Abstract
We tested the effects of insulin (2 nM, 30–60 min) on principal cells of isolated split-open rat cortical collecting ducts (CCD) using whole-cell current measurements. Insulin addition to the superfusate of the tubules enhanced Na pump (ouabain-sensitive) current from 18 ± 3 to 31 ± 3 pA/cell in control and from 74 ± 9 to 126 ± 11 pA/cell in high K-fed animals. It also more than doubled ROMK (tertiapin-Q-sensitive) K+ currents in control CCD from 320 ± 40 to 700 ± 80 pA/cell, although it did not affect this current in tubules from K-loaded rats. Insulin did not induce the appearance of amiloride-sensitive Na+ current in control animals, while in high K-fed animals the currents were similar in the presence (140 ± 30) and the absence (180 ± 70 pA/cell) of insulin. Intraperitoneal injection of insulin plus hypertonic dextrose decreased Na excretion, as previously reported. However, injection of dextrose alone, or the nonmetabolized sugar mannose, had similar effects, suggesting that they were largely the result of vascular volume depletion rather than specific actions of the hormone. In summary, we find no evidence for acute upregulation of the epithelial Na channel (ENaC) by physiological concentrations of insulin in the mammalian CCD. However, the hormone does activate both the Na/K pump and apical K+ channels and could, under some conditions, enhance renal K+ secretion.
Keywords: ENaC, ROMK, Kir1.1, Na-K-ATPase
the systems that control the excretion of K+ in the urine have not been fully identified. Although aldosterone has been implicated in the increased secretion of K+ under conditions of high K+ intake (35), even adrenalectomized animals can adapt to a dietary K+ load (30), implicating additional kaliuretic factors. Insulin is a candidate for one such factor, as this hormone is secreted in response to ingestion of a meal, when large amounts of K+ can be absorbed into the extracellular fluid. The hormone helps to control plasma K+ under these circumstances, at least in part by stimulating K+ uptake into cells (26).
At least three transporters are directly involved in effecting K+ secretion in the distal nephron; the basolateral Na/K pump brings K+ into the cell, apical K+ channels facilitate its exit from the cell into the urine, and apical Na+ channels help to provide an electrical driving force for this exit step. Insulin stimulates Na-K-ATPase in many cell types including the mammalian cortical collecting duct (CCD) (7, 8). The hormone also clearly upregulates the epithelial Na channel (ENaC) in amphibian model tissues, including frog skin (28), toad urinary bladder (3, 29), and A6 cells (2, 9). However, such effects have not been observed in mammalian kidney tubules (15, 34). The possible role of insulin in regulating K+ channels has not been directly tested.
Assessments of the effects of insulin on Na+ and K+ excretion in vivo have been inconsistent (5, 10, 18, 21, 27), in part due to difficulties secondary to changes in plasma glucose and K+ levels caused by the hormone. Recently, however, evidence has been presented supporting increased Na+ reabsorption through the epithelial Na channel (ENaC) in response to insulin (32, 33).
We have directly examined the actions of insulin on apical Na+ and K+ channels as well as on the Na/K pump. We also reevaluated the effects of hormone injection in vivo using the rat as a model system. We conclude that insulin can upregulate ROMK channels and the Na/K pump but find no evidence for an acute effect on ENaC. Stimulation of the K+-transporting system could help to excrete a K+ load after a meal.
METHODS
Animals.
All procedures using animals were approved by the Institutional Animal Care and Use Committee of Weill-Cornell Medical College. Sprague-Dawley rats (180–220 g) of either gender (Charles River Laboratories, Kingston, NY) raised free of viral infections were used for all experiments. Animals were fed either normal lab chow or a synthetic diet containing 10% KCl (Harlan Teklad) for 1 wk.
Electrophysiology.
Measurement of whole-cell Na+ currents (INa) in principal cells of the CCD followed procedures described previously (11, 12). Split-open tubules were superfused at a rate of ∼5 ml/min (mean velocity ∼ 4 mm/s) with solutions prewarmed to 37°C containing (in mM) 135 Na methanesulfonate, 5 KCl, 2 Ca methanesulfonate, 1 MgCl2, 2 glucose, 5 Ba methanesulfonate and 10 HEPES adjusted to pH 7.4 with NaOH.
Patch-clamp pipettes were filled with solutions containing (in mM) 7 KCl, 123 aspartic acid, 20 CsOH, 20 TEAOH, 5 EGTA, 10 HEPES, 3 MgATP, and 0.3 NaGDPβS, with the pH adjusted to 7.4 with KOH. The total concentration of K+ was ∼120 mM. Net Na+ fluxes through Na+ channels were measured as the difference in current with and without 10−5 M amiloride (Sigma-Aldrich) in the bath.
