Abstract
Collecting duct (CD) endothelin-1 (ET-1) is an important autocrine inhibitor of Na and water transport. CD ET-1 production is stimulated by extracellular fluid volume expansion and tubule fluid flow, suggesting a mechanism coupling CD Na delivery and ET-1 synthesis. A mouse cortical CD cell line, mpkCCDc14, was subjected to static or flow conditions for 2 h at 2 dyn/cm2, followed by determination of ET-1 mRNA content. Flow with 300 mosmol/l NaCl increased ET-1 mRNA to 65% above that observed under static conditions. Increasing perfusate osmolarity to 450 mosmol/l with NaCl or Na acetate increased ET-1 mRNA to ∼184% compared with no flow, which was not observed when osmolarity was increased using mannitol or urea. Reducing Na concentration to 150 mosmol/l while maintaining total osmolarity at 300 mosmol/l with urea or mannitol decreased the flow response. Inhibition of epithelial Na channel (ENaC) with amiloride or benzamil abolished the flow response, suggesting involvement of ENaC in flow-regulated ET-1 synthesis. Aldosterone almost doubled the flow response. Since Ca2+ enhances CD ET-1 production, the involvement of plasma membrane and mitochondrial Na/Ca2+ exchangers (NCX) was assessed. SEA0400 and KB-R7943, plasma membrane NCX inhibitors, did not affect the flow response. However, CGP37157, a mitochondrial NCX inhibitor, abolished the response. In summary, the current study indicates that increased Na delivery, leading to ENaC-mediated Na entry and mitochondrial NCX activity, is involved in flow-stimulated CD ET-1 synthesis. This constitutes the first report of either ENaC or mitochondrial NCX regulation of an autocrine factor in any biologic system.
Keywords: sodium-calcium exchanger, cortical collecting duct, epithelial sodium channel
collecting duct (CD)-derived endothelin-1 (ET-1) is an important regulator of arterial pressure and urinary Na excretion. The CD is the predominant nephron site of ET-1 production and receptor expression (14). ET-1 binding to the CD results in reduction of epithelial Na channel (ENaC) activity through inhibition of channel open probability and apical membrane abundance (4, 27), as well as decreased Na-K-ATPase activity (34). CD-specific deficiency of ET-1 causes marked salt-sensitive hypertension and impaired renal Na excretion (1). Hence, CD-derived ET-1, under normal physiologic conditions, exerts a natriuretic and hypotensive effect.
High-Na intake enhances urinary ET-1 excretion (5, 6, 11, 19, 23) and CD ET-1 production (17), indicating that the CD ET-1 system perceives alterations in extracellular fluid volume (ECFV). However, the mechanism by which Na loading regulates CD ET-1 production is poorly understood. Circulating hormones that are modified by ECFV status do not explain Na loading-induced increases in CD ET-1 (8, 14). Na loading can increase medullary osmolarity leading to increased thick ascending limb ET-1 synthesis (9); however, the effect of tonicity on CD ET-1 production is controversial—interstitial hypertonicity may even reduce CD ET-1 synthesis (13). Our group recently showed that increasing fluid flow augments CD ET-1 production (17); since Na loading increases tubule fluid flow, this suggests a mechanism by which ECFV status regulates CD ET-1 production.
The mechanisms by which tubule flow increases CD ET-1 production are only beginning to be understood. Flow-induced increases in CD ET-1 synthesis are dependent on Ca2+, protein kinase C, and phospholipase C (17). However, the mechanism by which increases in tubule fluid flow result in activation of these intracellular signaling systems is unknown. While flow may cause mechanotransduced signals, an intriguing possibility is that Na delivery per se may activate signaling systems within the CD that lead to enhanced ET-1 production. Consequently, the current study was undertaken to investigate the relationship between Na delivery and CD ET-1 production, as well as to determine mechanisms by which Na delivery might regulate CD ET-1 production.
MATERIALS AND METHODS
Materials.
SEA0400 was synthesized by Taisho Pharmaceutical (Saitama, Japan). KB-R7943 and CGP 37157 were obtained from Tocris Bioscience (Ellisville, MO). DMEM and F-12 were obtained from Invitrogen Life Technologies (Grand Island, NY). All other drugs and chemicals were obtained from Sigma (St. Louis, MO) unless stated otherwise.
