Abstract
Preterm neonates are commonly exposed postnatally to pharmacological treatments for a patent ductus arteriosus. Exposure of the developing kidney to nephrotoxic medications may adversely impact renal development. This study aimed to determine the effect of early postnatal ibuprofen treatment, both alone and in combination with a nitric oxide synthase inhibitor (NOSi), on renal development and morphology. Baboon neonates were delivered prematurely at 125-day (125d) gestation (term = 185d) and were euthanized at birth or postnatal day 6. Neonates were divided into four groups: 125d gestational controls (n = 8), Untreated (n = 8), Ibuprofen (n = 6), and ibuprofen (Ibu)+NOSi (n = 4). Animals in the Ibuprofen and Ibu+NOSi groups received five doses of ibuprofen, with the Ibuprofen+NOSi animals additionally administered a NOS inhibitor (NG-monomethyl-l-arginine). There was no difference among groups in body weight, kidney weight, or glomerular generation number. Nephrogenic zone width was significantly reduced in the Ibuprofen group (123.5 ± 7.4 μm) compared with the 125d gestational control (176.1 ± 6.9 μm) and Untreated animals (169.7 ± 78.8 μm). In the Ibu+NOSi group, nephrogenic zone width averaged 152.7 ± 3.9 μm, which was not significantly different from any other group. Morphologically abnormal glomeruli were present at a range of 0.0–22.9% in the Untreated group, 0.0–6.1% in the Ibuprofen group, and 0.0–1.4% in the Ibu+NOSi group. In conclusion, early postnatal ibuprofen exposure is associated with a reduced nephrogenic zone width, which may suggest the early cessation of nephrogenesis following treatment. Ultimately, this may impact the number of nephrons formed in the preterm kidney.
Keywords: nephrogenesis, NSAID, patent ductus arteriosus
nephrogenesis is normally completed in utero between 32- and 36-wk gestation (13). Therefore, infants born extremely preterm (<28-wk gestation) are born at a time when nephrogenesis is still ongoing. Although it has been shown that nephrogenesis continues postnatally after preterm birth (10, 31), there is evidence to suggest that it may be impaired with previous reports of reduced glomerular generation formation (24), accelerated postnatal maturation (31), and the presence of morphologically abnormal glomeruli in the outer renal cortex of the preterm kidney (10, 24, 31, 32). These glomeruli are cystic in appearance, exhibiting an enlarged Bowman's space and shrunken glomerular tuft (10, 31, 32). Through previous studies in a baboon model of preterm birth, we have demonstrated that the proportion of abnormal glomeruli varies considerably between neonates (10, 32), suggesting that the morphological abnormalities may not be attributed to preterm birth per se, but rather to factors in the postnatal clinical course of the preterm neonate (which varies with each neonate).
Exposure to medications commonly occurs during the period of postnatal nephrogenesis in the preterm neonate. NSAIDs are used in the treatment of a patent ductus arteriosus (PDA), a condition affecting up to 70% of extremely preterm neonates (11). NSAIDs, such as ibuprofen, act by preventing prostaglandin synthesis via the inhibition of cyclooxygenase (COX) enzyme activity (36). Following NSAID treatment, 30% of infants fail to close their PDA, or after an initially successful closure the PDA will reopen (8, 28). This failure to respond to treatment has been attributed to a compensatory increase in vasodilatory nitric oxide production (2, 3, 29). Recent studies in preterm baboon and human infants demonstrate that treatment of a PDA with a combination of NSAIDs and nitric oxide synthase inhibitors significantly increases the success of ductus closure compared with NSAID treatment alone (16, 26).
Importantly, there is experimental evidence to suggest that exposure to NSAIDs can adversely impact nephrogenesis. Komhoff et al. (19) and Olliges et al. (23) recently determined that NSAID exposure led to a significantly reduced cortical mass, reduced glomerular density, and reduced glomerular and tubular volumes in mice models of NSAID exposure during the period of postnatal renal development. Similarly, Kent et al. (17, 18) observed a significantly reduced glomerular density and also renal injury in neonatal rats with early postnatal NSAID exposure; however, there was no effect on total nephron endowment. Of particular importance, in human infants exposed in utero to NSAIDs, changes in renal morphology have been described (15, 33) which are similar to the glomerulocystic changes observed in the preterm baboon kidney. NOS inhibitors elicit a similar physiological response as NSAIDS, such as reduction in renal blood flow and urine output (27); however, the effects on nephrogenesis have not been previously investigated. Therefore, the aim of this study was to determine the effects of ibuprofen treatment on renal development and morphology in the preterm baboon kidney both alone and in combination with a NOS inhibitor.