For measurements of whole-cell K+ currents, Ba2+ was omitted from the superfusate. The pipette solutions contained (in mM) 7 KCl, 123 aspartic acid, 5 EGTA, and 10 HEPES, with the pH adjusted to 7.4 with KOH. The total concentration of K+ was ∼140 mM. Net K+ fluxes through ROMK channels were measured as the difference in current with and without 10−7 M tertiapin-Q (TPNQ; Sigma-Aldrich) in the bath.
For measurements of whole-cell Na/K-pump currents, the superfusate was the same as for the measurement of INa. The pipette solutions contained (in mM) 7 KCl, 123 aspartic acid, 20 CsOH, 20 TEAOH, 5 EGTA, 10 HEPES, 3 MgATP and 50 mM NaOH, with the pH adjusted to 7.4 with KOH. The total concentration of K+ was ∼70 mM. Pump currents were measured as the difference in current with and without 10−3 M ouabain (Sigma-Aldrich) in the bath.
Pipettes were pulled from hematocrit tubing, coated with Sylgard, and fire polished with a microforge. Pipette resistances ranged from 2 to 5 MΩ. Currents were measured with a List EPC-7 amplifier (Heka Elektronik, Lambrecht, Germany). Voltages were controlled and currents recorded using Pulse software (Heka) and an Instrutech ITC-16 interface (Instrutech, Mineola, NY).
Insulin injection.
To assess the effects of insulin in vivo, rats were placed individually in metabolic cages and urine was collected for two intervals of 2 h each. Animals were injected intraperitoneally (ip) with 0.5 U/kg body wt of human insulin (Humulin R) solution with 50% dextrose (2.5 g/kg body wt). Controls received 1 ml sterile H2O ip. In some cases insulin was injected with 1 ml of 5% dextrose. Other animals were given the same dose of hypertonic dextrose alone or an equivalent dose of mannose. After the final urine collection, a sample of blood was obtained from the aorta under anesthesia.
Analysis.
Na+ and K+ concentrations in urine and plasma were measured with a flame photometer (model 943, Instrumentation Laboratories, Lexington, MA). Creatinine in plasma and urine was measured using a commercial kit (Infinity, Fisher Diagnostics, Middletown, VA).
Modeling.
We used a previously developed mathematical model of Na+ and K+ transport by the CCD (16). For the basal state, we used an intermediate value of apical Na permeability (PNa) of 0.94 × 10−8 cm2/s corresponding to a modest level of stimulation by aldosterone (16). Other membrane parameters assumed were the apical K+ conductance (440 nS/mm tubule), basolateral K+ conductance (5,800 nS/mm), maximal basolateral Na/K pump activity of 17 nA/mm, and a nonspecific paracellular conductance of 300 nS/mm.
Statistical analysis.
Statistical significance was evaluated using the two-tailed Student's t-test.
RESULTS
Insulin increases the activity of the Na/K-ATPase in many cells including those of the CCD (7, 8). To see whether this could be detected electrically in the split-open tubule preparation, we measured the currents generated by the pump as the ouabain-sensitive component of the whole-cell current-voltage (I-V) relationship (22). The tubules were first opened and preincubated for 30 min in either control solution or with 2 nM (270 μU/ml) insulin. For comparison, plasma insulin levels in rats are ∼30 μU/ml and increase to 60–100 μU/ml with a glucose challenge (1, 19, 24, 25). Thus the dose chosen is somewhat higher than what the kidneys would normally experience in vivo.
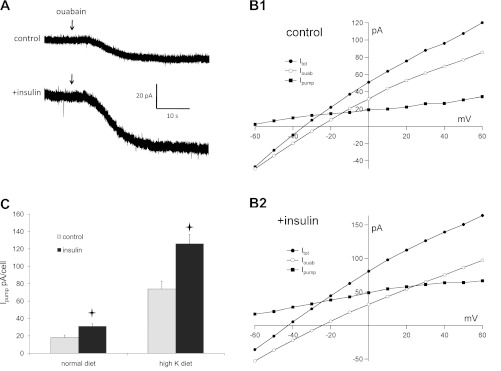
In most tubules, we assessed pump current by adding ouabain while monitoring membrane current in principal cells of CCDs while clamping the membrane voltage to 0 mV. Typical examples for rats on a normal diet are shown in Fig. 1A. In some cases, we measured I-V relationships before and after ouabain administration. Figure 1B shows typical examples of these I-V curves with and without insulin. As previously reported (22), the ouabain-sensitive currents were outward at voltages greater than or equal to −60 mV and tended to saturate at positive cell potentials. Thus currents measured at 0 mV are close to maximal under these conditions. Although using separate tubules for the measurements with and without hormone introduced some variability, the pump currents were consistently larger in the insulin-treated cells, with an average increase of 70% (Fig. 1C). There was no systematic effect of insulin on the extrapolated zero-current voltage or on the current in the presence of ouabain. We also tested effects of insulin on CCDs from rats adapted to a high-K diet. As found earlier (22), this adaptation process entailed a large increase in pump activity. Insulin further increased the pump currents by a factor of 1.7-fold, similar to cells from animals fed the control diet. Thus the effects of high K+ intake in vivo and insulin in vitro appeared to be independent.
