Abstract
Heart failure is a leading cause of morbidity and mortality in Western society. The cardiovascular transcription factor CHF1/Hey2 has been linked to experimental heart failure in mice, but the mechanisms by which it regulates myocardial function remain incompletely understood. The objective of this study was to determine how CHF1/Hey2 affects development of heart failure through examination of contractility in a myocardial knockout mouse model. We generated myocardial-specific knockout mice. At baseline, cardiac function was normal, but, after aortic banding, the conditional knockout mice demonstrated a greater increase in ventricular weight-to-body weight ratio compared with control mice (5.526 vs. 4.664 mg/g) and a significantly decreased ejection fraction (47.8 vs. 72.0% control). Isolated cardiac myocytes from these mice showed decreased calcium transients and fractional shortening after electrical stimulation. To determine the molecular basis for these alterations in excitation-contraction coupling, we first measured total sarcoplasmic reticulum calcium stores and calcium-dependent force generation in isolated muscle fibers, which were normal, suggesting a defect in calcium cycling. Analysis of gene expression demonstrated normal expression of most genes known to be involved in myocardial calcium cycling, with the exception of the ryanodine receptor binding protein FKBP12.6, which was expressed at increased levels in the conditional knockout hearts. Treatment of the isolated knockout myocytes with FK506, which inhibits the association of FKBP12.6 with the ryanodine receptor, restored contractile function. These findings demonstrate that conditional deletion of CHF1/Hey2 in the myocardium leads to abnormalities in calcium handling mediated by FKBP12.6 that predispose to pressure overload-induced heart failure.
Keywords: cardiac failure, gene regulation, hypertrophy, transcription factors
generation of contractile force in the heart is essential for the circulation of blood. Failure to generate sufficient force results in circulatory failure. At the cellular myocyte level, generation of contractile force is dependent on calcium-dependent activation of sarcomeric contraction, which, in turn, relies on cyclic, action potential-dependent release of calcium from the sarcoplasmic reticulum (SR). During diastole, or the relaxation phase of the cardiac cycle, contraction is terminated by re-uptake of cytosolic calcium into the SR. Alterations in calcium cycling have been implicated in the development of heart failure (2).
Cardiac hypertrophy is a common response to hemodynamic stress in the heart (9). Excessive overloading in diseases such as hypertension can cause a maladaptive response of the heart, referred to as pathological hypertrophy, a common precursor to heart failure, cardiac arrhythmia, and sudden death (16, 35). Congestive heart failure is a growing public health concern, as the incidence and prevalence continue to rise worldwide. In most heart failure patients, restoration of pump function remains an elusive goal. An understanding of the pathophysiological processes that lead to heart failure at the molecular level will likely facilitate the development of specific, novel, and improved therapies for treatment.
CHF1/Hey2 is a member of the hairy family of basic helix-loop-helix transcription factors and is a downstream target of the Notch signaling pathway (3, 11, 15, 25). Our laboratory has shown that CHF1/Hey2 loss of function results in ventricular septal defects, valvular abnormalities (tricuspid stenosis and mitral valve thickening), vascular smooth muscle defects (28–30), and coronary artery abnormalities (36). Others have reported similar findings (4, 7, 13). In the adult heart, CHF1/Hey2 overexpression can attenuate phenylephrine-induced hypertrophy (38) and can promote physiological hypertrophy instead of pathological hypertrophy induced by pressure overload (41, 42). Possible reported mechanisms by which CHF1/Hey2 affects cardiac hypertrophy include effects on GATA4 activity and through attenuation of apoptosis, although many cellular pathways appear to be affected (19, 38, 42, 43).
To better understand how CHF1/Hey2 affects the development of cardiac hypertrophy and heart failure and to mitigate the effects of congenital or vascular abnormalities, we generated CHF1/Hey2 myocardial conditional knockout (cKO) mice, performed in vitro assessment of excitation-contraction (EC) coupling in isolated myocytes and in vivo assessment of cardiac function after aortic banding. Here we report that loss of CHF1/Hey2 leads to altered EC coupling manifested as decreased calcium transients and fractional shortening, but does not affect overall SR calcium stores. Additional studies indicate normal calcium sensitivity in isolated myofibers, but increased susceptibility to heart failure after aortic banding. These defects are associated with an increase in expression of the ryanodine receptor binding protein FKBP12.6. FKBP12.6 binds ryanodine receptor 2 (RyR2), the predominant ryanodine receptor isoform in the heart, and stabilizes the channel in the closed position, thereby attenuating gain during EC coupling and preventing diastolic calcium leak (6, 39). Treatment of KO myocytes with FK506, an inhibitor of FKBP12.6 binding to RyR2, restored contractile function. Taken together, these findings indicate an important role for CHF1/Hey2 in regulating EC coupling and the development of heart failure through regulation of FKBP12.6.
MATERIALS AND METHODS
Animals.
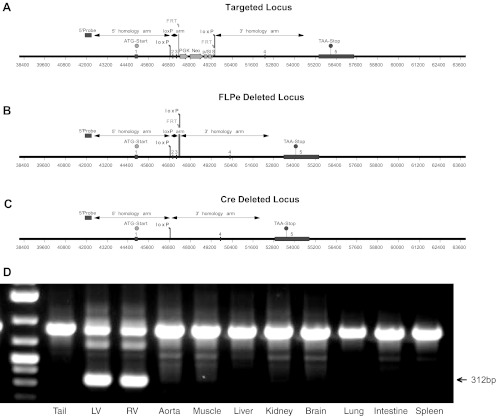
The CHF1/Hey2 conditional allele was generated by insertion of loxP sites surrounding exons 2 and 3, as shown in Fig. 1, using a commercially available service (Ozgene). A PGK Neo cassette flanked by FRT sites was also placed between the loxP sites downstream of exons 2 and 3. Targeted C57BL/6 embryonic stem cells were used to generate a pure inbred strain of C57BL/6 mice carrying the conditional allele, by standard methods. After germline transmission, mice carrying the conditional allele were crossed with a C57BL/6 FLPe deleter strain (27) to remove the PGK Neo cassette. Myocardial-specific cKO mice were generated by mating CHF1Flox/Flox and α-myosin heavy chain (MHC) Cre/CHF1Flox/+ mice, also on a pure C57BL/6 background. All studies were conducted according to guidelines and protocols for biohazards and animal care approved by the University of Washington Institutional Animal Care and Use Committee.
Fig. 1.
Generation of CHF1/Hey2 conditional knockout (cKO) mice. A: the endogeneous CHF1/Hey2 locus was targeted, as shown. B: mice containing the targeted allele were crossed with a FLPe deleter strain to remove the neomycin cassette, as shown. C: the floxed allele mice were then crossed with α-myosin heavy chain (MHC) Cre mice to remove exons 2 and 3 in the myocardium, resulting in the deleted locus shown. D: the specificity of myocardial deletion was demonstrated by PCR, as shown. The arrow indicates the KO band (size: 312 bp). LV, left ventricle; RV, right ventricle.