Cell culture.
The mouse cortical CD cell line mpkCCDc14 was generously provided by Prof. Alain Vandewalle at Institut National de la Santé et de la Recherche Médicale, France. Cells were grown to confluence on 10-cm2 plastic culture plates at 37°C in a 5% CO2 incubator. 50:50 DMEM/F-12 supplemented with 2 μg/ml dexamethasone, insulin, transferrin, selenium, 400 nM triiodothyronine, 1 μg/ml epidermal growth factor, 2 mM glutamine, 1 mg/ml penicillin, 1 mg/ml streptomycin, and 2% fetal bovine serum was used as a growth medium. In some cases, cells were grown under identical conditions in 24-well plates.
Flow studies.
Confluent cultures of mpkCCDc14 cells were rinsed with Hanks' balanced salt solution (HBSS) and flow chambers were attached to the plates. Rectangular parallel plate polycarbonate flow chambers [cat. no. 31–010, Glycotech, Gaithersburg, MD (shown at: www.glycotech.com/apparatus/parallel.html)] were sealed using vacuum and silastic gaskets onto individual culture plates to form a channel. The channel depth was 0.25 mm having a width of 1 cm and a length of 5.9 cm. Thus, the surface area of cells exposed to flow was 5.9 cm2. The flow chamber has two openings through which medium enters and exits the channel. The medium is recirculated through the channel by a peristaltic pump (Ismatec, Glattbrug, Switzerland) at a shear stress of 2 dyn/cm2 for 2 h. The perfusing medium used was HBSS (pH 7.4) for control experiments and supplemented with drugs and/or chemicals for other experiments. After flow, RNA was isolated from the cells within the perfusion chamber. RNA from cells exposed to flow was compared with RNA from control cells studied on the same day. Culture dishes with the control cells were placed into the flow chambers and exposed to the same perfusate solution as in the cells undergoing flow, with the exception that the perfusate was static (no flow). All experiments were performed at 37°C. Control cell RNA was isolated from cells within the area of the perfusion chamber.
ET-1 mRNA.
RNA was extracted from cells using RNeasy Mini Kit, reverse transcribed using Omniscript RT Kit (Qiagen, Valencia, CA), and cDNA levels were determined for ET-1 and GAPDH using real-time PCR (StepOne Plus, Applied Biosystems, Foster City, CA). PCR was performed according to instructions provided by the manufacturer using Taqman Gene Expression Assay (Applied Biosystems) with ET-1 (cat. no. Mm00438656_m1) and GAPDH (cat. no. Mm03302249_m1) primers.
Statistics.
Data are represented as means ± SE. Differences between groups were determined using two-way ANOVA. Bonferroni post hoc tests were used to compare individual means. P < 0.05 was taken as significant.
RESULTS
Effect of increased Na concentration on flow-stimulated ET-1 mRNA.
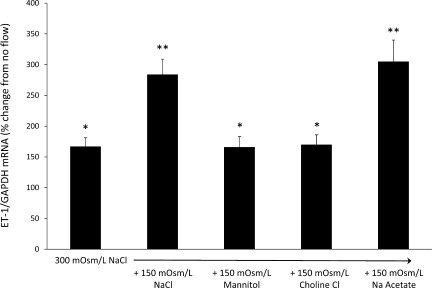
To determine the effect of increased Na delivery on CD ET-1 production, mpkCCDc14 cells were exposed to flow and static conditions for 2 h at 2 dyn/cm2 (Fig. 1). Flow with 300 mosmol/l NaCl increased ET-1 mRNA content by 67% over that seen with cells exposed to 300 mosmol/l NaCl under static (no flow) conditions. Perfusing with 450 mosmol/l NaCl increased ET-1 mRNA by 184% compared with static conditions; this was a significantly greater ET-1 flow response than seen with 300 mosmol/l NaCl. To determine the effect of increasing osmolarity per se, cells were perfused with 450-mosmol/l solution containing 300 mosmol/l NaCl and 150 mosmol/l mannitol; this yielded the same degree of increased ET-1 mRNA levels as seen with 300 mosmol/l NaCl, i.e., no augmentation of the flow response was observed by increasing perfusate osmolarity with mannitol. Increasing perfusate osmolarity to 450 mosmol/l using Na acetate (150 mosmol/l Na acetate plus 300 mosmol/l NaCl) increased ET-1 mRNA by 205%, i.e., similar to that seen with 450 mosmol/l NaCl and greater than that with 300 mosmol/l NaCl. Finally, increasing perfusate osmolarity to 450 mosmol/l using choline chloride (150 mosmol/l choline chloride plus 300 mosmol/l NaCl) increased ET-1 mRNA by 70%, similar to that seen with 300 mosmol/l NaCl, but not as great as observed with increasing osmolarity with NaCl or Na acetate. Hence, increasing perfusate Na concentration, but not perfusate osmolarity or chloride concentration, increased the ET-1 mRNA response to flow.
