Abstract
Maintaining tight control over body fluid and acid-base homeostasis is essential for human health and is a major function of the kidney. The collecting duct is a mosaic of two cell populations that are highly specialized to perform these two distinct processes. The antidiuretic hormone vasopressin (VP) and its receptor, the V2R, play a central role in regulating the urinary concentrating mechanism by stimulating accumulation of the aquaporin 2 (AQP2) water channel in the apical membrane of collecting duct principal cells. This increases epithelial water permeability and allows osmotic water reabsorption to occur. An understanding of the basic cell biology/physiology of AQP2 regulation and trafficking has informed the development of new potential treatments for diseases such as nephrogenic diabetes insipidus, in which the VP/V2R/AQP2 signaling axis is defective. Tubule acidification due to the activation of intercalated cells is also critical to organ function, and defects lead to several pathological conditions in humans. Therefore, it is important to understand how these “professional” proton-secreting cells respond to environmental and cellular cues. Using epididymal proton-secreting cells as a model system, we identified the soluble adenylate cyclase (sAC) as a sensor that detects luminal bicarbonate and activates the vacuolar proton-pumping ATPase (V-ATPase) via cAMP to regulate tubular pH. Renal intercalated cells also express sAC and respond to cAMP by increasing proton secretion, supporting the hypothesis that sAC could function as a luminal sensor in renal tubules to regulate acid-base balance. This review summarizes recent advances in our understanding of these fundamental processes.
Keywords: aquaporin 2, vacuolar ATPase, intercalated cell, principal cell, kidney, epididymis
it has been known for many years that the major functions of collecting duct principal cells and intercalated cells (Fig. 1) can be regulated by the recycling of aquaporin 2 (AQP2) and the vacuolar H+-ATPase (V-ATPase), respectively, between cytoplasmic vesicles and the plasma membrane (1, 26, 28, 40, 54, 67, 85, 142). A considerable amount of the earlier data illuminating these processes was generated using readily accessible model epithelial systems such as the toad urinary bladder (48, 83, 140) and the turtle bladder (3, 127, 128), in which cellular function and morphology could be correlated with measurements of water flux and acid-base transport. More recently, a variety of in vitro cell culture models have replaced the toad bladder as an experimental system for water channel trafficking and function. This review will show how cell cultures have provided novel and important information on aquaporin 2 trafficking that has been translatable to the in vivo situation and has allowed the development of strategies to bypass the vasopressin (VP)/vasopressin type 2 receptor (V2R) signaling axis that is defective in nephrogenic diabetes insipidus (NDI). We will go on to demonstrate how the epididymis and vas deferens (from the male reproductive tract) have proven to be powerful and accessible model proton-secreting epithelia that have provided new insights into the regulation of intercalated cells in the kidney collecting duct (21).
Fig. 1.
Medullary collecting duct from rat kidney, triple immunostained to show apical aquaporin 2 (AQP2; green) and basolateral AQP4 (red) in principal cells (PC), and apical vacuolar H+-ATPase (V-ATPase) (blue) in A-type intercalated cells (IC). Principal cells are responsible for vasopressin (VP)-sensitive water reabsorption from the lumen while intercalated cells are involved in acid secretion.
Aquaporin 2 Water Channel Trafficking
The aquaporin 2 water channel (AQP2) is the gatekeeper of water permeability in renal collecting duct principal cells (42). It accumulates in the apical plasma membrane of these cells (Fig. 1) under the action of the antidiuretic hormone VP and allows the osmotic extraction of water from the lumen into the renal interstitium in both cortical and medullary regions of the kidney (47, 88, 114). In the cortex, water flow out of the lumen is driven by a gradient between the dilute luminal fluid and an isotonic interstitium, while in the medulla the tubular fluid equilibrates osmotically with a hypertonic interstitium. At the cellular level, apical water permeability is regulated by the insertion and removal of AQP2 to and from the apical plasma membrane. Basolateral membranes in different collecting duct segments are believed to be constitutively water permeable due to the presence of AQP3 and/or AQP4 (Fig. 1) (55, 134). One interesting sidebar is that AQP2 itself is also expressed basolaterally in principal cells. In some regions such as the inner medulla, the level of basolateral expression can be equal to or greater than apical expression (36, 89, 137). Whether this basolateral AQP2 is key for water permeability of the cells or whether it has an additional biological function is not resolved. However, recent data from our group and others have shown that AQP2 is an integrin-binding protein via the RGD domain located in its second extracellular loop. While it has been proposed that integrin modulates AQP2 expression (133), we have shown that it is also involved in cell migration during tubulogenesis (34).
How does AQP2 accumulate in the apical membrane of principal cells when the body needs to reduce water loss in the urine? Under conditions in which the serum becomes slightly hypertonic or when a loss of volume is detected by various sensing mechanisms in the circulatory system and in the brain, the hormone VP is released from the posterior pituitary and interacts with basolateral vasopressin type 2 receptors (V2R) in principal cells (14). The V2R is a canonical G protein-coupled receptor (GPCR), which activates adenylyl cyclase via a Gsα protein, and increases intracellular cAMP levels (53). This results in activation of protein kinase A (PKA), which leads to phosphorylation of AQP2 on a serine 256 residue in its cytoplasmic COOH terminus (41, 59) as well as other COOH-terminal residues whose function is incompletely understood (50, 82, 106). As will be described in more detail below, activation of the V2R leads to a dual effect on AQP2 recycling by increasing exocytosis and decreasing endocytosis. Together, these processes lead to a significant accumulation of AQP2 in the apical membrane, resulting in increased water permeability of the collecting duct epithelium.
Role of Both Exocytosis and Endocytosis in AQP2 Membrane Accumulation
On the basis of earlier evidence from amphibian model systems, isolated perfused collecting ducts, and in vivo studies, it was proposed that VP acted by stimulating water channel insertion into the apical plasma membrane by VP-induced exocytosis of cytoplasmic vesicles (29, 45, 48, 57, 100, 141). While a modeling study in 1993 proposed that both the exocytotic and the endocytotic arms of the pathway were likely to be involved in the process leading to membrane accumulation of water channels (64), most attention was focused on the exocytotic pathway as the main regulatory event in the onset of VP action (141). However, the modeling predictions implicating the importance of regulated endocytosis were subsequently verified by experimental evidence. Notably, simply blocking endocytosis in AQP2-expressing cells in culture resulted in membrane accumulation of AQP2 in the absence of any hormonal stimulus. This was first achieved by coexpressing dominant negative dynamin, a GTPase that is necessary for clathrin-mediated endocytosis, in cultured cells (129). Subsequently, use of the drug methyl-β-cyclodextrin (MBCD), which reduces membrane cholesterol content and blocks clathrin-mediated endocytosis (110), confirmed this effect, but on a much more rapid (10–15 min) time scale after administration (72). Remarkably, there was no detectable difference in the extent of AQP2 membrane accumulation between cells treated for 15 min with VP and cells treated for 15 min with MBCD. Not only is AQP2 recycling constitutively, but the rate of recycling is sufficient to result in a massive membrane accumulation of the water channel upon inhibition of endocytosis. This effect was then shown in principal cells in situ, following treatment of isolated, perfused rat kidneys with MBCD (112).
Is a blockade of endocytosis involved in VP-induced membrane accumulation of AQP2? Yes—we showed that VP treatment of AQP2-expressing cells causes the water channel to become resistant to endocytosis. In fact, when the V2R and AQP2 are expressed in the same membrane domain in cultured cells, the V2R is removed from the membrane by clathrin-mediated endocytosis while AQP2 in the same membrane does not enter clathrin-coated pits and remains at the cell surface (18). We found that phosphorylation of AQP2 at the S256 residue prevents its direct interaction with heat shock proteins 70 (hsc/hsp70), which are central regulators of clathrin- mediated endocytosis (73). Importantly, expression of dominant negative hsc70 in AQP2-expressing cells results in membrane accumulation to a similar extent as that caused by expression of dominant negative dynamin. Other groups also confirmed this interaction with heat shock proteins 70, and showed that phosphorylation of AQP2 prevents the assembly of an endocytotic complex that is required for endocytosis (82, 148). Phosphorylated AQP2 also interacts with a protein called MAL (myelin and lymphocyte-associated protein), which could contribute to an inhibition of AQP2 internalization (58). Finally, inhibition of cyclooxygenase-2 (COX-2) has also been associated with a decrease in AQP2 endocytosis in various animal models of polyuria, including bilateral ureteral obstruction and lithium-induced polyuria (see below) (35, 60, 92).
The discovery that blockade of endocytosis is sufficient to induce membrane accumulation of AQP2 has been important for envisaging novel therapeutic measures that might increase urinary concentrating ability in the disease, NDI.
Nephrogenic Diabetes Insipidus
Diabetes insipidus (DI) from various causes results in the production of copious amounts of dilute urine, in excess of 15 liters per day in the most severe cases (12). It is commonly caused by a defect in the VP/V2R pathway in the collecting duct principal cells, but it can have other causes that include defective proximal tubule water reabsorption and inability to generate a hypertonic medullary interstitium (11, 16). The concentrating capacity of the collecting duct is low in the absence of VP, and one form of DI results from an absence of biologically active hormone. This is known as Central DI, and it is commonly treated by administration of VP via, for example, a nasal spray. In contrast, nephrogenic DI (NDI) occurs when the kidney is refractory to VP. Acquired forms of NDI are quite frequently encountered, the most common of which occurs in about 20% of patients receiving lithium treatment for bipolar disorder (135). Lithium induces a dramatic downregulation of AQP2 in principal cells, such that they no longer express adequate amounts of this critical water channel (78). The molecular mechanism of AQP2 downregulation is not fully understood, but it may involve inhibition of the GSK signaling pathway (66, 87, 147). Other acquired causes of NDI include hypokalemia, hypercalcemia, ureteral obstruction, and secondary hyperaldosteronism (25). Hereditary NDI is less common (about 1:200,000 live births); 90% of the cases are mutations in the V2R, while 10% result from AQP2 gene mutations (13). The V2R form is X-linked, affecting mostly the male population, while the AQP2 form is autosomal recessive and, infrequently, autosomal dominant. The reader is referred to excellent reviews in which the various forms of acquired and hereditary DI are discussed in more detail, including a description of the genetic mutations in AQP2 and the V2R that are found in the human population (13, 71, 116).