Transverse aortic constriction surgery, gravimetry, and histology.
C57BL/6 background mice (age: 12 wk; body weight: 20–30 g) were used in this study. The mice were anesthetized with ketamine (130 mg/kg ip) and xylazine (8.8 mg/kg ip) and then subjected to aortic banding surgery, as previously described (17, 19, 34, 42). Briefly, we banded the aorta using a 26G needle to tie the suture between the brachiocephalic and left common carotid branches. One week after surgery, mice were euthanized by carbon dioxide inhalation, followed by weighing, heart removal, and exsanguination. Hearts were rinsed in cold phosphate-buffered saline, trimmed of atrial and vascular tissue, dried briefly, and weighed. Heart tissue was fixed in paraformaldehyde, embedded in paraffin, sectioned, and stained with Masson's trichrome by standard methods. The fibrotic area was quantified as a percentage of total area in sections, as previously described (19).
Echocardiography.
Echocardiography to measure the left ventricular (LV) wall thickness, LV end-diastolic dimension (LVEDD), and LV ejection fraction (EF) was performed 1 wk before the surgery and 1 wk after the surgery using a Visual Sonics VEVO 770 system equipped with a 707B scan head, as previously described (17). Mice were lightly anesthetized with 1% isoflurane. The data were measured in M-mode from short axis. LVEF was calculated as follows: LVEF = [(LVIDd)3 − (LVIDs)3]/(LVIDd)3 × 100 (%) (where LVIDd is LV internal diastolic diameter, LVIDs is LV internal systolic diameter) (41).
Invasive hemodynamics.
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (2.5 mg/kg), intubated by tracheostomy, and connected to a rodent ventilator. To maintain constant body temperature at 37–39°C, a rectal probe was inserted following the tracheostomy and maintained throughout the procedure. The neck region was surgically cut using a surgical microscope, and the right internal jugular vein was carefully exposed to insert an intravenous catheter (24 gauge, BD Becton Dickson Angiocath) and secured in place. The animal then underwent an open lateral thoracotomy to insert a 1.4F high-fidelity micromanometer catheter into the LV via an apical puncture to obtain the pressure-volume measurements. After baseline measurements were obtained, 32 ng·g−1·min−1 of dobutamine were infused into the catheter to obtain additional measurements.
Myocardial strip dissection and Ca2+ sensitivity measurement.
Individual muscle strips were manually dissected from myocardial-specific cKO hearts for the measurement of Ca2+ sensitivity, as described (14, 24). Strips from CHF1Flox/Flox mice without α-MHC Cre were isolated as controls. For mechanical analysis, preparations were attached via aluminum T-clips and stretched between a force transducer (400A Aurora Scientific) and a length controller (model 300, Aurora Scientific) while bathed in relaxing solution. Force was measured at various concentrations of calcium to determine the contractile properties of control and KO cardiac muscle. Force vs. pCa data were plotted by expressing submaximal force (P) at each pCa as a fraction of maximal force measured at pCa 4.0 (P0), that is, P/P0, where P/P0 = Prel. The pCa50 and slope, Hill coefficient (nH), for each curve were determined by fitting the normalized data to the Hill equation:
Adult cardiac myocyte isolation, calcium fluorescence, and single-cell geometry measurements.
Adult control or cKO cardiac myocytes were isolated according to the Alliance for Cellular Signaling protocol (31). For all experiments, myocytes were cultured briefly and studied on the same day. Calcium transients induced by electrical stimulation at 1 Hz were measured in fura-2 loaded cells using IonOptix equipment as described (18). Cells were loaded with fura-2 AM at a concentration of 1 μM for 15 min. Ratiometric (360 nm/380 nm) fura-2 fluorescence was measured using an IonOptix spectrophotometer (Stepper Switch) attached to a fluorescence microscope. Emitted fura-2 fluorescence was collected by the ×40 objective, passed through a 510-nm filter, and detected by a photomultiplier tube. The velocities and times of Ca2+ rise and decay were calculated. Changes in cell shape over time were also assessed optically using edge detection software, to determine fractional shortening and shortening velocity. FK506 rescue experiments in which isolated myocytes were pretreated with FK506 (10 μM) for at least 10 min before IonOptix assessment were performed as previously described (22, 33). Cyclosporin A rescue experiments were performed similarly, but at a concentration of 0.2 μM for 20 min. SR calcium stores were measured by treatment of fluo-4 loaded cells with caffeine (10 mM), followed by measurement of the induced fluorescence-to-basal fluorescence ratio using a Nikon Swept Field confocal system, as described (44).
RNA isolation and quantitative real-time PCR.
Total RNA was isolated from LVs by homogenization and the use of TRIZOL reagent (Invitrogen). The extraction was done according to the manufacturer's instructions. RNA was reverse transcribed into cDNA using Superscript III (Invitrogen) and oligo(dT)20 primers (Invitrogen). Quantitative real-time PCR was used to assess FKBP12.6 expression, which was performed on the cDNA samples using SYBR green fluorescent reagent and an Applied Biosystems 7500 system based on the standard protocols. Other molecules involved in calcium cycling were assessed as well. GAPDH was used as a control. The primers used for real-time PCR are listed in Table 1. Data analyses were performed by the 2-ΔΔCT method, as described (20).
Table 1.
Expression of genes involved in calcium cycling
| Gene | Protein | Primers | cKO Relative Expression |
|---|---|---|---|
| Atp2a2 | Sarcoplasmic reticulum Ca2+-ATPase | TCAGCAGGAACTTTGTCACC | 1.04 ± 0.10 |
| GGGCAAAGTGTATCGACAGG | |||
| PLN | Phospholamban | AACAGGCAGCCAAATGTGA | 0.93 ± 0.06 |
| CCCAGCTAAGCTCCCATAAG | |||
| Ryr2 | Ryanodine receptor | CAAATCCTTCTGCTGCCAAG | 1.58 ± 0.67 |
| CGAGGATGAGATCCAGTTCC | |||
| Ppp1ca | Protein phosphatase 1 | GTCTGTGGGCCGCATAATAC | 0.97 ± 0.20 |
| CAGCCATTGTGGATGAGAAG | |||
| S100a1 A1 | S100 calcium binding protein | CACATTGATGAGGGTCTCCA | 1.12 ± 0.09 |
| GTGCCCTTCTGTCGAGAATC | |||
| Ppp1r1a | Protein phosphatase 1, regulatory (inhibitor) subunit 1A | ACAAGTGTGGCAGGGGTG | 0.89 ± 0.28 |
| CCCACGGAAGATCCAGTTTA | |||
| Ppp1r2 | Protein phosphatase 1, regulatory (inhibitor) subunit 2 | GCCATAGTCTTTGTCAGCGG | 0.99 ± 0.23 |
| CGATCGTGGAAGAGGAACTG | |||
| Fkbp1b | FK506 binding protein 12.6 kDa | TTCTTGCCCTTTTGAAGCAT | 1.82 ± 0.19* |
| ATCGAGACCATCTCCCCAG | |||
| Fkbp1a | FK506 binding protein (FKBP12) | TTTCCATCTTCAAGCATCCC | 0.95 ± 0.29 |
| GTGGAGACCATCTCTCCTGG | |||
| Camk2d | Calcium/calmodulin-dependent protein kinase II, delta | TACAGTGAAGCTGATGCCAG | 1.00 ± 0.20 |
| GAGCACAGGCAGACAAACAT | |||
| Asph | Junctin | CTGGTGGTGTGTCTTGCTGT | 1.15 ± 0.27 |
| CACGAAGGGGTCGAGATTT | |||
| Casq2 | Calsequestrin-2 | TCCTTGAATCCACCATCACA | 1.11 ± 0.39 |
| AAAACAGTTCCAGCTGAAGGA | |||
| Trdn | Triadin | ATGACTGAGATCACTGCTGAAGG | 0.97 ± 0.23 |
| ATGTTGTCACAATGTCTTCGGT | |||
| CACNA1C | Calcium channel, voltage-dependent, L type, α1C subunit | ATGAAAACACGAGGATGTACGTT | 1.73 ± 0.35 |
| ACTGACGGTAGAGATGGTTGC | |||
| NCX | Sodium-calcium exchanger | TTCACATTAACAGGCCAACC | 1.47 ± 0.36 |
| TCAGTGGCTGCTTGTCATCA |
cKO, conditional knockout.