Fig. 1.
Effect of increased media osmolarity, Na, or chloride concentration on flow-stimulated endothelin (ET)-1 mRNA content in mpkCCDc14 cells. Each data point represents the comparison of ET-1 mRNA in cells exposed to flow vs. cells not undergoing flow, with both flowed and stationary cells exposed to identical media osmolarity and electrolyte concentrations; n = 12 each data point. *P < 0.05 vs. cells not exposed to flow. **P < 0.05 vs. cells exposed to 300 mosmol/l NaCl.
Effect of reduced Na concentration on flow-stimulated ET-1 production.
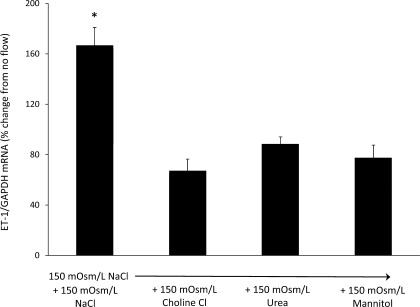
To determine the effect of reducing Na delivery on the ET-1 flow response (Fig. 2), cells were exposed to flow conditions with 150 mosmol/l NaCl. Perfusate osmolarity was maintained at 300 mosmol/l by using either 150 mosmol/l urea, mannitol, or choline chloride. The ET-1 flow response was decreased by 60, 47, and 54% when using choline chloride, urea, or mannitol, respectively. Thus, reducing perfusate NaCl concentration decreased flow-stimulated ET-1 mRNA content.
Fig. 2.
Effect of reduced Na concentration on flow-stimulated ET-1 mRNA content in mpkCCDc14 cells. Each data point represents the comparison of ET-1 mRNA in cells exposed to flow vs. cells not undergoing flow, with both flowed and stationary cells exposed to identical media osmolarity and electrolyte concentrations; n = 12 each data point. *P < 0.05 vs. cells not exposed to flow.
Role of ENaC in flow-stimulated ET-1 mRNA.
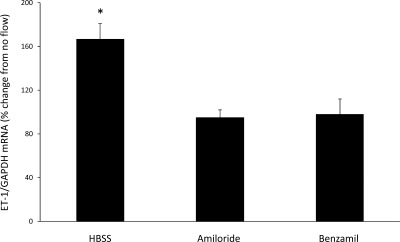
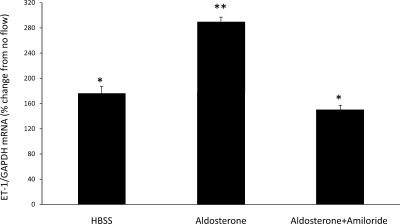
The above studies indicated that Na delivery is directly correlated with ET-1 mRNA accumulation. Since ENaC is the major channel through which Na enters CD cells, we evaluated the role of ENaC in the ET-1 response to flow (Fig. 3). When cells were treated with 1 μM amiloride, there was a complete loss of flow-stimulated ET-1 mRNA production. Similarly, 0.2 μM benzamil abolished the ET-1 flow response. To further explore a potential role of ENaC, cells were treated with 1 μM aldosterone [which has been shown to upregulate ENaC in mpkCCD cells (10)] for 48 h (Fig. 4). Aldosterone treatment markedly enhanced the ET-1 mRNA response to flow (190% increase in ET-1 mRNA compared with no flow). This increase was substantially greater than flow-stimulated ET-1 mRNA accumulation in cells not treated with aldosterone (67% increase in ET-1 mRNA compared with no flow). Addition of 1 μM amiloride for 30 min before flow experiments prevented the stimulatory effect of aldosterone on the ET-1 flow response. Taken together, these experiments indicate that ENaC is necessary for flow-induced ET-1 mRNA accumulation.
