Abstract
The transcription factor nuclear factor-E2-related factor 2 (Nrf2) is a key regulator for induction of hepatic detoxification and antioxidant mechanisms, as well as for certain hepatobiliary transporters. To examine the role of Nrf2 in bile acid homeostasis and cholestasis, we assessed the determinants of bile secretion and bile acid synthesis and transport before and after bile duct ligation (BDL) in Nrf2−/− mice. Our findings indicate reduced rates of biliary bile acid and GSH excretion, higher levels of intrahepatic bile acids, and decreased expression of regulators of bile acid synthesis, Cyp7a1 and Cyp8b1, in Nrf2−/− compared with wild-type control mice. The mRNA expression of the bile acid transporters bile salt export pump (Bsep) and organic solute transporter (Ostα) were increased in the face of impaired expression of the multidrug resistance-associated proteins Mrp3 and Mrp4. Deletion of Nrf2 also decreased ileal apical sodium-dependent bile acid transporter (Asbt) expression, leading to reduced bile acid reabsorption and increased loss of bile acid in feces. Finally, when cholestasis is induced by BDL, liver injury was not different from that in wild-type BDL mice. These Nrf2−/− mice also had increased pregnane X receptor (Pxr) and Cyp3a11 mRNA expression in association with enhanced hepatic bile acid hydroxylation. In conclusion, this study finds that Nrf2 plays a major role in the regulation of bile acid homeostasis in the liver and intestine. Deletion of Nrf2 results in a cholestatic phenotype but does not augment liver injury following BDL.
Keywords: Nrf2 knockout mice, obstructive cholestasis, glutathione, biliary excretion
nuclear factor -E2-related factor 2 (Nrf2) is a transcription factor that is a member of the basic leucine zipper family (24). Nrf2 functions as a crucial mediator of an adaptive response to counteract oxidative stress (17). Activation of Nrf2 signaling is controlled by the actin-associated kelch-domain protein 1 (Keap1), which binds and sequesters Nrf2 to the cytoplasm. In response to oxidative stress, Nrf2 escapes Keap1-facilitated ubiquitination and degradation, allowing for accumulation and translocation to the nucleus, where it binds to the antioxidant response elements (AREs) of target genes and subsequently initiates gene transcription (14, 15, 18). The oxidative stress response embraces the induction of endogenous antioxidant molecules and detoxification enzymes, including NAD(P)H quinone oxidoreductase 1 (Nqo1) (3), heme oxygenase-1 (HO-1) (29), glutathione-S-transferase (GST) (11), and the GSH synthesis enzymes glutamate cysteine ligases (17, 25). Oxidative stress has been implicated in chemical toxicity of the liver and in the pathogenesis of numerous hepatic diseases, including cholestasis (1). The importance of Nrf2 to liver toxicity has been demonstrated by studies in Nrf2 knockout (Nrf2−/−) mice. Several in vivo studies have shown that mice deficient in Nrf2 are more susceptible to acetaminophen (APAP) hepatotoxicity (6, 10, 26) as a result of alterations in pathways responsible for APAP bioactivation and detoxification, as well as impaired compensatory induction of Nrf2 target genes involved in cellular antioxidant defenses. Furthermore, Nrf2 mediates induction of the hepatic transporters multidrug resistance-associated proteins Mrp3 and Mrp4 after APAP liver toxicity (2). This evidence suggests that Nrf2 is an important transcription factor that regulates the detoxification and efflux transport pathways in the liver to mitigate cellular injury.
Increased liver expression of Nqo1 is also seen in cholestasis following bile duct ligation (BDL) in the mouse and is Nrf2-dependent (3). Thus Nrf2 would also seem to be part of the protective response to liver injury following cholestasis. However, these Nrf2−/− mice accumulate less hepatic bile acids and do not exhibit more severe hepatic injury after BDL (3). This is possibly related to reduced bile acid synthesis and/or changes in bile acid enterohepatic recycling and/or excretion in the Nrf2−/− mice. Although reduced hepatic levels of cytochrome P-450 (Cyp7b1 and Cyp8b1) have been observed in Nrf2−/− mice after BDL (3), it is unclear why Nrf2−/− mice accumulate lower levels of hepatic bile acids following BDL and why liver injury is not more severe in Nrf2−/− than wild-type mice. In addition, detailed information on the involvement of Nrf2 in bile acid homeostasis remains unknown. In the present study, we provide the first information on the role of Nrf2 in bile acid homeostasis and clarify why liver injury is not more severe after BDL in Nrf2−/− mice. Our findings indicate that Nrf2 plays a major role in the regulation of bile acid synthesis and the enterohepatic circulation of bile acids. Taken together, these alterations may attenuate the severity of liver injury reported in other animal models when Nrf2 is deleted.
MATERIALS AND METHODS
Materials.
All reagents were obtained from Sigma-Aldrich (St. Louis, MO), unless otherwise indicated. TaqMan primers/probes for real-time PCR were purchased from Applied Biosystems (Foster City, CA), and some gene probes were purchased from Integrated DNA Technologies (Coralville, IA) (Table 1). Rabbit polyclonal antibody K13 against Mrp2, sodium taurocholate cotransporting polypeptide (Ntcp), and organic anion-transporting polypeptide (Oatp1a1) were kindly provided by Dr. Bruno Stieger (University of Zurich). Rabbit polyclonal antibody against organic solute transporter (Ostα) was a gift from Dr. Nazzareno Ballatori (University of Rochester). Rabbit polyclonal antibody against apical sodium-dependent bile acid transporter (Asbt) was a generous gift from Dr. Paul A. Dawson (Wake Forest University School of Medicine). Rabbit polyclonal antibody against Mrp3 was developed in our laboratory (41). Goat polyclonal anti-Mrp4 was purchased from Everest Biotech (Oxfordshire, UK), and rabbit polyclonal anti-bile salt export pump (Bsep) antibody was purchased from Kamiya Biomedical (Seattle, WA). Rabbit polyclonal Src homology 2 domain-containing protein tyrosine phosphatase (SH-PTP1) antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Table 1.
