Abstract
Conclusive evidence is presented that an acid phosphatase catalyzes phosphate (oxygen)-water exchange. Studies conducted with human prostatic acid phosphatase by two independent methods have established that, despite earlier reports to the contrary, the enzyme catalyzes an exchange reaction between oxygen atoms of phosphate ion and of water. Kinetic data were obtained both by chemical conversion to trimethyl phosphate followed by mass spectroscopy and by a totally independent method involving 31P isotope shift nuclear magnetic resonance spectroscopy. Analysis showed that the enzyme catalyzes the exchange in a random, noncoupled process. If any coupled exchange occurs, it must represent less than 10% of the total. By mass spectral analysis, catalytic rate constants kcat = 0.14 sec-1 (4 degrees) and 1.8 sec-1 (37.5 degrees) were obtained. By 31P nuclear magnetic resonance kcat = 1.6 sec-1 (31 degrees) was obtained. The energy of activation for the exchange reaction is approximately 13kcal mol-1. The kcat value for exchange is about 10-fold greater than that observed with Escherichia coli alkaline phosphatase.
Full text
PDF
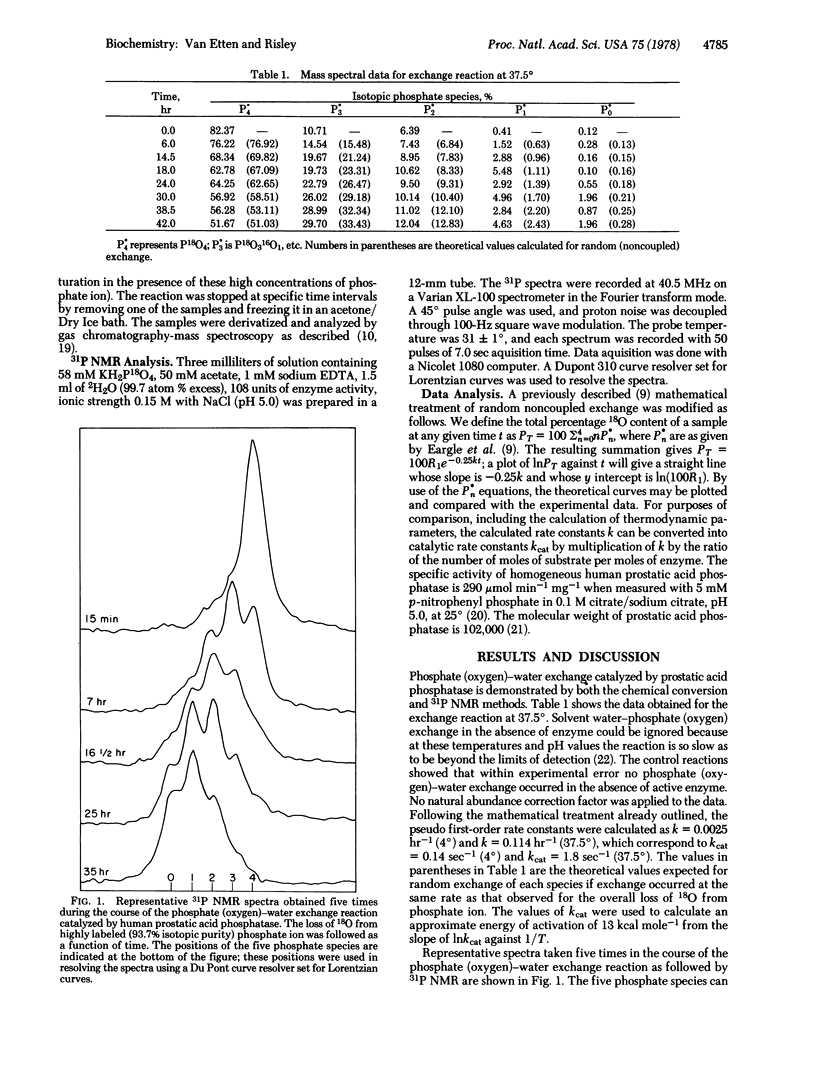
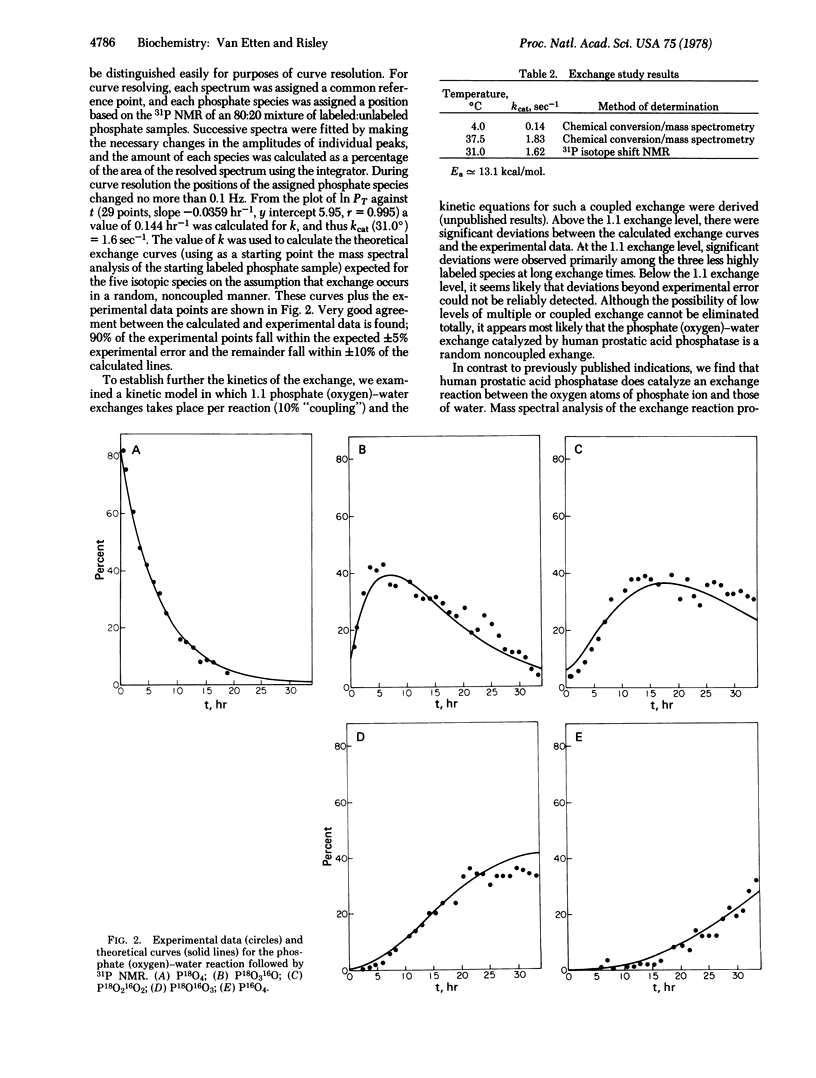

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Johnson B. P., Coleman J. E. Phosphate binding to alkaline phosphatase. Metal ion dependence. J Biol Chem. 1970 Oct 10;245(19):4968–4976. [PubMed] [Google Scholar]
- Bock J. L., Cohn M. Metal dependence of the phosphate (oxygen)-water exchange reaction of Escherichia coli alkaline phosphatase. Kinetics followed by 31P(18O) NMR. J Biol Chem. 1978 Jun 25;253(12):4082–4085. [PubMed] [Google Scholar]