Fig. 1.
Insulin upregulates Na/K pump activity in principal cells of the rat cortical collecting duct (CCD). A: responses to ouabain administration with the cell voltage clamped to 0 mV in a control cell and a cell treated with insulin from an animal on normal chow. Initial outward currents before ouabain addition were 21 and 65 pA, respectively. B: pump-mediated current-voltage (I-V) relationships from principal cells of an animal on normal chow. Data were obtained from a control cell (B1) and a cell treated with insulin (B2). Currents were measured at steady state in the absence (Itot; ●) and presence (Iouab; ○) of 1 mM ouabain. The difference current (Ipump; ■) represents charge moved through the pump. C. summary of Ipump measured at 0 mV in control and insulin-treated cells from animals on control or high-K diets. Values are means ± SE for 13 cells (control diet) and 9–11 cells (high-K diet). *Statistical significance (P < 0.05 vs. control values).
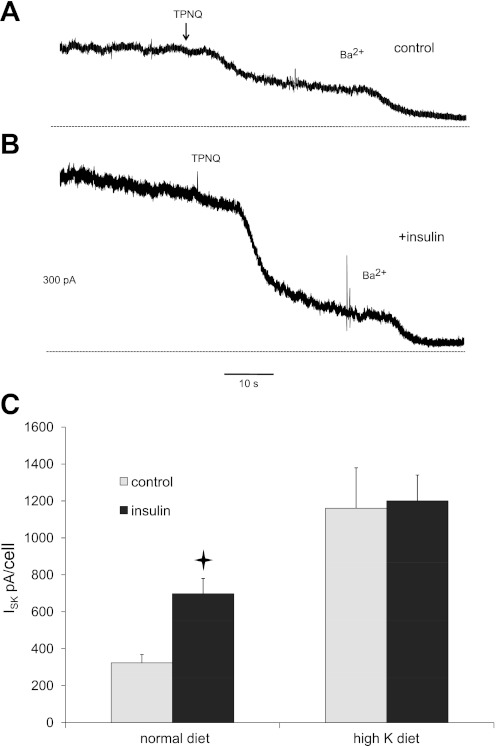
We next examined the effects of the hormone on the function of the renal secretory K+ channel Kir1.1 (ROMK). Activity of these channels was assessed as the outward whole-cell current inhibited by the modified honey bee venom peptide TPNQ (ISK) (14). Although this peptide can under some circumstances also block currents through BK (Maxi-K) channels (17), these channels will not be active under these conditions due to the high concentration of EGTA in the pipette solution, leading to very low levels of cytoplasmic Ca2+ (23). Typical tracings are shown in Fig. 2A and B. As with the pump currents, tubules were isolated, split open, and preincubated for 30 min with or without 2 nM insulin. In tubules from control animals, insulin increased ISK by about twofold (P = 0.0002) (Fig. 2C). Although there was again some variability in the magnitude of these currents from animal to animal, they were larger in the insulin-treated tubules in each of seven rats tested. When the mean values of ISK from individual rats were compared, the ratio of values from insulin-treated/controls was 2.42 ± 0.44 (P = 0.017). In tubules from animals fed a high-K diet, basal K+ currents were considerably larger, consistent with previous findings (14). In this case, however, insulin did not further increase ISK. Therefore in contrast to the actions on the Na/K pump, the effects of insulin and K loading were not additive.
Fig. 2.
Insulin upregulates ROMK channel activity in the rat CCD. A: typical trace from a principal cell of a control CCD. The animal was on normal chow. Currents were monitored at 0 mV while 10−7 M tertiapin-Q (TPNQ) and 5 mM Ba2+ were added sequentially to the superfusate. The dotted line indicates the 0-current level. B: similar trace from a principal cell of an insulin-treated CCD. C: summary of ISK measured as the change in current after addition of TPNQ in control and insulin-treated cells from animals on control or high-K diets. Values are means ± SE for 26 cells (control diet) and 7–10 cells (high-K diet). *Statistical significance (P < 0.05 vs. control values).