METHODS
Animal Care and Treatment Groups
All animal studies were performed at the Southwest Foundation for Biomedical Research (San Antonio, TX) and were approved by the institutional animal care and use committee. Baboon neonates were delivered prematurely by caesarean section at 125 days' gestation (term = 185 days), a time point equivalent to 27-wk gestation in humans. Animals in the gestational (fetal) control group (125d; n = 8) were euthanized at delivery. The remainder of animals were cared for in a primate intensive care nursery and were euthanized on postnatal day 6 (144 h after delivery), with the exception of one animal in the ibuprofen plus NOS inhibitor group, which was euthanized on postnatal day 5 (120 h after delivery).
Preterm baboon neonates received one of three treatment protocols, as have been previously described (26, 35): 1) no treatment (Untreated; n = 8); 2) ibuprofen alone (Ibuprofen; n = 6); and 3) ibuprofen plus nitric oxide synthase inhibitor (Ibu+NOSi; n = 4).
In the Ibuprofen group, ibuprofen lysine (Farmacon; Westport, CT) was administered intravenously at 10 mg/kg (over 20 min) at 24 h of age, followed by 5 mg/kg at 48, 72, 96, and 120 h of age. This dosing regime was based on the recommended dosage of ibuprofen for the treatment of a patent ductus arteriosus in human preterm infants (22). In the Ibu+NOSi group, ibuprofen was given in combination with the nitric oxide synthase inhibitor NG-monomethyl-l-arginine (l-NMMA). Treatment with l-NMMA (Calbiochem, San Diego, CA) was initiated at 50 h after delivery, and was continuously infused at a rate of 20 mg·kg−1·h−1 until the time of necropsy.
Physiological Measurements
Echocardiographic assessment of ductal patency was performed daily using an 8-mHz transducer interfaced with a Biosound AU3 echocardiographic system (Genoa, Italy). Animals were instrumented with an umbilical arterial catheter, which enabled measurement of blood pressure, and the administration of fluid requirements. Fluid intake and urine output were continuously recorded over the 6 days of life. Mean blood pressure, at 12, 24, 48, 96, and 120 h of life was determined by averaging three measured values of systolic and diastolic blood pressure (within a 4- to 6-h period) around the defined time point.
In the assessment of blood pressure, infusion rates of l-NMMA were reduced by 25% if mean systemic blood pressure was consistently >47 mmHg. In cases of significant hypotension (defined as a mean blood pressure of <25 mmHg, accompanied by either increasing base deficit or decreasing urine output), volume supplementation was first initiated (10–20 ml/kg, administered at least twice over a 1-h period) followed by the use of inotropic support. Dopamine was initially administered at a rate of 4–6 μg·kg−1·min−1 and further increased to a maximum rate of 20 μg·kg−1·min−1. If mean blood pressure failed to respond to volume and inotropic drugs within 2–4 h, then hydrocortisone (Soul-Cortef, Pharmacia & Upjohn, Kalamazoo, MI) at a dose of 0.5–1.0 mg/kg was administered at 6-h intervals until either mean blood pressure increased to >28 mmHg or a maximum of four doses of hydrocortisone was received.
Tissue Collection and Processing
Kidneys collected at necropsy from animals in the Ibuprofen and Ibu+NOSi groups, and from five animals from the Untreated group, were embedded in Tissue-Tek OCT compound and snap frozen in liquid nitrogen. Kidney tissue from the 125d gestational control group, and from three animals in the Untreated group, was formalin-fixed and embedded in paraffin. There was no significant difference observed in any measured parameters between the formalin-fixed paraffin-embedded tissue and the frozen tissue within the Untreated group.
Kidneys were halved along the coronal axis before embedding, and sectioned at a similarly central region for each kidney. Sections (5–7 μm) were stained with hematoxylin and eosin. In all assessments of renal development and morphology, the researcher was blinded to the experimental grouping of the animals.
Assessment of Renal Development and Morphology
Nephrogenic zone width.
The width of the nephrogenic zone, defined as the area in the outer renal cortex exhibiting developing glomerular structures in the form of comma- and S-shaped bodies, was measured using image analysis software (Image Pro Plus v6.0 for Windows; Media Cybernetics, Silver Spring, MD). This method has previously been utilized to assess renal maturity in both human (5, 31) and baboon (10) fetal and neonatal kidneys. One stained section from each kidney was viewed at ×200 magnification, and the width of the nephrogenic zone was measured in four separate regions. An average nephrogenic zone width was determined for each kidney.
Glomerular generation number.
The medullary ray glomerular generation counting method was utilized to estimate the number of glomerular generations formed within the kidney. This method has been validated by Hinchliffe and colleagues (12), and also utilized in previous studies to assess renal maturity in preterm human (6, 24, 31) and baboon neonates (10, 32). In one complete section from each kidney, five clearly distinguishable medullary rays from separate regions of the section were identified and the number of mature glomeruli along one side of the medullary ray was counted. An average number of glomerular generations was determined for each kidney.
