Abstract
The aim of this study was to evaluate the potential of two probiotic preparations, containing live lactic acid bacteria (Lactococcus lactis CECT 539 and Lactobacillus casei CECT 4043) and their products of fermentation (organic acids and bacteriocins), as a replacement for antibiotics in stimulating health and growth of broiler chickens. The effects of the supplementation of both preparations (with proven probiotic effect in weaned piglets) and an antibiotic (avilamycin) on body weight gain (BWG), feed intake (FI), feed consumption efficiency (FCE), relative intestinal weight, and intestinal microbiota counts were studied in 1-day posthatch chickens. The experiments were conducted with medium-growth Sasso X44 chickens housed in cages and with nutritional stressed Ross 308 broiler distributed in pens. Consumption of the different diets did not affect significantly the final coliform counts in Sasso X44 chickens. However, counts of lactic acid bacteria and mesophilic microorganisms were higher in the animals receiving the two probiotic preparations (P < 0.05). In the second experiment, although no differences in BWG were observed between treatments, Ross 308 broilers receiving the probiotic Lactobacillus preparation exhibited the lowest FCE values and were considered the most efficient at converting feed into live weight.
1. Introduction
Antibiotics have been extensively used in animal feed to improve production in poultry and piglet industries [1]. However, the use of these substances as growth promoters can lead to the development of antibiotic resistances. Such resistances can occur not only in pathogenic bacteria [2, 3], which can be transferred from poultry products to human population [4], but also in commensal bacteria [5], constituting a reservoir of resistance genes for pathogenic bacteria [6]. In recent years, the interest in finding alternatives to the use of antibiotics in animal feed has been increased due to the ban of subtherapeutic antibiotic usage in Europe. The research is mostly focused on incorporating into animal feeds, substances derived from plants, animals, bacteria and fungi, as well as organic acid, essential oils, and bacteriocins, that could interfere in colonisation of pathogens [7–9].
Due to their potential to reduce enteric disease in poultry, probiotics are considered to be a good alternative to the use of antibiotics [10]. The production of antimicrobial compounds (mainly organic acids and bacteriocins) by many lactic acid bacteria (LAB) into the intestine has provided these organisms with a competitive advantage over other microorganisms to be used as probiotics [11, 12]. Moreover, the presence of some Lactobacillus in the chicken gastrointestinal tract (GIT) has been described to be of great importance for regulating the composition of the intestinal microflora, developing immunity of the intestine, and promoting the health of chickens [13].
The administration of highly concentrated bacterial cultures, containing both the live cells and their products of fermentation, was an effective way to promote body weight gain (BWG) and improve feed conversion efficiency (FCE) in chickens [6, 14–16]. In fact, probiotics are used nowadays by compound feed industry to improve the poultry production [17, 18].
In a previous work [1], two potentially probiotic preparations, containing the live cells of Lactococcus lactis CECT 539 or Lactobacillus casei CECT 4043, as well as their fermentation products, were evaluated as probiotic additives to replace antibiotics in weaning pig diets. The administration of these potentially probiotic preparations improved BWG and feed intake (FI). In the same study, Guerra et al. [1] observed that the two above-mentioned LAB fulfil many of the probiotic criteria [19], because they are (i) nonpathogenic, (ii) able to survive during processing and storage, (iii) resistant to bile and acid environment, and (iv) producer of inhibitory compounds (organic acids and antibacterial activity).
Since probiotic effects are strain dependent [1] and may also depend on the host and their immunologic state [20], the observed probiotic effects of the L. lactis CECT 539 and Lact. casei CECT 4043 preparations in piglets might not be observed in other host entities. Therefore, in an attempt for testing the latter hypothesis, the L. lactis CECT 539 and Lact. casei CECT 4043 preparations (containing both the live cells and their products of fermentation) were evaluated as a replacement for antibiotics in stimulating health and growth of broilers.
2. Materials and Methods
2.1. Bacterial Strains
Lactobacillus casei subsp. casei CECT 4043 (a high lactic acid-producing strain) and Lactococcus lactis subsp. lactis CECT 539 (a nisin-producing strain) were obtained from the Spanish Type Culture Collection (CECT). Stock cultures of both LAB strains were maintained at −40°C in nutrient broth supplemented with 15% glycerol. Working cultures, maintained at 4°C on de Man, Rogosa, and Sharpe (MRS, Cultimed) agar, were prepared monthly from frozen stock cultures.
2.2. Production of the Potentially Probiotic Preparations of the Two LAB
The potentially probiotic preparations of the strains CECT 4043 and CECT 539 were obtained by using a fed-batch fermentation technique based on successive realkalizations of the culture media, which were prepared with cheese whey from a local dairy plant [21]. The fermentation medium was a diluted whey (DW: concentrated whey (CW) mixed with wash water), which contained (in g/L): total sugars, 20.54; total nitrogen, 0.45; total phosphorus, 0.25, and soluble proteins, 2.04 [1]. The substrates used as feeding media were a concentrated lactose solution (400 g/L) and CW medium. The latter substrate contained (in g/L): total sugars, 48.11; total nitrogen, 1.05; total phosphorus, 0.43, and soluble proteins, 5.02 [1].
The realkalized fed-batch cultures with each LAB strain were carried out at a controlled temperature of 30°C in a 10 L bench top bioreactor tailored at an agitation speed of 200 rpm and continuous record of pH as described before [1, 21, 22].
At the end of each realkalized fed-batch culture, the media were adjusted to pH 7.0 to facilitate the adsorption of the bacteriocin onto the producer strains [23]. Subsequently, the cultures (cells plus the fermentation products) were preserved at −20°C with skim milk powder (300 g/L of fermented medium) until further use, as indicated by Guerra et al. [1].
2.3. Preparation of the Experimental Feeds
The potentially probiotic preparations of the two LAB (composition in Table 1) were defrosted and mixed with the commercial mash broiler feed (named basal diet which composition is showed in Table 2) using an end-over-end mixer in a ratio of 20 mL probiotic preparation/kg feed. No pellet was made with the experimental feeds. This can contribute to increase feed wastage and, so, to increase feed conversion values; however for comparative purposes between treatments it is worth keeping high probiotic counts. The probiotic preparations were added weekly to the basal diets as recommended by Guerra et al. [1]. In the group receiving the antibiotic, the basal diet was supplemented with 10 mg of avilamycin per kg of feed.
