Abstract
Blood-brain barrier dysfunction is a serious consequence of inflammatory brain diseases, cerebral infections, and trauma. The proinflammatory cytokine interleukin (IL)-1β is central to neuroinflammation and contributes to brain microvascular leakage and edema formation. Although it is well known that IL-1β exposure directly induces hyperpermeability in brain microvascular endothelium, the molecular mechanisms mediating this response are not completely understood. In the present study, we found that exposure of the human brain microvascular endothelium to IL-1β triggered activation of novel PKC isoforms δ, μ, and θ, followed by decreased transendothelial electrical resistance (TER). The IL-1β-induced decrease in TER was prevented by small hairpin RNA silencing of PKC-θ or by treatment with the isoform-selective PKC inhibitor Gö6976 but not by PKC inhibitors that are selective for all PKC isoforms other than PKC-θ. Decreased TER coincided with increased phosphorylation of regulatory myosin light chain and with increased proapoptotic signaling indicated by decreased uptake of mitotracker red in response to IL-1β treatment. However, neither of these observed effects were prevented by Gö6976 treatment, indicating lack of causality with respect to decreased TER. Instead, our data indicated that the mechanism of decreased TER involves PKC-θ-dependent phosphorylation of the tight junction protein zona occludens (ZO)-1. Because IL-1β is a central inflammatory mediator, our interpretation is that inhibition of PKC-θ or inhibition of ZO-1 phosphorylation could be viable strategies for preventing blood-brain barrier dysfunction under a variety of neuroinflammatory conditions.
Keywords: neuroinflammatory conditions, edema, blood-brain barrier, microvascular permeability, tight junction proteins
brain inflammation is a pathological consequence of trauma, stroke, cerebral infection, multiple sclerosis, and other inflammatory conditions (4, 10, 30, 34). A serious consequence of brain inflammation is microvascular leakage and brain edema, leading to brain swelling, neuronal injury, and death. Microvascular leakage occurs due to misregulation of the protective interface between the blood and the brain tissue known as the blood-brain barrier (BBB; for review see Refs. 1, 12, 17, 22). During brain inflammation, microvascular barriers become compromised in response to changes in expression or organization of endothelial tight junction proteins permitting plasma components to leak across the BBB into the brain tissue interstitial space. This BBB hyperpermeability occurs in response to proinflammatory agents that are present in the brain tissue during inflammation.
The proinflammatory cytokine interleukin (IL)-1β is produced in the brain during neuroinflammation and contributes to brain microvascular leakage and brain edema (8, 10, 13, 30, 34). The proedematous effects of IL-1β are mediated through the IL-1 receptor (IL1-R1), in that direct inhibition of IL-1β binding to IL1-R1 prevented both brain edema and brain tissue injury in rodent models of experimental cerebral ischemia (38). Brain edema resulting from hypoxic/ischemic injury was also prevented in IL1-R1 knockout mice compared with wild-type mice (27). In addition, vascular effects result from direct exposure of endothelial cells to IL-1β, as demonstrated in studies (14) with cultured human brain microvascular endothelial cells where IL-1β treatment induced hyperpermeability in the absence of all other brain parenchymal cell types. While the effects of IL-1β on brain endothelial hyperpermeability have been clearly demonstrated, most of the intermediate cellular signaling events leading to hyperpermeability are unknown.
In many tissue types, microvascular hyperpermeability is signaled through classical (α, βI, βII, and γ), novel (δ, ε, η, θ, and μ), or atypical (ζ and λ/ι) protein kinase C (PKC) isoforms (32, 39–41). For example, microvascular leakage was mediated by PKC-βII (2, 3), -δ (25), and -ζ (6) at the blood-retinal barrier. Likewise, the isoform-selective PKC inhibitor Gö6976 [12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole] prevented microvascular leakage during ischemia-reperfusion injury in the rat heart, as well as hyperpermeability in human coronary microvascular endothelium in response to IL-1β exposure (36). In addition, BBB dysfunction was mediated by PKC-α in response to tumor necrosis factor (TNF)-α exposure in mouse brain endothelium (29) and involved activation of PKC-θ and possibly other PKC isoforms during hypoxia in the rat brain (18, 37). Based on these observations, we hypothesized that select PKC isoforms mediate hyperpermeability in human brain microvascular endothelium during IL-1β exposure.
In the present study, we examined the role of PKC in mediating barrier dysfunction in human brain microvascular endothelium in response to IL-1β exposure. Our data indicated that novel PKC isoforms (δ, θ, and μ) were activated in response to IL-1β exposure in human brain microvascular endothelial cells (hBMECs). Evidence from PKC isoform-specific inhibitors and gene silencing indicated that PKC-θ was necessary for IL-1β-induced barrier dysfunction [measured as decreased transendothelial electrical resistance (TER)] in hBMEC monolayers. We found that decreased TER was not accompanied by altered expression of junction proteins, increased cell contractility, or apoptotic mechanisms. Furthermore, immunoprecipitation experiments demonstrated that decreased TER in response to IL-1β involved PKC-θ-dependent phosphorylation of zona occludens (ZO)-1. Therefore, selective inhibition of PKC-θ under inflammatory conditions may prevent BBB leakage that is due to posttranslational modification of tight junction proteins and conformational modification of tight junctions in the brain microvascular endothelium.
METHODS
Reagents.
Chemicals were purchased from Sigma-Aldrich; pharmacological inhibitors were from Calbiochem (EMD Chemicals), unless otherwise stated. Anti-PKC antibodies were obtained from Cell Signaling Technology (Danvers, MA) or Santa Cruz Biotechnology (Santa Cruz, CA). Small hairpin (sh)RNA was from Santa Cruz Biotechnology. Human recombinant IL-1β was from Alexa Biochemicals.
Endothelial cell culture.
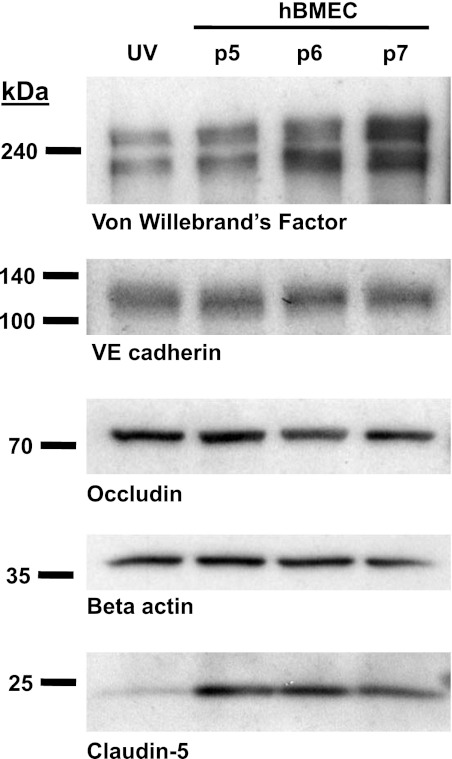
Primary hBMECs isolated from healthy adult cerebral cortex were obtained from Angio Proteomie (Boston, MA) and grown in complete EGM2 growth medium (Lonza, Walkersville, MD) in culture ware pretreated as recommended with Quick Coating Solution (cAP-01; Angio Proteomie). Cells were passaged at 50,000 cells per cm2 and grown for 7–10 days on plastic cell culture dishes or electrical cell impedance sensor (ECIS) arrays; exclusively passages 5–7 were used for experiments. The hBMECs were examined numerous times at passages 5–7 by immunoblotting and confirmed to express endothelial specific marker proteins: von Willebrand factor and vascular endothelial cadherin, and tight junction proteins: occludin and claudin-5 (Fig. 1).
