Abstract
Tubuloglomerular feedback (TGF) and connecting tubule glomerular feedback (CTGF) are mechanisms that control afferent arteriole (Af-Art) tone. TGF, initiated by increased NaCl at the macula densa, causes Af-Art constriction. Prolonged activation of TGF leads to an attenuation or “resetting” of its constrictor effect. The mechanisms of TGF resetting remain incompletely understood. CTGF is initiated by increased NaCl in the connecting tubule and Na+ entry via epithelial sodium channels (ENaC). Contrary to TGF, CTGF dilates the Af-Art. Here, we hypothesize that CTGF, in part, mediates TGF resetting. We performed micropuncture of individual rat nephrons while measuring stop-flow pressure (PSF), an index of glomerular filtration pressure and Af-Art tone. Increases in Af-Art tone cause PSF to decrease. TGF responses, measured as the decrease in PSF induced by switching late proximal tubule perfusion from 5 to 40 nl/min, were elicited before and after a 30-min period of sustained perfusion of the late proximal tubule at a rate of 40 nl/min designed to induce TGF resetting. TGF responses were 7.3 ± 0.3 and 4.9 ± 0.2 mmHg before and after resetting was induced (P < 0.001, n = 6). When CTGF was inhibited with the ENaC blocker benzamil (1 μM), TGF responses were 9.5 ± 0.3 and 8.8 ± 0.6 mmHg (NS, n = 6), thus resetting was abolished. In the presence of the carbonic anhydrase inhibitor acetazolamide (10 mM), TGF responses were 8.8 ± 0.6 and 3.3 ± 0.4 mmHg before and after resetting (P < 0.001, n = 6). With both acetazolamide and benzamil, TGF responses were 10.4 ± 0.2 and 8.4 ± 0.5 mmHg (P < 0.01, n = 6), thus resetting was attenuated. We conclude that CTGF, in part, mediates acutely induced TGF resetting.
Keywords: afferent arteriole, macula densa, glomerular filtration rate, Na+ transport, benzamil, stop-flow pressure
two segments of the distal nephron, the macula densa and the connecting tubule (CNT), return to their own glomerulus and come in close contact with the afferent arteriole (Af-Art). The macula densa contacts the Af-Art at the vascular pole of the glomerulus, while the CNT accompanies closely the Af-Art for some length, often more than half of its path from the branching of the interlobular artery to the glomerulus. This anatomical characteristic provides an opportunity for functional cross-talks between the tubules and the vasculature. Both the macula densa and CNT sense increases in NaCl in the lumen of the nephron and send signals that control the tone of the Af-Art, although with opposing effects. Increases in luminal NaCl at the macula densa cause constriction of the Af-Art, thus decreasing glomerular filtration rate (GFR) and favoring Na+ retention, a mechanism known as tubuloglomerular feedback (TGF) (15). On the other hand, increases in luminal NaCl cause the CNT to release prostaglandins and epoxyeicosatrienoic acids which dilate the Af-Art, a mechanism we called connecting tubule glomerular feedback (CTGF) (11, 12, 22). A decrease in Af-Art resistance increases both renal blood flow and GFR thus favoring Na+ excretion (12, 16, 21).
Sustained activation of TGF leads to an attenuation of TGF-induced Af-Art constriction, a phenomenon known as TGF resetting. Examples of physiological and pathophysiological conditions that induce TGF resetting include growth (2), contralateral nephrectomy (10), increased NaCl intake and volume expansion (3, 18).
Although several factors are known to modulate TGF, including nitric oxide (4, 14), cyclooxygenase (COX)-2 products (5), and the renin-angiotensin system (13), the mechanisms of TGF resetting remain incompletely understood. Briggs et al. (2) showed that sustained perfusion of a single nephron at 40 nl/min for 30 min attenuates the maximum decrease in stop-flow pressure (PSF), suggesting that TGF resetting occurs acutely and that the mechanism is intrinsic to the nephron in the absence of systemic changes.
