Abstract
Children raised in families with low socioeconomic status (SES) go on to have high rates of chronic illness in adulthood. However, a sizeable minority of low-SES children remain healthy across the lifecourse, raising questions about the factors associated with, and potentially responsible for, resilience. Using a sample of 1215 middle-aged Americans, we explored whether two characteristics - upward socioeconomic mobility and early parental nurturance – were associated with resilience to the health effects of childhood disadvantage. The primary outcome was presence of metabolic syndrome in adulthood. Analyses revealed that low childhood SES was associated with higher metabolic syndrome prevalence at midlife, independent of traditional risk factors. Despite this pattern, half the participants raised in low-SES households were free of metabolic syndrome at midlife. Upward mobility was not associated with resilience. However, analyses were consistent with a buffering scenario, whereby high levels of maternal nurturance offset the metabolic consequences of childhood disadvantage.
Poverty's deleterious influences on children's educational and psychosocial functioning have long been recognized (Duncan et al., 1998; McLoyd, 1998). But recently it has become evident that childhood poverty has even further-reaching consequences, in the form of heightened vulnerability to common medical illnesses across the lifecourse (Shonkoff et al., 2009). Indeed, children raised by families of lower socioeconomic status (SES) go on to have elevated rates of infectious, respiratory, metabolic, and cardiovascular diseases in adulthood, independent of traditional risk factors for those conditions (Cohen et al., 2010; Galobardes et al., 2008).
However, not all children who grow up poor go on to develop medical problems as adults. In fact, a sizeable minority of them stay in good health (Chen & Miller, 2011). This resilience was vividly illustrated in a study wherein adults were exposed to a rhinovirus, and then monitored in quarantine for emergence of the common cold (Cohen et al., 2004). Participants who had been reared in low-SES families were more likely to become infected with the virus and to develop colds. But even among those from the lowest SES category, fewer than 50% actually became sick. These findings highlight the existence of resilient individuals who, despite having been exposed to childhood socioeconomic adversity, managed to resist an infectious challenge in adulthood.
Presently, little is known about the pathways associated with resilience to health problems in persons from low-SES backgrounds. Two broad routes to good health have been hypothesized. The first involves upward mobility offsetting the influence of childhood disadvantage (Lynch & Smith, 2005). The idea here is that children who grow up poor can have varying socioeconomic trajectories. Some will become low-SES adults, in whom the continuing and cumulative effects of disadvantage will have deleterious health effects. Others will have upward mobility and the health benefits it confers. The assumption is that favorable socioeconomic conditions in adulthood mitigate whatever disease processes were instigated by the childhood environment (Cohen et al., 2010).
The other scenario highlights the role of parental nurturance in buffering children against poverty's sequelae. Research shows that nurturant parenting can offset many of the educational and psychosocial disadvantages that beset poor children (Luthar, 2006; Masten, 2001). There is also mounting evidence that aspects of nurturant parenting – particularly warmth and sensitivity - can favorably mold the stress-response tendencies of vulnerable children (Cicchetti & Blender, 2006; Gunnar & Quevedo, 2007). In doing so nurturant parents may help mitigate the wear-and-tear that low SES places on children's physiology (Chen et al., in press; Evans et al., 2007). That said, it remains unclear whether the benefits of nurturant parenting extend to health problems, particularly ones that do not manifest until the later years of life.
The goal of this paper was to begin evaluating the plausibility of these scenarios. In a sample of middle-aged Americans, we examined links between childhood SES and later presence of the metabolic syndrome (MetS), a cluster of signs that includes high blood pressure, impaired glucose control, abdominal adiposity, and lipid dysregulation. MetS is a precursor and contributor to a number of chronic diseases of aging, including diabetes, heart disease, and stroke. It is highly prevalent in the United States, with rates estimated at 25–39%, and is becoming even more so in recent decades (Cornier et al., 2008). Here, we examined three hypotheses about childhood SES and MetS. The first was that low childhood SES would be associated with higher MetS prevalence at midlife. The second was that despite this general trend, there would be a sizeable subgroup of resilient individuals, who came from low-SES backgrounds but did not show MetS. Lastly, we predicted that both upward mobility and parental nurturance would be associated with Met S resilience at midlife.