Fig. 1.
Western blot evidence for expression of endothelial specific and tight junction markers in human brain microvascular endothelial cells (hBMECs). Shown (left to right) are Western blot data for cell lysates of human umbilical vein endothelial cells (UV; lane 1), and hBMECs at passage 5, 6 and 7 (lanes 2, 3, and 4, respectively). Western blot bands correspond to von Willebrand's factor, vascular endothelial (VE)-cadherin, occludin, β-actin, and claudin-5, with positions of molecular mass markers shown at left (represents n ≥ 4 similar results).
TER.
Endothelial cell monolayer TER was assessed using ECIS (Applied Biophysics, NY) as described previously (19). Briefly, 105 cells were seeded onto 1-cm2 ECIS electrode arrays. A 1-V, 4,000-Hz alternating current was supplied through a 1-MΩ resistor to a constant current source, and in-phase and out-of-phase voltages were recorded using ECMS 1.0 software (CET). Endothelial barrier function was expressed as background subtracted TER normalized to baseline before the addition of IL-1β or other pharmacological agents. Only endothelial cell monolayers with ECIS resistances of 5,000 Ω-cm2 or greater were used for experiments. ECIS experiments were performed on ≥3 separate days at passages 5–7; ECIS data are shown as means ± SE; n ≥ 8 each.
Endothelial cell transfection.
hBMECs were grown to 90% confluence before transfection. Cells were transfected with plasmids encoding shRNA for either PKC-θ or scrambled sequence. Transfections were performed using a Nucleofector II (Amaxa Biosystems) electroporator and a basic Nucleofector kit (Amaxa, Lonza), according to the manufacturer's instructions. Electroporation was performed with 100,000 cells in a 100-μl suspension using instrument protocol T013. Transfected cells were plated onto ECIS arrays and grown to confluence in puromycin (10 mg/l) selection medium applied 24 h after transfection. ECIS arrays were used for TER measurements and subsequently for Western blot analysis after TER measurements were completed.
Immunoprecipitation of ZO-1.
Treated hBMECs were rapidly frozen in liquid nitrogen then thawed in the presence of (4°C) lysis buffer (PBS, pH 7.4, plus 30 mM sodium fluoride, 20 mM tetrasodium pyrophosphate, 5 mM EDTA, 2 mM EGTA, 1 mM orthovanadate, 40 mM β-glycerophosphate, and Mini Complete protease inhibitor; Roche). Following centrifugation (15 k g, 1 min, 4°C), pellets were washed again in lysis buffer by centrifugation and removal of aqueous supernatant. Resulting pellets were suspended in immunoprecipitation (IP) buffer [lysis buffer, 1× RIPA (Millipore) and 1 mM DTT] at 4°C, sonicated, incubated on wet ice for 20 min, and centrifuged (4°C, 15,000 g, 10 min). Supernatants containing detergent-soluble membranes were used for IP.
IP beads were prepared by covalently crosslinking anti-ZO1 antibody (MAb) to protein G-Sepharose beads (GE Healthcare) by treatment with dimethyl pimelimidate (DMP), essentially as described by Schneider et al. (31). Then, 25 μl of protein G beads were incubated with 3 μg of antibody in 0.1 M boric acid buffer (pH 8.2) on a rotator for 30 min at room temperature. Beads were washed twice in borate buffer and twice in triethanolamine (0.2 M, pH 8.2), followed by DMP (18 mg/ml in triethanolamine) on a rotator for 45 min. The reaction was quenched with ethanolamine (70 mM, pH 8.2), then washed in borate buffer, followed by PBS, and stored in IP buffer at 4°C for use the same day. Sample supernatants were precleared by incubation with DMP-treated beads (no antibody) for 30 min, and the resulting supernatants were added to the antibody-linked beads (4°C, rotating overnight). The following day, the beads were washed gently in IP buffer and harvested by addition of SDS-PAGE sample buffer. Harvested samples were run by SDS-PAGE (Tris-glycine 10% gels; Invitrogen) and transferred to PVDF membranes for immunoblotting.
Immunoblotting.
SDS-PAGE and Western blotting were performed according to the manufacturer's instructions (Novex; Invitrogen). Immunoprecipitated samples on PVDF membranes were analyzed by Western immunoblotting first in anti-phospho-serine/threonine antibody (MAb) and then stripped and reprobed with anti-ZO-1 antibody. Other Western blots were performed similarly from whole cell lysates (lysis buffer + RIPA) or detergent-soluble membranes, as described above, using anti-mouse or ant-rabbit primary antibodies and horseradish peroxidase-conjugated secondary antibodies. Chemiluminescent bands (ECL Supersignal; GE Healthcare) were exposed to film and developed, and images were scanned and saved as TIFF files. Integrated band intensities were quantified using NIH Image J software (W.S. Rasband, Image J, National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/) and expressed as the band intensity ratio (phospho-serine/threonine:ZO-1) normalized to the control condition. Western blot data shown represent n ≥ 4 independent experiments each.
Statistical analysis.
Data subjected to statistical analysis are expressed as means ± SE. ECIS experiments are n ≥ 8 per condition with experiments performed minimally on 3 separate days. Single treatment conditions were compared with control using a two-tailed unpaired Student's t-test, with significance indicated as P < 0.05. Grouped treatments were compared using one-way ANOVA or two-way ANOVA (for comparing multiple time points), followed by a Tukey posttest for multiple comparisons, a Bonferroni posttest for comparing predetermined pairs of samples, as indicated, or Dunnett's posttest when comparing with a single control condition; significance indicated as P < 0.05.
RESULTS
Effects of IL-1β on TER in human brain microvascular endothelium.
