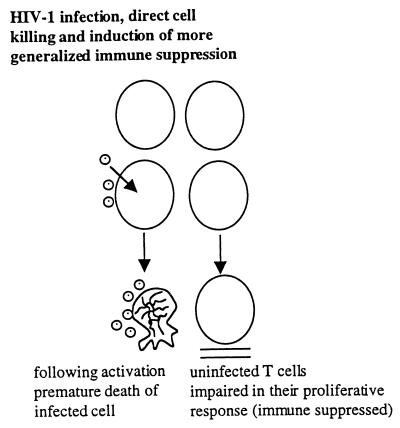
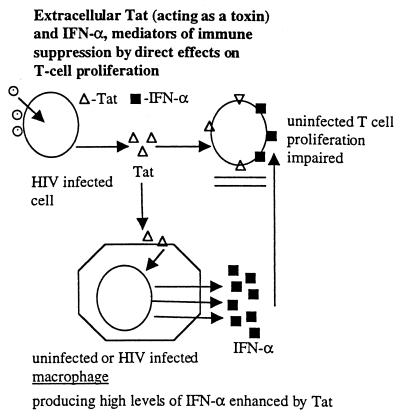
HIV-induced immune pathogenesis is more complex than simple infection of CD4+ T cells, their subsequent death, replacement by new CD4+ T cells, and eventual immune exhaustion. Even from the earliest period of HIV research, it was clear from in vitro results that the bulk of T cells did not respond properly to antigenic stimuli and even ultimately to mitogenic stimuli, despite the fact that only a small percent of T cells were infected (1, 2). These results, however, were not limited to in vitro experiments. Other studies showed abnormalities of proliferation and sometimes “bystander” apoptosis of uninfected cells in simian immunodeficiency virus-infected macaques (3) and, more recently, regenerative abnormalities that include more generalized effects on hematopoiesis of HIV infected people (4). Consequently, a direct infection and cell killing of CD+ T cells followed by their rapid replacement and ultimate immune exhaustion as suggested in some models does not encompass the overall reality of HIV-induced immune pathogenesis. HIV immune pathogenesis also clearly involves extracellular factors, which have an effect on uninfected cells (Fig. 1). Candidates may include the aberrant production of normal cellular factors, such as the over-production of some cytokines and/or their production at the wrong time and place. In fact, in studies chiefly performed by my collaborator D. Zagury, we have presented evidence that IFN-α may be one such candidate because of its over-production after HIV infection (5), its known anti-proliferative effects (6–8) (Fig. 2), and because we could partially restore T-cell proliferation in vitro to normal when HIV infected peripheral blood mononuclear cell cultures were treated with antibodies to IFN-α (8). Viral proteins, of course, also may be extracellular toxins, and D. Zagury, his collaborators, and I have proposed that Tat is one such toxin and perhaps the most important one (8) (Fig. 2).
The discovery of the HIV Tat protein as an essential regulatory gene for HIV replication was not unexpected to those familiar with earlier known human retroviruses (9). Analagous to the Tat protein of human T-cell leukemia virus 1 and 2 (9, 10), Tat is a small (14 kDa) nuclear protein product transcribed from complex spliced mRNAs, functions as a transacting transcriptional activator (9, 10), and is known to be involved in initiation of transcription and, more importantly, in RNA chain elongation (11) by complex processes involving interaction with cellular proteins and a specific region (TAR) of the viral RNA (11–13). More central to this perspective, however, are the results from studies that implied that Tat might be released from infected cells and might be capable of entering and/or binding other cells (14–16) (Fig. 2). Results of some early experiments suggested that extracellular Tat could activate otherwise quiescent HIV proviruses, leading to still more virus production. Obviously, questions were raised as to the potential physiological meaning of these results, particularly in view of the relatively high concentrations of Tat required for the transactivation effect, as generally determined by activation of virus expression from Tat defective proviruses.
