Abstract
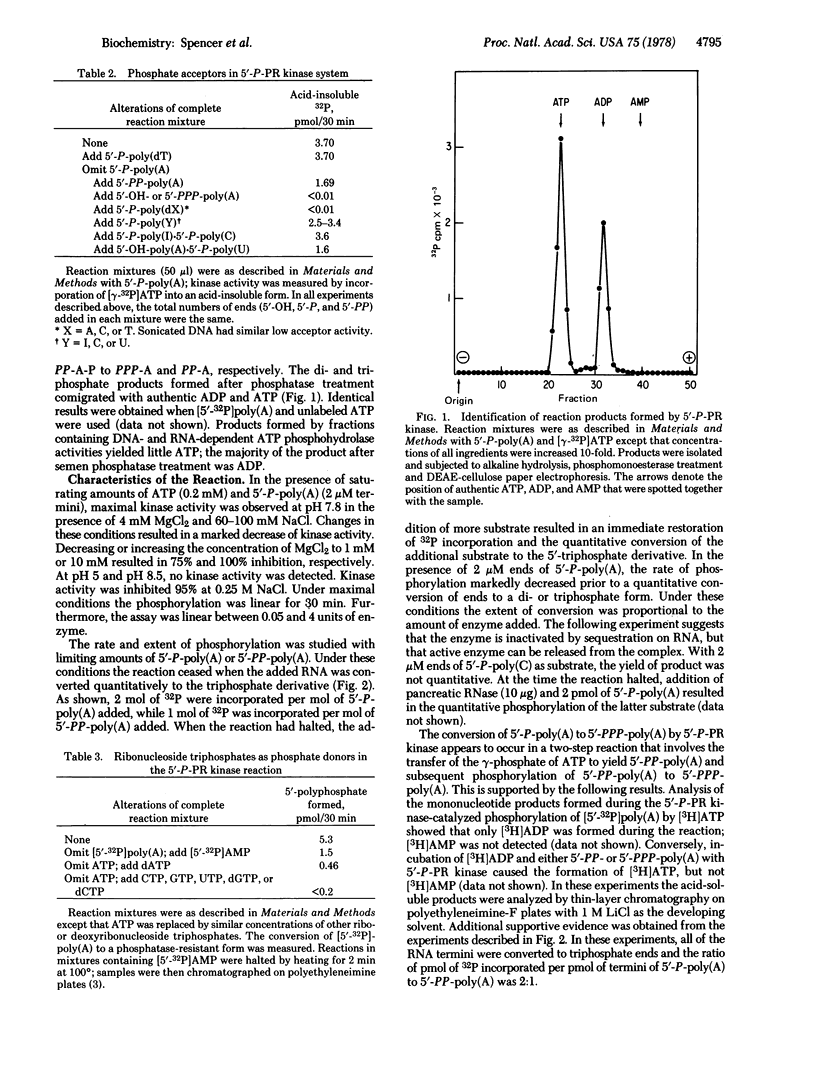
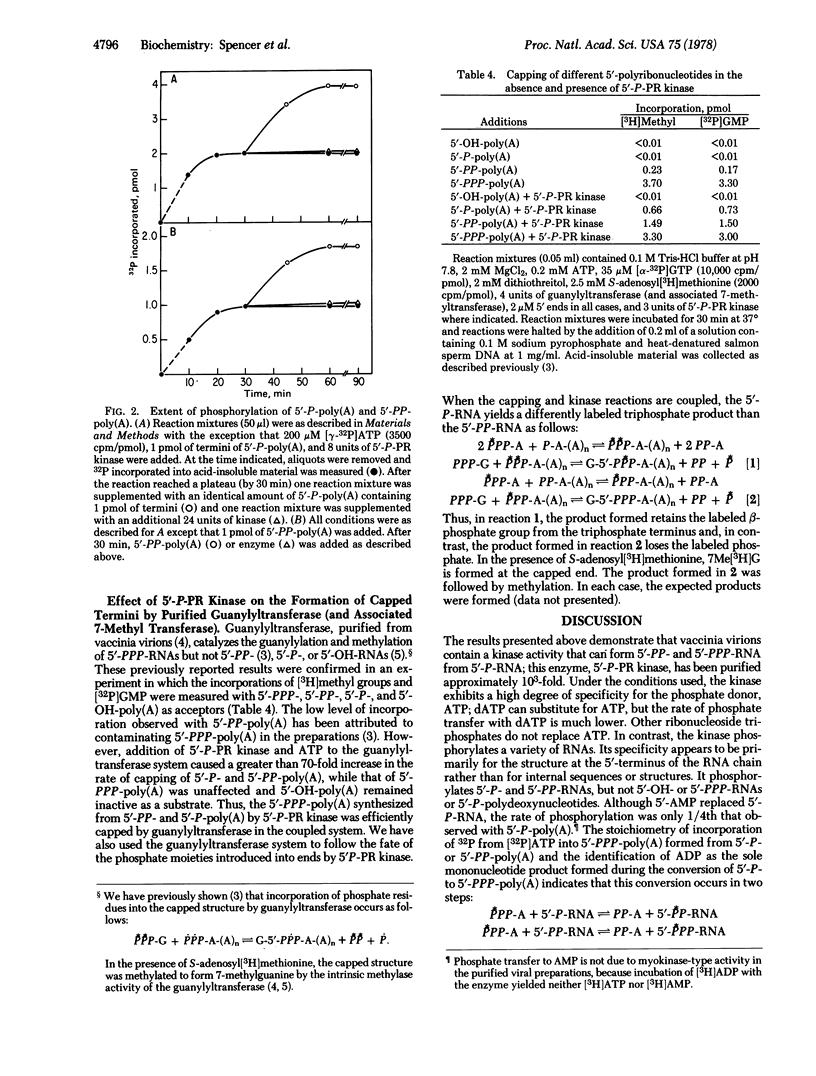
We have isolated from vaccinia virus cores an enzyme, 5'-phosphate-polyribonucleotide kinase, that in the presence of ATP and Mg2+ catalyzes the conversion of 5'-phosphate and 5'-diphosphate termini of RNA to the 5'-triphosphate species. With the exception of dATP, other nucleoside triphosphates were inactive as phosphate donors; activity with dATP was 10% of that observed with ATP. The purified enzyme did not phosphorylate 5'-hydroxyl- or 5'-monophosphate-terminated polydeoxyribonucleotides, although a variety of 5'- monophosphate-terminated RNA chains were active as phosphate acceptors. By using a coupled system of 5'-phosphate-polyribonucleotide kinase and guanylyltransferase in the presence of ATP, GTP, Mg2+, and S-adenosylmethionine, capping of 5'-P-, 5'-PP-, and 5'-PPP-RNA was demonstrated; in the absence of 5'-phosphate-polyribonucleotide kinase only 5'-PPP-RNA was capped by guanylyltransferase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Heil A., Zillig W. Reconstitution of bacterial DNA-dependent RNA-polymerase from isolated subunits as a tool for the elucidation of the role of the subunits in transcription. FEBS Lett. 1970 Dec;11(3):165–168. doi: 10.1016/0014-5793(70)80519-1. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Knapp G., Beckmann J. S., Johnson P. F., Fuhrman S. A., Abelson J. Transcription and processing of intervening sequences in yeast tRNA genes. Cell. 1978 Jun;14(2):221–236. doi: 10.1016/0092-8674(78)90109-5. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Paoletti E., Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9322–9329. [PubMed] [Google Scholar]