Mouse primers/probes used in TaqMan real-time PCR
| Gene | Gene Assay ID No. | Primer Sequences (5′ to 3′) | GenBank Accession No. |
|---|---|---|---|
| Cyp7a1 | Mm00484152_m1 | ||
| Cyp8b1 | Mm00501637_s1 | ||
| Cyp27a1 | Mm00470430_m1 | ||
| Ntcp | Mm00441421_m1 | ||
| Oatp1a1 | Mm00649796_m1 | ||
| Bsep | Mm00445168_m1 | ||
| Mdr1a | Mm00440761_m1 | ||
| Mdr1b | Mm00440736_m1 | ||
| Mrp2 | Mm00496899_m1 | ||
| Mrp3 | Mm00551550_m1 | ||
| Mrp4 | Forward: CATCAAGTCCAGGGAAAAGGTTG | AK052778 | |
| Reverse: GAGGGCCGAGATGAGGGAG | |||
| Probe: TGGGCAGAACCGGAGCTGGGAAA | |||
| Ostα | Forward: TGTTCCAGGTGCTTGTCATCC | NM_145932 | |
| Reverse: CCACTGTTAGCCAAGATGGAGAA | |||
| Probe: CCGCCCTGCAGCCTGCCAT | |||
| Ostβ | Forward: ATGCGGCTCCTTGGAATTA | NM_178933 | |
| Reverse: GGAGGAACATGCTTGTCATGAC | |||
| Probe: TCCATCCTGGTCCTGGCAGTCCTG | |||
| Asbt | Mm00488258_m1 | ||
| Fxr | Mm00436419_m1 | ||
| Shp | Mm00442278_m1 | ||
| Fgf15 | Mm00433278_m1 | ||
| Pxr | Mm00803095_m1 | ||
| Car | Mm00437986_m1 | ||
| Cyp3a11 | Mm00731567_m1 | ||
| Sult2a1 | Forward: GTCACTCGGAACTTATTTTGAATGGT | ||
| Reverse: AGCCAGCCACGAACATGCT | |||
| Probe: CCTCAAAGGAAATGTTCTATTCGG | |||
| Ugt1a1 | Forward: GAGGCTTTGGGCAGAATTCC | ||
| Reverse: TTTGCAAGATTCGATGGTCTAGTTC | |||
| Probe: CAGACGGTCCTGTGGCGCTACACC | |||
| Procollagen1α(I) | Mm00801666_m1 | ||
| Gapdh | Forward: GCCCAGAACATCATCCCTGC | NM_001001978 | |
| Reverse: CCGTTCAGCTCTGGGATGACC | |||
| Probe: TCCACTGGTGCTGCCAAGGCTGTG |
Except for mouse Gapdh, multidrug resistance-associated protein (Mrp4), organic solute transporters (Ostα and Ostβ), sulfotransferase (Sult2a1), and UDP-glucuronosyltransferase (Ugt1a1), which are designed according to the mouse sequence using Primer Express software (Applied Biosystems), all primers are proprietary to Applied Biosystems and labeled with 6-carboxyfluorescein (FAM). A labeled probe for Gapdh is 2,7-dimethoxy-4,5-dichloro-6-carboxyfluorescein (JOE), and labeled probes for Mrp4, Ostα, Ostβ, Sult2a1, and Ugt1a1 are FAM. Cyp, cytochrome P-450; Ntcp, sodium taurocholate-cotransporting polypeptide; Oatp, organic anion-transporting polypeptide; Bsep, bile salt export pump; Mdr, multidrug resistance; Asbt, apical sodium-dependent bile acid transporter; Fxr, farnesoid X receptor; Shp, small heterodimer partner; Pxr, pregnane X receptor; Car, constitutive androstane receptor.
Animals and experimental design.
Nrf2−/− mice, bred into a background of C57BL/6J mice, were generated and introduced from the laboratory of Dr. Thomas W. Kensler (Johns Hopkins University). The Nrf2−/− mice were backcrossed to a C57BL/6J background eight times when we received them from a line originally developed by Dr. Masayuki Yamamoto. Wild-type control, age-matched male C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were housed in a 12:12-h light-dark cycle and allowed food and water ad libitum. Only males were used for the study, and the animals were genotyped by PCR of tail DNA. Genotyping was performed using the following primers: Nrf2 (5′-TGGACGGGACTATTGAAGGCTG-3′), nLacZ (5′-GCGGATTGACCGTAATGGGATAGG- 3′), and NAS (5′-TGGACGGGACTATTGAAGGCTG-3′). Nrf2−/− and wild-type mice (10–13 wk of age) were randomly assigned to one of four groups as follows: 1) wild-type sham-operated control, 2) wild-type BDL, 3) Nrf2−/− sham-operated control, and 4) Nrf2−/− BDL. Mice underwent BDL or sham operation and were studied for 7 days under a study protocol that was approved by the Yale Animal Care and Use Committee and was in accordance with National Institutes of Health guidelines (protocol no. 2009-07458). Briefly, all surgical procedures were performed under aseptic conditions. Mice received buprenorphine (0.1 mg/kg body wt im) ∼15 min prior to surgery and then were anesthetized with the inhaled anesthetic agent isoflurane. The common bile duct was ligated in wild-type or knockout mice. Two ligatures separated by 2 mm were tied close to the liver hilus below the bifurcation, and a cut was made between two ligatures. Control mice underwent a sham operation that consisted of exposure but no ligation of the common bile duct. BDL animals were subcutaneously injected with 0.04 mg of vitamin K1 (Neogen, Lexington, KY) at 2, 4, and 6 days following ligation. Mice were fasted overnight, and each animal was housed in an individual cage for the last 14–16 h for collection of feces and euthanized between 9 and 11 AM to minimize circadian effect on bile acid metabolism (49). After anesthesia with isoflurane, bile and urine were collected from the gallbladder and urinary bladder, respectively. Blood was collected from the abdominal aorta, and plasma was isolated immediately. Mice were then euthanized by portal vein perfusion. Liver, kidney, and ileum tissues were snap frozen for biochemical assays or fixed in 4% neutral buffered formaldehyde solution for evaluation of histological damage and immunohistochemical analyses.
Liver histology and immunohistochemistry.
