Abstract
The sympathetic nervous system is thought to play a key role in genesis and maintenance of ventricular arrhythmias. The myocardial effect of sympathetic stimulation on myocardial repolarization in humans is poorly understood. The purpose of this study was to evaluate the effects of direct and reflex sympathetic stimulation on ventricular repolarization in patients with postinfarct cardiomyopathy (ICM). The effects of direct sympathetic stimulation were assessed using isoproterenol, while those of reflex sympathetic stimulation were assessed with nitroprusside infusion in ICM patients (n = 5). Five patients without cardiomyopathy were also studied. Local repolarization was measured from intracardiac electrograms that were used to calculate the activation recovery interval (ARI), a surrogate of action potential duration. Isoproterenol significantly increased heterogeneity in repolarization in patients with ICM; the decrease in ARI from baseline was 72.9 ± 9.1 ms in more viable regions, 64.5 ± 8.9 ms in the scar, and 54.9 ± 9.1 ms in border zones (P = 0.0002 and 0.014 comparing normal and scar to border zones, respectively). In response to nitroprusside, the ARI at the border zones decreased significantly more than either scar or surrounding viable myocardium, which showed an increase in ARI (P = 0.014 and 0.08 comparing normal tissue and scar to border zones, respectively). Furthermore, isoproterenol increased ARI dispersion by 70%, while nitroprusside increased ARI dispersion by 230% when ICM patients were compared to those with structurally normal hearts (P = 0.0015 and P < 0.001, respectively). In humans, both direct and reflex sympathetic stimulations increase regional differences in repolarization. The normal tissue surrounding the scar appears denervated. Dispersion of ARI in response to sympathetic stimulation is significantly increased in patients with ICM.
Keywords: ventricular arrhythmias, cardiomyopathy
myocardial infarct scars predispose to ventricular tachycardia (VT) and sudden cardiac death. The proclivity to these malignant arrhythmias may be secondary to both structural myocardial changes and heterogeneity in regional distribution and activity of sympathetic nerves (22, 31). Prior studies in animal models of myocardial infarction have shown that coronary occlusion causes nerve cell injury and death. This denervation is not localized to the infarct and can be seen distal to the infarcted territory (1). The normal myocardium distal to the infarct responds differently to sympathetic stimulation (via left and right stellate ganglia) than to direct stimulation with catecholamine infusion (1, 30). Denervation supersensitvity can potentially further increase the heterogeneity in response to circulating cathecholamines (1). Further, sympathetic nerve sprouting that occurs in response to nerve injury after infarction has also been shown to occur in a nonuniform fashion, predominantly at the borders of normal tissue and scar (6, 23, 24), implicating the border zones in the pathogenesis of ventricular arrhythmias. The border zones of scars have also been found to be frequent sites of origin of inducible VT in canine infarct models (8, 10). The predominant mechanism responsible for increased repolarization heterogeneity in humans, whether circulating catecholamines or stimulation of cardiac nerves, is unclear. Understanding the mechanisms that are responsible for these changes have direct therapeutic implications. Blockade of direct sympathetic stimulation via beta-blockers can reduce the risk of sudden cardiac death, while blockade of reflex sympathetic system via thoracic epidural anesthesia and cervicothoracic sympathectomy has been shown to reduce the risk of sudden cardiac death and the burden of ventricular arrhythmias in small studies (3, 13, 16, 20). However, there are no human data to determine the mechanism of sympathetic modulation in postinfarct scars with regards to either direct or reflex sympathetic stimulation.
The primary aim of this study was to assess whether sympathetic stimulation increases regional repolarization heterogeneity between scar, border zones, and unscarred myocardium in humans. We studied patients with postinfarct cardiomyopathy (ICM) undergoing ablation of ventricular arrhythmias. We hypothesized that the electrical heterogeneity in response to both direct sympathetic stimulation (via activation of β-adrenergic receptors) and reflex sympathetic stimulation (via activation of the sympathetic system in response to induced hypotension) in patients with ICM would be increased compared with that of patients who did not have cardiomyopathy.
METHODS
This study was approved by the University of California, Los Angeles, Human Institutional Review Board. Five consecutive patients with ICM and recurrent ventricular arrhythmias referred for catheter ablation were recruited. Patients with structurally normal hearts (n = 5) referred for electrophysiological study for possible supraventricular or focal ventricular tachycardia also underwent the same experimental protocol as patients with ICM. Data from these structurally normal hearts were analyzed to ensure that the effects of nitroprusside and isoproterenol on activation recovery interval (ARI) were not due to the experimental protocol, medications, or any other factors such as left ventricular (LV) strain. Detailed written informed consent for the study was obtained from all patients. Patients with hemodynamic instability were excluded from the protocol at the discretion of the investigator.
Electrophysiological study and electroanatomic mapping.
All patients were brought to the electrophysiological laboratory. All antiarrhythmic medications including beta-blockers were discontinued ≥12 h before the procedure. Single or double transseptal catheterization was performed in all patients with ICM but only in those normal patients whose electrophysiology (EP) study dictated the necessity for left atrial or LV access. When necessary, epicardial access was obtained in patients with ICM using the method described by Sosa et al. (21). An endocardial, and when necessary, an epicardial voltage map was created to assess for the presence of normal, scar, and border zone regions in all patients with ICM using either the CARTO (CARTO XP; Biosense-Webster, Diamond Bar, CA) or Nav-X (St. Jude Medical, St. Paul, MN) electroanatomic mapping systems. A multipolar catheter (2-mm spacing, 2-mm tip; St. Jude Medical) was then used to obtain unipolar recordings (filter bandwidth: 0.05–500 Hz) from each of its 20 electrodes. Electrodes in the distal inferior vena cava were used as the unipolar references for this catheter. These 20 electrodes were placed in the LV (endocardium or epicardium) so that simultaneous recordings from normal, scar, and border zone regions could be obtained. Scar was defined as areas with electrogram (EGM) amplitude <0.5 mV. Border zone regions were defined by areas with EGM amplitude between 0.5 and 1.5 mV. Viable myocardium was defined as areas with EGM amplitude >1.5 mV (17).