Fig. 3.
Effect of epithelial Na channel (ENaC) inhibition on flow-stimulated ET-1 mRNA content in mpkCCDc14 cells. Cells were pretreated for 30 min with 1 μM amiloride or 0.2 μM benzamil. Each data point represents the comparison of ET-1 mRNA in cells exposed to flow vs. cells not undergoing flow, with both flowed and stationary cells exposed to identical media and ENaC inhibitors. All studies were done in Hanks' balanced salt solution (HBSS; 300 mosmol/l); n = 12 each data point. *P < 0.05 vs. cells not exposed to flow.
Fig. 4.
Effect of aldosterone ± amiloride on flow-stimulated ET-1 mRNA content in mpkCCDc14 cells. Cells were pretreated with 1 μM aldosterone for 2 days and pretreated with 1 μM amiloride for 30 min. Each data point represents the comparison of ET-1 mRNA in cells exposed to flow vs. cells not undergoing flow, with both flowed and stationary cells exposed to identical media and reagents. All studies were done in HBSS (300 mosmol/l); n = 12 each data point. *P < 0.05 vs. cells not exposed to flow. **P < 0.05 vs. aldosterone alone.
Role of Na/Ca2+ exchange in flow-stimulated ET-1 mRNA.
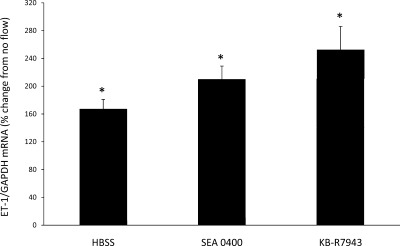
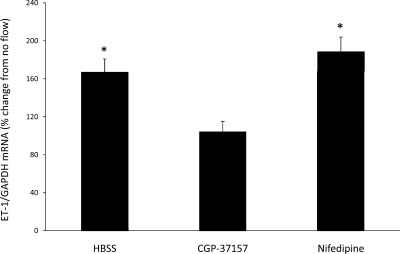
Since Na delivery and ENaC-mediated Na entry into cells are necessary for flow-stimulated ET-1 mRNA production, and since Ca2+ is required for CD ET-1 gene transcription (30), a potential relationship between Na and Ca2+ was investigated. The most apparent mechanism for such a relationship is the Na/Ca2+ exchanger (NCX) family. Since an increase in intracellular Na concentration ([Na]i) can potentially increase intracellular Ca2+ concentration ([Ca2+]i) through activation of plasma membrane NCX activity, the effect of inhibiting plasma membrane NCX was investigated. Treatment of cells with either 3 μM SEA0400, a highly selective inhibitor of plasma membrane NCX (20), as well as 10 μM KB-R7943, another plasma membrane NCX inhibitor, resulted in a flow-stimulated increase in the ET-1 mRNA content of 210 and 249%, respectively (Fig. 5). Thus, plasma membrane NCX does not appear to be involved in the ET-1 flow response. In contrast, treatment with CGP37157, the only known mitochondrial NCX inhibitor, completely prevented the ET-1 flow response (Fig. 6). While CGP37157 is specific for the mitochondrial NCX (as opposed to plasma membrane NCX isoforms), one report suggested that it may also inhibit L-type Ca2+ channels (31). To determine whether this mechanism was involved in the CGP37157 effect, cells were treated with 100 μM nifedipine. Nifedipine did not alter the ET-1 flow response (Fig. 6), indicating that the CGP37157 effect was not due to inhibition of L-type Ca2+ channels.
Fig. 5.
Effect of plasma membrane Na calcium exchanger inhibition on flow-stimulated ET-1 mRNA content in mpkCCDc14 cells under static (no flow) and flow conditions. Cells pretreated for 30 min with 3 μM SEA0400 (n = 7) or 10 μM KB-R7943 (n = 12). Each data point represents the comparison of ET-1 mRNA in cells exposed to flow vs. cells not undergoing flow, with both flowed and stationary cells exposed to identical media and reagents. All studies were done in HBSS (300 mosmol/l). *P < 0.05 vs. cells not exposed to flow.
