Abstract
Over the past decade, scientists, healthcare providers, the public, and policy makers have made substantial efforts to improve understanding of the sex/gender* differences in cardiovascular disease (CVD)† and to recognize the importance of heart disease in women. Federal and American Heart Association (AHA) initiatives to raise awareness and to reduce gender disparities in research and clinical care are listed in Table 1. There was a near doubling of the rate of awareness of heart disease as the leading cause of death in women between 1997, when the AHA launched its first campaign for women, and 2009; during that same period, the death rate resulting from CVD decreased by nearly half.2–4 The extent to which efforts to close research gaps and to heighten awareness of heart disease in women are causally linked to lower CVD mortality or have resulted in improved clinical outcomes for women is not established. The purposes of this article are to evaluate contemporary sex/gender differences in the burden of CVD, to assess the impact of recent clinical trials on recommendations for the prevention of CVD in women, and to examine factors that may facilitate or impede quality CVD preventive care in women. Recommendations for the design and analyses of future CVD clinical trials in women are also provided.
Keywords: cardiovascular diseases, clinical trials as topic, prevention, women
Gender Differences in the Burden of CVD
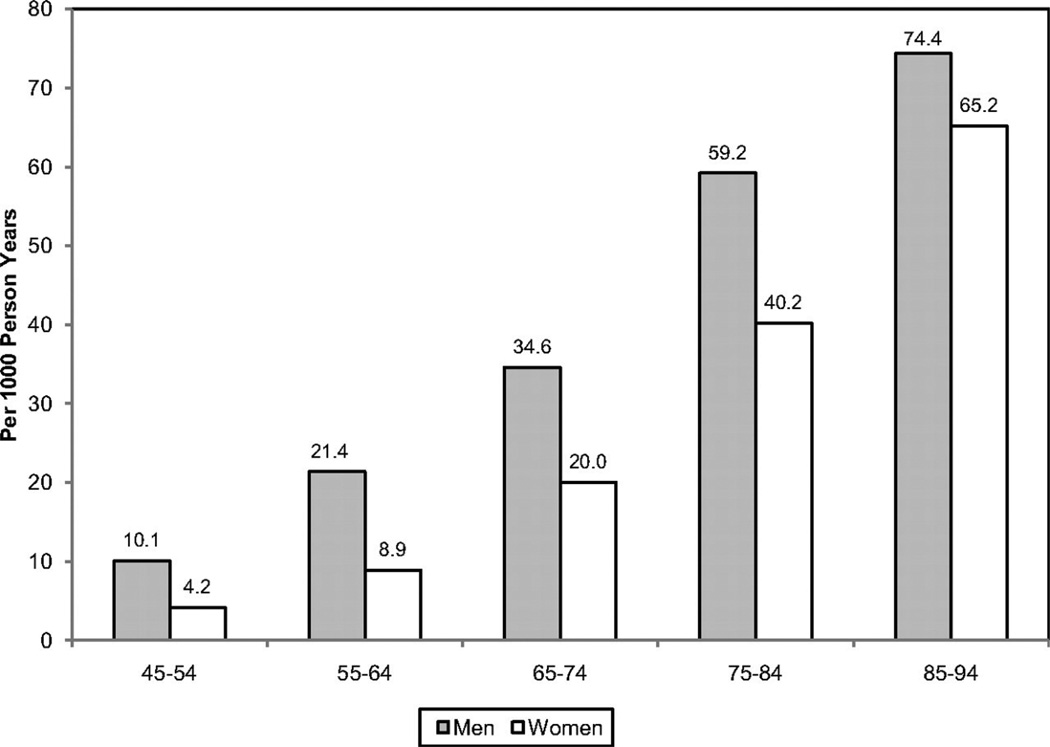
Although CVD remains the leading killer of both women and men in the United States, there are substantial sex/gender differences in the prevalence and burden of different CVDs, as outlined in Table 2. For both women and men, coronary heart disease (CHD) is the largest contributor to CVD morbidity and mortality. The absolute numbers of women living with and dying of CVD and stroke exceed those of men, as does the number of hospital discharges for heart failure and stroke.2 In 2007, women accounted for 60.6% of US stroke deaths.2 In contrast, more men are living with and dying of CHD than women and have more hospital discharges for CVD and CHD. As shown in Figure 1, the prevalence of CHD is higher in men within each age stratum until after 75 years of age, which may contribute to the perception that heart disease is a man’s disease. Sex differences in CVD and CHD mortality largely reflect sex differences in US demographics. Because female sex is associated with a longer life expectancy than male sex, women constitute a larger proportion of the elderly population in which the prevalence of CVD is greatest. Alarming statistics among younger women 35 to 44 years of age show that CHD mortality rates have increased an average of 1.3% annually between 1997 and 2002, a statistically significant trend.5
Table 2.
Sex/Gender Differences in the Burden of Cardiovascular Disease
| Men | Women | |
|---|---|---|
| Remaining lifetime risk for CVD at age 40 y | 2 in 3 | 1 in 2 |
| CVD | ||
| Deaths caused by CVD and congenital heart disease (2007), n | 391 886 | 421 918 |
| Age-adjusted CVD death rate per 100 000 (2007) | 300.3 | 211.6 |
| Prevalence of CVD (2008, age ≥20 y), n (%) | 39 900 000 (37.4) | 42 700 000 (35.0) |
| Hospital discharges for CVD (2007) | 3 016 000 | 2 874 000 |
| CHD | ||
| Deaths caused by CHD (2007), n | 216 050 | 190 301 |
| Age-adjusted CHD death rate per 100 000 (2007) | 165.4 | 95.7 |
| Prevalence of CHD (2008, age ≥20 y), n (%) | 8 800 000 (8.3) | 7 500 000 (6.1) |
| Prevalence of angina pectoris (2008, age ≥20 y), n (%) | 4 000 000 (3.8) | 5 000 000 (4.0) |
| Hospital discharges for CHD (2007), n | 965 000 | 607 000 |
| Stroke | ||
| Deaths resulting from stroke (2007, all ages) | 54 111 | 81 841 |
| Age-adjusted stroke death rate per 100 000 | 42.5 | 41.3 |
| Prevalence of stroke (2008, age ≥20 y), n (%) | 2 800 000 (2.7) | 4 200 000 (3.3) |
| Hospital discharges for stroke (2007), n | 371 000 | 458 000 |
| Heart failure | ||
| Prevalence of heart failure (2008, age ≥20 y), n (%) | 3 100 000 (3.0) | 2 600 000 (2.0) |
| Hospital discharges for heart failure (2007, all ages) | 470 000 | 520 000 |
| Economic burden | ||
| Percent in-hospital patient cost | 51.7 | 48.3 |
| Percent of $187 billion national cost | 57.2 | 42.8 |
CVD indicates cardiovascular disease; CHD, coronary heart disease. Source: Heart Disease and Stroke Statistics—2011 Update: A Report From the American Heart Association.2
Figure 1.
