Abstract
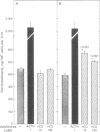
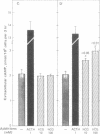
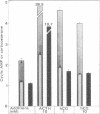
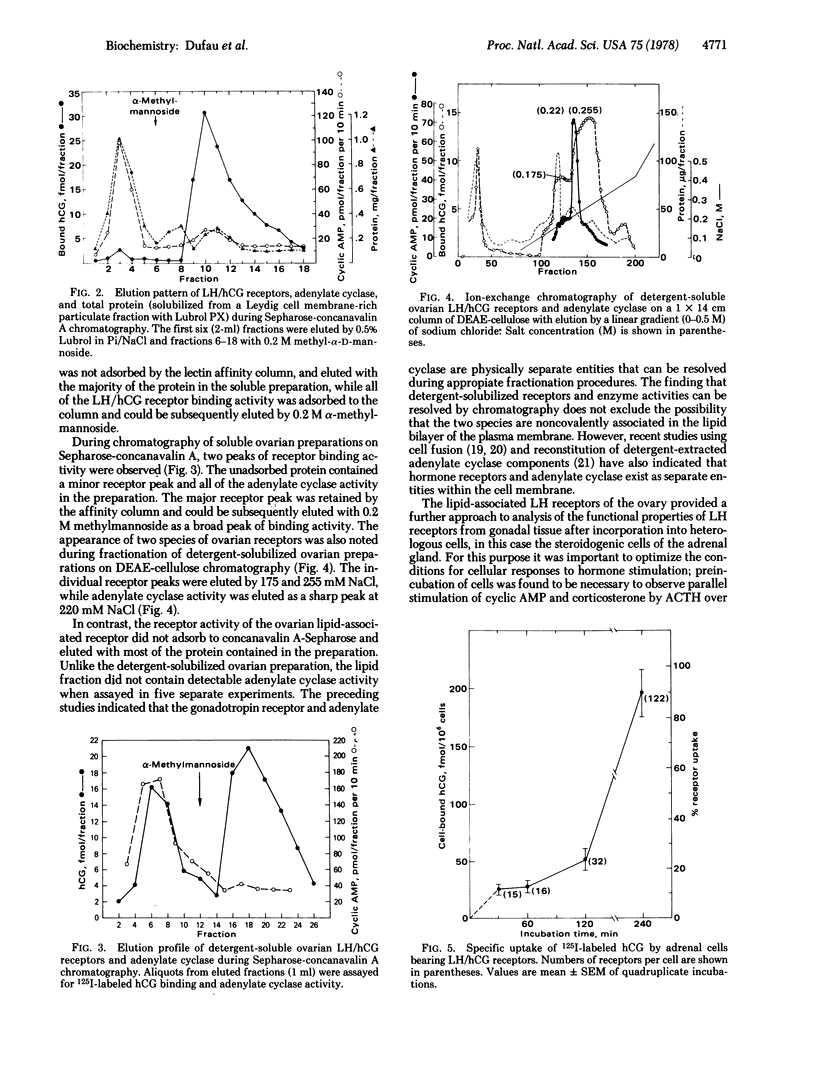
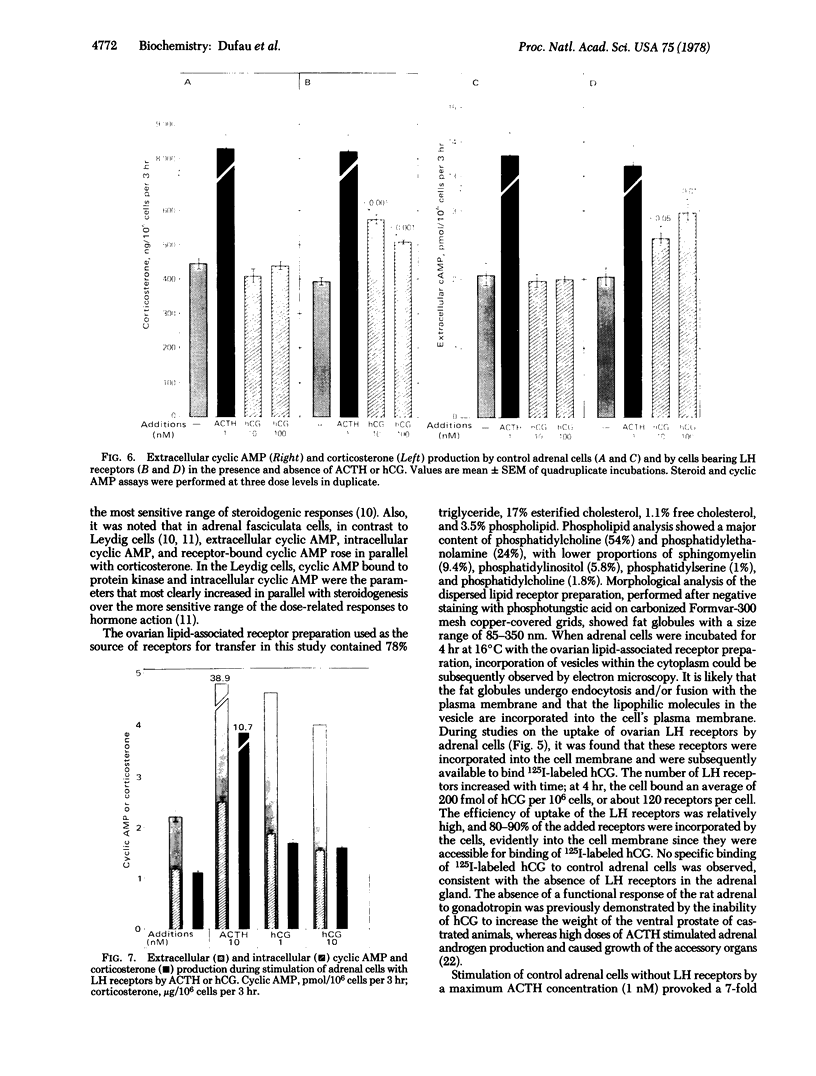
Luteinized rat ovaries contain a high concentration of particulate luteinizing hormone (lutropin, LH) receptors and a small quantity of lipid-associated receptors that float in the 360,000 X g supernatant fraction of ovarian homogenates. During fractionation of Lubrol-solubilized LH receptors and adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] from the ovary and testis, LH receptors and adenylate cyclase were coincident on gel filtration, but could be resolved during ion-exchange chromatography of soluble ovarian preparations and were completely separated by lectin-affinity chromatography on Sepharose-concanavalin A. For further analysis of receptor-adenylate cyclase coupling, the lipid-rich fraction of ovarian luteal cells was used to transfer gonadal LH receptors to isolated adrenal fasciculata cells. The lipid vesicles obtained from ovarian homogenates by flotation at 360,000 X g contained 5--10% of the ovarian LH receptors and were devoid of adenylate cyclase activity. During incubation of lipid-associated receptors with dispersed rat fasciculata cells at 16 degrees C, progressive incorporation of LH binding sites into the adrenal cells was observed. When adrenal cells bearing heterotopic LH receptors were incubated with 1 nM human choriogonadotropin, cyclic AMP production was consistently stimulated, with an accompanying increase in corticosterone production. These results indicate that LH receptors exist as separate entities from adenylate cyclase in the gonadal cell membrane and can become functionally coupled to adenylate cyclase to evoke cyclic AMP production and steroidogenesis in the host adrenal cells to which they are transferred.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Catt K. J., Tsuruhara T., Mendelson C., Ketelslegers J. M., Dufau M. L. Gonadotroping binding and activation of the interstitial cells of the testis. Curr Top Mol Endocrinol. 1974;1:1–30. doi: 10.1007/978-1-4684-2595-6_1. [DOI] [PubMed] [Google Scholar]
- Charreau E. H., Dufau M. L., Catt K. J. Multiple forms of solubilized gonadotropin receptors from the rat testis. J Biol Chem. 1974 Jul 10;249(13):4189–4195. [PubMed] [Google Scholar]
- Douglas J., Aguilera G., Kondo T., Catt K. Angiotensin II receptors and aldosterone production in rat adrenal glomerulosa cells. Endocrinology. 1978 Mar;102(3):685–696. doi: 10.1210/endo-102-3-685. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Baukal A. J., Ryan D., Catt K. J. Properties of detergent-solubilized adenylate cyclase and gonadotropin receptors of testis and ovary. Mol Cell Endocrinol. 1977 Feb;6(4-5):253–269. doi: 10.1016/0303-7207(77)90100-9. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Charreau E. H., Catt K. J. Characteristics of a soluble gonadotropin receptor from the rat testis. J Biol Chem. 1973 Oct 25;248(20):6973–6982. [PubMed] [Google Scholar]
