcium but not hydrogen ion concentration. J Membr Biol 113: 75– 92, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Peracchia C. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim Biophys Acta 1662: 61– 80, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Peracchia C, Bernardini G, Peracchia LL. Is calmodulin involved in the regulation of gap junction permeability? Pflügers Arch 399: 152– 154, 1983 [DOI] [PubMed] [Google Scholar]
- 43. Peracchia C, Peracchia LL. Gap junction dynamics: reversible effects of divalent cations. J Cell Biol 87: 708– 718, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peracchia C, Sotkis A, Wang XG, Peracchia LL, Persechini A. Calmodulin directly gates gap junction channels. J Biol Chem 275: 26220– 26224, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Peracchia C, Wang XG, Peracchia LL. Behavior of chemical- and slow voltage-sensitive gating of connexin channels: the “cork” gating hypothesis. In: Gap Junctions. Molecular Basis of Cell Communication in Health and Disease, edited by Peracchia C. San Diego, CA: Academic, 2000, p. 271– 295 [Google Scholar]
- 46. Reverdin EC, Weingart R. Electrical properties of the gap junctional membrane studied in rat liver cell pairs. Am J Physiol Cell Physiol 254: C226– C234, 1988 [DOI] [PubMed] [Google Scholar]
- 47. Richard G. Connexin disorders of the skin. Adv Dermatol 17: 243– 277, 2001 [PubMed] [Google Scholar]
- 48. Roe MW, Fiekers JF, Philipson LH, Bindokas VP. Visualizing calcium signaling in cells by digitized conventional wide-field and confocal fluorescence microscopy. Methods Mol Biol 319: 37– 66, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Roscoe W, Veitch GI, Gong XQ, Pellegrino E, Bai D, McLachlan E, Kidder GM, Laird DW. Oculodentodigital dysplasia-causing connexin43 mutants are non-functional and exhibit dominant effects on wild-type connexin43. J Biol Chem 280: 11458– 11466, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Rose B, Loewenstein WR. Permeability of a cell junction and the local cytoplasmic free ionized calcium concentration: a study with aequorin. J Membr Biol 28: 87– 119, 1976 [DOI] [PubMed] [Google Scholar]
- 51. Shibayama J, Paznekas W, Seki A, Taffet S, Jabs EW, Delmar M, Musa H. Functional characterization of connexin43 mutations found in patients with oculodentodigital dysplasia. Circ Res 96: e83– e91, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Spray DC, Harris AL, Bennett MV. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science 211: 712– 715, 1981 [DOI] [PubMed] [Google Scholar]
- 53. Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673– 4680, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Török K, Stauffer K, Evans WH. Connexin 32 of gap junctions contains two cytoplasmic calmodulin-binding domains. Biochem J 326: 479– 483, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Turin L, Warner AE. Carbon dioxide reversibly abolishes ionic communication between cells of early amphibian embryos. Nature 270: 56– 57, 1977 [DOI] [PubMed] [Google Scholar]
- 56. Tusnady GE, Simon I. The HMMTOP transmembrane topology prediction server. Bioinformatics 17: 849– 850, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Unwin PN, Ennis PD. Two configurations of a channel-forming membrane protein. Nature 307: 609– 613, 1984 [DOI] [PubMed] [Google Scholar]
- 58. Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, Mathias RT, Robinson RB, Rosen MR, Cohen IS, Brink PR. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol 568: 459– 468, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Veenstra R. Physiological modulation of cardiac gap junction channels. J Cardiovasc Electrophysiol 2: 168– 189, 1991 [Google Scholar]
- 60. Veenstra RD. Voltage clamp limitations of dual whole cell recordings of gap junction current and voltage recordings. I. Conductance measurements. Biophys J 80: 2231– 2247, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Veenstra RD, DeHaan RL. Cardiac gap junction channel activity in embryonic chick ventricle cells. Am J Physiol Heart Circ Physiol 254: H170– H180, 1988 [DOI] [PubMed] [Google Scholar]
- 62. Weingart R. The actions of ouabain on intercellular coupling and conduction velocity in mammalian ventricular muscle. J Physiol 264: 341– 365, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. White RL, Doeller JE, Verselis VK, Wittenberg BA. Gap junction conductance between pairs of ventricular myocytes is modulated synergistically by H+ and Ca2+. J Gen Physiol 95: 1061– 1075, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yap KL, Kim J, Truong K, Sherman M, Yuan T, Ikura M. Calmodulin target database. J Struct Funct Genomics 1: 8– 14, 2000 [DOI] [PubMed] [Google Scholar]
- 65. Zhang X, Qi Y. Role of intramolecular interaction in connexin50: mediating the Ca2+-dependent binding of calmodulin to gap junction. Arch Biochem Biophys 440: 111– 117, 2005 [DOI] [PubMed] [Google Scholar]
- 66. Zhou Y, Yang W, Lurtz MM, Chen Y, Jiang J, Huang Y, Louis CF, Yang JJ. Calmodulin mediates the Ca2+-dependent regulation of Cx44 gap junctions. Biophys J 96: 2832– 2848, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhou Y, Yang W, Lurtz MM, Ye Y, Huang Y, Lee HW, Chen Y, Louis CF, Yang JJ. Identification of the calmodulin binding domain of connexin 43. J Biol Chem 282: 35005– 35017, 2007 [DOI] [PubMed] [Google Scholar]