Abstract
The cAMP/PKA signaling system constitutes an inhibitory pathway in T cells and, although its biochemistry has been thoroughly investigated, its possible effects on ion channels are still not fully understood. KV1.3 channels play an important role in T-cell activation, and their inhibition suppresses T-cell function. It has been reported that PKA modulates KV1.3 activity. Two PKA isoforms are expressed in human T cells: PKAI and PKAII. PKAI has been shown to inhibit T-cell activation via suppression of the tyrosine kinase Lck. The aim of this study was to determine the PKA isoform modulating KV1.3 and the signaling pathway underneath. 8-Bromoadenosine 3′,5′-cyclic monophosphate (8-BrcAMP), a nonselective activator of PKA, inhibited KV1.3 currents both in primary human T and in Jurkat cells. This inhibition was prevented by the PKA blocker PKI6–22. Selective knockdown of PKAI, but not PKAII, with siRNAs abolished the response to 8-BrcAMP. Additional studies were performed to determine the signaling pathway mediating PKAI effect on KV1.3. Overexpression of a constitutively active mutant of Lck reduced the response of KV1.3 to 8-Br-cAMP. Moreover, knockdown of the scaffolding protein disc large 1 (Dlg1), which binds KV1.3 to Lck, abolished PKA modulation of KV1.3 channels. Immunohistochemistry studies showed that PKAI, but not PKAII, colocalizes with KV1.3 and Dlg1 indicating a close proximity between these proteins. These results indicate that PKAI selectively regulates KV1.3 channels in human T lymphocytes. This effect is mediated by Lck and Dlg1. We thus propose that the KV1.3/Dlg1/Lck complex is part of the membrane pathway that cAMP utilizes to regulate T-cell function.
Keywords: protein kinase A, T cell signaling, Jurkat
the activity of t lymphocytes is regulated by ion channels that control the Ca2+ response triggered by antigen presentation and necessary for cytokine production and proliferation (2). The main ion channels expressed in human T cells are the Ca2+-release activated Ca2+ channel (CRAC/Orai1), the voltage-dependent KV1.3, and the Ca2+-activated KCa3.1 K+ channels (2). The interplay between these channels allows Ca2+ signaling in T lymphocytes. KV1.3 channels regulate the cell membrane potential and maintain the membrane hyperpolarized, thus allowing Ca2+ entry through the CRAC channels (2). Consequently, KV1.3 inhibition reduces T-cell activation and blockers of KV1.3 channels have immunosuppressive properties (2, 34). Furthermore, upon T-cell activation, KV1.3 channels localize in the immune synapse (IS), the signaling zone that forms between a T cell and an antigen-presenting cell (2, 29, 30). The IS is a site of active signaling, and it is believed that KV1.3, once localized at this site, may be inhibited by signaling molecules also present at the IS. Indeed, blockade of KV1.3 entry to the IS results in an exaggerated Ca2+ response, which can be expected if KV1.3 inhibition is obstructed (29). Thus it is clear that KV1.3 undergoes a tight regulation during the T-cell activation process and that modulation of KV1.3 activity by kinases/phosphatases can ultimately have important consequences on T-cell function.
Various signaling molecules modulate KV1.3 activity including src-protein tyrosine kinases, protein kinase C (PKC) and A (PKA; Refs. 4, 7, 14, 17, 42, 43). PKA is of particular interest as it has been shown to downregulate T-cell activation and it has been implicated in the hypo- and hyperactivity of T lymphocytes related to HIV infection and systemic lupus erythematosus, respectively (20, 21, 47). KV1.3 has also been implicated in the pathology of these diseases (11, 30, 31). Yet, neither the PKA isoform regulating KV1.3 activity in human T lymphocytes nor the signaling pathways involved have been described. PKA is a holoenzyme composed of two catalytic (C) and two regulatory (R) subunits, and it exists in two isoforms defined by their respective R subunits (RIα/RIβ and RIIα/RIIβ; Ref.39). Each R subunit has two cooperative cAMP binding sites. The binding of cAMP to these sites results in the release of two free C subunits, which are then able to phosphorylate serine and threonine residues on specific substrates. Both isoforms of PKA, PKAI and PKAII, are expressed in T lymphocytes. PKAI, but not PKAII, downregulates T-cell function (45). Specifically, PKAI activation inhibits T-cell proliferation, cytolitic activity, and production of cytokines such as IL-2 and IFNγ. PKAI inhibits T-cell receptor (TCR) signaling by suppressing the activity of Lck (37). Specifically, PKAI activates Csk (COOH-terminal Src kinase), which in turn inhibits Lck via phosphroylation of its COOH-terminal inhibitory tyrosine Y505 (28).
It has been reported that KV1.3 in T lymphocytes exists in a macrocomplex with the membrane-associated guanylate kinase protein disc large 1 (Dlg1) and Lck (18). The true functional significance of the Dlg1 association to KV1.3 in T cells is not known. It has been speculated that Dlg1 is important to stabilize the channel membrane complex and serves as scaffolding for Lck activity, thus raising the possibility that KV1.3 may be functionally associated to PKA through the Dlg1/Lck complex.
The studies reported here where undertaken to determine the PKA isoform that modulates KV1.3 function and to define if the Dlg1/Lck complex regulates the functional interaction between KV1.3 and PKA in human T lymphocytes.
MATERIALS AND METHODS
Cells.
Primary CD3+ T cells were isolated from venous blood from consenting healthy donors using E-rosetting (StemCell Tech, Vancouver, Canada) followed by Ficoll-Paque density gradient centrifugation (ICN Biomedicals, Aurora, OH; Ref. 30). The cells were maintained in RPMI medium supplemented with 10% pooled male human AB serum (Intergen, Milford, MA), 200 U/ml penicillin, 200 μg/ml streptomycin, and 10 mM HEPES. To generate activated T cells, T cells were stimulated with 4 μg/ml phytohemaglutinin (Sigma-Aldrich, St. Louis, MO) in the presence of autologous peripheral blood mononuclear cells for >48 h. Jurkat T cells (American Tissue Culture Collection, Masassas, VA) were maintained in RPMI 1640 medium supplemented with 10% FBS (Fisher Scientific, Pittsburgh, PA), 200 U/ml penicillin, 200 μg/ml streptomycin, and 10 mM HEPES (10). These studies were approved by the University of Cincinnati Institutional Review Board.
Transfection of small interfering RNAs and clones.
