Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is a common inherited disease characterized by massive enlargement of fluid-filled cysts in the kidney. However, there is no effective therapy yet for this disease. To examine whether ginkgolide B, a natural compound, inhibits cyst development, a Madin-Darby canine kidney (MDCK) cyst model, an embryonic kidney cyst model, and a PKD mouse model were used. Interestingly, ginkgolide B significantly inhibited MDCK cyst formation dose dependently, with up to 69% reduction by 2 μM ginkgolide B. Ginkgolide B also significantly inhibited cyst enlargement in the MDCK cyst model, embryonic kidney cyst model, and PKD mouse model. To determine the underlying mechanisms, the effect of ginkgolide B on MDCK cell viability, proliferation, apoptosis, chloride transporter CFTR activity, and intracellular signaling pathways were also studied. Ginkgolide B did not affect cell viability, proliferation, and expression and activity of the chloride transporter CFTR that mediates cyst fluid secretion. Ginkgolide B induced cyst cell differentiation and altered the Ras/MAPK signaling pathway. Taken together, our results demonstrate that ginkgolide B inhibits renal cyst formation and enlargement, suggesting that ginkgolide B might be developed into a novel candidate drug for ADPKD.
Keywords: natural product, ADPKD, MAPK
autosomal dominant polycystic kidney disease (ADPKD) is a common inherited kidney disease with an incidence rate of 1:1,000, afflicting 5–6 million people worldwide. Cysts in ADPKD compromise normal renal parenchyma and cause renal failure. There is no effective therapy as yet to stop renal cyst development, and hemodialysis or kidney transplantation appears to be the only option available for ADPKD patients at end stage.
ADPKD is mainly caused by mutations in one of two genes, Pkd1 and Pkd2, encoding the proteins polycystin-1 (PC1) and polycystin-2 (PC2), respectively (10, 17). It has been reported that the mutant PC1 or PC2 leads to the reduction of intracellular Ca2+ and the augmentation of intracellular cAMP. Increased intracellular cAMP stimulates cyst epithelial cell proliferation and fluid secretion (35). Previous studies have demonstrated that the abnormal proliferation of cyst epithelial cells is mediated through multiple intracellular signaling pathways, including the MAPK pathway, mammalian target of rampamycin (mTOR) pathway, and Wnt pathway (3, 32). Additionally, cyst fluid secretion mainly depends on CFTR function in cyst epithelia (45).
Recently, a number of chemical compounds have been developed to treat ADPKD (27, 33). Somatostatin or its analog octreotide inhibits cyst expansion by suppressing the cAMP accumulation not only in the kidney but also in the liver in vitro and in vivo (22, 30). Recent studies by our group and others also demonstrated that curcumin, a polyphenol diferuloylmethane, inhibits renal cyst development in vitro and in vivo (7, 15). Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease markedly by inhibiting CFTR-mediated cystic fluid secretion (45). Others that have shown cyst inhibition effects in ADPKD orthologous animal models, including tolvaptan, src inhibitors, pioglitazone, etanercept, and triptolide (16, 18, 28). Ginkgolide B, a natural product isolated from the leaves of Ginkgo biloba, traditionally used in Chinese medicine, exerts multiple biological activities, including anti-inflammation (13), antiallergy, antioxidation, anticancer, and neuroprotection (11, 14, 38). In the present study, we examined whether ginkgolide B inhibits renal cyst development using an in vitro Madin-Darby canine kidney (MDCK) cyst model, embryonic kidney cyst model, and in vivo PKD mouse model. These cyst models have been used for screening candidate inhibitors of cyst formation and growth and exploring the inhibition mechanism (34, 37, 45). We found that ginkgolide B significantly inhibited renal cyst formation and growth.
METHODS
Ginkgolide B (G6910, Sigma), forskolin (F6886, Sigma), and 8-bromo (Br)-cAMP (B-1381, Sigma) were dissolved, respectively, in 100% DMSO to prepare a 100 mM stock solution and were stored at −20°C. Anti-H-Ras (sc-35), anti-B-Raf (sc-166), anti-Raf-1 (sc-227), anti-p-MEK-1/2 (sc-7995), anti-p-ERK (sc-7383), anti-ERK2 (sc-153), anti-Egr-1 (sc-110), anti-actin (sc-1615-R), donkey anti-goat IgG (sc-2020), and goat anti-rabbit IgG (sc-2004) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Goat anti-mouse IgG and goat anti-rat IgG were purchased from Sigma.
MDCK cyst model.
Type I MDCK cells (ATCC no. CCL-34) were cultured in an atmosphere of 5% CO2-95% air at 37°C in a 1:1 mixture of DMEM and Ham's F-12 nutrient medium supplemented with 10% fetal bovine serum (Hyclone), 100 U/ml penicillin, and 100 μg/ml streptomycin. For cyst generation, 400 MDCK cells were suspended in 0.4 ml of ice-cold MEM containing 2.9 mg/ml collagen (PureCol, Inamed Biomaterials, Fremont, CA), 10 mM HEPES, 27 mM NaHCO3, 100 U/ml penicillin, and 100 μg/ml streptomycin (pH 7.4). The cell suspension was plated onto 24-well plates. After incubation for ∼90 min at 37°C, collagen gel solutions were freeze-dried. Then, 1.5 ml of MDCK cell medium containing 10 μM forskolin was added to each well, and plates were maintained in a humidified atmosphere of 5% CO2-95% air at 37°C.
To evaluate the inhibition of ginkgolide B on cyst formation using the MDCK cyst model, 0.125, 0.5, and 2 μM ginkgolide B were added, respectively, in the culture medium with 10% fetal bovine serum in the presence of 10 μM forskolin from day 0 to day 6. The medium containing forskolin and ginkgolide B was changed every 12 h. On day 6, cysts (with diameters >50 μm) and noncyst cell colonies were counted by phase-contrast light microscopy. To study the effect of ginkgolide B on MDCK cyst growth, the established cysts (on day 4 cultured with forskolin) were exposed to different concentrations of ginkgolide B in the presence of forskolin from day 4 to day 12. The medium with 10% fetal bovine serum containing forskolin and ginkgolide B was changed every 12 h for 8 days. Furthermore, to test the reversibility of the inhibition on cyst growth by ginkgolide B, the established cysts were exposed to forskolin and ginkgolide B from day 4 to day 8, and then ginkgolide B was removed from day 8 to day 12. Micrographs showing the same cysts in collagen gels (identified by markings on plates) were obtained every 2 days. Cyst diameters were measured using Image-Pro Plus 6.0 to determine the growth rate of cysts. At least 10 cysts/well and 3 wells/group were measured for each condition.