Methods
Participants
Participants were from the Midlife in the United States (MIDUS) survey, which commenced in 1995–1996 with 7,108 non-institutionalized adults, selected via random-digit dialing from the 48 contiguous states. To allow genetically informed analyses, MIDUS included 957 pairs of twins and 950 non-twin siblings. An average of 9 years later, 75% of surviving respondents participated in a follow-up, known as MIDUS II. Biological data were collected from a subset of participants (n=1,255), who traveled to a General Clinical Research Centers (GCRCs) for an overnight visit. 43% of those invited for a biological assessment agreed to participate. These individuals had higher educational attainment than the overall MIDUS II sample, but were comparable on other demographic (age, sex, race, income) and biomedical characteristics (subjective health, chronic conditions, health behaviors; Dienberg Love et al., 2010). The mean age of the sample was 46.40 and 56.80 at the MIDUS I and II assessments, respectively.
Childhood SES
Childhood SES was indexed with MIDUS I data on parental educational attainment. Values ranged from “some grade school” through “doctoral degree.” When a respondent's parents had different levels of educational attainment, the higher value was used. For analysis, the sample was later stratified into four roughly equal groups. They corresponded to families where the highest educational achievement consisted of (a) less than a high school diploma, (b) graduation from high school or its equivalent, (c) some college education with or without an Associate's degree, and (d) a Bachelor's degree from a four-year institution (or more).
Metabolic Outcomes
The International Diabetes Federation provides a worldwide definition of MetS (Cornier et al., 2008). To qualify, an individual must show central adiposity, defined by ethnic and sex-specific cutoffs for waist circumference. (For those of Europid and African descent, who make up nearly all the MIDUS sample, cutoffs are ≥ 94 and ≥ 80 cm for men and women, respectively.) At least 2 of 4 additional components must also be present. They include (a) high blood pressure, defined as ≥ 130 systolic or ≥ 85 diastolic, (b) raised triglycerides, defined as ≥ 1.7 mmol/L, (c) raised fasting glucose, defined as ≥ 5.6 mmol/L, and (d) low high-density lipoprotein levels, defined as ≤ 0.9 and ≤ 1.1 mmol/L in men and women, respectively.
MetS components were assessed during GCRC visits. Waist circumference was measured at the narrowest point between the ribs and iliac crest. Blood pressure was assessed three times in a seated position, with 30-second intervals between measurements. The average of the two most similar readings was used for analysis. From fasting morning blood samples, a lipid panel and blood glucose were measured (using automated instruments from Roche Diagnostics, Indianapolis, IN).
For analyses, we constructed two outcome variables. One reflected the number of MetS components for which the participant met clinical cutoffs, and could range from 0–5. The other was a binary variable reflecting whether the participant met the IDF case definition for MetS.
Parental Nurturance
Parental nurturance was assessed at MIDUS I with a validated questionnaire (Rossi, 2001). It contained seven questions regarding the quality of the parental relationships during childhood. Mothers and fathers were rated separately. Sample items included: “How much did she/he understand your problems and worries?” and “How much time and attention did she give you when you needed it?” Responses to the items were averaged. Both the maternal and parental composites showed high internal consistency (α = .91 and .92, respectively).
Potential Confounders
We assessed several demographic and biobehavioral characteristics that might provide alternative explanations for any observed associations, and modeled them as covariates. The demographic covariates included age, sex, and race, coded as dummy variables reflecting European (White) or African-American (Black) descent. (In preliminary analyses we determined that none of these covariates interacted with childhood SES in predicting metabolic outcomes, p's > .14.) We also included current SES as a covariate, using a four-level education indicator with the same categories as above. This controlled for the possibility that childhood SES was proxying for more direct health effects of concurrent SES. The biobehavioral covariates were binary indicators that reflected current smoking, history of diabetes, and history of CVD.
Results
Preliminary Analyses
Table 1 presents descriptive statistics for the sample, stratified into four levels of childhood SES. The groups differed on all predictors, covariates, and endpoints, with disparities favoring those from more advantaged backgrounds. The exception was the scales tapping parental nurturance, where values were nearly identical across the childhood SES strata.
Table 1.