The Dlg1 small interfering (siRNA) was designed based on previously described Dlg1 siRNAs with the addition of a mismatch on the 3′-end of the sense strand to enhance the silencing effect (13, 38). The sense strand is gguccggaagauaucauu (note the mismatched bp at nucleotide 19), and the antisense sequence is gguaucuugcuuccggaccuu (Sigma Aldrich). PKAI and PKAII siRNAs were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). A scramble RNA (Santa Cruz Biotechnology) was used as the negative control. Transient transfection was performed using either the Amaxa Nucleofector technology (Lonza, Cologne, Germany) or X-tremeGENE transfection reagent (Roche, Indianapolis, IN). The Amaxa transfection was done using 10 × 106 T cells, siRNAs (0.6 μg siPKAI and siPKAII, and 3–30 μg siDlg1), and p-enhanced green fluorescent protein (EGFP) in a 10:1 ratio and program T-14 or U-14, according to the manufacturer's instructions. Constitutively active Lck (LckY505F; kind gift of C. T. Baldari, University of Siena, Italy) subcloned into the GFP-expressing vector pIRES2-eGFP was transected at 0.5 μg/10 × 106 cells, as previously described (43). The pIRES2-eGFP empty vector served as control (12). The efficiency of transfection of the nucleofection experiments was determined by flow cytometry using a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA) and FCS3 flow cytometric analysis software (DeNovo Software, Los Angeles, CA). The transfection efficiency of Jurkat cells transfected with PKAII siRNAs and pEGFP at a 10:1 ratio was 49.6 ± 8.4% (n = 4) of EGFP-positive cells. X-tremeGENE transfection was performed according manufacturer's instruction using PKAI siRNA and pEGFP in a 10:1 ratio. Jurkat cells transfected were ≤10th passage.
Electrophysiology.
Patch-clamp experiments were performed in whole cell configuration as previously described (10, 43). KV1.3 currents were recorded with an external solution of the following composition (in mM): 150 NaCl, 5 KCl, 2.5 CaCl2, 1.0 MgCl2, 10 glucose, and 10 HEPES pH 7.4. The pipette solution was composed of the following (in mM): 134 KCl, 1 CaCl2, 5 mM ATP-Na2, 10 EGTA, 2 MgCl2, and 10 HEPES pH 7.4, estimated free Ca2+ concentration of 10 nM (10). In some experiments ATP-Na2 was replaced with 10 mM NaCl. Experiments were performed using Axopatch 200B amplifier (Axon Instruments, Foster City, CA). The digitized signals were stored and analyzed using pClamp 9 software (Axon Instruments). Experiments were conducted at room temperature (22°C). The voltage-dependent activation was determined by converting the current into conductance (G) using the following equation: G = I/(V − EK), where the calculated EK, the Nernst K+ equilibrium potential, was −84 mV. The normalized conductances at different potentials were fitted to a Boltzmann equation G = Gmax/{1 + exp[−(V − V1/2)/k]}, where Gmax is the maximum conductance, V1/2 is the test potential at which the channel has a half-maximal conductance, and k is the parameter that represents the slope of the activation curve. To measure KV1.3 current inactivation, the current decay was fitted with a single exponential equation.
Semiquantitative RT-PCR.
Total RNA was isolated from siRNA transfected cells and RT was performed according to commercial instructions using 1–3 μg of total RNA (GeneAmp; Perkin Elmer; Ref. 9). PCR was performed at a number of cycles before saturation (30 cycles) with commercially designed specific primers for Dlg1 (Santa Cruz, no. sc-36452-PR). Custom-designed primers for GAPDH were purchased from Invitrogen (Carlsbad, CA). The GAPDH primer sequences are reported in Table 1. PCR was performed at an annealing temperature of 58°C. PCR products were quantified by densitometric analysis (Odyssey version 2.0 software; Licor Biosciences, Lincoln, NE).
Table 1.
Primer sequences
| Primer | Sequence |
|---|---|
| PKARI | AGAAAGCAGGCACTCGTACA (s) |
| PKARI | AAAGGAGACCGAAAACATGG (as) |
| PKARII | CTTGGAAGTTCCAGTTCCTAG (s) |
| PKARII | GTAGCAACAATGGTAGCAGCTC (as) |
| PKACα1 | AGAAGGGCAGCGAGCAGGAGA (s) |
| PKACα1 | CGCGCTTGGCGAAACCGAAG (as) |
| PKACβ1 | AATTGGAAGGTTTTGCTAGCCGGT (s) |
| PKACβ1 | ACCGTGCATGGGGCTCACTG (as) |
| PKACβ2 | AGTTGTCCCAGACTGTGGAGTGGC (s) |
| PKACβ2 | GCAGCAGGTTCCGTAGAAGGTCC (as) |
| GAPDH | ACCACAGTCCATGCCATCAC (s) |
| GAPDH | TCCACCACCCTGTTGCTGTA (as) |
s, Sense; as, antisense.
RT-quantative PCR.
RT-quantitative (q)PCR was performed to determine the mRNA levels of PKA-RI, PKA-RII, and the three catalytic subunits of PKA, PKACα, PKACβ1 and PKACβ2. The custom-made primers used are reported in Table 1. Primers were purchased from Integrated DNA Technologies (Coralville, IA). Total RNA was isolated using E.Z.N.A. total RNA isolation kit (Omega Biotek, Norcross, GA) as per the manufacturer's instructions. One microgram RNA was used to synthesize cDNA using TaqMan reverse transcription reagents (Applied Biosystems) as per the manufacturer's instructions. RT-qPCR reaction was set up in a 48-well plate by addition of 40 ng cDNA, 1× Fast SYBR Green Master Mix (Applied Biosystems), and 1 μl of the sense and anti-sense primers. All samples were run in quadruplicates. RT-qPCR reaction was cycled in Applied Biosystems StepOne real-time PCR system (Applied Biosystems). The reactions were cycled under the following conditions: 95°C for 20 s, followed by 40 cycles at 95°C for 3 s and 60°C for 30 s. Melting curves were performed to verify the specificity of the product, and GAPDH was used as an internal control. Quantification of the PKA subunits was done as previously described (5). Briefly, CT values were measured and were normalized against measured CT values for GAPDH and subsequently the ΔΔCT values were calculated. Relative quantity values, representing the fold change in PKA subunit gene expression compared with controls, were calculated as the 2−ΔΔCT values.
Immunohistochemistry.
Jurkat T cells, either nonactivated or preactivated on anti-CD3 antibody (Johnson & Johnson, New Brunswick, NJ)-precoated flasks, were plated on poly-l-lysine-coated coverslips. Cells were then fixed with 3.7% formaldehyde in PBS, permeabilized, and blocked using FBS and Triton X-100. Triple-protein staining was performed by incubating the cells simultaneously with primary antibodies: rabbit anti-Dlg1 (Santa Cruz, no. sc-25661), goat anti- KV1.3 (Santa Cruz, no. sc-17241), and either mouse anti-PKA-RI (BD Biosciences, no. 610165) or anti-PKA-RII (BD Biosciences, no. 612242) overnight at 4°C. After being washed, cells were incubated with the following secondary antibodies: Alexa Fluor 488 anti-rabbit, Alexa Fluor 546 anti-goat, and Alexa Fluor 647 anti-mouse (Invitrogen, Carlsbad, CA). Cells were visualized by confocal microscopy (Axioscope; Carl Zeiss Microimaging) using a ×63 oil objective lens. Data were obtained using the “Multi Track” option of the microscope to exclude cross talk between detection channels. Linescan analysis of the confocal images was performed with MetaMorph software (Molecular Devices, Sunnyvale, CA).