By the early 1990s, Tat was unequivocally shown to be released from acute infected CD4+ T lymphocytes (15), and, when it interacted with nearby activated uninfected T-cells, Tat inhibited proliferation of these cells (8, 17–19) (Fig. 2). Sometimes, apoptosis of these bystander cells also was observed (18, 19). The amount of Tat required for inhibiting T-cell proliferation is less than that needed for the proviral transactivation effects, perhaps because these effects on cells may only require interaction with the cell surface rather than cell entry and nuclear membrane penetration.
How Tat produces these effects, however, is not yet settled. It’s binding to cells is promiscuous probably because of its basic region interacting by electrostatic forces with the cell surface. However, Tat RGD region and other segments may bind specific integrins on some cells (20) and chemokine receptors on others (21). Indeed, Tat has been shown to have chemokine-like effects in promoting migration of some cells (15, 20–22). In addition, it was recently shown that extracellular Tat induces chemokine receptors expression on T-cells (23, 24). Whether solely binding and signaling mediate these effects of extracellular Tat is likely but unproven (25, 26). Several other toxic effects of extracellular Tat, including neurotoxicity (27), also have been described, but these cannot be discussed here.
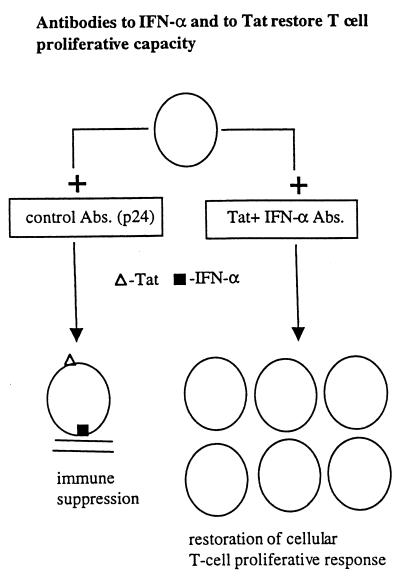
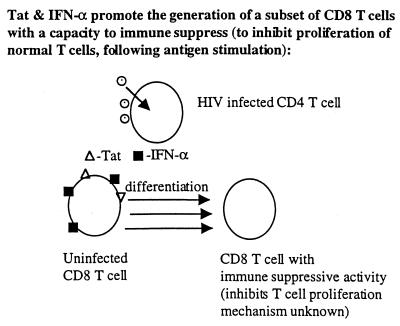
Collectively, these in vitro results prompted speculations on a possible role of extracellular Tat acting as a toxin in HIV-induced immune pathogenesis leading to AIDS. What is the evidence that Tat is involved in immune suppression, i.e., in inhibition of proliferation of uninfected T-cells and/or altering antigen-presenting cells? (i) Tat activates INF-α production from macrophages (8) (Fig. 2), INF-α is present in high levels in HIV-infected persons (5), and INF-α at high levels is immune suppressive as discussed above (6–8). (ii) Tat directly inhibits T-cell proliferation (8, 17) and promotes apoptosis of some cells (18, 19) probably by inducing aberrant activation (28), though with some cells and under certain conditions Tat can be anti-apoptotic (28). (iii) Data from 24 different experiments showed that full recovery of T-cell proliferation is achieved when HIV-1-infected peripheral blood mononuclear cells were cultured in the presence of antibodies to both Tat and IFN-α whereas only partial recovery was obtained with either antibody alone (Fig. 4). Thus, enhancing neutralizing antibodies to Tat, though likely insufficient when used as an exclusive immunogen, may be helpful as a component of a composite vaccine by reducing immunosuppression (8, 16, 17) and reducing the viral replication (32) induced by this toxin. (iv) Tat and INF-α promote the generation of a subset of CD8+ T-cells with immunosuppressive properties themselves (8) (Fig. 3). (v) Tat inhibits NK activity (29). (vi) Corroborating these in vitro results are a series of recent European clinical studies. First, a Dutch group reported that HIV-infected persons who rapidly progress to AIDS have less Tat-specific cytotoxic T-cells compared with those who progress slowly (30). In a French cohort of 250 HIV-infected nonprogressors (the GRIV cohort), the best serum correlate of nonprogression was a high titer of antibodies against Tat during 1–2 years of follow-up of these subjects (31). Michel La Placa, in Bologna, Italy, pioneered studies on the in vitro effects of Tat, being the first to show that extracellular Tat could markedly enhance viral replication through an autocrine-paracrine loop, and was the first to suggest targeting Tat by antibodies. He and his colleagues reported the first study showing a correlation of antibodies against Tat and slower disease progression, albeit in a smaller study than some of the recent reports (32). Finally, a very recent German study showed that only infected individuals without antibodies to Tat progressed to develop Kaposi’s sarcoma (I, Demirhan, A. Chandra, O. Hasselmayer, and P. Chandra, personal communication).