P < 0.001.
Protein extraction and Western blotting.
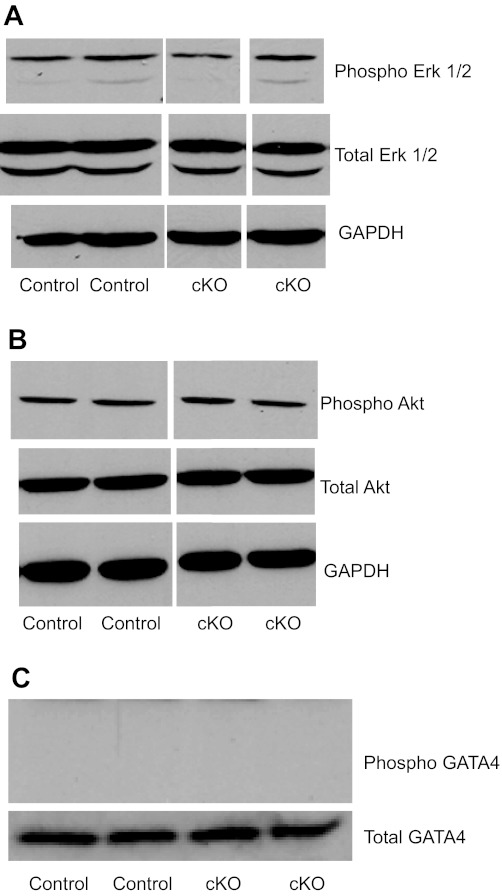
Total protein was isolated from mouse LV tissue by homogenization and lysis in 50 mM Tris·HCl (pH 8.0), 150 mM NaCl, 0.1% SDS, 1% IGEPAL, and 0.5% sodium deoxycholate with complete protease inhibitor (Roche), as previously described (10). Protein concentration was determined using the Bradford method, according to the kit manufacturer's instructions (Bio-Rad). Total proteins were loaded onto 12% SDS-PAGE gels and transferred to membranes for Western blot analysis. The following antibodies were used: anti-FKBP12.6 (Abnova); anti-GATA-4 (phospho-Ser105, Invitrogen); anti-GATA-4 (Santa Cruz Biotechnology) for the detection of total GATA4; anti-Phospho-ERK1/2, anti-total ERK1/2, anti-phospho-Akt, and anti-total Akt antibody (Cell Signaling); and anti-GAPDH (Cell Signaling) as a control.
Statistical analysis.
All data are reported as means ± SE. The comparison between the groups was made by one-way ANOVA with the post hoc Fisher protected least significant difference test for multiple paired comparisons. All of the analyses were performed using commercially available software (StatView, SAS Institute). A value of P < 0.05 was taken as the minimal level of significance.
RESULTS
Generation and characterization of myocardial-specific CHF1/Hey2 KO mice.
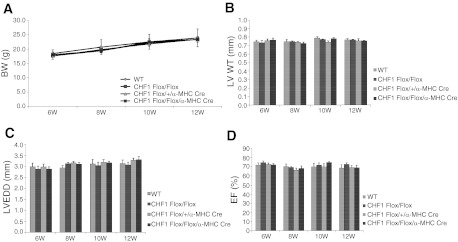
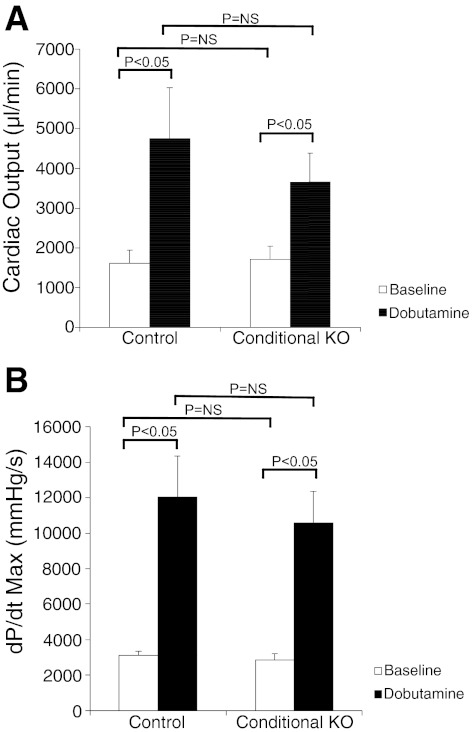
As described above, CHF1/Hey2 conditional allele mice were developed by targeting exons 2 and 3 and crossing these mice with first a FLPe deleter strain (27) to remove the PGK Neo cassette and then with an α-MHC Cre line (1) to remove exons 2 and 3, as shown in Fig. 1. Tissue-specific deletion was verified by genomic PCR (Fig. 1D). Animals were viable, survived to adulthood in expected Mendelian ratios, and did not develop spontaneous heart failure when observed by serial echocardiography up until 12 wk of age, in contrast to a previously reported study in which similar mice develop cardiomyopathy at 6 wk of age (40). We performed serial body weight and heart function measurements in CHF1/Hey2 myocardial cKO mice, together with floxed allele mice, from 6 wk to 12 wk of age. The results showed similar body weight, LV wall thickness, and heart function at all time points (Fig. 2). We also measured cardiac output and change in pressure over time at baseline and after dobutamine infusion and did not find a significant difference between control and cKO mice (Fig. 3).
Fig. 2.