Glomerular morphology.
One complete kidney section from each kidney was systematically sampled at a step length of 1 mm. At each field of view, the numbers of normal and abnormal mature glomeruli were recorded. Glomeruli were classified as abnormal if they exhibited a grossly enlarged Bowman's space and a shrunken glomerular tuft (10, 31, 32). The percentage of abnormal glomeruli per kidney was determined.
Statistical Analysis
Data were analyzed using GraphPad Prism software (v5.03 for Windows) and Intercooled Stata (v8.0 for Windows) and graphed as means ± SE. Analysis of physiological parameters (fluid intake, urine output, and blood pressure) was undertaken using a two-way repeated measures ANOVA, with the factors T (Treatment), A (Age), and TxA (Interaction). To determine differences between groups at individual time points, the two-way ANOVA was followed by a Bonferroni post hoc test. To determine differences in categorical variables among groups [including ductus closure, dopamine administration, hydrocortisone administration, oliguria, and a high (>5%) or low (<5%) percentage of abnormal glomeruli], a Fisher's exact test was performed. All other statistical comparisons among groups (body and kidney weights, assessments of renal morphology) were performed using a one-way ANOVA followed by a Bonferroni post hoc test. Statistical significance was accepted at the level of P ≤ 0.05.
RESULTS
Body Weight and Kidney Weight
Birth weight, necropsy weight, kidney weight, and kidney weight-to-body (necropsy) weight ratios for each group are shown in Table 1. There was no significant difference between any of the gestational control (125d) or preterm (Untreated, Ibuprofen, Ibu+NOSi) groups in any parameter of body and kidney weight.
Table 1.
Body and kidney weights of gestational control (125d) and preterm (Untreated, Ibuprofen, and Ibu+NOSi) baboons
| 125d (n = 8) | Untreated (n = 8) | Ibuprofen (n = 6) | Ibu+NOSi (n = 4) | |
|---|---|---|---|---|
| Birth wt, g | 382.9 ± 20.4 | 381.9 ± 10.7 | 388.8 ± 17.7 | 395.3 ± 16.4 |
| Necropsy wt, g | 382.9 ± 20.4 | 377.1 ± 15.8 | 352.8 ± 17.1 | 417.8 ± 42.2 |
| Combined kidney wt, g | 2.9 ± 0.2 | 3.2 ± 0.1 | 3.0 ± 0.3 | 3.4 ± 0.3 |
| Kidney wt-to-body wt ratio, g/kg | 7.9 ± 0.7 | 8.5 ± 0.3 | 8.3 ± 0.6 | 8.4 ± 0.8 |
Values are given as the mean ± SE. NOSi, nitric oxide synthase inhibitor.
Fluid Intake and Urine Output
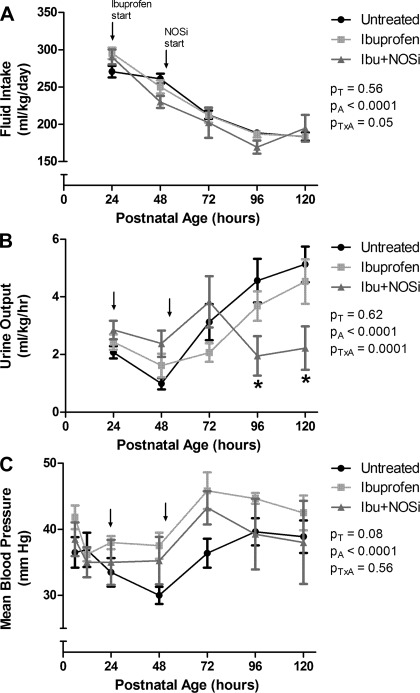
Fluid intake was reduced significantly with increasing postnatal age (Fig. 1A). There was no effect of treatment on fluid intake, but a significant interaction effect between postnatal age and treatment was evident.
Fig. 1.
Fluid intake (A), urine output (B), and mean blood pressure (C), up to 120 h of life, in preterm baboons in the Untreated, Ibuprofen, and ibuprofen plus nitric oxide synthase inhibitor (Ibu+NOSi) groups. Arrows mark the starting point for ibuprofen administration (24 h; Ibuprofen and Ibu+NOSi groups) and NOSi administration (50 h; Ibu+NOSi group). Values are means ± SE. PT, PA, and PTxA refer to results of ANOVA for treatment, age, and interaction. *P < 0.05 Ibu+NOSi vs. Untreated.