We showed that, in vivo, vasodilatory CTGF counters vasoconstrictor TGF. Furthermore, we showed that the relative contribution of CTGF to the control of glomerular hemodynamics is greater at high perfusion rates (22), which are similar to the tubular flow rates seen in volume-expanded animals (7), and previously shown to induce TGF resetting (2). Thus, here we hypothesize that CTGF, in part, mediates acute TGF resetting.
MATERIALS AND METHODS
All experiments were approved by the Henry Ford Health System Institutional Animal Care and Use Committee and were conducted in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and APS's Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training. Male Sprague-Dawley rats weighing 243–300 g were anesthetized with thiobutabarbital (125 mg/kg body wt ip). Micropuncture experiments were performed as previously described (22). Briefly, animals were maintained at 37.5°C, infused with 0.9% NaCl 1.5 ml/h, and their blood pressure was monitored continuously. The left kidney was immobilized and bathed in saline. An early loop of proximal tubule was blocked with grease, a pressure pipette was inserted upstream from the grease block, and a perfusion pipette attached to a nanoliter infusion pump was inserted downstream from the grease block.
Each perfusion rate was maintained for 1–5 min as required to observe a stable PSF. Two TGF responses were elicited by switching the tubular perfusion rate from 5 to 40 nl/min at the 5- and 45-min time points.
Protocol 1 (time control): During the intervening 30-min period between the first and second TGF responses, the tubular perfusion rate was maintained at 5 nl/min.
Protocol 2 (resetting): During the intervening 30-min period between the first and second TGF responses, the tubular perfusion rate was maintained at 40 nl/min. TGF resetting was calculated as the difference between the first and second TGF responses.
Protocols 3–5 tested the effect of benzamil, acetazolamide, or both, on TGF resetting induced by maintaining tubular perfusion rate at 40 nl/min for 30 min.
The ENaC inhibitor benzamil and the carbonic anhydrase inhibitor acetazolamide were purchased from Sigma and administered in the tubular perfusate.
Statistics.
Data are expressed as means ± SE. TGF was defined as the change in PSF when switching tubular perfusion from 5 to 40 nl/min. Paired t-test was used to compare the first and second TGF responses (i.e., before and after a 30-min period of sustained tubular perfusion). TGF resetting was defined as first TGF minus second TGF. ANOVA was used to compare TGF resetting from different experimental groups, followed by two sample Student's t-tests when significant interactions were found. Hochberg's step-up procedure was used to adjust the P values for multiple comparisons so that the family wise type I error rate, predefined as 0.05, was controlled.
RESULTS
We first performed a time control experiment. We changed the perfusion rate from 5 to 40 nl/min, causing PSF to decrease from 39.0 ± 0.6 to 31.5 ± 0.7 mmHg. We then returned the perfusion rate to 5 nl/min and maintained it for 30 min. After that, we again changed the perfusion rate from 5 to 40 nl/min, causing PSF to decrease from 39.4 ± 0.8 to 32.1 ± 0.8 mmHg (n = 6 rat/6 tubules; Fig. 1, top). We calculated the TGF responses as PSF at 40 nl/min − PSF at 5 nl/min. We found no difference between the first and second TGF responses (Fig. 1, bottom), showing reproducibility of TGF responses under time control conditions.
Fig. 1.
Time control experiment. Top: stop-flow pressure (PSF) measured while the late proximal tubule was perfused at 5 or 40 nl/min. Bottom: tubuloglomerular feedback (TGF) responses (calculated as PSF at 40 nl/min − PSF at 5 nl/min), before and after a 30-min period of sustained perfusion at 5 nl/min. There was no difference between the first and second TGF responses.