Myocardial deletion of CHF1/Hey2 has no effect on body weight (BW), heart size, or LV function at up to 12 wk of age. Wild-type (WT), CHF1/Hey2Flox/Flox, CHF1/Hey2Flox/Flox + α-MHC Cre, and CHF1/Hey2Flox/+ + α-MHC Cre mice were evaluated by echocardiography at 6, 8, 10, and 12 wk of age. BW (A), LV wall thickness (LVWT; B), LV end-diastolic dimension (LVEDD; C), and ejection fraction (EF; D) were as shown. Values are means ± SE; WT, n = 3; CHF1Flox/Flox, n = 4; CHF1Flox/+/α-MHC Cre, n = 4; CHF1Flox/Flox/α-MHC Cre, n = 4.
Fig. 3.
Hemodynamic analysis shows no difference between CHF1/Hey2 myocardial KO mice and control mice at baseline or after dobutamine infusion (32 ng·g−1·min−1). A: CHF1/Hey2 cKO mice and control mice have equivalent cardiac output at baseline and equivalent augmentation after dobutamine infusion. B: CHF1/Hey2 cKO mice and control mice have the equivalent maximum change in pressure over time (dP/dtmax) at baseline and equivalent augmentation after dobutamine infusion. NS, nonsignificant. Values are means ± SE; control, n = 8; cKO, n = 10.
Conditional deletion of CHF1/Hey2 in the myocardium predisposes to hypertrophy and heart failure after pressure overload.
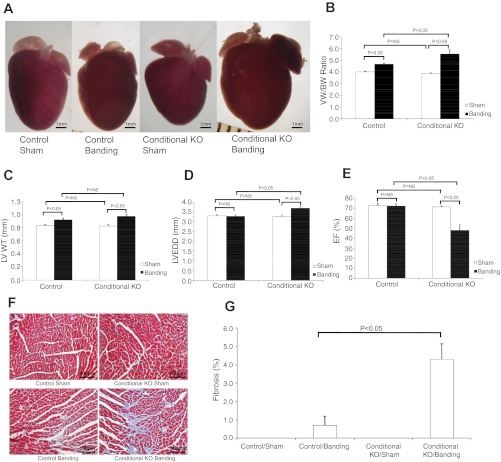
To determine whether myocardial deletion of CHF1/Hey2 predisposes to hypertrophy and heart failure, we performed transverse aortic constriction on 12-wk-old mice. As discussed above, this myocardial deletion did not affect baseline ventricular function measured in vivo by echocardiography. After aortic banding, however, gravimetric analysis demonstrated a greater increase in ventricular weight-to-body weight ratio in cKO mice compared with control mice (5.526 vs. 4.664 mg/g, Fig. 4B). cKO mice developed increased LVEDD compared with control (3.69 vs. 3.29 mm, Fig. 4D), which suggested eccentric hypertrophy in cKO mice compared with the concentric hypertrophy in control mice. cKO mice also demonstrated increased susceptibility to heart failure. One week after aortic banding, control flox/flox mice showed no decrement in EF (72.0 vs. 72.5% sham, Fig. 4E). cKO mice, in contrast, showed significantly decreased EF (47.8 vs. 71.2% sham and vs. 72.0% control banding, Fig. 4E). Masson's trichrome staining demonstrated increased fibrosis in the cKO hearts after aortic banding, verified by quantitative image analysis (Fig. 4, F and G). To determine whether baseline activation of ERK1/2, AKT, or GATA4 could explain the predisposition to pathological hypertrophy, we analyzed protein expression and phosphorylation for these important molecules. As shown in Fig. 5, no difference was seen for control and cKO animals at baseline.
Fig. 4.
Myocardial deletion of CHF1/Hey2 leads to LV hypertrophy, decreased ventricular function, and fibrosis after aortic banding. cKO and Flox/Flox control mice were subjected to mild aortic banding or sham operation for 1 wk and then evaluated by echocardiography, gravimetry, and Masson's trichrome staining. A: gross pathology of control and cKO hearts after sham operation and aortic banding. B: gravimetric analysis examining ventricular weight (VW)-to-BW ratio after sham operation and aortic banding. C: LVWT. D: LVEDD. E: LVEF. F: Masson's trichrome staining. G. quantification of fibrotic area by ImagePro analysis. Values are means ± SE; control/sham: n = 6; control/banding: n = 7; cKO/sham: n = 4; cKO/banding: n = 6.
Fig. 5.
Evaluation of ERK1/2, Akt, and GATA4 expression and phosphorylation in CHF1/Hey2 myocardial KO mice and control mice. Protein lysates were analyzed for protein expression by Western blot analysis using antibodies for phosphorylated GATA4 [phospho-Ser105 (pS105)] and total GATA4 (C), phospho-ERK1/2 and total ERK1/2 (A), phospho-Akt and total Akt (B), and GAPDH (A and B), as indicated. Duplicate representative lanes from control and cKO lysates run on the same gel are shown. The experiment was repeated three times.
CHF1/Hey2 loss of function affects myocyte contraction through alterations in calcium cycling, but not calcium sensitivity or calcium stores.
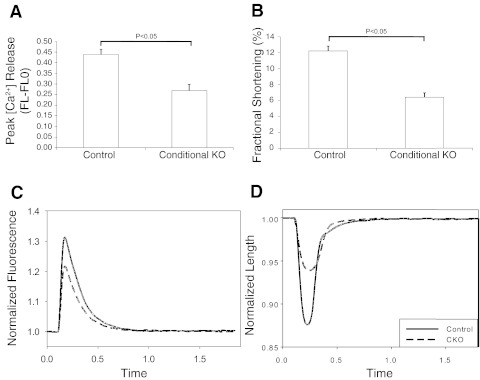
Previous reports have also indicated that CHF1/Hey2 loss of function predisposes to hypertrophy and heart failure (7, 19, 28, 38, 40, 42), but the primary mechanisms have not been completely elucidated. Since calcium cycling is essential for action potential-triggered EC coupling and normal contractile function, we examined the functional properties of cardiac myocytes isolated from myocardial cKO mice, using an IonOptix system to measure electrical field stimulated calcium fluxes in fura-2-loaded cells. Cells were isolated from 12-wk-old mice that were known to have normal ventricular function, as measured by echocardiography and invasive hemodynamics. Despite the appearance of normal cardiac function in vivo and normal hemodynamics after dobutamine infusion, isolated cardiac myocytes from cKO mice were found to have abnormal myocardial peak calcium transients that were decreased significantly compared with control myocytes, as shown graphically and by stimulated calcium fluorescence over time (Fig. 6, A and C). Fractional shortening was also decreased significantly in KO cells, as shown graphically and by observing stimulus-mediated change in cell length over time (Fig. 6, B and D). These results indicate decreased electrical stimulus-induced Ca2+ release in the cKO cells that is associated with decreased fractional shortening. Furthermore, these changes are present before the onset of overt heart failure in mice, suggesting that this defect in contraction is a primary defect resulting from CHF1/Hey2 loss of function.
Fig. 6.