- COHN M., DRYSDALE G. R. A study with O18 of adenosine triphosphate formation in oxidative phosphorylation. J Biol Chem. 1955 Oct;216(2):831–846. [PubMed] [Google Scholar]
- Christeller J. T., Tolbert N. E. Mechanism of phosphoglycolate phosphatase. Studies of hydrolysis and transphosphorylation, substrate analogs, and sulfhydryl inhibition. J Biol Chem. 1978 Mar 25;253(6):1791–1798. [PubMed] [Google Scholar]
- Cohn M., Hu A. Isotopic (18O) shift in 31P nuclear magnetic resonance applied to a study of enzyme-catalyzed phosphate--phosphate exchange and phosphate (oxygen)--water exchange reactions. Proc Natl Acad Sci U S A. 1978 Jan;75(1):200–203. doi: 10.1073/pnas.75.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derechin M., Ostrowski W., Galka M., Barnard E. A. Acid phosphomonesterase of human prostate. Molecular weight, dissociation and chemical composition. Biochim Biophys Acta. 1971 Oct;250(1):143–154. doi: 10.1016/0005-2744(71)90128-8. [DOI] [PubMed] [Google Scholar]
- Eargle D. H., Jr, Licko V., Kenyon G. L. Kinetic studies of 18O exchange of inorganic phosphate using mass spectral measurements on the tris-(trimethylsilyl) derivative. Anal Biochem. 1977 Jul;81(1):186–195. doi: 10.1016/0003-2697(77)90612-1. [DOI] [PubMed] [Google Scholar]
- Gallati H., Roth M. Aktivierung der sauren Prostataphosphatase durch 1-Pentanol. J Clin Chem Clin Biochem. 1976 Dec;14(12):581–587. [PubMed] [Google Scholar]
- McTigue J. J., Van Etten R. L. An essential active-site histidine residue in human prostatic acid phosphatase. Ethoxyformylation by diethyl pyrocarbonate and phosphorylation by a substrate. Biochim Biophys Acta. 1978 Apr 12;523(2):407–421. doi: 10.1016/0005-2744(78)90043-8. [DOI] [PubMed] [Google Scholar]