Embryonic kidney cyst model.
Mouse embryonic kidneys at embryonic day 13.5 (E13.5) were dissected and placed on transparent Falcon 0.4-μM diameter porous cell culture inserts as described previously (20). The lower chambers were filled with a 1:1 mixture of DMEM/Ham's F-12 nutrient medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 10 mM HEPES, 5 μg/ml insulin, 5 μg/ml transferrin, 2.8 nM selenium, 25 ng/ml prostaglandin E, 32 pg/ml T3, 250 U/ml penicillin, and 250 μg/ml streptomycin. Medium was replaced every 12 h. As indicated, 100 μM 8-Br-cAMP and ginkgolide B were added. Kidneys were photographed using a Nikon inverted microscope (Nikon TE 2000-S) equipped with ×2 objective lens, 520-nm band-pass filter, and high-resolution PixeLINK color CCD camera. Cyst area was calculated by dividing the total cyst area by total kidney area.
Pkd1/Ksp-Cre mouse model of ADPKD.
Pkd1flox mice (from Yale PKD Center) and Ksp-Cre transgenic mice (from UT Southwestern O'Brien Center) in a C57BL/6 background were generated as described previously (32). Ksp-Cre mice express Cre recombinase under the control of the Ksp-cadherin promoter. Pkd1−/−;Ksp-Cre mice were generated by cross-breeding Pkd1flox/flox mice with Pkd1+/−:Ksp-Cre mice. Neonatal mice (age 1 day) were genotyped by genomic PCR. Ginkgolide B (16 mg·kg−1·day−1) or a saline DMSO vehicle control (0.05 ml/injection) was administrated by subcutaneous injection on the back of neonatal mice two times a day using a 1-ml insulin syringe, beginning at age 1 day (5 mice/group). Pkd1flox/+;Ksp-Cre or Pkd1flox/− mice from the same litter were used as wild-type. Body weight was measured at the age of 4 days. Kidneys were removed and weighed and fixed for histological examination. Protocols were approved by the Peking University Health Center Committee on Animal Research.
MDCK tubule model.
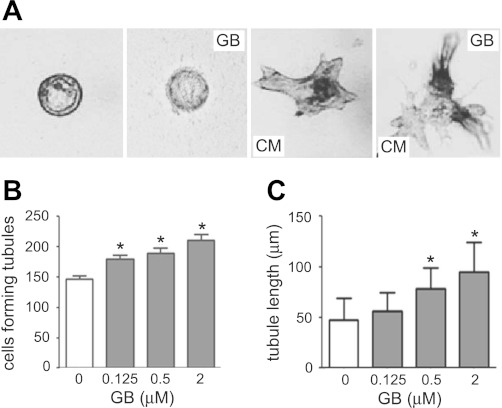
To determine whether ginkgolide B promotes MDCK cell to form tubules, MDCK cells were cultured in 3T3 conditioned medium (3T3 CM) with increasing doses of ginkgolide B (at 0.125, 0.5, or 2 μM) for 12 days. The medium containing ginkgolide B was changed every 12 h. On day 12, the numbers of cells forming tubule-like structures were counted at 20–35 random sites in each culture dish using phase-contrast light microscopy. Furthermore, to examine whether ginkgolide B induces tubulogenesis from MDCK cysts formed within three-dimensional collagen gels, the cysts were established by exposing them to forskolin for 4 days as mentioned above. Then, the medium was replaced with fresh 3T3 CM with or without different concentrations of ginkgolide B over the next 8 days. Tubules were monitored and photographed every 2 days. On day 12, the length of the longest tubule from each cyst was measured by Image-Pro Plus 6.0.
Cytotoxicity and apoptosis.
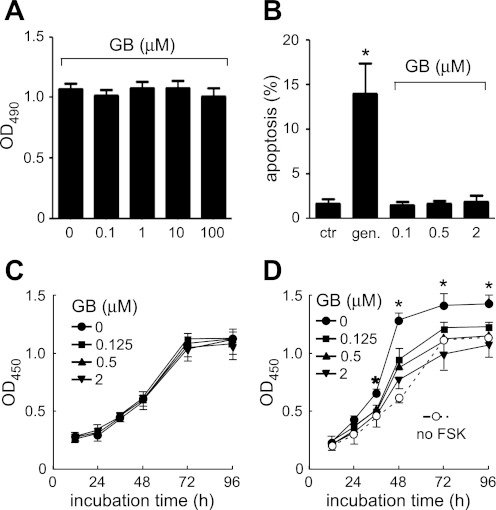
An MTT assay was used to assess ginkgolide B cytotoxicity. MDCK cells in a 96-well plate were exposed to ginkgolide B at 0, 0.1, 1, 10, or 100 μM for 24 h. Twenty microliters of MTT solution (5 mg/ml) was added and incubated for 4 h at 37°C. The medium was then removed, and 150 μl DMSO was added. The absorbance at 490 nm was measured. Cell viability was shown as the OD490 value.
The in situ cell death detection kit (Roche Diagnostics, Indianapolis, IN) was used to measure apoptosis. MDCK cells were seeded on eight-chamber polystyrene tissue culture-treated glass slides and incubated with ginkgolide B at 0.125, 0.5, or 2 μM. Gentamycin (2 mM) was used as a positive control. Three days later, the assay was performed according to the manufacturer's instructions. Five microscopic fields were analyzed per condition. The apoptotic index was calculated as follows: apoptotic index (%) = (apoptotic cell number/total cell number) × 100.
Cell proliferation.
Cell Counting Kit-8 (CCK-8) was used to assay the proliferation of MDCK cells incubated with or without ginkgolide B in the absence and presence of 10 μM forskolin at indicated time points (12, 24, 36, 48, 72, and 96 h). MDCK cells (1,000 cells·100 μl−1·well−1) in a 96-well plate were cultured in a 37°C humidified 5% CO2 incubator. Each well was used with 10 μl CCK-8 solution and was incubated for 1 h. The absorbance at 450 nm was measured. The cell proliferation rate was expressed as the OD450 value.
cAMP measurement.
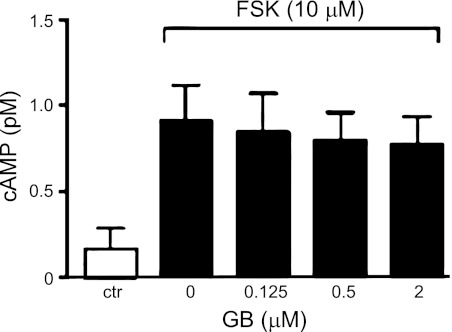
MDCK cells cultured in six-well plates were exposed to ginkgolide B (at 0.125, 0.5, or 2 μM) for 30 min with or without 10 μM forskolin stimulation. Intracellular cAMP content was determined by RIA following the procedure recommended in the cAMP RIA kit (Chinese People's Liberation Army General Hospital, Beijing, China).