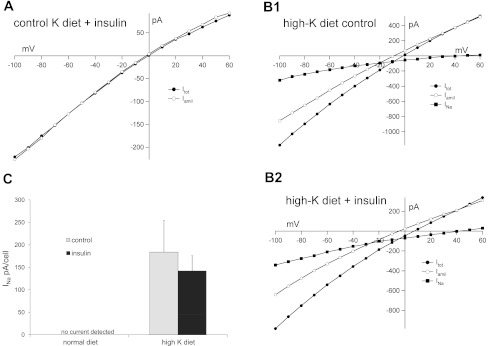
Finally, we tested the ability of insulin to regulate apical Na+ channels. Activity of these channels was measured as inward current inhibited by 10−5 M amiloride. In control animals, these currents were very low and difficult to detect (Fig. 3A), consistent with previous findings (11, 13). Pretreatment with insulin did not induce significant amiloride-sensitive currents (Fig. 3A). Similar negative results were obtained with 5 nM rat IGF1 (data not shown). One caveat is that basal currents under these conditions were very low, so that even a large fractional increase might be difficult to test. In contrast, when animals were fed a high-K diet Na+ currents were easily measurable (Fig. 3B). The amiloride-sensitive I-V relationship shows typical Goldman-type inward rectification. The reversal potentials at +40 to +50 mV presumably reflect accumulation of Na+ in the cell close to the membrane by diffusion through open channels. There was no significant difference in these currents in cells treated with 2 nM insulin (Fig. 3, B2 and C).
Fig. 3.
Insulin does not stimulate epithelial Na channel (ENaC)-mediated currents in the rat CCD. A: typical I-V relationships from a principal cell of a control CCD in the presence of insulin. The animal was on normal chow. Currents were measured at steady state in the absence (Itot; ●) and presence (Iamil; ○) of 10−5 M amiloride. B: I-V relationships from principal cells of CCDs from animals on a high-K diet. Perfusates were without (top) or with (bottom) 2 nM insulin. The difference currents (INa; ■) represent charge moved through the Na+ channels. C: summary of INa from control and insulin-treated cells from animals on control or high-K diets. Values are means ± SE for 9 cells (control diet) and 19–20 cells (high-K diet).
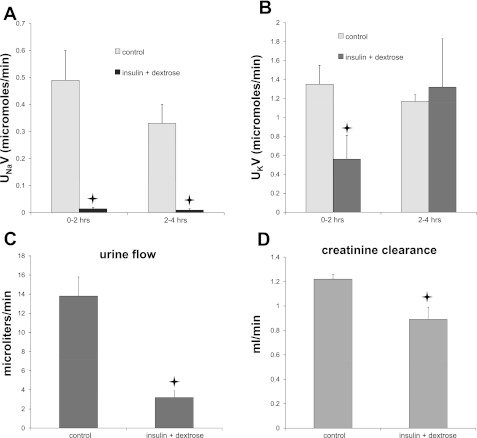
The absence of an effect of insulin on Na+ channels, although consistent with previous measurements using isolated-perfused tubules (see discussion), was surprising in light of a recent report (32) that insulin injection into mice resulted in an acute, substantial fall in Na excretion that could be reversed with amiloride. We therefore repeated some of these protocols in rats. As shown in Fig. 4A, ip injection of insulin together with 1 ml 50% dextrose to prevent hypoglycemia produced a dramatic decrease in Na+ excretion compared with H2O-injected controls that was maintained over 4 h. These results replicate those reported previously for mice (32).
Fig. 4.
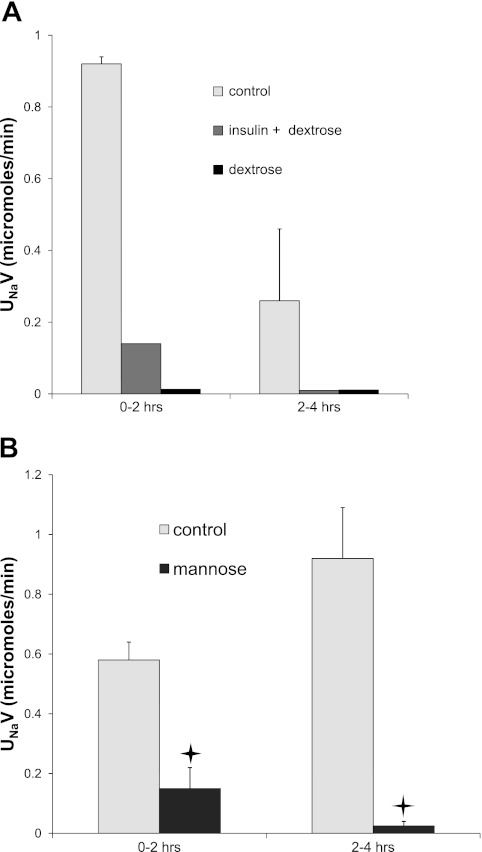
Injection of insulin+hypertonic dextrose decreases Na+, K+, and H2O excretion, and glomerular filtration rate. Rats were injected (ip) with 1 ml of either water or insulin in 50% dextrose solution. Urine was collected over two 2-h periods. A: Na+ excretion. B: K+ excretion. C: volume excretion. D: creatinine clearance was measured from the rate of creatinine excretion during the final 2-h period and the concentration of creatinine in plasma at the end of this collection. *Statistical significance (P < 0.05 vs. control values).