Characteristics of sample by childhood socioeconomic status, as indexed by parental educational attainment.
| Highest Educational Attainment by Mother or Father | Less than High School Diploma (n = 289) | High School Graduate or GED (n = 419) | Some College or Associates Degree (n = 217) | Bachelor's Degree and Higher (n = 280) | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age | 58.92 | 12.01 | 53.71 | 11.23 | 54.61 | 12.04 | 51.06 | 10.58 | < .001 |
| Female (%) | 58.80 | 51.80 | 62.70 | 55.70 | .05 | ||||
| White (%) | 68.50 | 81.90 | 82.90 | 89.60 | < .001 | ||||
| Black (%) | 26.60 | 15.00 | 11.50 | 7.10 | < .001 | ||||
| High School Graduate (%) | 89.10 | 94.30 | 98.10 | 98.60 | < .001 | ||||
| University Graduate (%) | 27.80 | 34.10 | 48.60 | 68.60 | < .001 | ||||
| Current Smoker (%) | 16.30 | 14.60 | 14.30 | 7.50 | .01 | ||||
| History of CVD (%) | 22.10 | 13.80 | 11.10 | 12.50 | < .001 | ||||
| History of Diabetes (%) | 14.20 | 9.30 | 8.80 | 7.50 | .04 | ||||
| Maternal Warmth (1–4) | 3.16 | 0.74 | 3.14 | 0.74 | 3.10 | 0.71 | 3.10 | 0.65 | .80 |
| Paternal Warmth (1–4) | 2.67 | 0.84 | 2.68 | 0.83 | 2.74 | 0.77 | 2.74 | 0.75 | .67 |
| MetS Components (0–5) | 2.53 | 1.31 | 2.29 | 1.35 | 2.21 | 1.25 | 1.93 | 1.38 | < .001 |
| MetS Diagnosis (%) | 49.80 | 41.80 | 39.20 | 31.10 | < .001 | ||||
Note. CVD = cardiovascular disease; MetS = metabolic syndrome.
Denotes the presence of significant differences between groups by one way ANOVA (for continuous outcomes) or χ2 analysis (for categorical outcomes).
Tests of Hypotheses
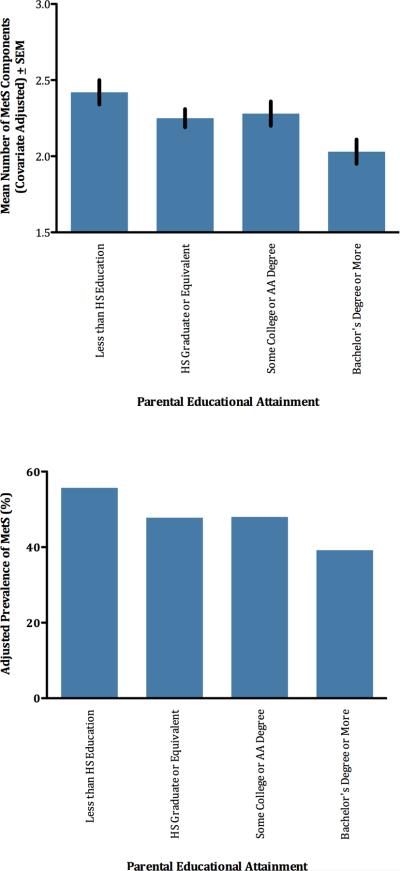
Our first hypothesis was that low childhood SES would be associated with MetS outcomes in adulthood. To examine counts of MetS components, we conducted an ANCOVA with childhood SES as the between-subjects variable, and the set of covariates described above. As Table S1 shows (see Online Supplement), those with less education and a diabetes history showed significantly higher counts of MetS components. There were also significant differences in counts of MetS components by childhood SES, F(3,1183) = 3.89, P = .009; see Figure 1. Follow-up contrasts showed that individuals from the most disadvantaged households, i.e., those from families where neither parent had a high school diploma, had significantly higher counts relative to participants from other socioeconomic backgrounds (p =.01). Similarly, individuals who were raised in the most advantaged circumstances, i.e., in families where one or both parents had a Bachelor's degree, displayed significantly lower MetS component counts than other participants (p = .002).
Figure 1.
Childhood SES is associated with number of MetS components (upper panel) and prevalence of diagnosable MetS (lower panel).
To examine whether childhood SES also predicted diagnosable MetS, we computed a binary logistic regression with diagnostic status as the outcome, and the same predictor and covariates as above (see Figure 1 and Table S2 in Supplement). Again, men and participants with less education and a diabetes history were more likely to have diagnosable MetS. There were also significant differences in MetS prevalence by childhood SES (Wald = 11.29, p = .01). MetS was most common among participants from households where neither parent completed high school. Contrasts showed that this disadvantaged population had higher MetS prevalence compared to participants whose parents had earned high-school diplomas (p = .05) and Bachelor's degrees (p = .001). They also had marginally higher MetS prevalence than participants from households with some college (p = .09).