Formalin-fixed liver was embedded in paraffin, sectioned, and stained with hematoxylin and eosin, Masson's trichrome, and Sirius red. The sections were examined and blindly scored (on a scale of 0–4) by J.L.B. for inflammation, necrosis, bile duct proliferation, and fibrosis. Liver immunohistochemistry was performed as previously described (32) to assess bile duct proliferation using an antibody to cytokeratin 19 (Troma-III) developed by R. Kemler and obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA) and processed using a diaminobenzidine peroxidase kit according to the manufacturer's instructions (Vector Laboratories, Burlingame, CA).
Bile acid measurements and liver function tests.
Bile acids were extracted from liver homogenates as previously described (23). The bile acid concentration in plasma, gallbladder, extracted liver, ileum, urine, and feces was measured using a commercial kit (Diazyme Laboratories, Poway, CA). Serum levels of alanine aminotransferase (ALT) and total bilirubin were measured as indicators of hepatic injury using standard diagnostic kits (Thermo Scientific, Middletown, VA).
Bile acid analysis.
Individual bile and serum samples were analyzed by nano-electrospray ionization (ESI) mass spectrometry. The bile acid analyses were performed on a mass spectrometer (PE Sciex API III) modified with a nano-ESI source (Protana), as previously described (32).
Analysis of mRNA expression by quantitative real-time PCR.
Total RNA was isolated from tissues using TRIzol reagent (Invitrogen, Carlsbad, CA) and purified using an RNeasy MiniElute Cleanup kit (Qiagen) according to the manufacturer's recommendations. Five micrograms of total RNA were reverse-transcribed into cDNA using the AffinityScript Multi Temperature cDNA synthesis kit (Stratagene, La Jolla, CA). Quantitative TaqMan real-time PCR was performed on a sequence detection system (Prism 7500, Applied Biosystems), as previously described (42). Expression of target genes was normalized to Gapdh, and quantification of relative expression was determined by Pfaffl's method (28).
Western blot analysis for bile acid transporter expression.
Membrane-enriched proteins from mouse liver, ileum, and kidney tissues were isolated as previously described (4). Briefly, tissues were minced with scissors in ice-cold buffer containing 10 mM Tris·HCl (pH 7.4), 10 mM KCl, 1.5 mM MgCl2, and Halt protease inhibitor cocktail with EDTA (Thermo Scientific, Rockford, IL) and 2 mM PMSF. Tissues were homogenized with a Dounce homogenizer on ice, and sucrose was added to a final concentration of 250 mM. Samples were then centrifuged at 800 g at 4°C for 20 min. The supernatant was ultracentrifuged at 100,000 g at 4°C for 60 min. The membrane-enriched pellet was resuspended in buffer containing 10 mM Tris·HCl (pH 7.4), 125 mM sucrose, and Halt protease inhibitor cocktail with EDTA and 2 mM PMSF and then stored at −80°C. The protein concentration of each sample was determined using a bicinchoninic acid protein assay kit (Pierce). Western blot analysis was performed using 4%-20% Bis-Tris gradient gel (Invitrogen) and transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA). The membrane was blocked with Odyssey blocking buffer (Li-Cor, Lincoln, NE) and then incubated overnight with the appropriate primary antibody, as described above. After the membrane was washed with PBS-Tween, it was incubated with IRDye680-conjugated anti-rabbit IgG or IRDye800-conjugated anti-goat IgG (Li-Cor) for 1 h at room temperature. The fluorescence signal on the membrane was scanned, and the relative quantities of protein expression were analyzed using the Odyssey infrared image system (Li-Cor). SH-PTP1 was used as a loading control.
Measurement of bile flow.
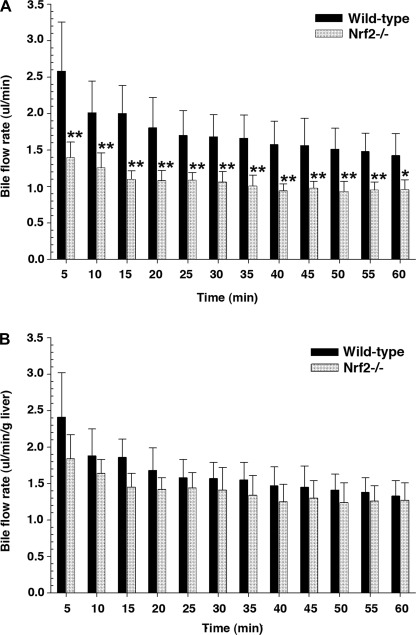
Bile flow was measured in mice as reported previously by this laboratory (20). Bile was collected every 5 min for a total of 60 min into preweighed Eppendorf tubes containing water or 6% 5-sulfosalicylic acid for determination of bile acid or GSH concentration, respectively, using a commercial GSH assay kit (Sigma) according to the manufacturer's recommendations. A previous study (12) and this study found that liver weight and liver weight relative to body weight were statistically less for Nrf2−/− than wild-type mice. Therefore, if we use liver weight to normalize the bile flow data, the actual bile flow rate in Nrf2−/− mice is overestimated, resulting in no statistical difference between the wild-type and Nrf2−/− mice. Therefore, in this study, bile flow rate is presented with and without the normalization of liver weight, as shown in Fig. 1.
Fig. 1.
Bile flow rate is decreased in nuclear factor-E2-related factor 2 knockout (Nrf2−/−) mice. A and B: bile flow rates without and with normalization to liver weight. Bile samples were collected every 5 min for a total of 60 min. Values are means ± SD of 6–7 independent experiments. *P < 0.05. **P < 0.01.
Statistical analysis.
In each group, five to seven animals were studied. Values are means ± SD. Significant differences between two groups were determined using Student's t-test. When multiple comparisons were made, one-way ANOVA was followed by Dunnett's test (SigmaStat, Jandel Scientific, San Rafael, CA).
RESULTS
Nrf2 deletion reduces biliary bile acid and GSH excretion and enzymatic determinants of bile acid synthesis.