Table 1.
Mean composition of the probiotic preparations obtained from realkalized fed-batch cultures of Lact. casei CECT 4043 and L. lactis CECT 539.
| Composition | CECT 4043 | CECT 539 |
|---|---|---|
| Total sugars (g/L) | 16.16 | 19.71 |
| Nitrogen (g/L) | 1.37 | 1.59 |
| Phosphorous (g/L) | 0.07 | 0.36 |
| Protein (g/L) | 6.09 | 9.15 |
| pH | 7.00 | 7.00 |
| Viable cells (CFU/mL)1 | 3.69 × 109 | 3.34 × 109 |
| Antibacterial activity (AU/mL)2 | 28.85 | 164.49 |
| Lactic acid (g/L) | 33.47 | 17.54 |
| Formic acid (g/L) | 0.10 | 0.85 |
1CFU: colony-forming units.
2AU: activity units.
Table 2.
Composition of the basal diets of both experiments.
| Composition (g/Kg of diet) | Experiment 1 | Experiment 2 |
|---|---|---|
| Barley | 20.0 | 70.0 |
| Wheat | 300.0 | 500.0 |
| Maize | 300.0 | 0.0 |
| Animal fat | 15.0 | 48.2 |
| Full fat soybean | 35.0 | 28.6 |
| Soybean meal 440 | 272.0 | 20.0 |
| Gluten feed | 20.0 | 0.0 |
| Soybean meal 470 | 0.0 | 287.4 |
| Sodium bicarbonate | 0.3 | 0.5 |
| Calcium carbonate | 14.0 | 11.2 |
| Monocalcium phosphate | 13.5 | 19.6 |
| Sodium chloride | 3.9 | 3.5 |
| L-Choline 75 | 0.9 | 0.9 |
| DL(+)-Methionine | 1.9 | 3.4 |
| L-Threonine | 0.0 | 0.6 |
| L-Lysine | 0.7 | 3.3 |
| Coccidiostatea | 0.6 | 0.6 |
| Mineral premixb | 1.1 | 1.1 |
| Vitamin premixc | 1.1 | 1.1 |
aC-Maxiban G150.
bPremix contained per kg of diet: Co 0.15 g, Cu 8 g, Fe 40 g, I 1.9 g, Mn 80 g, Se 0.25 g, Zn 65 g.
cPremix contained per kg of diet: Vitamin A 12000 IU, Vitamin D3 4000 IU, Vitamin E 50 IU, Vitamin K 3.5 mg, Vitamin B1 2.5 mg, Vitamin B2 7 mg, pantothenic acid 14 mg, Vitamin B6 3 mg, Vitamin B12 15 mg, nicotinic acid 55 mg, folic acid 1 mg, biotin 0.17 mg.
2.4. Analytical Determinations
The concentrations of total sugars, phosphorous, nitrogen, protein, lactic acid, formic acid and antibacterial activity in the probiotic preparations were determined by methods previously described [23, 24].
2.5. Experimental Animals and Management
The two experiments were carried out in the experimental farm of COREN, S.C.L. (Ourense, Spain). One-day-old males were used in both experiments and fed ad libitum. Four experimental groups were assayed: a control group fed with unsupplemented basal diet, a second group fed with the probiotic Lactobacillus casei preparation, a third group fed with the probiotic Lactococcus lactis preparation, and an antibiotic (avilamycin) supplemented fed group.
2.5.1. Experiment 1
A total of 120 medium-growth Sasso X44 chickens were distributed into 12 replicates of 10 birds each (three replicates per treatment) during an experimental period of 42 days. Each replicate was housed separately in individual metal cages (75 × 40 × 105 cm), situated at 80 cm from the floor to limit the consumption of faeces. The cycle of light was natural environment (12 h of daylight) and the temperature during the treatment, maintained with propane heating, was the following: first week, 32°C, second week, 30°C, and third week, 26°C. From day 21, the temperature was maintained between 20 and 24°C. The BWG, FI, and FCE were calculated at 7, 14, 21, and 42 days after the animals received the experimental diets.
2.5.2. Experiment 2
A total of 1200 commercial Ross 308 broiler chicks were distributed into 24 pens (12.7 animals/m2) of 50 birds (six replicates per treatment) during an experimental period of 31 days on wood shaving floor to simulate farm conditions. According to the good manufacturing practices of Coren S.C.L., the light program consisted in 24 h for the first 12 days, one hour alternating light and dark from day 15 to day 21, and 2 h alternating light and dark from day 21 until the end of the experiment. The temperature program was similar to that of experiment 1. BWG, FI, and FCE were determined at 16 and 31 days.
2.6. Sample Collection
At 7, 14, 21, and 42 days (in case of experiment 1) and at 7, 16, and 31 days (in case of experiment 2), 24 chickens (2 per replicate, so 6 per treatment) in experiment 1 and 48 chickens (2 per replicate, so 12 per treatment) in experiment 2 were chosen randomly and sacrificed. Animal management followed the animal care and welfare guidelines of COREN S.C.L. The abdominal cavity was opened and the gastrointestinal tract was excised to determine the number of bacterial counts. In experiment 1 the whole gastrointestinal tract, from crop to caeca, was used. In experiment 2 only the caeca were excised.
2.7. Bacterial Counts in Gastrointestinal Samples
All gastrointestinal samples emptied of faeces were weighed and placed in a sterile bag with sterile phosphate buffered saline (PBS, pH 7, 100 mM NaCl) in proportion 1 : 10 (w/v) and pummelled for 2 min in a Stomacher. Tenfold serial dilutions in sterile PBS were performed up to 107 and aliquots (0.1 mL) of each dilution were pour-plated in MRS agar (Cultimed), eosin methylene blue agar (EMB, Levine Formulation, Cultimed), and Tryptone Soy Agar (TSA, Cultimed) to determine the main representative cultivable LAB microbiota, coliforms, and total mesophilic counts, respectively. The plates were incubated at 37 ± 1°C for 24 h for coliform counts and at 30 ± 1°C for 24 h and 48 h for mesophilic bacteria and LAB counts, respectively. The results were expressed as the number of colony-forming units per gram (wet weight) of intestinal content (CFU/g).