- Monroy G., Spencer E., Hurwitz J. Characteristics of reactions catalyzed by purified guanylyltransferase from vaccinia virus. J Biol Chem. 1978 Jun 25;253(12):4490–4498. [PubMed] [Google Scholar]
- Monroy G., Spencer E., Hurwitz J. Purification of mRNA guanylyltransferase from vaccinia virions. J Biol Chem. 1978 Jun 25;253(12):4481–4489. [PubMed] [Google Scholar]
- O'Farrell P. Z., Cordell B., Valenzuela P., Rutter W. J., Goodman H. M. Structure and processing of yeast precursor tRNAs containing intervening sequences. Nature. 1978 Aug 3;274(5670):438–445. doi: 10.1038/274438a0. [DOI] [PubMed] [Google Scholar]
- Paolette E., Rosemond-Hornbeak H., Moss B. Two nucleid acid-dependent nucleoside triphosphate phosphohydrolases from vaccinia virus. Purification and characterization. J Biol Chem. 1974 May 25;249(10):3273–3280. [PubMed] [Google Scholar]
- Paoletti E. High molecular weight virion-associated RNA of vaccinia. A possible precursor to 8 to 12 S mRNA. J Biol Chem. 1977 Feb 10;252(3):872–877. [PubMed] [Google Scholar]
- Perry R. P., Bard E., Hames B. D., Kelly D. E., Schibler U. The relationship between hnRNA and mRNA. Prog Nucleic Acid Res Mol Biol. 1976;19:275–292. doi: 10.1016/s0079-6603(08)60925-3. [DOI] [PubMed] [Google Scholar]
- WITTENBERG J., KORNBERG A. Choline phosphokinase. J Biol Chem. 1953 May;202(1):431–444. [PubMed] [Google Scholar]