Isolated adult cardiac myocytes from CHF1/Hey2 cKO mice show alterations in calcium release and fractional shortening. Cells were isolated from 12-wk-old cKO and floxed allele mice that had no evidence of LV hypertrophy or heart failure. Cells were loaded with the calcium fluorophore fura-2 and stimulated electrically. Cell shape changes and calcium fluxes were tracked optically using a commercially available system (Ionoptix). A: calcium transient by fura-2 (peak height, defined as induced fluorescence FL minus basal fluorescence FLO). B: FS. C: calcium fluorescence vs. time tracing per cycle for control and cKO myocytes. D: cell length vs. time tracing per cycle for control and cKO myocytes. [Ca2+], calcium concentration. Values are means ± SE; control: n = 33; cKO: n = 29.
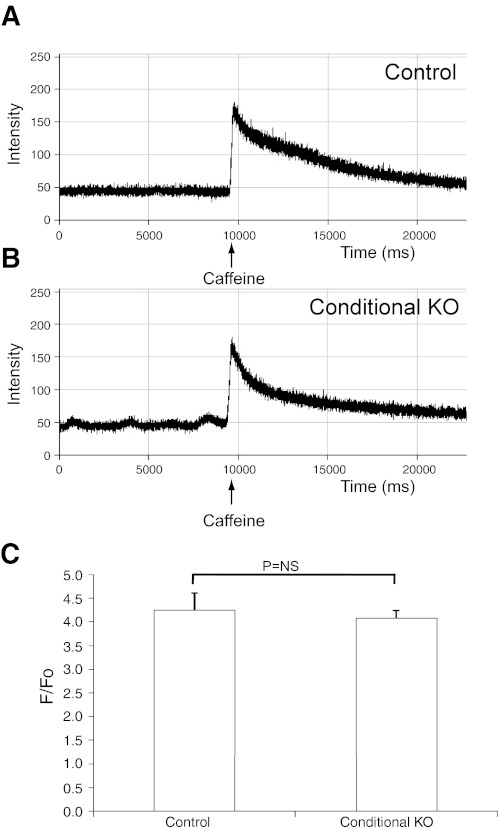
As calcium release in cardiac myocytes is related to calcium stores within the SR, we measured the SR stores by triggering calcium release via caffeine-mediated activation of the ryanodine receptor. Control floxed allele and cKO adult myocytes were loaded with fluo-4 and treated with caffeine (10 mM). As shown in Fig. 7, the Ca2+ flux from the SR was comparable in KO myocytes compared with wild type. The ratio of induced fluorescence to basal fluorescence was not significantly different, which indicates a comparable Ca2+ load in the KO myocytes. These findings indicate that calcium stores are normal in the KO cells.
Fig. 7.
Isolated adult cardiac myocytes from CHF1/Hey2 cKO mice show normal calcium stores. Cells were isolated from 12-wk-old cKO and floxed allele mice and loaded with the calcium fluorophore fluo-4. Cells were treated with caffeine (10 mM) to induce calcium release from the sarcoplasmic reticulum, and the ratio of induced fluorescence to basal fluorescence was measured by confocal fluorescence microscopy. A: fluorescence tracing for representative control cardiac myocyte before and after caffeine treatment. B: fluorescence tracing for representative cKO cardiac myocyte before and after caffeine treatment. C: graphical representation of the peak calcium-induced fluorescence (F)-to-basal fluorescence (F0) ratio for control and cKO myocytes after caffeine treatment. Values are means ± SE; control: n = 7; cKO: n = 7.
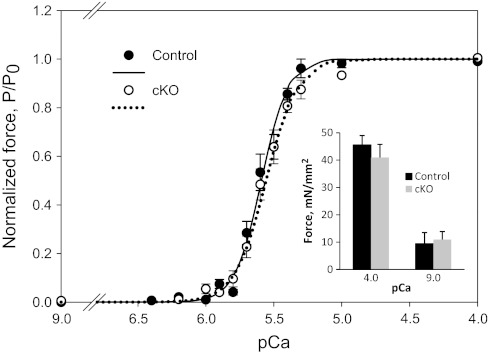
Although single CHF1/Hey2 KO myocytes demonstrated defects in Ca2+ cycling, an additional potential contributor to defective contraction could be alterations of the contractile apparatus that affect calcium sensitivity. Our laboratory's previous study has shown that loss of CHF1/Hey2 leads to ectopic expression of atrial genes in the LV compact myocardium, including the contractile proteins MLC1A and MLC2A (12). To address this possibility, we isolated adult heart strips from 12-wk-old floxed control and cKO animals that had normal LV function by echocardiography. We measured force generation as a function of calcium concentration in these skinned strips. As shown in Fig. 8, the force-calcium curves are indistinguishable between control and cKO strips (pCa50 5.60 in cKO vs. 5.64 in control mice), and there was also no difference in passive or maximally activated force (Fig. 8, inset).
Fig. 8.
Isolated heart muscle strips from CHF1/Hey2 cKO mice show no difference in calcium sensitivity or maximum contractile force. Skinned ventricular muscle strips were tethered to a force transducer and bathed in calcium solutions of varying concentrations. cKO muscle strips show no difference in force generated at given calcium concentrations compared with control strips, indicating that the contractile apparatus is functionally intact in these cells. The experiment was repeated 3 times. P/P0, submaximal force at each pCa as a fraction of maximal force measured at pCa 4.0. Inset: there was no difference in passive or maximally activated force. Values are means ± SE.
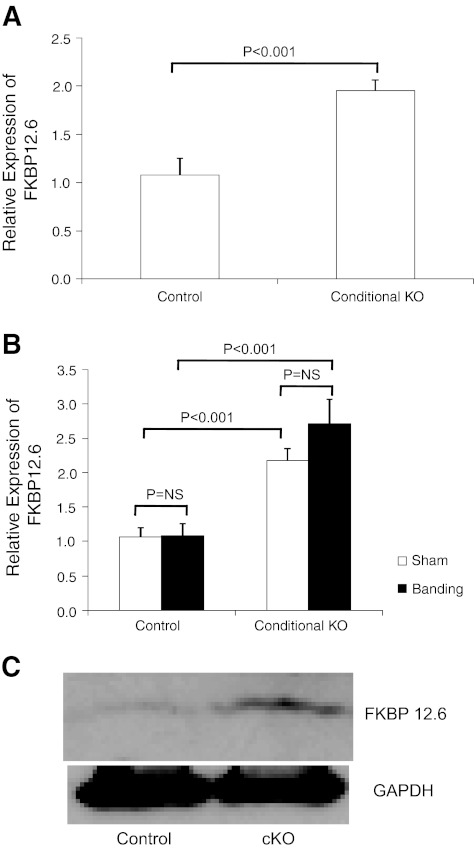
Conditional deletion of CHF1/Hey2 in myocardium is associated with increased expression of FKBP12.6.
To determine whether alterations in expression of genes known to control calcium cycling are altered in cKO hearts, we performed quantitative RT-PCR on RNA prepared from control and cKO hearts that had normal ventricular function. As shown in Table 1, the expression profiles of a variety of genes, such as Serca2a, phospholamban, calsequestrin, RyR2, etc., were unchanged. Expression of FKBP12.6 was increased significantly, however. We examined further its expression in hearts isolated from either CHF1/Hey2 floxed allele mice or CHF1/Hey2 myocardial cKO mice that either were sham operated or underwent aortic banding. FKBP12.6 expression was consistently elevated in cKO hearts after sham operation and after aortic banding compared with control hearts treated similarly (Fig. 9). These findings suggest a role for FKBP12.6 in the development of heart failure in these animals.
