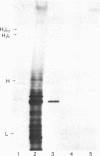
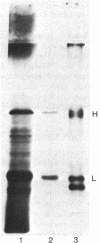
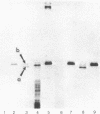
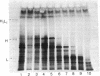
Abstract
Lymphoid cells obtained from the peripheral blood of a patient with heavy chain disease have been established in long-term culture. They continue to produce a protein antigenically identical to the deleted gamma3 heavy chain disease protein found in the patient's serum. The availability of the cell line has made it possible to analyze the mRNA coding for this protein. The primary in vitro translation product is 1500-2000 daltons larger than the polypeptide portion of the cytoplasmic or secreted protein and has methionine at the amino terminus. The mRNA sediments at 15.5 S on sucrose gradients and therefore appears to be smaller than the 17S message coding for normal-sized mouse gamma chains. It contains a base sequence that codes for a hydrophobic amino-terminal peptide not found in the cytoplasmic protein. There was no evidence for the synthesis of translatable light chain message by these cells. The present data suggest that this protein results from a primary somatic genetic event that gave rise to a cell product bearing a normal aminoterminus sensitive to limited proteolytic digestion. The serum protein thus appears to begin in the hinge region but, in fact, contains a normal heavy chain initiation site.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlersberg J. B., Franklin E. C., Frangione B. Repetitive hinge region sequences in human IgG3: isolation of an 11,000-dalton fragment. Proc Natl Acad Sci U S A. 1975 Feb;72(2):723–727. doi: 10.1073/pnas.72.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bernier G. M., Gunderman J. R., Ruymann F. B. Kappa-chain deficiency. Blood. 1972 Dec;40(6):795–805. [PubMed] [Google Scholar]
- Brack C., Tonegawa S. Variable and constant parts of the immunoglobulin light chain gene of a mouse myeloma cell are 1250 nontranslated bases apart. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5652–5656. doi: 10.1073/pnas.74.12.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G., Mendecki J., Lee S. Y. A procedure for the isolation of mammalian messenger ribonucleic acid. Biochemistry. 1972 Feb 15;11(4):637–641. doi: 10.1021/bi00754a027. [DOI] [PubMed] [Google Scholar]
- Buxbaum J. N., Preud'homme J. L. Alpha and gamma heavy chain diseases in man: intracellular origin of the aberrant polypeptides. J Immunol. 1972 Nov;109(5):1131–1137. [PubMed] [Google Scholar]
- Buxbaum J., Franklin E. C., Scharff M. D. Immunoglobulin M heavy chain disease: intracellular origin of the mu chain fragment. Science. 1970 Aug 21;169(3947):770–773. doi: 10.1126/science.169.3947.770. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Milstein C. The translation in vitro of mRNA for immunoglobulin heavy chains. Eur J Biochem. 1973 Jul 2;36(1):1–7. doi: 10.1111/j.1432-1033.1973.tb02877.x. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Secher D. S., Milstein C. Intracellular immunoglobulin chain synthesis in non-secreting variants of a mouse myeloma: detection of inactive light-chain messenger RNA. J Mol Biol. 1974 Dec 25;90(4):691–701. doi: 10.1016/0022-2836(74)90533-6. [DOI] [PubMed] [Google Scholar]
- Faust C. H., Jr, Diggelmann H., Mach B. Isolation of poly(adenylic acid)-rich ribonucleic acid from mouse myeloma and synthesis of complementary deoxyribonucleic acid. Biochemistry. 1973 Feb 27;12(5):925–931. doi: 10.1021/bi00729a021. [DOI] [PubMed] [Google Scholar]
- Frangione B., Franklin E. C. Heavy chain diseases: clinical features and molecular significance of the disordered immunoglobulin structure. Semin Hematol. 1973 Jan;10(1):53–64. [PubMed] [Google Scholar]
- Green M., Zehavi-Willner T., Graves P. N., McInnes J., Pestka S. Isolation and cell-free translation of immunoglobulin messenger RNA. Arch Biochem Biophys. 1976 Jan;172(1):74–89. doi: 10.1016/0003-9861(76)90049-7. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. The rabbit beta-globin gene contains a large large insert in the coding sequence. Cell. 1977 Dec;12(4):1097–1108. doi: 10.1016/0092-8674(77)90172-6. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Holland J. P., Dozy A. M., Varmus H. E. Demonstration of non-functional beta-globin mRNA in homozygous beta (0) thalassemia. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5140–5144. doi: 10.1073/pnas.72.12.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig D. F. Two adenovirus mRNAs have a common 5' terminal leader sequence encoded at least 10 kb upstream from their main coding regions. Cell. 1977 Sep;12(1):9–21. doi: 10.1016/0092-8674(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Valbuena O., Perry R. P. Isolation, purification, and properties of mouse heavy-chain immunoglobulin mRNAs. Biochemistry. 1978 May 2;17(9):1723–1733. doi: 10.1021/bi00602a022. [DOI] [PubMed] [Google Scholar]
- Melchers F. Biosynthesis, intracellular transport, and secretion of immunoglobulins. Effect of 2-deoxy-D-glucose in tumor plasma cells producing and secreting immunoglobulin G1. Biochemistry. 1973 Apr 10;12(8):1471–1476. doi: 10.1021/bi00732a001. [DOI] [PubMed] [Google Scholar]
- Mårtensson L. Genes and immunoglobulins. Vox Sang. 1966 Sep-Oct;11(5):521–545. doi: 10.1111/j.1423-0410.1966.tb04251.x. [DOI] [PubMed] [Google Scholar]
- Ramirez F., O'Donnell J. V., Marks P. A., Bank A., Musumeci S., Schilirò G., Pizzarelli G., Russo G., Luppis B., Gambino R. Abnormal or absent beta mRNA in betao Ferrara and gene deletion in delta beta thalassaemia. Nature. 1976 Oct 7;263(5577):471–475. doi: 10.1038/263471a0. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C. Inhibition of the multiplication of enveloped viruses by glucose derivatives. Curr Top Microbiol Immunol. 1975;70:101–119. doi: 10.1007/978-3-642-66101-3_4. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Tse T. P. Isolation of rat liver albumin messenger RNA. J Biol Chem. 1976 Dec 10;251(23):7461–7467. [PubMed] [Google Scholar]
- Terry W. D., Ohms J. Implications of heavy chain disease protein sequences for multiple gene theories of immunoglobulin synthesis. Proc Natl Acad Sci U S A. 1970 Jun;66(2):558–563. doi: 10.1073/pnas.66.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]