Abstract
Physiological studies of intact crypt epithelium have been limited by problems of accessibility in vivo and dedifferentiation in standard primary culture. Investigations of murine intestinal stem cells have recently yielded a primary intestinal culture in three-dimensional gel suspension that recapitulates crypt structure and epithelial differentiation (Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, Van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Nature 459: 262–265, 2009). We investigated the utility of murine intestinal crypt cultures (termed “enteroids”) for physiological studies of crypt epithelium by focusing on the transport activity of the cystic fibrosis transmembrane conductance regulator Cftr. Enteroids had multiple crypts with well-differentiated goblet and Paneth cells that degranulated on exposure to the muscarinic agonist carbachol. Modified growth medium provided a crypt proliferation rate, as measured by 5-ethynyl-2′-deoxyuridine labeling, which was similar to proliferation in vivo. Immunoblots demonstrated equivalent Cftr expression in comparisons of freshly isolated crypts with primary and passage 1 enteroids. Apparent enteroid differences in mRNA expression of other transporters were primarily associated with villous epithelial contamination of freshly isolated crypts. Microelectrode analysis revealed cAMP-stimulated membrane depolarization in enteroid epithelium from wild-type (WT) but not Cftr knockout (KO) mice. Morphological and microfluorimetric studies, respectively, demonstrated Cftr-dependent cell shrinkage and lower intracellular pH in WT enteroid epithelium in contrast to Cftr KO epithelium or WT epithelium treated with Cftr inhibitor 172. We conclude that crypt epithelium of murine enteroids exhibit Cftr expression and activity that recapitulates crypt epithelium in vivo. Enteroids provide a primary culture model that is suitable for physiological studies of regenerating crypt epithelium.
Keywords: cystic fibrosis transmembrane conductance regulator, cell proliferation, cell size, stem cells, intracellular pH
past investigations of transport physiology in intestinal crypt epithelium have been limited by the relative inaccessibility of the crypt in native intestine and failure to sustain crypt epithelial differentiation in primary culture using standard methods. Most previous physiological studies used either freshly isolated colonic crypts or imaged the base of crypts in muscle-stripped colonic sheets, which elucidated important features including the presence of absorptive function (22, 29), aspects of extracellular pH regulation (7), cell volume changes (39, 60), transepithelial NaCl movement, and second messenger Ca2+i/cAMP regulation (26). However, important variables in those experiments were changes in the structural and functional integrity of the crypt epithelium resulting from apoptosis (anoikis) initiated by the acute loss of contact with the extracellular matrix (ECM) and submucosal elements (32). In some studies (e.g., Ref. 22), microdissection of colonic crypts resulted in fairly uniform retention of the basement membrane thereby minimizing the effect due to loss of the ECM (49), whereas in other studies (e.g., Ref. 39), colonic crypt isolation by Ca2+ chelation was used, which disrupts the integrity of the basement membrane (49). Also, reports from one laboratory (48) indicate that apoptotic induction is overcome using short-term culture on collagen matrices where crypt features of proliferation/axial migration are maintained, but this methodology has not become widespread nor adapted to murine intestine for use with gene-targeted or transgenic models. Thus the available experimental preparations have limitations that affect our understanding of crypt physiology in the colon and to a greater extent in the small intestine.
Developmental biologists investigating intestinal stem cell identity have provided a model of regenerating intestinal crypts grown in three-dimensional (3D) gel culture (52). In a study validating the putative stem cell marker, leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5), it was shown that freshly isolated small intestinal crypts placed in collagen gels with specific growth factors would form self-organizing crypt organoids (enteroids). Crypts seal at the open end and begin a process whereby new crypts are formed by a process similar to crypt fission in vivo (14, 15). The enteroid epithelial cells migrate from the crypt to a central epithelial-lined cavity (villus-like domain) where they are eventually shed in an apoptotic process. The crypts produce all four cell lineages (Paneth, goblet, enteroendocrine, and absorptive) in roughly the same proportions as in vivo, show <2.0% variance in gene expression from freshly isolated crypts, retain euploidism, and can be passaged at regular intervals for months (52). Although enteroid culture has been investigated for stem cell characteristics and cell-cell interactions (51, 52), little is known about the physiology of the enteroid crypts and whether they provide a useful model of intestinal crypts in vivo.
Cftr is arguably the dominant ion transporter in crypt epithelium where it functions in transepithelial secretion of Cl− and HCO3−. Cftr is highly expressed in the crypt epithelium and at reduced levels in the villous epithelium in an expression gradient that decreases along the longitudinal axis of the small bowel (except for low numbers of CFTR high expressing cells; Refs. 3, 57). The conductive properties of Cftr exhibit a Cl−: HCO3−permeability ratio of ∼4:1 (45), which may be modulated by the concentration of extracellular anions (54, 63), activity of with-no-lysine kinases (44), and interactions with Slc26a anion exchangers (36). Cftr also provides a conductive Cl− “leak” pathway that facilitates the activity of luminal Cl−/HCO3− exchangers in the small intestine (56). The central role of Cftr in the cellular export of HCO3− is demonstrated by the alkaline intracellular pH (pHi) that develops in duodenal villous epithelium of Cftr-null mice under conditions designed to accentuate apical membrane transport (56). Cftr has also been shown to regulate other transport proteins, especially Na+ transporters (18, 28, 58), which include the Na+/H+ exchanger Nhe3 in the small intestine (20). Thus the multifunctional role that Cftr plays in processes of ion and acid-base transport is essential to normal crypt physiology. In the present study, we investigate the activity of Cftr in murine enteroids to determine the value of this culture model for physiological studies of intestinal crypt epithelium.
MATERIALS AND METHODS
Mice.
Mice with gene-targeted disruption of the murine homolog of Cftr [Abcc7, Cftr knockout (KO)] and wild-type (WT) littermates were used. All comparisons were made with sex- and age-matched (+/+ or +/−) siblings. The mutant mice were identified using a PCR-based analysis of tail snip DNA, as previously described (9). All mice were maintained ad libitum on standard laboratory chow (Formulab 5008, Rodent Chow; Ralston Purina) and distilled water containing Colyte laxative to avoid intestinal obstruction in the Cftr KO mice (9). Mice were housed individually in a temperature (22–26°C)- and light (12:12-h light-dark cycle)-controlled room in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facility at the Dalton Cardiovascular Research Center, University of Missouri. All experiments involving animals were approved by the University of Missouri Animal Care and Use Committee.
Enteroid culture.
The enteroid culture method was modified from Sato et al. (52). Mouse proximal small intestine (∼10 cm) was excised, opened longitudinally, and washed with ice-cold PBS. The intestine was cut into small pieces (∼4- to 5-mm diameter) and incubated in ice-cold PBS containing 2 mM EDTA for 30 min. After being rinsed once with ice-cold PBS to remove EDTA, the intestinal fragments were resuspended four times in ice-cold PBS (10 ml) by repeated, vigorous pipetting using a 10-ml pipette. After each resuspension, the tissue fragments were allowed to settle and the supernatant from the first two resuspensions was discarded. The supernatant from the last two resuspensions was collected and passed through a 70-μm cell strainer (Becton-Dickinson Bioscience, Franklin Lakes, NJ) to remove tissue fragments. Crypts in the strained solution were separated from suspended single cells by centrifugation (200 g, 1 min). The crypt pellet was resuspended with cold PBS and mixed 1:2.5 vol/vol with Matrigel (BD Bioscience) for plating (e.g., ∼100 μl/35-mm petri dish). After polymerization of the Matrigel, culture medium composed of Ham's F-12 containing 5% FBS, 50 μg/ml gentamicin, 125 ng/ml Rspondin1, 25 ng/ml noggin, and 12.5 ng/ml epidermal growth factor (EGF) was added and changed every 2–4 days. The percentage of FBS and concentrations of Rspondin1, noggin, and EGF are 25% of those used in the method by Sato et al. (52). Preliminary studies of WT enteroids grown in increasing dilutions of these growth factors indicated that enteroid development, as assessed by size and crypt numbers, was slowed by ∼1–2 days but not prevented using 25% of the original concentration (data not shown). For enteroid passage at 7–10 days postplating, culture dishes were rinsed twice with ice-cold PBS before addition of cell recovery solution (BD Biosciences). Matrigel containing the enteroids was scraped from the dish surface using a rubber cell scraper and, together with the cell recovery solution, transferred to 1.5-ml tubes for a 30-min incubation on ice. Enteroid fragments were centrifuged at 200 g (1 min), and supernatant containing Matrigel was aspirated. The enteroids were washed once with ice-cold PBS (∼1 ml), centrifuged, and resuspended in PBS for plating.
Proliferation and immunofluorescence.