NDI is not fatal per se if compensatory hydration is possible, but in inherited forms of the disease, early symptoms in infants include fever, vomiting, anorexia, growth retardation, and developmental delay that can result in serious health issues later in life, including even mental retardation in untreated cases. Some NDI patients may ultimately require dialysis or transplantation. Current treatment regimes for NDI, including a low-sodium diet and thiazide diuretics, are only partially successful in reducing the production of large volumes of urine. These strategies induce volume depletion, in an attempt to lower glomerular filtration rate and increase proximal fluid reabsorption via ANG-II stimulation. Thus, there is less delivery of fluid to the collecting duct, resulting in a lower urine output. However, some studies indicate that there may also be a more direct effect of thiazides to increase water permeability of collecting duct principal cells (30, 76).
Bypassing Defective V2R Signaling
The search for alternative therapeutic approaches to treat both acquired and inherited forms of NDI has resulted in some promising findings in the laboratory setting. Such strategies are based on the need to bypass the defective VP/V2R signaling pathway and induce VP-independent apical membrane accumulation of AQP2 in collecting duct principal cells. While this review will necessarily focus on work carried out in our own laboratory (summarized in Fig. 2)—which was the subject of the Davson Lecture—several other groups are involved in this quest, and their important contributions will be folded into the discussions as appropriate.
Fig. 2.
Diagram of signaling pathways in a principal cell that have been exploited to bypass the VP/VP type 2 receptor (V2R) pathway, and induce apical membrane accumulation of AQP2. The three pathways shown are 1) calcitonin-receptor signaling via cAMP to induce AQP2 phosphorylation via PKA, and membrane accumulation of AQP2; 2) the cGMP-mediated pathway that is activated by phosphodiesterase (PDE5) inhibition using sildenafil (Viagra); AQP2 phosphorylation in this case occurs via PKG; and 3) statin-mediated actin cytoskeleton depolymerization that results in AQP2 membrane accumulation. By inactivating RhoA, simvastatin depolymerizes actin and inhibits AQP2 endocytosis, leading to plasma membrane accumulation. AC, adenylyl cyclase.
Calcitonin - An Alternative GPCR Receptor Ligand That Increases AQP2 Membrane Accumulation and Urine Concentration
One obvious strategy to stimulate VP/V2R-independent AQP2 trafficking is to increase intracellular cAMP in principal cells by an alternative means. An antidiuretic effect of activating the calcitonin (CT) receptor with its ligand calcitonin was suggested many years ago (39). This receptor is present in several tissues, but its major effect is on the kidney (79, 117). CT stimulates the activity of adenylyl cyclase in various segments of the urinary tubule, including the thick ascending limb (TAL), distal convoluted tubule (DCT), and the cortical collecting duct (31, 32). On the basis of these data, we reevaluated the effect of CT on AQP2 membrane accumulation in both cell cultures and in VP-deficient Brattleboro rats in vivo (see Fig. 2). We found that, as expected, CT treatment of LLC-PK1 cells expressing AQP2 resulted in a significant increase in intracellular cAMP levels, and plasma membrane accumulation of the water channel (19). This effect, therefore, mimicked that of VP and was also blocked by the PKA inhibitor H-89. A mutated form of AQP2 that lacks the important PKA target site (called AQP2-S256A) did not accumulate on the membrane in response to CT. These data provided proof of principle that CT can mobilize AQP2 in a manner similar to VP. Next, we applied CT (100 nM) to slices of kidney incubated in vitro and found that AQP2 accumulated on the apical membranes of principal cells in situ, in kidney tissue. Finally, VP-deficient Brattleboro rats were implanted with Alzet osmotic minipumps delivering 2 mU (312 pg)·min−1·100 g body wt−1, and the effect on urine concentration was determined. Within 4 h of treatment, a significant reduction in urine output was measured, together with an increase in urine osmolality. After 12 h, the amount of urine produced was only 33% of that in the control group, and the osmolality had more than doubled. This effect wore off over the next 24 h, and their urinary output returned to that of the controls. They did, however, respond positively to a second hormone treatment given a few days later.
These data are encouraging, and show that increasing cellular cAMP via activation of an alternative GPCR—the calcitonin receptor—not only stimulates AQP2 membrane accumulation, but also increases the urine-concentrating ability of VP-deficient rats. The use of Brattleboro rats is important in such studies to eliminate the possible effect of endogenous VP on any treatment being envisaged. This alternative hormonal strategy has potential advantages and disadvantages. A major advantage is that calcitonin is approved for use in humans, and it will affect only those cells expressing an appropriate receptor. Therefore, it will not cause a global increase in cAMP in many cells in the body, as would be the case for a cAMP phosphodiesterase inhibitor, for example. However, the potential effects of long-term CT treatment on other organ systems, bone, for example, is unclear, especially in infants and children. Finally, the effect seems short-lived, since urine-concentrating ability of the rats diminished over time, despite the continued delivery of CT from the implanted minipumps. Further studies need to be aimed at optimizing the dose and treatment regime to maximize the duration of the antidiuretic effect without inducing unwanted side effects and without inducing significant receptor downregulation.
cGMP Phosphodiesterase Inhibition With Sildenafil Citrate (Viagra)
An earlier study from our laboratory showed that cGMP could induce AQP2 phosphorylation at the crucial S256 site, and that protein kinase G could phosphorylate AQP2 in vitro (17). Increasing cGMP levels in cells using sodium nitroprusside (SNP, an NO donor), l-arginine (a precursor of NO), and atrial natriuretic factor (ANF) all resulted in AQP2 membrane accumulation both in cell cultures and in slices of rat kidney tissue incubated in vitro. The effect of ANF on mobilization of AQP2 was confirmed in a subsequent study in vivo (145). All of these manipulations were proposed to work via stimulation of guanylate cyclase activity, thereby increasing cGMP concentration within the cells (Fig. 2). On the basis of these data, we examined the effect of cGMP-specific phosphodiesterase (mainly PDE5) inhibitors on AQP2 distribution in cells (Fig. 2), notably 4-{[3′,4′-(methylenedioxy)benzyl]amino}-6-methoxyquinazoline (MBMQ) and sildenafil citrate (Viagra), and found that both caused a significant plasma membrane accumulation of AQP2 in cell cultures (20). The next step was to test the agents on principal cells in tissue slices in vitro. It had been reported previously that PDE5 is expressed in principal cells, and indeed we showed that PDE5 inhibition had the desired effect of causing AQP2 apical plasma membrane accumulation. As for the calcitonin study, we then tested the effects of Viagra on Brattleboro rats in vivo. Upon immunocytochemical examination of the kidneys, AQP2 was clearly increased in the apical membrane of collecting duct principal cells in these rats in the outer medulla but not in the cortex or inner medulla. Disappointingly, however, there was only a small and nonsignificant increase in urine-concentrating ability throughout a 6-day testing period. We attribute this at least in part to the vasodilatory effect of Viagra increasing renal blood flow, and potentially having an adverse effect on the establishment of a medullary interstitial gradient. However, a very recent study has reported that over a 2- to 4-wk time frame, sildenafil/Viagra reverses the polyuria seen in lithium-treated rats, in part by upregulating levels of AQP2 and the UTA1 urea transporter in the renal medulla (115).
On the basis of earlier studies in mice, it was proposed that blocking cAMP degradation using rolipram, a specific inhibitor of a cAMP phosphodiesterase, may work to cause AQP2 membrane accumulation (51). This is a reasonable hypothesis based on the known V2R signaling pathway, but the target of rolipram has a more ubiquitous cellular expression than the PDE5 target of sildenafil and may cause undesired effects throughout the body. Nonetheless, inhibiting cAMP degradation in hypercalcemic mice ameliorated their polyuria and increased aquaporin expression (144). In humans, however, rolipram administration was not successful in increasing urine concentration in a limited number of subjects with NDI (15). The use of PDE inhibitors remains attractive, but this strategy will certainly require more work to find a way to circumvent the unwanted effect of these drugs on the renal vasculature and other organ systems.
Statins Mobilize AQP2 Trafficking and Increase Urinary Concentrating Ability
The hypothesis that statins might be effective in increasing the membrane accumulation of AQP2 in principal cells and lead to increased urine-concentrating ability derived from our combined understanding of statin and AQP2 biology. They are related by the involvement of the actin cytoskeleton in the process of VP-mediated AQP2 trafficking. It has been known for many years, from early work on the toad urinary bladder, that the actin cytoskeleton is remodeled upon VP-induced increase in epithelial cell water permeability (49, 56, 99). Furthermore, immunogold studies in collecting ducts showed that VP-induced actin depolymerization occurred mostly at the apical pole of principal cells, where AQP2 was found to accumulate in later studies (126). A series of reports then clearly demonstrated that simply depolymerizing actin caused membrane accumulation of AQP2, even in the absence of VP stimulation (63, 131, 132). This could be achieved by inhibiting the function of the RhoA signaling cascade. Conversely, preventing actin depolymerization by activating RhoA prevented AQP2 membrane accumulation. Some studies have shown that actin and actin-associated proteins interact with AQP2 to regulate its trafficking (91, 130). We have found that VP treatment depolymerizes actin only in cells that express AQP2, implying that the water channel somehow catalyzes this process (146). Indeed, a phosphorylation-dependent interaction between AQP2 and the actin-binding protein tropomyosin 5b may be at least partially responsible for this effect (90).