- Midelfort C. F., Rose I. A. A stereochemical method for detection of ATP terminal phosphate transfer in enzymatic reactions. Glutamine synthetase. J Biol Chem. 1976 Oct 10;251(19):5881–5887. [PubMed] [Google Scholar]
- Ostrowski W., Barnard E. A. Evidence for a phosphoryl-enzyme intermediate in the catalytic reaction of prostatic acid phosphatase. Biochemistry. 1973 Sep 25;12(20):3893–3898. doi: 10.1021/bi00744a016. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ J. H. The phosphorylation of alkaline phosphatase. Proc Natl Acad Sci U S A. 1963 Jun;49:871–878. doi: 10.1073/pnas.49.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten R. L., Hickey M. E. Phosphohistidine as a stoichiometric intermediate in reactions catalyzed by isoenzymes of wheat germ acid phosphatase. Arch Biochem Biophys. 1977 Sep;183(1):250–259. doi: 10.1016/0003-9861(77)90438-6. [DOI] [PubMed] [Google Scholar]
- Van Etten R. L., McTigue J. J. pH dependence and solvent isotope effects in the hydrolysis of phosphomonoesters by human prostatic acid phosphatase. Biochim Biophys Acta. 1977 Oct 13;484(2):386–397. doi: 10.1016/0005-2744(77)90094-8. [DOI] [PubMed] [Google Scholar]
- Van Etten R. L., Saini M. S. Selective purification of tartrate-inhibitable acid phosphatases: rapid and efficient purification (to homogeneity) of human and canine prostatic acid phosphatases. Clin Chem. 1978 Sep;24(9):1525–1530. [PubMed] [Google Scholar]
- Webb M. R., McDonald G. G., Trentham D. R. Kinetics of oxygen-18 exchange between inorganic phosphate and water catalyzed by myosin subfragment 1, using the 18O shift in 31P NMR. J Biol Chem. 1978 May 10;253(9):2908–2911. [PubMed] [Google Scholar]
- Wimmer M. J., Rose I. A. Mechanism for oxygen exchange in the chloroplast photophosphorylation system. J Biol Chem. 1977 Oct 10;252(19):6769–6775. [PubMed] [Google Scholar]