2.8. Statistical Analysis
Data on growth performance, bacterial counts, and relative intestine weight were statistically analyzed using the software package SPSS 13.0 for Windows (Release 13.0.1; SPSS Inc., Chicago, IL, 2004). Viable counts in the intestinal content were transformed by logarithm (log10) before statistical analysis. Normal distribution of data as well as the independence and homogeneity of variances among treatment groups was verified by looking at the distribution of the data and the Fisher F-test (which is included in the t-test output), respectively. A one-way analysis of variance (ANOVA) with the step-down multiple-stage F post hoc test (Ryan-Einot-Gabriel-Welsch multiple F-test (P = 0.05) was used to distinguish treatment mean differences [1].
3. Results
3.1. Probiotic Preparations
The mean compositions of the two probiotic preparations obtained from the realkalized fed-batch fermentations with Lact. casei and L. lactis on whey are shown in Table 1. As it can be observed, both probiotic preparations contained relatively high concentrations of inhibitory products (lactic acid and antibacterial activity) and relatively low concentrations of formic acid, which were accumulated at different concentrations in the culture media during the realkalized fed-batch fermentations.
In addition, both preparations were characterized with high concentrations of viable cells (3.69 × 109 CFU/mL in case of Lact. casei and 3.34 × 109 CFU/mL in case of L. lactis). Therefore, addition of the two potential probiotic preparations at the dose of 20 mL/kg of feed offered the possibility of preparing feeds with viable cells concentrations of 7.38 × 107 CFU of Lact. casei or 6.68 × 107 CFU of L. lactis per g of feed. Both concentrations are higher than that of the recommended dose of viable probiotic cells (106 CFU per g or mL) necessary to observe beneficial effects [25, 26].
3.2. Performance of Medium-Growth Sasso X44 Chickens in Cages (Experiment 1)
The BWG, FI, and FCE values of medium-growth Sasso X44 chickens fed with the different diets during the experimental period are shown in Table 3. Broilers fed the L. lactis CECT 539 preparation showed the lowest values of BWG and FI during the first 21 d of treatment (P < 0.05), but no differences were found for FCE among groups. In addition, for the entire experimental period, diets did not affect any of the growth performance parameters studied.
Table 3.
Effect of dietary probiotic preparations (CECT 4043, CECT 539) or antibiotic (avilamycin) on growth performance parameters of medium-growth Sasso X44 chickens during 42 days (experiment 1).
| Treatment1 | BWG (g per chicken) | FI (g per chicken) | FCE (g of FI/g of BWG) | Relative intestine weight (g of organ/g of BW) |
|---|---|---|---|---|
| Chicken performance (days 1–21) | ||||
|
| ||||
| Control | 436 ± 21a | 749 ± 16a | 1.72 ± 0.05 | 0.066 ± 0.006 |
| CECT 4043 | 425 ± 5ab | 754 ± 9a | 1.78 ± 0.01 | 0.064 ± 0.003 |
| CECT 539 | 395 ± 13b | 654 ± 80b | 1.65 ± 0.15 | 0.067 ± 0.007 |
| Avilamycin | 440 ± 17a | 766 ± 15a | 1.74 ± 0.05 | 0.060 ± 0.009 |
| F | 5.477 | 4.630 | 1.179 | 0.965 |
| d.f. (N)2 | 3 (12) | 3 (12) | 3 (12) | 3 (24) |
|
| ||||
| Chicken performance (days 1–42) | ||||
|
| ||||
| Control | 1336 ± 33 | 2864 ± 140 | 2.14 ± 0.07 | 0.046 ± 0.004a |
| CECT 4043 | 1348 ± 33 | 2858 ± 97 | 2.12 ± 0.05 | 0.045 ± 0.002a |
| CECT 539 | 1314 ± 16 | 2800 ± 120 | 2.13 ± 0.07 | 0.048 ± 0.006a |
| Avilamycin | 1503 ± 16 | 3084 ± 84 | 2.06 ± 0.14 | 0.040 ± 0.004b |
| F | 3.935 | 3.741 | 0.584 | 4.242 |
| d.f. (N) | 3 (12) | 3 (12) | 3 (12) | 3 (24) |
a–cMeans within columns followed by different letters are significantly different (P < 0.05).
1The chickens from the control group were not given probiotic preparations or antibiotic. The chickens in the CECT 4043, CECT 539, and avilamycin groups were fed with Lactobacillus casei CECT 4043 (7.38 × 1010 CFU/Kg diet), Lactococcus lactis CECT 539 (6.68 × 1010 CFU/Kg diet) preparations, and avilamycin (10 mg/Kg diet), respectively. BWG: body weight gain, FI: feed intake, FCE: feed conversion efficiency.
2d.f.: degree of freedom. N: number of samples.
Interestingly, at the end of the experiment (42 d), avilamycin feeding resulted in a reduction (P < 0.05) in the mean relative intestinal weight (0.040 g of organ/g of BW) in comparison to the control group (0.046 g of organ/g of BW) and the groups receiving Lact. casei (0.045 g of organ/g of BW) and L. lactis (0.048 g of organ/g of BW). However, no significant differences in the relative intestine weight were observed between the two groups receiving the two probiotic preparations and the control group (Table 3).
3.3. Effect of the Feeding with Probiotic Preparations on the Intestinal Microbiota of Medium-Growth Sasso X44 Chickens in Cages (Experiment 1)
The viable counts of the different groups of bacteria analyzed in this trial are shown in Figure 1. With regard to the coliforms (upper part of Figure 1), no significant differences were found in total counts among diets, probably because birds were placed in cages elevated 80 cm above the barn floor, where no reconsume of faeces occurred.
Figure 1.