Fig. 6.
Effect of mitochondrial Na calcium exchanger inhibition on flow-stimulated ET-1 mRNA content in mpkCCDc14 cells under static (no flow) and flow conditions. Cells were pretreated for 30 min with 1.2 μM CGP37157 (n = 12). Cells were pretreated with 100 μM nifedipine (n = 6) to control for an effect on L-type dihydropyridine Ca2+ channels. Each data point represents the comparison of ET-1 mRNA in cells exposed to flow vs. cells not undergoing flow, with both flowed and stationary cells exposed to identical media and reagents. All studies were done in HBSS (300 mosmol/l). *P < 0.05 vs. cells not exposed to flow.
Effect of treatments under no-flow conditions.
To determine whether the reagents or different media used in this study affected ET-1 mRNA levels in mpkCCD cells under no-flow conditions, cells were grown in 24-well dishes and the effect of changing media solute content or addition of various reagents was assessed (all experiments were done in HBSS for 2 h). None of the reagents (NCX or ENaC inhibitors) altered ET-1 mRNA content. Increasing osmolarity to 450 mosmol/l, regardless of the solute utilized, decreased ET-1 mRNA content by 51 ± 6% (n = 42) compared with cells exposed to 300 mosmol/l NaCl. Decreasing media NaCl concentration to 75 mM, while maintaining osmolarity at 300 mosmol/l, also decreased ET-1 mRNA content by 48 ± 9% (n = 27) compared with cells exposed to 300 mosmol/l NaCl, regardless of the solute used to maintain media osmolarity at 300 mosmol/l. Thus, the Na- and ENaC-dependent ET-1 flow response was not mimicked in cells under no-flow conditions. Finally, ET-1 mRNA content was similar between cells grown in 24-well plates and exposed to HBSS for 2 h compared with cells grown on 10-cm dishes, covered with perfusion chambers, and maintained under no-flow conditions for 2 h, indicating that the perfusion chamber conditions per se did not alter ET-1 mRNA levels.
DISCUSSION
Our previous study (17) demonstrated that flow increases CD ET-1 production; the goal of the current study was to explore mechanisms responsible for this effect. In the current study, we report that 1) Na delivery increases ET-1 mRNA accumulation in CD cells, 2) ENaC is necessary for flow-stimulated ET-1 mRNA augmentation, and 3) mitochondrial NCX is necessary for flow-stimulated CD ET-1 mRNA accumulation. Thus, while flow or shear stress per se may exert effects on CD ET-1 production, our findings indicate that the magnitude of Na delivery is an important factor in determining CD ET-1 production. This observation is consistent with the known stimulatory effect of a high-Na intake on CD ET-1 production in vivo (17). Since CD-derived ET-1 exerts an autocrine inhibitory effect on Na reabsorption (1), the above findings provide a rational system whereby increased Na intake leads to increased CD Na delivery, increased CD ET-1 production, ET-1 inhibition of Na transport, and ultimately enhanced natriuresis.