Fig. 9.
FKBP12.6 expression is increased in cKO hearts compared with controls. FKBP12.6 expression was measured by quantitative RT-PCR in control and cKO hearts at baseline (A) and in sham and banded hearts (B). Values are means ± SE. C: Western blotting demonstrates increased FKBP12.6 protein expression in cKO hearts at baseline. The experiment was repeated 3 times.
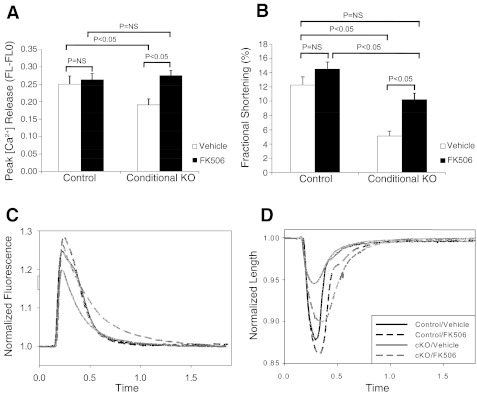
Treatment of cKO myocytes with FK506, an inhibitor of FKBP12.6, rescues defective contractile function.
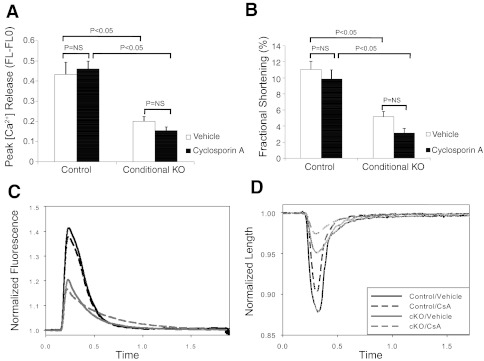
To determine whether the increased expression of FKBP12.6 accounts for the diminished contractile function observed in cKO myocytes, we treated the cKO cells with FK506, which is known to inhibit the association of FKBP12.6 with RyR2. As shown in Fig. 10, treatment with FK506 restored calcium transient amplitude (Fig. 10A) to levels not significantly different from that of FK506-treated control cells. Fractional shortening in FK506 treated cKO cells increased to a level similar to that of vehicle-treated control cells, but was decreased compared with FK506-treated control cells (Fig. 10B). Representative tracings for cell length vs. time and calcium fluorescence vs. time further demonstrate these defects in calcium release and fractional shortening in the cKO cells and also show the rescuing effects of FK506 (Fig. 10, C and D). Since FK506 also inhibits the activity of calcineurin, we treated cells with cyclosporin A, which inhibits calcineurin but does not affect FKBP12.6. As shown in Fig. 11, cyclosporin A treatment did not rescue the defects in calcium transient and fractional shortening observed in the KO myocytes. These findings demonstrate that regulation of FKBP12.6 activity has profound effects in this model of hypertrophy and heart failure, that alterations in FKBP12.6 expression likely explain the observed EC coupling defects, and that inhibition of FKBP12.6 activity can restore contractile function to normal levels in KO cardiac myocytes.
Fig. 10.
FK506 treatment restores contractile function in CHF1/Hey2 cKO myocytes. Cells were isolated from 12-wk-old cKO and floxed allele mice that had no evidence of LV hypertrophy or heart failure. Cells were loaded with the calcium fluorophore fura-2, treated with FK506 (10 μM) for at least 10 min, and stimulated electrically. Cell shape changes and calcium fluxes were tracked optically using a commercially available system (Ionoptix). A: calcium transient by fura-2 (peak height). B: fractional shortening. C: calcium fluorescence vs. time tracing per cycle for control and cKO myocytes treated with either vehicle or FK506. D: cell length vs. time tracing per cycle for control and cKO myocytes treated with either vehicle or FK506. Values are means ± SE; control/vehicle: n = 15; control/FK506: n = 22; cKO/vehicle: n = 20; cKO/FK506: n = 21.
Fig. 11.
Cyclosporin A treatment does not rescue contractile function in CHF1/Hey2 KO myocytes. Cells were isolated from 12-wk-old myocardial KO and floxed allele mice that had no evidence of LV hypertrophy or heart failure. Cells were loaded with the calcium fluorophore fura-2, treated with cyclosporin A (0.2 μM) for 20 min, and stimulated electrically. Cell shape changes and calcium fluxes were tracked optically using a commercially available system (Ionoptix). A: calcium transient by fura-2 (peak height). B: fractional shortening. C: calcium fluorescence vs. time tracing per cycle for control and cKO myocytes treated with either vehicle or cyclosporin A. D: cell length vs. time tracing per cycle for control and cKO myocytes treated with either vehicle or cyclosporin A. Values are means ± SE.
DISCUSSION
Our laboratory has previously reported that expression levels of CHF1/Hey2 profoundly affect the response of the heart to hypertrophic stimulation and control the development of physiological vs. pathological hypertrophy (19, 42). These studies have shown that CHF1/Hey2 affects the development of fibrosis and apoptosis in the heart, but they have also shown that many other potential pathways are affected (42, 43). A limitation of earlier studies is that many of the differences observed between wild-type and KO hearts were made after treatment with hypertrophic stimuli and may reflect downstream effects rather than causal changes.
In this study, we have found that CHF1/Hey2 loss of function leads to alterations in EC coupling that occur before any hypertrophic stimulus and before the development of clinically detectable hypertrophy or heart failure. Specifically, we find that loss of CHF1/Hey2 in cardiac muscle cells results in defective myocardial calcium release and diminished fractional shortening, while calcium stores and calcium sensitivity are normal, suggesting that CHF1/Hey2 fundamentally regulates calcium cycling. Interestingly, these cellular defects are readily detected in cells from our cKO mice before they manifest any defects in contraction in vivo and even before any changes are detectable with dobutamine challenge. We have not detected any decrement in ventricular function up until at least 12 wk of age, but others have reported that a similar myocardial deletion leads to spontaneous cardiomyopathy at ∼6 wk of age (40), and our laboratory has previously reported that global KO mice develop cardiomyopathy at ∼17 wk of age (28). Since the targeting construct and the genetic background (C57BL/6) in this study are similar, the likely explanation for this discrepancy is the use of different myocardial-specific promoters driving Cre recombinase expression. The Nkx2–5 Cre strain used in the study of Xin et al. (40) is likely to delete CHF1/Hey2 at an earlier developmental stage, which may lead to a more severe myocardial defect. Nevertheless, our observation that alterations in calcium cycling exist at baseline before the onset of heart failure provides a fundamental mechanism that can explain the previously published studies, demonstrating an association between CHF1/Hey2 loss of function and the development of hypertrophy and heart failure. Furthermore, our demonstration of the exaggerated hypertrophic and heart failure response to pressure overload in cKO mice supports the hypothesis that these alterations in calcium cycling predispose to hypertrophy and heart failure. We propose that a fundamental defect in EC coupling leads to excessive hypertrophy and ultimately myocardial failure in response to hemodynamic stress. The dichotomous observation that LV function is preserved in vivo at baseline and after dobutamine infusion, but isolated cKO myocytes are dysfunctional in vitro, is likely accounted for by the subtle nature of the defect that is only manifest at the cellular level and likely compensated for at the organ level. Our finding that these mice rapidly develop hypertrophy and heart failure after aortic banding is consistent with an EC coupling defect that is only apparent after a provocative stimulus.