However, several aspects of this response were not as expected for a specific activation of ENaC. First, K+ excretion fell (Fig. 4B), rather than being stimulated as would be predicted from increased Na+ channel activity. Second, urine volume flow was strongly depressed (Fig. 4C). Finally, the glomerular filtration rate, estimated from creatinine clearance, decreased by ∼30% in the insulin+dextrose-treated animals (Fig. 4D). We also noted in many cases the accumulation of fluid in the peritoneal cavity, suggesting that these effects might result in part from a reduction in extracellular fluids due to the presence of hyperosmotic dextrose.
To test this idea further, we injected dextrose alone, without added insulin. Urine volume and Na+ excretion fell, similar to those in the insulin+dextrose-treated animals (Fig. 5A), showing that insulin administration was not necessary to evoke the natriferic response. However, it was possible that the sugar elicited the secretion of endogenous insulin secretion. To test this, we injected four animals with insulin plus isotonic dextrose. This concentration of sugar prevented hypoglycemia, but Na+ excretion and urine volume did not decline under these conditions (data not shown), implying that insulin was not sufficient for the observed anti-natriuresis. Finally, we used 50% mannose instead of glucose. This sugar should not produce insulin release from the pancreas. Even in the absence of added insulin, administration of mannose strongly decreased Na+ excretion (Fig. 5B). Mannose treatment decreased urine volume flow less than did dextrose, probably due to an osmotic diuresis due to urinary excretion of the nonreabsorbable sugar. These results suggest that the reduction in Na+ excretion observed here and previously is at least in part an indirect effect of the presence of hypertonic fluid in the peritoneal cavity.
Fig. 5.
Injection of 50% dextrose or 50% mannose decreased Na+ excretion. A: rats were injected ip with 1 ml of water, dextrose+insulin, or dextrose alone. B: rats were injected with 1 ml water or mannose. Urine was collected over two 2-h periods. *Statistical significance (P < 0.05 vs. control values).
DISCUSSION
Effects of insulin on Na-K-ATPase.
The stimulation of Na+ pump activity by insulin that we observed is consistent with previous reports showing that the hormone increased ouabain-sensitive Rb+ uptake in isolated rat CCDs (7, 8). In those studies, a 30–50% stimulation was observed after 15-min incubation with 10 nM insulin. The effect was independent of the Na+ load on the cells. This activation is part of a general pattern of regulation of the pump by the hormone in tissues including skeletal muscle (4, 36), liver (6), adipocytes (20), and renal proximal tubules (7). The mechanism by which this stimulation occurs remains to be established. In adipocytes, the effect appears to involve an increased affinity of the enzyme for intracellular Na+ (20). However, in the CCD the hormone did not change the Na+ affinity for ATP hydrolysis, indicating an increased maximal turnover rate (8). Our data do not address this issue.
Effects of insulin on ROMK.
To our knowledge, this is the first report on the effects of insulin on ROMK channels, the major apical membrane pathway for K+ secretion by the distal nephron under most conditions. The increase in ROMK conductance, assessed as TPNQ-sensitive whole-cell current, contrasts with modest inhibition of net K+ secretion observed in rabbit CCDs (15). However, in that study Na+ transport was also somewhat reduced and the transepithelial voltage was depolarized, indicating that the driving force for K+ secretion was diminished. Thus effects on apical K+ conductance cannot be inferred from these data.
Effects of insulin on ENaC.
The effects of insulin in vitro on epithelial Na+ channels appear to be tissue dependent. In amphibian model systems, insulin clearly stimulates ENaC-mediated Na+ transport as shown in studies of frog skin (28), toad urinary bladder (3, 29), and A6 cells (2, 9). As discussed above, the rat CCD responds to insulin with an increase in Na-K-ATPase activity. However, in two studies of the isolated, perfused rabbit CCD, the hormone either modestly decreased (15) or did not affect (34) Na+ transport. Our failure to observe an effect of insulin on Na+ conductance in the rat CCD is consistent with these reports. However, the negative results should be interpreted with caution as we also saw no effect of IGF1 on whole-cell ENaC currents under these conditions, while a previous study found stimulation of Na channels by this hormone in cell-attached patches of rat CCD (31). We do not know the basis for this discrepancy.