In my view, these in vitro and in vivo results taken together, as well as animal experiments showing the safety and immunogenicity of inactivated Tat (35), are powerful arguments for clinical intervention. Indeed, they have already prompted a clinical therapeutic (33) and a preventive (34) vaccine study in humans utilizing a chemically detoxified but immunogenic Tat called Tat toxoid (33). Preliminary results of these studies in immune deficient patients show safety and immunogenicity (33). These clinical trials were complemented by earlier and ongoing studies that utilized a preparation of inactivated IFN-α as a therapeutic vaccine, i.e., used only in infected individuals (35, 36). As in the recent Tat studies, safety and immunogenicity were demonstrated, and CD4+ T cell numbers were stabilized (35, 36). The efficacy results, however, are anecdotal and demand larger and rigorously controlled trials. If clearly efficacious, this approach has great advantages, such as low cost, little or no toxicity, and feasibility for the Third World because only periodic injections are needed. In combination with anti-HIV chemotherapy, it also may be useful in the industrial world.
In the case of Tat, I think these studies also have major implications for the use of chemically inactivated but immunogenic (33, 35) Tat as an added component of an HIV-preventive vaccine. Indeed, we have already initiated trials in healthy uninfected individuals that have shown safety and striking immunogenicity of the Tat toxoid. The antibody titers ranged from 1:16,000 to 1:64,000 and lasted over 1 year after one inoculation (34).
On the other hand, it is also important to note reports of in vitro studies that show Tat might have beneficial effects by reducing HIV infection or expression. For instance, my collaborators and I reported that Tat can enhance production of IFN-α and β-chemokines (8), and, of course, both can inhibit either HIV entry into its target cells or HIV expression. However, the overriding effect of Tat over a period of time is to suppress T-cell proliferation, which ultimately negates the aforementioned beneficial effects, eventually including a decline in chemokine production. The net effect of several in vitro effects can only be determined from in vivo clinical results or in vivo experiments. As already discussed, several clinical–epidemiological studies verify that a stronger immune response against Tat correlates with better prognosis. This has now been strengthened by recent studies of simian immunodeficiency virus- or SHIV-infected monkeys, as described below.