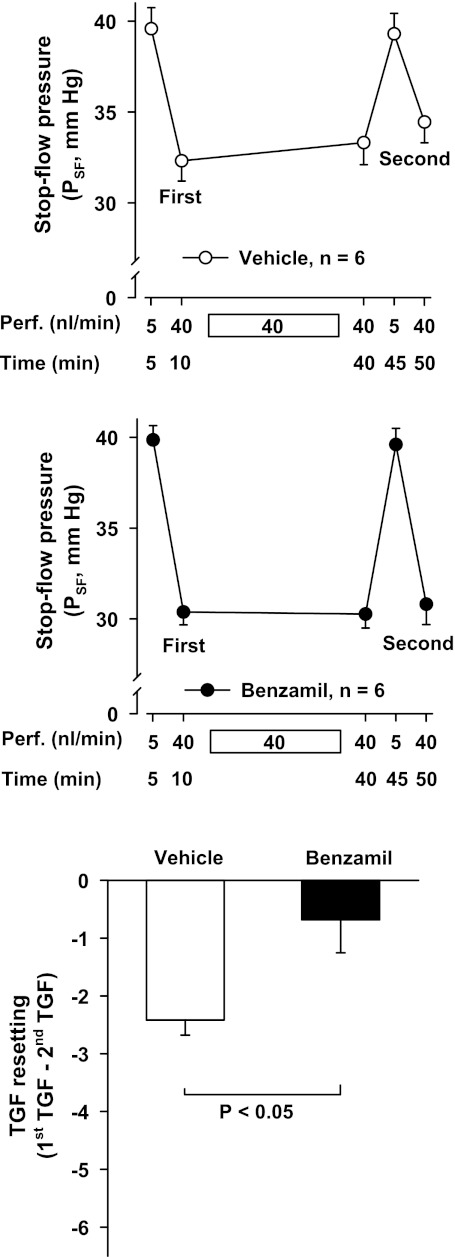
Next, we tested the effect of TGF resetting on PSF. We changed the perfusion rate from 5 to 40 nl/min, causing PSF to decrease from 39.6 ± 1.2 to 32.3 ± 1.1 mmHg. We then maintained the perfusion rate at 40 nl/min for 30 min. After that, we returned the perfusion rate to 5 nl/min and again changed it to 40 nl/min, causing PSF to decrease from 39.3 ± 1.1 to 34.5 ± 1.1 mmHg. Thus, the second TGF response was significantly smaller than the first one, showing TGF resetting (P < 0.001, n = 6 rat/6 tubules; Fig. 2, top). To test whether CTGF participates in the TGF resetting induced by a sustained increase in tubular perfusion, we repeated these experiments in the presence of the CTGF blocker benzamil (1 μM) added to the tubular perfusate. We found that benzamil enhanced the TGF response and prevented the attenuation of the second TGF response (n = 6 rats/6 tubules; Fig. 2, middle). We compared the degree of TGF resetting, calculated as the difference between the first and second TGF responses, in the absence and presence of benzamil. Benzamil significantly attenuated TGF resetting (Fig. 2, bottom). Furthermore, simply maintaining tubular perfusion at 40 nl/min made PSF drift upwards in the vehicle group (P < 0.05) but this did not occur in the benzamil group (note PSF at 10 and 40 min in Fig. 2). These results suggest that CTGF partly mediates TGF resetting induced by a sustained increase in tubular perfusion.
Fig. 2.
TGF resetting in the absence and presence of the connecting tubule glomerular feedback (CTGF) blocker benzamil. PSF measured while the late proximal tubule was perfused at 5 or 40 nl/min with either vehicle (top) or benzamil (middle). Bottom: TGF resetting, calculated as the difference between the 1st and 2nd TGF responses (i.e., before and after a 30-min period of sustained perfusion at 40 nl/min). Significant resetting was induced by sustained tubular perfusion in the vehicle group, but not in the benzamil group.
Since CTGF accounted for nearly all of the TGF resetting induced by sustained tubular perfusion, we decided to test whether a further enhancement of TGF resetting would also be modulated by CTGF. We first established a protocol to induce further TGF resetting by maintaining tubular perfusion rate at 40 nl/min for 30 min as described above, but with the addition of the carbonic anhydrase inhibitor acetazolamide (10 mM) to the tubular perfusate, which increases NaCl delivery to the distal nephron. In tubules perfused with vehicle, TGF resetting was 2.4 ± 0.6 mmHg. In tubules perfused with acetazolamide, TGF resetting was 5.5 ± 0.9 mmHg, indicating that acetazolamide potentiated TGF resetting (n = 6 rats/6 tubules; Fig. 3).
Fig. 3.
