Abstract
Leucine (Leu) and insulin both stimulate muscle protein synthesis, albeit at least in part via separate signaling pathways. While alcohol (EtOH) suppresses insulin-stimulated protein synthesis in cultured myocytes, its ability to disrupt Leu signaling and Rag GTPase activity has not been determined. Likewise, little is known regarding the interaction of EtOH and Leu on the AMPK/TSC2/Rheb pathway. Treatment of myocytes with EtOH (100 mM) decreased protein synthesis, whereas Leu (2 mM) increased synthesis. In combination, EtOH suppressed the anabolic effect of Leu. The effects of EtOH and Leu were associated with coordinate changes in the phosphorylation state of mTOR, raptor, and their downstream targets 4EBP1 and S6K1. As such, EtOH suppressed the ability of Leu to activate these signaling components. The Rag signaling pathway was activated by Leu but suppressed by EtOH, as evidenced by changes in the interaction of Rag proteins with mTOR and raptor. Overexpression of constitutively active (ca)RagA and caRagC increased mTORC1 activity, as determined by increased S6K1 phosphorylation. Furthermore, the caRagA-caRagC heterodimer blocked the inhibitory effect of EtOH. EtOH and Leu produced differential effects on AMPK signaling. EtOH enhanced AMPK activity, resulting in increased TSC2 (S1387) and eEF2 phosphorylation, whereas Leu had the opposite effect. EtOH also decreased the interaction of Rheb with mTOR, and this was prevented by Leu. Collectively, our results indicate that EtOH inhibits the anabolic effects that Leu has on protein synthesis and mTORC1 activity by modulating both Rag GTPase function and AMPK/TSC2/Rheb signaling.
Keywords: AMP-activated protein kinase, tuberous sclerosis protein complex 2, mammalian target of rapamycin complex 1, protein synthesis, Rag proteins, Ras homolog enriched in brain
muscle atrophy is a common manifestation of prolonged excessive alcohol consumption with a prevalence exceeding that of many hereditary myopathies (32). This alcoholic myopathy increases morbidity, and the erosion of lean body mass in other catabolic conditions is causally linked to increased mortality (29). Muscle protein represents a dynamic balance between the opposing processes of protein synthesis and degradation. While basal muscle protein degradation does not appear altered by alcohol (EtOH) (16, 53), we and others have reported that acute alcohol intoxication and chronic alcohol consumption decrease muscle protein synthesis (6, 16, 31, 54). At present, the etiology of alcoholic myopathy is not fully elucidated. However, the control of protein synthesis is typically integrated by both positive and negative effectors at the level of the mammalian target of rapamycin (mTOR).
mTOR exerts its effect as part of two biochemically and functionally distinct protein complexes termed mTOR complex (mTORC) 1 and 2 (33). In addition to mTOR, mLST8/GβL and Deptor (DEP domain-containing mTOR-interacting protein) (40) are members of both the mTORC1 and the mTORC2 complexes. Raptor (regulatory-associated protein of mTOR) and PRAS40 (proline-rich Akt substrate of 40 kDa), on the other hand, are unique members of mTORC1 (48, 52), whereas rictor (rapamycin-insensitive companion of mTOR), Sin1, and protor (protein observed with rictor) are distinct components of mTORC2 (9, 39, 49).
Multiple lines of evidence reveal that growth factors regulate mTOR via the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway. Activation of Akt leads to phosphorylation of the mTOR component PRAS40 (48, 52), as well as the tumor suppressor protein tuberous sclerosis protein complex 2 (TSC2), which is an important negative regulator of mTORC1 (22). In addition, TSC2 serves as a GTPase activating protein for Rheb (Ras homolog enriched in brain) (12, 21). Rheb, in turn, binds to mTORC1, thereby controlling its activity. Essential amino acids, and in particular leucine (Leu), also stimulate mTORC1 activity and protein synthesis (27, 50). Increased availability of these amino acids alters the phosphorylation state and the function of a number of proteins that regulate translation initiation and elongation. These include the eukaryotic initiation factor 4E binding protein 1 (4EBP1), ribosomal S6 kinase 1 (S6K1), and eukaryotic elongation factor 2 (eEF2). Despite progress in understanding how growth factors regulate mTORC1 signaling, it remains unclear how EtOH affects this pathway.
The mechanisms by which amino acids signal to mTORC1 remains enigmatic, although intracellular proteins such as vacuolar protein sorting (Vps)34, mitogen-activated protein (MAP)4K3, and Rag GTPases have been identified as amino acid sensors (4, 25, 38, 40). The Rag proteins are a family of four related small guanosine triphosphatases (GTPases) implicated in the regulation of mTORC1 (46). Rag proteins function as heterodimers, whereby a single RagA or RagB protein forms a complex with either RagC or RagD. The function of the Rag complex is controlled by the nucleotide loading state of RagA or RagB. For example, expression of RagBGTP/RagDGDP in HEK293T and L6 cells increases their activity, as judged by the phosphorylation state of the mTORC1 substrate S6K1 (46). It has been proposed that Rag GTPases bind to mTORC1 via a heterotrimeric protein complex termed the Ragulator (45). This complex links mTORC1 to the lysosomal membrane in an inducible manner, with the Rag heterodimer serving as the bridge between mTORC 1 and Ragulator. The association of mTORC1 with lysosomal membranes promotes mTORC1 activation by colocalizing mTORC1 with Rheb. This, in turn, mediates mTORC1 activation by amino acids. We posit that the EtOH-induced decrease in protein synthesis is modulated by the Rag proteins.