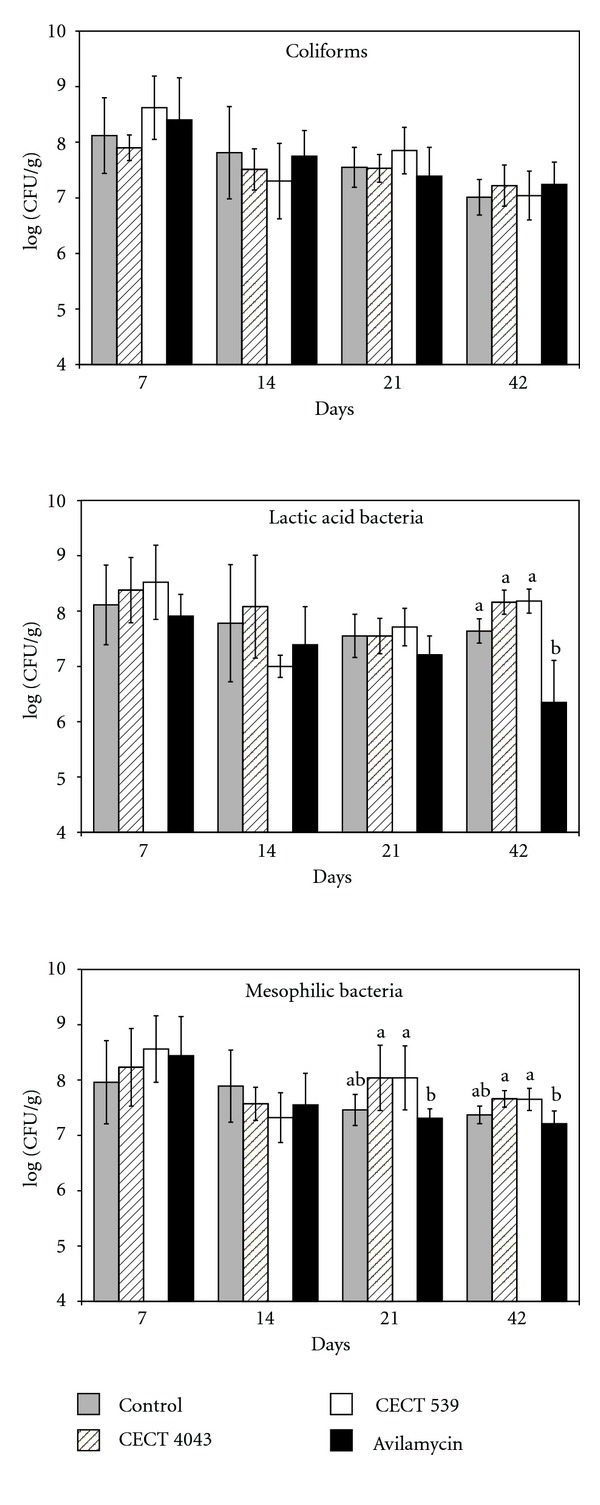
Viable plate counts (means ± standard deviations) of coliforms, lactic acid bacteria and mesophilic bacteria in the intestinal content of medium-growth Sasso X44 chickens fed with a non supplemented diet (control), or diets supplemented with the probiotic Lact. casei CECT 4043 or L. lactis CECT 539 preparations or avilamycin. a–cMeans within columns followed by different letters are significantly (P < 0.05).
However, in broilers fed the probiotic preparations, the final average LAB counts (8.16 log10 CFU/g in case of strain CECT 4043 and 8.18 log10 CFU/g in case of strain CECT 539) were higher (P < 0.10) than that (7.64 log10 CFU/g) of the control group (middle part of Figure 1). In chickens fed avilamycin, the average LAB counts decreased progressively until reaching a final value at the end of the experiment (6.35 log10 CFU/g) significantly lower (P < 0.05) than those final levels obtained in the other three groups (middle part of Figure 1).
As expected, the final average mesophilic counts in the groups fed the probiotic preparations (7.66 log10 CFU/g in case of strain CECT 4043 and 7.65 log10 CFU/g in case of strain CECT 539) by day 42 were significantly higher (P < 0.05) than that (7.21 log10 CFU/g) of the avilamycin group (lower part of Figure 1).
3.4. Performance of Broiler Chickens in Pens with Wood Shaving Litter and Subjected to Nutritional Stress (Experiment 2)
Table 4 summarizes the effect of dietary probiotic preparations or avilamycin on growth performance parameters in stressed chickens. With regard to the final BWG, no significant differences were observed among treatments during the whole experimental period. However, broilers fed avilamycin had higher FCE values (P < 0.05) than the other three groups. At 16 days of treatment, the FCE values in the groups fed the probiotic preparations (1.86 ± 0.08 in case of strain CECT 4043 and 1.82 ± 0.08 in case of strain CECT 539) were lower than those of the avilamycin (2.15 ± 0.18, P < 0.05) and control (2.04 ± 0.20) groups. Although the FCE values increased in the four groups at the end of the experimental period, broilers fed Lact. casei CECT 4043 preparation were more efficient than those fed avilamycin (P < 0.05) or unsupplemented feed (P < 0.10). In the present study, mortality was not significantly (P < 0.05) affected by probiotic treatment over the experimental period (data not shown).
Table 4.
Effect of dietary probiotic preparations (CECT 4043, CECT 539) or antibiotic (avilamycin) on growth performance parameters of Ross 308 broiler chickens subjected to nutritional stress (experiment 2).
| Treatment1 | BWG (g per chicken) | FI (g per chicken) | FCE (g of FI/g of BWG) | Relative caeca weight (g of organ/g of BW) |
|---|---|---|---|---|
| Chicken performance (days 1–16) | ||||
|
| ||||
| Control | 397 ± 34 | 805 ± 42a | 2.04 ± 0.20ba | 0.012 ± 0.003 |
| CECT 4043 | 387 ± 13 | 721 ± 9b | 1.86 ± 0.08cb | 0.011 ± 0.003 |
| CECT 539 | 388 ± 15 | 706 ± 18b | 1.82 ± 0.08c | 0.011 ± 0.002 |
| Avilamycin | 369 ± 39 | 789 ± 47a | 2.15 ± 0.18a | 0.012 ± 0.003 |
| F | 1.025 | 13.042 | 6.810 | 1.669 |
| d.f (N)2 | 3 (24) | 3 (24) | 3 (24) | 3 (48) |
|
| ||||
| Chicken performance (days 1–31) | ||||
|
| ||||
| Control | 1377 ± 82 | 2909 ± 154 | 2.11 ± 0.10ab | 0.009 ± 0.003 |
| CECT 4043 | 1388 ± 42 | 2802 ± 34 | 2.02 ± 0.05b | 0.009 ± 0.003 |
| CECT 539 | 1364 ± 46 | 2812 ± 56 | 2.06 ± 0.07ab | 0.008 ± 0.003 |
| Avilamycin | 1319 ± 87 | 2872 ± 33 | 2.18 ± 0.12a | 0.007 ± 0.003 |
| F | 1.221 | 2.135 | 3.689 | 3.446 |
| d.f (N) | 3 (24) | 3 (24) | 3 (24) | 3 (44) |
a–cMeans within columns followed by different letters are significantly different (P < 0.05).