Western blotting.
Total lysate was prepared using a lysis buffer of the following composition: 10 mM Tris, 1 mM EDTA, 1 mM PMSF, 1% Triton X-100, 2 μg/ml aprotinin, 2 μg/ml leupeptine, and 1 mM sodium orthovanadate, as previously described (43). Cell lysates were collected, sonicated for 30 s, and centrifuged at 14,000 rpm for 10 min at 4°C. Proteins were submitted to SDS-PAGE and immunoblotted, treated with fluorescent-conjugated secondary antibody, and scanned on an Odyssey near-infrared scanner. The antibodies used for western blotting were anti-PKA-RI (BD Biosciences, no. 610165), anti-PKA-RII (BD Biosciences, no. 612242), and anti-Dlg1 (Santa Cruz, no. sc-9961).
Data analysis.
All data are presented as means ± SE, unless otherwise indicated. Statistical analyses were performed using Student's t-test (paired or unpaired), unless otherwise specified; P ≤ 0.05 was defined as significant.
Chemicals.
8-Bromoadenosine 3′,5′-cyclic monophosphate (8-BrcAMP) and protein kinase inhibitor PKI6–22 were purchased from Sigma. ShK was purchased from Bachem (Torrence, CA). Chemicals were purchased from Sigma, unless indicated otherwise.
RESULTS
PKA modulates the activity of KV1.3 channels in human T lymphocytes.
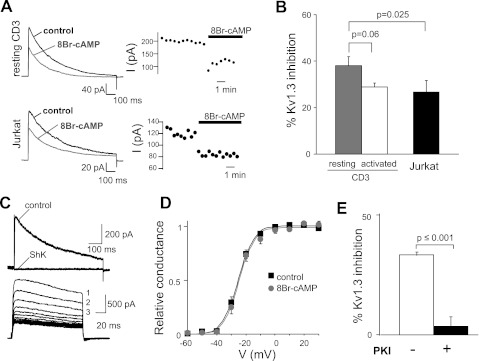
The effect of PKA on KV1.3 was tested in human T cells. Activation of PKA by 8-BrcAMP (a membrane-permeable cAMP analog) inhibits KV1.3 currents in resting and activated primary T and Jurkat cells (Fig. 1, A and B). KV1.3 currents are characterized by their sensitivity to the specific KV1.3 blocker ShK and their characteristic biophysical properties (voltage-dependence and cumulative inactivation; Fig. 1, C and D) (10, 43). KV1.3 inhibition by 8-BrcAMP was not due to a shift in voltage-dependent activation (Fig. 1D). Similar voltage-dependent activations were observed before (control) and after steady-state inhibition by 8-BrcAMP both in resting T and Jurkat cells. The V1/2, the voltage at which half of the channels are activated, and k values, indicative of the steepness of the voltage dependence, were similar in control and 8-BrcAMP treated cells. The values of V1/2 for resting T cells in control and 8-BrcAMP were −25.3 ± 1.1 mV (n = 12) and −24.5 ± 1.3 mV (n = 8; P = 0.6), respectively. The k values for resting T cells in control and 8-BrcAMP were 4.6 ± 0.3 mV (n = 12) and 4.7 ± 0.5 mV (n = 8; P = 0.8), respectively. The values of V1/2 for Jurkat cells in control and 8-BrcAMP were −39.9 ± 1.9 mV (n = 14) and −40.6 ± 2.6 mV (n = 9; P = 0.8), respectively. The k values for Jurkat cells in control and 8-BrcAMP were 4.7 ± 0.7 mV (n = 14) and 5.2 ± 0.5 mV (n = 9; P = 0.6), respectively. Furthermore, 8-BrcAMP did not alter KV1.3 current inactivation. The inactivation time constants (τ) for resting T cells in control and 8-BrcAMP were 199.9 ± 21.6 and 184.0 ± 21.9 ms (n = 7; P = 0.61), respectively. The τ for Jurkat cells in control and 8-BrcAMP were 339.9 ± 16.9 and 326.3 ± 19.2 ms (n = 12; P = 0.60), respectively. These values were similar to those previously reported in the literature (3, 27, 31). The effect of 8-BrcAMP was prevented by the PKA catalytic subunit specific inhibitor PKI6–22 both in primary T cells (Fig. 1E) and Jurkat (16, 48). In Jurkat cells, the percentage of KV1.3 current inhibition by 8-BrcAMP in the absence and presence of PKI6–22 was 50.1 ± 7.2% (n = 9) and 7.8 ± 11.8% (n = 8, P = 0.007), respectively.
Fig. 1.
Activation of PKA significantly decreases KV1.3 activity. A: representative macroscopic KV1.3 currents (I) in resting CD3+ T cells (left top) and Jurkat cells (left bottom) before and after extracellular application of 8-bromoadenosine 3′,5′-cyclic monophosphate (8-BrcAMP; 1 mM). Corresponding changes in KV1.3 peak currents with time after addition of 8-BrcAMP are shown at right. Currents were elicited by depolarizing pulses to +50 mV from a holding potential (HP) of −80 mV every 30 s. B: average inhibition of KV1.3 currents by 8-BrcAMP in resting (n = 7) and activated (n = 10) CD3+ cells and in Jurkat (n = 10) cells. C: characteristic electrophysiological and pharmacological properties of KV1.3 currents in Jurkat T cells. Top: representative KV1.3 currents recorded before and after steady-state inhibition by ShK (10 nM). KV1.3 currents were elicited by depolarizing voltage steps to +50 mV from −80-mV HP every 30 s. Bottom: cumulative inactivation of KV1.3 channels. Cumulative inactivation was induced by consecutive depolarizing pulses to +50 mV from −80-mV HP applied every s. Current amplitude progressively decreased with each successive pulse (indicated by progressive numbers). D: average conductance (relative to the maximum conductance at +50 mV)-voltage curves for KV1.3 in resting CD3+ cells before (control) and after addition of 8-BrcAMP. Currents were elicited by depolarizing steps from −60 mV in 10-mV increment (−80-mV HP) every 30 s. E: average inhibition of KV1.3 currents by 8-BrcAMP in resting CD3+ cells with and without addition of 25 μM of the protein kinase inhibitor PKI6–22 in the pipette solution (n = 10 without PKI6–22 and n = 8 with PKI6–22).