mTOR can be regulated by an Akt-independent pathway that utilizes the AMP-activated protein kinase (AMPK). AMPK is a major nutrient and cell energy sensing protein activated under unfavorable conditions (e.g., hypoxia, glucose starvation, EtOH) (14, 18). As such, this kinase downregulates energetically demanding processes such as protein synthesis. Mechanistically, the activation of AMPK decreases protein synthesis by inhibiting the ability of mTOR to signal to regulatory proteins associated with translational initiation and elongation. Along these lines, the phosphorylation of TSC2 (S1345 and T1227) by AMPK increases its activity (21). TSC2, which functions as a GTPase activator protein (GAP) for Rheb, modifies this protein, thereby affecting the ability of Rheb to interact with mTOR and impairing mTORC1 signaling (5, 15, 21, 34). In addition to its effect on TSC2, AMPK activation also increases the phosphorylation of the mTORC1 component raptor, which adversely affects the interaction of this protein with other components of mTORC1. This occurs in response to energy stress and exposure to EtOH via a process that promotes the interaction of raptor with cytosolic anchor proteins such as 14-3-3 (14, 19).
Owing to the fact that EtOH inhibits protein synthesis in C2C12 myocytes, we hypothesized that this drug would suppress the anabolic effects of Leu. To test this, we examined the individual and combined effects of EtOH and Leu on protein synthesis. We also investigated the signaling pathways involved in mediating the effects of these agents. EtOH suppressed the stimulatory effects of Leu on protein synthesis and the associated signaling components. EtOH and Leu signaled to mTORC1 via Rag GTPases by altering the interaction of these proteins with raptor and mTOR. These agents also affected mTORC1 function through the AMPK/TSC2 pathway. In addition, our findings suggest that EtOH decreased Rheb binding to mTOR, and this was mediated via the Rag GTPases. However, when given in combination, Leu counteracted the inhibitory effect that EtOH had on this association, thereby affecting mTORC1 activity.
MATERIALS AND METHODS
Materials.
Ethanol was purchased from Fisher Scientific (Springfield, NJ). The pRK5-HA GST RagA 66L (Addgene plasmid no. 19300) and pRK5-HA GST RagC 120L (Addgene plasmid no. 19306) plasmids were obtained from Addgene (Cambridge, MA; submitted by Dr. David M. Sabatini). Antibodies against rictor were from Bethyl Laboratories (Montgomery, TX). Total S6K1 and Rheb antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against phosphorylated (p-) mTOR (S2448), p-raptor (S792), p-rictor (T1135), p-S6K1 (T389), p-S6 ribosomal protein (S235/236), p-4EBP1 (T37/46), p-TSC2 (S1387), p-eEF2(T56), and p-AMPK (T172) were purchased from Cell Signaling Technology (Beverly, MA). Antibodies to RagA, RagC, total mTOR, raptor, TSC2, ribosomal protein S6, eEF2, and AMPK were obtained from the same source. l-Leucine, phenylmethanesulfonyl fluoride (PMSF), protease, phosphatase I and II inhibitor cocktails were from Sigma (St. Louis, MO). [35S]methionine/cysteine (>1,000 Ci/mol) was obtained from MP Biomedicals (Aurora, OH). Cell culture media and fetal bovine serum (FBS) were from GIBCO, Invitrogen (Carlsbad, CA).
Cell culture and transfection.
C2C12 mouse myoblasts (American Type Culture Collection; Manassas, VA) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin (25 μg/ml) at 37°C in 5% CO2. For transient expression, pRK5-HA-GST plasmids constitutively overexpressing RagA, RagC, and control plasmids were transfected into C2C12 myocytes using electroporation and the cell line nucleofector kit V (Amaxa, Germany) following the manufacturer's protocol. Primary studies using the same conditions indicated that transfection efficiency was 40–50%, as determined by counting GTP-positive and total cells from six random fields. Experiments were carried out 24–36 h posttransfection, and cells were harvested 18–24 h thereafter for immunoblot analysis.
The effect of EtOH and Leu on protein synthesis was determined as previously described (16, 20). One hundred millimolar EtOH was used because this concentration produces an optimal effect without being cytotoxic to myocytes. The concentration and the incubation time of Leu were chosen on the basis of dose- and time-response curves from our preliminary studies and the literature (1, 50). All experiments were conducted using cells at the early passage of the myoblast stage. For metabolic labeling, cells were pretreated with EtOH for 18–24 h in 1.5% FBS minimum essential medium eagle (MEM). The presence of serum was necessary because C2C12 myocyte survival is decreased when cells are cultured for extended periods in serum-free media. Thereafter, cells were changed to MEM for 1 h and then radioisotope labeled for 1 h with 10 μCi of [35S] in the presence or absence of EtOH and/or Leu. At the end of the experiment, cells were collected and precipitated in 10% trichloroacetic acid (TCA), and the incorporation of [35S]methionine/cysteine into TCA-precipitable protein was determined via liquid scintillation counting. Total protein was determined using the bicinchoninic acid reagent (BCA) protein assay kit with BSA as a standard (Pierce, Rockford, IL). The results were then normalized with total protein and compared with the control group. For the determination of cellular energy levels, ATP and AMP concentrations in myocytes were measured using standard fluorometric methods (36). Data are expressed as a percentage of the control value.
Immunoprecipitation and immunoblot analysis.