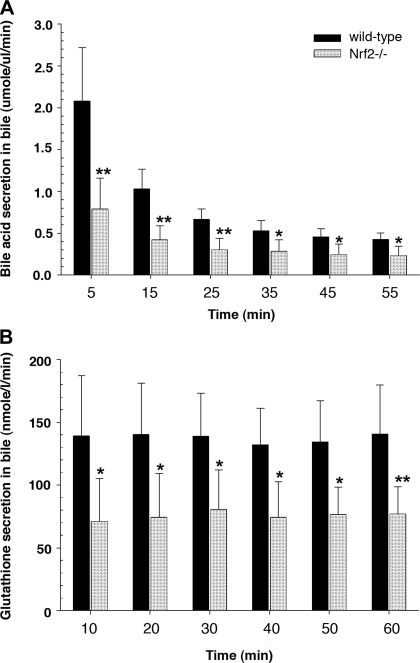
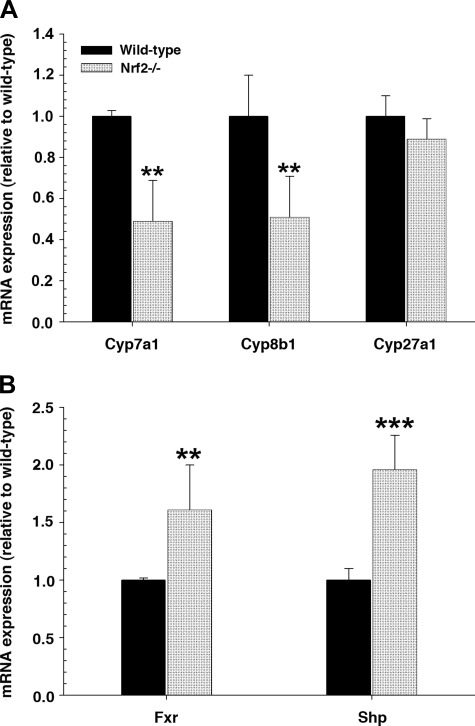
Nrf2 deletion resulted in lower absolute bile flow rates (Fig. 1) and lower rates of bile acid and GSH excretion than were observed in wild-type mice (Fig. 2). However, these differences are not apparent when normalized to liver weight. Nevertheless, the hepatic level of bile acids was increased approximately twofold in the Nrf2−/− mice (51.3 ± 31.4 vs. 96.1 ± 29.4 μmol/g liver, P < 0.05). However, there was no significant difference in bile acid pool size between wild-type and Nrf2−/− mice (178.8 ± 40.1 and 186.0 ± 58.5 μg, respectively). Because hepatic bile acid levels can influence bile acid synthesis, we next determined that there were significant reductions in mRNA levels for Cyp7a1 (the rate-limiting step for bile acid synthesis) and Cyp8b1 (de novo bile acid synthesis) in Nrf2−/− compared with wild-type mice (Fig. 3A). However, Cyp27a1 (the acidic bile acid synthesis enzyme) remained unchanged. Both key nuclear receptors that regulate bile acid synthetic enzymes, the farnesoid X receptor (Fxr) and the small heterodimeric partner (Shp), were significantly upregulated in Nrf2−/− mice (Fig. 3B), whereas hepatocyte nuclear factor-4α (HNF4α) mRNA expression remained unchanged (1.0 ± 0.1 and 0.83 ± 0.2 in wild-type and Nrf2−/− mice, respectively). These findings suggest that the elevated hepatic bile acid levels in Nrf2−/− mice led to the activation of Fxr, which activated the transcription of Shp, resulting in inhibition of Cyp7a1 and Cyp8b1 expression (7, 9, 21, 47).
Fig. 2.
Excretion of determinants of bile acid-dependent and -independent bile flow is decreased in Nrf2−/− mice. A and B: bile acid and GSH excretion rate in normal wild-type and Nrf2−/− mice. Content of bile acid and GSH was determined at 10-min intervals. Values are means ± SD of 6–7 independent experiments. *P < 0.05. **P < 0.01.
Fig. 3.
Enzymes that regulate bile acid synthesis are downregulated and farnesoid X receptor (Fxr) and small heterodimer partner (Shp) are upregulated in Nrf2−/− mice. A: relative mRNA expression of genes involved in bile acid synthesis [cytochrome P-450 (Cyp7a1, Cyp8b1, and Cyp27a1)] in liver of wild-type and Nrf2−/− mice. B: mRNA expression of the nuclear receptor Fxr and Shp in liver of wild-type and Nrf2−/− mice. Data are expressed as fold change relative to wild-type mice. Values are means ± SD of 5–6 animals. Amount of mRNA from wild-type mice is set as 1. **P < 0.01. ***P < 0.001.
Hepatic bile acid transporter gene expression is altered in Nrf2−/− mice.
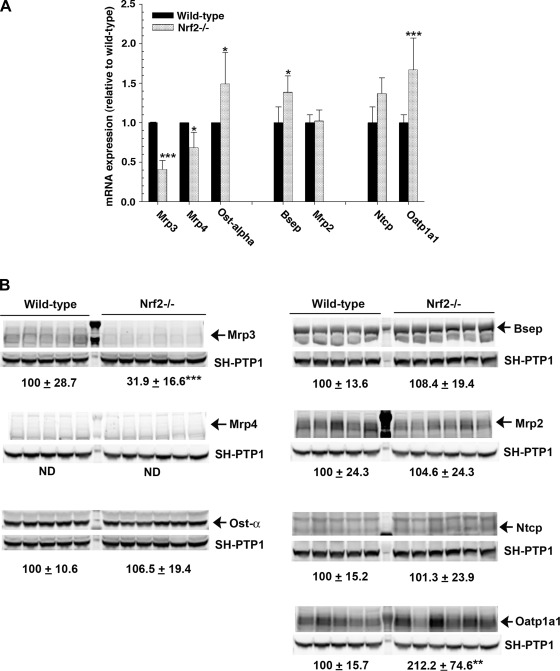
Apart from synthesis, a balance between uptake and elimination also affects hepatic bile acid concentrations. Therefore, we next examined the expression of bile acid transporters in the liver. As shown in Fig. 4, the mRNA expression levels of the efflux basolateral transporters Mrp3 and Mrp4 were reduced in Nrf2−/− mice. This is expected, since they are known to be Nrf2-dependent (22). Protein expression of Mrp3 was also decreased in Nrf2−/− mice; however, Mrp4 could not be accurately detected because of its very low expression in wild-type and Nrf2−/− mice. In contrast, Ostα mRNA was increased in the Nrf2−/− mice compared with the wild-type controls, although its protein expression was maintained. The mRNA level for the canalicular efflux transporter Bsep, but not Mrp2, was also increased in the Nrf2−/− mice compared with the wild-type controls, in agreement with a previous report (35). Nevertheless, there were no differences in Bsep and Mrp2 protein expression. Deletion of Nrf2 did not affect the mRNA or protein expression of Ntcp, the major basolateral transporter that transports conjugated bile acids from portal blood. However, the mRNA and protein expression of Oatp1a1 and the mRNA level of Oatp1b2 (1.0 ± 0.1 and 1.4 ± 0.1 in wild-type and Nrf2−/− mice, respectively, P < 0.05), both of which transport unconjugated bile acids, were significantly increased in the Nrf2−/− mice compared with the wild-type controls. These findings demonstrate that the genetic deletion of Nrf2 leads to changes in the expression of hepatic bile acid transporters, which together may contribute to the increase in hepatic bile acids in Nrf2−/− mice.