CFTR function assay.
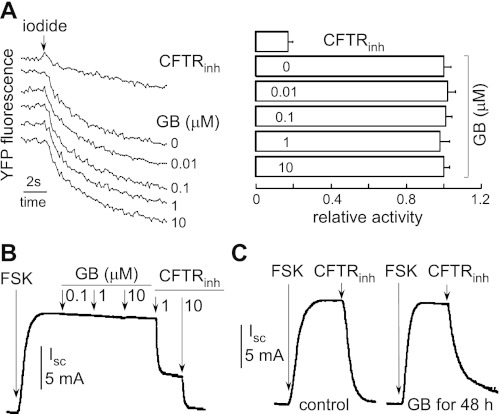
Fischer rat thyroid (FRT) epithelial cells stably coexpressing human CFTR and the high-sensitivity I−-sensing green fluorescent protein YFP-H148Q/I152L were used as described previously (6). At the time of the assay, cells were washed with PBS and then incubated with PBS containing forskolin (20 μM) and ginkgolide B (at 0.01, 0.1, 1, or 10 μM) for 20 min. Measurements were carried out using FLUOstar fluorescence plate readers (Optima; BMG LABTECH), equipped with 500 ± 10-nm excitation and 535 ± 15-nm emission filters (Chroma Technology). Each well was assayed individually for I− influx by recording fluorescence continuously (200 ms/point) for 2 s (baseline) and then for 12 s after rapid (<1 s) addition of 165 μl PBS in which 137 mM Cl− was replaced by I−. The I− influx rate was computed by fitting the final 11.5 s of the data to an exponential for extrapolation of initial slope and normalizing for background-subtracted initial fluorescence. All experiments contained negative control (DMSO vehicle) and positive control CFTRinh172.
Short-circuit current measurements.
MDCK cells in Snapwell inserts (transepithelial resistance 1,000–2,000 Ω) were cultured in media containing ginkgolide B (at 0.1, 1, or 10 μM) for 1 or 48 h. Ginkgolide B was washed out with medium 1 h before short-circuit current measurements. Snapwell inserts containing MDCK cells were mounted on a standard Ussing chamber system. Permeabilization reagent (250 μg/ml amphotericin B) was added on the basolateral membrane of the insert. The hemichambers were filled with 5 ml of 65 mM NaCl, 65 mM Na-gluconate, 2.7 mM KCl, 1.5 mM KH2PO4, 1 mM CaCl2, 0.5 mM MgCl2, Na-HEPES, and 10 mM glucose (apical), and 130 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 1 mM CaCl2, 0.5 mM MgCl2, Na-HEPES, and 10 mM glucose (basolateral) (pH 7.3). Short-circuit current was recorded continuously using a DVC-1000 voltage clamp (World Precision Instruments, Sarasota, FL) with Ag/AgCl electrodes and 1 M KCl agar bridges.
Western blotting.
MDCK cells were seeded in 6-well plates in a 1:1 mixture of DMEM and Ham's F-12 nutrient medium containing 10% FBS for 2 h, followed by serum starvation for 24 h. Then, MDCK cells were exposed to medium containing ginkgolide B (at 0.125, 0.5, or 2 μM) for 1 h with or without 10 μM forskolin stimulation. Western blot analysis was performed as described in the manufacturer's instructions (Santa Cruz Biotechnology).
Statistical analysis.
Results were expressed as means ± SD. Statistical analysis was performed by one-way ANOVA. A level of P < 0.05 was considered to be significant.
RESULTS
Ginkgolide B inhibits MDCK cyst formation and growth.
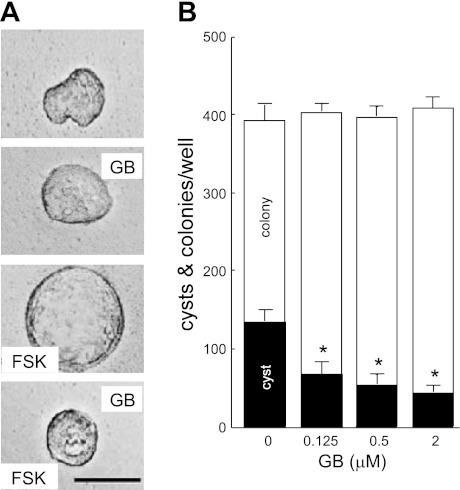
An MDCK cyst model was used to evaluate the effects of ginkgolide B on cyst formation. MDCK cells did not form cysts but grew into colonies in the absence of forskolin without or with ginkgolide B (Fig. 1A, top two panels). In the presence of 10 μM forskolin, however, cysts were seen on day 4 and progressively expanded over the next 8 days (Fig. 1A, third panel). When MDCK cells were incubated with ginkgolide B (at 0.125, 0.5, or 2 μM) in the presence of 10 μM forskolin for 6 days, cyst formation was significantly inhibited (Fig. 1A, bottom panel), and this effect of ginkgolide B was dose dependent with an inhibition by up to 69% at 2 μM ginkgolide B (Fig. 1B). The numbers of total colonies (cysts with diameter >50 μm plus noncyst colonies) were similar to the original seeded cell numbers in all groups, indicating that ginkgolide B was not toxic to MDCK cells.
Fig. 1.
Ginkgolide B (GB) inhibits Madin-Darby canine kidney (MDCK) cell cyst formation. A: representative light micrographs of MDCK cells cultured in collagen gels. Light micrographs were taken on day 6 after cell seeding. MDCK cells were cultured without forskolin (FSK; top) or with 2 μM GB and without FSK (the second from top) or with 10 μM FSK (third from top) or 10 μM forskolin plus 2 μM GB (bottom). Scale bar = 50 μm. B: MDCK cyst formation rate. Open bars show the total numbers of colonies (cysts colonies plus noncyst colonies) per well on day 6 after MDCK cells were incubated without (control) or with GB at indicated concentrations in the presence of 10 μM FSK. Filled bars show the numbers of cysts with a diameter >50 μm (means ± SD; n = 3). *P < 0.05 vs. control.