There was no significant effect of treatment on urine output over the 6 days of life (Fig. 1B). Overall, there was a significant effect of postnatal age on urine output, and a significant interaction effect between postnatal age and treatment. Urine output was significantly reduced in the Ibu+NOSi group compared with the Untreated group at both 96 and 120 h of age.
Oliguria (urine output <1 mg·kg−1·h−1) was exhibited in three of eight Untreated animals, one of six Ibuprofen animals, and one of four Ibu+NOSi animals, with no association between treatment group and oliguria (P = 0.80).
Ductus Closure and Blood Pressure
There was a strong association between treatment and ductus closure (P = 0.005). In all Ibuprofen- and Ibu+NOSi-treated animals, the ductus was closed on days 2–3 of life and remained closed until necropsy. Two of the Untreated animals achieved ductus closure, which occurred on day 4 of life. In the remainder of Untreated animals, the ductus remained open throughout the 6-day study period.
Overall, there was a significant effect of postnatal age on mean blood pressure (Fig. 1C). There was also a strong trend toward an effect of treatment on mean blood pressure; however, this did not quite reach statistical significance (P = 0.08).
There was a significant association between treatment group and dopamine administration (P = 0.002), where it was required in seven of eight Untreated, none of six Ibuprofen, and two of four Ibu+NOSi animals. Similarly, hydrocortisone administration was most common in the Untreated group, where it was required in four of eight animals, and was not administered to any of the Ibuprofen or Ibu+NOSi animals (P = 0.05).
Nephrogenic Zone Width
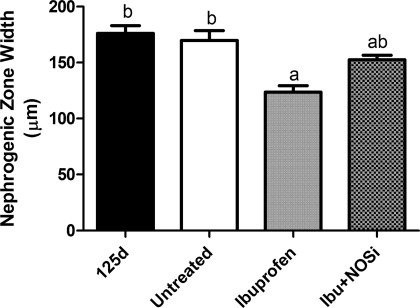
As shown in Fig. 2, the width of the nephrogenic zone averaged 176.1 ± 6.9 μm in the 125d gestational control group and was not different from the Untreated group at postnatal day 6 (169.7 ± 8.8 μm). Ibuprofen treatment alone significantly reduced nephrogenic zone width by 30% compared with the 125d group, and 27% compared with the Untreated group, with a mean of 123.5 ± 5.8 μm. Ibu+NOSi animals had a mean nephrogenic zone width of 152.7 ± 3.9 μm, which was not different to any other group.
Fig. 2.
Width of the nephrogenic zone in the kidneys of gestational control baboons (125d) and in preterm baboons (Untreated, Ibuprofen, and Ibu+NOSi) analyzed at postnatal day 6. Values are means ± SE. Significant differences among groups (P < 0.05) are indicated by the letters; a is different from b, but not from ab.
Glomerular Generation Number
In the 125d gestational control group, the number of glomerular generations averaged 6.8 ± 0.2. Similarly, in the Untreated group at postnatal day 6, the mean glomerular generation number was 6.4 ± 0.1. There was no effect of Ibuprofen (6.6 ± 0.1) or Ibu+NOSi (6.7 ± 0.2) treatment on glomerular generation number.
Glomerular Morphology
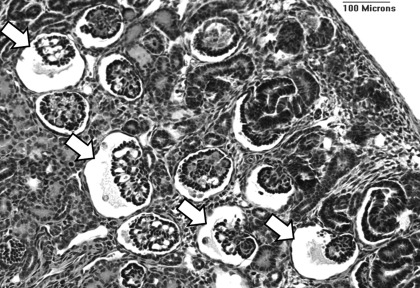
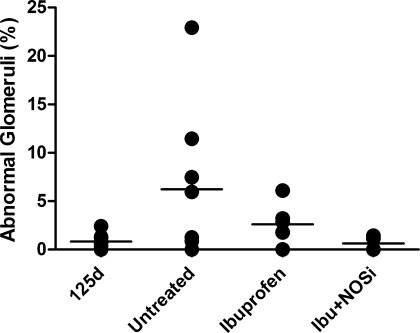
Morphologically abnormal glomeruli, with an enlarged Bowman's space and shrunken glomerular tuft (Fig. 3), were commonly observed in the outer renal cortex of the preterm kidneys at postnatal day 6, whereas the percentage of abnormal glomeruli was negligible (ranging from 0.0 to 2.4%) in the 125d gestational controls (Fig. 4). In the Untreated group, the percentage of abnormal glomeruli was markedly variable among individuals, ranging from 0.0 to 22.9%. Similarly, in the Ibuprofen group the range was 0.0 to 6.1%. In the Ibu+NOSi animals, however, the percentage of abnormal glomeruli ranged from 0.0 to 1.4%. The percentage of abnormal glomeruli was not statistically different among the four groups.
Fig. 3.