Our second hypothesis was that a sizeable minority of individuals from low-SES backgrounds would prove to be MetS resilient. This was indeed the case. As Table 1 shows, MetS prevalence in participants whose parents lacked a high school diploma was 50 percent, meaning that half of the most disadvantaged participants were free of the condition at midlife. A similar pattern was apparent in covariate-adjusted models, where MetS prevalence was estimated at 56 percent in participants whose parents lacked a high school diploma (versus 48, 48, and 39 percent in the other groups, respectively). Looking at counts, 4.5 percent of the most disadvantaged participants had a score of 0, meaning there were no MetS components for which they met clinical cutoffs (versus 9, 9, and 16 percent in the other groups, respectively).
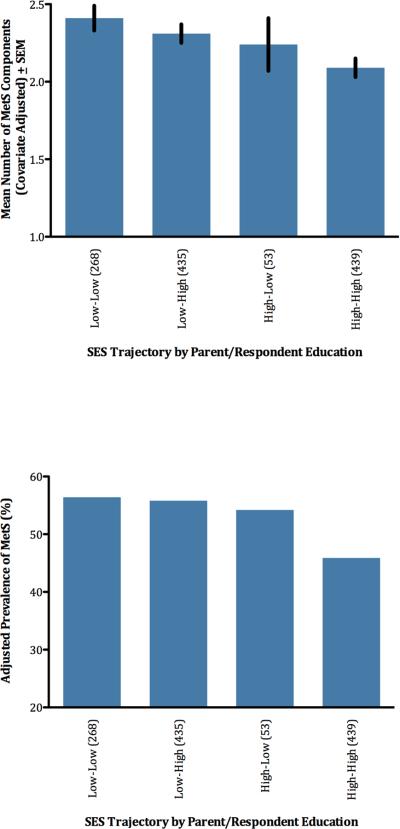
Our third hypothesis was that social mobility and parental nurturance would be associated with resilience to the health effects of low childhood SES. To examine social mobility, we split the sample into four groups reflecting SES in childhood (lower vs. higher) and adulthood (lower vs. higher). Categorization in both cases was based upon educational attainment, with high school diploma or less coded as relatively low and any college education or more coded as relatively high. An ANCOVA was then computed with SES trajectory as a between-subjects variable and the same covariates as above (see Table S1 and Figure 2). There were significant differences across groups, F(3,1183) = 3.87, p = .009. Subjects with stably low SES (L-L) had the highest counts, while those with stably high SES (H-H) had the lowest. The other groups fell in between, though closer in number to the L-L group. To more clearly examine social mobility, we performed contrast analyses comparing upwardly mobile (L-H) participants to other groups. Even though these individuals had made large SES gains over their lifetimes, in terms of MetS components they were statistically equivalent to L-L individuals (p=.35). And they had significantly higher counts than H-H individuals (p = .01). Supplemental analyses showed that L-L and H–L individuals did not differ (p = .40), though the latter group's size (n=53) limits power for this comparison.
Figure 2.
SES trajectory is associated with number of MetS components (upper panel) and prevalence of diagnosable MetS (lower panel).
These patterns were mirrored in diagnosable MetS (see Table S2 and Figure 2), whose prevalence varied across groups (Wald = 9.18, p = .03). MetS was most prevalent among those with stably low SES, and least among those with stably high SES. Critically for the social mobility hypothesis, participants in the L–H and L-L categories were statistically indistinguishable (p = .90). Further, L–H participants had higher MetS prevalence than H-H participants (p = .01). There were no differences between L-L and H–L individuals (p = .79), but again power is an issue here.
We ran two other sets of analyses to insure these results were robust. In one set we adopted a stricter definition of social mobility, which required participants to have obtained a university degree to qualify for the high-SES category. In the other set, we treated both childhood and adulthood SES as four-level indicators, using the same educational categories as above, and looked for an interaction between them. The results of both analyses mirrored those reported above; there was little evidence that upward mobility offset the MetS risks associated with low childhood SES.