Effects of insulin in vivo.
Several whole-kidney and in vivo studies suggested that insulin is antinatriuretic, although the site of action of the hormone was not clearly identified. Nizet et al. (21) found that insulin (80–300 μU/ml) reduced Na+, K+, and water excretion in perfused dog kidneys without any changes in glomerular filtration rate, plasma glucose, or K+. They suggested that the increase in Na+ reabsorption caused by insulin took place at a nephron segment different from that where Na+ is exchanged for K+. DeFronzo et al. (5) infused insulin, together with glucose to maintain euglycemia, into human subjects and reported decreases in Na+ and K+ excretion, while plasma K+ fell from 3.8 to 3.1 mM. They suggested that the increased Na+ reabsorption occurred in the diluting segment or more distally in the renal tubules, while decreased plasma K+ lowered K+ secretion. Kirchner (18) studied glucose-clamped, insulin-infused rats using free-flow micropuncture. He found that plasma insulin (up to 46 μU/ml) reduced Na+, Cl−, and water excretion. Insulin markedly increased Cl− reabsorption in the loop of Henle, but it had no effect on Cl− delivery out of the proximal convoluted tubule or on distal tubule fractional Cl− reabsorption.
Other investigations, however, failed to show direct insulin-induced increases in Na+ reabsorption.. In humans maintained euglycemic, Friedberg et al. (10) found that insulin decreased Na+ and K+ excretion but that this effect was abrogated when K+ was infused with insulin to maintain plasma K+. Briffeuil et al. (10) infused insulin directly into one dog renal artery. Fractional excretions of Na+ and K+ were not different from those in the contralateral renal artery infused with vehicle.
Rosseti et al. (27) assessed the effects of insulin on renal electrolyte excretion in saline-infused rats in which both plasma glucose and plasma K+ levels could be controlled. In euglycemic animals, elevation of plasma insulin levels to 380 μU/ml without administration of K+ caused a fall in plasma K+ of 0.35 mM and no change in K+ excretion. In animals infused with K+ to maintain constant plasma levels, insulin increased K+ excretion by 2.5-fold but did not affect Na+ excretion. Our results are in general accordance with these findings.
Most recently, Tiwari et al. (32) observed a strong decrease in Na+ excretion of mice treated with insulin plus hyperosmotic dextrose to maintain euglycemia. As discussed above, we believe that this effect is due at least in part to the hyperosmotic fluid added to the peritoneal cavity. Although we cannot rule out a natriferic response to insulin, we think it will be necessary to reevaluate this model, avoiding possible changes in extracellular volume and osmolarity associated with the dextrose administration.
Regulation of K+ excretion.
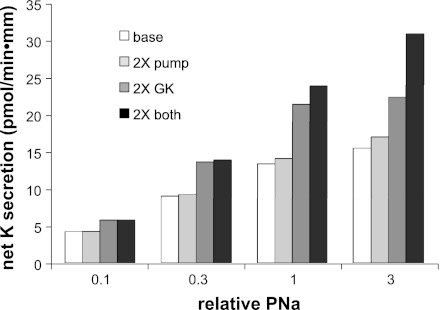
Although we could not document an effect of insulin on Na+ channels, believed to be the rate-limiting step in transepithelial Na+ transport, we did observe increases in the activities of transporters directly involved in K+ secretion, namely apical K+ (ROMK) channels and the Na-K-ATPase. To evaluate the impact of these changes on K+ secretion, we used a numerical model of Na+ and K+ transport developed previously (14, 16). Figure 6 shows the results of the analysis. Baseline values were chosen to mimic rates of Na+ and K+ transport observed under conditions of a modest level of ENaC activity (16). A twofold increase in apical K+ conductance (GK, a), similar to what we observed for rats on a normal rodent diet, increased net K+ secretion rates by 40–60%, depending on apical PNa. Increasing the pump activity by twofold induced smaller rises, from 1 to 10%, in K+ transport. Generally, the two effects of the two perturbations were additive. However, at the highest PNa, increasing GK, a and basolateral pump density were synergistic. This was the result of a large rise in intracellular Na+ from 19 to 48 mM with increased GK a, saturating the activity of the pump when its maximal turnover rate was limiting.
Fig. 6.
