identification of the genes involved in polycystic kidney disease (PKD) suggests that defects in ciliary function are a common feature (4). In renal tubular epithelia, primary cilia on the apical surface normally protrude into the tubular lumen where they can detect fluid flow, likely due to the bending of the cilium and the triggering of calcium transients. How the lack of a central cilium and the disruption of this sensing and signaling could lead to the development of a pathologic cystic phenotype remain a very active area of investigation.
The Oak Ridge polycystic kidney mouse (ORPK), first described through collaborative efforts of Avner and Woychik (7), displays renal cystic lesions that resemble cysts found in human autosomal recessive polycystic kidney disease (ARPKD). The phenotype arose from an insertional mutation in the Ift88 gene encoding the protein IFT88 (Polaris), a mutation that leads to the lack of a well-formed central cilium in multiple tissues. The study by Olteanu et al. (8) in this issue, a collaboration between a number of labs with complementary strengths in renal and epithelial physiology, utilizes both conditionally immortalized cortical collecting duct (CCD) principal cells (PCs) isolated from the ORPK mouse, as well as a kidney-specific conditional cilium knockout mouse generated from the ORPK mouse to assess altered renal transporter activity and polarity in these cilia-deficient cells. A previous study from this group in cilium-deficient CCD PCs reported a fourfold increase in apical epithelial Na+ channel (ENaC)-mediated sodium reabsorption (9), suggesting that the cilium-sensing pathway controls ENaC activity, a potential clinically relevant finding given the early onset of hypertension that is observed in the ARPKD population (3). The current study provides evidence that Na/H exchanger 1 (NHE1), normally expressed in a polarized fashion in the basolateral membrane of CCD PCs, is expressed in both the apical and the basolateral membranes of the ORPK CCD cells. Another recent study reports that vasopressin receptors are also mislocated to the apical membrane in CD cells from the ORPK mouse (10). These studies indicate that the lack of Polaris expression alters the polarized trafficking of membrane proteins which may lead to, directly or indirectly, elevated Na+ and water reabsorption in these cilium-deficient cells.
The major positive features of the Olteanu study include: 1) the use of a chamber that allowed precise independent control of the cultured CCD cells' apical and basolateral bathing fluids compartments coupled to, 2) recording of Na+/H+ exchanger activity in real time as changes in intracellular pH using a pH-sensitive dye and, 3) implementation of multiple Na+/H+ exchanger inhibitors and specific antibodies to identify apical NHE1, as well as, 4) the generation of a complementary HoxB7 cre-lox kidney-specific conditional cilium knockout mouse (ift88flox/Δ), which allowed the study of isolated perfused, intact cortical collecting tubular function with and without ciliated PCs. These genetic studies confirmed the results of the cellular studies and went on to show that knockout mice exhibited metabolic alkalosis consistent with excessive acid excretion via apical NHE1 in the cilium-deficient PC.
PKDs are the most common primary renal genetic disorders, affecting between 1 in 400–1,000 (over 13 million people worldwide), and producing hypertension and end-stage renal disease in children as well as adults (5). Despite major advances over the past decade in delineating the genetics and pathophysiology of PKD, therapeutic efforts are limited to treating the complications of the disease, as there is still no proven therapy for PKD (12).
The studies of Olteanu et al. (8, 9) are relevant to the growing body of literature on the relationships between ciliary structure/function and renal epithelial transport, and may be relevant to hypertension via control of ENaC abundance. However, careful evaluation is needed before concluding that the authors' meticulous studies in the ORPK model are applicable to human PKD. We summarize central issues for the readers' consideration:
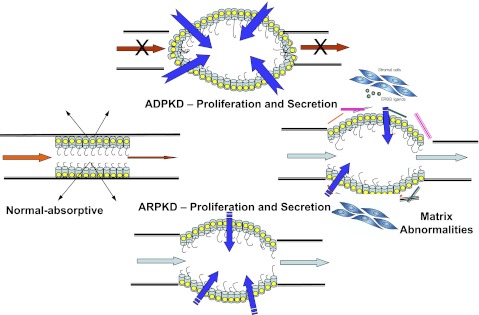
1) Data from many laboratories demonstrate that a secretory event is critical for cystic enlargement in PKD (Fig. 1). PKD epithelia are characterized by proliferative, extracellular matrix and secretory abnormalities. In ARPKD, although cysts remain open to tubular lumens, secretion may contribute to cyst enlargement with low flow states, distal obstruction, or by as-yet unknown mechanisms (5). It is unclear from the authors' study how increased Na+ reabsorption (via apical NHE and ENaC) leads to a secretory phenotype. In an era of orthologous models of PKD, the value of nonorthologous models is related to their overall phenotypic similarities, specifically the well-described, characteristic cystic epithelial cellular phenotype of PKD (5, 11, 12). However, the overall phenotype of the ORPK mouse may be more consistent with syndromic renal disease or “renal ciliopathies” (6), but further comment is difficult due to the heterogeneity and diverse pathophysiology of these disorders.