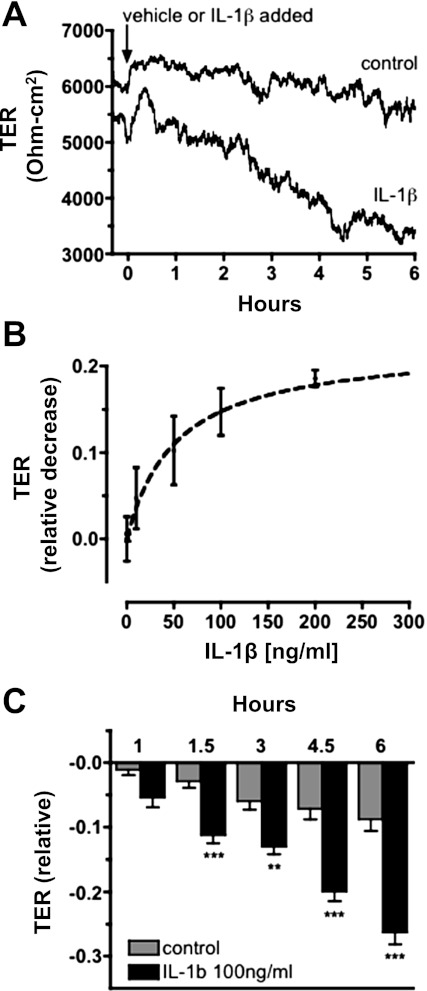
TER was measured across confluent hBMEC monolayers grown on ECIS arrays. A typical TER (ECIS) response of an hBMEC monolayer to treatment with vehicle (water 0.1% vol/vol) alone is shown in Fig. 2A. Additional hBMEC monolayers were examined following treatment with 100 ng/ml IL-1β. Addition of IL-1β caused TER to steadily decrease over several hours (Fig. 2A; represents n ≥ 30 similar results). This effect was further examined as time-course and dose-response data compiled from multiple experiments, expressed as the magnitude of IL-1β-dependent decrease in TER (relative to initial TER values at time zero) at 6 h after treatment with IL-1β. The dose-response data demonstrated that 100 ng/ml IL-1β is a near maximal effective concentration with respect to decreased TER (Fig. 2B). Time-course experiments following exposure to 100 ng/ml IL-1β revealed that TER was significantly (P < 0.001) decreased (relative to initial TER values at time zero) as early as 90 min relative to vehicle alone and continues to decrease for ≥6 h (Fig. 2C).
Fig. 2.
Transendothelial resistance (TER) is decreased in response to IL-1β in hBMEC monolayers. A: representative electrical cell impedance sensor (ECIS) trace showing a typical TER response. TER is minimally affected in response to treatment with vehicle alone (water 0.1% vol/vol; control); TER is steadily decreased in response to IL-1β treatment. Time of treatments added is indicated (arrow). Typical TER values for hBMEC monolayers were in the range of 5,000–8,000 Ω-cm2. B: IL-1β dose-response curve showing the decrease in TER relative to the time-matched control at 6 h after treatment with IL-1β; estimated half-maximal effective concentration (EC50) of IL-1β is 53 ng/ml. C: relative change in TER measured at specified intervals after addition of IL-1β or vehicle alone. TER data are compiled from multiple (n ≥ 30) ECIS determinations, and statistically significant differences (**P < 0.01, ***P < 0.001) are seen at 1.5–6 h after addition of IL-1β.
Activation of novel PKC isoforms in response to IL-1β treatment.
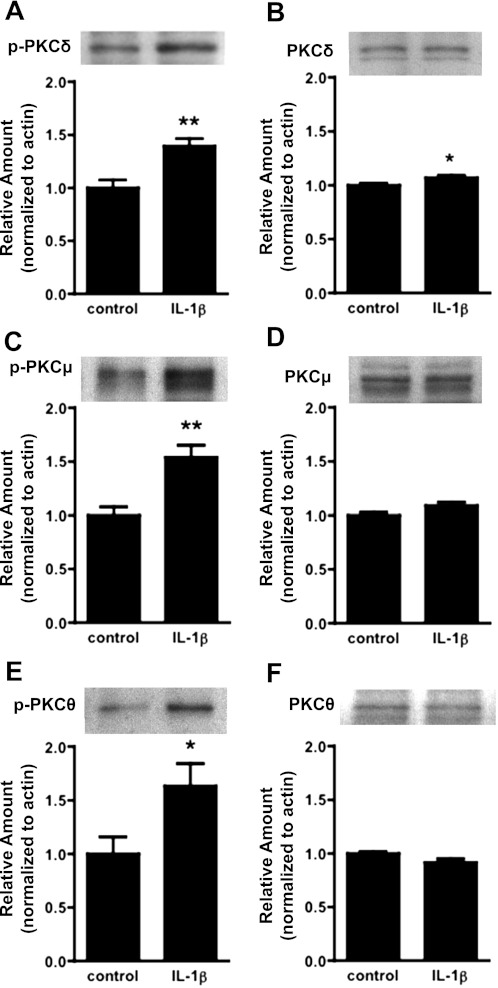
To investigate the involvement of specific PKC isoforms in the response to IL-1β, we examined expression levels and abundance of phosphorylated PKC isoforms. Because PKC signaling necessarily preceded or coincided with the onset of barrier dysfunction, we examined barrier dysfunction at 90 min, a time that corresponded to the earliest indication of barrier dysfunction in Fig. 2B. Immunoblotting with antibodies directed against phosphorylated (activated) forms of PKC showed significantly increased amounts of phosphorylated isoforms δ, μ, and θ (P < 0.05; n ≥ 5 each; Fig. 3, A, C, and F). Similar results were seen at 30 min after IL-1β exposure (data not shown). Because increased amounts of phosphorylated PKCs could be due to increased protein expression, the overall abundance of PKC isoforms δ, μ, and θ was also examined. Immunoblotting with antibodies against PKC isoforms revealed no change in protein expression levels of PKC isoforms θ or μ (Fig. 3, D and F). A small increase in total PKC-δ expression was detected (Fig. 3B), although this rise was too small to fully account for the much larger increase in abundance of phosphorylated PKC-δ (Fig. 3A). In contrast, the amount of phosphorylated PKC-ζ/λ did not increase, suggesting that atypical PKCs were not activated in response to IL-1β treatment (data not shown). The data for phosphorylated PKC-α/βII were suggestive of a modest (not significant) increase following IL-1β treatment (data not shown). However, this was similarly observed for total PKC-α and PKC-βII possibly reflecting a modest increase in protein expression for these isoforms (data not shown). Therefore, IL-1β-induced barrier dysfunction mainly coincided with activation of novel PKC isoforms δ, μ, and θ.
Fig. 3.
Activation of novel PKC isoforms in hBMECs in response to IL-1β exposure. Cells were treated for 1.5 h with IL-1β (100 ng/ml), and Western blots were performed with hBMEC lysates to probe for activation (phosphorylation) and expression of novel PKC isoforms δ, μ, and θ. There were significant increases in amounts of phosphorylated (p-)PKC δ, μ, and θ (A, C, and E). There were no substantial changes in expression of total PKC δ, μ, or θ (B, D, and F), although a modest increase in expression of PKC-δ was noted. Blots represent n ≥ 4 independent experiments, with quantitative data at bottom (band intensity normalized to β-actin). (*P < 0.05, **P < 0.01).
Role of selected PKC isoforms in IL-1β-induced barrier dysfunction.