Overall, these findings indicate that PKA inhibits KV1.3 channels in human T cells. Since multiple PKA isoforms are expressed in T cells, we proceeded to determine whether PKAI or PKAII was involved in the regulation of KV1.3 channels (32).
PKAI selectively inhibits the activity of KV1.3 channels in human T lymphocytes.
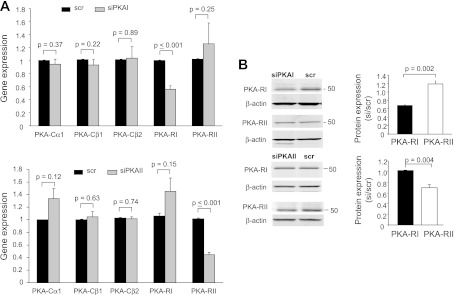
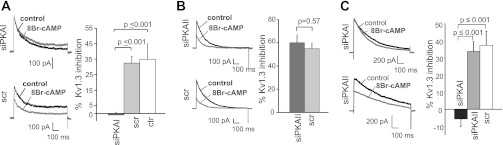
PKA is composed of two catalytic (C) and two regulatory (R) subunits, and it exists in two isoforms defined by their respective R subunits (RIα/RIβ and RIIα/RIIβ; Ref. 39). We used siRNAs designed against the regulatory subunit I-α (RIα) of PKAI (siPKAI) and the regulatory subunit II-α (RIIα) of PKAII (siPKAII) to knock down PKAI and PKAII, respectively (Fig. 2). The selectivity of siRNAs was established in Jurkat cells. Jurkat cells are ideal for biochemical experiments, as they constitute an unlimited source of cells providing material in sufficient quantity to make proteins and they are transfected more easily than primary T cells. RT-qPCR experiments indicated that PKAI siRNAs selectively decrease the mRNA levels of PKA-RI by 44% but not PKA-RII (Fig. 2A). Similarly, PKAII siRNAs selectively decrease the mRNA levels of PKA-RII by 55% but not PKA-RI (Fig. 2A). Furthermore, neither siRNAs affect the expression of the PKA catalytic subunits expressed in T lymphocytes: PKACα1, PKACβ1, and PKACβ2 (Fig. 2A) (15). Consequently, PKAI siRNAs inhibited the expression of PKA-RI but not PKA-RII protein (Fig. 2B). Similarly, PKAII siRNAs inhibited the expression of PKA-RII but not PKA-RI (Fig. 2B). Overall we measured a 35% average decrease in PKA-RI protein expression 48 h after transfection. PKA-RII protein expression was reduced by 30%. If we take into account that the efficiency of transfection by nucleoporation of Jurkat T cells is ∼50%, as reported in the materials and methods, we anticipate a significant downregulation of PKA-RIα and PKA-RIIα in the transfected cells. The response to 8-BrcAMP was then tested in Jurkat cells after 48 h transfection with siPKAI and compared with cells transfected with the scramble RNA (scr, control; Fig. 3A). The effect of siPKAII was determined in a separate set of experiments (Fig. 3B). Since the cells were transfected with siRNAs and a GFP plasmid at a 10:1 ratio, only the transfected cells, recognized by the GFP fluorescence, underwent patch clamping. Inhibition of KV1.3 by 8-BrcAMP was abolished in siPKAI transfected cells (Fig. 3A). On the contrary, the effect of 8-BrcAMP in siPKAII transfected cells was comparable with that of control cells (Fig. 3B). The selective inhibitory role of PKAI was confirmed in primary resting T cells with knockdown experiments (Fig. 3C). Electrophysiological experiments in primary T cells revealed that the inhibition of KV1.3 currents by 8-BrcAMP was abolished only in siPKAI-transfected cells, while siPKAII-transfected cells showed a KV1.3 inhibition comparable with control cells (Fig. 3C). Overall, these findings indicate that PKAI activation selectively inhibits KV1.3 current in T lymphocytes. This same isoform was shown to inhibit T-cell function (40, 44).
Fig. 2.
Specificity of small interfering (si)RNAs against PKA-RIα (siPKAI) and PKA-RIIα (siPKAII). A: siPKAI and siPKAII selectively downregulate the corresponding regulatory subunits but not the catalytic subunits. Gene expression of PKA catalytic (PKACα1, PKACβ1, and PKACβ2) and regulatory (PKA-RI and PKA-RII) subunits was determined by RT-quantitative (q)PCR in Jurkat cells 48 h after nucleofection with siRNA against PKA-RIα (siPKAI; top) and PKA-RIIα (siPKAII; bottom) or scrambled sequence siRNA (scr). Gene expression for the PKA subunits is represented as the fold change in PKA subunit gene compared with the housekeeping gene GAPDH. Data are the average of separate experiments each in quadruplicate: PKACα1 (n = 5), PKACβ1 (n = 4), PKACβ2 (n = 4), PKA-RI (n = 5), and PKA-RII (n = 3) in cells transfected with siPKAI. For siPKAII transfected cells, the expression of each PKA subunit was determined in 3 separate experiments. Data are normalized to scr controls (n = 6 for siPKAI and n = 3 for siPKAII). B: PKA-RI, but not PKAII, protein expression was downregulated by siPKAI. Left: immunoblotting of PKAI and PKAII in scr- and siPKAI-treated cells. Corresponding β-actin is shown at bottom. Predicted molecular weights for PKA-RI and PKA-RII are 48 and 51 kDa, respectively. Right: RI and RII protein levels (normalized to β-actin) are reported as relative to scr (n = 3; means ± SD). B: siPKAII selectively downregulates PKA-RII protein expression in Jurkat cells. Representative immunoblot of PKAI (top) and PKAII (bottom) in scr- and siPKAII-treated cells are shown at left. Corresponding β-actin is shown underneath. Average band intensities normalized to β-actin and relative to scr are reported at right (n = 3).
Fig. 3.