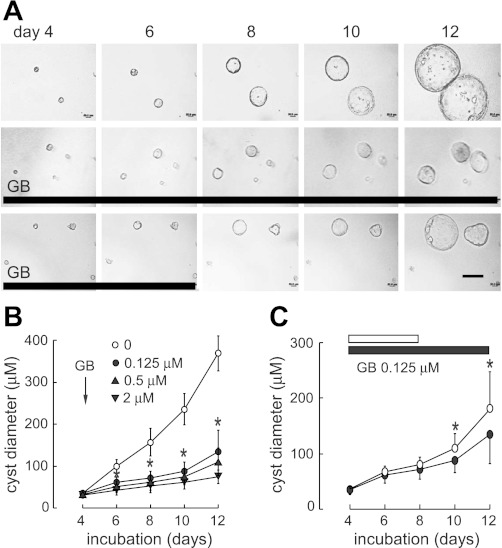
To determine the inhibition of ginkgolide B on cyst enlargement, the established cysts (on day 4 cultured with forskolin) were exposed to 0.125, 0.5, or 2 μM ginkgolide B in the presence of 10 μM forskolin. Cysts continuously enlarged with forskolin stimulation (Fig. 2A, top). Ginkgolide B remarkably inhibited cyst enlargement (Fig. 2A, middle, and B). This inhibition was abolished when ginkgolide B was removed after 4-day treatment (Fig. 2A, bottom, and C).
Fig. 2.
GB reversibly inhibits MDCK cyst growth. A: representative light micrographs of MDCK cyst growth in collagen gels. Light micrographs were taken at indicated days after cell seeding. MDCK cells were exposed continuously to 10 μM FSK (top). In some experiments, MDCK cysts (established on day 4 with FSK stimulation) were treated with GB from day 4 to day 12 (middle) or from day 4 to day 8 (bottom) in the presence of FSK. Scale bar = 200 μm. Thick lines indicate the culture time with GB. B: MDCK cell cyst enlargement shown as cyst diameters for GB at indicated concentration (means ± SD; >30 cysts analyzed/time point). *P < 0.05 vs. control. C: inhibition was reversible as shown by exposure to GB from day 4 to day 8 followed by washout (means ± SD, >30 cysts analyzed/time point). White bar and ○ represent GB group treated from day 4 to day 8; black bar and ● represent GB group treated from day 4 to day 12. *P < 0.05 vs. GB group treated from day 4 to day 12.
Ginkgolide B retards cyst development in embryonic kidney cyst model.
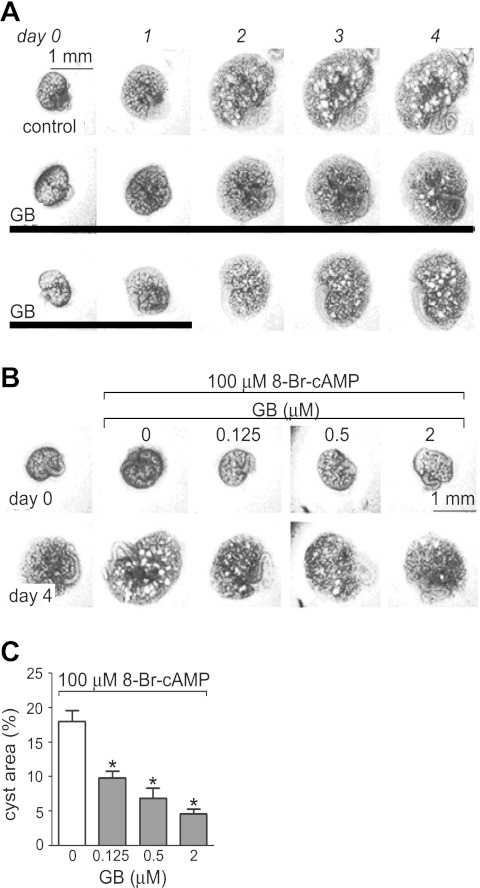
To evaluate the effect of ginkgolide B on renal cyst formation and growth in whole kidney organs, mouse embryonic kidneys at E13.5 were cultured on Transwell filters in the absence or presence of 100 μM 8-Br-cAMP as described previously. In the absence of 8-Br-cAMP, kidneys grew without cyst formation over 4 days, whereas numerous cystic structures were seen in the presence of 8-Br-cAMP as previously described (Fig. 3A, top) (37, 45). Ginkgolide B significantly inhibited cyst formation and growth in the embryonic kidneys (Fig. 3A, middle). Cysts grew and enlarged again following ginkgolide B washout after 2-day treatment, which suggests that the cyst inhibition of ginkgolide B was reversible (Fig. 3A, bottom). Moreover, the inhibition of ginkgolide B on cyst formation and growth was dose dependent (Fig. 3B), as shown by quantitative image analysis (Fig. 3C). Kidney growth with or without 8-Br-cAMP incubation was not affected by ginkgolide B (data not shown).
Fig. 3.
GB retards cyst development in embryonic kidney cyst model. A: representative light micrographs of embryonic kidneys cultured in Transwells and maintained from day 0 to day 4. Embryonic day 13.5 (E13.5) kidneys were exposed to 100 μM 8-bromo (Br)-cAMP as a positive control (top) or were treated with 2 μM GB from day 0 to day 4 (middle) or from day 0 to day 2 (bottom) in the presence of 100 μM 8-Br-cAMP. Each series of photographs shows the same kidney on successive days in culture. Scale bar = 1 mm. Thick solid lines indicate the culture time with GB. B: inhibition of 8-Br-cAMP-induced cyst growth by GB at indicated concentrations. Images show embryonic kidneys before (day 0) and after (day 4) continuous GB treatment. Scale bar = 1 mm. C: fractional cyst area (%) in positive control and GB-treated group (means ± SD; n = 6–10). *P < 0.05 vs. positive control.
Ginkgolide B inhibits renal cyst development in PKD mice.