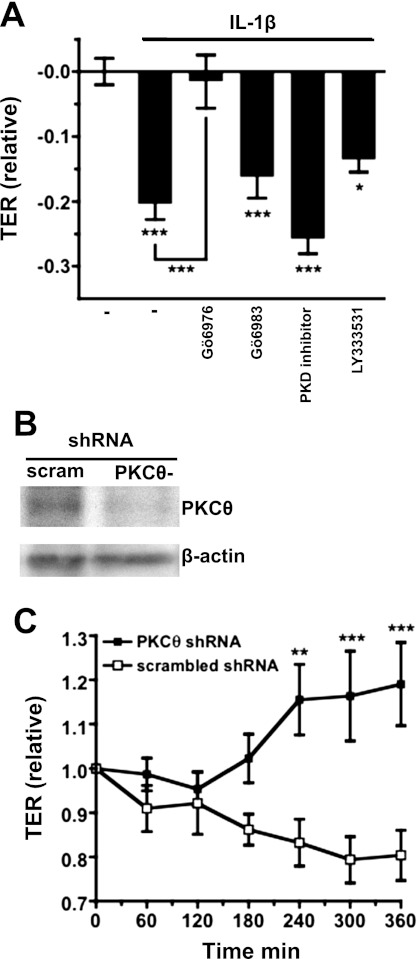
To determine if PKC activity was necessary for decreased TER in response to IL-1β treatment, we treated hBMECs with isoform-selective PKC inhibitors: 200 nM Gö6976 [selectively inhibits cPKCs and PKC-μ (aka. PKD1) at this concentration], 100 nM Gö6983 {2-[1-(3-dimethylaminopropyl)-5-methoxyindol-3-yl]-3-(1H-indol-3-yl)maleimide} (selectively inhibits cPKCs and PKCδ, ε, and ζ at this concentration), 100 nM LY333531 {(S)-13-[(dimethylamino)methyl]-10,11,14,15-tetrahydro-4,9:16,21-dimetheno-1H,13H-dibenzo[e,k]pyrrolo[3,4-h][1,4,13]oxadiazacyclohexadecene-1,3(2H)-dione} (selectively inhibits PKC-βI/II and -η at this concentration), or 2 μM PKD inhibitor CID755673 {7-hydroxy-2,3,4,5-tetrahydro-1H-[1]benzofuro[2,3-c]azepin-1-one} (potently inhibits PKC-μ/PKD) for 30 min before IL-1β. Treatment with these compounds alone did not significantly affect TER values relative to the control condition (vehicle: DMSO treated) at any time examined (from 30 min to 6 h; P > 0.05; data not shown). TER values were examined again after exposure to IL-1β. As in Fig. 1, IL-1β-treated hBMEC monolayers showed significantly decreased TER values relative to vehicle alone (P < 0.001; Fig. 4A). In addition, decreased TER in response to IL-1β was prevented by treatment with Gö6976 (P < 0.001; Fig. 4A), indicating involvement of either a classical PKC or PKD. However, decreased TER was not significantly affected by treatment with Gö6983, LY333531, or PKD inhibitor (Fig. 4A), inhibitors that collectively inhibit PKC isoforms α, β, γ, δ, ε, μ, η, and ζ. This logically eliminated all PKC isoforms except for θ or λ. Our Western blotting data (Fig. 3) showed that barrier dysfunction coincided with activation of novel PKC isoforms but not atypical or classical PKCs, which further suggested that only PKC-θ was likely to be involved in IL-1β-induced barrier dysfunction.
Fig. 4.
Effects of PKC isoform selective inhibitors on TER in hBMEC monolayers. A: monolayers were treated for 30 min with PKC isoform-selective pharmacological inhibitors before addition of IL-1β (100 ng/ml) for 6 h. Treatment with 200 nM Gö6976 prevented decreased TER in response to IL-1β treatment (P < 0.001). In contrast, treatment with 100 nM Gö6983, 2 μM PKD inhibitor, or 100 nM LY333531 failed to prevent decreased TER in response to IL-1β (decreased TER was significant compared with control; P < 0.001, 0.001, and 0.05, respectively). B: Western blots showing expression levels of PKC-θ in lysates of hBMECs stably transfected with PKC-θ or scrambled sequence (control) small hairpin (sh)RNA. PKC-θ protein expression is decreased in cells transfected with PKC-θ shRNA relative to control. C: TER (ECIS) measurements in monolayers of hBMECs stably transfected with shRNA. In response to addition of IL-1β (100 ng/ml), TER declines in hBMECs transfected with scrambled shRNA. In contrast, TER does not decline in response to IL-1β in hBMECs transfected with PKC-θ shRNA. TER values are significantly higher in hBMECs transfected with PKC-θ shRNA than in the scrambled shRNA controls at 4, 5, or 6 h after addition of IL-1β (*P< 0.05, **P < 0.01, ***P < 0.001).
To determine if PKC-θ was involved in IL-1β-induced barrier dysfunction, hBMECs were stably transfected with shRNA designed to silence/suppress expression of PKC-θ mRNA. Decreased expression of PKC-θ was confirmed by Western blotting compared with hBMECs transfected with a nonspecific scrambled shRNA (Fig. 4B). Next, confluent hBMEC monolayers were treated with IL-1β and examined by ECIS. Following treatment with IL-1β, monolayers of hBMECs transfected with scrambled shRNA experienced a drop in TER with a magnitude and time dependence similar to that of untreated hBMECs following IL-1β treatment (Fig. 4C). In contrast, monolayers of hBMECs transfected with PKC-θ shRNA showed no decrease in TER following IL-1β treatment, exhibiting TER values that were significantly above those of the scrambled shRNA transfected hBMECs at 4, 5, or 6 h (Fig. 4C). These findings confirm that PKC-θ is necessary for IL-1β-induced barrier dysfunction in human brain microvascular endothelium.
Increased phosphorylation of myosin light chain in response to IL-1β treatment.
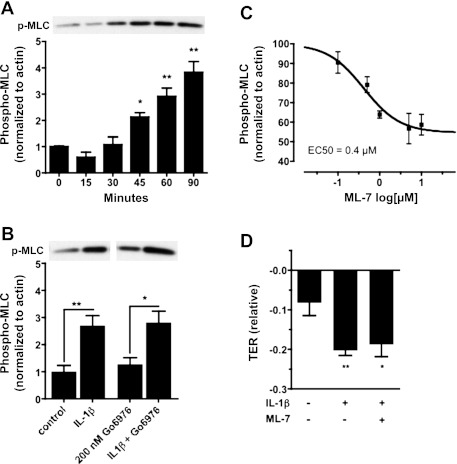
In microvascular endothelial cells and microvessels derived from many tissue types, actomyosin contractility is a principle mechanism mediating endothelial hyperpermeability. This contractile mechanism is rapid and coincides with retraction of cell-cell junctions in many types of endothelium. Increased actomyosin contractility is due to myosin light chain kinase (MLCK)-dependent phosphorylation of regulatory myosin light chain (MLC-2). Moreover, in cardiac endothelium, PKC-dependent hyperpermeability in response to IL-1β exposure required MLCK activity (36). Because of the possibility that MLCK was involved in PKC-dependent signaling in our study, we examined levels of phosphorylated MLC-2 in hBMEC lysates as an indicator of MLCK activity. Immunoblotting revealed significantly increased amounts of phosphorylated MLC-2 (normalized to β-actin) in response to IL-1β treatment (Fig. 5A), closely matching the time course of decreased TER shown in Fig. 2 (P < 0.05, 0.01 and 0.01, at 45, 60, and 90 min, respectively). Based on our observations of effects on TER, hBMECs were treated with 200 nM Gö6976; however, treatment with Gö6976 failed to prevent IL-1β-induced MLC phosphorylation, as determined at 90 min after treatment with IL-1β (Fig. 5B); similar results were also seen at 6 h (not shown). Finally, although dose-response data indicated that treatment of hBMECs with 5 μM ML-7 [1-(5-iodonaphthalene-1-sulfonyl) homopiperazine] (an MLCK-selective inhibitor) was sufficient to inhibit MLC phosphorylation (Fig. 5C), this treatment failed to prevent decreased TER in response to IL-1β exposure (Fig. 5D). Therefore, increased MLCK-dependent MLC phosphorylation coincides with but is not required for PKC-dependent barrier dysfunction in response to IL-1β exposure.