Proliferation of crypt epithelium in vivo and enteroids was measured using the Click-iT 5-ethynyl-2′-deoxyuridine (EdU) assay (Invitrogen) to label cells in S phase of the cell cycle, according to the manufacturer's protocol. Mice were injected with EdU (4.8 μg/g body wt) 1 h before euthanasia for the collection of duodenum. Duodenum was rinsed in ice-cold PBS, fixed in 4% paraformaldehyde overnight, embedded in paraffin, and cut into 5-μm sections. Fluorescence (AlexFluor 488) photomicrographs of crypt cross sections were counted for EdU+ cells. For enteroids, 4-day-old cultures (passages 1 and 2) were exposed in situ to EdU for 15 min and fixed in 4% paraformaldehyde (4°C, overnight). Fixed enteroids and Matrigel were scraped from the culture dishes, placed in 1.5-ml tubes, and centrifuged at 200 g (1 min), and supernatant containing Matrigel was aspirated. Enteroids were washed twice in PBS (∼1 ml) in the same manner before the Click-iT assay was performed. Labeled enteroids were concentrated by brief centrifugation (200 g) and sealed under glass coverslips on microscope slides. Crypts were imaged on a TCS SP5 Confocal-Multiphoton microscope built on a DMI6000 inverted platform (Leica, Wetzler, Germany). Z stacks were used to determine crypt cross sections for counting of EdU-labeled nuclei, avoiding sections where crypt fission was observed. For immunofluorescence of freshly isolated crypts, the crypts were fixed overnight in 4% paraformaldehyde, washed in PBS, and permeabilized using PBS + 0.5% Triton X-100. After blocking with PBS + 10% goat serum (30 min), the crypts were exposed overnight (4°C) to β-catenin antibody (sc-7199; Santa Cruz Biotechnology, Santa Cruz, CA) diluted in PBS + 0.1% Triton X-100. Crypts were washed three times in PBS + 2% goat serum before incubating overnight with anti-rabbit IgG Texas Red (sc-2780; Santa Cruz; 1:100 dilution). Washed crypts (PBS + 2% goat serum) were incubated with Hoechst 33342 diluted 1:2,000 for 1 h. Crypts were suspended in buffered Fluorogel (Electron Microscopy Sciences, Hatfield, PA), mounted under glass coverslips, and imaged on the TCS SP5 Confocal-Multiphoton microscope (Leica).
Immunoblot analysis.
Freshly isolated crypts and enteroids isolated by the method used for passaging (see Enteroid culture) were suspended in ice-cold PBS and lysed at 4°C by supersonication. Whole small intestinal epithelium from WT and Cftr KO mice was isolated by using the EDTA chelation technique, as describe previously (21), and processed similarly. Total lysate protein was loaded on 10% SDS-PAGE gels for electrophoresis, membrane transfer, and immunoblotting. Anti-Cftr (3G11; provided by CFTR Folding Consortium, Cystic Fibrosis Foundation Therapeutics) and anti-Pept1 (sc-20653; Santa Cruz) were used as primary antibodies. Anti-β-tubulin (sc-9104; Santa Cruz) was used as loading control.
Quantitative RT-PCR array.
Epithelial mRNA expression was measured in freshly isolated crypts, primary enteroids (p0, plated 7–10 days), and passage 1 enteroids (p1, plated 7–10 days) each from the same WT mouse. Freshly isolated crypts were placed immediately into RNA Later (Applied Biosystems, Foster City, CA) and refrigerated, whereas p0 and p1 enteroids were removed from Matrigel, rinsed twice with ice cold PBS, and placed in RNA Later for refrigeration. Samples in RNA Later were mixed with PBS (2:1 vol/vol) and pelleted before homogenization with QiShredder and total RNA extraction using RNeasy Plus (according to the manufacturer's protocol; Qiagen, Germantown, MD). Isolated RNA was reverse transcribed with Superscript III First Strand Synthesis Kit (Invitrogen, Carlsbad, CA) using oligo dT according to the manufacturer's protocol. cDNA was mixed with TaqMan Gene Expression Master Mix (Applied Biosystems), according to the manufacturer's protocol, and loaded onto customized mini-array 96-well plates containing TaqMan assays for the genes of interest (Table 1). In addition to the genes of interest, β-actin, serving as a −RT control, and three housekeeping genes (β-glucuronidase, hypoxanthine guanine phosphoribosyl transferase 1, and mitochondrial ribosomal protein L19) were assayed. A Mastercycler EP RealPlex thermocycler (Eppendorf, Hamburg, Germany) was used for quantitative PCR with the following protocol: 50°C (2 min), 95°C (10 min), and 40 cycles of 95°C (15 s), 60°C (1 min). The threshold cycle (Ct) of a gene of interest was subtracted from the geometric mean Ct of the housekeeping genes to yield ΔCt. The relative mRNA expression of the p0 or p1 enteroids vs. freshly isolated crypts was calculated using the ΔΔCt method (38).
Table 1.
Gene name, symbol, and Applied Biosystems assay ID for TaqMan mini-array
| Gene Name | Symbol | Assay ID |
|---|---|---|
| RNA, 18S ribosomal 1 (18S)* | RN18S1 | Hs03928990_g1 |
| Beta-glucuronidase† | Gusb | Mm00446953_m1 |
| Hypoxanthine guanine phosphoribosyl transferase 1† | Hprt1 | Mm00446968_m1 |
| Mitochondrial ribosomal protein L19† | Mrpl19 | Mm00452754_m1 |
| Actin, beta‡ | Actb | mm00607939_s1 |
| Na+/K+-ATPase, alpha-1 | Atp1a1 | Mm00523255_m1 |
| Carbonic anhydrase II (CaII) | Car2 | Mm00501572_m1 |
| Carbonic anhydrase IX (CaIX) | Car9 | Mm00519870_m1 |
| Cystic fibrosis transmembrane conductance regulator (Cftr) | Cftr | Mm00445197_m1 |
| Na+-K+-2Cl− cotransporter (Nkcc1) | Slc12a2 | Mm00436554_m1 |
| Down regulated in adenoma (Dra) | Slc26a3 | Mm00445313_m1 |
| Putative anion transporter 1 (Pat1) | Slc26a6 | Mm00506742_m1 |
| Anion exchanger 2 (Ae2) | Slc4a2 | Mm00436617_m1 |
| Na+ bicarbonate cotransporter electrogenic 1 (Nbce1) | Slc4a4 | Mm01347935_m1 |
| Sodium hydrogen exchanger 1 (Nhe1) | Slc9a1 | Mm00444270_m1 |
| Sodium hydrogen exchanger 2 (Nhe2) | Slc9a2 | Mm01237129_m1 |
| Sodium hydrogen exchanger 3 (Nhe3) | Slc9a3 | Mm01352473_m1 |
Manufacturing control;
housekeeping gene;
no reverse transcriptase control. For specific assay information, see the Applied Biosystems genomics product search page http://bioinfo.appliedbiosystems.com/genome-database/ and enter the assay ID.
Intracellular microelectrodes.
Enteroids from WT and Cftr KO littermate pairs (p0 and p1) were plated in Matrigel on chambered glass microscope slides (Fisher Scientific, Springfield, NJ) and cultured for 5–8 days. After removal of the culture chamber, the Matrigel was removed to within 0.5–1.0 mm from one side of individual enteroids using a #11 scalpel blade under stereomicroscopy. This typically exposed one to three crypts, leaving the remainder of the enteroid embedded in gel. The microscope slide was fitted with a polycarbonate perfusion chamber (Warner Instruments, Hamden, CT) to enable constant superfusion with a Kreb's bicarbonate Ringer (KBR) solution containing 5 mM N-tris(hydroxymethyl)-methyl-2-aminoethanesulfonic acid (TES) buffer and gassed with 95% O2-5% CO2 (pH 7.4, 37°C). Under magnification of an inverted microscope (×10 objective, Olympus IMT-2), residual Matrigel was aspirated from one side of individual crypts using a ∼100-μm (outer diameter) glass micropipette directed by a remote-controlled micromanipulator (MW-3; Narishige International, East Meadow, NY). Exposed enteroids were superperfused with KBR + TES containing vehicle (0.1% DMSO) or forskolin (10 μM) ± Cftr inhibitor 172 (Cftrinh172, 25 μM).
The method for construction and use of microelectrodes in intact intestinal epithelium was recently described (1). For these experiments, sharp microelectrodes were constructed from aluminum silicate glass capillary tubes (1.2-mm outer diameter) pulled on a horizontal puller (P-97 Flaming/Brown micropipette puller; Sutter Instruments, Novato, CA) to a tip resistance of 331 ± 14 MΩ when immersed in KBR + TES (n = 152). Microelectrodes were backfilled with 500 mM KCl and connected via an Ag-AgCl pelleted holder to a high-impedance amplifier (Duo 733; World Precision Instruments, Sarasota, FL). Cellular impalements were performed roughly perpendicular to the basolateral cell surface in cells (greater than +4 position) in individual crypts using light microscopy (×20 objective) and a remote-controlled micromanipulator. A 3M KCl agar bridge connected the bath to a calomel half-cell and served as ground. Signals were acquired using a Digidata 1332A A-D converter (Axon Instruments, Union City, CA) and pCLAMP 8.0 software (Molecular Devices, Sunnyvale, CA). The basolateral membrane potential (Vb) was indicated by an instantaneous shift in the microelectrode voltage that stabilized within ±5 mV by 10 s. Impalements were accepted if sustained longer than 30 s and returned to within ± 2 mV upon retraction. Sign convention is chosen so that Vb was referenced to the bath (basolateral side).
Measurement of cell shrinkage.