Calculation of the effects of increasing apical K+ conductance and Na/K pump activity on the rate of net K+ secretion in a model of transport by the rat CCD. Values of apical Na permeability (PNa) were varied over a factor of 30. At each value of PNa, net K+ secretion rates were calculated using basal values of apical K+ conductance (GK) and maximal pump rates, and after these parameters were increased by 2-fold either individually or together.
Overall, these calculations suggest that the observed actions of insulin could help to explain the findings of Rossetti et al. (27) discussed above. Administration of insulin to saline-loaded rats that were kept both euglycemic and eukalemic resulted in increased K+ excretion with little change in Na+ excretion. Although the calculated Na+ reabsorption through ENaC channels increases as a result of apical membrane hyperpolarization when K+ channels are activated, this effect is relatively small and might be compensated by other Na+ transporters.
Such a mechanism provides a means of excreting excess K+ after absorption of a meal, and the expected secretion of insulin. The hormone would be even more effective in this role if it also provoked a concomitant increase in Na+ channel activity, but we could not document such a stimulation. The accelerated K+ secretion would act together with increased uptake of K+ into cells in maintaining plasma K+ within normal limits.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK59659 and DK27847.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: G.F. and L.G.P. provided conception and design of research; G.F. and L.G.P. performed experiments; G.F. and L.G.P. analyzed data; G.F. and L.G.P. interpreted results of experiments; G.F. and L.G.P. edited and revised manuscript; G.F. and L.G.P. approved final version of manuscript; L.G.P. prepared figures; L.G.P. drafted manuscript.
REFERENCES
- 1. Bailey CJ, Matty AJ. Glucose tolerance and plasma insulin of the rat in relation to the oestrous cycle and sex hormones. Horm Metab Res 4: 266–270, 1972 [DOI] [PubMed] [Google Scholar]
- 2. Blazer-Yost BL, Esterman MA, Vlahos CJ. Insulin-stimulated trafficking of ENaC in renal cells requires PI3-kinase activity. Am J Physiol Cell Physiol 284: C1645–C1653, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Civan MM, Peterson-Yantorno K, O'Brien TG. Insulin and phrobol ester stimulate conductive Na+ transport through a common pathway. Proc Natl Acad Sci USA 85: 963–967, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clausen T, Kohn PG. The effect of insulin on the transport of sodium and potassium in rat soleus muscle. J Physiol 265: 19–42, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium and phosphate in man. J Clin Invest 55: 845–855, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fehlmann M, Freychet P. Insulin and glucagon stimulation of (Na+-K+)-ATPase transport activity in isolated rat hepatocytes. J Biol Chem 256: 7449–7453, 1981 [PubMed] [Google Scholar]
- 7. Feraille E, Marsy S, Cheval L, Barlet-Bas C, Khadouri C, Favre H, Doucet A. Sites of antinatriuretic action of insulin along rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 263: F175–F179, 1992 [DOI] [PubMed] [Google Scholar]
- 8. Feraille E, Rousselot M, Rajerison R, Favre H. Effect of insulin on Na,K-ATPase in rat collecting duct. J Physiol 488: 171–180, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fidelman ML, May JM, Biber TUL, Watlington CO. Insulin stimulation of Na+ transport and glucose metabolism in cultured kidney cells. Am J Physiol Cell Physiol 242: C121–C123, 1982 [DOI] [PubMed] [Google Scholar]
- 10. Friedberg CE, Koomans HA, Bijlsma JA, Rabelink TJ, Dorhout Mees EJ. Sodium retention by insulin may depend on decreased plasma potassium. Metabolism 40: 201–204, 1991 [DOI] [PubMed] [Google Scholar]
- 11. Frindt G, Masilamani S, Knepper MA, Palmer LG. Activation of epithelial Na channels during short-term Na deprivation. Am J Physiol Renal Physiol 280: F112–F118, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Frindt G, Palmer LG. Na channels in the rat connecting tubule. Am J Physiol Renal Physiol 286: F669–F674, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Frindt G, Sackin H, Palmer LG. Whole-cell currents in rat cortical collecting tubule: low-Na diet increases amiloride-sensitive conductance. Am J Physiol Renal Fluid Electrolyte Physiol 258: F562–F567, 1990 [DOI] [PubMed] [Google Scholar]