PKAI inhibits KV1.3 currents in human T cells. A: downregulation of PKAI abolishes the effect of 8-BrcAMP on KV1.3 channels in Jurkat cells. KV1.3 currents were recorded in Jurkat cells after a 48-h transfection with either siPKAI or scr. Representative traces before (black) and after application of 8-BrcAMP (1 mM, gray) are shown in the left panel. Currents were elicited by depolarizing pulses to +50 mV from a HP of −80 mV every 30 s. Average inhibition of KV1.3 current is shown at right for siPKAI (dark gray, n = 9)- and scr-transfected (light gray, n = 6) and nontransfected Jurkat cells (control, white; n = 6). B: downregulation of PKAII has no influence on the 8-BrcAMP effect on KV1.3 in Jurkat cells. Left: representative traces before (black) and after 8-BrcAMP (1 mM) application (gray) in siPKAII- and scr-transfected Jurkat cells. Currents were elicited as described in A. Right: average inhibition of KV1.3 currents in siPKAII (dark gray; n = 7)- and scr-transected cells (light gray; n = 6). C: PKAI inhibits KV1.3 currents in human primary resting T cells. KV1.3 currents were recorded in CD3+ cells after a 48-h transfection with siPKAI, siPKAII, or scr. Left: representative currents before (black) and after application of 8-BrcAMP (1 mM, gray). Currents were elicited as described in A. Average inhibition of KV1.3 currents are shown at left for siPKAI (dark gray; n = 8, 3 donors)-, siPKAII (light gray; n = 7, 3 donors)-, and scr-transfected CD3+ cells (white; n = 8, 3 donors).
It has been shown that PKAI effect on T-cell activity occurs via activation of Csk which in turn phosphorylates a COOH-terminal inhibitory tyrosine Y505 of Lck and inhibits it (28). Lck exists in active and inactive forms linked to the phosphorylation state of key tyrosines. Phosphorylation of Y394, located in the kinase domain, potentiates the kinase activity while phosphorylation of Y505 at the carboxy-terminal domain negatively regulates kinase activity. Since Lck is known to regulate KV1.3, we conduct further studies to determine whether the effect of PKAI on KV1.3 channels in T cells is mediated by Lck.
Lck mediates PKAI inhibition of KV1.3 channels.
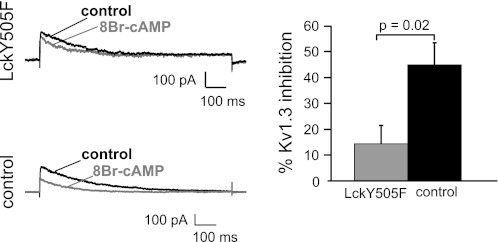
To study the role of Lck on the effect of PKAI on KV1.3 channels, we determined whether expression of a constitutively active mutant of Lck (LckY505F) reduced the inhibition of KV1.3 channels by 8-Br-cAMP (Fig. 4). This Lck mutant was obtained by replacing Y505 with F; thus it lacks the phosporylation site which is targeted by the PKAI/Csk pathway. This same construct was used by us to study the response to hypoxia of KV1.3 channels and was effective in conferring O2 sensitivity to KV1.3 (43). Our results indicated that Lck is required in the PKAI-regulation of KV1.3 channels since the effect of 8-BrcAMP was significantly decreased in LckY505F-transfected cells compared with the control (Fig. 4).
Fig. 4.
Constitutively active Lck (LckY505) reduces the effect of PKAI on KV1.3 channels. KV1.3 currents were recorded in Jurkat cell after a 24-h transfection with either LckY505F or the empty vector (control). Representative currents before (black) and after 8-BrcAMP application (1 mM, gray) are shown at left. Currents were elicited by depolarizing pulses to +50 mV every 30 s (HP = −80 mV). Average inhibition of KV1.3 current is shown in at right for LckY505F (gray; n = 5)- and empty vector-transfected Jurkat cells (control, black, n = 6).
The association of Lck to KV1.3 in Jurkat T cells is provided by Dlg1, a scaffolding proteins that binds KV1.3 at its PDZ domain and Lck at its SH3 domain (18). Further experiments were conduced to determine whether Dlg1 is also necessary for the response of KV1.3 channels to PKA.
Dlg1 is required for functional association of PKAI to KV1.3.
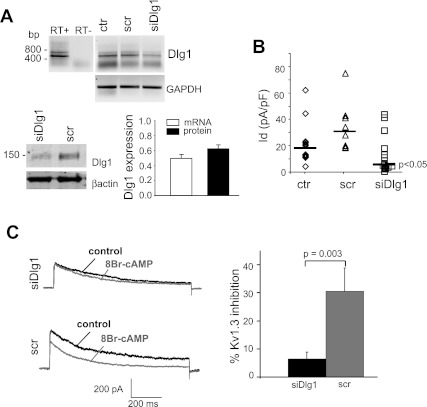
To determine whether Dlg1 is also involved in the response of KV1.3 to PKAI, we studied whether PKA activation is effective after Dlg1 downregulation by siRNA (Fig. 5). Transfection of Jurkat T cells with Dlg1 siRNA (siDlg1) reduced Dlg1 expression by 40% compared with cells transfected with a scrRNA (Fig. 5A). There was no difference in Dlg1 mRNA levels in control (ctr) and scr-transected cells. The average Dlg1 mRNA band intensities (normalized for GAPDH) were 0.36 ± 0.26 for ctr (n = 4) and 0.37 ± 0.22 for scr (n = 2; means ± SD). The KV1.3 currents in siDlg1-transfected cells, recognized by the eGFP expression, were also reduced by 50% (Fig. 5B). There was no change in cell capacitance in transfected cells. Scr-transfected and siDlg1-transfected cells had a capacitance of 3.2 ± 0.8 pF (n = 9) and 3.3 ± 1.5 pF (n = 19), respectively. The effect of 8-BrcAMP was then measured in siDlg1-transfected cells. We observed that the response to 8-BrcAMP was significantly reduced in siDlg1 transfected cells, indicating that Dlg1 is necessary for productive regulation of KV1.3 by PKAI. In this set of experiments, the KV1.3 currents were also reduced from 69.31 ± 13.8 pA/pF (n = 5) in scr to 44.9 ± 6.5 pA/pF (n = 9) in siDlg1, although this reduction was not significant, presumably because of the limited number of experiments.
Fig. 5.
PKAI effect on KV1.3 is reduced by Dlg1 downregulation. A: expression of Dlg1 is reduced by Dlg1 siRNAs. Top: RT-PCR amplifies a band of the predicated molecular size for Dlg1 (446 bp) with no genomic contamination (−RT). Representative RT-PCR bands for Dlg1 obtained at a number of cycles before saturation are reported for control cells (ctr, n = 4), and cells after a 48-h transfection with scr RNA (src, n = 2) and Dlg1siRNAs (n = 4). Control cells went through the transfection procedure without any RNAs. Dlg1 protein levels in scr and siDlg1-transfected cells are shown at bottom left. This blot represents 3 separate experiments. Average Dlg1 mRNA band intensity (normalized to GAPDH and reported as relative to control cells) and protein levels (normalized to β-actin and reported as relative to scr) are shown at right. B: KV1.3 current density in ctr and src- and siDlg1-treated cells (n = 19). Individual current densities and the corresponding median (solid line) are reported for ctr (◊) and scr (▵; n = 9)- and siDlg1-transfected cells (□; n = 14). Statistical analysis, performed with one-way ANOVA, showed significant differences between siDlg1 and both scr and ctr but no statistical difference between scr and ctr. C: KV1.3 currents were recorded in Jurkat cells transfected with either siDlg1 or scr. Left: representative traces before and after 8-BrcAMP (1 mM) application are shown. Average KV1.3 inhibition is shown at right (n = 9 for siDlg1 and 5 for scr). All these experiments were performed 48 h after transfection. Currents were elicited by depolarizing pulses to +50 mV from a HP of −80 mV every 30 s.