Fig. 5.
Phosphorylation of regulatory myosin light chain (MLC-2) in response to IL-1β treatment in hBMECs. A: Western blot data showing an increase in phosphorylated MLC-2 (p-MLC) over time in response to IL-1β treatment. A representative blot showing increasing p-MLC band intensity after IL-1β exposure is shown at top. Corresponding quantitative data (band intensity normalized to β-actin) are shown at bottom. Statistically significant increases are seen at 45, 60, and 90 min after IL-1β treatment (P < 0.05, 0.01, and 0.01, respectively). B: Western blot data showing increased p-MLC band reactivity at 90 min after IL-1β treatment. Increased p-MLC is not prevented by treatment with 200 nM Gö6976 for 30 min before IL-1β exposure. Corresponding quantitative data (band intensity normalized to β-actin) are shown below (P < 0.01 or 0.05 vs. control, respectively). C: Western blots show dose-dependent inhibition of MLC-2 phosphorylation in response to ML-7 treatment. Data are quantified (normalized to actin) and fit to a sigmoidal dose-response curve (IC50 = 0.4 μM). D: treatment with 5 μM ML-7 for 1 h fails to prevent the IL-1β (100 ng/ml)-induced decrease in TER at 6 h. Mean TER values are significantly decreased following IL-1β treatment in the presence or absence of ML-7 (P < 0.05 or 0.01, respectively) compared with the control condition. Data shown are means ± SE; represents n ≥ 4 independent experiments (*P< 0.05, **P < 0.01).
Activation of apoptotic signaling in hBMECs in response to IL-1β treatment.
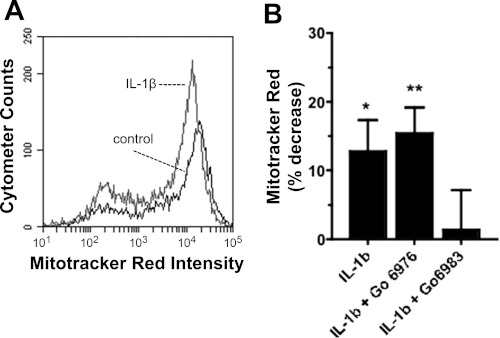
Novel PKC isoforms are shown to induce proapoptotic signaling in tumor cells (16). Proapoptotic signaling also induces barrier dysfunction in the gut mesentery microvasculature (11). Hence, it is possible that PKC-dependent barrier dysfunction in the brain microvascular endothelium is mediated through proapoptotic signaling. To test for the involvement of proapoptotic signaling, we used a flow cytometric assay and live cells labeled with mitotracker red (MTR; a fluorescent indicator of mitochondrial membrane potential polarization). MTR selectively partitions into the mitochondrial inner membrane in unperturbed living cells. Under proapoptotic conditions, mitochondrial membrane potential is depleted, causing decreased accumulation of MTR. In our experiments, confluent hBMEC monolayers treated with IL-1β for 90 min displayed a significant increase in proapoptotic signaling compared with vehicle alone, as indicated by decreased MTR fluorescence intensity (Fig. 6, A and B). We further tested the PKC dependence of this decreased fluorescence and found that proapoptotic signaling was prevented by treatment with Gö6983 but not by treatment with Gö6976. This indicates that proapoptotic signaling in hBMECs requires PKC isoforms that are inhibited exclusively by Gö6983 (e.g., δ or ε), and does not involve PKC isoforms that are inhibited by Gö6976. Thus proapoptotic signaling is not required for decreased TER in response to IL-1β.
Fig. 6.
Flow cytometric analysis of mitotracker red uptake by hBMECs following IL-1β treatment for 90 min. A: data showing decreased intensity of mitotracker red in hBMECs following treatment with IL-1β (represents similar results from n = 6 independent experiments). B: percent change in mean mitotracker red intensity of hBMECs relative to control, following treatment with IL-1β after 30 min of treatment with vehicle alone, 200 nM Gö6976, or 100 nM Gö6983. Mitotracker red intensity was significantly decreased (positive %change) by treatment with IL-1β alone or in the presence of Gö6976 (P < 0.05 or 0.01, respectively). In contrast, Gö6983 treatment prevented the decreased mitotracker red intensity in response to IL-1β (*P < 0.05, **P < 0.01).
Role of tight junction proteins in IL-1β-induced barrier dysfunction.
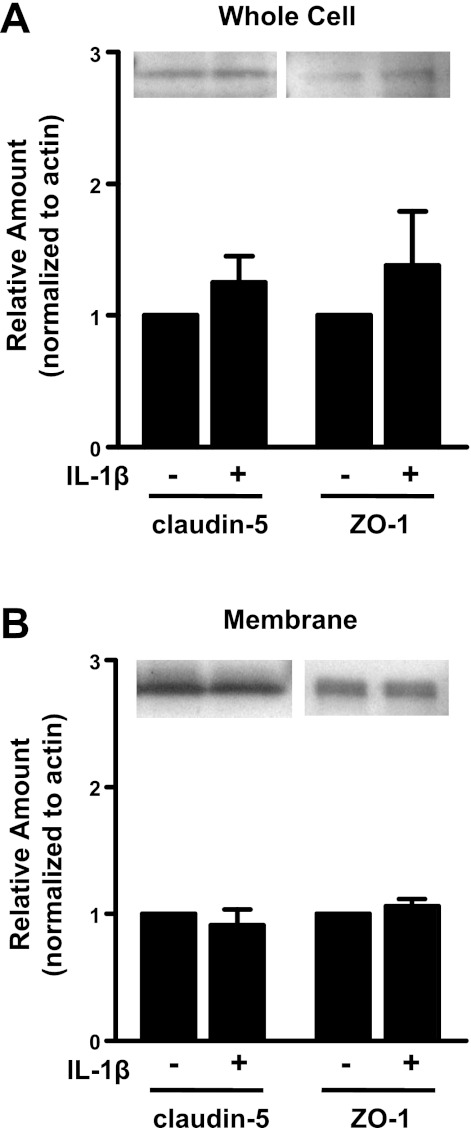
Several reports (20, 25, 37) indicated that increased expression or decreased expression of the tight junction proteins claudin-5 or ZO-1 were final effector mechanisms mediating brain microvascular barrier dysfunction. Because of this possibility, we examined expression of tight junction proteins in lysates or membrane fractions of hBMECs following treatment with IL-1β. Because the greatest decrease in TER occurred at 6 h after treatment, junction proteins were examined at this time point. Expression levels were examined for claudin-5 and ZO-1 both in cell lysates and in membrane fractions; no changes in expression were seen for either protein (Fig. 7, A and B). Immunocytostaining with antibodies directed against claudin-5 or ZO-1 similarly showed no apparent change in cellular or membrane expression of these proteins in response to IL-1β treatment (n = 3; not shown).