Statins are therapeutic agents that block the synthesis of cholesterol by inhibiting a key enzyme involved in this process, 3-hydroxy-3-methylglutaryl coenzyme A reductase (118). This enzyme is also involved in the production of isoprenoids, which are precursors of lipids that are required for the association of several proteins, including small GTPases, with membranes. By preventing or reducing the isoprenylation of RhoA, statins inhibit its activity, resulting in actin depolymerization (77). Since actin polymerization is important for endocytosis in various systems, we hypothesized that statins could induce AQP2 membrane accumulation by inhibiting the endocytotic arm of its recycling pathway, as described above (Fig. 2). It had been shown previously that statins inhibit endocytosis of FITC-albumin in proximal tubule cells in culture (125). When applied to cell cultures, we found that simvastatin induced a significant membrane accumulation of AQP2 within 60 min of exposure of the cells (70). This effect was far too rapid to be explained by a statin-induced reduction in membrane cholesterol, which requires several hours of statin exposure (107), and was not, therefore, mechanistically similar to the rapid effect of the cholesterol-depleting drug MBCD (see above). In fact, we showed that RhoA activity is rapidly inhibited in statin-treated cells, and that this is accompanied by actin depolymerization and an inhibition of endocytosis (70). An effect of another statin, lovastatin, on AQP2 membrane accumulation was shown by others after more chronic (days) exposure of cultured cells (101). We next treated rat kidney tissue slices with simvastatin in vitro and also found that AQP2 accumulated in the apical plasma membrane of principal cells. Finally, homozygous Brattleboro rats were treated with simvastatin by intraperitoneal injection (to give a calculated plasma concentration of 200 μM). Within 4 h of treatment, they showed an almost doubling of urinary concentration, and they produced half as much urine as vehicle-treated controls.
In combination with results from others showing a similar effect of lovastatin on urine concentration in normal mice (102), these data provide compelling evidence that a blockade of AQP2 endocytosis can be harnessed to increase urine-concentrating ability, perhaps ultimately in humans. There are of course several caveats before such therapy can be translated to the human condition. The dose of statin is high, and nonspecific effects on the endocytosis of other important proteins in the kidney and other organs are possible. The risk of inducing serious muscle pathologies in humans also increases with greater statin levels in the blood. Nonetheless, these encouraging results show proof of principle and provide guidance for future work in this area.
One general consideration with some strategies is that it is not clear whether the amount of AQP2 expressed by principal cells in NDI patients will be sufficient to allow any antidiuretic effect without a parallel increase in AQP2 protein expression. Thus, a successful therapeutic treatment may need to combine drugs that stimulate both targeting and expression of AQP2. That being said, the short-term effect of statins in VP-deficient rats, which do express lower levels of AQP2 in their kidneys, is encouraging in this respect.
Other Potential Strategies for Modulating AQP2 Trafficking
Basic research on the VP/V2R/AQP2 pathway has led to consideration of other novel approaches as potential therapeutic avenues for the treatment of urine concentration defects, in addition to the current thiazide/low-sodium diet treatments. These will not be discussed in detail here, but they include the development of chemical or peptide chaperones that allow mistargeted (but otherwise functional) V2R to be delivered to the plasma membrane of principal cells (108, 109). This would be useful in cases of congenital X-linked NDI in which point mutations in the V2R lead to a misfolded protein that cannot leave the rough endoplasmic reticulum or the Golgi. COX-2 inhibitors have also been shown to prevent or reduce the downregulation of AQP2 and Na-K-Cl cotransporter (NKCC2) that occurs in lithium-treated rats, and to increase their urine-concentrating ability compared with nontreated animals (60). This class of drugs inhibits the production of prostaglandins, which can reduce or inhibit the effect of vasopressin on urinary concentration (60). The effects of PGE2 on the urinary concentration mechanism are, however, complicated by the presence of at least four receptor subtypes through which PGE2 can act. In fact, stimulation of the EP4 prostanoid receptor with a specific agonist, ONO, partially ameliorated the effect of V2R knockout in mice (69), probably by increasing intracellular cAMP levels. Furthermore, prostanoid receptor agonists such as butaprost (EP2 agonist) and CAY10580 (an EP4 agonist) can also mobilize AQP2 to the cell surface and increase urine concentration in a rat model of NDI (93). Finally, lithium-induced NDI can be attenuated in rats with the administration of amiloride to block the entry of lithium into principal cells through the epithelial sodium channel (65).
V-ATPase Regulation in Proton-Secreting Cells
Several organ systems in the body contain very specialized cells in which the V-ATPase is highly expressed at the cell surface, where it plays a critical role in acidifying the extracellular milieu (23). This is in addition to its well-known “housekeeping” role in acidifying intracellular organelles such as endosomes, lysosomes, and parts of the Golgi network (80). For many years, we have been involved in studies aimed at elucidating the regulation of intercalated cells in the kidney, and of so-called clear cells in the epididymis and vas deferens. Intercalated cells respond to systemic acid-base variations by increasing or decreasing proton secretion into the collecting duct lumen (7, 9, 142). Clear cells, on the other hand, acidify the lumen of the epididymis, a process that is required for sperm maturation and storage (22, 124). In both of these cell types, a major factor that regulates proton secretion is the actual amount of V-ATPase in the plasma membrane. This is determined by a balance between exocytosis and endocytosis of characteristic vesicles that contain large amounts of V-ATPase in their limiting membranes. In this respect, the regulation of protein secretion resembles the trafficking and membrane accumulation of AQP2 to regulate water permeability, as described above. However, so far the extent to which the exo- and endocytotic arms of the V-ATPase recycling process are coregulated to cause cell surface accumulation of this pump remains unknown.
The Epididymis As a Model Proton-Secreting Epithelium
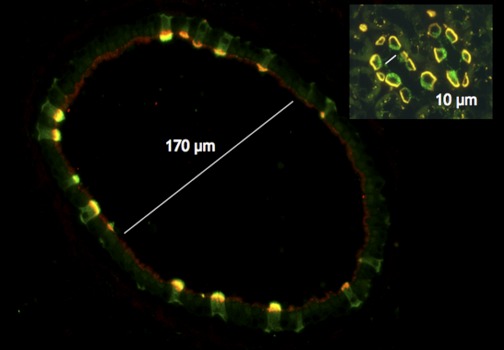
Throughout the history of renal physiology, major advances in our understanding of kidney function and dysfunction have been made using model organisms and tissues, such as the amphibian and turtle urinary bladder, Caenorhabditis elegans, Drosophila, and zebrafish to mention but a few. They all provide specific experimental advantages over the direct use of renal tissues. Over the past few years, we have shown that use of the epididymal tubule has contributed to many significant observations that have improved our understanding of how epithelial cells in the kidney sense and control local and systemic acid-base balance (24). This is, of course, in addition to the important information that has been obtained that is directly relevant to male reproductive physiology (123, 124). The epididymis is a powerful model system in which luminal acidification can be studied in the intact tissue, while epithelial cells reside in their native environment. The lumen of parts of the epididymal tubule and the vas deferens have a large diameter (between 150 and 400 μm) and are, therefore, much more accessible than the renal collecting duct, whose lumen diameter is approximately 10–20 μm (Fig. 3). This allows for the study of the epithelium by luminal factors via technically relatively simple in vivo/in situ perfusion. As mentioned above, the epididymal tubule and renal collecting duct contain similar V-ATPase-rich proton-secreting cells , clear and intercalated cells, respectively (Fig. 3) (21, 22). While studying the regulation of clear cells by luminal factors, we gained significant knowledge on how V-ATPase-dependent proton secretion in renal intercalated cells is regulated, and this information is now being applied directly to the kidney itself.
Fig. 3.
Comparison of proton-pumping clear cells in the male reproductive tract (in this case the vas deferens) and intercalated cells in a kidney collecting duct (inset) shown at the same magnification. The diameter of the tubule lumen is considerably greater in the vas deferens (and the adjacent cauda epididymis) than in the collecting duct (170 μm vs. 10 μm), allowing easier access to the luminal surface. Proton- secreting cells in the vas deferens are labeled with antibodies against the V-ATPase (green), and have endocytosed Texas-red dextran that was infused directly into the tubule lumen, producing a yellow color at the apical pole where overlap occurs. Adjacent principal cells also endocytose the dextran but to a lesser degree. In the inset, two collecting ducts from the medullary inner stripe of a rat kidney contain intercalated cells that are basolaterally stained to show the AE1 Cl−/HCO3− exchanger (yellow/orange). The rat was infused via the jugular vein with FITC-dextran (10,000 MW), which enters the tubule lumen and is avidly internalized at the apical pole of the type A intercalated cells (A-IC; green). Adjacent principal cells show some, but less, uptake of the dextran. Surrounding thick ascending limbs of Henle show spots of perinuclear AE1 staining that corresponds to the Golgi area. Insights into the regulation of proton secretion using the male reproductive tract have provided important information that can also be extended to kidney intercalated cell function.