Annual number of adults having diagnosed heart attack or fatal coronary heart disease (CHD) by age and sex. Reprinted with permission of the publisher.2 Copyright © 2011, American Heart Association, Inc.
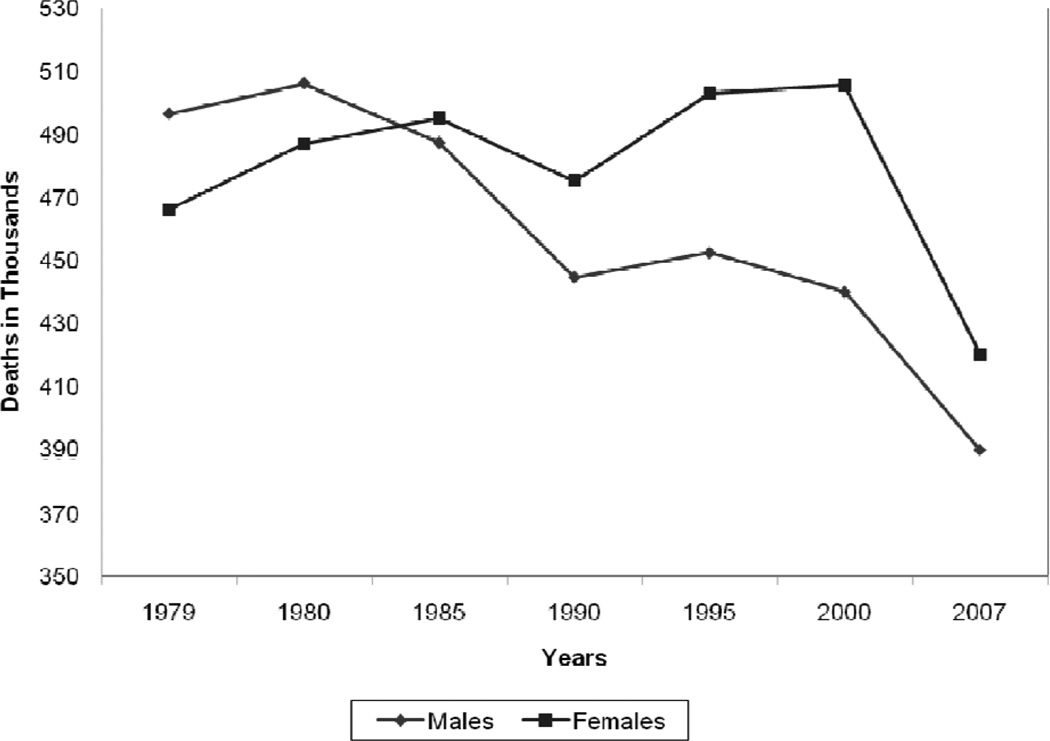
As illustrated in Figure 2 the absolute number of annual CVD deaths among the female sex has exceeded that of the male sex since 1984. These data are often confused with CVD mortality rates, which, when adjusted for differences in age distribution, reveal that the CVD mortality rate is substantially higher in men than women (Table 2). In 2007, the age-adjusted CVD death rate in men was 300 per 100 000 compared with 212 per 100 000 women. The 2007 CVD mortality rate in women represents a 43% reduction from the rate in 1997. From 1980 to 2000, the age-adjusted death rate for CHD fell from 263 to 134 per 100 000 women; during the same time period, the rate fell from 543 to 267 per 100 000 men.4
Figure 2.
Trends in the total annual number of deaths caused by cardiovascular disease according to gender, United States, 1979 to 2007. Reprinted with permission of the publisher.2 Copyright © 2011, American Heart Association, Inc.
The prevalence of CVD in women varies according to racial/ethnic minority status. The prevalence of CVD among women ≥20 years of age is 47% among blacks, 34% among whites, and 31% among Mexican Americans; the prevalence of CHD is 7.6%, 5.8%, and 5.6%, respectively.2 Asian women ≥18 years of age have the lowest prevalence of CHD (3.9%), according to the National Center for Health Statistics.2 The age-adjusted CHD death rate is highest among black women (122 per 100 000 compared with 94 per 100 000 in white women).2 The ominous trend for increasing rates of hypertension among black women is of particular concern because the increased risk for both CHD and stroke compared with white women could potentially widen the racial gap in CVD mortality.
Dr Bernadine Healy first introduced the concept of the Yentl syndrome in 1991, suggesting gender bias in the management of CHD.6 There is ongoing debate as to whether women have a poorer prognosis after a myocardial infarction (MI) than men, and why. Is any observed difference explained by delay in women seeking care, healthcare provider delay in recognition and treatment, underlying differences in pathophysiology, more comorbidities, or older ages at time of presentation among women compared with men?
Data over the past decade have shown that women have a higher 30-day mortality compared with men, and it is now recognized that the gender differences are largely explained by clinical differences at presentation.7,8 The higher mortality rate among women appears to be limited primarily to ST-segment–elevation MI.7,8 It has also been suggested that higher death rates may be restricted to younger women.9 Although women with acute coronary syndromes may have similar benefits from antiplatelet pharmacotherapy as men, they are more likely to have bleeding problems, possibly as a result of excess dosing.10 Women experience greater morbidity and mortality than men after coronary artery bypass grafting; this disparity may reflect technical difficulties resulting from differences in body size, more microvascular disease, and different risk factor profiles.11 More recently, it has been shown that increasing use of off-pump coronary artery bypass grafting has narrowed the gender disparity in outcomes.12 Early studies that examined gender differences in outcomes after MI and revascularization may no longer be relevant owing to temporal trends in management and risk factor profiles. Recent data from the National Registry of Myocardial Infarction showed that in-hospital mortality after an acute MI decreased more in women than in men between 1994 and 2006; the absolute reduction was 3 times larger in women than in men <55 years of age (2.7% versus 0.9%).13 This narrowing of the mortality gap was explained largely by greater improvements in risk factors in women than in men.
The classic risk factors for CVD are the same in women and men, but there are gender differences in the prevalence of risk factors. Although women and men overall have nearly equal percentages of hypertension (1 in 3 adults), data from the National Health and Nutrition Examination Survey (NHANES) showed that the prevalence of high blood pressure is greater in women >65 years of age.14 The highest rate of hypertension is among black women, 44%, and is increasing.15 The death rate caused by hypertension in 2007 was 37.0 per 100 000 for black women compared with 14.3 per 100 000 for white women.2
Diabetes mellitus is more prevalent among women than men ≥20 years of age (8.3% versus 7.2%).2 Type II diabetes mellitus imparts a greater risk of CHD in women than men and is not explained by differences in risk factors, but rather by the more favorable survival rate of women (than men) without diabetes mellitus.16 The prevalence of physician-diagnosed diabetes mellitus is highest among non-Hispanic black (14.7%) and Mexican American (12.7%) women.17 On the basis of the NHANES data, the age-adjusted prevalence of the metabolic syndrome is highest among Mexican American women (40.6%), which is ≈22% higher than in Mexican American men.18 The prevalence of total cholesterol ≥240 mg/dL in 2008 for those ≥20 years of age was 16.2% among women and 13.5% among men.2 In contrast, the percent of women with high-density lipoprotein cholesterol <40 mg/dL was 9.7% compared with 29.5% among men.