Fig. 7.
Expression and phosphorylation of tight junction proteins in hBMECs following 6 h of IL-1β treatment. Western blot data shows no significant (P > 0.05) change in expression of claudin-5 or zona occludens (ZO)-1 in hBMEC whole cell lysates (A) or membrane fractions (B). Representative Western blots are shown (top) together with quantified data normalized to β-actin (bottom) from n ≥ 4 independent experiments.
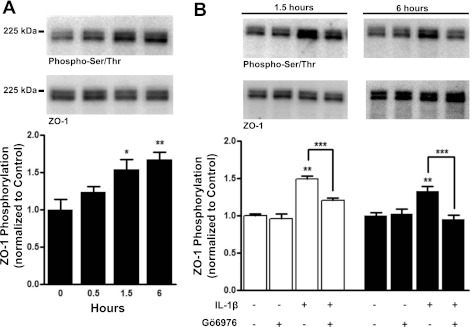
The tight junction protein ZO-1 is a guanylate kinase and PDZ domain adaptor protein that connects tight junction proteins to the actin cytoskeleton. Phosphorylation of ZO-1 affects the organization of tight junctions and the integrity of endothelial barriers (5, 21), which can occur without affecting ZO-1 expression. Hence, it is possible that phosphorylation of ZO-1 is a final effector mechanism in IL-1β-induced barrier dysfunction. To further investigate this, we examined the phosphorylation status of ZO-1 over 6 h following IL-1β treatment by immunoprecipitation of ZO-1 from hBMEC membrane fractions followed by immunoblotting with an anti-phospho-serine/threonine antibody. No significant change in ZO-1 phosphorylation was observed at 30 min (P > 0.05; n = 4). However, significant increases (1.5- or 1.7-fold) were seen at 1.5 and 6 h of exposure to IL-1β (P < 0.01 and 0.001, respectively; n = 4) (Fig. 8A). This corresponded well with the time course of decreased TER following IL-1β treatment and supports the interpretation that serine/threonine phosphorylation of ZO-1 is involved in barrier dysfunction. We also examined ZO-1 phosphorylation in the presence of Gö6976 after 1.5 or 6 h of exposure to IL-1β (Fig. 8B). Treatment with Gö6976 alone did not significantly affect basal serine/threonine phosphorylation of ZO-1 at 1.5 or 6 h (P > 0.05; n = 4 or 10, respectively). In contrast, inclusion of Gö6976 treatment fully prevented serine/threonine phosphorylation of ZO-1 induced by either 1.5 or 6 h of exposure to IL-1β (P < 0.01, n = 4 or 10, respectively) (Fig. 8B).
Fig. 8.
Western blots of ZO-1 immunoprecipitated from hBMEC membrane fractions show phosphorylated serine/threonine (p-Ser/Thr) reactive blots and the corresponding ZO-1 reactive blots detected at the same position on the same PVDF membrane stripped and reprobed with ZO-1 antibody (top). Quantitative data showing the ratio of p-Ser/Thr to total ZO-1 compiled from multiple experiments are also shown (bottom). A: hBMECs treated with 100 ng/ml IL-1β for 30 min, 1.5 h or 6 h, show that total serine/threonine phosphorylation of immunoprecipitated ZO-1 is significantly increased at 1.5 h and 6 h (P < 0.05 and 0.01, respectively; n = 4). B: there is a significant decrease in p-Ser/Thr reactivity of ZO-1 following treatment with Gö6976 and IL-1β relative to IL-1β alone following 1.5 h (left; open bars) or 6 h (right; closed bars) of IL-1β treatment (P < 0.001; n = 4 or 10, respectively) (*P < 0.05, **P < 0.01, ***P < 0.001).
DISCUSSION
The finding that IL-1β-induced BBB dysfunction was mediated through PKC-θ activation has not been previously reported and has important implications for clinical treatment of neuroinflammation. Currently existing therapies targeting inhibition of IL-1β in the brain have limited effectiveness, in part owing to biological complexities including the presence of endogenous IL-1 receptor antagonist (IL1-ra) as well as the presence of IL-1 receptor on nonendothelial cell types. Thus targeting downstream events more proximal to terminal events mediating barrier dysfunction is likely to be a more effective strategy for preventing BBB leakage under neuroinflammatory conditions. Kinase inhibition is known to be a viable therapeutic approach in humans, as many effective therapeutic agents are kinase inhibitors [e.g., LY333351 (ruboxystaurin)]. The finding that Gö6976, a PKC inhibitor, was capable of preventing IL-1β-induced barrier dysfunction in human brain endothelium suggests that PKC-θ inhibition could be an effective intervention for preventing brain edema in several neuroinflammatory conditions.
Role of PKC-θ in BBB dysfunction.
The finding that PKC-θ was involved in brain endothelial barrier dysfunction is well supported by the work of other laboratories (18, 37) showing that PKC-θ was activated in rat brain endothelium during hypoxic/ischemic (H/I) injury. In the present study, we found that IL-1β-induced decreased TER was mediated through PKC activity that was sensitive to inhibition by Gö6976. Based on the observed selective activation of novel PKC isoforms in response to IL-1β treatment, together with the relative isoform selectivity of pharmacological PKC inhibitors, we were able to logically exclude the participation of PKC isoforms α, β, γ, δ, ε, η, μ, ζ, and λ and were only unable to exclude PKC-θ as a mediator of decreased TER in response to IL-1β exposure. We then confirmed that PKC-θ was necessary for the response to IL-1β, in that gene silencing with PKC-θ shRNA completely prevented decreased TER in response to IL-1β. That coupled with the observation that PKC-θ was activated in response to IL-1β treatment strongly supported a role for PKC-θ activation in IL-1β-induced barrier dysfunction. However, the mechanisms for PKC-θ dependent barrier dysfunction in the present study appeared to be markedly different from those described by the results of H/I studies in rat brain endothelium. In the H/I studies, activation of PKC-θ resulted from increased PKC expression. In contrast, IL-1β exposure in hBMECs clearly resulted in increased activation (phosphorylation) of PKC-θ in the absence of changes in PKC protein expression. Although these mechanisms differ from each other, in either case the end result was increased abundance of activated PKC. However, this also implies that multiple strategies for PKC-θ inhibition may be possible for treating in vivo pathological conditions involving neuroinflammation and/or H/I injury.
PKC-dependent effects on tight junction proteins.