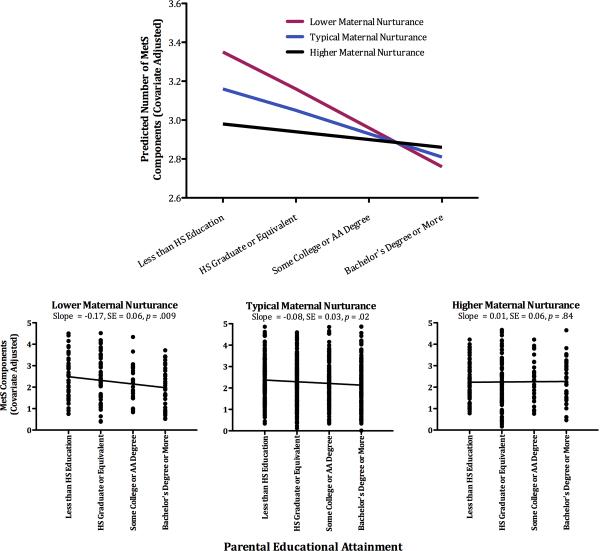
To examine the role of parental nurturance we constructed regression equations, wherein MetS counts were predicted from three successive blocks of variables: the set of covariates, variables reflecting main effects of childhood SES and parental nurturance, and a product term representing the interaction of the latter 2 constructs. The results of the maternal nurturance model indicated the presence of a significant interaction (B = 0.09, SE = 0.04, p = .03,ΔR2 = 0.005). To interpret this finding we plotted estimated MetS counts according to standard practices (Aiken & West, 1991). As Figure 3 shows the interaction was consistent with a buffering influence of maternal nurturance (also see Table S3). Childhood disadvantage was associated with higher counts among participants who reported low maternal nurturance (for simple slope, p < .001). But this association became progressively weaker as participants reported higher levels of maternal nurturance. In fact, among participants who reported maternal nurturance one standard deviation above the sample average, the association between childhood SES and MetS components was nonsignificant (for simple slope, p = .47).
Figure 3.
Maternal nurturance buffers against higher counts of MetS components in those from low-SES backgrounds. The top panel displays adjusted counts of MetS components as a function of childhood SES and maternal nurturance, which is depicted at lower (−1 SD), typical (sample mean), and higher (+1 SD) levels of the sample distribution. The bottom panel displays adjusted counts of MetS components by childhood SES. For illustration, the sample has been stratified into tertiles on maternal nurturance.
When a similar model was estimated for paternal nurturance, it did not yield evidence of a buffering influence (for interaction, B = 0.05, SE = 0.04, p = .28; Table S5 and Figure S1). This was not likely due to missing data, as 91% of participants completed paternal nurturance ratings. Finally, in logistic regressions predicting MetS diagnosis, neither maternal nor paternal nurturance interacted with childhood SES (p's > .34; Table S4 and S6).
Supplemental Analyses
As noted, sibling pairs are nested within MIDUS. Their presence violates the randomness assumption inherent in many statistical models, whereby observations within a sample are assumed to be independent and identically distributed (IID). To determine whether the siblings affected our results, we redid analyses using Generalized Estimating Equations (Hanley et al., 2003), specifying an exchangeable covariance matrix. This specification permits the IID assumption to be relaxed. In all cases, the conclusions emanating from these models were identical to those reported above, suggesting that familial clustering within MIDUS did not bias the results. As a second way to evaluate this issue, we redid the analyses after randomly selecting one sibling per family for inclusion. In this subset, we continued to observe links between childhood SES and midlife MetS, without any apparent offsetting by upward mobility. We also observed a buffering effect related to maternal nurturance. However, with 153 participants dropped from the analyses (12.70% of sample) the interaction term was only marginally significant (B = 0.07, SE = 0.04, p = .08).Nonetheless, plots of the effect revealed it to be identical to the one shown in Figure 3.
Experts disagree about how to best identify MetS, and several groups have offered diagnostic guidelines (Cornier et al., 2008). To insure that our results were robust, we redid the analyses identifying MetS by the standards of the National Cholesterol Education Program / Adult Treatment Panel III, the other commonly used guideline in North America. The analyses yielded identical conclusions.
At the time of MIDUS II, some participants already had chronic diseases to which MetS contributes. To determine whether our findings reflected susceptibility to MetS per se, we reconducted analyses after excluding participants with CHD and diabetes. Even though these analyses had less statistical power, they yielded identical conclusions.
Discussion
We found that low childhood SES was associated with poorer metabolic outcomes at midlife in a large cohort from across the United States. These disparities were fairly large in magnitude. Participants raised in families where neither parent finished high school were 1.4 times as likely to have MetS than their peers raised in university-educated households. The prevalence of MetS among participants whose parents earned high school diplomas or had some college education fell in between. This pattern of findings converges with previous research on the socioeconomic origins of MetS, which has generally reported greater prevalence of the condition among those reared in disadvantaged families, particularly when they are women (Chichlowska et al., 2009; Langenberg et al., 2006; Lawlor et al., 2002; but for exceptions, see Lucove et al., 2007; Parker et al., 2003).