Lifestyle risk factors also vary by gender, race, and ethnicity. Cigarette smoking has decreased overall in the United States, but remains more common among men than women (23.1% versus 18.1%).2 Non-Hispanic white women have a higher rate of smoking (20.7%) than black women (18.8%) and Hispanic women (9.4%). Age-adjusted rates of physical inactivity in 2009 were higher in women than men (34.5% versus 30.3%).19 Adverse trends in levels of physical activity (> 12 times a month) reveal a decline from 1988 to 2006 from 57% to 43% in men and from 49% to 43% in women.20 The decreasing levels of physical activity parallel the rising rates of overweight and obesity in the United States. Two thirds of Americans are overweight or obese (72% of men and 64% of women) as defined by body mass index.21 Among women, non-Hispanic blacks and Mexican Americans are more likely to be obese than non-Hispanic whites (50% versus 45% versus 33%, respectively). From 1999 to 2008, the increase in the prevalence of obesity was greater among men than women.21 Full adherence to 3 heart-healthy lifestyle behaviors (smoking abstinence, physical activity, and fruit and vegetable intake) was nearly 50% higher among women than men without CHD in a 2000 sample of the US population.22 Overall adherence was low (<20%) for both women and men. These data suggest that population-wide approaches are needed to reduce the burden of CVD in both genders.
Transformative CVD Prevention Research
Arguably, a major explanation for the decline in CVD death rates for women and men has been widespread application of evidence-based preventive strategies. An analysis from the Centers for Disease Control concluded that approximately half of the improvement in CHD death rates in the United States from 1980 and 2000 was due to better control of major risk factors, including reductions in total cholesterol, systolic blood pressure, and smoking prevalence.4 The other half was ascribed to evidence-based treatments, including secondary preventive therapies, which accounted for 11% of the improvement in mortality. In 2004, the AHA issued the first evidence-based guidelines for CVD prevention in women derived from a systematic search and review of the scientific literature designed to identify whether gender differences were present in response to preventive therapies.23 Only 75% of research articles that met the criteria to establish clinical recommendations included women, and inferences had to be drawn from trials that included only men. A 2011 update of the guidelines concluded that the evidence documented few gender differences in the efficacy of preventive interventions, but data evaluating parity in safety or cost-effectiveness were limited.24
Table 3 lists key clinical questions concerning CVD prevention in women that were unanswered in 2000. During the past decade, landmark clinical trials have transformed the practice of CVD prevention in women and men. Perhaps one of the most striking changes in medical practice occurred after the release of the Women’s Health Initiative (WHI) data in 2002 showing that menopausal hormone therapy did not prevent incident CHD and increased the risk of stroke in healthy women.25,26 Among women in the WHI with prior hysterectomy who were randomized to estrogen only, the increased risk of stroke did not persist in a postintervention 10-year follow-up study, and there were fewer CHD events among women 50 to 59 years of age randomized to estrogen compared with those randomized to placebo.27 The data are limited because of the observational nature of the extended follow-up study and the lack of data on the long-term safety regarding breast cancer; definitive conclusions about the balance of benefits and risks in this population are difficult to make. WHI findings were consistent with earlier secondary prevention data from the Heart and Estrogen/Progestin Replacement Study (HERS) showing no benefit from a combination of estrogen and progestin for the secondary prevention of CHD, an increased risk of MI in the first few months after initiation of hormone therapy, and an increased risk of venous thromboembolic events.28 Similarly, the selective estrogen receptor modulator raloxifene was not beneficial for the primary or secondary prevention of CHD and was associated with an increased risk of fatal stroke and venous thromboembolic events.29–31 The results of these trials led the AHA and the Food and Drug Administration to recommend against use of menopausal hormone therapy and selective estrogen receptor modulators for either the primary or secondary prevention of CVD in women.23,24,32
Table 3.
CVD Prevention in Women: What We Have Learned in the Past Decade
| Unknown in 2000 | Known in 2011 |
|---|---|
| Does menopausal hormone therapy prevent Incident CVD? | Menopausal hormone therapy does not prevent incident or recurrent CVD in women and increases risk of stroke25–28 |
| Do SERMs prevent incident or recurrent CVD? | SERMs do not prevent incident or recurrent CVD in women and increase risk of fatal stroke and VTE29–32 |
| Is aspirin effective for the primary prevention of CVD in women? | Aspirin does not prevent incident MI in women<65 y; aspirin prevents recurrent CVD and incident ischemic stroke and might prevent incident MI in women >65 y of age but increases risk of hemorrhagic strokes and GI bleeding33–38 |
| Do antioxidant supplements prevent incident or recurrent CVD? | Vitamins E and C and beta carotene do not prevent incident or recurrent CVD39–42 |
| Do folic acid and B vitamin supplements prevent incident or recurrent CVD? | Folic acid and B vitamin supplements do not prevent incident or recurrent CVD43–47 |
| Does omega-3 fatty acid supplementation prevent incident or recurrent CVD? | Omega-3 might prevent CVD in women with hypercholesterolemia but the absolute benefit is low48–51 |
| Does vitamin D and calcium supplementation prevent incident or recurrent CVD? | Combined vitamin D (400 IU daily) and calcium supplementation (1000 mg/d) do not reduce the risk of CVD, stroke, or mortality52,53 |
| Does intensive diabetic control prevent CVD? | Targeting HbA1C <6% does not prevent CVD events in patients with diabetes mellitus and increases the risk of death54 |
| Is LDL reduction effective for the primary prevention of CVD in women? | LDL reduction reduces recurrent events and might reduce incident events in women, but the absolute benefit for primary prevention is small55–61 |
CVD indicates cardiovascular disease; SERM, selective estrogen receptor modulator; VTE, venous thromboembolism; MI, myocardial infarction; GI, gastrointestinal; HbA1C, hemoglobin A1C; and LDL, low-density lipoprotein.