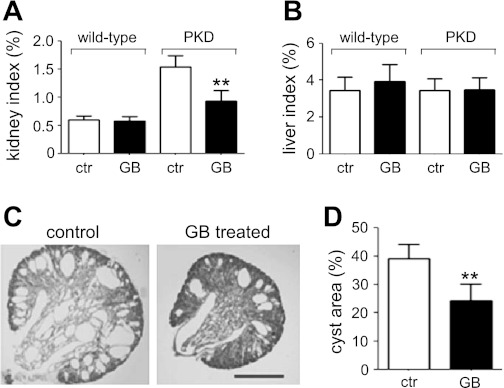
Kidney-specific Pkd1 knockout mice (Pkd1flox/−;Ksp-Cre) were used to determine whether ginkgolide B has any cyst inhibition activity in vivo. Ginkgolide B was subcutaneously injected into mice every 12 h at 16 mg·kg−1·day−1 from day 1 to day 4 of age. During the treatment period, wild-type mice and Pkd1flox/−;Ksp-Cre mice, with or without ginkgolide B treatment, were indistinguishable in their activity and behavior. After the treatment, there was no difference in body weight between the mouse groups (data not shown). Kidney sizes and weights in Pkd1flox/−;Ksp-Cre mice were greater than those in wild-type mice. However, ginkgolide B significantly reduced the kidney sizes and weights in Pkd1flox/−;Ksp-Cre mice (Fig. 4A). Ginkgolide B did not affect the liver sizes and weights in all groups (Fig. 4B). Figure 4C shows representative central coronal kidney sections. Kidney sections from ginkgolide B-treated Pkd1flox/−;Ksp-Cre mice showed fewer cysts of all sizes than those in untreated PKD mice. Image analysis of hematoxylin- and eosin-stained sections showed remarkably smaller fractional cyst areas per kidney in ginkgolide B-treated PKD mice compared with untreated PKD mice (Fig. 4D).
Fig. 4.
GB slows cyst growth in a Pkd1flox/−;Ksp-Cre mouse model of PKD. A: kidney weight indexes (age 4 days) of non-PKD mice (denoted wild-type) and Pkd1flox/−;Ksp-Cre mice treated for 3 days with vehicle (control) or GB (means ± SD; 5 mice per group). *P < 0.01. B: liver weight indexes of the same mice as in A. C: representative central coronal kidney sections from Pkd1flox/−;Ksp-Cre mice treated for 3 days with vehicle (left) or GB (16 mg·kg−1·day−1; right). Scale bar = 1 mm. D: fractional cyst area (%) in vehicle and GB-treated Pkd1flox/−;Ksp-Cre mice (means ± SD; n = 5). **P < 0.01 vs. control.
Ginkgolide B promotes tubule formation from MDCK cells and cysts.
Epithelial cells in ADPKD are less than terminally differentiated and continuously to proliferate (4, 39). The MDCK tubule model was used to study whether ginkgolide B promoted the differentiation of cyst epithelial cells. No tubule-like structure was formed from MDCK cells and cysts in the absence of 3T3 CM without or with ginkgolide B (Fig. 5A, left two panels). Renal tubule-like structures were formed from MDCK cells and cysts in the presence of 3T3 CM (Fig. 5A, third panel from the left) as described previously (1, 13, 29). When MDCK cells were incubated with ginkgolide B in the presence of 3T3 CM for 12 days, the numbers of MDCK cells forming tubule-like structures increased in a dose-dependent manner (Fig. 5B). When established MDCK cysts (on day 4 cultured with forskolin) were incubated with ginkgolide B in the presence of 3T3 CM from day 4 to day 12, the average lengths of the longest tubule from each MDCK cyst increased, and the tubules treated with 2 μM ginkgolide B were up to twofold longer than those in cysts cultured with 3T3 CM alone (Fig. 5A, right, and C). The results suggest that ginkgolide B may inhibit cyst formation and growth by promoting MDCK cell differentiation.
Fig. 5.
GB promotes the tubulogenesis in MDCK cells and MDCK cysts. A: representative light micrographs of tubule-like structures induced from the MDCK cysts in collagen gels. Light micrographs were taken on day 12 after MDCK cysts (established on day 4 with FSK stimulation) cultured without 3T3 conditioned media (CM; left) or with GB and without 3T3 CM (second from left) or with 3T3 CM (third from left), or with 3T3 CM and GB (right). Scale bar = 100 μm. B: numbers of MDCK cells forming tubule-like structures without or with GB treatment (means ± SD; n = 3). *P < 0.05 vs. control. C: average values of the longest length of tubule-like structures on each 3T3 CM-treated MDCK cyst without or with GB treatment (means ± SD; n = >30). *P < 0.05 vs. control.
Ginkgolide B does not induce cytotoxicity and apoptosis in MDCK cells but inhibits forskolin-induced MDCK cell proliferation.
An MTT assay was used to determine the cytotoxicity of ginkgolide B on MDCK cells. Ginkgolide B at up to 100 μM did not induce cytotoxicity as showed by OD490 (Fig. 6A). Ginkgolide B (at 0.125, 0.5, or 2 μM) did not induce apoptosis in MDCK cells analyzed by terminaluridine deoxynucleotidyl transferase-mediated dUTP nick-end labeling assays. As a positive control, gentamycin significantly increased apoptosis (Fig. 6B). In normal cell culture conditions, the proliferation rate of MDCK cells treated with ginkgolide B (at 0.125, 0.5, or 2 μM) was not significantly altered as measured by CCK-8 assay (showed as OD450 in Fig. 6C). However, ginkgolide B inhibited forskolin-induced the MDCK cell proliferation rate in a dose- and time-dependent manner (Fig. 6D).
Fig. 6.
GB does not induce cytotoxicity and apoptosis in MDCK cells. A: cytotoxicity assayed by MTT (means ± SD; n = 3). MDCK cells were treated without or with GB at indicated concentrations for 24 h. Cell viability is showed as OD490. B: apoptosis index of MDCK cells cultured with DMSO, gentamycin or GB (means ± SD; n = 3). *P < 0.05 vs. control. C: effect of GB on MDCK cell proliferation measured by CCK-8 kit and showed as OD450. MDCK cells were treated without or with GB at indicated concentrations. D: effect of GB on FSK-stimulated MDCK cell proliferation. MDCK cells were treated without or with GB at indicated concentrations in the presence of 10 μM FSK (means ± SD; n = 3). *P < 0.05 vs. GB-treated groups.
Ginkgolide B does not affect chloride transporter CFTR function.
To determine the effect of ginkgolide B on CFTR function, CFTR activity in FRT cells expressing CFTR was determined by an I−-sensitive fluorescence assay and in MDCK cells by an Ussing chamber assay. There was no difference in CFTR-mediated I− secretion between ginkgolide B-treated and untreated FRT cells (Fig. 7A). Figure 7B shows that ginkgolide B did not reduce the short-circuit current in MDCK cells with forskolin stimulation. Ginkgolide B did not alter CFTR function as seen by short-circuit current in MDCK cells after 1- vs. 48-h incubation with 0.1, 1, or 10 μM ginkgolide B followed by washout. The representative curves are showed in Fig. 7C.
Fig. 7.
Effect of GB on the function of CFTR. A: representative original fluorescence data from individual wells showing CFTR inhibitor control and GB-treated group. After addition of CFTR inhibitor or GB, I− influx was induced by adding an I−-containing solution. B: effect of GB on short-circuit current in MDCK cell monolayer stimulated by 20 μM FSK. C: short-circuit current in MDCK cell monolayer treated without or with 10 μM GB for 1 or 48 h. GB was washed out for 1 h before measurements. CFTR chloride current was stimulated by 20 μM FSK.