TGF resetting in the absence and presence of the carbonic anhydrase inhibitor acetazolamide. PSF measured while the late proximal tubule was perfused at 5 or 40 nl/min with either vehicle (top) or acetazolamide (middle). Bottom: TGF resetting, calculated as the difference between the 1st and 2nd TGF responses (i.e., before and after a 30-min period of sustained perfusion at 40 nl/min). Acetazolamide potentiated TGF resetting induced by sustained tubular perfusion at 40 nl/min.
We then tested the effect of CTGF inhibition with benzamil on TGF resetting in the presence of acetazolamide. Under these conditions, TGF resetting was only 2.1 ± 1.2 mmHg, indicating that benzamil attenuated TGF resetting in the presence of acetazolamide (n = 6 rats/6 tubules; Fig. 4).
Fig. 4.
TGF resetting induced by the combined effect of sustained tubular perfusion at 40 nl/min and the addition of acetazolamide to the perfusate was assessed in the presence and absence of benzamil. PSF measured while the late proximal tubule was perfused at 5 or 40 nl/min with either acetazolamide (top) or acetazolamide plus benzamil (middle). Bottom: TGF resetting, calculated as the difference between the 1st and 2nd TGF responses (i.e., before and after a 30-min period of sustained perfusion at 40 nl/min). Benzamil attenuated TGF resetting in the presence of acetazolamide.
DISCUSSION
Our data support the hypothesis that CTGF participates in acute TGF resetting. We found that sustained perfusion of the late proximal tubule at 40 nl/min for 30 min induced TGF resetting. This perfusion rate mimics the tubular flow rate reported in healthy, volume-expanded rats (7), but delivered to a single nephron, thus avoiding systemic confounding factors. To determine whether CTGF is involved in TGF resetting, we used benzamil to block CNT Na+ transport (and thus CTGF). Benzamil potentiated TGF, in keeping with our previous report (22), and prevented TGF resetting, indicating that CTGF antagonizes TGF and participates in acute TGF resetting.
In addition, we tested the role of CTGF in an experiment designed to further enhance TGF resetting. For this, we added the carbonic anhydrase inhibitor acetazolamide, thus decreasing proximal NaCl absorption and increasing NaCl delivery to the distal nephron. Carbonic anhydrase inhibitors have been used for the purpose of inducing TGF resetting (17), the rationale being that increased distal NaCl delivery would initially tend to increase TGF, but if maintained in time it would also increase TGF resetting. Acetazolamide in the tubular perfusate slightly increased the first TGF response, although without reaching statistical significance. More importantly, addition of acetazolamide significantly enhanced TGF resetting. Finally, we tested the effect of CTGF inhibition with benzamil on TGF resetting in the presence of acetazolamide. We found that benzamil significantly prevented the exaggerated TGF resetting, further confirming that CTGF was involved in acute TGF resetting. We acknowledge that acetazolamide may have multiple effects along the nephron independent of its increase in NaCl delivery to the macula densa and CNT; however, such effects would be present in both the first and second TGF responses. Furthermore, most of the acetazolamide-induced TGF resetting was blocked by benzamil, suggesting most of the effect of acetazolamide was mediated by CTGF.
The physiological relevance of TGF resetting has been postulated to be twofold (14, 19). First, if TGF was continuously maximally activated, it would lose its ability to buffer changes in glomerular perfusion, thus TGF resetting acts to maintain TGF efficiency. Second, TGF resetting may help maintain salt homeostasis. Indeed, without resetting, TGF may be viewed as anti-homeostatic as it would reduce glomerular filtration (and salt excretion) in response to a salt load, sensed as an increase in NaCl delivery to the macula densa. Thus, attenuation of TGF-induced vasoconstriction of the Af-Art can favor salt homeostasis.
Of note, our studies have dealt with acute TGF resetting, such as one may expect in the setting of increased salt consumption or other acute causes of volume expansion. A number of studies have focused on chronic forms of TGF resetting when glomerular filtration is persistently increased, including physiologic conditions such as pregnancy (1) and growth (2) and pathological conditions such as diabetes (20) and uninephrectomy (10). Chronic TGF resetting may have different mechanisms from acute TGF resetting, and the role of CTGF in chronic TGF resetting remains to be elucidated.