Drug infusion protocol.
All data recordings for the study were performed after completion of electroanatomic mapping but before induction of ventricular arrhythmias or ablation in the ICM patients. In patients without cardiomyopathy referred for possible supraventricular arrhythmias, the recordings were obtained at the end of the EP study and possible ablation procedure (in these patients ablative lesions were anatomically far removed from the ventricular recording sites). In ICM patients, unipolar recordings were obtained at baseline from normal, scar, and border zone regions. Catheter stability was confirmed multiple times throughout the study before and after each intervention. An infusion of isoproterenol was then begun to achieve an increase in heart rate of >20 beats/min above baseline. Heart rate and blood pressure were monitored throughout the study. At peak heart rate, simultaneously recorded unipolar recordings were obtained from the same regions as baseline. Isoproterenol infusion was then stopped, and the heart rate and blood pressure allowed to return to baseline. Catheter position was then reconfirmed. Subsequently, to test the reflex arc of the autonomic nervous system, a nitroprusside infusion was administered, at an initial low dose of 0.03 mcg·kg−1·min−1, and then increased to achieve a decrease in systolic blood pressure (SBP) of >20 mmHg from baseline. Blood pressure, rather than heart rate, was used as an endpoint for nitroprusside, given the lack of a heart rate response in many of the patients with ICM to nitroprusside. Unipolar recordings were obtained at peak infusion. Nitroprusside was discontinued if SBP decreased to <80 mmHg, and phenylephrine was administered to support blood pressure if SBP decreased to <75 mmHg. However, ARIs were only analyzed before phenylephrine infusion.
Analysis of ARI.
ARI was used to assess spatial heterogeneity of sympathetic innervation and its effect on repolarization in humans. ARI has been corroborated to be a reliable a surrogate for action potential duration (APD) by use of simultaneously acquired intracellular recordings and mono-APD recordings and has been validated in both animal models and humans (7, 12, 14, 18, 27, 29). Briefly, in each EGM, activation time is measured as the time of minimum dV/dt in the depolarization wave, the intrinsic deflection, and repolarization time is measured as the time of maximum dV/dt near the peak of the repolarization wave (T wave); the difference, ARI, reflects APD at the electrode site. This method has the advantage of allowing for simultaneous repolarization measurements in a human heart. More importantly, for the purposes of this study, changes in ARI at a given site during an intervention correlate extremely well with changes in APD measured with microelectrodes (7, 12, 14, 18).
ARIs were measured from 30 EGMs recorded at each electrode site under each condition for each patient for an average of 1,455 ± 315 (means ± SD) ARIs per patient. Electrograms with unclear repolarization waves, biphasic T waves, or electrodes with noise prohibiting accurate ARI analysis were excluded after visual confirmation. A mean of 14.2 electrodes per cardiomyopathy patients [mean of 3.6 scar electrodes, 6.0 border zone electrodes, and 5.6 electrodes from the surrounding viable myocardium (CM-NL) per patient] and 14.4 electrodes from the normal patients were analyzed. ScaldynM (University of Utah, Salt Lake City, UT), a customized program written for ARI analysis, was used to calculate ARI from unipolar EGMs. Measurements were also confirmed manually. Subsequently, the change in ARI (delta-ARI) with each condition was calculated for each region after each intervention by subtracting the postintervention ARI from the baseline ARI, allowing for correction of baseline conditions, which may have existed in each patient. Based on its electrode location on the electroanatomic map, and as confirmed by fluoroscopy, the delta-ARI was categorized as reflecting changes in the normal, border zone, or scarred myocardium with each intervention. In the case of no-cardiomyopathy patients, the delta-ARI from all electrodes reflected the change in ARI from normal myocardium.
Unipolar recordings obtained at baseline, peak isoproterenol, and peak nitroprusside infusion were exported and analyzed offline to measure ARI, a surrogate for APD 90.
Statistical analysis.
For comparison of baseline characteristics, the Wilcoxon ranks sum test was used to compare variables. For ARI analysis, a mixed repeated-measures ANOVA was used to compare the mean ARIs and adjusted mean change in ARI (delta-ARI) from baseline, a more accurate comparison when baselines are not the same and measurements were nested within a patient. The repeated-measures model recognizes that intrapatient vs. interpatient comparisons are distinct. Adjusted mean ARIs were calculated after confirming a Gaussian distribution with similarity of means to medians. For analysis of ARI dispersion, the variance in ARI comparing normal to cardiomyopathic subjects was assessed using the Bartlett's test. SAS 9.1 was used for statistical analysis. A P value <0.05 was considered significant. The P values under the repeated-measures ANOVA model were judged significant using the Fisher Tukey least significant difference criterion.
RESULTS
Patient characteristics.