Crypt epithelial volume and epithelial cell height measured on optical cross sections were used as an index of cell volume, as previously described for polarized epithelium (16, 20, 61). Enteroids from sex-matched, littermate WT and Cftr KO mouse pairs were grown on glass chamber slides. One-half of an enteroid was removed from the gel after bisection using the bevel of a 30-gauge needle under stereomicroscopy. The glass slide with bisected enteroids remaining within the gel was fitted with a polycarbonate horizontal perfusion chamber and superfused in a direction opposite the open side of the enteroids using KBR + TES (pH 7.4, gassed with 95% O2-5% CO2 at 37°C). A single crypt per culture was imaged using a ×40 water immersion objective on an Olympus BX-50WI microscope. Images were acquired at 1-min intervals for 20 min using a Sensi-Cam digital camera (Cooke, Auburn Heights, MI) and processed postacquisition using Slidebook 5.0 software (Intelligent Imaging Innovations, Denver, CO). Enteroids were constantly superfused with KBR + TES containing one of the following: 0.1% DMSO (vehicle), forskolin (10 μM), or carbachol (100 μM). For experiments with CFTRinh172, cultures were treated with CFTRinh172 (25 μM) for 1 h in the incubator before the experiment and CFTRinh172 (25 μM) was included in the superfusate during the experiment. Due to the hydrophobic nature of CFTRinh172, a longer incubation for diffusion is required for enteroids embedded within the Matrigel and preliminary studies indicated that a 1-h period provided optimal inhibition of forskolin-induced WT crypt cell shrinkage (data not shown). Cell height, i.e., distance from the apical to basolateral membrane, and the mean height (h) and width (2r) of the crypt and its corresponding crypt lumen in the midcrypt region (+8 to +15 cell position) were measured before and 10 min after treatment from captured images using ImagePro Plus 6.3 software (Media Cybernetics, Bethesda, MD). The percent change in cell height was calculated by dividing the change in cell height by the basal (pretreatment) cell height * 100. The percent change in crypt epithelial volume was calculated by dividing the change in crypt epithelial volume by the basal (pretreatment) crypt epithelial volume *100. Crypt epithelial volume at both points was calculated, assuming a cylindrical shape of the crypt and crypt lumen, by subtracting the crypt lumen volume from the total crypt volume, using the formula for a cylinder volume π·h·r2. Stable, morphological landmarks were used to determine the cylinder height at locations within the +8 to +15 cell position above the crypt base before and after treatments. Cell height and epithelial volume at the crypt base were not determined because nearly complete Paneth cell degranulation during some treatments, e.g., carbachol, confounded accurate measurement of the parameters.
Confocal microscopy measurement of intracellular pH.
Enteroids were cultured for 4–5 days on glass-bottomed 35-mm Fluorodishes (World Precision Instruments), as described in Enteroid culture. Enteroid epithelium was loaded with 40 μM ratiometric fluorescent pH indicator SNARF-5F (Invitrogen) and 2.5 μM quinicrine (Sigma-Aldrich, St. Louis, MO) in culture medium for 30 min at 37°C. Enteroid cultures were mounted on the stage of a TCS SP5 Confocal-Multiphoton microscope built on a DMI6000 inverted platform (Leica) and fitted with a temperature-controlled incubator containing a 95% O2-5% CO2 atmosphere (Life Imaging Services, Basel, Switzerland). Organoids were continuously superfused with KBR + TES (pH 7.4, gassed with 95% O2-5% CO2, 37°C). The excitation source for quinicrine was a 488-nm argon laser, and images were collected at an emission wavelength of 500–540 nm. The excitation source for SNARF 5F was a 514 nm argon laser and images were collected at dual emission wavelengths (580 ± 30 and 640 ± 30 nm). Z stacks (∼30 1-μm slices) of individual crypts were acquired using the two sets of excitation-emission wavelengths (avoiding optical contamination of SNARF 5F fluorescence by quinicrine). Avoiding granulated (quinicrine stained) cells, enterocytes were selected postacquisition for measurement of pHi by placing a 3D measurement sphere within the confines of individual cells using Imaris software (Bitplane, South Windsor, CT). The 580-to-640-nm ratio was converted to pHi using a standard curve generated by K+/nigericin technique (56).
Materials.
SNARF 5F acetoxymethyl ester was obtained from Invitrogen. Forskolin and Cftrinh172 were obtained from Enzo Life Sciences (Farmingdale, NY). EGF and noggin were obtained from R&D Systems (Minneapolis, MN). Recombinant Rspondin1 was isolated as described previously (43). All other materials were of analytical grade and obtained from either Sigma-Aldrich or Fisher Scientific.
Statistics.
All values are reported as means ± SE. Data between two groups were compared using a two-tailed Student t-test assuming equal variances between groups. Data from multiple treatment groups were compared using a one-way ANOVA with a post hoc Tukey's t-test. A probability value of P < 0.05 was considered statistically significant.
RESULTS
The enteroid model.
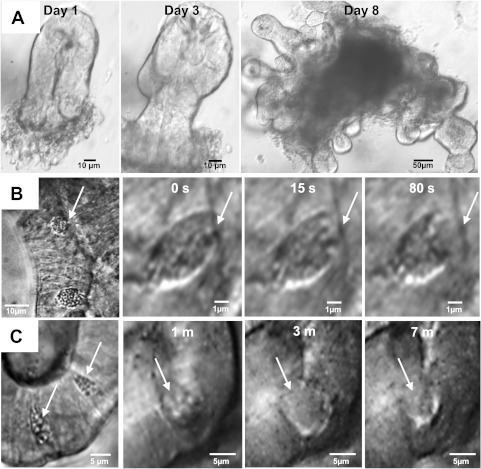
With the use of modifications of Sato et al. (52; see Enteroid culture), freshly isolated crypts initiated a process of crypt fission within 1–2 days to form an organoid with multiple crypts and a central, epithelial-lined cavity containing sloughed apoptotic cells (Fig. 1A). As shown in Fig. 1, B and C, goblet and Paneth cells were well differentiated and readily identified morphologically by light microscopy based on the characteristics of granule size, theca position, and location within the crypt, as described previously (8, 59). Goblet cell numbers in the WT enteroid crypts approximated WT small intestine crypts (WT enteroid: 4.8 ± 0.2 goblet cells/crypt cross section; WT small intestine: 5.2 ± 0.3 goblet cells/crypt cross section, NS, n = 6–7 mice). Paneth cell numbers were not estimated due to the difficulty of distinguishing individual cell borders in the histological sections. Goblet and Paneth cell functional activity was demonstrated by induction of degranulation upon basolateral exposure to the muscarinic agonist carbachol (100 μM; Fig. 1, B and C).
Fig. 1.
Enteroid model. A: development over 8 days of an enteroid structure from a single, freshly isolated murine crypt in Matrigel suspension culture (magnification: ×10 and ×20). B: goblet cell in enteroid crypt (left) and time course of goblet cell degranulation in response to treatment with 100 μM carbachol (right; magnification: ×60). C: Paneth cells in enteroid crypt (left) and time course of Paneth cell degranulation and partial recovery after treatment with 100 μM carbachol (right; magnification: ×60).
Rapid development of the enteroids raised the question of whether the epithelial proliferation rate of the enteroids recapitulates that of crypts in vivo. To estimate crypt epithelial proliferation rate in vivo, EdU labeling was used to identify S-phase cells in crypt cross sections of duodenal sections from WT mice (1 h post-EdU injection ip). As shown by the histogram in Fig. 2A, the number of EdU+ cells per crypt exhibited a wide range of proliferation, varying from quiescent crypts (0 EdU+ cells) to highly proliferative crypts (26 EdU+ cells). The number of EdU+ cells per crypt cross section was normally distributed with the means = 10.4 ± 2.2 EdU+ cells/crypt. With the use of enteroids cultured with modified growth medium, proliferation in WT enteroids was assessed in gel culture by in situ exposure to EdU (15 min) and counting of EdU+ cells in confocal images of enteroid crypts (Fig. 2B, inset). Mature enteroid crypt cross sections with 25–40 total nuclei were used for proliferation measurements. EdU+ cells at new sites of crypt fission (“buds”) were not included in the counts because most cells in a crypt bud are EdU+ and a distinct crypt lumen is not always apparent. As shown by the graph in Fig. 2B, the EdU+ cells/crypt cross section of WT enteroids also demonstrated a wide range of proliferation (2–23 EdU+ cells) that was normally distributed with a mean almost identical to in vivo (means = 10.8 ± 0.9 EdU+ cells/crypt cross section). Thus proliferation in the enteroid model is similar to in vivo but, based on observation, differs in the number and extent of crypt fission events. In vivo, crypt fission is a rare but normal biological process (14, 15) that we observed in <5% of freshly isolated small intestinal crypts (examples shown in Fig. 2C). Although crypt fission in the enteroids is difficult to visualize in all planes, a longitudinal study found that 57.6% of crypt cross sections have evidence of a budding event, i.e., bulging of cells at a confined site, within 1–3 days after mature crypt formation (n = 20 enteroids, 1–2 crypts/enteroid, 6 WT mice).
Fig. 2.