Inhibiting CTGF did not completely prevent TGF resetting, particularly in the presence of acetazolamide, thus it is possible that under conditions that strongly stimulate TGF resetting, other mechanisms may also participate. ANG II, nitric oxide (NO), COX-2 products, and the primary cilia have all been postulated as mediators of TGF resetting, ANG II toward TGF becoming more sensitive, the other factors toward desensitizing TGF. ANG II (either systemic, interstitial, or intraluminal) is known to potentiate TGF, and blocking AT1 receptors can blunt TGF, but whether ANG II plays a role in TGF resetting is disputed. TGF that has been desensitized by volume expansion can be restored by ANG II (13); however, in a model of TGF resetting induced by a carbonic anhydrase inhibition, plasma and kidney ANG II levels remain unchanged as long as fluid losses are replaced (5), and AT1 blockers do not prevent the recovery of renal blood flow (an index of TGF resetting at the whole kidney level) (4). A role for NO derived from neuronal NO synthase (NOS1) in TGF resetting is well-supported by data from both micropuncture and whole kidney studies. After volume expansion, TGF can be sensitized by intratubular or systemic NOS1 inhibition (3). Furthermore, NOS1 inhibition can prevent the decrease in maximum TGF and the recovery of renal blood flow during TGF resetting induced by a carbonic anhydrase inhibitor (4, 5, 17). In addition, COX-2 products have been implicated in TGF resetting, on the basis that a COX-2 inhibitor prevents the recovery of renal blood flow during systemic infusion of a carbonic anhydrase inhibitor (5). And more recently, a role in TGF resetting has been suggested for the primary cilia of the macula densa, as removal of the primary cilia by a siRNA against polaris prevents TGF resetting induced by high-salt diet (6).
We acknowledge that high concentrations of benzamil can inhibit the Na/Ca exchanger, which is present in the CNT (9); however, the IC50 for this effect is 100-fold greater than the concentration we used (8). We also acknowledge that it is unlikely that acetazolamide would cause a marked increase in distal NaCl delivery because there is only a short segment of proximal tubule downstream from the puncture site for acetazolamide to act on. However, our data indicate that acetazolamide, if maintained for 30 min, can reset TGF, and that much of this resetting is due to CTGF. It must be noted that at the perfusion rate we used, which is equivalent to the tubular flow rate seen in volume-expanded animals (7), TGF is saturated; and further increases in distal sodium delivery do not cause further constriction of the adjacent Af-Art (15), but CTGF is still operational, and its contribution to the control of Af-Art hemodynamics is indeed greatest at the high end of the physiological tubular flow range.
In summary, our studies provide evidence that a sustained perfusion of the nephron can cause TGF resetting and that inhibition of CTGF blocks TGF resetting. Even when TGF resetting was pharmacologically enhanced, most of it was still prevented by blocking CTGF.
CTGF is a novel regulatory mechanism of the renal microcirculation that may help explain the dilation of the Af-Art and the increase in GFR observed during high-salt intake by causing TGF resetting.
GRANTS
This study was supported by National Institutes of Health Program Project Grants HL028982 and HL088036.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.W. performed experiments; H.W. and M.A.D. analyzed data; H.W. drafted manuscript; H.W., M.A.D., J.L.G., Y.R., and O.A.C. approved final version of manuscript; M.A.D., J.L.G., Y.R., and O.A.C. interpreted results of experiments; M.A.D. prepared figures; M.A.D., J.L.G., and O.A.C. edited and revised manuscript; J.L.G. and O.A.C. conception and design of research.