- 14. Frindt G, Shah A, Edvinsson JM, Palmer LG. Dietary K regulates ROMK channels in connecting tubule and cortical collecting duct of rat kidney. Am J Physiol Renal Physiol 296: F347–F354, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Furuya H, Talei K, Muto S, Asano Y. Effect of insulin on potassium secretion in rabbit cortical collecting ducts. Am J Physiol Renal Fluid Electrolyte Physiol 262: F30–F35, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Gray DA, Frindt G, Palmer LG. Quantification of K+ secretion through apical low-conductance K channels in the CCD. Am J Physiol Renal Physiol 289: F117–F126, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Kanjhan R, Coulson EJ, Adams DJ, Bellingham MC. Tertiapin-Q blocks recombinant and native large conductance K+ channels in a use-dependent manner. J Pharmacol Exp Ther 314: 1353–1361, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Kirschner KA. Insulin increases loop segment chloride reabsorption in the euglycemic rat. Am J Physiol Renal Fluid Electrolyte Physiol 255: F1206–F1213, 1988 [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi M, Olefsky JM. Effects of streptozotocin-induced diabetes on insulin binding, glucose transport, and intracellular glucose metabolism in isolated rat adipocytes. Diabetes 28: 87–95, 1979 [DOI] [PubMed] [Google Scholar]
- 20. Lytton J. Insulin affects the sodium affinity of the rat adipocyte (Na+,K+)-ATPase. J Biol Chem 260: 10075–10080, 1985 [PubMed] [Google Scholar]
- 21. Nizet A, Lefebve P, Crabbé J. Control by insulin of sodium and water excretion by the isolated dog kidney. Pflügers Arch 323: 11–20, 1971 [DOI] [PubMed] [Google Scholar]
- 22. Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol 104: 693–710, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palmer LG, Frindt G. High-conductance K channels in intercalated cells of the rat distal nephron. Am J Physiol Renal Physiol 292: F966–F973, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Reaven E, Wright D, Mondon CE, Solomon R, Ho H, Reaven GM. Effect of age and diet on insulin secretion and insulin action in the rat. Diabetes 32: 175–180, 1983 [DOI] [PubMed] [Google Scholar]
- 25. Reaven EP, Reaven GM. Structure and function changes in the endocrine pancreas of aging rats with reference to the modulating effects of exercise and caloric restriction. J Clin Invest 68: 75–84, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosa RM, Williams ME, Epstein FH. Extrarenal potassium metabolism. In: The Kidney: Physiology and Pathophysiology, edited by Seldin DW, Giebisch G. New York: Raven, 1992, p. 2165–2190 [Google Scholar]
- 27. Rossetti L, Klein-Robbenhaar G, Giebisch G, Smith D, DeFronzo R. Effect of insulin on renal potassium metabolism. Am J Physiol Renal Fluid Electrolyte Physiol 252: F60–F64, 1987 [DOI] [PubMed] [Google Scholar]
- 28. Schoen HF, Erlij D. Insulin action on electrophysiological properties of apical and basolateral membrane of frog skin. Am J Physiol Cell Physiol 252: C411–C417, 1987 [DOI] [PubMed] [Google Scholar]
- 29. Siegel B, Civan MM. Aldosterone and insulin effects on driving force of Na+ pump in toad bladder. Am J Physiol 230: 1603–1608, 1976 [DOI] [PubMed] [Google Scholar]
- 30. Stanton B, Giebisch G, Klein-Robbenhaar G, Wade J, DeFronzo RA. Effects of adrenalectomy and chronic adrenal corticosteroid replacement on potassium transport in rat kidney. J Clin Invest 75: 1317–1326, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Staruschenko A, Pochynyuk O, Vandewalle A, Bugaj V, Stockand JD. Acute regulation of the epithelial Na+ channel by phosphatidylinositide 3-OH kinase signaling in native collecting duct principal cells. J Am Soc Nephrol 18: 1652–1661, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Tiwari S, Nordquist L, Halagappa VK, Ecelbarger CA. Trafficking of ENaC subunits in response to acute insulin in mouse kidney. Am J Physiol Renal Physiol 293: F178–F185, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Tiwari S, Riazi S, Ecelbarger CA. Insulin's impact on renal sodium transport and blood pressure in health, obesity, and diabetes. Am J Physiol Renal Physiol 293: F974–F984, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Vehaskari VM, Hering-Smith KS, Moskowitz DW, Weiner ID, Hamm LL. Effect of epidermal growth factor on sodium transport in the cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 256: F803–F809, 1989 [DOI] [PubMed] [Google Scholar]
- 35. Wright FS, Giebisch G. Regulation of potassium secretion. In: The Kidney: Physiology and Pathophysiology (2nd ed.), edited by Seldin DW, Giebisch G. New York: Raven, 1992, p. 2209–2247 [Google Scholar]
- 36. Zierler KL. Effect of insulin on membrane potential and potassium content of rat muscle. Am J Physiol 197: 515–523, 1959 [DOI] [PubMed] [Google Scholar]