Our findings indicate that ENaC is necessary for flow-stimulated ET-1 production as evidenced by inhibition of the flow response by amiloride or benzamil. To our knowledge, this constitutes the first report of ENaC-mediated regulation of an autocrine or paracrine factor. It is likely that ENaC-mediated increases in [Na]i are involved in flow-stimulated ET-1 production. Relatively little is known about [Na]i regulation of intracellular signaling processes in the CD. Increases in [Na]i may alter ENaC activity through modulation of G proteins (15). In addition, increased [Na]i leads to augmented Na-K-ATPase activity through modulation of salt-inducible kinase-1 (SIK-1) (29). Activation of SIK-1 is thought to be due to elevations in [Na]i driving increases in [Ca2+]i, which in turn activate calmodulin leading to activation of SIK-1 (29). This latter effect is relevant to possible mechanisms involved in ET-1 production; CD ET-1 synthesis is critically dependent on [Ca2+]i (30), while flow-stimulated CD ET-1 mRNA accumulation is dependent on [Ca2+]i and Ca2+-regulated pathways, including protein kinase C and phospholipase C (17). An obvious mechanism coupling changes in [Na]i to changes in [Ca2+]i involves NCX. Three plasma membrane isoforms of NCX have been described that can transport Ca2+ into cells in response to increases in [Na]i (18, 25). However, we found that pharmacologic blockade of all isoforms of plasma membrane NCX with either SEA0400 or KB-R7943, inhibitors of plasma membrane NCX (3, 16, 20), did not affect the ET-1 flow response. In addition to plasma membrane NCX, another NCX isoform, NCLX, is expressed exclusively in mitochondria and extrudes Ca2+ from mitochondria in response to increases in [Na]i (26). Blockade of NCLX with CGP37157 (26) prevented flow-induced CD ET-1 mRNA accumulation, suggesting that mitochondrial NCLX-mediated increases in [Ca2+]i are involved in flow stimulation of ET-1 production. Such a scenario of flow-stimulated mitochondrial Ca2+ release is not unprecedented; shear stress-induced increases in [Ca2+]i in cardiac myocytes have been demonstrated to be dependent on release of Ca2+ from mitochondrial stores (2). Taken together, the above studies suggest that ENaC-mediated increases in [Na]i lead to increases in [Ca2+]i through augmentation of mitochondrial NCLX activity; the increased [Ca2+]i ultimately leads to stimulation of ET-1 production. It should be noted that we have not directly measured [Ca2+]i or [Na]i; however, we believe that our data strongly support the proposed changes in [Ca2+]i and [Na]i without having directly measured their levels.
One might argue that Na delivery-induced increases in ET-1 would ultimately lead to ET-1 inhibition of ENaC and reduction of the signal driving ET-1 production. While this might indeed be the case, it should be noted that it takes at least an hour of increased flow to increase ET-1 mRNA in the CD and that ET-1 has prolonged effects once bound to its receptors (17). Thus, while ET-1 may ultimately reduce the signal driving its own production, it would still have several hours to exert its effects. It is perhaps not surprising that such a negative feedback system exists—one would not want the drive for ET-1 synthesis to continue for too long given that the peptide exerts such sustained effects.
This study did not examine how Ca2+ increases CD ET-1 production; however, our group has studied this previously. As mentioned earlier, flow-induced increases in CD ET-1 synthesis depend on Ca2+ (17). The Ca2+ effect is due, at least in part, to calmodulin (CaM) and CaM kinase (30). Blockade of CaM decreased ET-1 production by rat inner medullary CD cells; this effect was associated with unchanged ET-1 mRNA stability, but decreased activity of a transfected ET-1 promoter-reporter construct. Notably, CaM inhibition did not affect ET-1 release or transfected ET-1 promoter-reporter activity in rat aortic endothelial cells, indicating that the Ca2+ dependency of ET-1 gene transcription is at least relatively unique to CD cells.
Na delivery and ENaC are necessary for the flow response, but are not in of themselves sufficient to account completely for flow-stimulated ET-1 production. The finding that increasing or decreasing media Na concentration in cells under no-flow conditions reduced ET-1 mRNA content suggests that an additional sensor mechanism is necessary to confer the ET-1 flow response to extracellular Na. In addition, ENaC inhibition in cells under no-flow conditions did not change ET-1 mRNA levels, again indicating that ENaC is necessary but not sufficient to account for the ET-1 flow response. Other flow sensors exist in the apical membrane of the CD. Transient receptor potential (TRP) channels are expressed in the CD [including TRPV5 as well as TRPC1, 3, and 6 (7, 12)], can sense flow, and can function as Na as well as Ca2+ channels (33). In addition, P2X receptors are located in the CD, can sense flow, and can function as Na channels (24, 32). Finally, cilia in CD, which are well-recognized flow sensors through activation of polycystins leading to augmented [Ca2+]i (28), could be involved in the ET-1 flow response. Clearly, additional studies are needed to evaluate the role of these systems in mediating the ET-1 response to flow.
It should be noted that ET-1 mRNA, but not ET-1 protein, was measured in the current study. As previously reported (17), ET-1 protein, either released into the perfusate or within cells, was undetectable by RIA or ELISA. Since ET-1 release parallels ET-1 mRNA levels in almost every condition under which this has been measured (14, 30), ET-1 mRNA was taken as an accurate index of ET-1 production. In addition, ET-1 mRNA is very short-lived (∼15 min) due to the presence of destabilizing AUUUA sequences in the 3-UTR of ET-1 mRNA; this is very characteristic of gene products that are primarily regulated at the transcriptional level (21, 22).