Proliferation in native intestine and enteroid culture. A: proliferation rate in native wild-type (WT) duodenal crypts as measured by 5-ethynyl-2′-deoxyuridine (EdU) labeling of S-phase cells in fixed sections. Cumulative data of EdU-positive (EdU+) cells/crypt are normally distributed; n = 6 WT mice (8–12 crypts/mouse). B: proliferation rate in WT enteroid crypts (4 days in culture, passages 1 and 2) as measured in situ by EdU labeling of S-phase cells. Cumulative data of EdU-positive (+) cells/crypt are normally distributed; n = 5 WT mice (14–26 enteroid crypts/mouse). Inset: merged confocal image of enteroid crypts in situ with nuclear staining using EdU (green) and Hoechst 33342 (blue; magnification: ×63, n.a. 1.2). Arrows indicate EdU+ cells at crypt base and transit amplifying region. C: examples of spontaneous crypt fission in freshly isolated crypts from WT (left) and cystic fibrosis transmembrane conductance regulator (Cftr) knockout (KO; right) mouse small intestines. Shown are merged images of crypts stained with anti-β-catenin (red) and nuclear dye Hoechst 33342 (blue; magnification: ×63, n.a. 1.2).
Cftr and other transporter expression.
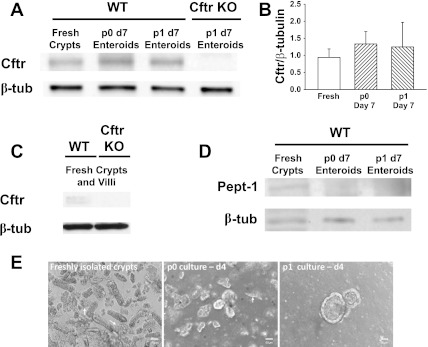
To assess the fidelity of the enteroid model with regard to Cftr activity, Cftr protein expression was measured by immunoblot analysis for comparison of freshly isolated crypts with primary (p0) and passage 1 (p1) cultured enteroids, all from the same WT mice. Cftr expression in the intestine is at highest levels in the crypts (2). As shown in Fig. 3, A and B, Cftr protein expression in p0 and p1 WT enteroids is equivalent to Cftr expression in freshly isolated WT crypts. Specificity of the Cftr immunoblot is demonstrated by the absence of Cftr detection in p1 enteroids from Cftr KO mice (Fig. 3A). Although the cumulative data did not indicate a statistical difference, a slight trend towards lower mean Cftr expression in the freshly isolated crypts was noted (mean Cftr densitometry of fresh vs. p0 day 7 enteroids, P = 0.395). It was reasoned that this may be an artifact resulting from contamination of the freshly isolated crypts with villous epithelium, which have much lower Cftr expression (2), and likely the stability of Cftr protein in apoptotic cells of the enteroid central lumen. Consistent with villous contamination, Cftr immunoblots on freshly isolated whole small intestinal epithelium, i.e., crypt and villous epithelium, from WT mice show very low levels of Cftr expression, presumably due to protein lysate “dilution” with villous epithelium (Fig. 3C). To verify villous epithelial contamination in our immunoblots, freshly isolated crypts were compared with p0 and p1 enteroids for Pept1, a H+ di-/tripeptide transporter which is primarily expressed in villous epithelium (27). As shown in Fig. 3D, Pept1 protein was detected in freshly isolated crypts relative to p0 and p1 enteroids, consistent with villous cell contamination of the freshly isolated crypt lysates. As shown in Fig. 3E, sheets of villous epithelium were typically observed in freshly isolated crypt preparations and, to a lesser extent, the p0 enteroid cultures.
Fig. 3.
Comparison of gene expression between freshly isolated crypts and primary (p0) or passage 1 (p1) enteroids cultured from crypts of the same WT mice. A: immunoblot for Cftr in freshly isolated crypts (fresh), p0 enteroids, and p1 enteroids (top). Enteroids were cultured for 7 days (d7). Immunoblot for β-tubulin (β-tub) was used as a loading control and lysates from Cftr KO enteroids demonstrate antibody specificity for murine Cftr. B: densitometry for immunoblots of Cftr normalized to β-tubulin in freshly isolated crypts, p0 enteroids and p1 enteroids from the same mice. n = 5 mice. C: immunoblot of Cftr in freshly isolated whole small intestinal epithelium from WT and Cftr KO mice (representative of n = 3). D: immunoblot for the H+ di-/tripeptide cotransporter Pept1 to assess villous epithelial contamination in the freshly isolated crypts, p0, and p1 enteroids. E: villous epithelial contamination in freshly isolated crypts and p0 enteroid cultures. Left: small sheets of villous epithelium (arrows) are inadvertently collected with freshly isolated crypts; middle: contaminating villous epithelium (arrow) appears viable for ∼4 days in p0 cultures; right: extraneous cellular debris is primarily retained with the gel solution during passage, yielding p1 and subsequent passaged enteroid cultures with minimal contamination. Magnification: ×10.
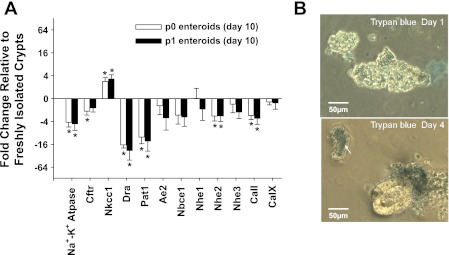
The mRNA expression of other acid-base transporters and relevant carbonic anhydrases measured by quantitative RT-PCR mini-arrays was compared among freshly isolated crypts, p0 enteroids and p1 enteroids from individual WT mice (Fig. 4A; Table 1). With the exception of Nkcc1, several transporters in the p0 and p1 enteroids had a mean mRNA abundance that was lower than in the freshly isolated crypts (which were assigned a value of zero in Fig. 4A). Significant decreases were found for the alpha-1 subunit of the Na-K Atpase, Nhe2, Dra, Pat-1, and CaII. Each of these transporters is expressed at greater levels in villous compared with crypt epithelium in mammalian intestine (11, 23, 33, 34, 50, 62), suggesting villous epithelial cell contamination of the RNA in the freshly isolated crypts. Since villous epithelium is considered terminally differentiated, we sequentially assessed the viability of noncrypt epithelium in p0 enteroid cultures from WT mice by trypan blue exclusion. Approximately 50% of the contaminating noncrypt epithelium failed to exclude trypan blue after 1 day and >90% failed to do so after 4 days (Fig. 4B). Given the relative instability of mRNA, it is unlikely that villous RNA significantly contaminated the samples from the p0 enteroids at day 10. In contrast to the above transporters, Nkcc1, which showed a significantly increased expression in the p0 and p1 enteroids, has greater expression in the crypt relative to villous epithelium (35). Curiously, Cftr mRNA, which should show a similar trend, tended to be reduced and levels attained a significant difference in the p0 enteroids. The reason for this variance from the protein expression may relate to the relative stability of Cftr protein vs. Cftr mRNA in the apoptotic cells of the enteroid central lumen. However, the departure from our interpretation of the mRNA expression data suggests that cftr transcription is slightly reduced in the enteroid cultures. This will likely require additional investigation because lower Cftr mRNA expression may result from the effect of mild hypoxia on Cftr expression due to enteroid/gel submersion in culture medium (30). Ae2, Nbce1, Nhe1, Nhe3, and CaIX in the p0 and p1 enteroids were not significantly different from the freshly isolated crypts. Direct comparisons between the p0 and p1 enteroids did not reveal a significant difference in mRNA expression for any of the genes of interest (data not shown).
Fig. 4.
Comparison of mRNA expression of Cftr and other electrolyte transport-relevant proteins between freshly isolated crypts and enteroids (p0, p1) from the same WT mice. A: enteroids were cultured for 7–10 days. Shown is the fold change in mRNA expression of the p0 and p1 enteroids relative to that in freshly isolated crypts based on the ΔΔCt method. Zero represents no change from freshly isolated crypts (note: the ordinate axis is log base 2 converted to log base 10 scale). *P < 0.05, significantly different from freshly isolated crypts; n = 6 WT mice. No statistically significant differences were found between p0 and p1 enteroid preparations. Na+-K+-Atpase, alpha-1 subunit (Atpa1); Cftr, Abcc7; Nkcc1, Na+/K+/2Cl− cotransporter (Slc12a2); Dra, downregulated in adenoma (Slc26a3); Pat-1, putative anion transporter-1 (Slc26a6); Ae2, anion exchanger 2 (Slc4a2); Nbce1, Na+ bicarbonate cotransporter electrogenic 1 (Slc4a4); Nhe1, Na+/H+ exchanger 1 (Slc9a1); Nhe2, Na+/H+ exchanger 2 (Slc9a2); Nhe3, Na+/H+ exchanger 3 (Slc9a3); CaII, carbonic anhydrase II (Car2); and CaIX, carbonic anhydrase IX (Car9). B: viability of contaminating villous epithelium in a p0 enteroid culture. Top: trypan blue exclusion from ∼50% of cells in sheets of villous epithelium in p0 culture. Bottom: trypan blue exclusion from <10% of cells in villous sheets (top left, arrow) and apoptotic cells in enteroid (representative of 3 experiments). Note exclusion of dye from crypt cells of enteroid. Magnification = ×10.
Functional activity: Cftr-dependent membrane depolarization.