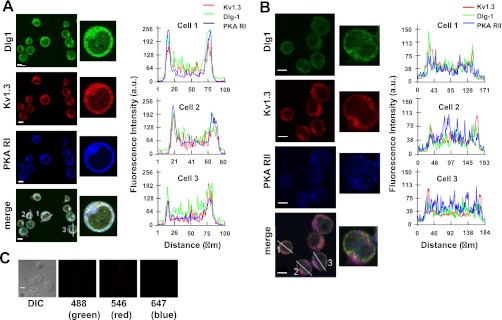
It is well established that spatial proximity is necessary for PKAI to regulate its target proteins (37, 44). Furthermore, it is known that Dlg1 links KV1.3 to Lck. It is thus possible that the KV1.3/Dlg1 macrocomplex in T cells is in close proximity with PKAI. Immunohistochemistry experiments showed the colocalization of Dlg1, KV1.3, and PKAI but not PKAII, in Jurkat cells (Fig. 6). This colocalization is clearly visible in the merge images and it is further confirmed by the correspondence of the peak fluorescence intensities of Dlg1, KV1.3, and PKAI in the linescan graphs (Fig. 6A).
Fig. 6.
Colocalization of Dlg1 and KV1.3 with PKA-RI. A: Dlg1 and KV1.3 colocalize with PKA-RI. Left: Jurkat cells were stained simultaneously with KV1.3 (red), Dlg1 (green) and PKA-RI (blue) antibodies. Merge image is shown at bottom. White color denotes colocalization of the three proteins. A representative cell (#1) is shown at higher magnification in the middle. Colocalization of Dlg1, KV1.3, and PKA-RI was determined by linescan analysis as described in materials and methods. Right: line scan analyses are plotted as Dlg1 (green), KV1.3 (red), and PKA-RI (blue) fluorescent intensity (y-axis) vs. the length of the lines drawn across the cells and shown in the merge image where the corresponding cells are also labeled. In this graph, the outmost peaks correspond to the cell membrane. Correspondence of the peak for each line is indicative of the colocalization of the proteins. B: Dlg1 and KV1.3 do not colocalize with PKA-RII. Left: Jurkat cells were stained with KV1.3 (red), Dlg1 (green), and PKAII (blue) antibodies as outlined in materials and methods. Merge image is shown at bottom. A representative cell (#2) is shown at higher magnification at middle. Colocalization of Dlg1, KV1.3, and PKA-RII was determined by linescan analysis and plotted as described in A, right. As seen in the line scan graphs, the peaks of PKA-RII fluorescence intensity do not correspond to those of Dlg1 and KV1.3. C: background fluorescence. Cells underwent the same experimental procedure as used in A and B but without primary antibodies. Differential interference contrast image and the corresponding images for each Alexa secondary antibody (reported at the bottom) are shown. For A-C, the scale bars = 5 μm.
DISCUSSION
In this study, we showed that PKAI inhibits KV1.3 channels in T cells and that this effect is mediated by the Dlg1-Lck complex. Overall, these findings indicate that KV1.3 is part of the immunosuppressive signaling cascade downstream of cAMP/PKA.
We herein report that PKA, and specifically its PKAI isoform, inhibits KV1.3 channels in human T cells. Although an inhibitory effect of cAMP on KV1.3 has been reported by others (6, 33, 41), the opposite (8) and no effect (8, 22) have also been reported. Differences in recording conditions, cell types, and activators of cAMP can account for the different results. We observed a comparable inhibition by 8-BrcAMP of KV1.3 currents in Jurkat as well as primary resting and activated T cells in the same experimental conditions. Furthermore, we report for the first time that the PKAI isoform is solely responsible for KV1.3 inhibition. This effect may contribute to the immunosuppressive properties of PKAI because inhibition of KV1.3 reduces Ca2+ signaling (2).
Our findings indicate that the effect of PKAI on KV1.3 requires Dlg1, which provides association with Lck (18). Indeed it has been shown that KV1.3 contains a PDZ binding site at the carboxy-terminal domain for binding to Dlg1 and that Lck binds to the SH3 domain of Dlg1 thus guaranteeing physical proximity between Lck and its effector KV1.3 (18). While in other cell types membrane-associated guanylate kinase proteins like Dlg1 have been shown to regulate localization and clustering of Kv1 channels, our studies indicate that in T cells Dlg1 allows KV1.3 regulation by PKAI. Moreover, these data show that downregulation of Dlg1 expression is associated with a decrease in KV1.3 current density. A possible explanation is that Dlg1 may be important to stabilize KV1.3 surface expression. Although there are other examples of PDZ proteins regulating Kv1 channel surface expression via a clustering mechanism that suppresses their internalization, it appears that this phenomenon might be different in different cell types because PDZ proteins display different channel targeting and subcellular localization (19, 26, 46). However, it is also possible that reduced KV1.3 currents in siDlg1 transfected cells may be due to the loss of constitutive Lck regulation, which was shown to increase KV1.3 activity in human T cells (43). Our data clearly show that the effect of PKAI on KV1.3 is mediated by Lck. A role for Lck on the effect of cAMP/PKAI on TCR-proximal signaling has been clearly identified. It is well established that in T lymphocytes PKAI exists in a macrocomplex with Csk (28). Csk is a negative regulator of Lck as it phosphorylates a COOH-terminal inhibitory tyrosine Y505 and through this mechanism downregulates T-cell activity. Our finding indicates that a similar mechanism mediates the effect of PKAI on KV1.3. Indeed, overexpression of a constitutively active Lck mutant lacking the phosphorylation site targeted by Csk significantly reduced the response of KV1.3 to 8-BrcAMP.