Intercalated Cell Function in Acidosis and Alkalosis
Intercalated cells are the critical mediators of urine acidification and proton secretion by the kidney. Located in the late distal tubule, connecting tubule, and collecting duct, they “sense” body acid-base status and adjust their capacity to secrete protons in a way that provides fine control of systemic acid-base balance (8, 113, 121, 138, 142). There are two types of intercalated cell: type A cells (A-IC) are proton-secreting cells that are found throughout the “distal” nephron and collecting duct, and type B cells (B-IC), which can be either acid or base secreting cells, are found exclusively in the cortical regions of these tubular segments (138). A-IC express an apical V-ATPase and basolateral Cl−/HCO3− exchanger AE1, while B-IC express basolateral V-ATPase (but see below) and a different apical Cl−/HCO3− exchanger, pendrin (4, 27, 111). Cells with the appearance of A-IC are more common after acid loading in laboratory animals, and cells with the appearance of B-IC are more common (but only in the cortex) in alkalotic animals (8, 75, 113). In assessing the “phenotype” of ICs under these conditions by immunostaining, it is clear that there is considerable plasticity of function and transporter distribution between these cells (8, 27, 119, 121), which has resulted in a nomenclature that includes an intermediate intercalated cell subtype, often referred to as a non-A, non-B cell because they express apical V-ATPase, apical pendrin, and no basolateral AE1 (61, 62). Furthermore, some ICs appear to have little or no plasma membrane V-ATPase; it is mostly located on intracellular vesicles. These cells might be in the process of moving V-ATPases from one membrane domain to the other, and they have been caught (by fixation) in the middle of this transition. They also might be A-IC or B-IC that have been inactivated (by removal of cell surface V-ATPase) by alkalosis or acidosis, respectively. Thus, we believe that the presence or absence of AE1 or pendrin should be the predominant criterion used to classify A-IC and B-IC. A growing body of evidence from the Al-Awqati group and collaborators indicates that the extracellular matrix protein hensin is necessary for the development of A-IC in mouse kidney (2). In hensin knockout mice, the intercalated cells are either mostly pendrin-expressing B-IC in the cortex, or an apparently novel type of intercalated cell with intermediate characteristics in the medulla, expressing the V-ATPase, but neither pendrin nor AE1 (43). These mice have, as expected, severe metabolic acidosis due to the paucity of proton-secreting A-IC in their kidneys. In vitro studies on perfused tubules also suggest that hensin is involved in the short-term adaptation of IC to acid or base secretion (120). As technology develops, it will be interesting to monitor, in real time, a single IC in situ in the process of adapting to an acid or base challenge.
Regulation of Proton Secretion by Soluble Adenylyl Cyclase: Lessons From the Epididymis
As discussed above, intercalated cells are equipped to respond rapidly to changes in acid-base status of the body. However, the sensing mechanisms that they use to survey their environment are poorly understood. These cells are exposed to luminal fluid, whose composition varies with metabolic and hydration status, and they are simultaneously exposed to interstitial fluid at their basolateral, blood-facing surface. How they monitor and respond to cues from apical and basolateral surfaces is an intriguing physiological issue. It is here that the use of the epididymal system has provided important insights, because this tubular organ can be easily perfused, either in vitro or in situ, with fluids containing drugs, hormones or ionic and pH compositions of choice. This can, of course, be achieved using isolated perfused renal tubules, but this is technically much more demanding, and data analysis is more difficult due to the small size of the tubule.
Using the epididymis, we showed that proton-secreting clear cells respond to an increase in luminal pH from its normal acidic value of 6.5 up to the slightly alkaline 7.8 (both in phosphate-buffered saline) by accumulating V-ATPase on their apical membrane, and by extending long, fingerlike projections (microvilli) that are enriched in the V-ATPase (94). A similar effect was induced by perfusing the epididymis with a bicarbonate buffer compared with phosphate buffer at the same pH. Importantly, microvillar extension and V-ATPase accumulation, which are correlated with increased proton secretion, was prevented by infusing the lumen with catechol estrogen, an inhibitor of the soluble adenylyl cyclase, sAC. This study provided the first evidence that sAC could be a luminal pH/bicarbonate sensor that could regulate the activity of proton-secreting epithelial cells. Previous studies by the Levin/Buck group had identified sAC as an enzyme whose cyclase activity is stimulated directly by bicarbonate, with further regulation by intracellular calcium to increase its bicarbonate sensitivity (33, 136). Immunocytochemical studies showed that sAC is expressed at high levels in clear cells compared with the surrounding principal cells (94), consistent with its central role in proton secretion by this cell type. In the epididymis, the physiological role of this mechanism is to maintain the lumen at the low pH that is necessary for sperm maturation and storage. In the same epithelium, epididymal principal cells secrete bicarbonate during sexual arousal to activate sperm (122, 124). Proton secretion by clear cells balances this alkalinization, and restores luminal pH to its resting acidic state. Thus, clear and principal cells work together to provide a suitable environment for sperm storage, but also allow the sporadic priming of sperm for fertilization while still in the epididymal tubule (124).
Because of the involvement of an adenylyl cyclase, a rise in intracellular cAMP was suspected to be the mediator of increased proton secretion. Indeed, perfusion of the epididymal lumen with permeant cAMP analogs also stimulated clear cells, even at acidic pH when they would normally be quiescent (94). A subsequent study showed that PKA was involved in this process and not the alternative cAMP-activated protein, Epac (95).
Soluble Adenylyl Cyclase, cAMP, and V-ATPase Regulation in Kidney Intercalated Cells
On the basis of these initial observations in the epididymis, we went on to show that sAC is also highly expressed in both A-IC and B-IC in the kidney. Its localization showed significant overlap with the V-ATPase in these cells, including at the basolateral pole of B-IC (96). This tight colocalization suggested that the two proteins might cooperate in the proton secretion activity of intercalated cells. An interaction was confirmed using coimmunoprecipitation assays, but it is currently not known whether the association is direct or indirect. Other acid-base transporting proteins including the electroneutral Na+-HCO3− cotransporter NBC3 are also complexed with the V-ATPase (104), but whether sAC is part of the same complex is also unknown.
There is at present no direct evidence showing that an elevation in luminal HCO3− increases V-ATPase membrane accumulation and proton secretion by A-IC. However, acetazolamide treatment of rats causes a marked increase in apical V-ATPase and sAC in these cells. It also results in a greatly increased apical surface area due to extension and growth of V-ATPase-labeled microvilli (6). This carbonic anhydrase-inhibiting drug causes systemic metabolic acidosis by blocking HCO3− reabsorption in the proximal tubule, thus increasing distal delivery and potentially increasing the sAC activity in A-IC. The activation of A-IC observed under these conditions is an appropriate response to correct the acidosis.
We further examined the pathway by which renal intercalated cells are activated, by infusing cell-permeant cAMP into rats. Morphological studies showed that A-IC in such animals develop an elaborate network of microvilli/microplicae at their luminal pole, together with an increased density of V-ATPase in the membrane domain (97). Thus, these data closely resemble those from the epididymal clear cell model. Finally, a fluorescence-based intracellular pH recovery assay using isolated collecting ducts showed that cAMP increased functional protein secretion by these cells (97). Subsequent correlated functional and immunocytochemical studies have shown that the cAMP effect on proton secretion is mediated by both PKA and PKC, although the crosstalk between these two pathways remains to be dissected in detail (143).
Isolated Intercalated Cells From B1-Enhanced Green Fluorescent Protein-Expressing Mice
In collaboration with Raoul Nelson and Lance Miller, we generated a novel transgenic mouse that expresses soluble enhanced green fluorescent protein (EGFP) in proton-secreting cells, driven by the promoter of the V-ATPase B1 subunit (B1-EGFP mice) (Fig. 4) (81). Previous work had shown that the B subunit, which forms part of the catalytic V1 domain of the V-ATPase, has two distinct isoforms known originally as the kidney isoform (B1) and the brain isoform (B2) (86, 103). The B1 variant is highly expressed in kidney intercalated cells and epididymal clear cells, whereas the B2 isoform is considered to be a more ubiquitous housekeeping isoform that is associated with acidification of intracellular organelles. This distinction is not absolute, however, because we showed that the B2 isoform can at least partially compensate for the loss of B1 in B1-knockout mice, to allow urine acidification and epididymal function to be maintained, at least under nonstressed, baseline conditions (38, 98). Furthermore, the B2 isoform is also expressed at the plasma membrane in some other cell types, including renal proximal tubule cells (86) and osteoclasts (68).
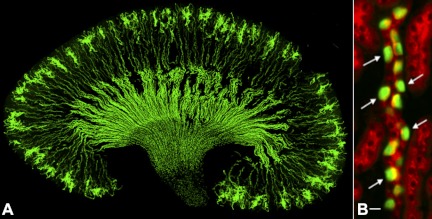
Fig. 4.
A: section of kidney from a B1 subunit enhanced green fluorescent protein (B1-EGFP) mouse expressing EGFP in intercalated cells, driven by the promoter of the B1-subunit of the V-ATPase. B: higher-magnification detail in which the mosaic arrangement of intercalated cells (arrows - green) and principal cells can be easily appreciated. Cells from these mice can be isolated by FACS and used for structural and functional analysis (see Fig. 5). Bar, 10 μm. [From Miller et al. (81).]
The B1-EGFP mice, which express EGFP in intercalated cells (Fig. 4) and epididymal clear cells, has allowed these cells to be isolated by FACS and examined in the absence of potentially confounding systemic conditions. In this way, we used spinning disk confocal microscopy to study the regulation of single, isolated A-IC in vitro and found that cAMP induces a dramatic elaboration of “microvilli,” which in some cases are several microns in length (Fig. 5) (97). The intracellular mechanisms involved in the growth of these remarkable microvilli/microplicae structures is not yet understood. However, intercalated cells (and epididymal clear cells) express large amounts of the actin-regulating protein gelsolin (74), and this protein is involved in regulating the plasma membrane accumulation of V-ATPase in these cells in the epididymis (10). One unusual feature of these extensions is that they do not appear to contain significant amounts of polymerized actin (10, 123). In this respect, the mechanism of formation appears to be quite different from, for example, the rapid formation of actin-rich projections that appear during platelet activation (46).
Fig. 5.