A meta-analysis of randomized controlled trials of aspirin available to 1997 supported the use of low-dose aspirin to prevent CVD in both high-risk men and women.33 The first large-scale clinical trial was the Physicians Health Study of men without CHD; aspirin reduced the incidence of MI and had no effect on stroke risk.34 In contrast, the Women’s Health Study (WHS) showed that aspirin had no overall effect on CVD, the primary end point, although a reduction in ischemic stroke was observed.35 In a post hoc analysis of women ≥65 years of age, there was a significant reduction in the risk of ischemic stroke and a small reduction in risk of MI; among women <65 years of age, there was no MI benefit and a small benefit in reduction of stroke risk.35 Overall, the risk of hemorrhagic stroke and gastrointestinal bleeding appeared to mitigate much of the beneficial effects of aspirin in the WHS. The AHA suggests that aspirin to prevent CVD in older women be considered as long as blood pressure is controlled and the potential benefits are likely to outweigh potential risks.24 The US Preventive Services Task Force recommends that aspirin use be encouraged only among women when the potential benefit of reduction in ischemic stroke outweighs the potential harm of an increase in gastrointestinal hemorrhage, a situation difficult to define for primary prevention.36 The gender differences in benefits associated with aspirin may reflect the later onset of CVD in women, the greater proportion of ischemic strokes among women compared with men, the relatively small incidence of MI among women and stroke among men, gender differences in aspirin metabolism, and the fact that aspirin resistance is more common in women than men.37,38
Based on a wealth of epidemiological data and support from basic science, there was widespread interest in the 1990s in evaluating the use of vitamin supplements for CVD prevention. The overwhelming majority of participants in early clinical trials were men. Supplementation with α-to-copherol and beta carotene showed no CVD protection and an increased risk of lung cancer among Finnish male smokers.39 Similarly, beta carotene supplementation showed no CVD benefit among 22 071 US male physicians40 or among 18 314 smokers or ex-smokers exposed to asbestos.41 Approximately 10 years later, the Women’s Antioxidant Cardiovascular Study (WACS) showed no benefit of ascorbic acid, vitamin E, or beta carotene on CVD events among 8171 female health professionals with CVD or risk factors.42 The folic acid supplementation arm of WACS also revealed no CVD benefit despite significant homocysteine lowering.43 Several other large-scale international clinical trials showed no benefit associated with folic acid and B vitamins on the incidence of CVD or stroke in patients with vascular disease,44 recent cerebrovascular disease,45 and advanced chronic kidney disease.46 A recent meta-analysis showed that dietary supplementation with folic acid had no benefit on cardiovascular events or cancer.47 On the basis of the totality of evidence, the AHA advised against the use of antioxidant vitamin supplements and folic acid with or without B vitamin supplementation for the primary or secondary prevention of CVD.24
In 1999, a large clinical trial (11 432 patients, <15% women) conducted in Italy demonstrated a protective effect of dietary supplementation with n-3 polyunsaturated fatty acids in patients with prior MI on the rate of death, nonfatal MI, and stroke but confirmed no benefit for vitamin E supplementation.48 Similarly, the Japan EPA Lipid Intervention Study (JELIS) showed that eicosapentaenoic acid was associated with a 19% reduction in the incidence of major coronary events among individuals with hypercholesterolemia and was effective for the secondary prevention of CHD, but the absolute benefit among women was low.49,50 A recent clinical trial in the Netherlands among nearly 5000 patients (78% men) with prior MI and receiving state-of-the-art preventive care showed that low-dose supplementation with eicosapentaenoic acid–docosahexaenoic acid or α-linolenic acid did not significantly reduce the rate of major CVD events.51 The 27% reduction in major CVD events with α-linolenic acid among women was not statistically significant. Because of the limited number of women in the trials, low absolute benefits, and possible increased risk of hemorrhagic stroke, an AHA expert panel concluded that the usefulness of omega-3 supplementation in women is not established; they recommended that consumption in the form of fish or capsule (eicosapentaenoic acid 1800 mg/d) be considered for women with dyslipidemia for primary and secondary prevention.24
More data on the role of omega-3 supplementation in primary prevention are expected from the Vitamin D and Omega-3 Trial (VITAL), which began recruitment of 20 000 US men and women in January 2010 to test whether daily supplements of vitamin D (2000 IU cholecalciferol) or fish oil (1 g omega-3 fatty acids) reduce the risk of CVD and cancer.52 The WHI did not show a reduction in coronary events, stroke, or mortality associated with combined vitamin D (400 IU daily) and calcium supplementation (1000 mg/d) compared with placebo.53 The dose of vitamin D tested in the WHI may have been too low, and CVD was not the primary end point. VITAL should inform the risk-benefits of vitamin D and fish oil supplementation for primary prevention of CVD in women.
Intensive control of blood sugar to nearly normal levels among patients with type 2 diabetes mellitus with prior CVD or ≥2 risk factors did not reduce the risk of fatal or nonfatal CHD or stroke and increased the risk of death 22% compared with standard treatment in the Action to Control Cardiovascular Risk in Diabetes Trial (ACCORD).54 The median level of glycohemoglobin in the standard strategy group was 7.5% at 1 year; in contrast, half of the intensive control group attained a hemoglobin AIC level <6.4%. Hypoglycemia requiring assistance was more frequent in the intensive therapy group, as was the use of diabetes medications (52% on 3 oral medications plus insulin compared with 16% in the standard therapy group). Death rates were consistently higher in the intensive therapy group regardless of gender, age, or race/ethnicity. These findings led the AHA to downgrade its strength of recommendation to target a hemoglobin AIC <7% in diabetic women, if it can be accomplished without significant hypoglycemia, from a Class 1 to a Class IIa recommendation.24,32 The panel acknowledged the importance of prevention of microvascular complications with appropriate glycemic control.32
The benefit of secondary prevention associated with a reduction of low-density lipoprotein cholesterol levels in both women and men is well established. The proportional reduction in vascular risk has been linked to the absolute reduction in low-density lipoprotein cholesterol achieved regardless of initial level of low-density lipoprotein cholesterol and is similar for women and men.55 The impact of lipid management among women without CHD is a topic of ongoing debate. Primary prevention lipid trials included few female participants until the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) study and Justification for the Use of Statins in Primary Prevention (JUPITER) trial, both reported in 2008.56,57 The MEGA study, which enrolled more women (5356) than men (2476), showed that pravastatin was associated with a significant benefit in men only; the nonsignificant 37% reduction in risk of all CVD in women was suggested to reflect the approximately one-third-lower incidence of events in women compared with men.56 JUPITER randomized 6801 women ≥60 years of age and 11 001 men ≥50 years of age without CHD and with low-density lipoprotein cholesterol <130 mg/dL and high-sensitivity C-reactive protein levels ≥2 mg/L to rosuvastatin 20 mg/d compared with placebo.57 The trial was stopped prematurely after a median of 1.9 years because of a significant 44% reduction in the primary end point (MI, stroke, arterial revascularization, hospitalization for unstable angina, or death caused by CVD) associated with statin therapy. The hazard reduction was similar for women and men, although the absolute benefit in women was lower, reflecting their lower baseline risk. The methods and results of the JUPITER trial have fueled the ongoing debate on gender differences in the use of statins for primary prevention.58,59 It has been suggested that lower-cost generic statins with wider eligibility criteria be applied unless long-term safety issues emerge.60 Although uncertainty remains, a recent Cochrane review concluded that current evidence does not support the use of statins in individuals with an annual all-cause mortality risk <1% or an annual CVD event rate <2%.61
Closing the Gap in Preventive Care
Adherence to guidelines for the prevention of CVD is suboptimal for women and men. The extent to which physician behaviors, patient behaviors, and environmental factors explain nonadherence is not established. The limited systematic evaluation of provider performance in CVD preventive care makes it difficult to document gender differences in the delivery of care. Etiologic explanations for any observed gender differences in adherence to preventive recommendations are even more elusive. Most studies are conducted in select settings, use a variety of quality indicators, and report limited data on confounding or effect-modifying variables. Despite these research limitations, several themes consistently emerge regarding barriers to optimal preventive care.