Representative photomicrograph of abnormal glomeruli (arrows), exhibiting an enlarged Bowman's space and shrunken glomerular tuft, in the outer renal cortex of a preterm baboon kidney (Untreated).
Fig. 4.
Percentage of morphologically abnormal glomeruli in the kidneys of gestational control baboons (125d) and in preterm baboons (Untreated, Ibuprofen, and Ibu+NOSi) analyzed at postnatal day 6.
Five animals (Untreated: n = 4, Ibuprofen: n = 1) had a percentage of abnormal glomeruli >5% (Fig. 4). At 24 h of age, mean blood pressure was significantly reduced in the group of animals with >5% of abnormal glomeruli compared with those with a low percentage of abnormal glomeruli (P = 0.01). There was also a trend toward decreased urine output at 48 h of age in those animals with a high percentage of abnormal glomeruli (P = 0.06). Two of the five animals with >5% abnormal glomeruli, and 4 of the 13 with <5% abnormal glomeruli exhibited oliguria during the study period (P = 1.00).
Four of the five animals with >5% abnormal glomeruli were treated with dopamine; this was not significantly different from the group of animals with a low percentage of abnormal glomeruli where 5 of 13 animals required dopamine treatment (P = 0.29). The number of baboons that required hydrocortisone treatment was significantly greater (P = 0.04) in the group of animals with >5% abnormal glomeruli (3 of 5 animals; 1 of 3 receiving 1 dose, and 2 of 3 receiving 3 doses) compared with the animals that exhibited a low percentage of abnormal glomeruli (1 of 13 animals receiving 1 dose).
DISCUSSION
Using a baboon model of preterm birth, where nephrogenesis is still ongoing postnatally, we have demonstrated that early postnatal exposure to the NSAID ibuprofen does not influence glomerular generation number or the percentage of morphologically abnormal glomeruli at postnatal day 6. Of concern, however, ibuprofen treatment led to a significantly reduced nephrogenic zone width, which may be indicative of the early cessation of nephrogenesis.
Pharmacological treatments for PDA have significant renal side effects in the preterm neonate. NSAID treatment increases renal vascular resistance, reduces renal blood flow (14, 30), decreases glomerular filtration rate (1, 22), and is an independent risk factor for the development of acute renal failure (4). Similarly, NOS inhibition has been shown to significantly reduce renal blood flow, glomerular filtration rate, and alter tubular function in neonatal animals (27). Furthermore, a combination of a NOS inhibitor and NSAID treatment has been shown to produce significant renal side effects (such as increased serum creatinine) in human preterm neonates (16). In the current study, fluid intakes were reduced in all preterm neonates over the course of the study (Fig. 1), likely in response to improved cardiovascular function and reductions in insensible fluid loss with increasing postnatal age. Urine output was significantly lower in the Ibu+NOSi group compared with the Untreated group at the 96- and 120-h time points, indicative that renal function was somewhat affected by the treatment; however, there was no effect in the Ibuprofen or Ibu+NOSi treatment groups on the number of animals that presented with oliguria during the study period.
As expected, there was a strong association between treatment and ductus closure, with all animals in the Ibuprofen and Ibu+NOSi groups achieving ductus closure by postnatal day 4 compared with just two animals in the Untreated group. The effect of treatment on mean blood pressure did not quite reach statistical significance (Fig. 1); however, dopamine and hydrocortisone treatment for hypotension was most common within the Untreated group. The lack of a substantial effect of treatment on blood pressure in this study may be explained by the strict maintenance of fluid requirements, and the adjustment of NOS inhibitor, dopamine, and hydrocortisone dosage in cases of significant hypo- and hypertension. Hence, it is to be noted that exposure to dopamine and hydrocortisone treatments is a confounding variable which could not be controlled for in this study.
Importantly, the results indicate that early postnatal exposure to ibuprofen leads to a significant reduction in nephrogenic zone width (Fig. 2). The width of the nephrogenic zone measured just 123.5 ± 5.8 μm in the Ibuprofen group, reflecting a 27% reduction in width compared with the Untreated animals. A reduced nephrogenic zone width is suggestive of either an early cessation of nephrogenesis and/or an increase in renal maturation following treatment. Such an effect on nephrogenesis has the potential to result in a nephron deficit, which in turn has long-term consequences for renal health (9). The finding of a reduced nephrogenic zone width is in accordance with previous studies in rodent models, where exposure to NSAIDs during the period of postnatal nephrogenesis (a longer time period of exposure than in the current study) resulted in impaired nephrogenesis (19) and also renal injury (18). These effects may be mediated via the inhibition of COX enzyme activity within the developing kidney. COX expression has been shown to be essential for renal development, with COX-2 (but not COX-1) knockout mice exhibiting severe renal dysplasia at birth (21). In addition, adult rats following both prenatal and early postnatal exposure to a COX-2 inhibitor exhibited a significant nephron deficit, with associated glomerular hypertrophy and glomerulosclerosis (25). The exact role of COX-2 in renal development, however, has not been fully elucidated.