Composite plate showing a single EGFP-positive intercalated cell affixed to a substrate, treated with 8-(4-chlorophenylthio)-cAMP (1 mM), and followed for 60 min by spinning disk confocal microscopy. Over time, the initial smooth surface of the cell extends numerous, fingerlike microvilli from the apical pole that in some cases measure several microns in length. A: 0 min. B: 12 min. C: 24 min. D: 36 min. E: 48 min. F: 60 min. This cAMP effect is part of the response that allows these cells to increase proton secretion by increasing their surface area and the number of V-ATPase pumps at the plasma membrane. Bar, 10 μm. [From Paunescu et al. (97).]
Role of V-ATPase Phosphorylation in cAMP-Mediated Trafficking
In a manner similar to the regulation of AQP2 trafficking by phosphorylation of COOH-terminal residues, it is likely that posttranslational modification is also involved in V-ATPase trafficking and/or function. There are some reports in the literature describing phosphorylation of selected V-ATPase subunits, and their role in membrane accumulation of V-ATPase. These include PKA-mediated phosphorylation of the A subunit in HEK-293 cells, which occurs at residue S175 (5, 44). Mutation of the A subunit to preclude phosphorylation interfered with its cell surface localization in these cells, and an S175D mutation to mimic constitutive phosphorylation resulted in increased acidification of the culture medium (5). The C and A subunits are also phosphorylated by PKA in the blowfly salivary gland during stimulation of trafficking to the membrane (105, 139). There are other scattered reports of V-ATPase subunit phosphorylation of the B2 isoform by AP50 (a clathrin assembly protein) (84), and of the Arabidopsis V-ATPase C-subunit by a With No Lysine (K) (WNK) kinase (52). We reported data from a large-scale proteomic screening of V-ATPase subunit phosphorylation sites, showing that several subunits including a2, B2, G3, H, and A contain putative target sites for several kinases including PKA, PKG, AKT, ATM, and CKI (97). Dissecting the relevance of all of these sites to various signaling events and to V-ATPase function will, of course, require a considerable amount of work as we move forward.
Proteomics Analysis of Kidney and Epididymal Proton-Secreting Cells
Our ability to harvest a pure population of EGFP-expressing proton-secreting epithelial cells by FACS from the kidney and the epididymis provided a unique opportunity to perform a full proteomic analysis on these cell types. In collaboration with Mark Knepper and Trairak Pisitkun at the National Institutes of Health (NIH), and Nicolas Da Silva in our own program, we determined the proteomic signature of epididymal clear cells and renal intercalated cells from the collecting duct and connecting segments of the mouse kidney (37). The idea behind this study was to provide a framework to inform future studies on specific proteins and pathways that are common to the proton-secreting function of these cells. The identified proteins were compared with those expressed in EGFP-negative cells from their respective tissues. In addition, by comparing proteins that are selectively expressed in these cells from different organs, we sought to identify those that might have a more tissue-specific function. In summary, we found about 200 proteins in the EGFP-positive cells that were not detectable in the negative cell population, and of these about 25% were common to both EGFP-cell populations from the two organs. This indicates that these are indeed different cells that have evolved to perform the same proton-secreting function.
A description of these proteins is beyond the scope of this review, but the complete list of proteins that were identified in the proteomic screen is publicly available (http://dir.nhlbi.nih.gov/papers/lkem/kevcpd/). A discussion of some of the more interesting proteins that were identified, including gelsolin, the progesterone receptor Pgrmc1, and the PKA-binding protein Lrba, can also be found in our published article (37).
Summary
Over the past decade or so, our understanding of aquaporin 2 biology and V-ATPase trafficking in intercalated cells has advanced owing to the efforts of many outstanding investigators in these research areas. The regulation of both proteins occurs in large part by modulating their trafficking to and from the plasma membrane, via modulation of exocytosis and endocytosis. Experimental data have revealed that AQP2 constitutively traffics to and from the cell surface, and that blocking endocytosis is sufficient to induce membrane accumulation and increased epithelial water permeability. It is likely, but not definitively proven, that a similar dual regulatory pathway occurs for the V-ATPase. Also, phosphorylation of AQP2 and V-ATPase is mediated by PKA and probably other kinases to regulate protein interactions involved in their trafficking. The use of a variety of cell culture models and in situ systems has enabled us to accelerate progress in these areas. In particular, the epididymis/vas deferens has proven to be a remarkable system in which to dissect V-ATPase trafficking in proton-secreting epithelial cells. Our findings using this organ have so far been directly applicable to intercalated cells in the kidney, and have of course also provided valuable information on the importance of epididymal epithelial cell function in male fertility.
This review is based mainly on the work from the laboratories of the coauthors, which was presented by one of us (D. Brown) in the 2011 Davson Distinguished Lecture at the Experimental Biology Meeting in Washington, DC. It is not meant to be a comprehensive review of the literature. As such, we apologize to those investigators who have made many important contributions to the fields of fluid homeostasis and acid-base balance whose work is not adequately presented here.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.B., R.B., T.G.P., S.B., and H.A.J.L. conception and design of the research; D.B., R.B., T.G.P., S.B., and H.A.J.L. performed the experiments; D.B., R.B., T.G.P., S.B., and H.A.J.L. analyzed the data; D.B., R.B., T.G.P., S.B., and H.A.J.L. interpreted the results of the experiments; D.B. prepared the figures; D.B. drafted manuscript; D.B., R.B., T.G.P., S.B., and H.A.J.L. edited and revised the manuscript; D.B., R.B., T.G.P., S.B., and H.A.J.L. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the important contributions of all of their past and present lab members in the MGH Program in Membrane Biology/Division of Nephrology. They also thank the NIH for continuous funding of these projects over the years, currently via NIH Grants DK-38452 (to D. Brown and S. Breton), DK-42956 (to D. Brown), DK-HD40793 (to S. Breton), DK-73266 (to T. G. Pǎunescu), DK-075940 (to H. A. J. Lu), and an Investigator Award from the National Kidney Foundation (to R. Bouley). S. Breton is the recipient of an MGH Scholars Award and H. A. J. Lu is an MGH Claflin Distinguished Fellow. We thank Yechuan Sharon Ruan from the Breton lab for preparing the illustration shown in Fig. 2. Finally, we are grateful for our continued interactions with many other labs throughout the world whose invaluable insights and collegiality make research so rich and rewarding.
REFERENCES
- 1. Al-Awqati Q. 2007 Homer W. Smith award: control of terminal differentiation in epithelia. J Am Soc Nephrol 19: 443–449, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Al-Awqati Q. Terminal differentiation in epithelia: the role of integrins in hensin polymerization. Annu Rev Physiol 73: 401–412, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Al-Awqati Q, Gluck S, Reeves W, Cannon C. Regulation of proton transport in urinary epithelia. J Exp Biol 106: 135–141, 1983 [DOI] [PubMed] [Google Scholar]
- 4.Alper SL, Natale J, Gluck S, Lodish HF, Brown D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci USA 86: 5429–5433, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alzamora R, Thali RF, Gong F, Smolak C, Li H, Baty CJ, Bertrand CA, Auchli Y, Brunisholz RA, Neumann D, Hallows KR, Pastor-Soler NM. PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the a subunit in kidney cells. J Biol Chem 285: 24676–24685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagnis C, Marshansky V, Breton S, Brown D. Remodeling the cellular profile of collecting ducts by chronic carbonic anhydrase inhibition. Am J Physiol Renal Physiol 280: F437–F448, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Bastani B, Gluck SL. New insights into the pathogenesis of distal renal tubular acidosis. Miner Electrolyte Metab 22: 396–409, 1996 [PubMed] [Google Scholar]
- 8.Bastani B, McEnaney S, Yang L, Gluck S. Adaptation of inner medullary collecting duct vacuolar H-adenosine triphosphatase to chronic acid or alkali loads in the rat. Exp Nephrol 2: 171–175, 1994 [PubMed] [Google Scholar]