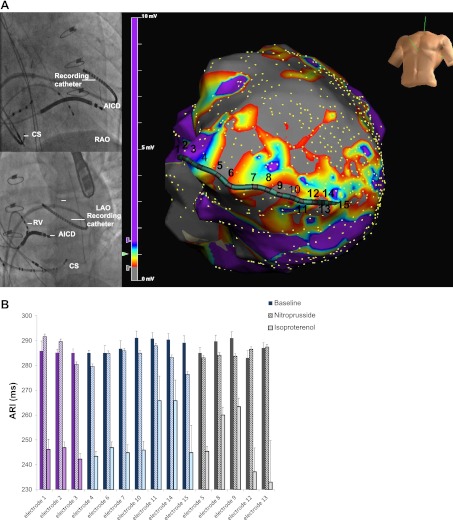
Five patients with ICM (age 71.8 ± 4.6 yr) underwent the experimental protocol (Table 1). Five patients with structurally normal hearts (age 43.6 ± 17.2 yr) also underwent the protocol. The mean LV ejection fraction in patients with ICM was 24 ± 4%, while that in the normal patients was 65 ± 5%. The hemodynamic data and peak drug dosages to achieve effect for the ICM and normal patients are shown in Table 2. Figure 1A demonstrates the location of the multipolar catheter and electroanatomic map of scar (grey), border zone (blue-red), and viable normal voltage tissue (CM-NL; purple), while the corresponding ARIs from each of the electrodes are shown in Fig. 1B. Figure 2 shows catheter position and mean ARIs obtained for a patient with structurally normal heart. Figure 3 shows examples of ARIs obtained from scar, border zone, and the more normal tissue in a patient with cardiomyopathy and the response to nitroprusside and isoproterenol.
Table 1.
Baseline characteristics of cardiomyopathy and normal patients
| Subjects | Age | Gender | LVEF | Findings on EPS | Location of ARI Recordings | Antiarrhythmics |
|---|---|---|---|---|---|---|
| ICM | ||||||
| 1 | 72 | Male | 18% | Inferolateral scar | Ventricular endocardium | Carvedilol, mexiletine, amiodarone |
| 2 | 69 | Male | 22% | Inferior scar | Ventricular endocardium | Carvedilol, amiodarone |
| 3 | 74 | Male | 25% | Anteroseptal and apical scar | Ventricular endocardium | Carvedilol, amiodarone |
| 4 | 66 | Male | 25% | Inferolateral scar | Ventricular endocardium | Sotalol |
| 5 | 78 | Male | 30% | Inferolateral scar | Ventricular epicardium | Carvedilol, mexiletine, amiodarone |
| Normal | ||||||
| 1 | 22 | Male | 55% | No arrhythmias | Ventricular endocardium | None |
| 2 | 47 | Female | 55% | Superior mitral annulus VT | Ventricular endocardium | Sotalol |
| 3 | 44 | Female | 55–60% | RVOT VT | Ventricular endocardium | Sotalol |
| 4 | 69 | Female | 70% | AVNRT | Ventricular endocardium | Metoprolol |
| 5 | 36 | Female | 60% | AVNRT | Ventricular endocardium | Sotalol |
ICM, postinfarct cardiomyopathy; LVEF, left ventricular ejection fraction; EPS, electrophysiological study; ARI, activation recovery intervals; RVOT, right ventricular outflow tract; AVNRT, atrioventricular nodal reentrant tachycardia; VT, ventricular tachycardia.
Table 2.
Hemodynamic response of cardiomyopathy and normal patients to each intervention
| Patients | Baseline HR, beats/min | Baseline BP, mmHg | Peak Iso Dosage, mcg/min | Peak HR on Iso, beats/min | BP at Peak Iso, mmHg | Peak Nitro Dosage, mcg·kg−1·min−1 | HR at Peak Nitro, mmHg | BP at Peak Nitro, mmHg |
|---|---|---|---|---|---|---|---|---|
| ICM hearts | ||||||||
| 1 | 60 | 105/60 | 15 | 92 | 110/50 | 1 | 60 | 75/35 |
| 2 | 64 | 100/53 | 15 | 95 | 106/78 | 0.3 | 63 | 80/30 |
| 3 | 58 | 116/57 | 20 | 94 | 100/51 | 0.5 | 58 | 87/40 |
| 4 | 60 | 136/89 | 16 | 83 | 164/85 | 2 | 59 | 93/51 |
| 5* | 48 pre-Iso | 97/53 pre-Iso | 20 | 69 | 141/51 | 0.3 | 58 | 80/40 |
| 58 pre-Nitro | 110/58 pre-Nitro | |||||||
| Normal hearts | ||||||||
| 1 | 70 | 93/55 | 2 | 100 | 80/40 | 0.5 | 84 | 80/48 |
| 2 | 65 | 119/59 | 6 | 114 | 167/55 | 1.0 | 76 | 106/46 |
| 3 | 83 | 130/78 | 6 | 120 | 110/42 | 1.0 | 111 | 88/41 |
| 4 | 61 | 144/48 | 3 | 104 | 144/51 | 1.0 | 88 | 110/45 |
| 5 | 80 | 100/49 | 2 | 101 | 85/34 | 0.5 | 94 | 81/46 |
ICM, postinfarct cardiomyopathy; HR, heart rate; BP, blood pressure; Iso, isoporterenol; Nitro, nitroprusside.
There was significant lapse in time before the initiation of nitroprusside in this patient during the procedure; therefore, prenitroprusside, baseline hemodynamic, and ARI recordings were reobtained.
Fig. 1.