After the in vitro results of the immunosuppressive effects of Tat discussed above and the initiation of the above-mentioned trials in humans, three supportive vaccine studies were conducted in monkeys. First, A. Osterhaus and his colleagues in Lyon, in collaboration with G. Sutter and V. Erfle in Munich, have reported marked suppression (absence of viremia) in cynamalgus monkeys infected with SIVmac 32h (pJ5) if the animals were vaccinated with a recombinant modified vaccinia expressing Tat and Rev (37). More recent studies of D. Pauza (in collaboration with D. Zagury and investigators at the Institute of Human Virology) indicate induction of delayed type hypersensitivity and other indicators of a marked CD8+ T cellular immune response in monkeys vaccinated with the same chemically modified Tat used in the human trials described above (see refs. 34 and 35). Moreover, Pauza’s experiments show that the infection with SHIV up-regulates expression of CXCR4 and CCR5 and that, in the animals vaccinated with Tat toxoid, this is diminished (D. Pauza, P. Trivedi, M. Wallace, T. Ruckwardt, H. LeBuanec, A. Burny, D. Zagury, and R.C.G., unpublished results). These in vivo results corroborate the earlier in vitro results showing that Tat is able to up-regulate CCR5 (23) and CXCR4 (23, 24). Independently, my former coworker, B. Ensoli, and her collaborators initiated a study of monkeys infected with the highly pathogenic strain SHIV 89.6P (42). One group of these animals received a commercial preparation of native Tat as a candidate vaccine. In these studies, five of seven monkeys showed no viremia, no detectable virus in peripheral blood mononuclear cells, or disease development. Unfortunately, on several occasions, presentation of these results has resulted in sensational headlines in Europe that a protective vaccine was in hand before publication of the results. Furthermore, these findings are regarded as still limited in that (i) only seven monkeys were studied and two exhibited signs of disease progression, and in this model, inexplicably, 50% of infected animals spontaneously showed a nonprogression profile with little or no viremia (38); (ii) all animals were infected as indicated by persistent serum antibodies (no sterilizing immunity); and (iii) use of native Tat (as used in these monkey studies) might be hazardous to people whose immune system is compromised by chronic infections, parasitic disease, or as a result of malnutrition or of autoimmune predisposition. Nonetheless, collectively, the three sets of results in monkeys are of interest and support the prior concepts, in vitro experiments, and early clinical trials summarized in this report.
There is one notable conflicting observation. Recent unpublished in vitro results of K. Teh-Jeang and P. Murphy show that Tat can block CXCR4 by both SDF-1 and CXCR4 utilizing HIV-1 strains but had no effect on CCR5 function. They speculate that Tat may provide selective pressure at initial infection, accounting in part for CXCR4 tropic HIV-1 variants being excluded at this stage and favoring emergence of CCR5 viruses as the dominant disease transmitting strain (P. Murphy and K. Teh-Jeang, unpublished results). These interesting results cannot be taken lightly and may very well have some in vivo consequence like our earlier results showing Tat can augment IFN-α and β-chemokine production, both of which inhibit HIV infection (8). However, I again emphasize that, eventually, high levels of Tat are immune suppressive and indirectly lead to greater HIV replication. Additionally, the three primate studies show suppression of virus, no emergence of CXCR4 tropic variants, and a reduction in expression of both CCR5 and CXCR4. Furthermore, in clinical studies and late stage HIV-1 infection with greater replication of HIV-1 (and hence greater Tat production), CXCR4 HIV-1 variants tend to emerge rather than being suppressed. I would conclude from this phenomenon that the in vivo data strongly indicate that the net effect of Tat is highly detrimental.
In my view, a “detoxified” Tat will be an important component of a prophylactic or therapeutic vaccine coupled with a vector delivery of HIV-1 structural proteins. Such a composite vaccine will trigger long-lasting Tat-antibodies, which may facilitate control of Tat-included immune suppression during acute infection and allow development of cell mediated immunity to HIV-1 structural antigens. This includes the release of β-chemokines, substances that may be viewed as quasi-immediate effectors of the cellular immune response, and, because they specifically inhibit infection by the macrophage tropic strains of HIV-1 (39), they also may be important in protection from HIV infection (40). We further think that, in infected persons, the effect of controlling extracellular Tat also will enhance the pool of anti-HIV-1 active memory cytotoxic T lymphocytes, which may lyse infected cells and thereby contain HIV-1 replication and prevent evolution toward AIDS (41). My long-time collaborator D. Zagury (Paris), has been involved in all of the concepts I have discussed here and is spearheading this approach in Europe. Other major collaborators include A. Bizzini, A. Lachgar, and J.-F. Zagury (Paris); A. Gringeri (Milan); and A. Burny (Brussels).
Acknowledgments
I am grateful to D. Zagury (Paris), G. Zauli (Ferrara), and especially to my colleague P. Secchiero for reading the manuscript and providing helpful comments.