- 9.Bastani B, Purcell H, Hemken P, Trigg D, Gluck S. Expression and distribution of renal vacuolar proton-translocating adenosine triphosphatase in response to chronic acid and alkali loads in the rat. J Clin Invest 88: 126–136, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaulieu V, Da Silva N, Pastor-Soler N, Brown CR, Smith PJ, Brown D, Breton S. Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+-ATPase recycling. J Biol Chem 280: 8452–8463, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Bichet DG. Hereditary polyuric disorders: new concepts, and differential diagnosis. Semin Nephrol 26: 224–233, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Bichet DG. Nephrogenic diabetes insipidus. Adv Chronic Kidney Dis 13: 96–104, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Bichet DG. V2R mutations, and nephrogenic diabetes insipidus. Prog Mol Biol Transl Sci 89: 15–29, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Bichet DG. Vasopressin receptor mutations in nephrogenic diabetes insipidus. Semin Nephrol 28: 245–251, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Bichet DG, Ruel N, Arthus MF, Lonergan M. Rolipram, a phosphodiesterase inhibitor, in the treatment of two male patients with congenital nephrogenic diabetes insipidus. Nephron 56: 449–450, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Boone M, Deen PM. Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflügers Arch 456: 1005–1024, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouley R, Breton S, Sun T, McLaughlin M, Nsumu NN, Lin HY, Ausiello DA, Brown D. Nitric oxide and atrial natriuretic factor stimulate cGMP-dependent membrane insertion of aquaporin 2 in renal epithelial cells. J Clin Invest 106: 1115–1126, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouley R, Hawthorn G, Russo LM, Lin HY, Ausiello DA, Brown D. Aquaporin 2 (AQP2) and vasopressin type 2 receptor (V2R) endocytosis in kidney epithelial cells: AQP2 is located in ‘endocytosis-resistant’ membrane domains after vasopressin treatment. Biol Cell 98: 215–232, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Bouley R, Lu HA, Nunes P, Da Silva N, McLaughlin M, Chen Y, Brown D. Calcitonin has a vasopressin-like effect on aquaporin-2 trafficking and urinary concentration. J Am Soc Nephrol 22: 59–72, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouley R, Pastor-Soler N, Cohen O, McLaughlin M, Breton S, Brown D. Stimulation of AQP2 membrane insertion in renal epithelial cells in vitro and in vivo by the cGMP phosphodiesterase inhibitor sildenafil citrate (Viagra). Am J Physiol Renal Physiol 288: F1103–F1112, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Breton S, Brown D. New insights into the regulation of V-ATPase-dependent proton secretion. Am J Physiol Renal Physiol 292: F1–F10, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Breton S, Smith PJ, Lui B, Brown D. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat Med 2: 470–472, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Brown D, Breton S. Mitochondria-rich, proton-secreting epithelial cells. J Exp Biol 199: 2345–2358, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Brown D, Breton S, Ausiello DA, Marshansky V. Sensing, signaling and sorting events in kidney epithelial cell physiology. Traffic 10: 275–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown D, Fenton RA. The cell biology of vasopressin action. In: Brenner and Rector's The Kidney, edited by Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu ASL, Brenner BM. Philadelphia, PA: Elsevier Saunders, 2012, p. 353–383 [Google Scholar]
- 26.Brown D, Hasler U, Nunes P, Bouley R, Lu HA. Phosphorylation events and the modulation of aquaporin 2 cell surface expression. Curr Opin Nephrol Hypertens 17: 491–498, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown D, Hirsch S, Gluck S. An H+-ATPase in opposite plasma membrane domains in kidney epithelial cell subpopulations. Nature 331: 622–624, 1988 [DOI] [PubMed] [Google Scholar]
- 28.Brown D, Paunescu TG, Breton S, Marshansky V. Regulation of the V-ATPase in kidney epithelial cells: dual role in acid-base homeostasis and vesicle trafficking. J Exp Biol 212: 1762–1772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown D, Stow JL. Protein trafficking and polarity in kidney epithelium: from cell biology to physiology. Physiol Rev 76: 245–297, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Cesar KR, Magaldi AJ. Thiazide induces water absorption in the inner medullary collecting duct of normal and Brattleboro rats. Am J Physiol Renal Physiol 277: F756–F760, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Chabardes D, Imbert-Teboul M, Montegut M, Clique A, Morel F. Distribution of calcitonin-sensitive adenylate cyclase activity along the rabbit kidney tubule. Proc Natl Acad Sci USA 73: 3608–3612, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chai SY, Christopoulos G, Cooper ME, Sexton PM. Characterization of binding sites for amylin, calcitonin, and CGRP in primate kidney. Am J Physiol Renal Physiol 274: F51–F62, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289: 625–628, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Rice W, Li W, Kong Y, Fenton RA, Li J, Hsu V, Brown D, Lu HAJ. AQP2 affects renal epithelial cell adhesion, migration and tubule formation by interacting with integrin b1 via an external RGD motif (Abstract). J Am Soc Nephrol Renal Week Archives SA-PO2112, 2246, 2010 [Google Scholar]
- 35.Cheng X, Zhang H, Lee HL, Park JM. Cyclooxygenase-2 inhibitor preserves medullary aquaporin-2 expression and prevents polyuria after ureteral obstruction. J Urol 172: 2387–2390, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Coleman RA, Wu DC, Liu J, Wade JB. Expression of aquaporins in the renal connecting tubule. Am J Physiol Renal Physiol 279: F874–F883, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Da Silva N, Pisitkun T, Belleannee C, Miller LR, Nelson R, Knepper MA, Brown D, Breton S. Proteomic analysis of V-ATPase-rich cells harvested from the kidney and epididymis by fluorescence-activated cell sorting. Am J Physiol Cell Physiol 298: C1326–C1342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Da Silva N, Shum WW, El-Annan J, Paunescu TG, McKee M, Smith PJ, Brown D, Breton S. Relocalization of the V-ATPase B2 subunit to the apical membrane of epididymal clear cells of mice deficient in the B1 subunit. Am J Physiol Cell Physiol 293: C199–C210, 2007 [DOI] [PubMed] [Google Scholar]
- 39.De Rouffignac C, Elalouf JM. Effects of calcitonin on the renal concentrating mechanism. Am J Physiol Renal Fluid Electrolyte Physiol 245: F506–F511, 1983 [DOI] [PubMed] [Google Scholar]
- 40.Fenton RA, Knepper MA. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev 87: 1083–1112, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361: 549–552, 1993 [DOI] [PubMed] [Google Scholar]
- 43.Gao X, Eladari D, Leviel F, Tew BY, Miro-Julia C, Cheema F, Miller L, Nelson R, Paunescu TG, McKee M, Brown D, Al-Awqati Q. Deletion of hensin/DMBT1 blocks conversion of beta- to alpha-intercalated cells and induces distal renal tubular acidosis. Proc Natl Acad Sci USA 107: 21872–21877, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong F, Alzamora R, Smolak C, Li H, Naveed S, Neumann D, Hallows KR, Pastor-Soler NM. Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. Am J Physiol Renal Physiol 298: F1162–F1169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harmanci MC, Stern P, Kachadorian WA, Valtin H, DiScala VA. Vasopressin and collecting duct intramembranous particle clusters: a dose-response relationship. Am J Physiol Renal Fluid Electrolyte Physiol 239: F560–F564, 1980 [DOI] [PubMed] [Google Scholar]
- 46.Hartwig JH. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol 118: 1421–1442, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi M, Sasaki S, Tsuganezawa H, Monkawa T, Kitajima W, Konishi K, Fushimi K, Marumo F, Saruta T. Expression and distribution of aquaporin of collecting duct are regulated by vasopressin V2 receptor in rat kidney. J Clin Invest 94: 1778–1783, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hays RM. Alteration of luminal membrane structure by antidiuretic hormone. Am J Physiol Cell Physiol 245: C289–C296, 1983 [DOI] [PubMed] [Google Scholar]
- 49.Hays RM, Condeelis J, Gao Y, Simon H, Ding G, Franki N. The effect of vasopressin on the cytoskeleton of the epithelial cell. Pediatr Nephrol 7: 672–679, 1993 [DOI] [PubMed] [Google Scholar]
- 50.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Homma S, Gapstur SM, Coffey A, Valtin H, Dousa TP. Role of cAMP-phosphodiesterase isozymes in pathogenesis of murine nephrogenic diabetes insipidus. Am J Physiol Renal Fluid Electrolyte Physiol 261: F345–F353, 1991 [DOI] [PubMed] [Google Scholar]
- 52.Hong-Hermesdorf A, Brux A, Gruber A, Gruber G, Schumacher K. A WNK kinase binds and phosphorylates V-ATPase subunit C. FEBS Lett 580: 932–939, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Innamorati G, Sadeghi H, Birnbaumer M. Phosphorylation and recycling kinetics of G protein-coupled receptors. J Recept Signal Transduct Res 19: 315–326, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Ishibashi K, Kuwahara M, Sasaki S. Molecular biology of aquaporins. Rev Physiol Biochem Pharmacol 141: 1–32, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Ishibashi K, Sasaki S, Fushimi K, Uchida S, Kuwahara M, Saito H, Furukawa T, Nakajima K, Yamaguchi Y, Gojobori T, Marumo F. Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc Natl Acad Sci USA 91: 6269–6273, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iyengar R, Lepper KG, Mailman DS. Involvement of microtubules and microfilaments in the action of vasopressin in canine renal medulla. J Supramol Struct 5: 521(373)–530(382), 1976 [DOI] [PubMed] [Google Scholar]
- 57.Jo I, Harris HWJ. Molecular mechanisms for the regulation of water transport in amphibian epithelia by antidiuretic hormone. Kidney Int 48: 1088–1096, 1995 [DOI] [PubMed] [Google Scholar]
- 58.Kamsteeg EJ, Duffield AS, Konings IB, Spencer J, Pagel P, Deen PM, Caplan MJ. MAL decreases the internalization of the aquaporin-2 water channel. Proc Natl Acad Sci USA 104: 16696–16701, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katsura T, Gustafson CE, Ausiello DA, Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol Renal Physiol 272: F817–F822, 1997 [PubMed] [Google Scholar]