1The chickens from the control group were not given probiotic preparations or antibiotic. The chickens in the CECT 4043, CECT 539, and avilamycin groups were fed with Lactobacillus casei CECT 4043 (7.38 × 1010 CFU/Kg diet), Lactococcus lactis CECT 539 (6.68 × 1010 CFU/Kg diet) preparations, and avilamycin (10 mg/Kg diet), respectively. BWG: body weight gain, FI: feed intake, FCE: feed conversion efficiency.
2d.f.: degree of freedom. N: number of samples.
Although, at the end of the experiment, the relative weight of caeca values in all the groups were approximately 1% of the animal weight, as described by Redig [27], no significant differences were found between broilers fed with the different diets.
3.5. Total Coliform Counts in the Caeca of Ross 308 Broiler Chickens (Experiment 2)
Coliform counts in the caeca were highly variable throughout the assay, with large variance of values within individual treatment groups and variations observed in counts at different time points (Figure 2). Consequently, no significant difference in fecal coliform counts (P < 0.05) was observed in the four groups during the experimental period.
Figure 2.
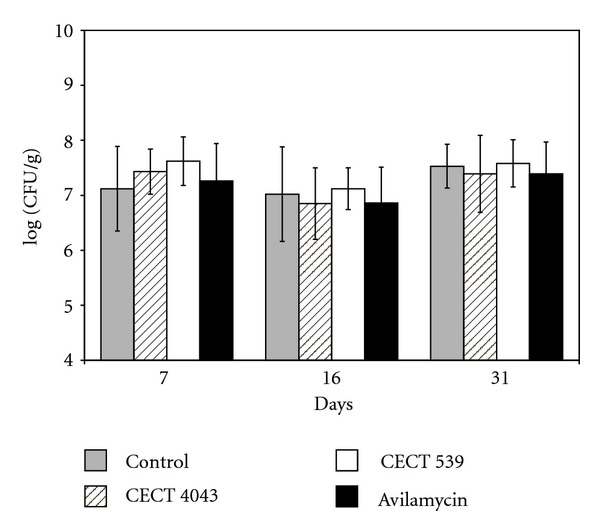
Viable plate counts (means ± standard deviations) of coliforms in the caeca of Ross 308 broilers fed with a non supplemented diet (control) or diets supplemented with the probiotic Lact. casei CECT 4043 or L. lactis CECT 539 preparations or avilamycin.
4. Discussion
The results obtained in experiment 1 showed that, at the end of the experimental period (42 days), consumption of the different diets did not affect any of the growth performance parameters of medium-growth Sasso X44 chickens. However, a significant decrease in the relative intestinal weight (P < 0.05) was observed in the group receiving avilamycin as compared with the nontreated control group and the groups fed diets with the probiotic preparations. A similar result was observed when bacitracin and virginiamycin, two antibiotic growth promoters, were assayed as additives in corn-soybean meal diets for Ross × Ross broiler chicks [28]. This decrease in the intestinal weight because of consumption of antibiotics has been documented by other authors [29, 30] before knowing their positive effect as growth promoters in chicks. Reduction in intestinal weight has been associated to a thinning of the gastrointestinal walls tract probably due to an inhibition of the microbial production of polyamines and volatile fatty acids [31].
Interestingly, in the present study, the groups receiving the two probiotic preparations had similar relative intestine weights than the group fed control diet (Table 3), suggesting that the mechanism of action of avilamycin and the probiotic preparations was different.
In the first experiment, no significant differences were found in total coliform counts among groups (Figure 1). In this way, when a pathogen colonizes the intestine, infection in the other chickens happens by horizontal transmission through faeces [32], but, in this assay, chickens had a minimum contact with their faeces and, consequently, the growth of enteric bacterial contamination was avoided.
At the end of the experimental period, LAB counts in the probiotic fed groups were higher than that of the control group. The strains CECT 4043 and CECT 539 used in this experiment have proven to be resistant to acid and bile salts in vitro under conditions that mimic the animal GIT environment [1]. Therefore, the higher LAB counts found in chickens fed probiotics could be due to the presence of the Lact. casei CECT 4043 and L. lactis CECT 539 in the GIT, permanently as a result of the colonization of the intestinal mucus, or temporarily and dependent on the diet consumed by the birds. Another possible reason to explain the increase in LAB counts could be the growth of other epiphytic LAB due to the probiotic cells supplemented in the diet. Contrarily, chickens fed with avilamycin showed a continuous decrease in LAB counts, which were at the end of the experiment (42 days), significantly lower than those of the groups receiving the two probiotic preparations. These results suggest that the feeding with avilamycin inhibits the development of LAB. Also the highest counts of mesophilic bacteria were found in chicks fed the two probiotic preparations. As mesophilic bacteria also include LAB, the increase in mesophilic counts observed in the probiotic groups could be due to the same reasons previously discussed for LAB counts. Yu et al. [33] also reported that Arbor Acres broiler fed a Lactobacillus reuteri Pg4 transformant in pens showed higher total aerobic and Lactobacillus spp. counts in the ileum and caecum than unsupplemented control birds. In conclusion, the experimental system chosen in this first trial for the handling of animals, which reduces their contact with faeces, could mask a potential protective effect of probiotic or antibiotic. These results suggested the need of choosing other handling conditions.