C2C12 myocytes, cultured in either 10 cm or six-well plates, were incubated in the presence of EtOH and/or Leu. Thereafter, cells were rinsed once with phosphate-buffered saline and lysed in ice-cold 0.3% CHAPS {3-[(3-cholamindopropyl) dimethylammonio]-1-propanesulfonate} buffer containing PMSF and a cocktail of protease and phosphatase inhibitors. Soluble fractions of cell extracts were isolated by centrifugation at 14,000 rpm for 5 min at 4°C. For immunoprecipitation, a 1:50 dilution of primary antibody (Ab) was added to equal amounts of protein (300–500 μg) from cell lysates and rotated overnight at 4°C. A total of 40 μl of a 50% slurry of protein A Sepharose was then added for an additional 1 h. Immunoprecipitates were washed three times with lysis buffer, and the precipitated proteins were denatured by the addition of 2 × Laemmli sample buffer. Equal amounts of protein from cell lysates were electrophoresed on denaturing polyacrylamide gels and transferred to nitrocellulose membranes. The resulting blots were blocked with 5% nonfat dry milk and incubated with the antibodies of interest (1:1,000). Unbound primary antibody was removed by washing with Tris-buffered saline containing 0.05% Tween-20 (ICI Americas, Wilmington, DE), and blots were incubated with anti-rabbit immunoglobulin (1:5,000) conjugated with horseradish peroxidase (HRP). On occasions where the denatured and reduced rabbit IgG light or heavy chains obscured protein bands of similar molecular weights, mouse anti-rabbit IgG (conformation specific L27A9) mAb (Cell Signaling) was added as a bridging antibody before incubation with an anti-mouse IgG, HRP-linked secondary antibody (Cell Signaling). Blots were developed with an enhanced chemiluminescence detection system (Amersham, Little Chalfont, UK) and exposed to MiDSci X-ray film (St. Louis, MO). The film was scanned (ScanMaker 4, Microtek, Los Angeles, CA) and analyzed with Scion image version 3b software (National Institutes of Health, Bethesda, MD).
Statistical analysis.
For experimental protocols with more than two groups, statistical significance was determined using one-way ANOVA followed by the Dunnett's test to compare all data to the appropriate control group. For experiments with only two groups, an unpaired Student's t-test was performed. Data are presented as means ± SE. A value of P < 0.05 was considered statistically significant.
RESULTS
Differential effect of EtOH and Leu on protein synthesis and mTOR signaling.
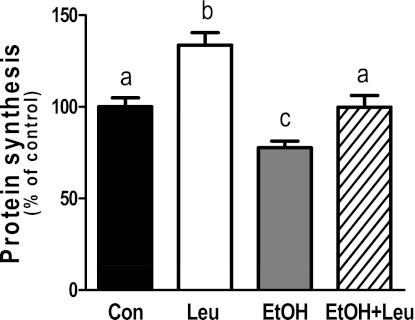
C2C12 myocytes were treated with EtOH, Leu, or a combination of both agents and examined for changes in global protein synthesis. Incubation of myocytes with 100 mM EtOH decreased protein synthesis by 22% compared with untreated control cells (Fig. 1). In contrast, protein synthesis increased by 34% in response to a maximally stimulatory dose (2 mM) of Leu. The combined effect of EtOH and Leu was intermediary in nature, with EtOH blunting the full anabolic response to Leu.
Fig. 1.
Alcohol (EtOH) impairs leucine-stimulated protein synthesis. C2C12 myocytes were incubated in the presence or absence of EtOH (100 mM) for 18–24 h. Cells were subsequently treated with Leu (2 mM) for 1 h with labeling media containing [35S]methionine/cysteine. The amount of trichloroacetic acid precipitable radioactivity was determined as described in materials and methods. Results are expressed as a percentage of basal control level. Each bar graph represents the mean ± SE of 3 independent experiments consisting of 4 replicate samples per experiment (n = 12). Groups with different letters are significantly different from each other (P < 0.05). Groups with the same letters are not significantly different.
The serine-threonine protein kinase mTOR plays an important role in the translational control of protein synthesis and is regulated by phosphorylation. As illustrated in Fig. 2, A and B, Leu increased mTOR phosphorylation relative to control values, whereas EtOH decreased mTOR phosphorylation by 34%. The addition of Leu ameliorated the inhibitory effect of EtOH. In contrast to its effect on mTOR, EtOH increased and Leu decreased the phosphorylation of raptor. However, the presence of EtOH largely prevented the Leu-induced decrease in raptor phosphorylation (Fig. 2C). As an indirect marker of mTOR kinase activity, we next assessed the phosphorylation of 4EBP1. Leu increased the phosphorylation of 4EBP1 by 57% (Fig. 2D), whereas EtOH decreased phosphorylation. Again, EtOH attenuated the anabolic effect of Leu.
Fig. 2.
EtOH attenuates leucine-induced changes in the phosphorylation of mTOR, raptor (regulatory-associated protein of mTOR) and 4E binding protein 1 (4EBP1). C2C12 myocytes were incubated with EtOH and/or Leu as described in Fig. 1. Equal amounts of protein from cell lysates were analyzed via Western blotting using antibodies against phosphorylated and total forms of the indicated proteins (A). The protein levels were quantified as bar graphs (B–D). Results were normalized to total cell lysate protein and are expressed as percentage of control levels. Each bar graph represents the mean ± SE of 4–5 independent experiments consisting of 3–4 replicate samples per experiment (n = 12–20). Groups with different letters are significantly different from each other (P < 0.05). Groups with the same letters are not significantly different. mTOR, mammalian target of rapamycin.