Fig. 4.
Hepatic bile acid transporter genes are differentially regulated in Nrf2−/− mice. A: relative mRNA expression of multidrug resistance-associated protein (Mrp3 and Mrp4), organic solute transporter (Ostα), bile salt export pump (Bsep), multidrug resistance (Mrp2), sodium-taurocholate-cotransporting polypeptide (Ntcp), and organic anion-transporting polypeptide (Oatp1a1) in liver of wild-type and Nrf2−/− mice. Data are expressed as fold change relative to wild-type mice. Values are means ± SD of 5–6 animals. B: immunoblots of membrane-enriched fractions from wild-type and Nrf2−/− mouse liver for Mrp3, Mrp4, Ostα, Bsep, Mrp2, Ntcp, and Oatp1a1. Values represent ratio of protein expression in wild-type mice to that in Nrf2−/− mice. Data are normalized to Src homology 2 domain-containing protein tyrosine phosphatase (SH-PTP1) and expressed in arbitrary units. Values are means ± SD of 5–6 individual animals in each group. Amount of protein from wild-type mice is set as 100. *P < 0.05. **P < 0.01. ***P < 0.001.
Enterohepatic circulation of bile acids is impaired in Nrf2−/− mice.
Because biliary bile acid secretion was reduced in Nrf2−/− mice, we examined the determinants of bile acid transport in the ileum. Interestingly, there was a significant twofold decrease in mRNA and protein level of Asbt in Nrf2−/− mice (Fig. 5, A and B). Bile acid concentrations in ileal tissue were also decreased by approximately twofold (data not shown), while fecal bile acids were significantly increased 50% in Nrf2−/− compared with wild-type mice (Fig. 5C). Furthermore, mRNA levels of ileal Fxr, Fgf15, ileal bile acid-binding protein (Ibabp), and Ostα were significantly lower in Nrf2−/− than wild-type mice (Fig. 5D). All these findings reflect a marked disruption of the enterohepatic circulation of bile acids through alterations in determinants of bile acid homeostasis in the ileum and the liver.
Fig. 5.
Downregulation of intestinal apical sodium-dependent bile acid transporter (Asbt), Fxr, Fgf15, ileal bile acid-binding protein (Ibabp), and Ostα in Nrf2−/− mice results in increased fecal bile acid excretion. A and B: expression of membrane transporter gene (Abst) and protein expression of Asbt in ileum of wild-type and Nrf2−/− mice. SH-PTP1 was used as an internal control, and densitometry was performed using the Li-Cor Odyssey infrared imaging system. Data are expressed as arbitrary units. Values are means ± SD of 5–6 animals. Amount of protein from wild-type mice is set as 100. C: fecal bile acid levels. Altered enterohepatic circulation in Nrf2−/− mice results in more fecal excretion of bile acids in Nrf2−/− mice. D: relative mRNA expression for Fxr, Fgf15, Ibabp, and Ostα from ileum of wild-type and Nrf2−/− mice. Data are expressed as fold change relative to wild-type mice. Values are means ± SD of 5–6 animals. Amount of mRNA from wild-type mice is set as 1. *P < 0.05. ***P < 0.001.
Gallbladder bile composition is altered in Nrf2−/− mice.
Because biliary bile acid levels were significantly higher in Nrf2−/− than wild-type mice, the bile composition in gallbladder bile was assessed. ESI-mass spectrometry revealed fourfold higher taurine dihydroxy bile acids in bile of Nrf2−/− than wild-type mice, while there was no significant difference in taurine trihydroxy bile acids (Table 2). In addition, although taurine tetrahydroxy bile acid levels were low in Nrf2−/− and wild-type mice, they were present at a higher level in Nrf2−/− than wild-type mice (Table 2). Bile acid sulfates were present at very low levels, which were not significantly different between the two phenotypes (Table 2).
Table 2.
Analysis of biliary bile acids by mass spectrometry in wild-type and Nrf2−/− mice
| Amount, μg | |||
|---|---|---|---|
| Bile Acids | m/z | Wild-type | Nrf2−/− |
| Conjugated taurine | |||
| C24olc(OH)2·taurine | 498.4 | 0.25 ± 0.1 | 1.13 ± 0.7* |
| C24olc(OH)3·taurine | 514.4 | 1.79 ± 0.5 | 1.93 ± 0.6 |
| C24olc(OH)4·taurine | 530.6 | Undetectable | 0.18 ± 0.1* |
| Conjugated sulfate | |||
| ΔC27ol(OH)2·SO4 | 481.4 | 0.01 ± 0.005 | Undetectable |
| Stigmasterol·SO4 | 491.4 | 0.03 ± 0.04 | Undetectable |
| C26ol(OH)4·SO4 | 501.4 | 0.01 ± 0.005 | Undetectable |
| C27ol(OH)4·SO4 | 514.4 | 0.02 ± 0.004 | Undetectable |
| C26ol(OH)5·SO4 | 517.4 | 0.01 ± 0.004 | Undetectable |
| C27ol(OH)5·SO4 | 531.4 | 0.01 ± 0.005 | Undetectable |
| ΔC27ol(OH)6·SO4 | 545.4 | 0.01 ± 0.004 | Undetectable |
| C27ol(OH)6·SO4 | 547.2 | 0.01 ± 0.002 | Undetectable |
Values are means ± SD (n = 5 mice per group). Biliary bile acid composition was analyzed by nano-electrospray ionization mass spectrometry. Amount of bile acid composition in wild-type and nuclear factor-E2-related factor 2 knockout (Nrf2−/−) mice was calculated relative to amount of internal standard (nor-ursocholic acid, 1 μg). Nrf2−/− mice demonstrated a significant increase in taurine dihydroxy, but not taurine trihydroxy, bile acids. Although its level was low, taurine tetrahydroxy bile acid was observed in Nrf2−/−, but not wild-type, mice. Bile alcohol sulfate as listed and stigmasterol sulfate were found in wild-type mice but were undetectable in Nrf2−/− mice. Convention for abbreviations is as follows: C27ol(OH)·SO4 is a 27-carbon bile alcohol with 1 OH group conjugated with sulfate; Δ indicates that 2 protons are missing, typical of compounds containing a double bond or ketone in place of the hydroxyl. m/z, Mass-to-charge ratio.