Ginkgolide B does not affect intracellular cAMP content.
Figure 8 shows the intracellular cAMP levels in MDCK cells incubated with forskolin in the presence or absence of ginkgolide B (at 0.125, 0.5, or 2 μM). Intracellular cAMP content was significantly increased in MDCK cells by forskolin. There was no difference in intracellular cAMP concentration between ginkgolide B-treated and untreated MDCK cells with forskolin stimulation.
Fig. 8.
Effect of GB on intracellular cAMP in MDCK cells. Intracellular cAMP concentration was measured by cAMP RIA kit (means ± SD; n = 6). MDCK cells were treated without or with GB in the presence of 10 μM FSK for 30 min.
Ginkgolide B regulates intracellular signaling pathways.
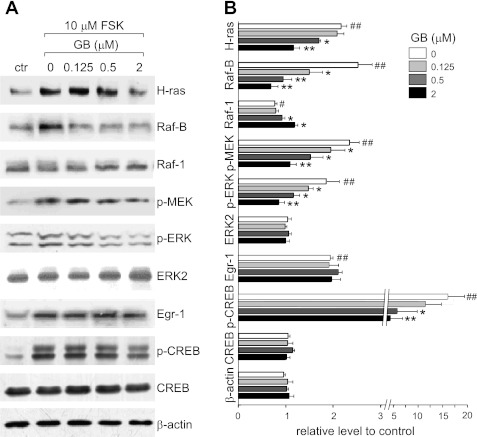
To study the mechanism by which ginkgolide B inhibits cysts, the signaling proteins involved in the Ras/MAPK pathway were analyzed by Western blotting. The highest p-ERK expression levels were found in serum-starved MDCK cells exposed to 10 μM forskolin for 60 min (data not shown). Based on this time course, serum-starved MDCK cells were treated with ginkgolide B at 0.125, 0.5, and 2 μM for 60 min in the presence of 10 μM forskolin. Ginkgolide B significantly decreased levels of Ras, B-raf, p-MEK, and p-ERK but increased Raf-1 levels in MDCK cells with forskolin stimulation. Ginkgolide B did not affect the expression of Egr-1. We also examined the effect of ginkgolide B on CREB phosphorylation in MDCK cells cotreated with 10 μM forskolin for 60 min. Forskolin-mediated CREB activation was significantly inhibited by 0.5 and 2 μM ginkgolide B, whereas total CREB content was not affected (Fig. 9).
Fig. 9.
GB regulates intracellular pathways in MDCK cells. A: representative Western blots of signaling proteins in MDCK cells treated with GB at indicated concentrations in the presence of 10 μM FSK for 60 min. Control refers to MDCK cells without GB and FSK treatment. Each lane was loaded with 20 μg protein. B: quantitative analysis of signaling protein expression in MDCK cells as described above. Relative level means the ratio of Western blotting band density in testing groups (with FSK or FSK plus GB) to that in control group (means ± SD; n = 6). #P < 0.05, ##P < 0.01 vs. control group. *P < 0.05, **P < 0.01 vs. FSK-treated group.
DISCUSSION
Ginkgolide B, the major bioactive component of G. biloba extracts, has been reported to play multiple pharmacological roles as mentioned above (11, 14, 19, 21, 38, 40). Recent studies have found that ginkgolide B may have anticancer properties in molecular, cellular, and whole animal models. Ginkgolide B specifically inhibits nonmucinous ovarian cancer cell proliferation (46). Considering these effects of ginkgolide B, this study was designed to determine whether ginkgolide B has any effects on renal cyst formation and enlargement.
First, ginkgolide B was found to significantly inhibit cyst formation and expansion in the MDCK cyst model. The MDCK cyst model is a classic in vitro model of renal cysts, which are created by exposing MDCK type I cells to forskolin in three-dimensional collagen gels to generate polarized, single-layer cystic structures. MDCK cysts undergo abnormal proliferation and fluid secretion, which are cAMP dependent, as seen in tubular epithelial cells cultured from ADPKD kidneys. MDCK cysts have been widely used as an in vitro model of cystogenesis and cyst enlargement in response to cAMP (9, 23–25). Our results showed that ginkgolide B significantly inhibited MDCK cyst formation and growth in a dose-dependent manner.
To determine the effect of ginkgolide B on cyst formation and enlargement at the organ level, we used a metanephric organ culture system in which embryonic kidneys are removed from mice during the midfetal stage (E13.5) (20). In this model, 8-Br-cAMP treatment resulted in dramatically expanded cystlike structures throughout the embryonic kidneys. Our results showed ginkgolide B reversibly inhibited renal cyst formation and growth induced by 8-Br-cAMP (cAMP analog) in embryonic kidneys.
Pkd1flox/−;Ksp-Cre mice are a useful PKD mouse model for evaluating PKD therapeutic agents. The renal epithelium-specific cysts form after birth and develop quickly in these mice. Interestingly, we found ginkgolide B significantly inhibited renal cyst development in the PKD mice at a dose of 16 mg/kg. These results suggest that ginkgolide B can reach the renal cyst epithelial cells and inhibit renal cyst development in PKD.
To elucidate the mechanism of cyst inhibition, the effects of ginkgolide B on cell viability, proliferation, apoptosis, and CFTR-mediated fluid secretion were examined. Our results did not show any effect of ginkgolide B on cytotoxicity and apoptosis. However, we found that ginkgolide B inhibited forskolin-stimulated MDCK cell proliferation, although it did not affect proliferation of MDCK cells cultured under normal conditions.
Similar to fetal epithelial cells, cyst epithelial cells are less than terminally differentiated, exhibiting increased expression and apical localization of abnormal EGFR and Na+-K+-ATPase. The MDCK tubule model was used for evaluating the differentiation activity of epithelial cells. The results showed that ginkgolide B significantly promoted MDCK cells or cysts to form tubule-like structure. These results suggest that the cyst inhibition activity of ginkgolide B may be attributed at least partially to its effect on epithelial cell differentiation.
Cyst formation and differentiation are thought to be controlled by intracellular signaling pathways (3, 36). Recent findings indicate that activation of the adenylate cyclase/cAMP pathway may trigger cell proliferation via an extracellular signal-related kinase cascade. We measured the intracellular cAMP level in MDCK cells treated with or without ginkgolide B in the presence of forskolin. Forskolin significantly increased the intracellular cAMP concentration by activating adenylate cyclase. However, ginkgolide B did not affect the forskolin-induced intracellular cAMP, which indicates that ginkgolide B may inhibit cyst development through downstream but not upstream of cAMP signaling.