In spite of these trends, not all participants from low-SES backgrounds had MetS at midlife. In fact, nearly half of them proved resilient to MetS, a result consistent with findings in other disease contexts (Chichlowska et al., 2009; Cohen et al., 2004; Dong et al., 2004). (That said, some participants free of MetS could have other medical problems, raising questions about whether they were resilient to disease in a more general sense.) To identify characteristics associated with resilience, we examined the influence of upward social mobility, but analyses suggested it offered little in the way of metabolic advantages. Indeed, MetS prevalence was equivalent in L-L and L–H participants (56 vs. 55 percent), despite the latter individuals having made substantial gains in SES over their lifetimes. These findings converge with results from a large study of older British women, which found that upward mobility did little to offset the metabolic risks of childhood poverty (Lawlor et al., 2002). Benefits of upward mobility have sometimes been seen in studies of other health outcomes, but they are typically modest in size (Kuh et al., 2002; Pensola & Martikainen, 2003; Power et al., 2005; Smith et al., 1998).
Collectively, these findings suggest that socioeconomic disadvantage may get embedded in children's physiology, and do so in a manner that is not reversed by a transition into more favorable circumstances. It remains unclear what exactly this residue of childhood adversity is, and how it contributes to the emergence of metabolic syndrome decades later. Biological programming of systems that regulate neuroendocrine or inflammatory signaling is one plausible mechanism. Children from low-SES families go on to have elevated cortisol output in adulthood, irrespective of their achieved social standing (Li et al., 2007; Miller et al., 2009). With persistent exposure to high cortisol, MetS components like central adiposity, insulin resistance, and blood pressure can worsen (Bjorntorp & Rosmond, 1999). Inflammation is another candidate mechanism of embedding. Youth raised in low-SES families go on to have mild, chronic inflammation as adults (Phillips et al., 2009). This embedding is sustained by selective modulation of gene networks in white blood cells, marked by activation of pro-inflammatory and repression of anti-inflammatory signaling pathways (Miller et al., 2009). Inflammation plays a key pathogenic role in the metabolic syndrome (Hotamisligil, 2006), and thus could be a mechanism underlying the findings seen here.
We also examined parental nurturance as a correlate of resilience. These analyses suggested a buffering scenario, wherein maternal nurturance offset the metabolic consequences of childhood disadvantage. The benefits were evident across the spectrum of maternal nurturance. As participants recalled higher levels of nurturance, the association between childhood SES and MetS components became progressively weaker. It is not clear why this pattern did not extend to diagnosable MetS. Due to the binary nature of this outcome, we may have lacked sufficient power. Nevertheless, the analyses focused on components suggests that maternal nurturance is an important contributor to resilience among persons from low-SES backgrounds. In this manner, our results converge with evidence that nurturant parenting has educational, psychosocial, and biobehavioral benefits for children facing adversity (Cicchetti & Blender, 2006; Gunnar & Quevedo, 2007; Luthar, 2006; Masten, 2001). They extend this corpus of evidence to components of a health problem, MetS, that typically manifests decades later in the middle years of adulthood.
It remains unclear how maternal nurturance buffers against the long-term health effects of childhood disadvantage. Nurturant caregivers imbue children with the sense that the world is a safe place and others can be trusted (Cassidy & Shaver, 2008). These beliefs may enable disadvantaged youngsters to read less threat into their social worlds, with a consequent reduction in the wear-and-tear such vigilance can place on bodily systems (Chen & Miller, 2011). Nurturant parents also help children to learn emotion-regulation strategies, so that when they do encounter stress the physiological consequences are attenuated (Repetti et al., 2002). Mechanistically, the benefits of nurturance could occur through increased expression of oxytocin, a peptide released when people experience warmth and security (Grewen et al., 2005). Research in animals suggests that oxytocin counteracts some pathogenic mechanisms involved with MetS (Camerino, 2009). Future research that elucidates the role of these presumptive mechanisms would be valuable. It also will be important to understand why paternal nurturance does not seem to confer lasting health benefits. Mothers could have a unique influence on their offspring's health trajectories. Alternatively, our findings could reflect a cohort effect. MIDUS participants were generally born after World War 2, when cultural norms were such that fathers were not heavily engaged in childrearing. Shifting cultural norms may also explain why childhood SES and parental nurturance were unrelated in this cohort, whereas they tend to cluster in today's young families (Repetti et al., 2002).