Then, in experiment 2, the effect of the probiotic preparations containing Lact. casei CECT 4043 and L. lactis CECT 539 cultures was evaluated in wood shaving floor, using the similar conditions of temperature, cycle of light, and handling as the farm conditions. With this approach, the transmission of microorganisms between animals could be facilitated. The medium-growth Sasso X44 chickens previously used in the first trial were replaced by Ross 308 broilers in the second trial. In order to obtain a more accurate analysis, both the number of animals per treatment and the number of replicates were increased. Additionally, a feed based on barley and wheat was used to promote intestinal adverse conditions that could be reversed or improved by using a probiotic treatment. These cereals contain high amounts of nonstarch polysaccharides (NSPs), which have “anti-nutritive effects” and increase the viscosity of the intestinal content [34]. The use of barley can deteriorate the intestinal structure, causing decrease of length and width of villi and their atrophy [35, 36]. Otherwise, Hofshagen and Kaldhusdal [37] observed that the counts of Lactobacillus and Streptococcus strains were higher in chickens fed with diets based on corn than chickens fed diets with barley. Moreover, Dalloul et al. [38] observed an increase in resistance to coccidiosis in chickens fed with a Lactobacillus-based probiotic diet. These results support the hypothesis that these bacteria could control the necrotic enteritis and it could be expected that the probiotic feed used in this trial would protect against this infection.
In this second experiment the FCE values of broilers fed with Lact. casei CECT 4043 preparation were lower (P < 0.10) than that of the broilers fed with the control diet at 16 and 31 days. With respect to avilamycin, the decrease of FCE was statistically significant (P < 0.05) in both periods. This positive effect of Lactobacillus-supplemented diets on FCE has been previously reported. Thus, laying hens, that received L. acidophilus supplemented diets for a 48-week period [39], had significantly better FCE than control birds. In the same way, the addition of either the single L. acidophilus or a mixture of 12 Lactobacillus cultures significantly improved FCE in broilers [14].
The use of the wheat and barley diet did not increase mortality in any of the treatments. Thus, in the second experiment, slighter and subclinic necrotic enteritis could occur, as this type of enteritidis is asymptomatic [40]. Consequently, it is probable that the nutritional stress induced in this trial was not enough to see the growth-stimulating effects of the probiotic bacteria in animal exposed to stress reported by other authors [41, 42].
In experiment 1, no significant differences were found between treatments in total coliform counts in the whole intestinal tract (Figure 1). However, it has been reported that bacterial distribution along the gastrointestinal tract is not uniform [33]. The high acidity in the stomach as well as the concentration of bile components in the proximal intestine determines a strain selection [43] and reduces the bacteria counts in the proximal segments of intestine, crop, and ileum, in comparison with the caecum. But the homogenization of the contents of the whole intestine could minimize differences among the different segments. In this manner, Jin et al. [14] did not observe significant differences in the coliform population in the small intestine of broilers fed diets supplemented with Lactobacillus during the experimental period, although the number of coliforms was significantly reduced in the caeca. For these reasons, and considering that the caecum is a distal part where more favourable conditions for bacterial development exist, in experiment 2, this part was used to analyze the number of total coliform counts. However, no significant differences (P < 0.05) were found in total coliform counts between the treatments (Figure 2).
Probiotics inhibit the adhesion of certain pathogenic bacteria such as E. coli and Salmonella enterica to the epithelial cells in vitro [44]. Competitive binding to receptors or the stimulation of host factors such as the production of mucin has been proposed as possible reasons to explain this inhibition [45]. However, not always inhibitory effects of probiotic strains on the growth of coliforms are observed in in vivo trials because host-dependent mechanisms are important in reducing the coliform level [46]. Guerra et al. [1], reported that viable coliform counts in pigs fed L. lactis CECT 539 and Lact. casei CECT 4043 preparations dropped on average for 1.8 and 1.4 log units, respectively mean; while viable coliform counts did not change in the control group. However, in the current experiment, the above discussed coliform counts reduction was not observed. The host-dependent theory suggested by Meimandipour et al. [46] could be used to explain this fact.
On the other hand, when Lact. casei CECT 4043 preparation was tested as probiotic in pigs, the mean final BWG and FI values were higher than those observed in the control group [1]. However, these researchers did not observe significant differences in FCE between the two groups receiving probiotic preparations and the control group. In contrast, the results of present study showed that, by day 31, the final BWG values in chickens receiving the different diets were not significantly different (Table 4), but the animals fed Lact. casei CECT 4043 preparation improved their final FCE in comparison to chickens fed avilamycin.
However, it is worth highlighting that differences usually found in BWG between chickens fed with antibiotics and chickens fed with diets without growth promoters were not present in this case (Tables 3 and 4). Patterson and Burkholder [10] recommended that studies in which there is no response to the growth promotant antibiotics should not be considered negative for the probiotic treatment. Then, the lack of differences between antibiotic and control groups in both experiments (medium-growth Sasso X44 chickens and Ross 308 broilers) suggests that the probiotic effect of Lact. casei CECT 4043 preparation observed in chickens has been of minor magnitude because of the good condition of the animals.
5. Conclusions
The ability of Lact. casei CECT 4043 to improve the FCE in chickens, together with its capability to stimulate the growth and to reduce coliform counts in the faeces of postweaning piglets, indicates that the probiotic Lact. casei CECT 4043 preparation could be successfully used as a feed additive for the animal feed industry. In addition the different probiotic effect observed in pigs and broilers supports the hypothesis that probiotic mechanisms are host dependent. The results obtained in this study reinforce previous reports on the probiotic effect of Lactobacillus sp. on chicken and pig growth.
Acknowledgments
This research was financially supported by the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (project CAL01-045-C2-2) and The Xunta de Galicia, Spain (project PGIDIT02PXIC38). The authors thank COREN, S.C.L. (Ourense, Spain) for its collaboration in the elaboration of this work.