Interestingly, nephrogenic zone width in the group of animals that received the combined Ibu+NOSi treatment was not significantly reduced compared with the Untreated control group. This result suggests that the inhibition of nitric oxide synthesis may ameliorate the effects of ibuprofen treatment on width of the nephrogenic zone. The reasons for this are unknown; however, there is known to be much cross talk between the prostaglandin and nitric oxide pathways. In some tissues, for example, prostaglandin inhibition leads to a significant increase in NOS expression and nitric oxide production (29, 34). Increased nitric oxide levels have been shown to inhibit cellular adhesion, extracellular matrix synthesis, and proliferation of cultured mesangial cells (7), and also to promote glomerular apoptosis (20). An analysis of prostaglandin and nitric oxide levels within the preterm kidney, particularly within the developing outer renal cortex, would help to elucidate the mechanisms underlying the response of nephrogenesis to NSAID and NOS inhibitor treatment.
Despite the reduced nephrogenic zone width, glomerular generation number was not affected; this likely relates to the early timing of examination at just 5 days following the onset of ibuprofen treatment. Furthermore, we would expect the potentially adverse effects of ibuprofen exposure may be of short duration given that the recommended treatment for patent ductus arteriosus is only three doses of either indomethacin or ibuprofen, at 12- to 24-h intervals (22). Therefore, it is possible that any adverse effect NSAID exposure has on the kidney may be short-lived and therefore would not significantly influence final nephron endowment. Indeed Kent et al. (17), in a stereological assessment of nephron endowment following in vivo NSAID exposure, demonstrated in a neonatal rat model that total nephron endowment was not altered following extended postnatal exposure to NSAIDs. In that study, the NSAIDs were administered over a period equivalent to 24- to 30-wk gestation in humans (17), a much longer time period of exposure than in the current study. An examination of the baboon kidneys at a later postnatal time point, after the treatments and nephrogenesis have ceased, would be required to fully describe the long-term effects of NSAID exposure on renal development.
Consistent with previous studies in this model (10, 32), and also in the human preterm neonate (31), morphologically abnormal glomeruli were commonly present in the outer renal cortex of the preterm kidneys (Fig. 3). The proportion of abnormal glomeruli was highly variable; there was no statistically significant difference in the percentage of abnormal glomeruli among treatment groups (Fig. 4). In the Untreated animals, the percentage of abnormal glomeruli ranged from as low as 0% to as high as 22.9% (a very abnormal kidney). Importantly, our findings demonstrate that ibuprofen treatment does not lead to the glomerular abnormalities associated with preterm birth. In the ibuprofen-treated animals, the number of abnormal glomeruli ranged from 0 to 6%; the animals that received the combined ibuprofen and NOS inhibitor treatment exhibited the lowest percentage of abnormal glomeruli, at 1.4% or less per kidney. Although glomerular abnormalities have been described in the human kidney following antenatal NSAID exposure (15, 33), the results of this study clearly indicate that early postnatal exposure to ibuprofen is certainly not the cause of the abnormal glomeruli commonly observed in the outer renal cortex of the preterm kidney.
The cause of the glomerular abnormalities, therefore, remains unknown. In the current study, animals with a high percentage (>5%) of abnormal glomeruli had significantly lower blood pressure at 24 h of age, and also a trend toward a reduced urine output at 48 h of age, compared with those animals with a low percentage of abnormal glomeruli. Furthermore, there was a significant association between a high percentage of abnormal glomeruli and a requirement for hydrocortisone treatment. From these results it may be speculated that changes in blood pressure and perhaps renal blood flow may be involved in the formation of the morphologically abnormal glomeruli in the preterm kidney. An analysis of renal blood flow changes following preterm birth would be required in the future, however, to definitively establish whether this is the cause of the glomerular abnormalities.
Alternatively, it may be the hydrocortisone treatment itself (administered following persistent hypotension in baboon neonates that did not respond to dopamine treatment) that may be linked to the formation of abnormal glomeruli. It is to be noted, however, that we have previously shown in a separate cohort of animals that dopamine and hydrocortisone administration were not associated with a high percentage of abnormal glomeruli (32); in a group of 12 baboons delivered preterm at 125d gestation, the percentage of abnormal glomeruli per kidney ranged from 0.00 to 16.03% in baboons not exposed to dopamine (mean: 8.06 ± 2.7%), and from 0.55 to 2.56% in baboons that were exposed (mean: 1.3 ± 0.4%). When hydrocortisone was additionally administered, the percentage of abnormal glomeruli was very low (1.14%) (32).