A: location of the recording multi-electrode catheter on fluoroscopy (top left) and electroanatomic map (bottom right) in this patient with postinfarct cardiomyopathy (ICM) and a large antero-apical scar is shown. On the electroanatomic map, the purple areas represent unscarred tissue with normal voltage while the dense grey represents dense scar. All other colors represent border zones (tissue with voltage >0.5 mV but <1.5 mV). B: corresponding mean activation recovery intervals (ARIs) obtained from 30 electrograms (EGMs) from each electrode (1–15) seen on the electroanatomic map is shown at bottom at baseline and with each intervention. Electrodes have been grouped by type of tissue with purple bars representing unscarred tissue (CM-NL), blue representing border zones, and grey representing scar. The first bar represents the baseline ARI value while the second bar represents response to nitroprusside and the third bar shows the response to isoproterenol in all cases. Note the shortening of ARI in all regions with isoproterenol and the heterogeneous response, even within each region, to nitroprusside. In response to nitroprusside, the border zone ARIs slightly shorten, compared with other regions, although none of the changes are as significant as those with isoproterenol. Note that electrodes 16–20 were not analyzed due to either noise, poor contact, or both. RV, right ventricle; CS, coronary sinus; LAO, left anterior oblique view; RAO, right anterior oblique view; AICD, automated implantable cardiac defibrillation.
Fig. 2.
Locations of the recording multipolar catheter on fluoroscopy and the corresponding mean ARIs from each electrode in this patient without cardiomyopathy are shown. Note the overall uniform shortening of ARI in response to both isoproterenol and nitroprusside. Electrodes 1 and 16–20 were not analyzed in this patient either due to noise or poor contact. HRA, high right atrium; HIS, His bundle catheter.
Fig. 3.
Examples of ARIs obtained from a cardiomyopathy patient from areas with normal voltage (CM-NL), border zone (CM-BZ), and scar (CM-Scar) are shown. Note that in the CM-NL and CM-Scar electrodes in this patient, nitroprusside prolongs ARI while minimally shortening the electrode located along the border zone. Isoproterenol shortens ARI in all regions, although to different degrees. Scale: each of the smaller hash marks represents an interval of 50 ms.
Regional heterogeneity in ARI.
The adjusted mean ARI and delta-ARI with each intervention is shown in Table 3 and Fig. 4, respectively. Isoproterenol significantly decreased ARI in ICM patients as well as structurally normal hearts (P < 0.001 in all cases; Table 4).
Table 3.
Adjusted mean change in ARI from baseline in response to isoproterenol and nitroprusside in each group/site
| Group-Site | Isoproterenol Adjusted Mean ΔARI | Pooled SE | P Value (Comparing with Baseline)* | P Value (Comparing with Normals)† | Nitroprusside Adjusted Mean ΔARI | Pooled SE | P Value (Comparing with Baseline)‡ | P Value (Comparing with Normals)§ |
|---|---|---|---|---|---|---|---|---|
| Normal hearts | −51.1 | 8.5 | <0.001 | N/A | −27.1 | 8.5 | 0.002 | N/A |
| ICM-viable regions | −72.9 | 9.1 | <0.001 | 0.08 | 12.4 | 9.1 | 0.2 | 0.002 |
| ICM-border zones | −54.1 | 8.9 | <0.001 | 0.8 | −0.78 | 8.8 | 0.17 | 0.025 |
| ICM-scar | −64.9 | 8.9 | <0.001 | 0.3 | 8.0 | 8.9 | 0.6 | 0.005 |
ΔARI, change in ARI from baseline (postintervention adjusted mean ARI-baseline adjusted mean ARI); N/A, not applicable.
This P value reflects the comparison between the adjusted mean ARI on isoproterenol as compared with baseline.
This P value reflects the comparison between the pooled mean ΔARI with isoporterenol in cardiomyopathy patients vs. normals.
This P value reflects the comparison between the adjusted mean ARI on nitroprusside as compared with baseline.
This P value reflects the comparison between the pooled mean ΔARI with nitroprusside in cardiomyopathy vs. noncardiomyopathy patients.
Fig. 4.
Delta-ARI (change in ARI from baseline) within the cardiomyopathic and normal hearts is shown. Note that in response to isoproterenol, the delta-ARI is greatest in the CM-NL and scar regions of the cardiomyopathic heart. Border zones within each patient are the least responsive to isoproterenol. On the other hand, in response to nitroprusside, the scar and the CM-NL tissue appear to be the least responsive, even paradoxically increasing their ARI compared with the border zone regions. Therefore, it appears that the most denervated regions have the greatest response to catecholamines. Error bars represent the pooled standard error in each group.
Table 4.
Regional differences in the mean change in ΔΑRI within patients with cardiomyopathy
| Intervention | Site | Mean Difference in ΔARI, ms | SE | P Value |
|---|---|---|---|---|
| Isoproterenol | CM-NL vs. border zones | 18.8 | 4.9 | 0.0002 |
| Isoproterenol | CM-NL vs. scar | 8.0 | 4.8 | 0.096 |
| Isoproterenol | Border zones vs. scar | −11.0 | 4.3 | 0.014 |
| Nitroprusside | CM-NL vs. border zones | −12.0 | 4.8 | 0.016 |
| Nitroprusside | CM-NL vs. scar | −4.4 | 4.8 | 0.36 |
| Nitroprusside | Border zones vs. scar | 7.3 | 4.2 | 0.08 |
CM-NL, viable tissue with normal voltage in postinfarct cardiomyopathy patients.
When the change in ARI (delta-ARI) within ICM patients was compared at different locations, the viable tissue and scarred regions showed a decrease in ARI that was significantly more than in the border zone locations (with each patient serving as their own control pre- and postintervention). Specifically, the mean delta-ARI was −72.9 ± 9.1 ms in the CM-NL myocardium of the cardiomyopathic heart, −54.1 ± 8.9 ms in the border zones, and −64.9 ± 8.9 ms in the scar. The differences in delta-ARI of 18.8 ± 4.9 ms between the border zones and CM-NL tissue and the differences in delta-ARI of 11.0 ± 4.3 ms between the CM-NL and scar were statistically significant (P = 0.002 and 0.014, respectively; Fig. 4). There was a trend for a further decrease in delta-ARI of CM-NL tissue compared with that of scar (difference of 8.0 ± 4.8 ms), although this did not reach statistical significance (P = 0.096). Hence, isoproterenol significantly increases the heterogeneity in repolarization by significantly shortening repolarization duration, particularly in the CM-NL regions of the cardiomyopathic heart compared with the scar and border zones areas (although all regions showed a trend for statistically significant difference when compared with each other).