Statistically significantly different from wild-type.
Nrf2−/− mice are not more susceptible to hepatic injury after BDL.
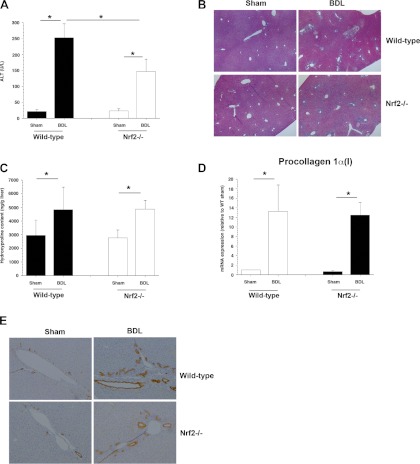
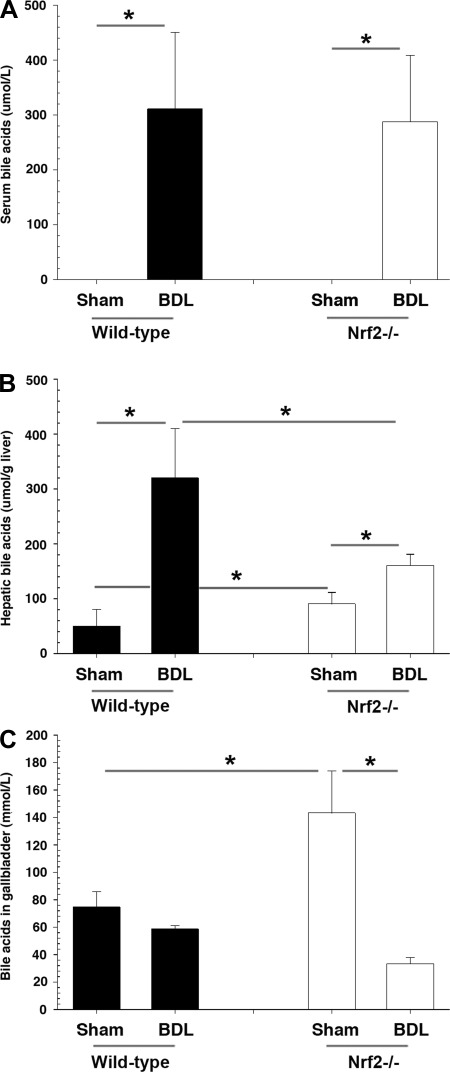
BDL leads to accumulation of bile acids in the liver and results in liver injury. Since Nrf2 reduces oxidative stress in the liver, greater liver injury might be expected in Nrf2−/− BDL mice. Paradoxically, however, serum ALT levels, although elevated in all BDL mice, were statistically lower in Nrf2−/− than wild-type mice (Fig. 6A). While blinded histopathological analysis of liver sections from Nrf2−/− BDL mice also tended to show less necrosis and bile duct proliferation, these differences were not statistically significant (Fig. 6B). There was no difference in fibrosis as assessed by Sirius red staining (data not shown), hepatic hydroxyproline content, or mRNA levels of procollagen1(α)I in Nrf2−/− compared with wild-type mice after BDL (Fig. 6, C and D). Morphometric analysis of cytokeratin 19 immunohistochemical staining of liver sections also showed less, but not statistically significant, bile duct proliferation (Fig. 6E). Serum bile acids were also similar between the two groups (Fig. 7A). However, while hepatic bile acid accumulation was significantly increased in both BDL groups, levels of hepatic bile acids were significantly less in Nrf2−/− mice (Fig. 7B), findings consistent with a previous report (3). We also found significantly lower bile acid concentrations in the gallbladder of Nrf2−/− mice following BDL (Fig. 7C).
Fig. 6.
After bile duct ligation (BDL), degree of hepatic injury is similar in Nrf2−/− and wild-type mice. A: serum levels of alanine aminotransferase (ALT). B: representative images of liver sections from sham-operated and BDL wild-type and Nrf2−/− mice. Sections were stained with hematoxylin and eosin, and liver injury and necrosis were analyzed. Original magnification ×4. C: hepatic collagen deposition, as determined biochemically as relative hydroxyproline content, did not differ in wild-type and Nrf2−/− mice. Values are means ± SD of 5–6 animals. D: mRNA expression of procollagen1α(I) is significantly increased to the same extent in wild-type and Nrf2−/− mouse liver after BDL. Data are expressed as fold change relative to sham-operated wild-type (WT) mice. Multiple comparisons were made. *P < 0.05. E: representative images of immunohistochemical staining of liver for cytokeratin 19. Note bile duct proliferation in sham-operated and BDL wild-type and Nrf2−/− mice. Original magnification ×10.
Fig. 7.
Altered levels of hepatic and biliary bile acids result in less susceptibility to hepatic injury after BDL in Nrf2−/− mice. A, B, and C: serum, total hepatic, and biliary bile acid concentrations in sham-operated and BDL wild-type and Nrf2−/− mice. Serum bile acid was below detection levels in sham-operated mice. Values are means ± SD of 5–6 animals. Multiple comparisons were made. *P < 0.05.
Compensatory changes in bile acid transporters and nuclear receptors in Nrf2−/− BDL mice may limit cholestatic liver injury.