A fundamental barrier to implementation of prevention guidelines may be the guidelines themselves. Shaneyfelt et al62 evaluated the guidelines process and found that longer guidelines included more standards than shorter guidelines but were more often ignored in practice. Evidence-based recommendations were used more often than recommendations for practice not based on research evidence, and controversial recommendations were followed less often than those that were noncontroversial.63 A study of AHA/American College of Cardiology Guidelines showed that adherence was higher when the recommendations were supported by randomized, controlled clinical trials.64 Guidelines are more likely to be followed if they are easy to implement and come from a highly respected source.65 The AHA has published 3 women-specific evidence-based guidelines between 2004 and 2011 for the prevention of CVD, but the extent to which these guidelines changed physician behavior or affected any gender gap in risk factor management is not known.23,24,32 The most recent AHA women’s guideline 2011 update emphasized the importance of risk assessment to improve the quality of preventive care and highlighted challenges of available risk assessment tools: short-term focus, relevance of outcome measures (CVD versus CHD), and underestimation of risk in women.24 Further research is needed to determine whether improved risk assessment is associated with improved clinical outcomes.
Cabana et al66 evaluated 76 studies describing barriers to adherence to clinical practice guidelines; lack of awareness, lack of familiarity, lack of agreement, lack of self-efficacy, lack of outcome expectancy, and inertia of previous practice were recurring thematic barriers for following guidelines. It was suggested that AHA guidelines for the prevention of CVD in women are heterogeneous, and consequently there are different barriers to implementation of individual recommendations. 67 In a national AHA study of 500 randomly selected physicians, the most commonly cited barriers to implementation of CVD prevention guidelines were time, insurance coverage, and the patient.68 This study also revealed that physician assessment of CVD risk of the patient was the primary driver of quality preventive care. Gender disparities in treatment were explained largely by the provider’s lower perceived CVD risk in women, despite a similar calculated risk compared with men. A subanalysis of this study suggested that solo practitioners and older physicians should be targeted to help promote the use of the guidelines.69 In a program designed to improve screening and management of CHD risk factors in women, internists and obstetricians/gynecologists were queried about barriers to primary prevention; physician time was perceived as a major barrier to the provision of preventive care.70 The authors suggest that the current structure and reimbursement system for health care must be addressed if the gender gap in CVD preventive care is to be reduced.
In a nationally representative sample of women, the most frequently cited barriers to heart health were confusion in the media (49%), the belief that health is determined by a higher power (44%), and caretaking responsibilities (36%).71 Psychosocial factors may also contribute to nonadherence to preventive recommendations in women. For example, depression and social isolation have been linked to CVD risk and may be mediated by nonadherence to preventive recommendations, although there is a lack of clinical trials to document that treatment of psychosocial risk improves patient outcomes.24 The roles of body image and other psychological, social, and cultural factors as mediators of nonadherence deserve further study. Systems approaches to CVD prevention have the potential to improve outcomes and to reduce disparities. The Get With the Guidelines Quality Improvement Program has shown improved adherence to secondary prevention guidelines over time for both women and men, but the data are subject to selection bias and secular trends.72
Recommendations for Design and Conduct of Future CVD Trials
Progress in the inclusion of women in CVD trials reveals divergent interpretations. A study conducted in 2000 of NHLBI-funded studies of CVD concluded that federal efforts to increase the representation of women in clinical trials had been moderately successful, primarily because of the initiation of a small number of large single-sex trials that enrolled women. It also noted little progress in the sex composition of cohorts in the majority of CVD studies.73 The lack of inclusion of women in CVD trials was highlighted as a barrier to making clinical recommendations in the initial AHA evidence-based guidelines for the prevention of CVD in women in 2007.23 Among randomized, controlled trials cited by the women’s guidelines, 87% included both women and men; the proportion of women increased significantly from 9% in 1970 to 41% in 2006.74 A 2004 review of published randomized, controlled trials found that federally funded trials inconsistently adhered to National Institutes of Health standards to include women: on average women made up only 37% of study participants.75 An updated analysis of trials published in 2009 that included both genders revealed that the median enrollment of women was unchanged since 2004.76 Moreover, presentation of analyses by sex was evident in only 35% of studies, 11% did not analyze by sex but provided an explanation, and 64% did not include sex in the analysis, did not provide an explanation, or both.77 Similarly, a recent systematic review of reporting of results of high-risk cardiovascular devices that received premarket approval by the Food and Drug Administration between 2000 and 2007 showed no report of sex of enrollees in 28% of studies and no increase in the enrollment of women over time (average of 67% men). The lack of gender-specific safety and effectiveness is a barrier to optimal CVD care for women. More research has to be conducted on effective lifestyle methods to prevent CVD, especially those approaches that have the potential for long-term sustainability among diverse groups of women.
Table 4 lists recommendations for the design, conduct, and reporting of future CVD trials in women. A 2011 report from the Institute of Medicine Committee on Women’s Health Research recommended that the government ensure adequate participation of women in trials and analyses and reporting of data, both efficacy and adverse effects, by sex.1 The availability of such data will inform future guidelines and facilitate the translation of research findings into practice. It will be important to determine to what extent these data and their dissemination can reduce gender disparities in preventive care and improve clinical CVD outcomes for women.
Table 4.
Recommendations for Future Cardiovascular Trials in Women
| Include equal representation of women and men unless adequately justified |
| Limit exclusion criteria and remove upper age limit to improve the generalizability of results and the projection of effectiveness in clinical practice |
| Women-only trials should be limited to the study of conditions unique to or predominate in women |
| Cardiovascular end points should include the scope of outcomes significant for women, including all acute coronary syndromes, fatal coronary heart disease, stroke (thromboembolic and hemorrhagic), and heart failure |
| Quality-of-life measures should be part of outcomes evaluated by gender |
| Gender-specific analyses should be conducted and published for both efficacy and safety |
| Reasons for nonadherence to interventions should be documented according to gender |
| Cost-effectiveness analysis should be conducted and gender-specific data published |
| Gender-specific power calculations should be conducted and published |
| Dissemination of results should include communication regarding any significant gender differences in efficacy and adverse effects |
Table 1.