- 60.Kim GH, Choi NW, Jung JY, Song JH, Lee CH, Kang CM, Knepper MA. Treating lithium-induced nephrogenic diabetes insipidus with a COX-2 inhibitor improves polyuria via upregulation of AQP2 and NKCC2. Am J Physiol Renal Physiol 294: F702–F709, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Kim J, Kim YH, Cha JH, Tisher CC, Madsen KM. Intercalated cell subtypes in connecting tubule and cortical collecting duct of rat and mouse. J Am Soc Nephrol 10: 1–12, 1999 [DOI] [PubMed] [Google Scholar]
- 62.Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, Nielsen S. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol 283: F744–F754, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Klussmann E, Tamma G, Lorenz D, Wiesner B, Maric K, Hofmann F, Aktories K, Valenti G, Rosenthal W. An inhibitory role of Rho in the vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J Biol Chem 276: 20451–20457, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Knepper MA, Nielsen S. Kinetic model of water and urea permeability regulation by vasopressin in collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 265: F214–F224, 1993 [DOI] [PubMed] [Google Scholar]
- 65.Kortenoeven ML, Li Y, Shaw S, Gaeggeler HP, Rossier BC, Wetzels JF, Deen PM. Amiloride blocks lithium entry through the sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int 76: 44–53, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Kortenoeven ML, Schweer H, Cox R, Wetzels JF, Deen PM. Lithium reduces aquaporin-2 transcription independent of prostaglandins. Am J Physiol Cell Physiol 302: C131–C140, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Kwon TH, Nielsen J, Moller HB, Fenton RA, Nielsen S, Frokiaer J. Aquaporins in the kidney. Handb Exp Pharmacol 2009: 95–132, 2009 [DOI] [PubMed] [Google Scholar]
- 68.Lee BS, Holliday LS, Ojikutu B, Krits I, Gluck SL. Osteoclasts express the B2 isoform of vacuolar H(+)-ATPase intracellularly and on their plasma membranes. Am J Physiol Cell Physiol 270: C382–C388, 1996 [DOI] [PubMed] [Google Scholar]
- 69.Li JH, Chou CL, Li B, Gavrilova O, Eisner C, Schnermann J, Anderson SA, Deng CX, Knepper MA, Wess J. A selective EP4 PGE2 receptor agonist alleviates disease in a new mouse model of X-linked nephrogenic diabetes insipidus. J Clin Invest 119: 3115–3126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li W, Zhang Y, Bouley R, Chen Y, Matsuzaki T, Nunes P, Hasler U, Brown D, Lu HA. Simvastatin enhances aquaporin-2 surface expression and urinary concentration in vasopressin-deficient Brattleboro rats through modulation of Rho GTPase. Am J Physiol Renal Physiol 301: F309–F318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loonen AJ, Knoers NV, van Os CH, Deen PM. Aquaporin 2 mutations in nephrogenic diabetes insipidus. Semin Nephrol 28: 252–265, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Lu H, Sun TX, Bouley R, Blackburn K, McLaughlin M, Brown D. Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am J Physiol Renal Physiol 286: F233–F243, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Lu HA, Sun TX, Matsuzaki T, Yi XH, Eswara J, Bouley R, McKee M, Brown D. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J Biol Chem 282: 28721–28732, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Lueck A, Brown D, Kwiatkowski DJ. The actin-binding proteins adseverin and gelsolin are both highly expressed but differentially localized in kidney and intestine. J Cell Sci 111: 3633–3643, 1998 [DOI] [PubMed] [Google Scholar]
- 75.Madsen KM, Verlander JW, Kim J, Tisher CC. Morphological adaptation of the collecting duct to acid-base disturbances. Kidney Int Suppl 33: S57–S63, 1991 [PubMed] [Google Scholar]
- 76.Magaldi AJ. New insights into the paradoxical effect of thiazides in diabetes insipidus therapy. Nephrol Dial Transplant 15: 1903–1905, 2000 [DOI] [PubMed] [Google Scholar]
- 77.Maher BM, Dhonnchu TN, Burke JP, Soo A, Wood AE, Watson RW. Statins alter neutrophil migration by modulating cellular Rho activity–a potential mechanism for statins-mediated pleotropic effects? J Leukoc Biol 85: 186–193, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Marples D, Christensen S, Christensen EI, Ottosen PD, Nielsen S. Lithium-induced downregulation of aquaporin-2 water channel expression in rat kidney medulla. J Clin Invest 95: 1838–1845, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marx SJ, Woodward C, Aurbach GD, Glossmann H, Keutmann HT. Renal receptors for calcitonin. Binding and degradation of hormone. J Biol Chem 248: 4797–4802, 1973 [PubMed] [Google Scholar]
- 80.Mellman I. The importance of being acid: the role of acidification in intracellular membrane traffic. J Exp Biol 172: 39–45, 1992 [DOI] [PubMed] [Google Scholar]
- 81.Miller RL, Zhang P, Smith M, Beaulieu V, Paunescu TG, Brown D, Breton S, Nelson RD. V-ATPase B1-subunit promoter drives expression of EGFP in intercalated cells of kidney, clear cells of epididymis and airway cells of lung in transgenic mice. Am J Physiol Cell Physiol 288: C1134–C1144, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Moeller HB, Praetorius J, Rutzler MR, Fenton RA. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci USA 107: 424–429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muller J, Kachadorian WA, DiScala VA. Evidence that ADH-stimulated intramembrane particle aggregates are transferred from cytoplasmic to luminal membranes in toad bladder epithelial cells. J Cell Biol 85: 83–95, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Myers M, Forgac M. The coated vesicle vacuolar (H+)-ATPase associates with and is phosphorylated by the 50-kDa polypeptide of the clathrin assembly protein AP-2. J Biol Chem 268: 9184–9186, 1993 [PubMed] [Google Scholar]
- 85.Nedvetsky PI, Tamma G, Beulshausen S, Valenti G, Rosenthal W, Klussmann E. Regulation of aquaporin-2 trafficking. Handb Exp Pharmacol 2009: 133–157, 2009 [DOI] [PubMed] [Google Scholar]
- 86.Nelson RD, Guo XL, Masood K, Brown D, Kalkbrenner M, Gluck S. Selectively amplified expression of an isoform of the vacuolar H(+)-ATPase 56-kilodalton subunit in renal intercalated cells. Proc Natl Acad Sci USA 89: 3541–3545, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA. Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci USA 105: 3634–3639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA 92: 1013–1017, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci USA 90: 11663–11667, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noda Y, Horikawa S, Kanda E, Yamashita M, Meng H, Eto K, Li Y, Kuwahara M, Hirai K, Pack C, Kinjo M, Okabe S, Sasaki S. Reciprocal interaction with G-actin and tropomyosin is essential for aquaporin-2 trafficking. J Cell Biol 182: 587–601, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Noda Y, Horikawa S, Katayama Y, Sasaki S. Water channel aquaporin-2 directly binds to actin. Biochem Biophys Res Commun 322: 740–745, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Norregaard R, Jensen BL, Li C, Wang W, Knepper MA, Nielsen S, Frokiaer J. COX-2 inhibition prevents downregulation of key renal water and sodium transport proteins in response to bilateral ureteral obstruction. Am J Physiol Renal Physiol 289: F322–F333, 2005 [DOI] [PubMed] [Google Scholar]
- 93.Olesen ET, Rutzler MR, Moeller HB, Praetorius HA, Fenton RA. Vasopressin-independent targeting of aquaporin-2 by selective E-prostanoid receptor agonists alleviates nephrogenic diabetes insipidus. Proc Natl Acad Sci USA 108: 12949–12954, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, Buck J, Levin LR, Breton S. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem 278: 49523–49529, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pastor-Soler NM, Hallows KR, Smolak C, Gong F, Brown D, Breton S. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol Cell Physiol 294: C488–C494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paunescu TG, Da Silva N, Russo LM, McKee M, Lu HA, Breton S, Brown D. Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. Am J Physiol Renal Physiol 294: F130–F138, 2008 [DOI] [PubMed] [Google Scholar]
- 97.Paunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, Brown D. cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Physiol 298: F643–F654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Paunescu TG, Russo LM, Da Silva N, Kovacikova J, Mohebbi N, Van Hoek AN, McKee M, Wagner CA, Breton S, Brown D. Compensatory membrane expression of the V-ATPase B2 subunit isoform in renal medullary intercalated cells of B1-deficient mice. Am J Physiol Renal Physiol 293: F1915–F1926, 2007 [DOI] [PubMed] [Google Scholar]
- 99.Pearl M, Taylor A. Role of the cytoskeleton in the control of transcellular water flow by vasopressin in amphibian urinary bladder. Biol Cell 55: 163–172, 1985 [DOI] [PubMed] [Google Scholar]
- 100.Phillips ME, Taylor A. Effect of nocodazole on the water permeability response to vasopressin in rabbit collecting tubules perfused in vitro. J Physiol 411: 529–544, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Procino G, Barbieri C, Carmosino M, Rizzo F, Valenti G, Svelto M. Lovastatin-induced cholesterol depletion affects both apical sorting and endocytosis of aquaporin 2 in renal cells. Am J Physiol Renal Physiol 298: F266–F278, 2010 [DOI] [PubMed] [Google Scholar]
- 102.Procino G, Barbieri C, Carmosino M, Tamma G, Milano S, De Benedictis L, Mola MG, Lazo-Fernandez Y, Valenti G, Svelto M. Fluvastatin modulates renal water reabsorption in vivo through increased AQP2 availability at the apical plasma membrane of collecting duct cells. Pflügers Arch 462: 753–766, 2011 [DOI] [PubMed] [Google Scholar]
- 103.Puopolo K, Kumamoto C, Adachi I, Magner R, Forgac M. Differential expression of the “B” subunit of the vacuolar H(+)-ATPase in bovine tissues. J Biol Chem 267: 3696–3706, 1992 [PubMed] [Google Scholar]