References
- 1.Zagury D, Bernard J, Leonard R, Cheynier R, Feldman M, Sarin P S, Gallo R C. Science. 1986;231:850–853. doi: 10.1126/science.2418502. [DOI] [PubMed] [Google Scholar]
- 2.Clerici M, Stocks N I, Zajac R A, Boswell R N, Lucey D R, Via C S, Shearer G M. J Clin Invest. 1989;84:1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins M, Hanley M B, Moreno M B, Wieder E, McCune J. Blood. 1998;91:2672–2678. [PubMed] [Google Scholar]
- 5.Ambrus J L, Poiesz B J, Lillie M A, Stadler I, Di Berardino L A, Chadha K C. Am J Med. 1989;87:405–407. doi: 10.1016/s0002-9343(89)80822-8. [DOI] [PubMed] [Google Scholar]
- 6.Petricoin E F, III, Ito S, Williams B L, Audet S, Stancato L F, Gamero A, Clouse K, Grimley P, Weiss A, Beeler J, et al. Nature (London) 1995;390:629–632. doi: 10.1038/37648. [DOI] [PubMed] [Google Scholar]
- 7.Lachgar A, Bizzini B. Mol Cell Biol. 1995;41:431–437. [PubMed] [Google Scholar]
- 8.Zagury D, Lachgar A, Chasms V, Fall L S, Bernard J, Zagury J F, Bizzini B, Gringeri A, Santagostino E, Rappaport J, et al. Proc Natl Acad Sci USA. 1998;95:3851–3856. doi: 10.1073/pnas.95.7.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong-Staal F, Gallo R C. Nature (London) 1985;317:395–403. doi: 10.1038/317395a0. [DOI] [PubMed] [Google Scholar]
- 10.Fujisawa J, Seiki M, Kiyokawa T, Yoshida M. Proc Natl Acad Sci USA. 1985;82:2277–2281. doi: 10.1073/pnas.82.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cullen B R. Cell. 1990;63:655–657. doi: 10.1016/0092-8674(90)90129-3. [DOI] [PubMed] [Google Scholar]
- 12.Dayton A I, Sodroski J G, Rosen C A, Goh W C, Haseltine W A. Cell. 1986;44:941–947. doi: 10.1016/0092-8674(86)90017-6. [DOI] [PubMed] [Google Scholar]
- 13.Wei P, Garber M E, Fang S M, Fischer W H, Jones K A. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 14.Frankel A D, Pabo C O. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 15.Ensoli B, Barillari G, Salahuddin S Z, Gallo R C, Wong-Staal F. Nature (London) 1990;345:84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- 16.Zauli G, La Placa M, Vignoli M, Re M C, Gibellini D, Furlini G, Milani D, Marchisio M, Mazzoni M, Capitani S. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;10:306–316. [PubMed] [Google Scholar]
- 17.Viscidi R P, Mayur K, Lederman H M, Frankel A D. Science. 1989;246:1606–1608. doi: 10.1126/science.2556795. [DOI] [PubMed] [Google Scholar]
- 18.Li C J, Friedman D J, Wang C, Metelev V, Pardee A B. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 19.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K M, Krammer P H. Nature (London) 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 20.Barillari G, Gendelman R, Gallo R C, Ensoli B. Proc Natl Acad Sci USA. 1993;90:7941–7945. doi: 10.1073/pnas.90.17.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albini A, Ferrini S, Benelli R, Sforzini S, Giunciuglio D, Aluigi M G, Proudfoot A E, Alouani S, Wells T N, Mariani G, et al. Proc Natl Acad Sci USA. 1998;95:13153–13158. doi: 10.1073/pnas.95.22.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitola S, Sozzani S, Luini W, Primo L, Borsatti A, Weich H, Bussolino F. Blood. 1997;90:1365–1372. [PubMed] [Google Scholar]