Initiatives Affecting Women and Cardiovascular Disease
| Year | Event |
|---|---|
| 1985 | Women’s Health: Report of the Public Health Service Task Force on Women’s Health Issues addresses lack of research focus on women’s health concerns |
| 1986 | NHLBI publishes proceedings of Coronary Heart Disease in Women: Reviewing the Evidence, Identifying the Needs |
| 1987 | NIH Guide to Grants and Contracts urges inclusion of women in clinical trial studies |
| 1990 | NIH Office of Research on Women’s Health established |
| 1991 | HHS establishes Office on Women’s Health |
| 1992 | NIH Women’s Health Initiative launched |
| 1992 | American Heart Association publishes the first scientific statement on women and CVD |
| 1992 | US Government Accountability Office issues a report on the inclusion of women in clinical trials used by the FDA to evaluate drugs for marketing approval |
| 1993 | NIH Revitalization Act requires the inclusion of women in all clinical research and analyses of results by sex for phase III clinical trials |
| 1993 | FDA reverses its 1977 guidelines barring women of childbearing potential from participating in clinical research |
| 1994 | FDA and Centers for Disease Control and Prevention establish OWH |
| 1994 | NIH guidelines state that women are to be included in all human subject research and that valid analyses of differences in intervention effects are to be conducted; cost is not an allowable reason for exclusion, and recruitment outreach programs are initiated and supported |
| 1996 | HHS OWH funds the National Centers of Excellence in Women’s Health |
| 1997 | AHA launches national Take Wellness to Heart awareness campaign |
| 1998 | Agenda for Research on Women’s Health Report released by the NIH Office of Research on Women’s Health |
| 1999 | AHRQ Center for Education and Research of Therapeutics program begins including the topics of irregular heart rhythms and menstrual and hormone therapy |
| 1999 | AHA publishes the first women-specific clinical recommendations for prevention of CVD, “A Guide to Preventive Cardiology in Women” |
| 2001 | NHLBI’s Heart Truth campaign launched |
| 2003 | FDA OWH study finds that nearly equal numbers of men and women participate in clinical trials but that studies do not publish gender-specific results |
| 2003 | AHRQ releases the first annual National Healthcare Quality Report and the National Healthcare Disparities Report |
| 2004 | AHA publishes first evidence-based guidelines for preventing CVD in women |
| 2004 | The Red Dress becomes the symbol of heart disease in women |
| 2005 | AHA releases a consensus statement on the role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease |
| 2010 | Patient Protection and Affordable Care Act establishes an HHS coordinating committee on women’s health |
| 2010 | Institute of Medicine releases Women’s Health Research: Progress, Pitfalls, and Promise |
| 2011 | HHS OWH launches the Make the Call. Don’t Miss a Beat campaign to raise awareness of heart disease symptoms in women and the need to call 9-1-1 |
| 2011 | AHA issues effectiveness-based guidelines on the prevention of CVD in women |
NHLBI indicates National Heart, Lung, and Blood Institute; NIH, National Institutes of Health; HHS, Department of Health and Human Services; CVD, cardiovascular disease; FDA, US Food and Drug Administration; OWH, Offices on Women’s Health; and AHRQ, Agency for Healthcare Research and Quality. Reproduced and adapted with permission from the Institute of Medicine’s report, Women’s Health Research: Progress, Pitfalls, and Promise. Washington DC: National Academies Press; 2010. Courtesy of National Academies Press, Washington, DC.1
Acknowledgments
We wish to thank Lisa Rehm for her assistance with literature searches and manuscript submission.
Sources of Funding
Dr Mosca is supported in part by an National Institutes of Health Research Career Development Award (K24 2HL076346-06).
Footnotes
Gender is referred to throughout this document as a person’s self-representation as male or female or how that person is responded to in social situations on the basis of the individual’s gender representation. Gender is shaped by environment and experience. Sex is the classification of female or male according to reproductive organs and functions assigned by chromosomal complement.1
Throughout this document, CVD is inclusive of coronary heart disease, stroke, and other atherosclerotic conditions except when national statistics are provided in which CVD encompasses International Classification of Diseases, 10th revision, codes 100 to 199.2
Disclosures
Drs Mosca and Wenger were members of the AHA expert panel on the prevention of heart disease in women. Dr Mosca was a member of the Institute of Medicine Committee on Women’s Health Research and has served as a consultant for Advise and Consent, Rowpar, Gilead Sciences, Inc, and Sanofi-aventis. Drs Mosca, Barrett-Connor, and Wenger were members of the Raloxifene Use for the Heart Study Executive Committee. Dr Wenger was a HERS and WHI investigator and a member of writing groups for these studies and chairs the Data Safety Monitoring Board for the Physicians Health Study, WACS, WHS, and VITAL trials. Dr Barrett-Connor was a principal investigator for the HERS and the Postmenopausal Estrogen/Progestin Interventions trials.
References
- 1.Adler NE, Adashi EY, Aguilar-Gaxiola S, Amaro H, Anthony M, Brown DR, Col N, Cu-Uvin S, Faustman DL, Finnegan JR, Hazzard WR, Hefner JE, Miranda J, Mosca L, Peterson H, Pisano ED, Salganicoff A, Snetselaar LG Institute of Medicine’s (IOM) Committee on Women’s Health Research. Women’s Health Research: Progress, Pitfalls, and Promise. Washington DC: National Academies Press; 2010. [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosca L, Mochari-Greenberger H, Dolor RJ, Newby LK, Robb K. Twelve-year follow up of American women’s awareness of cardiovascular disease (CVD) risk and barriers to heart health. Circ Cardiovasc Qual Outcomes. 2010;3:120–127. doi: 10.1161/CIRCOUTCOMES.109.915538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50:2128–2132. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 6.Healy B. The Yentyl syndrome. N Engl J Med. 1991;325:274–276. doi: 10.1056/NEJM199107253250408. [DOI] [PubMed] [Google Scholar]
- 7.Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, Van De Werf F, Aylward P, Topol EJ, Calif RM Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. Sex, clinical presentation, and outcomes in patients with acute coronary syndromes. N Engl J Med. 1999;341:226–232. doi: 10.1056/NEJM199907223410402. [DOI] [PubMed] [Google Scholar]
- 8.Berger JS, Elliott L, Gallup D, Roe M, Granger CB, Armstrong PW, Simes RJ, White HD, Van de Werf F, Topol EJ, Hochman JS, Newby LK, Harrington RA, Califf RM, Becker RC, Douglas PS. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302:874–882. doi: 10.1001/jama.2009.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction: National Registry of Myocardial Infarction 2 participants. N Engl J Med. 1999;34:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 10.Alexander KP, Chen AY, Newby LK, Schwartz JB, Redberg RF, Hochman JS, Roe MT, Gibler WB, Ohman EM, Peterson ED CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress aDverse outcomes with Early implementation of the ACC/AHA guidelines) Investigators. Sex differences in major bleeding with glycoprotein IIb/IIIa inhibitors: results from the CRUSADE initiative. Circulation. 2006;114:1380–1387. doi: 10.1161/CIRCULATIONAHA.106.620815. [DOI] [PubMed] [Google Scholar]