When comparing the overall delta-ARI in ICM in response to nitroprusside, no significant change in response to nitroprusside was seen, likely due to increases in some regions and decreases in other regions (Table 3). However, when comparing regional differences in delta-ARI in each patient (with the baseline for each patient serving as their control), the change in delta-ARI within each patient in the CM-NL myocardium and scar was 11.6 ± 4.8 and 7.3 ± 4.8 ms greater (P = 0.02 and P = 0.08, respectively) compared with the border zones (Table 4 and Fig. 3). Hence, although less than isoproterenol, a heterogeneity in repolarization still exists after reflex sympathetic stimulation in various regions of the cardiomyopathic myocardium, with prolongation of ARI in the normal and scarred regions compared with the border zone regions. This is especially notable as changes seen with nitroprusside cannot be attributed to heart rate in ICM, as there was no significant chronotropic response in these patients to nitroprusside (Table 2). As expected, nitroprusside did significantly decrease the ARI in normal hearts by 27.1 ± 8.5 ms compared with baseline (P = 0.002).
Comparison of repolarization dispersion.
In response to isoproterenol, the variance in delta-ARI in structurally normal hearts was 106.4 ms2 compared with 182 ms2 in ICM (P = 0.015). Hence, the dispersion in ARI was 71% greater (when comparing variances) in cardiomyopathic hearts compared with the hearts of subjects without cardiomyopathy (Fig. 5).
Fig. 5.
Dispersion in delta-ARI in response to direct and reflex sympathetic stimulation within cardiomyopathy compared with normal patients is shown. Variance in delta-ARI increases significantly more in ICM patients in response to isoproterenol and nitroprusside compared with that in patients with structurally normal hearts.
In response to nitroprusside, the variance in delta-ARI in ICM patients was also greater than that of noncardiomyopathy patients (variance 103.2 vs. 45.7 ms2; P = 0.0005; Fig. 5). Therefore, the dispersion in ARI was significantly greater in cardiomypathic hearts compared with structurally normal hearts (220% greater when comparing variances and 50% greater when comparing SD) in response to nitroprusside. While certain regions of the cardiomyopathic hearts showed an increase in ARI in response to reflex sympathetic stimulation, others demonstrated a decrease, all regions of the normal myocardium in normal hearts uniformly decreased their repolarization time.
DISCUSSION
Major findings.
This is the first human study to evaluate the heterogeneity in repolarization in patients with ICM in response to direct sympathetic stimulation with isoproterenol and reflex sympathetic stimulation with nitroprusside. Our data show that heterogeneity of repolarization exists at baseline in ICM patients and that is further exacerbated by sympathetic stimulation. In addition, the degree of the response to circulating catecholamines in cardiomyopathy patients is a reflection of the degree of underlying innervation, with border zone regions appearing less denervated compared with scar and the surrounding viable myocardium, which appear predominantly denervated. Finally, in response to either direct or reflex sympathetic stimulation, there is a significant dispersion of repolarization in cardiomyopathic hearts compared with structurally normal hearts, with a 50–200% increase in overall variance of repolarization.
Regional variations in ARI in response to sympathetic stimulation.
Direct sympathetic stimulation with isoproterenol significantly shortened ARI in all regions in ICM. However, the degree of the decrease in duration of ARI was significantly different between these regions. The adjusted change in delta-ARI was 72.9 ms in the surrounding CM-NL tissue compared with 54.1 ms in the border zones and 64.9 ms in the scar. This important finding demonstrates that direct sympathetic stimulation with catecholamines causes significant heterogeneity in repolarization in various regions of the myocardium, setting up the substrate required for initiation and maintenance of reentry and ventricular arrhythmias in humans. Furthermore, this is the first study to demonstrate that the change in delta-ARI is greater in the CM-NL and scarred myocardium compared with the border zone regions (P value = 0.0002 when comparing border zone to normal tissue and 0.01 when comparing border zones to scars) in humans. This difference in shortening of repolarization suggests that the repolarization gradient between border zone and more viable myocardium plays a key role in genesis and maintenance of arrhythmias in humans. Beta-blocker and angiotensin converting enzyme inhibitors have been shown to reduce the risk of sudden cardiac death in large clinical trials (13, 15). Yet, the mechanistic basis for these observations has previously proved elusive. This study suggests that these medications, by blocking the effect of direct catecholamines, may decrease the heterogeneity in the duration of repolarization in the various regions of the myocardium in response to sympathetic stimulation, thereby decreasing the likelihood of differential conduction and refractoriness required for reentry.