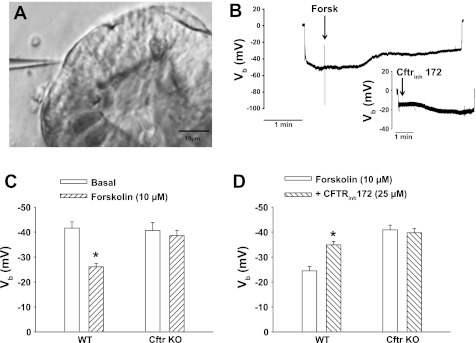
Cftr is the predominant anion channel in the apical membrane of crypt epithelium and is regulated by intracellular cyclic nucleotide activity (4, 35). Stimulation of Cftr activity by agonists increasing intracellular cAMP results in cell membrane depolarization resulting from an inward current carried by Cl− and HCO3− conduction (45). To evaluate the activity of Cftr in the enteroid crypts, conventional microelectrodes were used to assess changes in the membrane potential of crypt enterocytes (Fig. 5A). As shown by the experiment on a p1 WT enteroid (Fig. 5B), impalement across the enterocyte basolateral membrane resulted in an abrupt deflection from ground (0 mV) to approximately −50 mV. During sustained impalement recording, the cAMP agonist forskolin (10 μM) was introduced into the superfusate, resulting in depolarization of the membrane potential (Vb). Retraction of the microelectrode abruptly returned the electrode voltage to near ground (0 mV), indicating a successful impalement. As shown in Fig. 5B, inset, subsequent impalement of another enterocyte during exposure to forskolin (∼10 min) revealed that the crypt enterocytes remained depolarized. However, addition of Cftrinh172 (25 μM) to the superfusate resulted in partial repolarization of Vb, indicative of Cftr-dependent conductive activity. To investigate this further, the membrane potential of enterocytes under basal and cAMP-stimulated conditions was measured in p0 and p1 enteroids from sex-matched WT and Cftr KO littermate mice. As shown in Fig. 5C, enterocytes from WT and Cftr KO enteroids demonstrate a similar membrane potential, averaging approximately −40 mV. Following exposure to forskolin in the superfusate (10–20 min postaddition), Vb in WT enterocytes was depolarized to approximately −25 mV, whereas Vb was unchanged in Cftr KO enterocytes and significantly greater than the forskolin-treated WT cells (P < 0.05). In a second series of experiments (Fig. 5D), p0 and p1 enteroids from paired WT and Cftr KO mice were superfused with a forskolin-containing solution. Multiple cell impalements between 10 and 20 min after forskolin demonstrated Vb depolarization only in the WT enterocytes. Subsequent treatment with Cftrinh172 for 10–30 min resulted in ∼10- to 15-mV repolarization of Vb in the WT enterocytes but had no effect of Vb in the Cftr KO enterocytes.
Fig. 5.
Microelectrode analysis of Cftr-dependent changes in membrane potential in enteroid crypt epithelium. A: micrograph of enteroid crypt epithelial cell impaled with a conventional microelectrode (magnification: ×20). B: representative recording of the basolateral membrane potential (Vb) of an impaled cell before and after exposure to 10 μM forskolin. Inset: representative recording of Vb in an impaled cell from the same forskolin-treated enteroid crypt before and after exposure to 25 μM Cftrinh172. Note abrupt voltage change from 0 mV upon microelectrode impalement and return towards 0 mV upon microelectrode retraction in both recordings. However, there was a −4-mV electrode drift during the prolonged impalement showing forskolin-induced depolarization. C: cumulative data of mean Vb measured in WT and Cftr KO enteroid crypt epithelial cells before (basal) and after exposure to 10 μM forskolin. Impalements during forskolin were performed 10–30 min after treatment. Enteroids were from WT and Cftr KO sex-matched littermate mice. *P < 0.05, significantly different from basal within genotype; n = 33–34 impalements, 4–6 p0-p1 enteroids from 3 mice pairs. Mean Vb for Cftr KO, both basal and forskolin, is significantly greater than WT treated with forskolin, P < 0.05. D: cumulative data of mean Vb measured in WT and Cftr KO forskolin-treated enteroid crypt epithelial cells before (forskolin: 10 μM) and after exposure to 25 μM Cftrinh172. Enteroids were exposed to forskolin for 10 min before microelectrode impalements. Impalements during Cftrinh172 were performed 10–30 min after treatment. Enteroids were from WT and Cftr KO sex-matched littermate mice. *P < 0.05, significantly different from forskolin within genotype; n = 16–26 impalements, 3–4 p0-p1 enteroids from 3 mice pairs.
Functional activity: Cftr-dependent cell shrinkage.
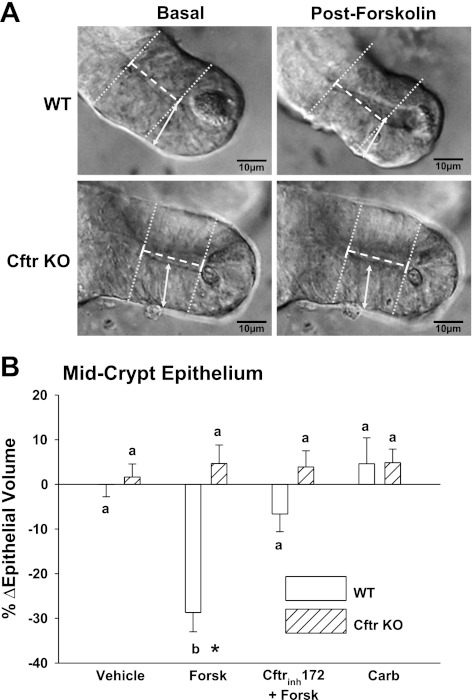
Previous studies of freshly isolated crypts from WT and Cftr KO mice have demonstrated Cftr-dependent reductions in cell volume during cAMP stimulation (60). Moreover, Cftr-dependent cell shrinkage of villous epithelium in the duodenum (which has significant levels of Cftr expression; Ref. 2) plays an important physiological role in inhibiting Nhe3 activity during cAMP stimulation (20). To determine whether Cftr-dependent cell shrinkage occurs in enteroid crypts, WT and Cftr KO enteroids were exposed to 10 μM forskolin and changes in crypt epithelial volume and epithelial cell height, indexes of enterocyte cell volume in intact intestine (20, 61), were measured using light microscopy. Initial studies with intact WT enteroids found global epithelial flattening after forskolin treatment, presumably due to backpressure from fluid secretion within the enteroid (data not shown). Further evidence of cAMP-stimulated fluid secretion by enteroid crypts was inferred from the movement of debris from the lumen of a micro-dissected crypt (see Supplemental Video S1; Supplemental Material for this article is available online at the Am J Physiol Cell Physiol website). Therefore, to ensure the accuracy of the epithelial volume and cell height measurements, the enteroid cultures were bisected manually to prevent backpressure and enable exposure of the enteroid interior to the superfusate. As shown in Fig. 6A, forskolin treatment induced a significant decrease in crypt epithelial cell height at midcrypt in WT enteroids, whereas minimal changes in cell height were measured in Cftr KO enteroids. As shown in Fig. 6B, the calculations for the percent change in crypt epithelial volume (−28.7%) in the WT enteroid crypts treated with forskolin were similar to those previously measured as total crypt volume (−27%) in freshly isolated WT mouse crypts exposed to the cAMP agonist VIP (60). Cftr dependence for WT epithelial cell shrinkage was shown by the lack of significant forskolin-induced crypt epithelial volume changes in Cftr KO enteroids and in WT enteroids pretreated for >1 h with 25 μM Cftrinh172. Changes in cell height at the +8 to +15 cell position were similar to the changes in crypt epithelial volume for the various treatments (WT cell height: %Δvehicle = +2.7 ± 2.5; %Δforskolin = −21.8 ± 4.0*; %Δforskolin + Cftrinh172 = −4.7 ± 3.4; %Δcarbachol = −1.0 ± 6.6, *P < 0.05 vs. other treatments. Cftr KO cell height: %Δvehicle = +4.5 ± 1.4; %Δforskolin = 0.0 ± 3.5+; %Δforskolin + Cftrinh172 = 2.9 ± 1.7; %Δcarbachol = −2.2 ± 1.3, +P < 0.05 vs. WT). Volume changes induced by cAMP-stimulated Cftr activation were sustained relative to the transient effect induced by the Ca2+ mobilizing agonist carbachol (100 μM), which was not apparent after a 10-min treatment. To further assess the transient volume change induced by carbachol treatment, images of crypts from WT enteroids were acquired at high rate (5 images/s). Maximal epithelial volume change was −11.9 ± 2.5%, which occurred ∼4 min following carbachol addition (n = 3).
Fig. 6.
Cftr-dependent cell shrinkage in enteroid crypts. A: photomicrographs of WT and Cftr KO enteroid crypts before (basal) and after 10-min exposure to 10 μM forskolin (postforskolin). Arrowed bar indicates measurement of epithelial cell height as an index of cell shrinkage before forskolin treatment. Dotted white and solid white lines indicate diameters (2r) of crypt and crypt lumen, respectively, and dashed white line indicates height (h) for calculation of epithelial volume before forskolin treatment (magnification: ×40). Enteroids were from WT and Cftr KO sex-matched littermate mice. Mean cell height before treatments were WT = 21.1 ± 0.9 μm and Cftr KO = 21.3 ± 0.9 μm; n = 18. B: cumulative data of % change in epithelial volume after vehicle (Vehicle), forskolin (Forsk, 10 μM), pretreatment with 25 μM Cftrinh172 + forskolin (Cftrinh172 + Forsk), or carbachol (Carb, 100 μM) in mid-crypt epithelium (between position +8 to +15) of paired WT and Cftr KO enteroids (p0-p1). Epithelial volume was calculated by subtracting the crypt luminal volume from the total crypt volume between cell positions +8 to +15, assuming each with a cylindrical shape, using the formula π·h·r2 and averaged measurements of the height (h) and radius (r) of each in optical cross sections. %ΔEpithelial volume was calculated from the formula: change in epithelial volume (in μm3)/basal epithelial volume (in μm3) * 100. a,bMeans with the same letter are not significantly different within genotype. *P < 0.05, significantly different from Cftr KO; n = 6 WT and Cftr paired enteroids.