References
- 1.Guerra NP, Bernárdez PF, Méndez J, Cachaldora P, Pastrana Castro L. Production of four potentially probiotic lactic acid bacteria and their evaluation as feed additives for weaned piglets. Animal Feed Science and Technology. 2007;134(1-2):89–107. [Google Scholar]
- 2.Aarestrup FM, Bager F, Andersen SJ. Association between the use of avilamycin for growth promotion and the occurrence of resistance among Enterococcus faecium from broilers: epidemiological study and changes over time. Microbial Drug Resistance. 2000;6(1):71–75. doi: 10.1089/mdr.2000.6.71. [DOI] [PubMed] [Google Scholar]
- 3.Randall LP, Ridley AM, Cooles SW, et al. Prevalence of multiple antibiotic resistance in 443 Campylobacter spp. isolated from humans and animals. Journal of Antimicrobial Chemotherapy. 2003;52(3):507–510. doi: 10.1093/jac/dkg379. [DOI] [PubMed] [Google Scholar]
- 4.van den Bogaard AE, Stobberingh EE. Epidemiology of resistance to antibiotics: links between animals and humans. International Journal of Antimicrobial Agents. 2000;14(4):327–335. doi: 10.1016/s0924-8579(00)00145-x. [DOI] [PubMed] [Google Scholar]
- 5.Lukášová J, Šustáčková. A. Enterococci and antibiotic resistance. Acta Veterinaria Brno. 2003;72:315–323. [Google Scholar]
- 6.Apata DF. Antibiotic resistance in poultry. International Journal of Poultry Science. 2009;8(4):404–408. [Google Scholar]
- 7.Yang Y, Iji PA, Choct M. Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. World’s Poultry Science Journal. 2009;65(1):97–114. [Google Scholar]
- 8.Hume ME. Historic perspective: prebiotics, probiotics, and other alternatives to antibiotics. Poultry Science. 2011;90(11):2663–2669. doi: 10.3382/ps.2010-01030. [DOI] [PubMed] [Google Scholar]
- 9.Ganan M, Silván JM, Carrascosa AV, Martínez-Rodríguez AJ. Alternative strategies to use antibiotics or chemical products for controlling Campylobacter in the food chain. Food Control. 2012;24(1-2):6–14. [Google Scholar]
- 10.Patterson JA, Burkholder KM. Application of prebiotics and probiotics in poultry production. Poultry Science. 2003;82(4):627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- 11.Marteau P, Pochart P, Bouhnik Y, Rambaud JC. The fate and effects of transiting, nonpathogenic microorganisms in the human intestine. World Review of Nutrition and Dietetics. 1993;74:1–21. doi: 10.1159/000422599. [DOI] [PubMed] [Google Scholar]
- 12.Salminen S, Deighton MA, Benno Y, Gorbach SL. Lactic acid bacteria in health and disease. In: Salminen S, von WrightA, editors. The Lactic Acid Bacteria: Microbiology and Functional Aspects. 2nd edition. New York, NY, USA: Marcel Dekker; 1998. pp. 211–253. [Google Scholar]
- 13.Muir WI, Bryden WL, Husband AJ. Immunity, vaccination and the avian intestinal tract. Developmental and Comparative Immunology. 2000;24(2-3):325–342. doi: 10.1016/s0145-305x(99)00081-6. [DOI] [PubMed] [Google Scholar]
- 14.Jin LZ, Ho YW, Abdullah N, Jalaludin S. Growth Performance, Intestinal Microbial Populations, and Serum Cholesterol of Broilers Fed Diets Containing Lactobacillus Cultures. Poultry Science. 1998;77(9):1259–1265. doi: 10.1093/ps/77.9.1259. [DOI] [PubMed] [Google Scholar]
- 15.Kabir SML, Rahman MM, Rahman MB, Rahman MM, Ahmed SU. The dynamics of probiotics on growth performance and immune response in broilers. International Journal of Poultry Sciences. 2004;3:361–364. [Google Scholar]
- 16.Willis WL, Reid L. Investigating the effects of dietary probiotic feeding regimens on broiler chicken production and Campylobacter jejuni presence. Poultry Science. 2008;87(4):606–611. doi: 10.3382/ps.2006-00458. [DOI] [PubMed] [Google Scholar]
- 17.Griggs JP, Jacob JP. Alternatives to antibiotics for organic poultry production. Journal of Applied Poultry Research. 2005;14(4):750–756. [Google Scholar]
- 18.Nava GM, Bielke LR, Callaway TR, Castañeda MP. Probiotic alternatives to reduce gastrointestinal infections: the poultry experience. Animal Health Research Reviews. 2005;6(1):105–118. doi: 10.1079/ahr2005103. [DOI] [PubMed] [Google Scholar]
- 19.Simmering R, Blaut M. Pro- and prebiotics—the tasty guardian angels? Applied Microbiology and Biotechnology. 2001;55(1):19–28. doi: 10.1007/s002530000512. [DOI] [PubMed] [Google Scholar]
- 20.Delcenserie V, Martel D, Lamoureux M, Amiot J, Boutin Y, Roy D. Immunomodulatory effects of probiotics in the intestinal tract. Current Issues in Molecular Biology. 2008;10(1):37–54. [PubMed] [Google Scholar]
- 21.Pérez Guerra N, Fajardo Bernárdez P, Torrado Agrasar A, López Macías C, Pastrana Castro L. Fed-batch pediocin production by Pediococcus acidilactici NRRL B-5627 on whey. Biotechnology and Applied Biochemistry. 2005;42(1):17–23. doi: 10.1042/BA20040146. [DOI] [PubMed] [Google Scholar]
- 22.Bernárdez PF, Amado IR, Castro LP, Guerra NP. Production of a potentially probiotic culture of Lactobacillus casei subsp. casei CECT 4043 in whey. International Dairy Journal. 2008;18(10-11):1057–1065. [Google Scholar]
- 23.Guerra NP, Rua ML, Pastrana L. Nutritional factors affecting the production of two bacteriocins from lactic acid bacteria on whey. International Journal of Food Microbiology. 2001;70(3):267–281. doi: 10.1016/s0168-1605(01)00551-7. [DOI] [PubMed] [Google Scholar]
- 24.Pérez Guerra N, Pastrana Castro L. Enhancement of nisin production by Lactococcus lactis in periodically re-alkalized cultures. Biotechnology and Applied Biochemistry. 2003;38(2):157–167. doi: 10.1042/BA20030059. [DOI] [PubMed] [Google Scholar]