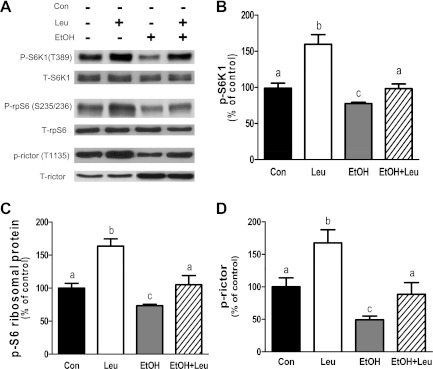
The phosphorylation of S6K1, another target of mTORC1, was also examined. Figure 3, A and B, illustrates that Leu increased S6K1 phosphorylation at T389, which was prevented by EtOH. To verify the effect of EtOH and Leu on S6K1 activity, we assessed the phosphorylation state of rpS6, a physiologically relevant S6K1 substrate. The effect of EtOH and/or Leu on rpS6 phosphorylation was comparable to the changes detected for S6K1 phosphorylation (Fig. 3C). S6K1 also directly regulates the phosphorylation of rictor, a component of mTORC2 that functions as part of a negative feedback loop for mTORC1 (3). In this regard, Leu increased rictor phosphorylation by ∼70%, compared with control values (Fig. 3D). In contrast, EtOH decreased the phosphorylation of rictor by 50% and attenuated the Leu-induced increase in rictor phosphorylation.
Fig. 3.
EtOH attenuates leucine-induced changes in the phosphorylation of S6 kinase 1 (S6K1), rpS6, and rictor (rapamycin-insensitive companion of mTOR). C2C12 myocytes were incubated with EtOH and/or Leu as described in Fig. 1. Equal amounts of protein from cell extracts were analyzed via Western blotting using antibodies against phosphorylated and total forms of the indicated proteins (A). The protein levels were quantified as bar graphs (B–D). Results were normalized to total cell lysate protein and are expressed as a percentage of control levels. Each bar graph represents the mean ± SE of 4 independent experiments consisting of 3–4 replicate samples per experiment (n = 12–16). Groups with different letters are significantly different from each other (P < 0.05). Groups with the same letters are not significantly different.
EtOH and Leu increase the association of RagA and RagC with mTOR.
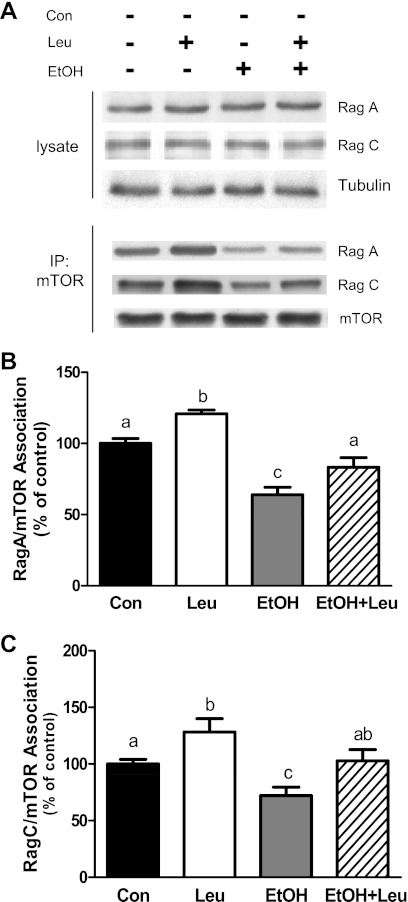
The heterodimeric Rag GTPases have been implicated in amino acid signaling to mTORC1 (2, 26). Exposure of myocytes to EtOH and/or Leu did not affect the total amount of RagA or RagC, compared with control values (Fig. 4A). Therefore, the effects of these agents on protein synthesis and signaling cannot be attributed to a change in the total cellular content of these Rag proteins. Because protein-protein interactions are important in mediating signaling events, the association of Rag proteins with mTORC1 components was assessed. For these experiments, mTOR and raptor were immunoprecipitated from cell lysates and the interacting proteins were evaluated by Western analysis. Treatment of cells with Leu increased the interaction of RagA (Fig. 4B) and RagC (Fig. 4C) with mTOR. In contrast, EtOH decreased the association of RagA and Rag C with mTOR, and it blocked the stimulatory effect of Leu on binding. Similar results were also observed for the association between raptor and RagA and RagC (data not shown). Thus these data are consistent with a model in which both EtOH and Leu mediate their effects on protein synthesis by modulating the interaction of Rag proteins with mTORC1.
Fig. 4.
EtOH and leucine differentially regulate the interaction of RagA and RagC with mTOR complex 1 (mTORC1). C2C12 myocytes were incubated with EtOH and/or Leu as described in Fig. 1. Equal amounts of protein from cell extracts were analyzed via Western blotting using antibodies against RagA and RagC (A). mTOR was immunoprecipitated from equal amounts of cell extract and then immunoblotted with RagA (B) or RagC (C). Results were normalized with immunoprecipitated mTOR which was assessed by immunoblotting. Data are means ± SE of 6 independent experiments consisting of 3–4 replicate samples per experiment (n = 18–24). Groups with different letters are significantly different form each other (P < 0.05). Groups with the same letters are not significantly different. IP, immunoprecipitation.
Stimulatory effects of constitutively active RagAQ66L and RagCQ120L on mTORC1 signaling.