In summary, our findings suggest a link between Na delivery, ENaC, mitochondrial NCLX activity, and ET-1 mRNA accumulation. This system provides, at least teleologically, a link between Na intake and urinary Na excretion. In essence, high-Na intake, leading to ECFV expansion and increased Na delivery to the CD, activates the above system to ultimately induce autocrine inhibition of Na reabsorption by the CD. Novel findings in the current study include ENaC-dependent regulation of a regulatory peptide as well as the dependency of ET-1 production upon mitochondrial NCLX activity. Further exploration of the pathways involved in flow stimulation of ET-1 production, as well as the potential roles of ENaC and mitochondrial NCLX in regulating CD function, is warranted.
GRANTS
This research was supported by National Institutes of Health Grant P01 HL095499 (to D. E. Kohan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.M.P., K.A.S., T.M., and D.E.K. conception and design of research; M.M.P. and K.A.S. performed experiments; M.M.P., K.A.S., T.M., and D.E.K. analyzed data; M.M.P., K.A.S., T.M., and D.E.K. interpreted results of experiments; M.M.P. and D.E.K. prepared figures; M.M.P. and D.E.K. drafted manuscript; M.M.P., T.M., and D.E.K. edited and revised manuscript; M.M.P., K.A.S., T.M., and D.E.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Prof. Alain Vandewalle for generously providing the mpkCCDc14 cells.
REFERENCES
- 1. Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest 114: 504–511, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belmonte S, Morad M. Shear fluid-induced Ca2+ release and the role of mitochondria in rat cardiac myocytes. Ann NY Acad Sci 1123: 58–63, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Birinyi P, Acsai K, Banyasz T, Toth A, Horvath B, Virag L, Szentandrassy N, Magyar J, Varro A, Fulop F, Nanasi PP. Effects of SEA0400 and KB-R7943 on Na+/Ca2+ exchange current and L-type Ca2+ current in canine ventricular cardiomyocytes. Naunyn Schmiedebergs Arch Pharmacol 372: 63–70, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol 295: F1063–F1070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chu T, Wu MS, Hsieh BS. Urinary endothelin-1 in patients with renal disease. J Formos Med Assoc 97: 667–672, 1998 [PubMed] [Google Scholar]
- 6. Cuzzola F, Mallamaci F, Tripepi G, Parlongo S, Cutrupi S, Cataliotti A, Stancanelli B, Malatino LS, Bellanuova I, Ferri C, Galletti F, Filigheddu F, Glorioso N, Strazzullo P, Zoccali C. Urinary adrenomedullin is related to ET-1 and salt intake in patients with mild essential hypertension. Am J Hypertens 14: 224–230, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Goel M, Sinkins WG, Zuo CD, Estacion M, Schilling WP. Identification and localization of TRPC channels in the rat kidney. Am J Physiol Renal Physiol 290: F1241–F1252, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Gumz ML, Popp MP, Wingo CS, Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol Renal Physiol 285: F664–F673, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Herrera M, Garvin J. A high-salt diet stimulates thick ascending limb eNOS expression by raising medullary osmolality and increasing release of endothelin-1. Am J Physiol Renal Physiol 288: F58–F64, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Hill WG, Butterworth MB, Wang H, Edinger RS, Lebowitz J, Peters KW, Frizzell RA, Johnson JP. The epithelial sodium channel (ENaC) traffics to apical membrane in lipid rafts in mouse cortical collecting duct cells. J Biol Chem 282: 37402–37411, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Hwang YS, Hsieh TJ, Lee YJ, Tsai JH. Circadian rhythm of urinary endothelin-1 excretion in mild hypertensive patients. Am J Hypertens 11: 1344–1351, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Irnaten M, Blanchard-Gutton N, Praetorius J, Harvey BJ. Rapid effects of 17beta-estradiol on TRPV5 epithelial Ca2+ channels in rat renal cells. Steroids 74: 642–649, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Kohan DE, Padilla E. Osmolar regulation of endothelin-1 production by rat inner medullary collecting duct. J Clin Invest 91: 1235–1240, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev 91: 