Although to a lesser degree than isoproterenol, the response to reflex sympathetic stimulation in various regions of the heart also proved heterogeneous. Within each patient, there was a significant difference in ARI between border zones compared with the nonscarred and scarred myocardium in response to reflex sympathetic stimulation (a difference of 11.6 ± 4.8 ms, P = 0.016, when comparing CM-NL tissue to border zones, and a difference of 7.3 m ± 4.8 ms when comparing scar to border zone regions, P = 0.08; Table 4). Therefore, within each patient, the CM-NL and scar showed an ARI increase that was significantly more than the border zone tissue. Previous studies (5, 6) in transplanted human hearts have shown that the border zones contain localized areas of nerve sprouting. Furthermore, an association between nerve sprouting and sudden cardiac death has been shown in animal models of myocardial infarction (5). These regions with areas of nerve sprouting would not be expected to show an increase in ARI as much as other denervated areas in the border zones, scar, and normal myocardium around the scar, given that the areas of nerve sprouting seen on histopathology are very localized (6), even within the border zones. This could explain why in some border zone regions, such as those with nerve sprouting, ARI decreased, while in others (denervated regions), ARI increased. This also provided the impetus for analyzing the dispersion or variance in ARI within the cardiomyopathy patients. Moreover, although certain regions within the normal myocardium, border zones, or scar showed shortening of ARI in the same patient, the predominant finding was that of an increase in mean ARI in the scar and normal tissue, compared with the border zones (Fig. 2). This paradoxical increase in ARI was previously noted in a study (28) of rabbit hearts. In response to left stellate ganglion stimulation, certain denervated regions of the rabbit heart increased their ARI, although the reasons for this finding remain unclear. However, it appears that even the normal tissue close to scarred regions behaves in a denervated fashion and does not shorten its APD in response to reflex sympathetic stimulation. This finding is supported by previous canine studies (1) of myocardial infarction demonstrating that certain regions of the normal myocardium distal to an infarct do not respond to left and right stellate ganglion stimulation, suggesting denervation of these regions. Of note, in patients with structurally normal hearts, nitroprusside consistently shortened ARI.
Results of studies (4, 14, 25) in canine models of myocardial infarction have suggested that the normal myocardium distal to an infarct, in addition to behaving in a denervated fashion, can exhibit denervation supersensitivity or an exaggerated response to direct sympathetic stimulation with norepinephrine infusion. In this study, we found that within each patient, the CM-NL tissue around the scar of the cardiomyopathic heart did indeed show an ARI decrease considerably more than other regions (a mean difference of 18.8 ± 4.8 ms compared with border zone regions, P = 0.0002, and 8.0 ± 4.3 ms compared with the scar, P = 0.02) in response to isoproterenol. Furthermore, there was a trend for shorter repolarization times in CM-NL tissue of the cardiomyopathic hearts compared with hearts of noncardiomypathy patients (mean difference of 21.8 ± 12.5 ms; P value = 0.08). Therefore, this is the first study of ischemic cardiomyopathy patients to suggest that denervation supersensitivity does appear to exist in humans, particularly in the normal denervated myocardium around the scar.
In 1994, Beau and Saffitz (2) showed that the heterogeneity of norepinephrine uptake in failing hearts appeared to be a reflection of heterogenous innervation. In our study, the areas that appeared the least denervated, i.e., the border zones, had the least response to isoproterenol, suggesting that regions with the most innervation have decreased beta-adrenergic receptor density compared with more denervated regions.
This study demonstrates that with reflex sympathetic nerve stimulation, a significant heterogenous response in repolarization is observed in cardiomyopathy patients in vivo. Although to a lesser degree than direct sympathetic stimulation, reflex sympathetic stimulation also creates increased heterogeneity in repolarization duration in the various regions of the diseased myocardium that can serve as the substrate for reentry and occurrence of ventricular arrhythmia. Dispersion in repolarization is a requirement for reentry, and dispersion in ARI has been previously linked to ventricular arrhythmias (9, 19, 22, 26). Left cervicothoracic sympathectomy and thoracic epidural anesthesia can decrease the burden of ventricular arrhythmias in patients presenting in VT storm (3, 16, 20). The results of this study provide insight into the therapeutic benefit of these procedures, which may work by decreasing the effect of the sympathetic nervous system on ARI dispersion.
Dispersion in repolarization.
In patients without cardiomyopathy, all regions of the heart significantly and uniformly exhibited ARI shortening in response to both isoproterenol and nitroprusside. However, in cardiomyopathic hearts, there was significant variation in response to sympathetic stimulation, even within the same regions such as the border zones. In fact, the overall variance in ARI in cardiomyopathic hearts was 70% more in response to isoproterenol and 230% more in response to nitroprusside compared with that in structurally normal hearts. These findings are limited by the younger age of the patients without cardiomyopathy.
Limitations.
The number of patients enrolled in this study is small, and it is possible that certain regional differences did not reach statistical significance given this small number. However, despite small number of patients, this study utilized high-density sampling and was able to show regional differences in ARI between normal, scar, and border zone tissue in response to sympathetic stimulation, the primary aim of this study.
Many of the patients with ICM were on amiodarone and beta-blockers. Given the long half-life of amiodarone and possible residual effect of beta-blockers despite a 12-h discontinuation period, it is possible that the antiadrenergic effects of these medications affected the ARI measurements. For this reason, ARI measurements were obtained at baseline and postsympathetic stimulation and the delta-ARI (or the difference between postintervention ARI and baseline ARI) was used to calculate regional differences at each electrode in each patient. Importantly, amiodarone and beta-blockers have sympatholytic effects that should have decreased dispersion in ARI. Despite these medications, significant heterogeneity in delta-ARI was noted in response to sympathetic stimulation. Moreover, beta-blockers have not been able to block the effect of sympathetic nerve stimulation on ventricular arrhythmias.(11).
Repolarization measurements in scar can be difficult to obtain, due to low T waves and poorly defined T-wave upstroke. In this study, only electrodes with reliable depolarization and repolarization waves were used and each point was sampled 30 times to assure that the ARI from that location was consistent.
Nitroprusside can reduce wall strain, which may complicate the effect of the reflex sympathetic nervous system. In this study, nitroprusside caused a consistent decrease in all patients with structurally normal hearts while patients with ICM had a significantly different response. Therefore, a decrease in LV strain as the reason for ARI changes observed is unlikely.