- Dufau M. L., Podesta E. J., Catt K. J. Physical characteristics of the gonadotropin receptor-hormone complexes formed in vivo and in vitro. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1272–1275. doi: 10.1073/pnas.72.4.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufau M. L., Tsuruhara T., Horner K. A., Podesta E., Catt K. J. Intermediate role of adenosine 3':5'-cyclic monophosphate and protein kinase during gonadotropin-induced steroidogenesis in testicular interstitial cells. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3419–3423. doi: 10.1073/pnas.74.8.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufau M. L., Watanabe K., Catt K. J. Stimulation of cyclic AMP production by the rat testis during incubation with hCG in vitro. Endocrinology. 1973 Jan;92(1):6–11. doi: 10.1210/endo-92-1-6. [DOI] [PubMed] [Google Scholar]
- Gross H. A., Ruder H. J., Brown K. S., Lipsett M. B. A radioimmunoassay for plasma corticosterone. Steroids. 1972 Dec;20(6):681–695. doi: 10.1016/0039-128x(72)90051-7. [DOI] [PubMed] [Google Scholar]
- Han S. S., Rajaniemi H. J., Cho M. I., Hirshfield A. N., Midgley A. R., Jr Gonadotropin receptors in rat ovarian tissue. II. Subcellular localization of LH binding sites by electron microscopic radioautography. Endocrinology. 1974 Aug;95(2):589–598. doi: 10.1210/endo-95-2-589. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Mendelson C., Dufau M., Catt K. Gonadotropin binding and stimulation of cyclic adenosine 3':5'-monophosphate and testosterone production in isolated Leydig cells. J Biol Chem. 1975 Nov 25;250(22):8818–8823. [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Resolution of some components of adenylate cyclase necessary for catalytic activity. J Biol Chem. 1977 Oct 25;252(20):6966–6969. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schramm M., Orly J., Eimerl S., Korner M. Coupling of hormone receptors to adenylate cyclase of different cells by cell fusion. Nature. 1977 Jul 28;268(5618):310–313. doi: 10.1038/268310a0. [DOI] [PubMed] [Google Scholar]
- Schulster D., Orly J., Seidel G., Schramm M. Intracellular cyclic AMP production enhanced by a hormone receptor transferred from a different cell. beta-adrenergic responses in cultured cells conferred by fusion with turkey erythrocytes. J Biol Chem. 1978 Feb 25;253(4):1201–1206. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- TULLNER W. W. HORMONAL FACTORS IN THE ADRENAL-DEPENDENT GROWTH OF THE RAT VENTRAL PROSTATE. Natl Cancer Inst Monogr. 1963 Oct;12:211–223. [PubMed] [Google Scholar]