This study has three principal limitations. First, its observational design makes it impossible to render causal inferences. By covarying out a host of demographic and biobehavior confounders, we have addressed some plausible alternative interpretations. However, this strategy is fallible when relevant covariates are overlooked or mismeasured. That said, there are a host of animal studies that manipulate early stress experimentally and document lasting health consequences (Avitsur et al., 2006; Kruschinski et al., 2008), proving that effects like those seen here can be causal in nature. A second limitation is the study's retrospective assessments. Some participants will surely have misreported their childhood SES and these errors would lead to misestimation of associations. With parental nurturance misreporting seems even more likely, as participants may provide what they viewed as socially desirable responses. And even if they were not attempting to do so, their reports are likely reconstructed versions of family life, rather than veridical representations of what actually transpired. That said, such reports predict the occurrence of important biomedical outcomes, both here and elsewhere (Dong et al., 2004). Thus, research is needed to identify what exactly these scales are tapping and how it confers protection against disease. Finally, our analyses focused on a single dimension of SES (education) and how it relates to single biomedical outcome (MetS). Future research will need to determine whether similar patterns emerge when other SES indicators are considered in relation to other disease outcomes. For example, analyses of upward mobility in other SES domains, like wealth, might yield stronger evidence of health benefits, or other relevant life outcomes.
Rates of childhood poverty have been climbing steadily in the United States (Chou et al., 2010), fueling concerns about the long-term political, economic, and biomedical costs. Against this backdrop our findings provide some cause for optimism, documenting an impressively high rate of resilience to childhood economic adversity, and identifying maternal nurturance as a factor that promotes it. Research that identifies other factors that promote resilience, especially ones that are amenable to intervention, would be an extremely valuable contribution to America's future. That said, the SES gradient arises from a complex mixture of historical, political, economic, social, and biological forces. To ameliorate it, we will need multifaceted interventions that operate at multiple junctures along the causal pathway.
Supplementary Material
Acknowledgements
Support for MIDUS came from the National Institute on Aging (RO1 AG-032271, PO1 AG-020166). Preparation of this article was supported by R01 HD-058502 from the National Institute of Child Health and Human Development.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage Publications; London: 1991. [Google Scholar]
- Avitsur R, Hunzeker J, Sheridan JF. Role of early stress in the individual differences in host response to viral infection. Brain, Behavior, and Immunity. 2006;20:339–348. doi: 10.1016/j.bbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. Hypothalamic origin of the metabolic Syndrome X. Annals of the New York Academy of Sciences. 1999;892:297–307. doi: 10.1111/j.1749-6632.1999.tb07803.x. [DOI] [PubMed] [Google Scholar]
- Camerino C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity (Silver Spring) 2009;17:980–984. doi: 10.1038/oby.2009.12. [DOI] [PubMed] [Google Scholar]
- Cassidy J, Shaver PR. Handbook of Attachment: Theory, Research and Clinical Applications. Second Edition Guilford; New York: 2008. [Google Scholar]
- Chen E, Miller GE. “Shift-and-Persist” strategies: Why being low in socioeconomic status isn't always bad for your health. Manuscript Under Review. 2011 doi: 10.1177/1745691612436694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. doi: 10.1038/mp.2010.53. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichlowska KL, Rose KM, Diez-Roux AV, Golden SH, McNeill AM, Heiss G. Life course socioeconomic conditions and metabolic syndrome in adults: the Atherosclerosis Risk in Communities (ARIC) Study. Annals of Epidemiology. 2009;19:875–883. doi: 10.1016/j.annepidem.2009.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou M, Thampi K, Wight VR. Basic facts about low-income children, 2009. National Center for Children in Poverty, Mailman School of Public Health, Columbia University; New York: 2010. [Google Scholar]
- Cicchetti D, Blender JA. A multiple-levels-of-analysis perspective on resilience: implications for the developing brain, neural plasticity, and preventive interventions. Annals of the New York Academy of Sciences. 2006;1094:248–258. doi: 10.1196/annals.1376.029. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Annals of the New York Academy of Sciences. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosomatic Medicine. 2004;66:553–558. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocrine Reviews. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienberg Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS National Study: Protocol, Measures, Sample, and Comparative Context. Journal of Aging and Health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: Adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Yeung WJ, Brooks-Gunn J, Smith JR. How much does childhood poverty affect the life chances of children? American Sociological Review. 1998;63:406–423. [Google Scholar]
- Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology. 2007;43:341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. Journal of Epidemiology and Community Health. 2008;62:387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosomatic Medicine. 2005;67:531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. American Journal of Epidemiology. 2003;157:364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Kruschinski C, Skripuletz T, Bedoui S, Raber K, Straub RH, Hoffmann T, Grote K, Jacobs R, Stephan M, Pabst R, von Horsten S. Postnatal life events affect the severity of asthmatic airway inflammation in the adult rat. Journal of Immunology. 2008;180:3919–3925. doi: 10.4049/jimmunol.180.6.3919. [DOI] [PubMed] [Google Scholar]
- Kuh D, Hardy R, Langenberg C, Richards M, Wadsworth ME. Mortality in adults aged 26–54 years related to socioeconomic conditions in childhood and adulthood: post war birth cohort study. BMJ. 2002;325:1076–1080. doi: 10.1136/bmj.325.7372.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg C, Kuh D, Wadsworth ME, Brunner E, Hardy R. Social circumstances and education: Life course origins of social inequalities in metabolic risk in a prospective national birth cohort. American Journal of Public Health. 2006;96:2216–2221. doi: 10.2105/AJPH.2004.049429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Ebrahim S, Davey Smith G. Socioeconomic position in childhood and adulthood and insulin resistance: cross sectional survey using data from British women's heart and health study. BMJ. 2002;325:805. doi: 10.1136/bmj.325.7368.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Power C, Kelly S, Kirschbaum C, Hertzman C. Life-time socio-economic position and cortisol patterns in mid-life. Psychoneuroendocrinology. 2007;32:824–833. doi: 10.1016/j.psyneuen.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Lucove JC, Kaufman JS, James SA. Association between adult and childhood socioeconomic status and prevalence of the metabolic syndrome in African Americans: the Pitt County Study. American Journal of Public Health. 2007;97:234–236. doi: 10.2105/AJPH.2006.087429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthar SS. Resilience in development: A synthesis of research across five decades. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, 2nd ed., Vol. 3. Risk, disorder, and adaptation (pp. 739-795) John Wiley & Sons; New York: 2006. [Google Scholar]
- Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annual Review of Public Health. 2005;26:1–35. doi: 10.1146/annurev.publhealth.26.021304.144505. [DOI] [PubMed] [Google Scholar]
- Masten AS. Ordinary magic. Resilience processes in development. American Psychologist. 2001;56:227–238. doi: 10.1037//0003-066x.56.3.227. [DOI] [PubMed] [Google Scholar]
- McLoyd VC. Socioeconomic disadvantage and child development. American Psychologist. 1998;53:185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok A, Walker H, Lim A, Nicholls EP, Cole SW, Kobor MS. Low early-life social class leaves a biological residue manifest by decreased glucocorticoid and increased pro-inflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L, Lamont DW, Unwin N, Pearce MS, Bennett SM, Dickinson HO, White M, Mathers JC, Alberti KG, Craft AW. A lifecourse study of risk for hyperinsulinaemia, dyslipidaemia and obesity (the central metabolic syndrome) at age 49–51 years. Diabetes Medicine. 2003;20:406–415. doi: 10.1046/j.1464-5491.2003.00949.x. [DOI] [PubMed] [Google Scholar]
- Pensola TH, Martikainen P. Cumulative social class and mortality from various causes of adult men. Journal of Epidemiology and Community Health. 2003;57:745–751. doi: 10.1136/jech.57.9.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JE, Marsland AL, Flory JD, Muldoon MF, Cohen S, Manuck SB. Parental education is related to C-reactive protein among female middle-aged community volunteers. Brain, Behavior, and Immunity. 2009;23:677–683. doi: 10.1016/j.bbi.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Hypponen E, Smith GD. Socioeconomic position in childhood and early adult life and risk of mortality: A prospective study of the mothers of the 1958 British Birth Cohort. American Journal of Public Health. 2005;95:1396–1402. doi: 10.2105/AJPH.2004.047340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Rossi A. Developmental roots of adult social responsibility. In Caring and doing for others: Social responsibility in the domains of family, work, and community (pp. 227-320) University of Chicago Press; Chicago: 2001. [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. Journal of the American Medical Association. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Smith GD, Hart C, Blane D, Hole D. Adverse socioeconomic conditions in childhood and cause specific adult mortality: prospective observational study. BMJ. 1998;316:1631–1635. doi: 10.1136/bmj.316.7145.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.