Although patients underwent perfusion scans and myocardial ischemia was excluded, these tests could have missed subtle differences in perfusion in smaller regions of the myocardium (below the test resolution). Therefore, the observed responses could have been underestimated due to heterogeneity of perfusion.
Although the ARI method allows for simultaneous assessment of all tissues studied, the mapping in this study was limited to electrodes feasible under the approved protocol. Nevertheless, the regional differences noted were sufficient to achieve statistical significance, despite sampling different regions in different patients. Additionally, with the use of the delta-ARI and obtaining simultaneous measurements for each condition from each region, any underlying patient related and anesthesia-related factors were adjusted for in the model used (each site in each patient served as a baseline for comparison).
Heterogeneity in remodeling of adrenergic signaling pathways adds further complexity to the response to adrenergic stimulation. A differential response based on postreceptor signaling mechanisms that ultimately transduce adrenergic stimulation into changes in membrane currents could be responsible for the experimental results. For instance, the nonlinear relationship between calcium homeostasis during adrenergic stimulation could play an important role. These factors could not be controlled for or tested in this type of a study. Further studies are needed to probe these important issues.
Conclusion.
This is the first in vivo human study to assess regional difference in repolarization between normal tissue, scar, and border zones in ICM patients. Significant heterogeneity in repolarization exists in these patients in response to sympathetic stimulation. Based on this data, sympathetic nerve stimulation in humans can promote ventricular arrhythmias by further exacerbating the regional differences in repolarization of the underlying myocardial substrate. Overall, the CM-NL and border zone regions around the scar and the scar itself behave in a denervated fashion compared with normal hearts. However, this denervation is not homogenous within each patient, with the border zones appearing the least denervated. The relatively normal tissue around the scar in cardiomyopathy patients exhibits denervation supersensitivity and has an exaggerated response to isoproterenol. Finally, the dispersion in repolarization is significantly greater in ICM patients compared with noncardiomyopathy patients. The increased dispersion in repolarization seen in ICM in response to sympathetic stimulation points to the important role the sympathetic nervous system plays in the control of myocardial excitability in human disease.
APPENDIX
Electrophysiological study and electroanatomic mapping.
After informed consent was obtained, all patients were brought to the electrophysiological laboratory. All antiarrhythmic medications including beta-blockers were discontinued ≥12 h before the procedure. Single or double transseptal catheterization was performed in all patients with ICM. In patients with structurally normal hearts, transseptal catheterization was performed only as dictated by the EP study. When necessary, epicardial access was obtained in patients with ICM using the method described by Sosa et al. (21). An endocardial, and when necessary, an epicardial voltage map was created to assess for the presence of normal, scar, and border zone regions in all patients with ICM using either the CARTO (CARTO XP, Biosense-Webster) or Nav-X (St. Jude Medical) electroanatomic mapping systems.
Transseptal catheterization.
Anticoagulation before transseptal catheterization was achieved with heparin to keep the activated clotting time > 300 ms. Under fluoroscopic guidance, a Mullins sheath was positioned in the superior vena cava over J tipped guide wire, which was then replaced with a Brockenbrough needle. The Brockenbrough needle was positioned inside the distal end of the sheath, and the entire system was then positioned along the foramen ovale. With the use of fluoroscopic guidance and pressure monitoring, the needle was advanced into the left atrium. The position of the sheath and needle in the left atrium was confirmed with contrast injection and pressure monitoring. The dilator and sheath were then advanced over the needle and positioned in the left atrium. A Toray valvuloplasty guide wire (Toray Industries, Tokyo, Japan) was placed across the interatrial septum, and the dilator and sheath were pulled back into the inferior vena cava. A second transseptal puncture was performed in a similar manner when necessary. Intracardiac echocardiography was used for anatomic guidance and to monitor the development of a pericardial effusion.
Epicardial access.
The subxiphoid area was anesthetized and through a Tuohy epidural needle, a small amount of contrast was injected to ascertain the position of the needle as it was advanced toward the LV in the left anterior oblique projection. Once access was obtained, a J-tipped guidewire was advanced through the needle, and then an 8-French SL-0 sheath was advanced over the wire into the pericardial space.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-084261 (to K. Shivkumar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.V., A.M., and K.S. performed experiments; M.V., R.L.L., A.M., and K.S. analyzed data; M.V., R.L.L., A.M., and K.S. interpreted results of experiments; M.V. and K.S. prepared figures; M.V. and K.S. drafted manuscript; M.V., R.L.L., A.M., and K.S. edited and revised manuscript; M.V., R.L.L., A.M., and K.S. approved final version of manuscript; K.S. conception and design of research.
ACKNOWLEDGMENTS
We thank the fellows and faculty of the University of California, Los Angeles, Cardiac Arrhythmia Center for helping with clinical care of patients who participated in these studies. We thank Shelly Cote for help with the research coordination of this study. We also thank the University of California, Los Angeles Statistical Biomathematical Core, specifically Jeffery Gornbein, for help in the statistical analysis of this paper.