Overall, these findings presented herein point toward KV1.3 being part of the TCR-proximal pathway that cAMP utilizes to regulate T-cell immune function. The questions thus arise as to when and where do optimal conditions occur during the T-cell activation process for KV1.3 to undergo PKAI regulation. It is well established that the compartmentalization of the cAMP/PKA system is important to determine the specificity of this pathway (44). The IS appears to be the ideal site for PKAI regulation of KV1.3. Interestingly, it has been shown that all the molecules involved in PKA-I/ KV1.3 interaction localize at the IS, which is also a site of lipid raft aggregation (23, 35–37, 50). In addition, the IS is also a site of localized increase in Ca2+. It has been shown that stimulation of the TCR leads to transient elevation in local cAMP concentrations and the recruitment of PKA in the IS (1, 24). Localized production of cAMP at the IS might be facilitated by the elevated Ca2+ influx at this site, as Ca2+ is a known regulator of adenylyl cyclase (AC) activity, which leads to the production of cAMP (49). Maximum Ca2+ influx occurs at the IS where CRAC channels accumulate (25). It has been speculated that in T cells there might be a direct association between Ca2+-sensitive ACs and specific components of the CRAC channel (e.g., STIM 1 or Orai1) that controls AC activity upon TCR stimulation (49). However, no direct evidence for any such interaction has yet been obtained. Therefore, the IS may indeed provide the correct compartmentalization of PKAI and KV1.3 and/or a tightly controlled cAMP generation.
Overall, these studies indicate that KV1.3 is downregulated by the cAMP pathway and raise the possibility that the PKAI/ KV1.3 functional interaction may be important to regulate T-cell immune function.
GRANTS
This project was supported by National Institutes of Health Grants 2R01-CA-095286, 1R21-AI-083076, and AHA-0855457D.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.K., V.K., and L.C. conception and design of research; Z.K., V.K., S.M.G., L.N., A.A.C., and A.H.F. performed experiments; Z.K., V.K., S.M.G., L.N., A.A.C., and A.H.F. analyzed data; Z.K., V.K., A.A.C., and L.C. interpreted results of experiments; Z.K., V.K., S.M.G., L.N., and A.A.C. prepared figures; Z.K. and L.C. drafted manuscript; Z.K., V.K., S.M.G., L.N., A.A.C., A.H.F., and L.C. approved final version of manuscript; A.A.C., A.H.F., and L.C. edited and revised manuscript.
ACKNOWLEDGMENTS
We acknowledge the contribution of Dr. S. A. Nicolaou with the initial experiments with Dlg1 siRNAs. We also thank Kelsey Dillehay and Sue Vergamini for technical assistance.
REFERENCES
- 1. Abrahamsen H, Baillie G, Ngai J, Vang T, Nika K, Ruppelt A, Mustelin T, Zaccolo M, Houslay M, Tasken K. TCR- and CD28-mediated recruitment of phosphodiesterase 4 to lipid rafts potentiates TCR aignaling. J Immunol 173: 4847– 4858, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev 231: 59– 87, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cahalan MD, Chandy KG, DeCoursey TE, Gupta S. A voltage-gated potassium channel in human T lymphocytes. J Physiol 358: 197– 237, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cayabyab FS, Khanna R, Jones OT, Schlichter LC. Suppression of the rat microglia Kv1.3 current by src-family tyrosine kinases and oxygen/glucose deprivation. Eur J Neurosci 12: 1949– 1960, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Chimote AA, Kuras Z, Conforti L, Disruption of Kv1.3 Channel forward vesicular trafficking by hypoxia in human T lymphocytes. J Biol Chem 287: 2055– 2067, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choquet D, Sarthou P, Primi D, Cazenave PA, Korn H. Cyclic AMP-modulated potassium channels in murine B cells and their precursors. Science 235: 1211– 1214, 1987 [DOI] [PubMed] [Google Scholar]
- 7. Chung I, Schlichter LC, Native Kv1.3 channels are upregulated by protein kinase C. J Membr Biol 156: 73– 85, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Chung I, Schlichter LC. Regulation of native Kv1.3 channels by cAMP-dependent protein phosphorylation. Am J Physiol Cell Physiol 273: C622– C633, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Conforti L, Millhorn DE. Selective inhibition of a slow-inactivating voltage-dependent K+ channel in rat PC12 cells by hypoxia. J Physiol 502: 293– 305, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conforti L, Petrovic M, Mohammad D, Lee S, Ma Q, Barone S, Filipovich AH. Hypoxia regulates expression and activity of Kv1.3 channels in T lymphocytes: a possible role in T cell proliferation. J Immunol 170: 695– 702, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Dellis O, Bouteau F, Guenounou M, Rona JP. HIV-1 gp160 decreases the K+ voltage-gated current from Jurkat E6.1 T cells by up-phosphorylation. FEBS Lett 443: 187– 191, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Di Somma MM, Nuti S, Telford JL, Baldari CT. p56lck plays a key role in transducing apoptotic signals in T cells. FEBS Lett 363: 101– 104, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Donze O, Picard D. RNA interference in mammalian cells using siRNAs synthesized with T7 RNA polymerase. Nucleic Acids Res 30: e46, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fadool DA. Tyrosine phosphorylation downregulates a potassium current in rat olfactory bulb neurons and a cloned Kv1.3 channel. Ann NY Acad Sci 855: 529– 532, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Funderud A, Aas-Hanssen K, Aksaas AK, Hafte TT, Corthay A, Munthe LA, Ørstavik S, Skålhegg BS. Isoform-specific regulation of immune cell reactivity by the catalytic subunit of protein kinase A (PKA). Cell Signal 21: 274– 281, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Glass DB, Lundquist LJ, Katz BM, Walsh DA. Protein kinase inhibitor-(6–22)-amide peptide analogs with standard and nonstandard amino acid substitutions for phenylalanine 10. Inhibition of cAMP-dependent protein kinase. J Biol Chem 264: 14579– 14584, 1989 [PubMed] [Google Scholar]
- 17. Gulbis JM, Mann S, MacKinnon R. Structure of a voltage-dependent K+ channel beta subunit. Cell 97: 943– 952, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Hanada T, Lin L, Chandy KG, Oh SS, Chishti AH. Human homologue of the drosophila discs large tumor suppressor binds to p56lck tyrosine kinase and shaker type Kv1.3 potassium channel in T lymphocytes. J Biol Chem 272: 26899– 26904, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Jugloff DG, Khanna R, Schlichter LC, Jones OT. Internalization of the Kv1.4 potassium channel is suppressed by clustering interactions with PSD-95. J Biol Chem 275: 1357– 1364, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Kammer GM. Deficient protein kinase a in systemic lupus erythematosus: a disorder of T lymphocyte signal transduction. Ann NY Acad Sci 968: 96– 105, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Khan IU, Laxminarayana D, Kammer GM. Protein kinase A RIβ subunit deficiency in lupus T lymphocytes: bypassing a block in RIβ translation reconstitutes protein kinase A activity and augments IL-2 production. J Immunol 166: 7600– 7605, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Krause D, Lee SC, Deutsch C. Forskolin effects on the voltage-gated K+ conductance of human T cells. Pflügers Arch 412: 133– 140, 1988 [DOI] [PubMed] [Google Scholar]