In conclusion, early postnatal ibuprofen treatment does not influence the percentage of morphologically abnormal glomeruli in the preterm kidney. Of concern, however, ibuprofen treatment led to a significantly reduced nephrogenic zone width, which may be indicative of the early cessation of nephrogenesis. Ultimately, this may impact on the number of nephrons formed in the preterm kidney.
GRANTS
This project was supported by a National Health and Medical Research Council (NHMRC) of Australia project grant, and also by the National Institutes of Health Grant HL-52636. M. R. Sutherland was the recipient of an Australian Postgraduate Award while undertaking this study.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.R.S., B.A.Y., D.M., S.S., R.I.C., and M.J.B. provided conception and design of research; M.R.S., B.A.Y., D.M., S.S., L.G., and R.I.C. performed experiments; M.R.S., B.A.Y., D.M., S.S., and R.I.C. analyzed data; M.R.S., B.A.Y., D.M., S.S., R.I.C., and M.J.B. interpreted results of experiments; M.R.S. prepared figures; M.R.S. drafted manuscript; M.R.S., B.A.Y., L.G., R.I.C., and M.J.B. edited and revised manuscript; M.R.S., B.A.Y., D.M., S.S., L.G., R.I.C., and M.J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Professor Kurt Albertine for the use of his laboratory facilities, and also Professor Jacqueline Coalson and Vicki Winter for providing some of the baboon kidney tissue.
REFERENCES
- 1. Allegaert K. The impact of ibuprofen or indomethacin on renal drug clearance in neonates. J Matern Fetal Neonatal Med 22, Suppl 3: 88– 91, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Clyman RI, Waleh N, Black SM, Riemer RK, Mauray F, Chen YQ. Regulation of ductus arteriosus patency by nitric oxide in fetal lambs: the role of gestation, oxygen tension, and vasa vasorum. Pediatr Res 43: 633– 644, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Coceani F, Kelsey L, Seidlitz E. Occurrence of endothelium-derived relaxing factor–nitric oxide in the lamb ductus arteriosus. Can J Physiol Pharmacol 72: 82– 88, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Cuzzolin L, Fanos V, Pinna B, di Marzio M, Perin M, Tramontozzi P, Tonetto P, Cataldi L. Postnatal renal function in preterm newborns: a role of diseases, drugs and therapeutic interventions. Pediatr Nephrol 21: 931– 938, 2006 [DOI] [PubMed] [Google Scholar]
- 5. dos Santos AM, Fonseca Ferraz ML, Pinto Rodriguez ML, Dos Reis MA, Miranda Correa RR, de Paula Antunes Teixeira V, da Cunha Castro EC. Assessment of renal maturity by assisted morphometry in autopsied fetuses. Early Hum Dev 82: 709– 713, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Faa G, Gerosa C, Fanni D, Nemolato S, Locci A, Cabras T, Marinelli V, Puddu M, Zaffanello M, Monga G, Fanos V. Marked interindividual variability in renal maturation of preterm infants: lessons from autopsy. J Matern Fetal Neonatal Med 23, Suppl 3: 129– 133, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Garg UC, Hassid A. Inhibition of rat mesangial cell mitogenesis by nitric oxide-generating vasodilators. Am J Physiol Renal Fluid Electrolyte Physiol 257: F60– F66, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS. Effects of indomethacin in premature infants with patent ductus arteriosus: results of a national collaborative study. J Pediatr 102: 895– 906, 1983 [DOI] [PubMed] [Google Scholar]
- 9. Gubhaju L, Sutherland MR, Black MJ. Preterm birth and the kidney: implications for long-term renal health. Reprod Sci 18: 322– 333, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Gubhaju L, Sutherland MR, Yoder BA, Zulli A, Bertram JF, Black MJ. Is nephrogenesis affected by preterm birth? Studies in a non-human primate model. Am J Physiol Renal Physiol 297: F1668– F1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hermes-DeSantis ER, Clyman RI. Patent ductus arteriosus: pathophysiology and management. J Perinatol 26, Suppl 1: S14– S18, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Hinchliffe SA, Sargent PH, Chan YF, van Velzen D, Howard CV, Hutton JL, Rushton DI. “Medullary ray glomerular counting” as a method of assessment of human nephrogenesis. Pathol Res Pract 188: 775– 782, 1992 [DOI] [PubMed] [Google Scholar]
- 13. Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest 64: 777– 784, 1991 [PubMed] [Google Scholar]