Functional activity: Cftr-dependent regulation of intracellular pH.
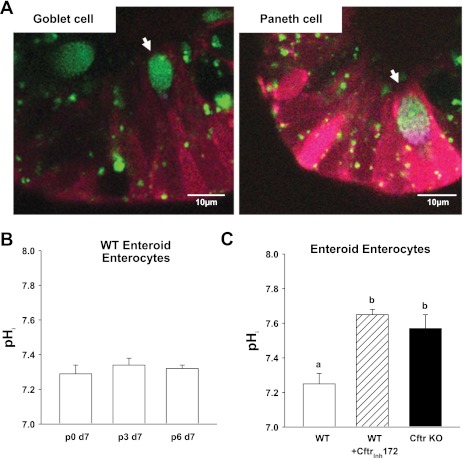
Previous studies of CFTR-null pancreatic duct, mammary epithelial cell lines or Chinese hamster ovary cells found pHi to be alkaline relative to these cells expressing recombinant CFTR (5, 17, 24), an effect that is likely due to Cftr's role in cellular HCO3− export (5, 10, 45). Moreover, WT duodenal villous epithelium is acidic relative to the villous epithelium of Cftr-null mice (56). The pH-sensitive dye SNARF 5F and fluorescence confocal microscopy were used to measure pHi in the enterocytes of WT and Cftr KO enteroids. Secretory cells were identified and avoided by using quinicrine to stain the granules of goblet and Paneth cells (Fig. 7A). A longitudinal study was first performed to evaluate the consistency of enterocyte pHi in WT enteroids. As shown in Fig. 7B, WT enteroids demonstrated a remarkably consistent pHi range of ∼7.2–7.3 over six passages when measured during superfusion with a physiological solution (KBR + TES gassed with 5% CO2-95% O2; 37°C). Under these same conditions (Fig. 7C), enterocyte pHi of WT enteroid crypts was found to be significantly more acidic by ∼0.2–0.3 pH units than Cftr KO enterocytes or WT enterocytes treated with Cftrinh172.
Fig. 7.
Cftr-dependent effect on basal pHi in enteroid crypt epithelium. A: merged confocal images of enteroid crypt epithelium stained with quinicrine (green) to identify granulated secretory cells (goblet and Paneth) and the pH-sensitive dye SNARF 5F (dual emission red and blue) for measurement of pHi (magnification: ×63, n.a. 1.2). B: mean enterocyte pHi measured in p0, p3, and p6 enteroids after day 7 (d7) in culture. Each group of enteroids were from the same WT mice (n = 3). C: mean enterocyte pHi measured in WT, WT pretreated for 1 h with 25 μM Cftrinh172 (WT + Cftrinh172) and Cftr KO enteroid crypts. Enteroids (p0-p1) were from WT and Cftr KO sex-matched littermate mice. a,bP < 0.05, means with the same letter are not significantly different; n = 6 mouse pairs.
DISCUSSION
The present study demonstrates the utility of enteroid culture for physiological studies of well-differentiated crypt epithelium. In the past, real-time characterization of crypt epithelial physiology was limited by restricted visual and physical access to crypts in the intact intestine. Other problems have plagued primary intestinal cell culture including rapid anoikis/apoptosis of freshly isolated crypts (32) and dedifferentiation of intestinal cells cultured by standard methods. These limitations are largely overcome by the enteroid culture method developed by Sato et al. (52). The organotypic structure of the enteroid crypts and distribution of cell types within the crypt appear to recapitulate native intestinal epithelium. Differentiation and functionality of secretory cells (goblet and Paneth) are preserved as demonstrated morphologically and by the capability to follow the degranulation process induced by a standard muscarinic agonist. Thus the model system presents an opportunity for physiological and pathophysiological studies of coordinated epithelial reactions. Of particular importance, enteroid culture makes it possible to distinguish intrinsic responses of the isolated epithelium independent of its interactions with the intestinal luminal milieu and submucosal cellular elements (fibroblasts, immune cells, and neurons). Advantages would include monitoring intestinal cell-specific activity in transgenic mouse models or evaluating epithelial responses after reintroduction of other cell types, humoral factors, or microbial agents.
The enteroid system is amenable to studies of cell proliferation and perhaps neoplastic progression. Modification of the growth medium in this study provided a level of crypt epithelial proliferation that recapitulates intercrypt variation (i.e., quiescent to high) and overall mean rates as measured in vivo. Endogenous Wnt production and signaling by the enteroid crypt epithelium were apparently sufficient for normal proliferation but required the addition of the mesenchymal-derived Wnt cofactor Rspondin1 (52). A major difference in the enteroid system is the high frequency of crypt fission events. We estimate that ∼5% of crypts freshly isolated from WT murine proximal small intestine show evidence of crypt fission, which is consistent with observations in other species (14, 15), whereas multiple crypt fission events in the enteroid cultures are observed during the period 3–8 days postplating. Newly forming crypts are readily recognized as “buds” from existing crypts and therefore can be avoided or specified for study. It is likely that the addition of noggin to the enteroid cultures contributes to the higher rate of crypt fission since transgenic overexpression of noggin induces ectopic crypt foci in mouse intestine (31). Crypt fission is of interest because it represents increased stem cell clonal expansion in intestinal dysplasia and models of familial adenomatous polyposis (40, 46).
The native crypt epithelium is the major site for Cftr expression in both small and large intestines (2, 35, 57). Cftr protein expression in WT enteroid cultures was robust and largely unchanged from that in freshly isolated WT crypts. The crypt isolation procedure using EDTA chelation of divalent cations also dislodges sheets of villous epithelium from the small intestine, which confounds comparison of gene expression between freshly isolated crypts and the enteroid cultures. Consequently, we found expression in the freshly isolated crypts differed from that in enteroids by higher expression of Na+-K+ Atpase, Dra, Pat-1, Pept1, Nhe2, and CaII, which are, collectively, known to be enriched in villous epithelium and lower expression of Nkcc1 that is primarily expressed in crypts (11, 23, 27, 33–35, 50, 62). Unlike Nkcc1, freshly isolated crypts had equivalent or higher Cftr mRNA expression compared with the enteroids, suggesting that Cftr mRNA is slightly reduced in the cultures. Enteroids, which have a greatly reduced villus-like surface area (i.e., villi do not form), likely exhibit predominantly crypt epithelial expression patterns. Villous epithelium from the initial crypt isolation may also contaminate protein lysates from p0 enteroids. However, upon passage, most cellular debris is trapped within the gel and separates from the enteroid structures during centrifugation. Thus p1 enteroids appear to be a preferable preparation for comparing expression between treatments because cellular debris is greatly reduced and plating density is more controlled relative to the p0 enteroids. For quantitative comparisons with in vivo crypts, it is likely that techniques such as laser-capture microdissection of intestinal sections will be required to avoid the problem of villous epithelial contamination in freshly isolated crypts.
A major physiological function of the crypt epithelium is vectorial anion secretion mediated by Cftr channel activity. Cftr conducts both Cl− and HCO3− (45), facilitates the activity of Cl−/HCO3− exchangers by providing a Cl− leak pathway (56), and may directly interact with members of the Slc26a multifunctional anion exchange family (36). Activation of Cftr channel function by agonists stimulating intracellular cyclic nucleotide concentration results in a net inward current of anion efflux and cell membrane depolarization (1, 12, 37). As shown in the present study, enteroids express similar levels of Cftr and demonstrate cell membrane depolarization upon cAMP stimulation. Cftr specificity was demonstrated by the absence of depolarization in Cftr KO enteroid epithelium and repolarization of cAMP-stimulated WT enteroid crypt epithelium treated with the Cftr-selective inhibitor Cftrinh172. An important intestinal response to acute Cftr activation is epithelial cell volume reduction, which facilitates crypt flushing and contributes to cytoskeletal-specific signaling (60). In the present study, measurements of crypt epithelial volume and cell height as indexes of cell shrinkage (20, 56) were performed on bisected enteroids to eliminate backpressure during fluid secretion. Under this condition, crypt enterocytes in WT enteroids responded to forskolin with reductions in epithelial volume that attained a magnitude very similar to the decrease in total crypt volume of freshly isolated WT mouse crypts treated with VIP, i.e., −28.7 vs. −27%, respectively (60). Cftr dependence of these cell volume changes were demonstrated by minimal epithelial volume or cell height changes in Cftr KO enteroids or WT enteroids pretreated with Cftrinh172. Indirect evidence of net fluid secretion induced by cAMP stimulation in WT enteroid crypts was inferred from observations of global epithelial flattening during forskolin stimulation of intact enteroids and the movement of debris from the lumen of microdissected cultured crypts. The Ca2+-mobilizing agonist carbachol also induced epithelial cell shrinkage in the WT enteroid crypts, but the change was smaller (−11.9%) and transient, lasting <6 min. The carbachol-induced change in volume is consistent with the transient secretory response occurring ex vivo in WT murine small intestine mounted in Ussing chambers (53). However, both observations contrast with Valverde et al. (60) who found that carbachol at the same concentration induced larger, sustained epithelial volume decreases (−27%) in freshly isolated murine crypts, leading to speculation that loss of crypt attachment/contact to the extracellular matrix may alter the epithelial volume response to Ca2+ mobilizing agonists.