1–77, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Komwatana P, Dinudom A, Young JA, Cook DI. Cytosolic Na+ controls and epithelial Na+ channel via the Go guanine nucleotide-binding regulatory protein. Proc Natl Acad Sci USA 93: 8107–8111, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luciani DS, Ao P, Hu X, Warnock GL, Johnson JD. Voltage-gated Ca2+ influx and insulin secretion in human and mouse beta-cells are impaired by the mitochondrial Na+/Ca2+ exchange inhibitor CGP-37157. Eur J Pharmacol 576: 18–25, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Lyon-Roberts B, Strait KA, van Peursem E, Kittikulsuth W, Pollock JS, Pollock DM, Kohan DE. Flow regulation of collecting duct endothelin-1 production. Am J Physiol Renal Physiol 300: F650–F656, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lytton J. Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem J 406: 365–382, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Malatino LS, Bellanuova I, Cataliotti A, Cuzzola F, Mallamaci F, Tripepi G, Parlongo S, Cutrupi S, Mangiafico RA, Ferri C, Galletti F, Gloriso N, Strazzullo P, Zoccali C. Renal endothelin-1 is linked to changes in urinary salt and volume in essential hypertension. J Nephrol 13: 178–184, 2000 [PubMed] [Google Scholar]
- 20. Matsuda T, Arakawa N, Takuma K, Kishida Y, Kawasaki Y, Sakaue M, Takahashi K, Takahashi T, Suzuki T, Ota T, Hamano-Takahashi A, Onishi M, Tanaka Y, Kameo K, Baba A. SEA0400, a novel and selective inhibitor of the Na+-Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J Pharmacol Exp Ther 298: 249–256, 2001 [PubMed] [Google Scholar]
- 21. Mawji IA, Marsden PA. Perturbations in paracrine control of the circulation: role of the endothelial-derived vasomediators, endothelin-1 and nitric oxide. Microsc Res Tech 60: 46–58, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Mawji IA, Robb GB, Tai SC, Marsden PA. Role of the 3′-untranslated region of human endothelin-1 in vascular endothelial cells. Contribution to transcript lability and the cellular heat shock response. J Biol Chem 279: 8655–8667, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Modesti PA, Cecioni I, Costoli A, Poggesi L, Galanti G, Serneri GG. Renal endothelin in heart failure and its relation to sodium excretion. Am Heart J 140: 617–622, 2000 [DOI] [PubMed] [Google Scholar]
- 24. North RA. Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Ottolia M, John S, Xie Y, Ren X, Philipson KD. Shedding light on the Na+/Ca2+ exchanger. Ann NY Acad Sci 1099: 78–85, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, Khananshvili D, Sekler I. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci USA 107: 436–441, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pavlov TS, Chahdi A, Ilatovskaya DV, Levchenko V, Vandewalle A, Pochynyuk O, Sorokin A, Staruschenko A. Endothelin-1 inhibits the epithelial Na+ channel through betaPix/14–3-3/Nedd4–2. J Am Soc Nephrol 21: 833–843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Praetorius H, Spring K. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens 12: 517–520, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Sjostrom M, Stenstrom K, Eneling K, Zwiller J, Katz AI, Takemori H, Bertorello AM. SIK1 is part of a cell sodium-sensing network that regulates active sodium transport through a calcium-dependent process. Proc Natl Acad Sci USA 104: 16922–16927, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Strait KA, Stricklett PK, Kohan JL, Miller MB, Kohan DE. Calcium regulation of endothelin-1 synthesis in rat inner medullary collecting duct. Am J Physiol Renal Physiol 293: F601–F606, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Thule T, Ahn JR, Woo SH. Inhibition of L-type Ca2+ channel by mitochondrial Na+-Ca2+ exchange inhibitor CGP-37157 in rat atrial myocytes. Eur J Pharmacol 552: 15–19, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol 294: F10–F27, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev 62: 381–404, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeidel ML, Brady HR, Kone BC, Gullans SR, Brenner BM. Endothelin, a peptide inhibitor of Na+-K+-ATPase in intact tubular epithelial cells. Am J Physiol Cell Physiol 257: C1101–C1107, 1989 [DOI] [PubMed] [Google Scholar]