An interesting difference between the present study and studies (18, 37) of H/I in rat brain endothelium was the endpoint mechanism of barrier dysfunction. In rat brain endothelium, hypoxia caused increased expression of the tight junction proteins, ZO-1, occludin, and claudin-5. This was in sharp contrast to studies by other investigators (23, 24) showing that exposure to the cytokine IL-17 caused BBB dysfunction coupled with decreased expression of occludin and ZO-1. In the present study, we observed no significant changes in expression of claudin-5, ZO-1 (Fig. 7A), or occludin (data not shown). Instead, we found increased serine/threonine phosphorylation of ZO-1 in response to IL-1β treatment. ZO-1 is a known phosphoprotein, although the functional consequences of ZO-1 phosphorylation vary according to cell type. In kidney inner medullary epithelial cells, tyrosine phosphorylation of ZO-1 increased binding of ZO-1 to the actin cytoskeleton and thereby strengthened tight junctions (35). In contrast, ZO-1 tyrosine phosphorylation coincided with vascular dysfunction in bovine retinal endothelial cells in response to treatment with vascular endothelial growth factor (5). Direct injection of IL-1β into the brains of juvenile rats was also shown to increase overall amounts of phosphotyrosine in the brain, coinciding with decreased expression of the tight junction proteins occludin and ZO-1 (9). In parallel experiments with human umbilical vein endothelial cell monolayers, phosphatase inhibitor treatment induced barrier dysfunction coinciding with increased tyrosine phosphorylation of ZO-1, suggesting that phosphorylation of ZO-1 and decreased ZO-1 expression are general mechanisms of barrier dysfunction in vascular endothelium.
In the present study, we examined serine/threonine phosphorylation of ZO-1, based on observations of PKC-dependent barrier dysfunction, and found that IL-1β-induced barrier dysfunction coincided with PKC-dependent serine/threonine phosphorylation of ZO-1. We found significant increases in ZO-1 phosphorylation in response to IL-1β treatment that followed a similar time course to that of decreased barrier dysfunction. In addition, Gö6976 treatment strongly inhibited IL-1β-induced phosphorylation of ZO-1. To our knowledge, this is the first reported evidence of serine/threonine phosphorylation of ZO-1 in human brain microvascular endothelial cells. The similarities in the time course and inhibition by Gö6976 implicated serine/threonine phosphorylation of ZO-1 as a downstream event in IL-1β-induced barrier dysfunction in human brain microvascular endothelium. Further investigations are necessary to determine the exact mechanism(s) by which phosphorylation of ZO-1 affects tight junction integrity, as well as the specific phosphorylation sites that are involved in endothelial barrier dysfunction. Multiple putative phosphorylation sites exist within the ZO-1 protein sequence and hence complex patterns of phosphorylation and dephosphorylation are expected. Complete understanding of these events will require careful dissection of the roles of individual phosphorylation sites by examining the functional effects of point mutations at critical serine/threonine residues on ZO-1.
Decreased TER and MLC phosphorylation.
In many examples, microvascular barrier dysfunction was mediated through increased endothelial MLCK-dependent actomyosin contractility (32, 33, 41). Likewise, in several studies (20, 23, 26, 28) of BBB dysfunction during focal brain tissue injury, hypoxia, and other neuroinflammatory conditions, BBB dysfunction was shown to depend on MLCK activity. For example, in bovine brain microvascular endothelial cells, intercellular gap formation, altered tight junction protein expression, and increased MLC phosphorylation in response to ethanol exposure and were prevented by treatment with ML-7 (20). MLCK-dependent BBB dysfunction also occured in mice and rats in response to hypoxia, brain tissue injury, or IL-17A exposure (23, 26, 28). In the present study, we found that MLC phosphorylation was strongly increased in response to exposure to IL-1β, suggestive of a role for actomyosin contractility in barrier dysfunction. Furthermore, the time course of MLC phosphorylation corresponded well with the onset of decreased TER. However, treatment with Gö6976 failed to prevent MLC phosphorylation, and treatment with 5 μM ML-7 failed to inhibit decreased TER (Fig. 5D) in response to IL-1β treatment. Therefore, although MLC phosphorylation coincides with barrier dysfunction, MLCK-dependent MLC phosphorylation is not causally related to PKC-mediated barrier dysfunction in response to IL-1β exposure in human brain endothelium.
BBB dysfunction and proapoptotic signaling.
Other investigators (11) have suggested that endothelial barrier dysfunction was caused by activation of proapoptotic signaling. Because of this, we examined cell accumulation of MTR, an indicator of compromised mitochondrial membrane potential (dysfunction) that occurs in proapoptotic signaling. We found that MTR uptake was decreased after treatment with IL-1β, consistent with induction of proapoptotic signaling. This is important in that that damaged mitochondria are a major source of reactive oxygen species (ROS), and ROS are strong inducers of MLC phosphorylation and brain endothelial hyperpermeability (20, 23, 26). In our study, proapoptotic signaling was prevented by Gö6983 and not by Gö6976, indicating that mitochondrial dysfunction is not necessary for decreased TER in response to IL-1β treatment. We also note that MLC phosphorylation was unaffected by Gö6983 treatment (data not shown), indicating that proapoptotic signaling is also not necessary for MLC phosphorylation in response to IL-1β treatment. Because we did not directly examine ROS generation in response to IL-1β treatment, further experiments are necessary to determine if ROS production from other enzymatic sources is involved in the response to IL-1β treatment.
Conclusion.
IL-1β plays a central role in the neuroinflammation in response to brain trauma, stroke, and many diseases of the brain. The damaging effects of IL-1β in the brain and at the BBB are clearly demonstrated in rodent models. Early clinical trials (15) have also shown evidence of improved outcome in acute stroke patients with use of IL-1ra and with no adverse effects attributed to this treatment. However, the effectiveness of IL-1ra as a protective agent in acute stroke has yet to be clearly demonstrated (7). Challenges in the use of IL-1β competitive inhibitors may arise from the complex actions of IL-1β on different cell types in the brain and from the observation that IL-1β plays both damaging and neuroprotective roles under inflammatory conditions in the brain. To design more effective therapies for preventing the damaging effects of IL-1β, it is necessary to understand the molecular mechanisms downstream of IL-1R activation that are specifically involved in the pathological consequences of neuroinflammation. In this study, we focused on the barrier response of human brain microvascular endothelial cells to IL-1β. Our strategy was to identify endothelial cellular signaling mechanisms triggered by IL-1β exposure that are responsible for BBB dysfunction. By selectively targeting these signaling molecules, it should be possible to inhibit the pathological effects of IL-1β at the BBB and to protect the brain from edema formation. In the present study, we determined that IL-1β exposure induced endothelial barrier dysfunction through activation of PKC-θ and PKC-dependent phosphorylation of ZO-1. These results were significant in that very little was previously known about brain endothelial cell signaling responses to IL-1β exposure or more generally about mechanisms of BBB dysfunction in response to inflammatory mediators in the human brain. This information suggests that PKC-θ inhibition is a reasonable strategy for inhibiting or preventing BBB leakage during neuroinflammation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-61507, HL-70753, HL-96640, and HL-84852 (S. Y. Yuan). R. R. Rigor was supported by National Institutes of Health T32 Training Grant Fellowship HL-086350.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.R.R. and S.Y.Y. conception and design of research; R.R.R., R.S.B., and O.P.L. performed experiments; R.R.R., R.S.B., and O.P.L. analyzed data; R.R.R. and R.S.B. interpreted results of experiments; R.R.R. prepared figures; R.R.R. drafted manuscript; R.R.R., R.S.B., and S.Y.Y. edited and revised manuscript; R.R.R. and S.Y.Y. approved final version of manuscript.