Given its role in HCO3− efflux, functional Cftr is expected to exert an acidifying influence on epithelial pHi regulation. Cftr expression has also been shown to affect transcriptional regulation of Cl−/HCO3− exchange proteins (25, 45, 55, 56). Studies in epithelial cell lines, heterologous cell types, and duodenal villous epithelium demonstrate that Cftr expression reduces pHi relative to Cftr-null cells (5, 17, 24, 56). The present study extends this observation to intestinal crypt epithelium, which was shown in the enteroid model to demonstrate a more alkaline pHi (∼0.2 U) when Cftr was either genetically ablated (Cftr KO) or pharmacologically inhibited. The pHi difference was measured in the enterocyte population, which is the predominant cell type of the crypt (13). Thus Cftr may set the upper limit of pHi in WT crypt epithelium. An alkaline pHi in Cftr-deficient epithelium would negatively impact processes of apoptosis by diminishing cellular activity of acid endonucleases (5) and positively influence cell proliferation by facilitating G2/M transition in the cell cycle and enhancing membrane biogenesis (47, 64). Previous studies (19) have shown that Cftr KO mice exhibit hyperproliferation of the intestinal epithelium, and it is known that cystic fibrosis patients have an approximately six-fold risk of gastrointestinal neoplasia despite being a relative young population (41). Thus the alkaline pHi in CF crypt epithelium may be a potential contributor to the propensity for gastrointestinal cancer in CF patients. Why other acid-base transporters in the Cftr KO epithelium do not fully compensate for an alkaline pHi is a question that will require further investigation. However, the propensity to maintain an alkaline pHi in the enterocyte population may have less innocuous consequences compared with the effects of an acidic pHi (increased apoptosis/reduced proliferation). Cell acidification evokes allosteric changes in the Na+/H+ exchanger Nhe1 that maximally increase its activity, whereas Nhe1 is largely inactive at pHi ≥7.2 (6). It is also been shown that CF crypt epithelium maintains lower Na+ and higher Cl− content relative to normal, which may affect the activity of other acid-base transporters (42). For example, these gradients would favor H+ efflux by Nhe1 and reduce HCO3− efflux by the anion exchanger AE2 at the basolateral membrane of the crypt cell.
In summary, the enteroid culture system developed by Sato et al. (52) provides a highly differentiated epithelium for physiological and biological studies of the intestinal crypt. A modification of the enteroid growth medium attained epithelial proliferation similar to native intestine, but the model differs in demonstrating a greater rate of crypt fission events than found in vivo. Cftr-mediated anion secretion is a major function of the intestinal crypt and is robustly preserved in the enteroid model. Moreover, Cftr regulation of important aspects of cell physiology, i.e., cell volume and cell pHi, is recapitulated in the crypts of the enteroid model. Enteroids are a primary culture of intestinal epithelium, which is largely isolated from the influences of luminal microflora, submucosal cell elements, or systemic factors. Therefore, the model provides the opportunity to investigate responses that are intrinsic to the epithelium and perhaps more proximate to pathogenic processes in epithelial diseases such as cystic fibrosis.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-48816 (to L. L. Clarke) and Cystic Fibrosis Foundation Grant Clarke11G0 (to L. L. Clarke).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L., N.M.W., A.O., and L.L.C. conception and design of research; J.L., N.M.W., M.T.C., and L.L.C. performed experiments; J.L., N.M.W., M.T.C., and L.L.C. analyzed data; J.L., N.M.W., M.T.C., and L.L.C. interpreted results of experiments; J.L., N.M.W., and L.L.C. prepared figures; J.L., N.M.W., and L.L.C. drafted manuscript; J.L., N.M.W., and L.L.C. edited and revised manuscript; J.L., N.M.W., and L.L.C. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jennifer M. Brazill and Erin H. Hoover for expert technical assistance and the Dalton Live Cell Imaging Core (director Dr. Luis Martinez-Lemus) for helpful advice and assistance in the confocal imaging studies.
REFERENCES
- 1. Alper SL, Stewart AK, Vandorpe DH, Clark JS, Horack RZ, Simpson JE, Walker NM, Clarke LL. Native and recombinant Slc26a3 (downregulated in adenoma, Dra) do not exhibit properties of 2Cl−/HCO3− exchange. Am J Physiol Cell Physiol 300: C276–C286, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ameen NA, Alexis J, Salas P. Cellular localization of the cystic fibrosis transmembrane conductance regulator in mouse intestinal tract. Histochem Cell Biol 114: 69–75, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Ameen NA, Ardito T, Kashgarian M, Marino CR. A unique subset of rat and human intestinal villus cells express the cystic fibrosis transmembrane conductance regulator. Gastroenterology 108: 1016–1023, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Anderson MP, Sheppard DN, Berger HA, Welsh MJ. Chloride channels in the apical membrane of normal and cystic fibrosis airway and intestinal epithelia. Am J Physiol Lung Cell Mol Physiol 263: L1–L14, 1992 [DOI] [PubMed] [Google Scholar]
- 5. Barrière H, Poujeol C, Tauc M, Blasi JM, Counillon L, Poujeol P. CFTR modulates programmed cell death by decreasing intracellular pH in Chinese hamster lung fibroblasts. Am J Physiol Cell Physiol 281: C810–C824, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11: 50–61, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Chu SY, Montrose MH. Extracellular pH regulation in microdomains of colonic crypts - effects of short-chain fatty acids. Proc Natl Acad Sci USA 92: 3303–3307, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clarke LL, Gawenis LR, Bradford EM, Judd LM, Boyle KT, Simpson JE, Shull GE, Tanabe H, Ouellette AJ, Franklin CL, Walker NM. Abnormal Paneth cell granule dissolution and compromised resistance to bacterial colonization in the intestine of CF mice. Am J Physiol Gastrointest Liver Physiol 286: G1050–G1058, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Clarke LL, Gawenis LR, Franklin CL, Harline MC. Increased survival of CFTR knockout mice using an oral osmotic laxative. Lab Anim Sci 46: 612–618, 1996 [PubMed] [Google Scholar]
- 10. Clarke LL, Harline MC. Dual role of CFTR in cAMP-stimulated HCO3− secretion across murine duodenum. Am J Physiol Gastrointest Liver Physiol 274: G718–G726, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Collins JF, Honda T, Knoble S, Bulus NM, Conary J, DuBois R, Ghishan FK. Molecular cloning, sequencing, tissue distribution, and functional expression of a Na+/H+ exchanger (NHE-2). Proc Natl Acad Sci USA 90: 3938–3942, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cotton CU, Stutts MJ, Knowles MR, Gatzy JT, Boucher RC. Abnormal apical cell membrane in cystic fibrosis respiratory epithelium. An in vitro electrophysiologic analysis. J Clin Invest 79: 80–85, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet 7: 349–359, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Cummins AG, Catta-Smith AG, Cameron DJ, Couper RT, Davidson GP, Day AS, Hammond PD, Moore DJ, Thompson FM. Crypt fission peaks early during infancy and crypt hyperplasia broadly peaks during infancy and childhood in the small intestine of humans. J Pediatr Gastroenterol Nutr 47: 153–157, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Cummins AG, Jones BJ, Thompson FM. Postnatal epithelial growth of the small intestine in the rat occurs by both crypt fission and crypt hyperplasia. Dig Dis Sci 51: 718–723, 2006 [DOI] [PubMed] [Google Scholar]
- 16. De Smet P, Simaels J, Declercq PE, Van Driessche W. Regulatory volume decrease in cultured kidney cells (A6): role of amino acids. J Gen Physiol 106: 525–542, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elgavish A. High intracellular pH in CFPAC: a pancreas cell line from a patient with cystic fibrosis is lowered by retrovirus-mediated CFTR gene transfer. Biochem Biophys Res Commun 180: 342–348, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Gabriel SE, Clarke LL, Boucher RC, Stutts MJ. CFTR and outward rectifying chloride channels are distinct proteins with a regulatory relationship. Nature 363: 263–266, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Gallagher AM, Gottlieb RA. Proliferation, not apoptosis, alters epithelial cell migration in small intestine of CFTR null mice. Am J Physiol Gastrointest Liver Physiol 281: G681–G687, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Gawenis LR, Franklin CL, Simpson JE, Palmer BA, Walker NM, Wiggins TM, Clarke LL. cAMP inhibition of murine intestinal Na+/H+ exchange requires CFTR-mediated cell shrinkage of villus epithelium. Gastroenterology 125: 1148–1163, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Gawenis LR, Hut H, Bot AGM, Shull GE, De Jonge HR, Stein X, Miller ML, Clarke LL. Electroneutral sodium absorption and electrogenic anion secretion across murine small intestine are regulated in parallel. Am J Physiol Gastrointest Liver Physiol 287: G1140–G1149, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Geibel JP, Rajendran VM, Binder HJ. Na+-dependent fluid absroption in intact perfused rat colonic crypts. Gastroenterology 120: 144–150, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Giannella RA, Orlowski J, Jump ML, Lingrel JB. Na+-K+-ATPase gene expression in rat intestine and Caco-2 cells: response to thyroid hormone. Am J Physiol Gastrointest Liver Physiol 265: G775–G782, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Gottlieb RA, Dosanjh A. Mutant cystic fibrosis transmembrane conductance regulator inhibits acidification and apoptosis in C127 cells: possible relevance to cystic fibrosis. Proc Natl Acad Sci USA 93: 3587–3591, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Greeley T, Shumaker H, Wang Z, Schweinfest CW, Soleimani M. Downregulated in adenoma and putative anion transporter are regulated by CFTR in cultured pancreatic duct cells. Am J Physiol Gastrointest Liver Physiol 281: G1301–G1308, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Greger R, Bleich M, Leipziger J, Ecke D, Mall M, Kunzelmann K. Regulation of ion transport in colonic crypts. News Physiol Sci 12: 62–66, 1997 [Google Scholar]