REFERENCES
- 1. Barber MJ, Mueller TM, Henry DP, Felten SY, Zipes DP. Transmural myocardial infarction in the dog produces sympathectomy in noninfarcted myocardium. Circulation 67: 787–796, 1983 [DOI] [PubMed] [Google Scholar]
- 2. Beau SL, Saffitz JE. Transmural heterogeneity of norepinephrine uptake in failing human hearts. J Am Coll Cardiol 23: 579–585, 1994 [DOI] [PubMed] [Google Scholar]
- 3. Bourke T, Vaseghi M, Michowitz Y, Sankhla V, Shah M, Swapna N, Boyle NG, Mahajan A, Narasimhan C, Lokhandwala Y, Shivkumar K. Neuraxial modulation for refractory ventricular arrhythmias: value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation 121: 2255–2262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bristow M. Changes in myocardial and vascular receptors in heart failure. J Am Coll Cardiol 22: 61A–71A, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, Chen PS. Nerve sprouting and sudden cardiac death. Circ Res 86: 816–821, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, Chen PS, Chen LS. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 101: 1960–1969, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Chinushi M, Tagawa M, Kasai H, Washizuka T, Abe A, Furushima H, Aizawa Y. Correlation between the effective refractory period and activation-recovery interval calculated from the intracardiac unipolar electrogram of humans with and without dl-sotalol treatment. Jpn Circ J 65: 702–706, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Dillon SM, Allessie MA, Ursell PC, Wit AL. Influences of anisotropic tissue structure on reentrant circuits in the epicardial border zone of subacute canine infarcts. Circ Res 63: 182–206, 1988 [DOI] [PubMed] [Google Scholar]
- 9. el-Sherif N, Caref EB, Yin H, Restivo M. The electrophysiological mechanism of ventricular arrhythmias in the long QT syndrome. Tridimensional mapping of activation and recovery patterns. Circ Res 79: 474–492, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Simvastatin therapy normalizes sympathetic neural control in experimental heart failure: roles of angiotensin II type 1 receptors and NAD(P)H oxidase. Circulation 112: 1763–1770, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Gillis RA, Pearle DL, Hoekman T. Failure of beta-adrenergic receptor blockade to prevent arrhythmias induced by sympathetic nerve stimulation. Science 185: 70–72, 1974 [DOI] [PubMed] [Google Scholar]
- 12. Haws CW, Lux RL. Correlation between in vivo transmembrane action potential durations and activation-recovery intervals from electrograms. Effects of interventions that alter repolarization time. Circulation 81: 281–288, 1990 [DOI] [PubMed] [Google Scholar]
- 13. Hjalmarson A. Effects of beta blockade on sudden cardiac death during acute myocardial infarction and the postinfarction period. Am J Cardiol 80: 35J–39J, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Kammerling JJ, Green FJ, Watanabe AM, Inoue H, Barber MJ, Henry DP, Zipes DP. Denervation supersensitivity of refractoriness in noninfarcted areas apical to transmural myocardial infarction. Circulation 76: 383–393, 1987 [DOI] [PubMed] [Google Scholar]
- 15. Kober L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, Videbaek J, Cole DS, Auclert L, Pauly NC. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med 333: 1670–1676, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Mahajan A, Moore J, Cesario DA, Shivkumar K. Use of thoracic epidural anesthesia for management of electrical storm: a case report. Heart Rhythm 2: 1359–1362, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation 101: 1288–1296, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Millar CK, Kralios FA, Lux RL. Correlation between refractory periods and activation-recovery intervals from electrograms: effects of rate and adrenergic interventions. Circulation 72: 1372–1379, 1985 [DOI] [PubMed] [Google Scholar]
- 19. Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest 115: 2305–2315, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwartz PJ, Motolese M, Pollavini G, Lotto A, Ruberti Y, Trazzi R, Bartorelli C, Zancehtti A. Prevention of sudden cardiac death after a first myocardial infarction by pharmacologic or surgical antiadrenergic interventions. J Cardiovasc Electrophysiol 3: 2–16, 1992 [Google Scholar]
- 21. Sosa E, Scanavacca M, d'Avila A, Oliveira F, Ramires JA. Nonsurgical transthoracic epicardial catheter ablation to treat recurrent ventricular tachycardia occurring late after myocardial infarction. J Am Coll Cardiol 35: 1442–1449, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Vaseghi M, Shivkumar K. The role of the autonomic nervous system in sudden cardiac death. Prog Cardiovasc Dis 50: 404–419, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vracko R, Thorning D, Frederickson RG. Fate of nerve fibers in necrotic, healing, and healed rat myocardium. Lab Invest 63: 490–501, 1990 [PubMed] [Google Scholar]
- 24. Vracko R, Thorning D, Frederickson RG. Nerve fibers in human myocardial scars. Hum Pathol 22: 138–146, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Warner MR, Wisler PL, Hodges TD, Watanabe AM, Zipes DP. Mechanisms of denervation supersensitivity in regionally denervated canine hearts. Am J Physiol Heart Circ Physiol 264: H815–H820, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Watanabe T, Yamaki M, Yamauchi S, Minamihaba O, Miyashita T, Kubota I, Tomoike H. Regional prolongation of ARI and altered restitution properties cause ventricular arrhythmia in heart failure. Am J Physiol Heart Circ Physiol 282: H212–H218, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Xiong W, Tian Y, DiSilvestre D, Tomaselli GF. Transmural heterogeneity of Na+-Ca2+ exchange: evidence for differential expression in normal and failing hearts. Circ Res 97: 207–209, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Yoshioka K, Gao DW, Chin M, Stillson C, Penades E, Lesh M, O'Connell W, Dae M. Heterogeneous sympathetic innervation influences local myocardial repolarization in normally perfused rabbit hearts. Circulation 101: 1060–1066, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Yue AM, Paisey JR, Robinson S, Betts TR, Roberts PR, Morgan JM. Determination of human ventricular repolarization by noncontact mapping: validation with monophasic action potential recordings. Circulation 110: 1343–1350, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Zipes DP, Barber MJ, Takahashi N, Gilmour RF., Jr Influence of the autonomic nervous system on the genesis of cardiac arrhythmias. Pacing Clin Electrophysiol 6: 1210–1220, 1983 [DOI] [PubMed] [Google Scholar]
- 31. Zipes DP, Wellens HJ. What have we learned about cardiac arrhythmias? Circulation 102: IV52–57, 2000 [DOI] [PubMed] [Google Scholar]