- 11.Aldea GS, Gaudiani JM, Shapira OM, Jacobs AK, Weinberg J, Cupples AL, Lazar HL, Shemin RJ. Improved clinical outcomes in patients undergoing coronary artery bypass grafting with coronary endarterectomy. Ann Thorac Surg. 1999;67:1097–1103. doi: 10.1016/s0003-4975(99)00055-7. [DOI] [PubMed] [Google Scholar]
- 12.Puskas JD, Kilgo PD, Kutner M, Pusca SV, Lattouf O, Guyton RA. Off-pump techniques disproportionately benefit women and narrow the gender disparity in outcomes after coronary artery bypass surgery. Circulation. 2007;116(suppl I):I-192–I-199. doi: 10.1161/CIRCULATIONAHA.106.678979. [DOI] [PubMed] [Google Scholar]
- 13.Vaccarino V, Parsons L, Peterson ED, Rogers WJ, Kiefe CI, Canto J. Sex differences in mortality after acute myocardial infarction. Arch Intern Med. 2009;169:1767–1774. doi: 10.1001/archinternmed.2009.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Center for Health Statistics. Health, United States, 2009: with special feature on medical technology. [Accessed March 8, 2011]; http://www.cdc.gov/nchs/data/hus/hus09.pdf. Published 2010. [PubMed]
- 15.Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness and management. Arch Intern Med. 2005;165:2098–2104. doi: 10.1001/archinte.165.18.2098. [DOI] [PubMed] [Google Scholar]
- 16.Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes a stronger risk factor for fatal ischemic heart disease in women than in men? JAMA. 1991;265:627–631. [PubMed] [Google Scholar]
- 17.Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah Sh. Full accounting of diabetes and pre-diabetes in the US population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ervin RB. National Health Statistics Reports. No. 13. National Center for Health Statistics; [Accessed March 8, 2011]. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. http://www.cdc.gov/nchs/data/nhsr/nhsr013.pdf. Published 2009. [PubMed] [Google Scholar]
- 19.Pleis JR, Ward BW, Lucas JW. Vital and Health Statistics. No. 249. Series 10. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; [Accessed March 8, 2011]. Summary health statistics for U.S. adults: National Health Interview Survey, 2009. DHHS publication No. (PHS) 2011-1577. http://www.cdc.gov/nchs/data/series/sr_10/sr10_249.pdf. Published 2010. [Google Scholar]
- 20.King DE, Mainous AG, 3rd, Carnemolla M, Everett CJ. Adherence to healthy lifestyle habits in US adults, 1988–2006. Am J Med. 2009;122:528–534. doi: 10.1016/j.amjmed.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 22.Miller RR, Sales AE, Kopjar B, Fihn SD, Bryson CL. Adherence to heart-healthy behaviors in a sample of the U.S. population. [Accessed March 14, 2011];Prev Chronic Dis. http://www.cdc.gov/pcd/issues/2005/apr/04_0115.htm. Published 2005. [PMC free article] [PubMed]
- 23.Mosca L, Appel LJ, Benjamin EJ, Berra K, Chandra-Strobos N, Fabunmi RP, Grady D, Haan CK, Hayes SN, Judelson DR, Keenan NL, McBride P, Oparil S, Ouyang P, Oz MC, Mendelsohn ME, Pasternak RC, Pinn VW, Robertson RM, Schenck-Gustafsson K, Sila CA, Smith SC, Jr, Sopko G, Taylor AL, Walsh BW, Wenger NK, Williams CL. American Heart Association Guidelines: Evidence-Based Guidelines for Cardiovascular Disease Prevention in Women. Circulation. 2004;109:672–669. doi: 10.1161/01.CIR.0000114834.85476.81. [DOI] [PubMed] [Google Scholar]
- 24.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Piña IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC, Jr, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation. 2011;123:1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy post-menopausal women: principal results of the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 26.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ WHI Investigators. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 27.LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, Margolis KL, Stefanick ML, Bryzyski R, Curb JD, Howard BV, Lewis CE, Wactawski-Wende J WHI Investigators. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women: Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 29.Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK Raloxifene Use for the Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 30.Collins P, Mosca L, Geiger MJ, Grady D, Kornitzer M, Amewou-Atisso MG, Effron MB, Dowsett SA, Barrett-Connor E, Wenger NK. Effects of the selective estrogen receptor modulator raloxifene on coronary outcomes in the Raloxifene Use for the Heart trial: results of subgroup analyses by age and other factors. Circulation. 2009;119:922–930. doi: 10.1161/CIRCULATIONAHA.108.817577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosca L, Grady D, Barrett-Connor E, Collins P, Wenger N, Abramson BL, Paganini-Hill A, Geiger MJ, Dowsett SA, Amewou-Atisso M, Kornitzer M. Effect of raloxifene on stroke and venous thromboembolism according to subgroups in postmenopausal women at increased risk of coronary heart disease. Stroke. 2009;40:147–155. doi: 10.1161/STROKEAHA.108.518621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosca L, Banka CL, Benjamin EJ, Berr K, Bushnell C, Ganiats T, Gomes AS, Gornick H, Gracia C, Gulati M, Haan CK, Judelson DR, Keenan N, Kelepouris E, Michos E, Oparil S, Ouyang P, Oz MC, Petitti D, Pinn VW, Redberg R, Scott R, Sherif K, Smith S, Jr, Sopko G, Steinhorn R, Stone NJ, Taubert K, Todd BA, Urbina E, Wenger N. 2007 Update: American Heart Association evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2007;115:1481–1501. doi: 10.1161/CIRCULATIONAHA.107.181546. [DOI] [PubMed] [Google Scholar]
- 33.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM, Cook NR, Lee I-M, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 36.U.S. Preventive Services Task Force. Aspirin for the Prevention of Cardiovascular Disease: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 37.Antithrombotic Trialists’ Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomized trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosca L. Aspirin chemoprevention: one size does not fit all. Circulation. 2008;117:2844–2846. doi: 10.1161/CIRCULATIONAHA.108.778407. [DOI] [PubMed] [Google Scholar]
- 39.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers: the Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 40.Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II: a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10:125–134. doi: 10.1016/s1047-2797(99)00042-3. [DOI] [PubMed] [Google Scholar]
- 41.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Jr, Valanis B, Williams JH, Jr, Barnhart S, Cherniack MG, Brodkin CA, Hammar S. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88:1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 42.Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167:1610–1618. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, Genest J, Jr Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 45.VITATOPS Trial Study Group. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins To Prevent Stroke (VITATOPS) trial: a randomized, double-blind, parallel, placebo-controlled trial. Lancet Neurol. 2010;9:855–865. doi: 10.1016/S1474-4422(10)70187-3. [DOI] [PubMed] [Google Scholar]