We next determined whether compensatory changes in bile acid transporters might be occurring to a greater extent in Nrf2−/− mice after BDL. Similar to wild-type mice, the hepatic bile acid uptake transporters Oatp1a1 and Ntcp were downregulated, with Oatp1a1 expression nearly eliminated. The percent changes from sham operation to BDL were similar for Mrp3 and Mrp4 between wild-type and Nrf2−/− mice. The alternative basolateral efflux transporter Ostα was increased to a greater extent after BDL in Nrf2−/− than wild-type mice. The canalicular efflux transporter Bsep, but not Mrp2 and Mdr1a, was also significantly increased after BDL in Nrf2−/− compared with wild-type mice (data above not shown). Thus, in addition to reduced bile acid biosynthesis under basal conditions, these increases in bile acid efflux transporters after BDL may further contribute to reduction of intrahepatic bile acid accumulation in Nrf2−/− BDL mice.
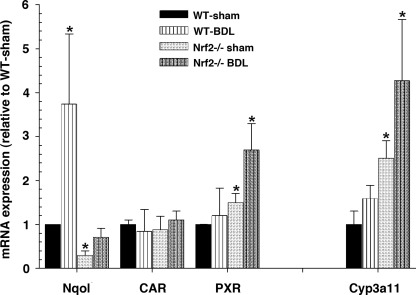
Figure 8 further illustrates that Cyp3a11 and its nuclear receptor agonist pregnane X receptor (Pxr) were significantly increased in the livers of Nrf2−/− mice and increased further after BDL. Increases in expression of Cyp3a11 hepatic mRNA after BDL would be expected to enhance bile acid hydroxylation and also contribute to reduced hepatic injury. As also shown in Fig. 8, mRNA levels of the prototypical Nrf2 target gene Nqo1 were substantially reduced in Nrf2−/− BDL mice, while there was no difference in the mRNA level of constitutive androstane receptor (Car) between wild-type BDL and Nrf2−/− BDL mice.
Fig. 8.
Cyp3a11, a phase I detoxifying enzyme involved in hydroxylation of bile acids, and its activating nuclear receptor pregnane X receptor (Pxr) are augmented in Nrf2−/− mice and after BDL. Data are expressed as fold change relative to sham-operated wild-type mice. Car, constitutive androstane receptor. Values are means ± SD of 5–6 animals. Amount of mRNA from wild-type mice is set as 1. Multiple comparisons were made. *P < 0.05.
Renal expression of bile acid transporters, including Asbt, Mrp2, Mrp3, Mrp4, and Ostα, was similar between wild-type and Nrf2−/− mice under basal conditions. However, adaptive responses to cholestasis occurred in transporters in the kidney after BDL. Mrp3 and Mrp4 expression increased after BDL as expected, although the increase in Mrp4 expression was somewhat less in Nrf2−/− mice, while Mrp2 and Ostα remained unchanged in the two groups. In contrast, expression of the apical transporter Asbt was significantly downregulated in wild-type BDL mice but was maintained in Nrf2−/− BDL mice (data above not shown). Together, the adaptive changes in renal bile acid transporters after BDL in Nrf2−/− mice do not appear to play much of a role in this model, as also reflected by the lack of changes in serum (Fig. 7A) and urinary bile acid excretion between the two phenotypes (data not shown).
DISCUSSION
The present study provides the first evidence that Nrf2 plays a role in the homeostasis of the enterohepatic circulation of bile acids and bile secretion. These alterations are sufficiently robust when Nrf2 is deleted in the mouse that they also prevent more serious liver injury from developing following BDL-induced cholestasis compared with the wild-type BDL mice. Specifically, deletion of Nrf2 in mice results in a decrease in the biliary excretion of bile acid and GSH (Figs. 1 and 2), an increase in hepatic and gallbladder bile acid concentrations, an increase in expression of hepatic Fxr and Shp mRNA, and a decrease in mRNA for the bile acid synthetic enzymes Cyp7a1 and Cyp8b1 (both of which are inhibited by Fxr- and Shp-mediated transcriptional events) (Fig. 3). Elevations in hepatic Fxr are also associated with increased production of the Fxr-regulated transporters Bsep and basolateral Ostα (Fig. 4). However, the basolateral membrane efflux transporters Mrp3 and Mrp4 are downregulated as anticipated, as they are known to be Nrf2-dependent (Fig. 4). In addition, there is downregulation of Asbt in the terminal ileum, which results in increased fecal excretion of bile acids and a reduction in ileal bile acid concentrations. This in turn reduces ileal Fxr and its target Fgf15, as expected (13) (Fig. 5).
Previous reports indicate that Nrf2 protects the liver from hepatotoxicity by upregulating expression of many detoxifying enzymes and hepatic efflux transporters (17), particularly Mrp3 and Mrp4, which play a key role in the adaptive response to cholestatic liver injury (23, 31). We therefore hypothesized that BDL in Nrf2−/− mice would have more detrimental effects. However, Aleksunes and co-workers (3) found that Nrf2−/− mice did not exhibit more severe hepatic injury 3 days after BDL and speculated that a reduction in bile acid production in Nrf2−/− mice might account for the lack of differences between Nrf2−/− and wild-type mice. In the present study, in which we subjected Nrf2−/− mice to BDL for 7 days, we provide confirmatory evidence that deletion of Nrf2 does not lead to liver injury beyond that seen in the wild-type mice. Indeed, there was a tendency toward less injury in Nrf2−/− mice, but these findings did not reach statistical significance, except for lower serum ALT levels (Fig. 6A). This lack of difference in liver injury between Nrf2−/− and wild-type mice has also been reported after α-naphthylisothiocyanate treatment (37). Tanaka and co-workers (37) suggest that Nrf2−/− mice have a compensatory mechanism for protecting the liver from injury via Nrf2-independent mechanisms by preferential upregulation of Shp mRNA and downregulation of HNF1α. We found a similar induction of Shp mRNA (Fig. 3), but not HNF1α (data not shown), expression in the liver, supporting the notion that an Nrf2-independent signaling mechanism is involved in limiting liver injury in Nrf2−/− mice. In addition to increased Shp expression in the liver, deletion of Nrf2 also resulted in downregulation of Fxr, Fgf15, Ibabp, and Ostα in the ileum (Fig. 5).