- 23.Huang L, Bosch I, Hofmann W, Sodroski J, Pardee A B. J Virol. 1998;72:8952–8960. doi: 10.1128/jvi.72.11.8952-8960.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Secchiero P, Zella D, Capitani S, Gallo R C, Zauli G. J Immunol. 1999;162:2427–31. [PubMed] [Google Scholar]
- 25.Li C J, Ueda Y, Shi B, Borodyansky L, Huang L, Li Y Z, Pardee A B. Proc Natl Acad Sci USA. 1997;94:8116–8120. doi: 10.1073/pnas.94.15.8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibellini D, Bassini A, Pierpaoli S, Bertolaso L, Milani D, Capitani S, La Placa M, Zauli G. J Immunol. 1998;160:3891–3898. [PubMed] [Google Scholar]
- 27.Conant K, Garzino-Demo A, Nath A, McArthur J C, Halliday W, Power C, Gallo R C, Major E O. Proc Natl Aad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zauli G, Gibellini D, Celeghini C, Mischiati C, Bassini A, La Placa M, Capitani S. J Immunol. 1996;157:2216–2224. [PubMed] [Google Scholar]
- 29.Zocchi M R, Rubartelli A, Morgavi P, Poggi A. J Immunol. 1998;161:2938–2943. [PubMed] [Google Scholar]
- 30.van Baalen C A, Pontesilli O, Huisman R C, Geretti A M, Klein M R, de Wolf F, Miedema F, Gruters R A, Osterhaus A D. J Gen Virol. 1997;8:1913–1918. doi: 10.1099/0022-1317-78-8-1913. [DOI] [PubMed] [Google Scholar]
- 31.Zagury J-F, Sill A, Blattner W, Lachgar A, Le Buanec H, Richardson M, Rappaport J, Hendel H, Bizzini B, Gringeri A, et al. J Hum Virol. 1998;1:282–292. [PubMed] [Google Scholar]
- 32.Re M C, Furlini G, Vignoli M, Ramazzotti E, Roderigo G, De Rosa V, Zauli G, Lolli S, Capitani S, La Placa M. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;10:408–416. doi: 10.1097/00042560-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Gringeri A, Santagostino E, Muca-Perja M, Mannucci P M, Zagury J F, Bizzini B, Lachgar A, Carcagno M, Rappaport J, Criscuolo M, et al. J Hum Virol. 1998;1:293–298. [PubMed] [Google Scholar]
- 34.Gringeri, A. (1999) J. Acquired Immune Defic. Syndr., in press. [DOI] [PubMed]
- 35.Gringeri A, Santagostino E, Cusini M, Muca-Perja M, Marinoni A, Mannucci P M, Burny A, Criscuolo M, Lu W, Andrieru J M, et al. J Acquired Immune Defic Syndr Hum Retrovirol. 1996;13:55–67. doi: 10.1097/00042560-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Gringeri, A. (1999) J. Acquired Immune Defic. Syndr. Hum. Retrovirol., in press. [DOI] [PubMed]
- 37.Osterhaus, A. D. M. E. (1999) Vaccine, in press.
- 38.Steger K K, Dykhuizen M, Mitchen J L, Hinds P W, Preuninger B L, Wallace M, Thomson J, Montefiori D C, Lu Y, Pauza C D. J Virol. 1998;72:1600–1605. doi: 10.1128/jvi.72.2.1600-1605.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 40.Zagury D, Lachgar A, Chams V, Fall L S, Bernard J, Zagury J F, Bizzini B, Gringeri A, Santagostino E, Rappaport J, et al. Proc Natl Acad Sci USA. 1998;95:3857–3861. doi: 10.1073/pnas.95.7.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goulder P J, Phillips R E, Colbert R A, McAdams S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, et al. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 42.Cafaro A, Caputo A, Fracasso C, Maggiorella M T, Goletti D, Baroncelli S, Pace M, Sernicola L, Koanga-Mogtomo M C, Betti M, et al. Nat Med. 1999;5:643–650. doi: 10.1038/9488. [DOI] [PubMed] [Google Scholar]