- 23. Krummel MF, Cahalan MD. The immunological synapse: a dynamic platform for local signaling. J Clin Immunol 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ledbetter JA, Parsons M, Martin PJ, Hansen JA, Rabinovitch PS, June CH. Antibody binding to CD5 (Tp67) and Tp44 T cell surface molecules: effects on cyclic nucleotides, cytoplasmic free calcium, and cAMP-mediated suppression. J Immunol 137: 3299– 3305, 1986 [PubMed] [Google Scholar]
- 25. Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, Sen D, Roos J, Stauderman KA, Cahalan MD. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc Natl Acad Sci USA 105: 2011– 2016, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marks DR, Fadool DA. Post-synaptic density perturbs insulin-induced Kv1.3 channel modulation via a clustering mechanism involving the SH3 domain. J Neurochem 103: 1608– 1627, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martel J, Dupuis G, Deschenes P, Payet MD. The sensitivity of the human Kv1.3 (hKv13) lymphocyte K+ channel to regulation by PKA and PKC is partially lost in HEK 293 host cells. J Membr Biol 161: 183– 196, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Mosenden R, Taskén K. Cyclic AMP-mediated immune regulation–overview of mechanisms of action in T cells. Cell Signal 23: 1009– 1016, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Nicolaou SA, Neumeier L, Steckly A, Kucher V, Takimoto K, Conforti L, Localization of Kv1.3 Channels in the immunological synapse modulates the calcium response to antigen stimulation in T lymphocytes. J Immunol 183: 6296– 6302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nicolaou SA, Neumeier L, Takimoto K, Lee SM, Duncan HJ, Kant SK, Mongey AB, Filipovich AH, Conforti L. Differential calcium signaling and Kv1.3 trafficking to the immunological synapse in systemic lupus erythematosus. Cell Calcium 47: 19– 28, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nicolaou SA, Szigligeti P, Neumeier L, Lee SM, Duncan HJ, Kant SK, Mongey AB, Filipovich AH, Conforti L. Altered dynamics of Kv1.3 channel compartmentalization in the immunological synapse in systemic lupus erythematosus. J Immunol 179: 346– 356, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Orstavik S, Funderud A, Hafte TT, Eikvar S, Jahnsen T, Skalhegg BS. Identification and characterization of novel PKA holoenzymes in human T lymphocytes. FEBS J 272: 1559– 1567, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Payet MD, Dupuis G. Dual regulation of the N type K+ channel in Jurkat T lymphocytes by protein kinases A and C. J Biol Chem 267: 18270– 18273, 1992 [PubMed] [Google Scholar]
- 34. Robbins JR, Lee SM, Filipovich AH, Szigligeti P, Neumeier L, Petrovic M, Conforti L. Hypoxia modulates early events in T cell receptor-mediated activation in human T lymphocytes via Kv1.3 channels. J Physiol 564: 131– 143, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roumier A, Olivo-Marin JC, Arpin M, Michel F, Martin M, Mangeat P, Acuto O, Dautry-Varsat A, Alcover A. The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity 15: 715– 728, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Round JL, Tomassian T, Zhang M, Patel V, Schoenberger SP, Miceli MC. Dlgh1 coordinates actin polymerization, synaptic T cell receptor and lipid raft aggregation, and effector function in T cells. J Exp Med 201: 419– 430, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ruppelt A, Mosenden R, Gronholm M, Aandahl EM, Tobin D, Carlson CR, Abrahamsen H, Herberg FW, Carpen O, Tasken K. Inhibition of T cell activation by cyclic adenosine 5′-monophosphate requires lipid raft targeting of protein kinase a type I by the a-kinase anchoring protein Ezrin. J Immunol 179: 5159– 5168, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell 115: 199– 208, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Skalhegg BS, Funderud A, Henanger HH, Hafte TT, Larsen AC, Kvissel AK, Eikvar S, Ostavik S. Protein kinase A (PKA)–a potential target for therapeutic intervention of dysfunctional immune cells. Current Drug Targets 6: 655– 664, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Skalhegg BS, Landmark BF, Doskeland SO, Hansson V, Lea T, Jahnsen T. Cyclic AMP-dependent protein kinase type I mediates the inhibitory effects of 3′,5′-cyclic adenosine monophosphate on cell replication in human T lymphocytes. J Biol Chem 267: 15707– 15714, 1992 [PubMed] [Google Scholar]
- 41. Soliven B, Nelson DJ. Beta-adrenergic modulation of K+ current in human T lymphocytes. J Membr Biol 117: 263– 274, 1990 [DOI] [PubMed] [Google Scholar]
- 42. Szabo I, Gulbins E, Apfel H, Zhang X, Barth P, Busch AE, Schlottmann K, Pongs O, Lang F. Tyrosine phosphorylation-dependent suppression of a voltage-gated K+ channel in T lymphocytes upon Fas stimulation. J Biol Chem 271: 20465– 20469, 1996 [DOI] [PubMed] [Google Scholar]
- 43. Szigligeti P, Neumeier L, Duke E, Chougnet C, Takimoto K, Lee SM, Filipovich AH, Conforti L. Signalling during hypoxia in human T lymphocytes–critical role of the src protein tyrosine kinase p56Lck in the O2 sensitivity of Kv1.3 channels. J Physiol 573: 357– 370, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tasken K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev 84: 137– 167, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Tasken K, Stokka AJ. The molecular machinery for cAMP-dependent immunomodulation in T-cells. Biochem Soc Trans 34: 476– 479, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Tiffany AM, Manganas LN, Kim E, Hsueh YP, Sheng M, Trimmer JS. PSD-95 and SAP97 exhibit distinct mechanisms for regulating K(+) channel surface expression and clustering. J Cell Biol 148: 147– 158, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Torgersen KM, Vang T, Abrahamsen H, Yaqub S, Taskén K. Molecular mechanisms for protein kinase A-mediated modulation of immune function. Cell Signal 14: 1– 9, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Vang T, Torgersen KM, Sundvold V, Saxena M, Levy FO, Skalhegg BS, Hansson V, Mustelin T, Tasken K. Activation of the COOH-terminal Src kinase (Csk) by cAMP-dependent protein kinase inhibits signaling through the T cell receptor. J Exp Med 193: 497– 507, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Willoughby D, Cooper DMF. Organization and Ca2+ regulation of adenylyl cyclases in cAMP Microdomains. Physiol Rev 87: 965– 1010, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Xavier R, Rabizadeh S, Ishiguro K, Andre N, Ortiz JB, Wachtel H, Morris DG, Lopez-Ilasaca M, Shaw AC, Swat W, Seed B. Discs large (Dlg1) complexes in lymphocyte activation. J Cell Biol 166: 173– 178, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]