- 104.Pushkin A, Abuladze N, Newman D, Muronets V, Sassani P, Tatishchev S, Kurtz I. The COOH termini of NBC3 and the 56-kDa H+-ATPase subunit are PDZ motifs involved in their interaction. Am J Physiol Cell Physiol 284: C667–C673, 2003 [DOI] [PubMed] [Google Scholar]
- 105.Rein J, Voss M, Blenau W, Walz B, Baumann O. Hormone-induced assembly and activation of V-ATPase in blowfly salivary glands is mediated by protein kinase A. Am J Physiol Cell Physiol 294: C56–C65, 2008 [DOI] [PubMed] [Google Scholar]
- 106.Rice WL, Zhang Y, Chen Y, Matsuzaki T, Brown D, Lu HA. Differential, Phosphorylation Dependent Trafficking of AQP2 in LLC-PK1 cells. PLos One 7: e32843, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ridsdale A, Denis M, Gougeon PY, Ngsee JK, Presley JF, Zha X. Cholesterol is required for efficient endoplasmic reticulum-to-Golgi transport of secretory membrane proteins. Mol Biol Cell 17: 1593–1605, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Robben JH, Kortenoeven ML, Sze M, Yae C, Milligan G, Oorschot VM, Klumperman J, Knoers NV, Deen PM. Intracellular activation of vasopressin V2 receptor mutants in nephrogenic diabetes insipidus by nonpeptide agonists. Proc Natl Acad Sci USA 106: 12195–12200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Robben JH, Sze M, Knoers NV, Deen PM. Functional rescue of vasopressin V2 receptor mutants in MDCK cells by pharmacochaperones: relevance to therapy of nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 292: F253–F260, 2007 [DOI] [PubMed] [Google Scholar]
- 110.Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell 10: 961–974, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98: 4221–4226, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Russo LM, McKee M, Brown D. Methyl-βb-cyclodextrin induces vasopressin-independent apical accumulation of aquaporin-2 in the isolated, perfused rat kidney. Am J Physiol Renal Physiol 291: F246–F253, 2006 [DOI] [PubMed] [Google Scholar]
- 113.Sabolic I, Brown D, Gluck SL, Alper SL. Regulation of AE1 anion exchanger and H(+)-ATPase in rat cortex by acute metabolic acidosis and alkalosis. Kidney Int 51: 125–137, 1997 [DOI] [PubMed] [Google Scholar]
- 114.Sabolic I, Katsura T, Verbavatz JM, Brown D. The AQP2 water channel: effect of vasopressin treatment, microtubule disruption, and distribution in neonatal rats. J Membr Biol 143: 165–175, 1995 [DOI] [PubMed] [Google Scholar]
- 115.Sanches TR, Volpini RA, Massola Shimizu MH, Braganca AC, Oshiro-Monreal F, Seguro AC, Andrade L. Sildenafil reduces polyuria in rats with lithium-induced NDI. Am J Physiol Renal Physiol 302: F216–F225, 2012 [DOI] [PubMed] [Google Scholar]
- 116.Sands JM, Bichet DG. Nephrogenic diabetes insipidus. Ann Intern Med 144: 186–194, 2006 [DOI] [PubMed] [Google Scholar]
- 117.Scarpace PJ, Parthemore JG, Deftos LJ. The distribution of biologically active and inactive radioiodinated human calcitonin in the rat. Endocrinology 103: 128–132, 1978 [DOI] [PubMed] [Google Scholar]
- 118.Schachter M. Chemical, pharmacokinetic, and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol 19: 117–125, 2005 [DOI] [PubMed] [Google Scholar]
- 119.Schwartz GJ, Al-Awqati Q. Carbon dioxide causes exocytosis of vesicles containing H+ pumps in isolated perfused proximal and collecting tubules. J Clin Invest 75: 1638–1644, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schwartz GJ, Al-Awqati Q. Role of hensin in mediating the adaptation of the cortical collecting duct to metabolic acidosis. Curr Opin Nephrol Hypertens 14: 383–388, 2005 [DOI] [PubMed] [Google Scholar]
- 121.Schwartz GJ, Barasch J, Al-Awqati Q. Plasticity of functional epithelial polarity. Nature 318: 368–371, 1985 [DOI] [PubMed] [Google Scholar]
- 122.Shum WW, Da Silva N, Brown D, Breton S. Regulation of luminal acidification in the male reproductive tract via cell-cell crosstalk. J Exp Biol 212: 1753–1761, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shum WW, Da Silva N, McKee M, Smith PJ, Brown D, Breton S. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell 135: 1108–1117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shum WW, Ruan YC, Da Silva N, Breton S. Establishment of cell-cell cross talk in the epididymis: control of luminal acidification. J Androl 32: 576–586, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sidaway JE, Davidson RG, McTaggart F, Orton TC, Scott RC, Smith GJ, Brunskill NJ. Inhibitors of 3-hydroxy-3-methylglutaryl-CoA reductase reduce receptor-mediated endocytosis in opossum kidney cells. J Am Soc Nephrol 15: 2258–2265, 2004 [DOI] [PubMed] [Google Scholar]
- 126.Simon H, Gao Y, Franki N, Hays RM. Vasopressin depolymerizes apical F-actin in rat inner medullary collecting duct. Am J Physiol Cell Physiol 265: C757–C762, 1993 [DOI] [PubMed] [Google Scholar]
- 127.Stetson DL, Steinmetz PR. Correlation between apical intramembrane particles and H+ secretion rates during CO2 stimulation in turtle bladder. Pflügers Arch 407, Suppl 2: S80–S84, 1986 [DOI] [PubMed] [Google Scholar]
- 128.Stetson DL, Steinmetz PR. Role of membrane fusion in CO2 stimulation of proton secretion by turtle bladder. Am J Physiol Cell Physiol 245: C113–C120, 1983 [DOI] [PubMed] [Google Scholar]
- 129.Sun TX, Van Hoek A, Huang Y, Bouley R, McLaughlin M, Brown D. Aquaporin-2 localization in clathrin-coated pits: inhibition of endocytosis by dominant-negative dynamin. Am J Physiol Renal Physiol 282: F998–F1011, 2002 [DOI] [PubMed] [Google Scholar]
- 130.Tajika Y, Matsuzaki T, Suzuki T, Ablimit A, Aoki T, Hagiwara H, Kuwahara M, Sasaki S, Takata K. Differential regulation of AQP2 trafficking in endosomes by microtubules and actin filaments. Histochem Cell Biol 124: 1–12, 2005 [DOI] [PubMed] [Google Scholar]
- 131.Tamma G, Klussmann E, Maric K, Aktories K, Svelto M, Rosenthal W, Valenti G. Rho inhibits cAMP-induced translocation of aquaporin-2 into the apical membrane of renal cells. Am J Physiol Renal Physiol 281: F1092–F1101, 2001 [DOI] [PubMed] [Google Scholar]
- 132.Tamma G, Klussmann E, Oehlke J, Krause E, Rosenthal W, Svelto M, Valenti G. Actin remodeling requires ERM function to facilitate AQP2 apical targeting. J Cell Sci 118: 3623–3630, 2005 [DOI] [PubMed] [Google Scholar]
- 133.Tamma G, Lasorsa D, Ranieri M, Mastrofrancesco L, Valenti G, Svelto M. Integrin signaling modulates AQP2 trafficking via Arg-Gly-Asp (RGD) motif. Cell Physiol Biochem 27: 739–748, 2011 [DOI] [PubMed] [Google Scholar]
- 134.Terris J, Ecelbarger CA, Marples D, Knepper MA, Nielsen S. Distribution of aquaporin-4 water channel expression within rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 269: F775–F785, 1995 [DOI] [PubMed] [Google Scholar]
- 135.Timmer RT, Sands JM. Lithium intoxication. J Am Soc Nephrol 10: 666–674, 1999 [DOI] [PubMed] [Google Scholar]
- 136.Tresguerres M, Parks SK, Salazar E, Levin LR, Goss GG, Buck J. Bicarbonate-sensing soluble adenylyl cyclase is an essential sensor for acid/base homeostasis. Proc Natl Acad Sci USA 107: 442–447, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Van Balkom BW, van Raak M, Breton S, Pastor-Soler N, Bouley R, van der Sluijs P, Brown D, Deen PM. Hypertonicity is involved in redirecting the aquaporin-2 water channel into the basolateral, instead of the apical, plasma membrane of renal epithelial cells. J Biol Chem 278: 1101–1107, 2003 [DOI] [PubMed] [Google Scholar]
- 138.Verlander JW, Madsen KM, Tisher CC. Structural and functional features of proton and bicarbonate transport in the rat collecting duct. Semin Nephrol 11: 465–477, 1991 [PubMed] [Google Scholar]
- 139.Voss M, Vitavska O, Walz B, Wieczorek H, Baumann O. Stimulus-induced phosphorylation of vacuolar H(+)-ATPase by protein kinase A. J Biol Chem 282: 33735–33742, 2007 [DOI] [PubMed] [Google Scholar]
- 140.Wade JB, Coleman RA. Direct visualization of the interrelationship between intramembrane and extracellular structures. Proc Natl Acad Sci USA 86: 2723–2727, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wade JB, Stetson DL, Lewis SA. ADH action: evidence for a membrane shuttle mechanism. Ann NY Acad Sci 372: 106–117, 1981 [DOI] [PubMed] [Google Scholar]
- 142.Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar H+-ATPase. Physiol Rev 84: 1263–1314, 2004 [DOI] [PubMed] [Google Scholar]
- 143.Wagner CA, Mohebbi N, Uhlig U, Giebisch GH, Breton S, Brown D, Geibel JP. Angiotensin II stimulates H-ATPase activity in intercalated cells from isolated mouse connecting tubules and cortical collecting ducts. Cell Physiol Biochem 28: 513–520, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang W, Li C, Kwon TH, Knepper MA, Frokiaer J, Nielsen S. AQP3, p-AQP2, and AQP2 expression is reduced in polyuric rats with hypercalcemia: prevention by cAMP-PDE inhibitors. Am J Physiol Renal Physiol 283: F1313–F1325, 2002 [DOI] [PubMed] [Google Scholar]
- 145.Wang W, Li C, Nejsum LN, Li H, Kim SW, Kwon TH, Jonassen TE, Knepper MA, Thomsen K, Frokiaer J, Nielsen S. Biphasic effects of ANP infusion in conscious, euvolumic rats: roles of AQP2 and ENaC trafficking. Am J Physiol Renal Physiol 290: F530–F541, 2006 [DOI] [PubMed] [Google Scholar]
- 146.Yui N, Lu HJ, Bouley R, Brown D. AQP2 is necessary for vasopressin- and forskolin-mediated filamentous actin depolymerization in renal epithelial cells. Biology Open 1: 101–108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang F, Phiel CJ, Spece L, Gurvich N, Klein PS. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J Biol Chem 278: 33067–33077, 2003 [DOI] [PubMed] [Google Scholar]
- 148.Zwang NA, Hoffert JD, Pisitkun T, Moeller HB, Fenton RA, Knepper MA. Identification of phosphorylation-dependent binding partners of aquaporin-2 using protein mass spectrometry. J Proteome Res 8: 1540–1554, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]