- 14. Kang NS, Yoo KH, Cheon H, Choi BM, Hong YS, Lee JW, Kim SK. Indomethacin treatment decreases renal blood flow velocity in human neonates. Biol Neonate 76: 261– 265, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Kaplan BS, Restaino I, Raval DS, Gottlieb RP, Bernstein J. Renal failure in the neonate associated with in utero exposure to non-steroidal anti-inflammatory agents. Pediatr Nephrol 8: 700– 704, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Keller RL, Tacy TA, Fields S, Ofenstein JP, Aranda JV, Clyman RI. Combined treatment with a nonselective nitric oxide synthase inhibitor (l-NMMA) and indomethacin increases ductus constriction in extremely premature newborns. Pediatr Res 58: 1216– 1221, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Kent AL, Douglas-Denton R, Shadbolt B, Dahlstrom JE, Maxwell LE, Koina ME, Falk MC, Willenborg D, Bertram JF. Indomethacin, ibuprofen and gentamicin administered during late stages of glomerulogenesis do not reduce glomerular number at 14 days of age in the neonatal rat. Pediatr Nephrol 24: 1143– 1149, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Kent AL, Maxwell LE, Koina ME, Falk MC, Willenborg D, Dahlstrom JE. Renal glomeruli and tubular injury following indomethacin, ibuprofen, and gentamicin exposure in a neonatal rat model. Pediatr Res 62: 307– 312, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Komhoff M, Wang JL, Cheng HF, Langenbach R, McKanna JA, Harris RC, Breyer MD. Cyclooxygenase-2-selective inhibitors impair glomerulogenesis and renal cortical development. Kidney Int 57: 414– 422, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Muhl H, Sandau K, Brune B, Briner VA, Pfeilschifter J. Nitric oxide donors induce apoptosis in glomerular mesangial cells, epithelial cells and endothelial cells. Eur J Pharmacol 317: 137– 149, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Norwood VF, Morham SG, Smithies O. Postnatal development and progression of renal dysplasia in cyclooxygenase-2 null mice. Kidney Int 58: 2291– 2300, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev 4: CD003481, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Olliges A, Wimmer S, Nusing RM. Defects in mouse nephrogenesis induced by selective and non-selective cyclooxygenase-2 inhibitors. Br J Pharmacol 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodriguez MM, Gomez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol 7: 17– 25, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Saez F, Reverte V, Salazar F, Castells MT, Llinas MT, Salazar FJ. Hypertension and sex differences in the age-related renal changes when cyclooxygenase-2 activity is reduced during nephrogenesis. Hypertension 53: 331– 337, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Seidner SR, Chen YQ, Oprysko PR, Mauray F, Tse MM, Lin E, Koch C, Clyman RI. Combined prostaglandin and nitric oxide inhibition produces anatomic remodeling and closure of the ductus arteriosus in the premature newborn baboon. Pediatr Res 50: 365– 373, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Sener A, Smith FG. Glomerular and tubular responses to NG-nitro-l-arginine methyl ester are age dependent in conscious lambs. Am J Physiol Regul Integr Comp Physiol 282: R1512– R1520, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Siassi B, Blanco C, Cabal LA, Coran AG. Incidence and clinical features of patent ductus arteriosus in low-birthweight infants: a prospective analysis of 150 consecutively born infants. Pediatrics 57: 347– 351, 1976 [PubMed] [Google Scholar]
- 29. Sodini D, Baragatti B, Barogi S, Laubach VE, Coceani F. Indomethacin promotes nitric oxide function in the ductus arteriosus in the mouse. Br J Pharmacol 153: 1631– 1640, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Speziale MV, Allen RG, Henderson CR, Barrington KJ, Finer NN. Effects of ibuprofen and indomethacin on the regional circulation in newborn piglets. Biol Neonate 76: 242– 252, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Sutherland MR, Gubhaju L, Moore L, Kent AL, Dahlstrom JE, Horne RS, Hoy WE, Bertram JF, Black MJ. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol 22: 1365– 1374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sutherland MR, Gubhaju L, Yoder BA, Stahlman MT, Black MJ. The effects of postnatal retinoic acid administration on nephron endowment in the preterm baboon kidney. Pediatr Res 65: 397– 402, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van der Heijden BJ, Carlus C, Narcy F, Bavoux F, Delezoide AL, Gubler MC. Persistent anuria, neonatal death, and renal microcystic lesions after prenatal exposure to indomethacin. Am J Obstet Gynecol 171: 617– 623, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Vassalle C, Domenici C, Lubrano V, L'Abbate A. Interaction between nitric oxide and cyclooxygenase pathways in endothelial cells. J Vasc Res 40: 491– 499, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Waleh N, Seidner S, McCurnin D, Yoder B, Liu BM, Roman C, Mauray F, Clyman RI. The role of monocyte-derived cells and inflammation in baboon ductus arteriosus remodeling. Pediatr Res 57: 254– 262, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci USA 96: 7563– 7568, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]