- 46.Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, Gaziano JM Veterans Affairs Site Investigators. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA. 2007;298:1163–1170. doi: 10.1001/jama.298.10.1163. [DOI] [PubMed] [Google Scholar]
- 47.Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, Manson JE, Bønaa KH, Spence JD, Nygård O, Jamison R, Gaziano JM, Guarino P, Bennett D, Mir F, Peto R, Collins R B-Vitamin Treatment Trialists’ Collaboration. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: meta-analysis of 8 randomized trials involving 37,485 individuals. Arch Intern Med. 2010;170:1622–1631. doi: 10.1001/archinternmed.2010.348. [DOI] [PubMed] [Google Scholar]
- 48.GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:446–455. [PubMed] [Google Scholar]
- 49.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K Japan EPA Lipid Intervention Study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomized open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 50.Matsuzaki M, Yokoyama M, Saito Y, Origasa H, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimad K, Shirato K, Matsuzawa Y JELIS Investigators, Japan. Incremental effects of eicosapentaenoic acid on cardiovascular events in statin-treated patients with coronary artery disease: secondary prevention analysis from JELIS. Circ J. 2009;73:1283–1290. doi: 10.1253/circj.cj-08-1197. [DOI] [PubMed] [Google Scholar]
- 51.Kromhout D, Giltay EJ, Geleijnse JM Alpha Omega Trial Group. n-3 Fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 52.Manson JE. Vitamin D and the heart: why we need large-scale clinical trials. Cleve Clin J Med. 2010;77:903–910. doi: 10.3949/ccjm.77gr.10004. [DOI] [PubMed] [Google Scholar]
- 53.Hsai J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, Heckbert SR, Johnson KC, Manson JE, Sidney S, Trevisan M Women’s Health Initiative Investigators. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846–854. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 54.Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2345–2359. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cholesterol Treatment Trialists (CIT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 56.Mizuno K, Nakaya N, Ohashi Y, Tajima N, Kushiro T, Teramoto T, Uchiyama S, Nakamura H MEGA Study Group. Usefulness of pravastatin in primary prevention of cardiovascular events in women: analysis of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA Study) Circulation. 2008;117:494–502. doi: 10.1161/CIRCULATIONAHA.106.671826. [DOI] [PubMed] [Google Scholar]
- 57.Ridker P, Danielson E, Fonseca FAH, Genest J, Gotto AM, Jr, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 58.de Lorgeril M, Salen P, Abramson J, Dodin S, Hamazaki T, Kostucki W, Okuyama H, Pavy B, Rabaeus M. Cholesterol lowering, cardiovascular diseases, and the rosuvastatin-JUPITER controversy: a critical reappraisal. Arch Intern Med. 2010;170:1032–1036. doi: 10.1001/archinternmed.2010.184. [DOI] [PubMed] [Google Scholar]
- 59.Kaul S, Morrissey RP, Diamond GA. By Jove! What is a clinician to make of JUPITER? Arch Intern Med. 2010;170:1073–1077. doi: 10.1001/archinternmed.2010.189. [DOI] [PubMed] [Google Scholar]
- 60.Hingorani A, Hemingway H. How should we balance individual and population benefits of statins for preventing cardiovascular disease? BMJ. 2011;342:c624. doi: 10.1136/bmj.c6244. [DOI] [PubMed] [Google Scholar]
- 61.Taylor F, Ward K, Moore THM, Burke M, Davey Smith G, Casas JP, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews. 2011 doi: 10.1002/14651858.CD004816.pub4. CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shaneyfelt TM, Mayo-Smith MF, Rothwangl J. Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer-reviewed medical literature. JAMA. 1999;281:1900–1905. doi: 10.1001/jama.281.20.1900. [DOI] [PubMed] [Google Scholar]
- 63.Van der Weijden T, Grol R. Preventing recurrent coronary heart disease: we need to attend more to implementing evidence based practice. BMJ. 1998;316:1400–1401. doi: 10.1136/bmj.316.7142.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leape LL, Weissman JS, Schneider EC, Piana RN, Gatsonis C, Epstein AM. Adherence to practice guidelines: the role of specialty society guidelines. Am Heart J. 2003;145:19–26. doi: 10.1067/mhj.2003.35. [DOI] [PubMed] [Google Scholar]
- 65.Cook D, Giacomini M. The trials and tribulations of clinical practice guidelines. JAMA. 1999;281:1950–1951. doi: 10.1001/jama.281.20.1950. [DOI] [PubMed] [Google Scholar]
- 66.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud P-AC, Rubin HR. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 67.Cabana MD, Kim C. Physician adherence to preventive cardiology guidelines for women. Womens Health Issues. 2003;13:142–149. doi: 10.1016/s1049-3867(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 68.Mosca L, Linfante AH, Benjamin EJ, Berra K, Hayes SN, Walsh BW, Fabunmi RP, Kwan J, Mills T, Simpson SL. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation. 2005;111:499–510. doi: 10.1161/01.CIR.0000154568.43333.82. [DOI] [PubMed] [Google Scholar]
- 69.Christian AH, Mills T, Simpson SL, Mosca L. Quality of cardiovascular disease preventive care and physician/practice characteristics. J Gen Intern Med. 2006;21:231–237. doi: 10.1111/j.1525-1497.2006.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barnhart J, Lewis V, Houghton JL, Charney P. Physician knowledge levels and barriers to coronary risk prevention in women: survey results from the women and heart disease physician education initiative. Womens Health Issues. 2007;17:93–100. doi: 10.1016/j.whi.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Mosca L, Mochari H, Christian A, Berra K, Taubert K, Mills T, Arrowood Burdick K, Simpson SL. National study of women’s awareness, preventive action, and barriers to cardiovascular health. Circulation. 2006;113:525–534. doi: 10.1161/CIRCULATIONAHA.105.588103. [DOI] [PubMed] [Google Scholar]
- 72.Lewis WR, Ellrodt AG, Peterson E, Hernandez AF, LaBresh KA, Cannon CP, Pan W, Fonarow GC. Trends in the use of evidence-based treatments for coronary artery disease among women and the elderly: findings from the get with the guidelines quality-improvement program. Circ Cardiovasc Qual Outcomes. 2009;2:633–641. doi: 10.1161/CIRCOUTCOMES.108.824763. [DOI] [PubMed] [Google Scholar]
- 73.Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. N Engl J Med. 2000;343:475–480. doi: 10.1056/NEJM200008173430706. [DOI] [PubMed] [Google Scholar]
- 74.Melloni C, Berger JS, Wang TY, Gunes F, Stebbins A, Pieper KS, Dolor RJ, Douglas PS, Mark DB, Newby LK. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes. 2010;3:135–142. doi: 10.1161/CIRCOUTCOMES.110.868307. [DOI] [PubMed] [Google Scholar]
- 75.Geller SE, Adams MG, Carnes M. Adherence to federal guidelines for reporting sex and race/ethnicity in clinical trials. J Womens Health. 2006;15:1123–1131. doi: 10.1089/jwh.2006.15.1123. [DOI] [PubMed] [Google Scholar]
- 76.Geller SE, Koch A, Pellettieri B, Carnes M. Inclusion, analysis, and reporting of sex and race/ethnicity in clinical trials: have we made progress? J Womens Health. 2011;20:215–220. doi: 10.1089/jwh.2010.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dhruva SS, Bero LA, Redberg RF. Gender bias in studies for Food and Drug Administration premarket approval of cardiovascular devices. Circ Cardiovasc Qual. Outcomes. 2011;4:165–171. doi: 10.1161/CIRCOUTCOMES.110.958215. [DOI] [PubMed] [Google Scholar]