Our finding that FKBP12.6 expression is elevated in cKO myocytes and inhibits contractile function is consistent with a role for FKBP12.6 in regulation of EC coupling. In a prior publication, it has been postulated that the role of FKBP12.6 in heart failure is to decrease diastolic calcium leakage, thereby preserving intracellular calcium stores and mitigating the heart failure phenotype, based on viral overexpression that resulted in an increase in contractility in vitro (26). FKBP12.6 has also been shown to affect calcium-induced calcium release, however, and overexpression in vivo has been shown to decrease calcium transients in isolated myocytes, thereby decreasing EC coupling gain (6). Loss of function has been associated with hypertrophy in male mice, increased amplitude and duration of calcium sparks, and increased calcium-induced calcium release, resulting in greater myocyte contraction (39). Our findings are more consistent with these studies demonstrating that FKBP12.6 expression level affects EC coupling in vivo, rather than affecting diastolic calcium leak and overall calcium stores. Although the increase in expression in the cKO is modest (approximately twofold), a significant effect is expected, given the recent report that, although only 10–20% of RyR2 is bound by FKBP12.6, all FKBP12.6 is bound to RyR2 and is sufficient to inhibit overall activity at that level of binding (8).
A potential confounder of our study is that FK506 has also been shown to inhibit the activity of calcineurin, thereby affecting the activity of nuclear factor of activated T cell transcription factors and the development of cardiac hypertrophy (23, 37). While we cannot formally exclude the possibility that effects of FK506 in our study may be mediated in part by effects on calcineurin rather than the interaction between FKBP12.6 and RyR2, this possibility is unlikely, in light of our finding that cyclosporin A, an inhibitor of calcineurin, did not rescue the calcium transient and fractional shortening defects in cKO myocytes. This finding is consistent with an earlier study showing that treatment of isolated rat ventricular myocytes with either cyclosporin A or FK506 inhibited calcineurin activity, but only FK506 affected the calcium transient, indicating that the effect of FK506 on ventricular myocyte calcium release is not mediated by calcineurin (5). Furthermore, loss of calcineurin activity is associated with decreased contractility (21, 32), which is opposite to the observed effect of FK506 in our study.
Another limitation of our study is that we have not yet demonstrated rescue of the heart failure phenotype by FKBP12.6 inhibition in vivo. In an attempt to rescue the heart failure observed in the myocardial KO mice after aortic banding, we infused either FK506 (10 mg·kg−1·day−1) or vehicle by osmotic minipump at the time of banding, but did not see any improvement in LVEF after 1 wk (data not shown). This finding is difficult to interpret, however, as we have no way of measuring the degree of inhibition of FKBP12.6 in vivo. Our present study is significant, however, in that it is the first to link CHF1/Hey2 to transcriptional regulation of FKBP12.6 and regulation of EC coupling gain in experimental heart failure. Our hope is that therapies based on the regulation of FKBP12.6 by CHF1/Hey2 will lead to improvements in the management of human heart failure.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant HL081088 to M. T. Chin. This work was also supported in part by an National Institute of Environmental Health Sciences Discover Grant P50ES015915. The invasive hemodynamic experiments were performed by Dr. Elina Minami though the NIH sponsored Mouse Metabolic Phenotyping Core at the University of Washington. F. Moussavi-Harami was supported by NIH Training Grant T32 HL07828.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.L., F.S.K., F.M.-H., M.Y., and M.V.R. performed experiments; Y.L., F.S.K., F.M.-H., M.Y., M.V.R., M.R., and M.T.C. analyzed data; Y.L., F.S.K., F.M.-H., M.Y., M.V.R., M.R., and M.T.C. interpreted results of experiments; Y.L., F.S.K., F.M.-H., M.Y., and M.T.C. prepared figures; Y.L., F.S.K., F.M.-H., M.V.R., M.R., and M.T.C. edited and revised manuscript; Y.L., F.S.K., F.M.-H., M.Y., M.V.R., M.R., and M.T.C. approved final version of manuscript; M.R. and M.T.C. conception and design of research; M.T.C. drafted manuscript.
REFERENCES
- 1. Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest 100: 169–179, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chien KR, Ross J, Jr, Hoshijima M. Calcium and heart failure: the cycle game. Nat Med 9: 508–509, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Chin MT, Maemura K, Fukumoto S, Jain MK, Layne MD, Watanabe M, Hsieh CM, Lee ME. Cardiovascular basic helix loop helix factor 1, a novel transcriptional repressor expressed preferentially in the developing and adult cardiovascular system. J Biol Chem 275: 6381–6387, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Donovan J, Kordylewska A, Jan YN, Utset MF. Tetralogy of Fallot and other congenital heart defects in Hey2 mutant mice. Curr Biol 12: 1605–1610, 2002 [DOI] [PubMed] [Google Scholar]
- 5. duBell WH, Gaa ST, Lederer WJ, Rogers TB. Independent inhibition of calcineurin and K+ currents by the immunosuppressant FK-506 in rat ventricle. Am J Physiol Heart Circ Physiol 275: H2041–H2052, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Gellen B, Fernandez-Velasco M, Briec F, Vinet L, LeQuang K, Rouet-Benzineb P, Benitah JP, Pezet M, Palais G, Pellegrin N, Zhang A, Perrier R, Escoubet B, Marniquet X, Richard S, Jaisser F, Gomez AM, Charpentier F, Mercadier JJ. Conditional FKBP12.6 overexpression in mouse cardiac myocytes prevents triggered ventricular tachycardia through specific alterations in excitation-contraction coupling. Circulation 117: 1778–1786, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Gessler M, Knobeloch KP, Helisch A, Amann K, Schumacher N, Rohde E, Fischer A, Leimeister C. Mouse gridlock: no aortic coarctation or deficiency, but fatal cardiac defects in Hey2−/− mice. Curr Biol 12: 1601–1604, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Guo T, Cornea RL, Huke S, Camors E, Yang Y, Picht E, Fruen BR, Bers DM. Kinetics of FKBP12.6 binding to ryanodine receptors in permeabilized cardiac myocytes and effects on Ca sparks. Circ Res 106: 1743–1752, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7: 589–600, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Hetz C, Lee AH, Gonzalez-Romero D, Thielen P, Castilla J, Soto C, Glimcher LH. Unfolded protein response transcription factor XBP-1 does not influence prion replication or pathogenesis. Proc Natl Acad Sci U S A 105: 757–762, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iso T, Sartorelli V, Chung G, Shichinohe T, Kedes L, Hamamori Y. HERP, a new primary target of Notch regulated by ligand binding. Mol Cell Biol 21: 6071–6079, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koibuchi N, Chin MT. CHF1/Hey2 plays a pivotal role in left ventricular maturation through suppression of ectopic atrial gene expression. Circ Res 100: 850–855, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Kokubo H, Miyagawa-Tomita S, Tomimatsu H, Nakashima Y, Nakazawa M, Saga Y, Johnson RL. Targeted disruption of hesr2 results in atrioventricular valve anomalies that lead to heart dysfunction. Circ Res 95: 540–547, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Kreutziger KL, Piroddi N, Scellini B, Tesi C, Poggesi C, Regnier M. Thin filament Ca2+ binding properties and regulatory unit interactions alter kinetics of tension development and relaxation in rabbit skeletal muscle. J Physiol 586: 3683–3700, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leimeister C, Externbrink A, Klamt B, Gessler M. Hey genes: a novel subfamily of hairy- and enhancer of split related genes specifically expressed during mouse embryogenesis. Mech Dev 85: 173–177, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA 275: 1557–1562, 1996 [PubMed] [Google Scholar]