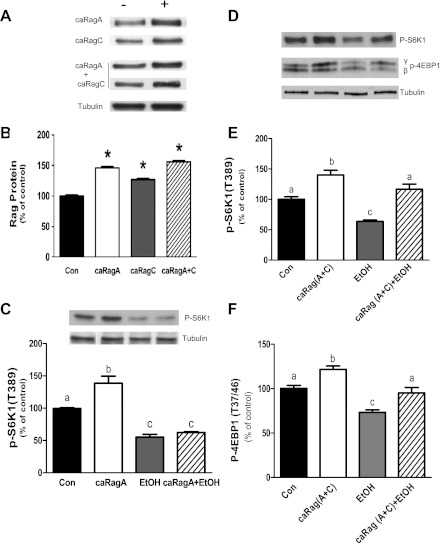
To further characterize the role that Rag GTPases play in mTORC1 regulation, myocytes were transfected with plasmids expressing constitutively active (ca)RagA and caRagC. Transfection with caRagA, caRagC, or the combination of both increased the amount of these proteins by 30–45% relative to control values (Fig. 5, A and B). Furthermore, similar levels were observed for both proteins when they were transfected simultaneously. To determine the effect of caRag proteins on mTORC1 activity, we examined the phosphorylation of S6K1. Overexpression of caRagA increased T389 phosphorylation of S6K1 (Fig. 5C). However, this stimulation did not overcome the inhibitory effect of EtOH. A similar increase of S6K1 and 4EBP1 phosphorylation was observed in myocytes overexpressing both caRagA and caRagC (Fig. 5, D–F). However, in contrast to cells expressing caRagA alone, the combined stimulatory effect of caRagA and caRagC was effective at maintaining levels of S6K1 and 4EBP1 phosphorylation in the presence of EtOH, suggesting the importance of both caRagA and caRagC.
Fig. 5.
Effects of exogenous expression of constitutionally active (ca)RagA and caRagA/caRagC on S6K1 phosphorylation. C2C12 myocytes were transfected with plasmids expressing caRagA, caRagC, or a combination of caRagA and caRagC (A and B). At 24–36 h after transfection, cells were incubated in the presence or absence of EtOH for 18 h. mTORC1 activity was then assessed by Western blot analysis of S6K1 and 4EBP1 phosphorylation at T389 and T37/46, respectively (C and D). The phosphorylation levels S6K1 and 4EBP1 in cells expressing the caRagA and caRagC combination were quantified as bar graphs (E and F). Results were normalized to total cell lysate protein and are expressed as a percentage of control levels. Each bar graph represents the mean ± SE of 3 independent experiments consisting of 3 replicate samples per experiment (n = 9). Groups with different letters are significantly different form each other (P < 0.05). Groups with the same letters are not significantly different. *P < 0.05, compared with control values.
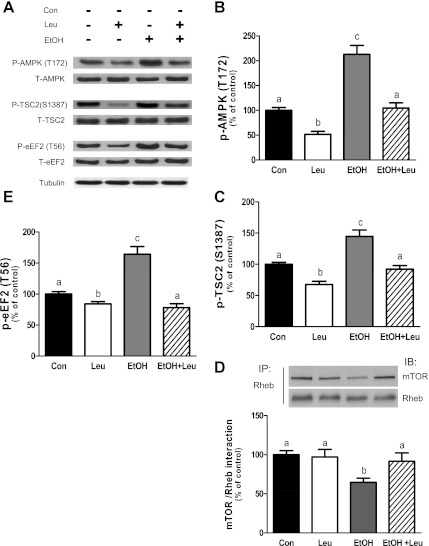
EtOH and Leu affect AMPK/TSC2, eEF2, and the Rheb-mTOR interaction.
Previous studies have reported increased AMPK activity in response to various stressors. This increase, in turn, negatively regulates mTORC1 by enhancing the phosphorylation of raptor, eEF2, and TSC2 (14, 18, 23). AMPK phosphorylation was elevated following EtOH exposure. These levels were reduced to control values when EtOH was given in combination with Leu, whereas they were below control levels for Leu alone (Fig. 6, A and B). Because TSC2 is also a downstream target of AMPK under stress conditions (21), we assessed whether Leu and EtOH affected TSC2 phosphorylation. Leu decreased TSC2 phosphorylation at the S1387 by 30%, relative to control values (Fig. 6, A and C). In contrast, EtOH enhanced TSC2 phosphorylation and this increase was largely prevented by the addition of Leu. These differences in AMPK activation could not be attributed to changes in the cellular concentration of AMP, ATP, or the AMP/ATP ratio, as these parameters were not altered in myocytes incubated with Leu or EtOH (Table 1).
Fig. 6.
EtOH and leucine affect the AMP-activated protein kinase (AMPK)/tuberous sclerosis protein complex 2 (TSC2), eukaryotic elongation factor 2 (eEF2), and Rheb-mTOR interaction. C2C12 myocytes were incubated with EtOH and/or Leu as described in Fig. 1. Equal amounts of protein from cell extracts were analyzed via Western blotting using antibodies against phosphorylated and total forms of the indicated proteins (A). The protein levels were quantified as bar graphs (B, C, and E). Rheb was immunoprecipitated from equal amounts of cell extract and then immunoblotted with mTOR (D). Results were normalized with immunoprecipitated Rheb which was assessed by Western blotting. Data are means ± SE of 3 independent experiments consisting of 3 replicate samples per experiment (n = 9). Groups with different letters are significantly different from each other (P < 0.05). Groups with the same letters are not significantly different.
Table 1.