2) Neither hypertension nor left ventricular hypertrophy (a pathological marker of hypertension) has been documented in the ORPK mouse on any genetic background (to our knowledge). In addition, increased activity of the renin-angiotensin system (RAS) is implicated as the major cause of hypertension in PKD, rather than elevated sodium reabsorption (2). Another critical point is that the results collected in immortalized cystic PCs from another nonorthologous model report that Na+ absorption via ENaC is reduced (rather than stimulated) when compared with cells from age-matched wild-type littermates (11, 13). Reduced ENaC is consistent with both the secretory phenotype seen in PKD and elevated RAS.
3) Neither autosomal dominant (ADPKD) nor ARPKD patients have been reported to be alkalotic at any stage of their disease. Indeed, PKD patients have systemic acidosis in direct proportion to their degree of renal failure.
Fig. 1.
This figure illustrates how a normal tubule becomes cystic in genetic polycystic kidney disease (PKD). A primary factor in both autosomal dominant (ADPKD) and autosomal recessive (ARPKD) is tubular cell proliferation. Such proliferation must occur with the conversion of an absorptive to a secretory phenotype. Complex (and incompletely characterized) abnormalities in the matrix biology of the cystic microenviroment not only contribute to proliferation and secretion in cyst formation and enlargement, but also may be a major factor in renal fibrosis and progression to end-stage renal disease (ESRD). [From Avner et al. (1).]
In sum, the authors have presented valuable new data regarding the relationship between renal cellular cilia expression and renal cellular transport. The major limitation of the study is the authors' extrapolation of data from a nonorthologous model of PKD in which neither the animal model nor the cellular phenotype is consistent with findings in human ARPKD. This collaborative group of authors is in an excellent position to take the steps to apply their elegant methodologies to characterize the transport abnormalities of PKD in orthologous models or fresh renal tissue obtained from patients with ADPKD and/or ARPKD.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant DK 5P50DK079306 and Children's Research Institute of Wisconsin (to E. D. Avner and W. E. Sweeney) and by NIH Grant DK 083785 and the University Kidney Research Organization (to A. McDonough).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.D.A. and W.E.S. drafted the manuscript; E.D.A., A.A.M., and W.E.S. edited and revised the manuscript; E.D.A., A.A.M., and W.E.S. approved the final version of the manuscript.
REFERENCES
- 1. Avner ED, Harmon WE, Niaudet P. Pediatric Nephrology (5th ed.), New York: Lippincott, Williams and Wilkins, 2004 [Google Scholar]
- 2. Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med 323: 1091– 1096, 1990 [DOI] [PubMed] [Google Scholar]
- 3. Guay-Woodford LM, Desmond RA. Autosomal recessive polycystic kidney disease: the clinical experience in North America. Pediatrics 111: 1072– 1080, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Harris PC. 2008 Homer W. Smith Award: insights into the pathogenesis of polycystic kidney disease from gene discovery. J Am Soc Nephrol 20: 1188– 1198, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med 60: 321– 337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med 364: 1533– 1543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moyer JH, Lee-Tischler MJ, Kwon HY, Schrick JJ, Avner ED, Sweeney WE, Godfrey VL, Cacheiro NL, Wilkinson JE, Woychik RP. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science 264: 1329– 1333, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Olteanu D, Liu X, Liu W, Roper VC, Sharma N, Yoder BK, Satlin LM, Schwiebert EM, Bevensee MO. Increased Na+/H+ exchanger activity on the apical surface of a cilium-deficient cortical collecting duct principal cell model of polycystic kidney disease. Am J Physiol Cell Physiol (February 1, 2012). doi:10.1152/ajpcell.00063.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olteanu D, Yoder BK, Liu W, Croyle MJ, Welty EA, Rosborough K, Wyss JM, Bell PD, Guay-Woodford LM, Bevensee MO, Satlin LM, Schwiebert EM. Heightened epithelial Na+ channel-mediated Na+ absorption in a murine polycystic kidney disease model epithelium lacking apical monocilia. Am J Physiol Cell Physiol 290: C952– C963, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Saigusa T, Reichert R, Guare J, Siroky BJ, Gooz M, Steele SL, Fenton RA, Bell PD, Kolb RJ. Collecting duct cells that lack normal cilia have mislocalized vasopressin-2-receptors. Am J Physiol Renal Physiol 302: F801– F808, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sweeney WE, Jr, Kusner L, Carlin CR, Chang S, Futey L, Cotton CU, Dell KM, Avner ED. Phenotypic analysis of conditionally immortalized cells isolated from the BPK model of ARPKD. Am J Physiol Cell Physiol 281: C1695– C1705, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Torres VE. Treatment strategies and clinical trial design in ADPKD. Adv Chronic Kidney Dis 17: 190– 204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Veizis EI, Carlin CR, Cotton CU. Decreased amiloride-sensitive Na+ absorption in collecting duct principal cells isolated from BPK ARPKD mice. Am J Physiol Renal Physiol 286: F244– F254, 2004 [DOI] [PubMed] [Google Scholar]