- 17. Liao R, Jain M, Cui L, D'Agostino J, Aiello F, Luptak I, Ngoy S, Mortensen RM, Tian R. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation 106: 2125–2131, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Lindegger N, Niggli E. Paradoxical SR Ca2+ release in guinea-pig cardiac myocytes after beta-adrenergic stimulation revealed by two-photon photolysis of caged Ca2+. J Physiol 565: 801–813, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Y, Yu M, Wu L, Chin MT. The bHLH transcription factor CHF1/Hey2 regulates susceptibility to apoptosis and heart failure after pressure overload. Am J Physiol Heart Circ Physiol 298: H2082–H2092, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Maillet M, Davis J, Auger-Messier M, York A, Osinska H, Piquereau J, Lorenz JN, Robbins J, Ventura-Clapier R, Molkentin JD. Heart-specific deletion of CnB1 reveals multiple mechanisms whereby calcineurin regulates cardiac growth and function. J Biol Chem 285: 6716–6724, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCall E, Li L, Satoh H, Shannon TR, Blatter LA, Bers DM. Effects of FK-506 on contraction and Ca2+ transients in rat cardiac myocytes. Circ Res 79: 1110–1121, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moreno-Gonzalez A, Korte FS, Dai J, Chen K, Ho B, Reinecke H, Murry CE, Regnier M. Cell therapy enhances function of remote non-infarcted myocardium. J Mol Cell Cardiol 47: 603–613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakagawa O, Nakagawa M, Richardson JA, Olson EN, Srivastava D. HRT1, HRT2, and HRT3: a new subclass of bHLH transcription factors marking specific cardiac, somitic, and pharyngeal arch segments. Dev Biol 216: 72–84, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Prestle J, Janssen PM, Janssen AP, Zeitz O, Lehnart SE, Bruce L, Smith GL, Hasenfuss G. Overexpression of FK506-binding protein FKBP12.6 in cardiomyocytes reduces ryanodine receptor-mediated Ca(2+) leak from the sarcoplasmic reticulum and increases contractility. Circ Res 88: 188–194, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet 25: 139–140, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Sakata Y, Kamei CN, Nakagami H, Bronson R, Liao JK, Chin MT. Ventricular septal defect and cardiomyopathy in mice lacking the transcription factor CHF1/Hey2. Proc Natl Acad Sci U S A 99: 16197–16202, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sakata Y, Koibuchi N, Xiang F, Youngblood JM, Kamei CN, Chin MT. The spectrum of cardiovascular anomalies in CHF1/Hey2 deficient mice reveals roles in endocardial cushion, myocardial and vascular maturation. J Mol Cell Cardiol 40: 267–273, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Sakata Y, Xiang F, Chen Z, Kiriyama Y, Kamei CN, Simon DI, Chin MT. Transcription factor CHF1/Hey2 regulates neointimal formation in vivo and vascular smooth muscle proliferation and migration in vitro. Arterioscler Thromb Vasc Biol 24: 2069–2074, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Sambrano GR, Fraser I, Han H, Ni Y, O'Connell T, Yan Z, Stull JT. Navigating the signalling network in mouse cardiac myocytes. Nature 420: 712–714, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Schaeffer PJ, Desantiago J, Yang J, Flagg TP, Kovacs A, Weinheimer CJ, Courtois M, Leone TC, Nichols CG, Bers DM, Kelly DP. Impaired contractile function and calcium handling in hearts of cardiac-specific calcineurin b1-deficient mice. Am J Physiol Heart Circ Physiol 297: H1263–H1273, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Su Z, Sugishita K, Li F, Ritter M, Barry WH. Effects of FK506 on [Ca2+]i differ in mouse and rabbit ventricular myocytes. J Pharmacol Exp Ther 304: 334–341, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics 16: 349–360, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Wachtell K, Okin PM, Olsen MH, Dahlof B, Devereux RB, Ibsen H, Kjeldsen SE, Lindholm LH, Nieminen MS, Thygesen K. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: the LIFE Study. Circulation 116: 700–705, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Watanabe T, Koibuchi N, Chin MT. Transcription factor CHF1/Hey2 regulates coronary vascular maturation. Mech Dev 127: 418–427, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun 322: 1178–1191, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Xiang F, Sakata Y, Cui L, Youngblood JM, Nakagami H, Liao JK, Liao R, Chin MT. Transcription factor CHF1/Hey2 suppresses cardiac hypertrophy through an inhibitory interaction with GATA4. Am J Physiol Heart Circ Physiol 290: H1997–H2006, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xin HB, Senbonmatsu T, Cheng DS, Wang YX, Copello JA, Ji GJ, Collier ML, Deng KY, Jeyakumar LH, Magnuson MA, Inagami T, Kotlikoff MI, Fleischer S. Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature 416: 334–338, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Xin M, Small EM, van Rooij E, Qi X, Richardson JA, Srivastava D, Nakagawa O, Olson EN. Essential roles of the bHLH transcription factor Hrt2 in repression of atrial gene expression and maintenance of postnatal cardiac function. Proc Natl Acad Sci U S A 104: 7975–7980, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol 42: 441–448, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu M, Liu Y, Xiang F, Li Y, Cullen D, Liao R, Beyer RP, Bammler TK, Chin MT. CHF1/Hey2 promotes physiological hypertrophy in response to pressure overload through selective repression and activation of specific transcriptional pathways. OMICS 13: 501–511, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu M, Xiang F, Beyer RP, Farin FM, Bammler TK, Chin MT. Transcription factor CHF1/Hey2 regulates specific pathways in serum stimulated primary cardiac myocytes: implications for cardiac hypertrophy. Curr Genomics 11: 287–296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhu WZ, Santana LF, Laflamme MA. Local control of excitation-contraction coupling in human embryonic stem cell-derived cardiomyocytes. PLos One 4: e5407, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]