Effects of EtOH or Leu on AMP, ATP, and AMP/ATP ratio in C2C12 myocytes
| Control | Leu | EtOH | |
|---|---|---|---|
| AMP, μmol/g protein | 0.42 ± 0.06 | 0.38 ± 0.04 | 0.43 ± 0.04 |
| ATP, μmol/g protein | 18.4 ± 1.2 | 18.0 ± 0.9 | 17.5 ± 2.1 |
| AMP/ATP ratio | 0.023 ± 0.002 | 0.021 ± 0.003 | 0.024 ± 0.002 |
Values are means ± SE; n = 6–8 per group. C2C12 myocytes were incubated in the presence or absence of EtOH (100 mM) or Leu (2 mM) for 18–24 h and 1 h, respectively. As a positive control, cells were incubated with oligomycin (40 μM) for 24 h, with these cells having an ATP concentration of 2.1 ± 1.8 μmol/g protein.
Recently, it was demonstrated that the association of Rag and raptor proteins results in the translocation of mTORC1 to the lysosome. There, mTOR interacts with Rheb, resulting in the activation of the mTOR kinase (47). Hence, we examined whether Leu and EtOH affected the formation of this complex. Treatment with Leu did not alter the extent of mTOR and Rheb binding (Fig. 6D). In contrast, EtOH exposure was observed to decrease this interaction. Interestingly, the addition of Leu attenuated the negative effects of EtOH on the mTOR/ Rheb interaction.
Finally, we examined whether Leu or the combination of Leu and EtOH regulated the phosphorylation of eEF2. Leu caused a small (∼18%), albeit significant, decrease in the phosphorylation of eEF2 relative to the control value (Fig. 6E). Conversely, EtOH increased phosphorylation of eEF2 by ∼70%, with this increase being completely prevented by Leu.
DISCUSSION
In this study, Leu increased protein synthesis in C2C12 myocytes, whereas EtOH had an inhibitory effect. Moreover, EtOH suppressed the anabolic effect of Leu. These results in myocytes are consistent with the EtOH-induced Leu resistance observed in rat skeletal muscle (30). Collectively, these data suggest that there may be common mechanisms involved in the action of each of these mediators. For example, alterations in protein synthesis following ETOH or Leu exposure were associated with changes in mTOR and raptor phosphorylation, which is consistent with reports from our lab and others (28, 30, 42). These changes resulted in posttranslational modifications and alterations in interactions between various members of mTORC1, as well as changes in interaction between mTORC1 and cytosolic anchor proteins such as 14-3-3 (18, 52). Taken together, these factors affected the ability of mTORC1 to signal to several key components of the translation machinery including 4EBP1, S6K1, ribosomal S6 protein, and eEF2.
Recent studies report that the association of Rag proteins with mTORC1 is necessary for the activation of the mTORC1 pathway in response to amino acids (26, 46). In this regard, treatment of C2C12 myocytes with Leu increased the interaction of Rag proteins with raptor and mTOR. In contrast, EtOH attenuated this association, suggesting that these agents mediate their signal through Rag GTPase. Because Rag GTPase activity is regulated by changes in its nucleotide state, it is possible that EtOH may affect Rag function via the induction of changes in the GTP status of this protein. Indeed, overexpression of the constitutively active form of GTP-bound Rag heterodimer increased the mTOR activity as judged by the enhanced phosphorylation of S6K1 at T389, with this occurring even in the absence of Leu. However, the suppressive effect of EtOH was overcome by overexpressing GTP-bound Rag proteins, indicating that the active Rag heterodimer can counteract the negative effects of EtOH.
A common nexus for many of the mTOR-related signaling pathways is TSC2, which acts as a convergence point for signaling inputs from the AMPK, Akt, and ERK/p38 MAPK pathways. Signals from these pathways promote phosphorylation of multiple residues on TSC2, which can either activate or repress the function of this protein toward Rheb, thereby regulating mTOR activity. For example, Akt and ERK mediate phosphorylation of TSC2 at S939, T1462, and S664 in response to insulin, insulin-like growth factor (IGF)-I, or phenylephrine. This inhibits the function of TSC2 and, consequently, stimulates mTOR activity, protein synthesis, and promotes cell growth (37, 41, 43, 55, 56). However, EtOH did not affect TSC2 phosphorylation at T1462, even though it increased Akt phosphorylation (S473) as part of a mTORC1-dependent negative feedback loop with mTORC2 (3, 8, 16, 18). Hence, these data suggest that EtOH does not signal to mTORC1 via the PI3K/Akt/TSC2 pathway.
Previously, we reported that EtOH increases AMPK activity, as determined using an in vitro kinase assay with raptor or eEF2 as substrates (16, 17). In the present study, activation of AMPK by EtOH increased TSC2 phosphorylation, albeit at a different site (S1387). Thus it appears that this AMPK activation alters the phosphorylation state of the downstream targets TSC2, eEF2, and raptor. This EtOH-induced change is consistent with reports of AMPK-mediated phosphorylation of TSC2 at S1345 and T1227 under conditions of energy starvation (21). In contrast to EtOH, Leu decreased AMPK and TSC2 phosphorylation, and it suppressed the stimulatory effects of EtOH on the phosphorylation of these proteins. The former result is in agreement with previous studies in human and rat skeletal muscle where Leu decreased AMPK phosphorylation (10, 44). However, our results for TSC2 are in contrast with findings where Leu had no affect on TSC2 phosphorylation (44).
Because TSC2 functions as a GAP for Rheb, the activation of TSC2 by EtOH may change the nucleotide status of Rheb. As such, this could decrease mTOR activity because Rheb activity is inhibited when it is in the GDP state. Alternatively, decreased mTOR activity may be due to changes in the interaction of Rheb and mTOR. Along these lines, EtOH decreased the binding of these two proteins, similar to the effect observed with amino acid withdrawal (35). Although Leu alone did not affect this association, the addition of Leu to EtOH-treated cells restored the level of interaction between Rheb and mTOR. At present, the exact mechanisms regulating the Rheb/mTOR interaction are unclear. As reported previously (46), activation of the Rag protein translocates the mTORC1 complex to the lysosome, thereby facilitating its association with Rheb. However, it is unknown whether other factors directly affect the binding ability of Rheb. The activation of the AMPK/TSC2 pathway could potentially play some role in this process, although the nucleotide status of Rheb is not thought to affect its ability to bind to mTOR (34).