REFERENCES
- 1. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 37: 13– 25, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M, Smith LE, King GL. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes 46: 1473– 1480, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Aiello LP, Clermont A, Arora V, Davis MD, Sheetz MJ, Bursell SE. Inhibition of PKC beta by oral administration of ruboxistaurin is well tolerated and ameliorates diabetes-induced retinal hemodynamic abnormalities in patients. Invest Ophthalmol Vis Sci 47: 86– 92, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Allan SM, Rothwell NJ. Inflammation in central nervous system injury. Philos Trans R Soc Lond B Biol Sci 358: 1669– 1677, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem 274: 23463– 23467, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Aveleira CA, Lin CM, Abcouwer SF, Ambrosio AF, Antonetti DA. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes 59: 2872– 2882, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banwell V, Sena ES, Macleod MR. Systematic review and stratified meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke. J Stroke Cerebrovasc Dis 18: 269– 276, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res 78: 151– 156, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience 86: 1245– 1257, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Chakraborty S, Kaushik DK, Gupta M, Basu A. Inflammasome signaling at the heart of central nervous system pathology. J Neurosci Res 88: 1615– 1631, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Childs EW, Tharakan B, Hunter FA, Tinsley JH, Cao X. Apoptotic signaling induces hyperpermeability following hemorrhagic shock. Am J Physiol Heart Circ Physiol 292: H3179– H3189, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Coisne C, Engelhardt B. Tight junctions in brain barriers during central nervous system inflammation. Antioxid Redox Signal 15: 1285– 1303, 2011 [DOI] [PubMed] [Google Scholar]
- 13. de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol 64: 37– 43, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Didier N, Romero IA, Creminon C, Wijkhuisen A, Grassi J, Mabondzo A. Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J Neurochem 86: 246– 254, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, Tyrrell PJ. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry 76: 1366– 1372, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Endo K, Oki E, Biedermann V, Kojima H, Yoshida K, Johannes FJ, Kufe D, Datta R. Proteolytic cleavage and activation of protein kinase C [micro] by caspase-3 in the apoptotic response of cells to 1-beta-d-arabinofuranosylcytosine and other genotoxic agents. J Biol Chem 275: 18476– 18481, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 31: 497– 511, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Fleegal MA, Hom S, Borg LK, Davis TP. Activation of PKC modulates blood-brain barrier endothelial cell permeability changes induced by hypoxia and posthypoxic reoxygenation. Am J Physiol Heart Circ Physiol 289: H2012– H2019, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Guo M, Breslin JW, Wu MH, Gottardi CJ, Yuan SY. VE-cadherin and β-catenin binding dynamics during histamine-induced endothelial hyperpermeability. Am J Physiol Cell Physiol 294: C977– C984, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haorah J, Heilman D, Knipe B, Chrastil J, Leibhart J, Ghorpade A, Miller DW, Persidsky Y. Ethanol-induced activation of myosin light chain kinase leads to dysfunction of tight junctions and blood-brain barrier compromise. Alcohol Clin Exp Res 29: 999– 1009, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol 36: 1206– 1237, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57: 173– 185, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, Bechmann I, Becher B, Luhmann HJ, Waisman A, Kuhlmann CR. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J 24: 1023– 1034, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13: 1173– 1175, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim JH, Jun HO, Yu YS, Kim KW. Inhibition of protein kinase C delta attenuates blood-retinal barrier breakdown in diabetic retinopathy. Am J Pathol 176: 1517– 1524, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuhlmann CR, Tamaki R, Gamerdinger M, Lessmann V, Behl C, Kempski OS, Luhmann HJ. Inhibition of the myosin light chain kinase prevents hypoxia-induced blood-brain barrier disruption. J Neurochem 102: 501– 507, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Lazovic J, Basu A, Lin HW, Rothstein RP, Krady JK, Smith MB, Levison SW. Neuroinflammation and both cytotoxic and vasogenic edema are reduced in interleukin-1 type 1 receptor-deficient mice conferring neuroprotection. Stroke 36: 2226– 2231, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Luh C, Kuhlmann CR, Ackermann B, Timaru-Kast R, Luhmann HJ, Behl C, Werner C, Engelhard K, Thal SC. Inhibition of myosin light chain kinase reduces brain edema formation after traumatic brain injury. J Neurochem 112: 1015– 1025, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Peng J, He F, Zhang C, Deng X, Yin F. Protein kinase C-alpha signals P115RhoGEF phosphorylation and RhoA activation in TNF-alpha-induced mouse brain microvascular endothelial cell barrier dysfunction. J Neuroinflammation 8: 28, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmidt OI, Heyde CE, Ertel W, Stahel PF. Closed head injury–an inflammatory disease? Brain Res Brain Res Rev 48: 388– 399, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Schneider C, Newman RA, Sutherland DR, Asser U, Greaves MF. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem 257: 10766– 10769, 1982 [PubMed] [Google Scholar]
- 32. Shen Q, Rigor RR, Pivetti CD, Wu MH, Yuan SY. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc Res 87: 272– 280, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shen Q, Wu MH, Yuan SY. Endothelial contractile cytoskeleton and microvascular permeability. Cell Health Cytoskelet 2009: 43– 50, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Simi A, Tsakiri N, Wang P, Rothwell NJ. Interleukin-1 and inflammatory neurodegeneration. Biochem Soc Trans 35: 1122– 1126, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Then C, Bergler T, Jeblick R, Jung B, Banas B, Kramer BK. Hypertonic stress promotes the upregulation and phosphorylation of zonula occludens 1. Nephron Physiol 119: p11– p21, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Tinsley JH, Hunter FA, Childs EW. PKC and MLCK-dependent, cytokine-induced rat coronary endothelial dysfunction. J Surg Res 152: 76– 83, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Willis CL, Meske DS, Davis TP. Protein kinase C activation modulates reversible increase in cortical blood-brain barrier permeability and tight junction protein expression during hypoxia and posthypoxic reoxygenation. J Cereb Blood Flow Metab 30: 1847– 1859, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke 26: 676– 680; discussion 681, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Yuan SY. Protein kinase signaling in the modulation of microvascular permeability. Vascul Pharmacol 39: 213– 223, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Yuan SY. Signal transduction pathways in enhanced microvascular permeability. Microcirculation 7: 395– 403, 2000 [PubMed] [Google Scholar]
- 41. Yuan SY, Rigor RR. Regulation of Endothelial Barrier Function. San Rafael, CA: Morgan and Claypool Life Sciences, 2011 [PubMed] [Google Scholar]