REFERENCES
- 1. Baylis C, Blantz RC. Tubuloglomerular feedback activity in virgin and 12-day-pregnant rats. Am J Physiol Renal Fluid Electrolyte Physiol 249: F169–F173, 1985 [DOI] [PubMed] [Google Scholar]
- 2. Briggs JP, Schubert G, Schnermann J. Quantitative characterization of the tubuloglomerular feedback response: effect of growth. Am J Physiol Renal Fluid Electrolyte Physiol 247: F808–F815, 1984 [DOI] [PubMed] [Google Scholar]
- 3. Brown R, Ollerstam A, Persson AE. Neuronal nitric oxide synthase inhibition sensitizes the tubuloglomerular feedback mechanism after volume expansion. Kidney Int 65: 1349–1356, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Deng A, Hammes JS, Thomson SC. Hemodynamics of early tubuloglomerular feedback resetting during reduced proximal reabsorption. Kidney Int 62: 2136–2143, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Deng A, Wead LM, Blantz RC. Temporal adaptation of tubuloglomerular feedback: effects of COX-2. Kidney Int 66: 2348–2353, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Fu Y, Roman RJ, Lu D, Zhu X, Liu R. High salt intake-induced TGF suppression is partially mediated by primary cilia on the macula densa (Abstract). Hypertension 56: e159, 2010 [Google Scholar]
- 7. Good DW, Wright FS. Luminal influences on potassium secretion: sodium concentration and fluid flow rate. Am J Physiol Renal Fluid Electrolyte Physiol 236: F192–F205, 1979 [DOI] [PubMed] [Google Scholar]
- 8. Kleyman TR, Cragoe EJ., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol 105: 1–21, 1988 [DOI] [PubMed] [Google Scholar]
- 9. Loffing J, Loffing-Cueni D, Valderrabano V, Kläusli L, Hebert SC, Rossier BC, Hoenderop JGJ, Bindels RJM, Kaissling B. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol 281: F1021–F1027, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Muller-Suur R, Norlen BJ, Persson AE. Resetting of tubuloglomerular feedback in rat kidneys after unilateral nephrectomy. Kidney Int 18: 48–57, 1980 [DOI] [PubMed] [Google Scholar]
- 11. Ren Y, D'Ambrosio MA, Garvin JL, Wang H, Carretero OA. Possible mediators of connecting tubule glomerular feedback. Hypertension 53: 319–323, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ren Y, Garvin JL, Liu R, Carretero OA. Crosstalk between the connecting tubule and the afferent arteriole regulates renal microcirculation. Kidney Int 71: 1116–1121, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Schnermann J, Briggs JP. Restoration of tubuloglomerular feedback in volume-expanded rats by angiotensin II. Am J Physiol Renal Fluid Electrolyte Physiol 259: F565–F572, 1990 [DOI] [PubMed] [Google Scholar]
- 14. Schnermann J, Briggs JP. The macula densa is worth its salt. J Clin Invest 104: 1007–1009, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schnermann J, Briggs JP. Function of the juxtaglomerular apparatus: control of glomerular hemodynamics and renin secretion. In: Seldin and Giebisch's The Kidney, edited by Alpern RJ, Hebert SC. Burlington, MA: Academic, 2007, p. 589–626. [Google Scholar]
- 16. Steinhausen M, Endlich K, Wiegman DL. Glomerular blood flow. Kidney Int 38: 769–784, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Thomson SC, Bachmann S, Bostanjoglo M, Ecelbarger CA, Peterson OW, Schwartz D, Bao D, Blantz RC. Temporal adjustment of the juxtaglomerular apparatus during sustained inhibition of proximal reabsorption. J Clin Invest 104: 1149–1158, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thomson SC, Blantz RC, Vallon V. Increased tubular flow induces resetting of tubuloglomerular feedback in euvolemic rats. Am J Physiol Renal Fluid Electrolyte Physiol 270: F461–F468, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Thomson SC, Vallon V, Blantz RC. Resetting protects efficiency of tubuloglomerular feedback. Kidney Int Suppl 67: S65–S70, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Vallon V, Blantz RC, Thomson S. Homeostatic efficiency of tubuloglomerular feedback is reduced in established diabetes mellitus in rats. Am J Physiol Renal Fluid Electrolyte Physiol 269: F876–F883, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Wagner C, de WC, Kurtz L, Grunberger C, Kurtz A, Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res 100: 556–563, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Wang H, Garvin JL, D'Ambrosio MA, Ren Y, Carretero OA. Connecting tubule glomerular feedback antagonizes tubuloglomerular feedback in vivo. Am J Physiol Renal Physiol 299: F1374–F1378, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]