Taken together, our results indicate that EtOH signals to mTORC1 through both the Rag GTPase and AMPK/TSC2/Rheb pathways. As such, the observed decrease in the Rag-raptor interaction may account for a reduced binding of this complex with the lysosomal compartment, where Rheb is localized (45, 46). This, in turn, may be responsible for the decreased Rheb-mTOR interaction and mTORC1 activity. In addition to protein-protein interaction, the TSC2 and AMPK pathways may play a role in mediating Rheb activity in the presence of EtOH. Activation of AMPK increased the phosphorylation of TSC2 at S1387, with the phosphorylation of TSC2 at this site stimulating its GAP activity towards Rheb. Thus this would decrease the ability of mTORC1 to phosphorylate 4EBP1 and S6K1, key proteins in the regulation of protein synthesis. The observed EtOH-mediated regulation of mTORC1 through both pathways may be atypical, since cellular stressors such as metformin and cysteine oxidants exert their effects on mTORC1 via only one or the other pathway (24, 58). Along these lines, Leu was observed to signal mTORC1 through the Rag GTPase, but not Rheb (2). However, Leu did modulate the effects that EtOH had on AMPK/TSC2/Rheb. Thus Leu can impact both signaling pathways, albeit only under stress conditions.
A number of unanswered questions remain regarding the mechanisms by which EtOH regulates mTOR and protein synthesis. In particular, the initial upstream signaling elements have not been identified. One potential regulator of this process is phospholipase D (PLD). PLD signals to mTOR via the generation of lipid second messenger phosphatidic acid (PA) (51). PA is a positive regulator of cell growth and proliferation, and levels are upregulated in response to amino acids, glucose, and growth factors (55, 57). In contrast, EtOH has been shown to have an antagonistic effect on this process (11). For example, EtOH completes with water as a substrate for PLD, and as such, this results in the preferred production of phosphatidyl-ethanol (7, 13). Decreased levels of PA can lead to the suppression of mTOR activity and may cause disturbances in cell function. Thus, in future studies, it will be interesting to determine how EtOH exerts its effects on this and other aspects of the protein synthetic signaling machinery.
GRANTS
This study was supported by National Institutes of Health Grant AA-11290.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.Q.H.-B., C.R.B., and C.H.L. conception and design of the research; L.Q.H.-B. and M.N. performed the experiments; L.Q.H.-B., C.R.B., and A.A.K. analyzed the data; L.Q.H.-B. and C.R.B. interpreted the results of the experiments; L.Q.H.-B. prepared the figures; L.Q.H.-B. drafted the manuscript; L.Q.H.-B., C.R.B., A.A.K., M.N., and C.H.L. approved the final version of the manuscript; C.R.B., A.A.K., M.N., and C.H.L. edited and revised the manuscript.
ACKNOWLEDGMENTS
We thank Danuta S. Huber for technical support and Dr. Margaret L. Shumate for critical review of the manuscript.
REFERENCES
- 1. Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 38: 1533–1539, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Dennis MD, Baum JI, Kimball SR, Jefferson LS. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem 286: 8287–8296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol 29: 5657–5670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dickinson JM, Rasmussen BB. Essential amino acid sensing, signaling, and transport in the regulation of human muscle protein metabolism. Curr Opin Clin Nutr Metab Care 14: 83–88, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Findlay GM, Harrington LS, Lamb RF. TSC1–2 tumour suppressor and regulation of mTOR signalling: linking cell growth and proliferation? Curr Opin Genet Dev 15: 69–76, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Fogle RL, Lynch CJ, Palopoli M, Deiter G, Stanley BA, Vary TC. Impact of chronic alcohol ingestion on cardiac muscle protein expression. Alcohol Clin Exp Res 34: 1226–1234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Foster DA. Reduced mortality and moderate alcohol consumption: the phospholipase D-mTOR connection. Cell Cycle 9: 1291–1294, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem 285: 14071–14077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol 16: 1865–1870, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol 582: 813–823, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fujita Y, Hiroyama M, Sanbe A, Yamauchi J, Murase S, Tanoue A. ETOH inhibits embryonic neural stem/precursor cell proliferation via PLD signaling. Biochem Biophys Res Commun 370: 169–173, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, Kozma SC, Hafen E, Bos JL, Thomas G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell 11: 1457–1466, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Gustavsson L. ESBRA 1994 Award Lecture. Phosphatidylethanol formation: specific effects of ethanol mediated via phospholipase D. Alcohol Alcohol 30: 391–406, 1995 [PubMed] [Google Scholar]
- 14. Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 18: 1926–1945, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Hong-Brown LQ, Brown CR, Huber DS, Lang CH. Alcohol and indinavir adversely affect protein synthesis and phosphorylation of MAPK and mTOR signaling pathways in C2C12 myocytes. Alcohol Clin Exp Res 30: 1297–1307, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Hong-Brown LQ, Brown CR, Huber DS, Lang CH. Alcohol regulates eukaryotic elongation factor 2 phosphorylation via an AMP-activated protein kinase-dependent mechanism in C2C12 skeletal myocytes. J Biol Chem 282: 3702–3712, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Hong-Brown LQ, Brown CR, Kazi AA, Huber DS, Pruznak AM, Lang CH. Alcohol and PRAS40 knockdown decrease mTOR activity and protein synthesis via AMPK signaling and changes in mTORC1 interaction. J Cell Biochem 109: 1172–1184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hong-Brown LQ, Brown CR, Navaratnarajah M, Huber DS, Lang CH. Alcohol-induced modulation of rictor and mTORC2 activity in C2C12 myoblasts. Alcohol Clin Exp Res 35: 1445–1453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong-Brown LQ, Frost RA, Lang CH. Alcohol impairs protein synthesis and degradation in cultured skeletal muscle cells. Alcohol Clin Exp Res 25: 1373–1382, 2001 [PubMed] [Google Scholar]