- 25.Charteris WP, Kelly PM, Morelli L, Collins JK. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. Journal of Applied Microbiology. 1998;84(5):759–768. doi: 10.1046/j.1365-2672.1998.00407.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee YK, Lim CY, Teng WL, Ouwehand AC, Tuomola EM, Salminen S. Quantitative approach in the study of adhesion of lactic acid bacteria to intestinal cells and their competition with enterobacteria. Applied and Environmental Microbiology. 2000;66(9):3692–3697. doi: 10.1128/aem.66.9.3692-3697.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redig PT. The avian ceca: obligate combustion chambers or facultative afterburners? The conditioning influence of diet. Journal of Experimental Zoology. 1989;(3):66–69. doi: 10.1002/jez.1402520511. [DOI] [PubMed] [Google Scholar]
- 28.Miles RD, Butcher GD, Henry PR, Littell RC. Effect of antibiotic growth promoters on broiler performance, intestinal growth parameters, and quantitative morphology. Poultry Science. 2006;85(3):476–485. doi: 10.1093/ps/85.3.476. [DOI] [PubMed] [Google Scholar]
- 29.Coates ME, Davies MK, Kon SK. The effect of antibiotics on the intestine of the chick. British Journal of Nutrition. 1955;9:110–119. doi: 10.1079/bjn19550016. [DOI] [PubMed] [Google Scholar]
- 30.Hill CH, Keeling AD, Kelly. JW. Studies on the effect of antibiotics on the intestinal weights of chicks. Journal of Nutrition. 1957;62:255–267. doi: 10.1093/jn/62.2.255. [DOI] [PubMed] [Google Scholar]
- 31.Bedford M. Removal of antibiotic growth promoters from poultry diets: implications and strategies to minimise subsequent problems. World’s Poultry Science Journal. 2000;56(4):362–365. [Google Scholar]
- 32.Gast RK, Holt PS. Experimental horizontal transmission of Salmonella enteritidis strains (phage types 4, 8, and 13a) in chicks. Avian Diseases. 1999;43(4):774–778. [PubMed] [Google Scholar]
- 33.Yu B, Liu JR, Hsiao FS, Chiou PWS. Evaluation of Lactobacillus reuteri Pg4 strain expressing heterologous β-glucanase as a probiotic in poultry diets based on barley. Animal Feed Science and Technology. 2008;141(1-2):82–91. [Google Scholar]
- 34.Meng X, Slominski BA, Guenter W. The effect of fat type, carbohydrase, and lipase addition on growth performance and nutrient utilization of young broilers fed wheat-based diets. Poultry Science. 2004;83(10):1718–1727. doi: 10.1093/ps/83.10.1718. [DOI] [PubMed] [Google Scholar]
- 35.Viveros A, Brenes A, Pizarro M, Castaño M. Effect of enzyme supplementation of a diet based on barley, and autoclave treatment, on apparent digestibility, growth performance and gut morphology of broilers. Animal Feed Science and Technology. 1994;48(3-4):237–251. [Google Scholar]
- 36.Teirlynck E, Bjerrum L, Eeckhaut V, et al. The cereal type in feed influences gut wall morphology and intestinal immune cell infiltration in broiler chickens. British Journal of Nutrition. 2009;102(10):1453–1461. doi: 10.1017/S0007114509990407. [DOI] [PubMed] [Google Scholar]
- 37.Hofshagen M, Kaldhusdal M. Barley inclusion and avoparcin supplementation in broiler diets. 1. Effect on small intestinal bacterial flora and performance. Poultry Science. 1992;71(6):959–969. doi: 10.3382/ps.0710959. [DOI] [PubMed] [Google Scholar]
- 38.Dalloul RA, Lillehoj HS, Shellem TA, Doerr JA. Enhanced mucosal immunity against Eimeria acervulina in broilers fed a Lactobacillus-based probiotic. Poultry Science. 2003;82(1):62–66. doi: 10.1093/ps/82.1.62. [DOI] [PubMed] [Google Scholar]
- 39.Haddadin MSY, Abdulrahim SM, Hashlamoun EAR, Robinson RK. The effect of Lactobacillus acidophilus on the production and chemical composition of hen’s eggs. Poultry Science. 1996;75(4):491–494. doi: 10.3382/ps.0750491. [DOI] [PubMed] [Google Scholar]
- 40.Kaldhusdal M, Hofshagen M. Barley inclusion and avoparcin supplementation in broiler diets. 2. Clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis. Poultry Science. 1992;71(7):1145–1153. doi: 10.3382/ps.0711145. [DOI] [PubMed] [Google Scholar]
- 41.Abe F, Ishibashi N, Shimamura S. Effect of administration of bifidobacteria and lactic acid bacteria to newborn calves and piglets. Journal of Dairy Science. 1995;78(12):2838–2846. doi: 10.3168/jds.S0022-0302(95)76914-4. [DOI] [PubMed] [Google Scholar]
- 42.Bogovic Matijasic B, Stojković S, Salobir J, Malovrh Š, Rogelj I. Evaluation of the Lactobacillus gasseri K7 and LF221 strains in weaned piglets for their possible probiotic use and their detection in the faeces. Animal Research. 2004;53(1):35–44. [Google Scholar]
- 43.Hyronimus B, Le Marrec C, Hadj Sassi A, Deschamps A. Acid and bile tolerance of spore-forming lactic acid bacteria. International Journal of Food Microbiology. 2000;61(2-3):193–197. doi: 10.1016/s0168-1605(00)00366-4. [DOI] [PubMed] [Google Scholar]
- 44.Gill HS. Probiotics to enhance anti-infective defences in the gastrointestinal tract. Bailliere’s Best Practice and Research in Clinical Gastroenterology. 2003;17(5):755–773. doi: 10.1016/s1521-6918(03)00074-x. [DOI] [PubMed] [Google Scholar]
- 45.Lee YK, Puong KY. Competition for adhesion between probiotics and human gastrointestinal pathogens in the presence of carbohydrate. British Journal of Nutrition. 2002;88(1):S101–S108. doi: 10.1079/BJN2002635. [DOI] [PubMed] [Google Scholar]
- 46.Meimandipour A, Shuhaimi M, Hair-Bejo M, et al. In vitro fermentation of broiler cecal content: the role of Lactobacilli and pH value on the composition of microbiota and end products fermentation. Letters in Applied Microbiology. 2009;49(4):415–420. doi: 10.1111/j.1472-765X.2009.02674.x. [DOI] [PubMed] [Google Scholar]