Cystogenesis involves increased cAMP-dependent cell proliferation, but cyst expansion is associated with concomitant transepithelial fluid secretion. Previous studies demonstrate that CFTR Cl− channels play an important role in cAMP-driven liquid excretion in cyst-lining epithelial cells. CFTR is also an effective drug target for ADPKD (45) and cystic fibrosis (2, 26). In the present study, CFTR function was examined in cells treated with ginkgolide B. The data showed that ginkgolide B did not affect the function of CFTR with forskolin stimulation. This result is consistent with our finding that ginkgolide B did not affect a forskolin-induced cAMP increase. However, Na+-K+-ATPase, Na+-Cl−, and Na+-K+-2Cl− cotransporters are also involved in cyst fluid secretion. Therefore, the possibility is not excluded that ginkgolide B affects fluid secretion mediated by other membrane transporters. Unfortunately, we do not have suitable techniques that allow us to determine cyst fluid secretion directly.
Increased intracellular cAMP stimulates ADPKD cyst epithelial cell proliferation mainly by activating the MAPK/ERK signaling pathway, whereas it has an inhibitory effect in normal kidney cells (43). Previous studies show that the opposite effect of cAMP in ADPKD cells and normal kidney cells may be related to the different regulation of B-Raf and Raf-1 (44). In ADPKD cells, cAMP stimulates the MAPK signaling pathway by activating B-Raf (42, 43). We examined the effect of ginkgolide B on the Ras/MAPK pathway by detecting the expression and/or phosphorylation levels of signaling proteins including Ras, Raf, MEK, and ERK. Our results showed that ginkgolide B downregulated the levels of B-Raf, but upregulated the levels of Raf-1 in MDCK cells exposed to forskolin. Ginkgolide B reduced levels of p-MEK and p-ERK directly or through downregulating Ras and B-Raf. The opposite regulation of B-Raf and Raf-1 may be the key mechanism responsible for the cyst inhibition caused by ginkgolide B. As an important regulator of cellular proliferation, apoptosis, and mediator of inflammation, Egr-1 was also examined in this study. We found that ginkgolide B did not affect the expression of Egr-1, indicating that Egr-1 may be not involved in the mechanism of cyst inhibition. A number of studies have shown that CREB is a downstream target of the MAPK cascade and some Ca2+-activated kinase pathways, including PKA and Ca2+/calmodulin kinase IV (8, 12, 31, 41). The activation of ERK is required for both CREB phosphorylation and its transcriptional activity. Davies' studies (5) also showed that ERK MAP kinases were active in the branching ureteric bud. These results suggest that the Ras/MAPK signaling pathway may be involved in the inhibition of abnormal proliferation in cyst cells by ginkgolide B. Whether other signaling pathways, such as those for mTOR and Wnt, are also involved in these processes remains to be investigated.
In summary, our study demonstrated for the first time that ginkgolide B significantly inhibited cyst development. Ginkgolide B induced no apparent toxicity and apoptosis, but inhibited forskolin-induced cyst epithelial cell proliferation and promoted epithelial cell differentiation. The cyst-inhibitory activity of ginkgolide B may be mediated through the Ras/MAPK signaling pathway. Further evaluation is needed for ginkgolide B as a novel promising candidate drug for ADPKD.
GRANTS
This work was supported by National Natural Science Foundation of China Grants 30870921 and 81170632, Drug Discovery Program Grant 2009ZX09301-010-30, The Research Fund for the Doctoral Program of Higher Education 20100001110047, Grant 2012DFA11070 from the Ministry of Science and Technology, and Beijing Natural Science Foundation Grant 7102105. Dr. Stefan Somlo was supported by Grant P30 DK090744.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.Z., J.G., L.Z., Xin Li, W.L., Xuejun Li, Y.X., and B.Y. performed experiments; H.Z., J.G., L.Z., Xin Li, W.L., Xuejun Li, Y.X., and B.Y. analyzed data; H.Z., J.G., L.Z., Xin Li, W.L., Xuejun Li, Y.X., and B.Y. interpreted results of experiments; H.Z., J.G., and B.Y. prepared figures; H.Z., J.G., and B.Y. drafted manuscript; H.Z., J.G., and B.Y. edited and revised manuscript; H.Z., J.G., L.Z., Xin Li, W.L., Xuejun Li, Y.X., and B.Y. approved final version of manuscript; B.Y. provided conception and design of research.
ACKNOWLEDGMENTS
We thank Fei Li for technical support. We acknowledge the Yale PKD Center for Pkd1flox mice and Peter Igarashi and the UT Southwestern O'Brien Center for Ksp-Cre mice.