- 21. Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 17: 1829–1834, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4: 648–657, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Kalender A, Selvaraj A, Kim SY, Gulati P, Brule S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, Marette A, Kozma SC, Thomas G. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab 11: 390–401, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 10: 935–945, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim E, Guan KL. RAG GTPases in nutrient-mediated TOR signaling pathway. Cell Cycle 8: 1014–1018, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr 136: 227S–231S, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Kimball SR, Shantz LM, Horetsky RL, Jefferson LS. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J Biol Chem 274: 11647–11652, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Kotler DP, Tierney AR, Wang J, Pierson RN., Jr Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr 50: 444–447, 1989 [DOI] [PubMed] [Google Scholar]
- 30. Lang CH, Frost RA, Deshpande N, Kumar V, Vary TC, Jefferson LS, Kimball SR. Alcohol impairs leucine-mediated phosphorylation of 4E-BP1, S6K1, eIF4G, and mTOR in skeletal muscle. Am J Physiol Endocrinol Metab 285: E1205–E1215, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Lang CH, Frost RA, Kumar V, Wu D, Vary TC. Impaired protein synthesis induced by acute alcohol intoxication is associated with changes in eIF4E in muscle and eIF2B in liver. Alcohol Clin Exp Res 24: 322–331, 2000 [PubMed] [Google Scholar]
- 32. Lang CH, Frost RA, Summer AD, Vary TC. Molecular mechanisms responsible for alcohol-induced myopathy in skeletal muscle and heart. Int J Biochem Cell Biol 37: 2180–2195, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 10: 457–468, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol 15: 702–713, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Long X, Ortiz-Vega S, Lin Y, Avruch J. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J Biol Chem 280: 23433–23436, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Lowry OH, Passonneau JV. A collection of metabolite assays. In: A Flexible System of Enzymatic Analysis, edited by Lowry OH, Passonneau JV. New York: Academic, 1972, p. 146–220 [Google Scholar]
- 37. Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121: 179–193, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, Zwartkruis FJ, Thomas G. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA 102: 14238–14243, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pearce LR, Huang X, Boudeau J, Pawlowski R, Wullschleger S, Deak M, Ibrahim AF, Gourlay R, Magnuson MA, Alessi DR. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem J 405: 513–522, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137: 873–886, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol 4: 658–665, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Proud CG. Amino acids and mTOR signalling in anabolic function. Biochem Soc Trans 35: 1187–1190, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Rolfe M, McLeod LE, Pratt PF, Proud CG. Activation of protein synthesis in cardiomyocytes by the hypertrophic agent phenylephrine requires the activation of ERK and involves phosphorylation of tuberous sclerosis complex 2 (TSC2). Biochem J 388: 973–984, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saha AK, Xu XJ, Lawson E, Deoliveira R, Brandon AE, Kraegen EW, Ruderman NB. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes 59: 2426–2434, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290–303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sancak Y, Sabatini DM. Rag proteins regulate amino-acid-induced mTORC1 signalling. Biochem Soc Trans 37: 289–290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell 25: 903–915, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14: 1296–1302, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Talvas J, Obled A, Fafournoux P, Mordier S. Regulation of protein synthesis by leucine starvation involves distinct mechanisms in mouse C2C12 myoblasts and myotubes. J Nutr 136: 1466–1471, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Toschi A, Lee E, Xu L, Garcia A, Gadir N, Foster DA. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol Cell Biol 29: 1411–1420, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9: 316–323, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Vary TC, Frost RA, Lang CH. Acute alcohol intoxication increases atrogin-1 and MuRF1 mRNA without increasing proteolysis in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 294: R1777–R1789, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vary TC, Lynch CJ, Lang CH. Effects of chronic alcohol consumption on regulation of myocardial protein synthesis. Am J Physiol Heart Circ Physiol 281: H1242–H1251, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Winter JN, Fox TE, Kester M, Jefferson LS, Kimball SR. Phosphatidic acid mediates activation of mTORC1 through the ERK signaling pathway. Am J Physiol Cell Physiol 299: C335–C344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Winter JN, Jefferson LS, Kimball SR. ERK and Akt signaling pathways function through parallel mechanisms to promote mTORC1 signaling. Am J Physiol Cell Physiol 300: C1172–C1180, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xu L, Salloum D, Medlin PS, Saqcena M, Yellen P, Perrella B, Foster DA. Phospholipase D mediates nutrient input to mammalian target of rapamycin complex 1 (mTORC1). J Biol Chem 286: 25477–25486, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yoshida S, Hong S, Suzuki T, Nada S, Mannan AM, Wang J, Okada M, Guan KL, Inoki K. Redox regulates mTORC1 activity by modulating the TSC1/TSC2-Rheb pathway. J Biol Chem 286: 32651–32660, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]