- 27. Groneberg DA, Doring F, Eynott PR, Fischer A, Daniel H. Intestinal peptide transport: ex vivo uptake studies and localization of peptide carrier PEPT1. Am J Physiol Gastrointest Liver Physiol 281: G697–G704, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Grubb BR, Gabriel SE. Intestinal physiology and pathology in gene-targeted mouse models of cystic fibrosis. Am J Physiol Gastrointest Liver Physiol 273: G258–G266, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Guan Y, Dong J, Tackett L, Meyer JW, Shull GE, Montrose MH. NHE2 is the main apical NHE in mouse colonic crypts but an alternative Na+-dependent acid extrusion mechanism is upregulated in NHE2-null mice. Am J Physiol Gastrointest Liver Physiol 291: G689–G699, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Guimbellot J, Fortenberry J, Siegal G, Moore B, Wen H, Venglarik C, Chen YF, Oparil S, Sorscher E. Role of oxygen availability in CFTR expression and function. Am J Respir Cell Mol Biol 39: 514–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haramis AP, Begthel H, Van Den Born M, Van Es JH, Jonkheer S, Offerhaus GJA, Clevers H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 303: 1684–1686, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Hofmann C, Obermeier F, Artinger M, Hausmann M, Falk W, Schoelmerich J, Rogler G, Grossmann J. Cell-cell contacts prevent anoikis in primary human colonic epithelial cells. Gastroenterology 132: 587–600, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun CH, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse CM, Donowitz M. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. Am J Physiol Gastrointest Liver Physiol 270: G29–G41, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Jacob P, Rossmann H, Lamprecht G, Kretz A, Neff C, Lin-Wu E, Gregor M, Groneberg DA, Kere J, Seidler U. Down-regulated in adenoma mediates apical Cl−/HCO3− exchange in rabbit, rat, and human duodenum. Gastroenterology 122: 709–724, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Jakab RL, Collaco AM, Ameen NA. Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am J Physiol Gastrointest Liver Physiol 300: G82–G98, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ko SBH, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol 6: 343–350, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lazarowski ER, Mason SJ, Clarke LL, Harden TK, Boucher RC. Characterization of adenosine receptors and their relationship to chloride secretion in human airway epithelia. Br J Pharmacol 106: 774–782, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and 2ΔΔCt method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Mignen O, Le Gall C, Harvey BJ, Thomas S. Volume regulation following hypotonic shock in isolated crypts of mouse distal colon. J Physiol 515.2: 501–510, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Milicic A, Harrison LA, Goodlad RA, Hardy RG, Nicholson AM, Presz M, Sieber O, Santander S, Pringle JH, Mandir N, East P, Obszynska J, Sanders S, Piazuelo E, Shaw J, Harrison R, Tomlinson IP, McDonald SA, Wright NA, Jankowski JA. Ectopic expression of P-cadherin correlates with promoter hypomethylation early in colorectal carcinogenesis and enhanced intestinal crypt fission in vivo. Cancer Res 68: 7760–7768, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Neglia J, Fitzsimmons S, Maisonneuve P, Schoni MH, Schoni-Affolter F, Corey M, Lowenfels A. The risk of cancer among patients with cystic fibrosis. N Engl J Med 332: 494–499, 1995 [DOI] [PubMed] [Google Scholar]
- 42. O'Loughlin EV, Hunt DM, Bostrom TE, Hunter D, Gaskin KJ, Gyory A, Cockayne DJH. X-ray microanalysis of cell elements in normal and cystic fibrosis jejunum: evidence for chloride secretion in villi. Gastroenterology 110: 411–418, 1996 [DOI] [PubMed] [Google Scholar]
- 43. Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, Sugihara H, Fujimoto K, Weissman IL, Capecchi MR, Kuo CJ. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med 15: 701–706, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, Lee MG. Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterology 139: 620–631, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA 91: 5340–5344, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Preston SL, Leedham SJ, Oukrif D, Deheregoda M, Goodlad RA, Poulsom R, Alison MR, Wright NA, Novelli M. The development of duodenal microadenomas in FAP patients: the human correlate of the Min mouse. J Pathol 214: 294–301, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Putney LK, Barber DL. Na-H exchange-dependent increase in intracellular pH times G2/M entry and transition. J Biol Chem 278: 44645–44649, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Reynolds A, Parris A, Evans LA, Lindqvist S, Sharp P, Lewis M, Tighe R, Williams MR. Dynamic and differential regulation of NKCC1 by calcium and cAMP in the native human colonic epithelium. J Physiol 582.2: 507–524, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Robert ME, Singh SK, Ikuma M, Jain D, Ardito T, Binder HJ. Morphology of isolated colonic crypts. Cells Tissues Organs 168: 246–251, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Saarnio J, Parkkila S, Parkkila AK, Waheed A, Casey MC, Zhou XY, Pastorekova S, Pastorek J, Karttunen T, Haukipuro K, Kairaluoma MI, Sly WS. Immunohistochemistry of carbonic anhydrase isozyme IX (MN/CA IX) in human gut reveals polarized expression in the epithelial cells with the highest proliferative capacity. J Histochem Cytochem 46: 497–504, 1998 [DOI] [PubMed] [Google Scholar]
- 51. Sato T, Van Es JH, Snippert HJ, Stange DE, Vries RG, Van Den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–419, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, Van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structure in vitro without a mesenchymal niche. Nature 459: 262–265, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge WH, Evans M, Ratcliff R, Gregor M. A functional CFTR protein is required for mouse intestinal cAMP-,cGMP- and Ca2+-dependent HCO3− secretion. J Physiol 505: 411–423, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shcheynikov N, Kim KH, Kim K, Dorwart MR, Ko SBH, Goto H, Naruse S, Thomas PJ, Muallem S. Dynamic control of cystic fibrosis transmembrane conductance regulator Cl−/HCO3− selectivity by external Cl−. J Biol Chem 279: 21857–21865, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Shumaker H, Burnham C, Shull GE, Yankaskas JR, Soleimani M. CFTR induces the expression of DRA along with Cl−/HCO3− exchange activity in tracheal epithelial cells. Am J Physiol Cell Physiol 279: C62–C71, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Simpson JE, Gawenis LR, Walker NM, Boyle KT, Clarke LL. Chloride conductance of CFTR facilitates Cl−/HCO3− exchange in the villous epithelium of intact murine duodenum. Am J Physiol Gastrointest Liver Physiol 288: G1241–G1251, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Strong TV, Boehm K, Collins FS. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest 93: 347–354, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science 269: 847–850, 1995 [DOI] [PubMed] [Google Scholar]
- 59. Trahair J, Neutra M, Gordon J. Use of transgenic mice to study the routing of secretory proteins in intestinal epithelial cells: analysis of human growth hormone compartmentalization as a function of cell type and differentiation. J Cell Biol 109: 3231–3242, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Valverde MA, O'Brien JA, Sepulveda FV, Ratcliff R, Evans MJ, Colledge WH. Inactivation of the murine cftr gene abolishes cAMP-mediated but not Ca2+-mediated secretagogue-induced volume decrease in small intestinal crypts. Pflügers Arch 425: 434–438, 1993 [DOI] [PubMed] [Google Scholar]
- 61. Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL. Role of down-regulated in adenoma anion exchanger in HCO3− secretion across murine duodenum. Gastroenterology 136: 893–901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestine transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol 288: C957–C965, 2005 [DOI] [PubMed] [Google Scholar]
- 63. Wright AM, Gong X, Verdon B, Linsdell P, Mehta A, Riordan JR, Argent BE, Gray MA. Novel regulation of cystic fibrosis transmembrane conductance regulator (CFTR) channel gating by external chloride. J Biol Chem 279: 41658–41663, 2004 [DOI] [PubMed] [Google Scholar]
- 64. Young BP, Shin JJ, Orij R, Chao JT, Li SC, Guan XL, Khong A, Jan E, Wenk MR, Prinz WA, Smits GJ, Loewen CJR. Phosphatidic acid is a pH biosensor that links membrane biogenesis to metabolism. Science 329: 1085–1088, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.