REFERENCES
- 1. Battini L, Fedorova E, Macip S, Li X, Wilson PD, Gusella GL. Stable knockdown of polycystin-1 confers integrin-alpha2beta1-mediated anoikis resistance. J Am Soc Nephrol 17: 3049–3058, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Belibi FA, Edelstein CL. Novel targets for the treatment of autosomal dominant polycystic kidney disease. Expert Opin Investig Drugs 19: 315–328, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calvet JP. Strategies to inhibit cyst formation in ADPKD. Clin J Am Soc Nephrol 3: 1205–1211, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Calvet JP, Grantham JJ. The genetics and physiology of polycystic kidney disease. Semin Nephrol 21: 107–123, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Fisher CE, Michael L, Barnett MW, Davies JA. Erk MAP kinase regulates branching morphogenesis in the developing mouse kidney. Development 128: 4329–4338, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Galietta LJ, Haggie PM, Verkman AS. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Let 499: 220–224, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Gao J, Zhou H, Lei T, Zhou L, Li W, Li X, Yang B. Curcumin inhibits renal cyst formation and enlargement in vitro by regulating intracellular signaling pathways. Eur J Pharmacol 654: 92–99, 2011 [DOI] [PubMed] [Google Scholar]
- 8. Gonzalez MV, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59: 611–617, 1989 [DOI] [PubMed] [Google Scholar]
- 9. Grantham JJ, Mangoo-Karim R, Uchic ME, Grant M, Shumate WA, Park CH, Calvet JP. Net fluid secretion by mammalian renal epithelial cells: Stimulation by cAMP in polarized cultures derived from established renal cells and from normal and polycystic kidneys. Trans Assoc Am Physicians 102: 158–162, 1989 [PubMed] [Google Scholar]
- 10. Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med 60: 321–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang SH, Duke RK, Chebib M, Sasaki K, Wada K, Johnston GA. Ginkgolides, diterpene trilactones of Ginkgo biloba, as antagonists at recombinant alpha1beta2 gamma2L GABAA receptors. Eur J Pharmacol 494: 131–138, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron 21: 869–883, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Jiang ST, Chuang WJ, Tang MJ. Role of fibronectin deposition in branching morphogenesis of Madin-Darby canine kidney cells. Kidney Int 57: 1860–1867, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Jin GH, Huang Z, Tan XF, Tian ML, Zhang XH, Qin JB, Xu HJ, Yew DT, Mak YT. Effects of ginkgolide on the development of NOS and AChE positive neurons in the embryonic basal forebrain. Cell Biol Int 30: 500–504, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Leonhard WN, van der Wal A, Novalic Z, Kunnen SJ, Gansevoort RT, Breuning MH, de Heer E, Peters DJ. Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: in vivo evidence from a Pkd1-deletion model. Am J Physiol Renal Physiol 300: F1193–F1202, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Leuenroth SJ, Bencivenga N, Igarashi P, Somlo S, Crews CM. Triptolide reduces cystogenesis in a model of ADPKD. J Am Soc Nephrol 19: 1659–1662, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leuenroth SJ, Crews CM. Targeting cyst initiation in ADPKD. J Am Soc Nephrol 20: 1–3, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Leuenroth SJ, Okuhara D, Shotwell JD, Markowitz GS, Yu Z, Somlo S, Crews CM. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc Natl Acad Sci USA 104: 4389–4394, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li R, Chen B, Wu W, Bao L, Li J, Qi R. Ginkgolide B suppresses intercellular adhesion molecule-1 expression via blocking nuclear factor-kappa B activation in human vascular endothelial cells stimulated by oxidized low-density lipoprotein. J Pharmacol Sci 110: 362–369, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Magenheimer BS, John PL, Isom KS, Abrahamson DR, De Lisle RC, Wallace DP, Maser RL, Grantham JJ, Calvet JP. Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na+, K+, 2Cl−, co-transporter-dependent cystic dilation. J Am Soc Nephrol 17: 3424–3437, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Mahmoud FF, Haines DD, Abul HT, Abal AT, Onadeko BO, Wise JA. In vitro effects of astaxanthin combined with ginkgolide B on T lymphocyte activation in peripheral blood mononuclear cells from asthmatic subjects. J Pharmacol Sci 94: 129–136, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3',5′-cyclic monophosphate. Gastroenterology 132: 1104–1116, 2007 [DOI] [PubMed] [Google Scholar]
- 23. McAteer JA, Dougherty GS, Gardner KD, Jr, Evan AP. Scanning electron microscopy of kidney cells in culture: surface features of polarized epithelia. Scan Electron Microsc Pt 3: 1135–1150, 1986 [PubMed] [Google Scholar]
- 24. McAteer JA, Dougherty GS, Gardner KD, Jr, Evan AP. Polarized epithelial cysts in vitro: a review of cell and explant culture systems that exhibit epithelial cyst formation. Scanning Microsc 2: 1739–1763, 1988 [PubMed] [Google Scholar]
- 25. McAteer JA, Evan AP, Gardner KD. Morphogenetic clonal growth of kidney epithelial cell line, MDCK. Anat Rec 217: 229–239, 1987 [DOI] [PubMed] [Google Scholar]
- 26. Murthy M, Pedemonte N, MacVinish L, Galietta L, Cuthbert A. 4-Chlorobenzo[F]isoquinoline (CBIQ), a novel activator of CFTR and DeltaF508 CFTR. Eur J Pharmacol 516: 118–124, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Park EY, Woo YM, Park JH. Polycystic kidney disease and therapeutic approaches. BMB Rep 44: 359–368, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Patel V, Chowdhury R, Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr Opin Nephrol Hypertens 18: 99–106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pollack AL, Runyan RB, Mostov KE. Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev Biol 204: 64–79, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Ruggenenti P, Remuzzi A, Ondei P, Fasolini G, Antiga L, Ene-Iordache B, Remuzzi G, Epstein FH. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int 68: 206–216, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 4: 477–485, 1990 [DOI] [PubMed] [Google Scholar]
- 32. Shibazaki S, Yu Z, Nishio S, Thomson RB, Mitobe M, Louvi A, Velazquez H, Ishibe S, Cantley LG, Igarashi P, Somlo S. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet 17: 1505–1516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sun Y, Zhou H, Yang B. Drug discovery for polycystic kidney disease. Acta Pharmacol Sin 32: 805–816, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taide M, Kanda S, Igawa T, Eguchi J, Kanetake H, Saito Y. Human simple renal cyst fluid contains a cyst formation-promoting activity for Madin-Darby canine kidney cells cultured in collagen gel. Eur J Clin Invest 26: 506–513, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Torres VE. Cyclic AMP, at the hub of the cystic cycle. Kidney Int 66: 1283–1285, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Torres VE. Vasopressin antagonists in polycystic kidney disease. Semin Nephrol 28: 306–317, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tradtrantip L, Sonawane ND, Namkung Verkman AS W. Nanomolar potency pyrimido-pyrrolo-quinoxalinedione CFTR inhibitor reduces cyst size in a polycystic kidney disease model. J Med Chem 52: 6447–6455, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang SJ, Chen HH. Ginkgolide B, a constituent of Ginkgo biloba, facilitates glutamate exocytosis from rat hippocampal nerve terminals. Eur J Pharmacol 514: 141–149, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Wilson PD. Polycystic kidney disease: new understanding in the pathogenesis. Int J Biochem Cell Biol 36: 1868–1873, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Xia SH, Fang DC. Pharmacological action and mechanisms of ginkgolide B. Chin Med J (Engl) 120: 922–928, 2007 [PubMed] [Google Scholar]
- 41. Xing J, Ginty DD, Greenberg ME. Coupling of the Ras-MAPK pathway to gene activation by RSK2, a growth factor regulated CREB kinase. Science 273: 959–963, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, Wallace DP. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol 17: 178–187, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley B5D, Pelling JC, Grantham JJ. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 63: 1983–1994, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 279: 40419–40430, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Yang B, Sonawane ND, Zhao D, Somlo S, Verkman AS. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J Am Soc Nephrol 19: 1300–1310, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ye B, Aponte M, Dai Y, Li L, Ho MC, Vitonis A, Edwards D, Huang TN, Cramer DW. Ginkgo biloba and ovarian cancer prevention: epidemiological and biological evidence. Cancer Lett 251: 43–52, 2007 [DOI] [PubMed] [Google Scholar]