It is known that bile acids induce Fgf15 in the ileum, which subsequently signals the liver via fibroblast growth factor receptor 4 to repress hepatic 7α-hydroxylase (CYP7A1/Cyp7a1), which limits bile acid production (13, 16). Paradoxically, our findings showed that knocking down Nrf2 caused a downregulation of ileal Fgf15, as well as Cyp7a1, expression. However, unlike rats and humans, the mechanism of Cyp7a1 regulation in mice appears to be predominantly Shp-dependent (40), since Fgf15 does not repress Cyp7a1 in Shp−/− mice (13). This is consistent with our findings. The increase in hepatic bile acids in Nrf2−/− mice also contributes to Cyp7a1 and Cyp8b1 inhibition as part of feedback regulation of bile acid synthesis by bile acids (7). The decrease in bile acid synthesis, the conversion of the bile acid pool to more hydrophilic bile acids, and the loss of bile acids in the feces should work together to diminish the degree of hepatic toxicity following BDL in Nrf2−/− mice.
Deletion of Nrf2 also resulted in a significant increase in Cyp3a11 expression, which is a direct target gene of Pxr, the expression of which was also increased in Nrf2−/− mice before and after BDL (Fig. 8). Cyp3a11 is the predominant phase I enzyme for bile acid hydroxylation reactions, which increase the solubility of primary and secondary bile acids in mice and, thus, reduce their toxicity (39). Mass spectrometry analyses demonstrated a more than fourfold increase in hydroxylation of bile acids conjugated with taurine in the bile from Nrf2−/− mice (Table 2). Since hydroxylation is the first line of defense against bile acid toxicity in biliary obstruction, it is reasonable to speculate that the increase in Cyp3a11 expression may result in more rapid and efficient hydroxylation, thus leaving the mice less susceptible to cholestatic liver injury than they otherwise might be (33, 39, 44).
After phase I detoxification, bile acids undergo phase II reactions to produce even more hydrophilic and less toxic molecules by sulfation and glucuronidation conjugation reactions. However, our mass spectrometry analysis showed fewer bile acids conjugated with sulfate in bile from Nrf2−/− mice (Table 2), consistent with a reduction in Nrf2-dependent sulfotransferase (Sult2a1) expression (data not shown). In contrast, UDP-glucuronosyltransferase (Ugt1a1) was unaltered in Nrf2−/− mice (data not shown). Although Ugt1a1 is normally a minor metabolic pathway for bile acid conjugation reactions in mice (48), maintenance of its expression might help compensate for the diminished sulfation reactions in the Nrf2−/− mice.
Normally, Mrp3, Mrp4, and Ostα-Ostβ are upregulated in cholestasis and are critical alternative routes for the efflux of bile acids from the liver into the systemic circulation in an attempt to limit the hepatic accumulation of bile acids and attenuate bile acid-induced liver injury. Nrf2 binds to AREs at −9919 bp in the mouse Mrp3 gene and to AREs/antioxidant response-like elements at −3753 to −3767 bp in the mouse Mrp4 gene (22). Therefore, Mrp3 and Mrp4 are Nrf2-dependent, and their expression is downregulated in Nrf2−/− mice (22). Nevertheless, Mrp3 and Mrp4 were induced to the same extent following BDL in the Nrf2−/− mice, possibly because the increase in expression of Pxr in Nrf2−/− mice may compensate for the loss of Nrf2. In addition, the basolateral expression of Ostα-Ostβ (an Nrf2-independent and Fxr-dependent bile acid transporter) should provide an additional compensatory mechanism for limiting hepatic bile acid accumulation.
Finally, determinants of bile acid-dependent (BADF) and bile acid-independent bile flow (BAIF) were reduced in Nrf2−/− mice (Figs. 1 and 2). Fxr−/− mice are protected from developing bile infarcts after BDL because of reduced BADF and intrabiliary pressure as a result of decreased expression of Bsep (38). In contrast, more severe liver necrosis is seen in BDL mice treated with an Nrf2 activator, oltipraz, that stimulates BAIF (43). In the present study, bile flow rates were lower in Nrf2−/− mice as a result of decreased bile salt and GSH excretion, the latter providing the driving osmotic force for BAIF (5). Previous studies also show that Nrf2 influences bile flow and GSH excretion in bile (30). For example, the biliary excretion of sulfobromophthalein, which is conjugated with GSH, is 42% less in Nrf2−/− than wild-type mice (30). Thus reductions in the driving forces for BADF and BAIF may also contribute to reductions in liver toxicity following BDL in the Nrf2−/− mouse.
The findings in this study and those of Aleksunes et al. (3) and Tanaka et al. (37) are at variance with other liver injury models, where 1) Nrf2 prevents alcohol-induced fulminant liver injury (19), 2) Nrf2 protects against APAP hepatotoxicity (6, 10), 3) Nrf2 prevents the onset and progression of nonalcoholic steatohepatitis (8, 34), and 4) lithocholic acid (LCA)-induced liver injury is enhanced in Nrf2−/− mice (35). However, Nrf2−/− mice have low γ-glutamylcysteine synthase and GSH levels (27), whereas normal levels of hepatic GSH are needed to protect the liver via conjugation with N-acetyl-p-benzoquinone imine, a reactive toxic metabolite of APAP. Ethanol-fed Nrf2−/− mice also progressively lose GSH in mitochondria, reducing the capacity to metabolize acetaldehyde, which results in functional and structural mitochondrial changes. In nonalcoholic steatohepatitis induced by a methione- and choline-deficient diet, Nrf2−/− mice develop liver failure, because of the inability to induce the expression of Nqo1, GST, and GSH necessary for scavenging lipid peroxidation products and toxic metabolites. LCA-induced liver injury is also associated with oxidative stress, and Nrf2−/− mice develop more severe liver injury, because hepatic cytoprotective genes [glutamate-cysteine ligase, catalytic subunit (Gclc), thioredoxin (Trx1), HO-1, and GSH] and hepatic antioxidant activities (GST) are repressed in these mice (35).
Nevertheless, the differences in the two models of cholestasis (LCA and BDL) need to be explained. 1) Inherent differences in bile acid synthesis Nrf2−/− mice have been noted by Tan et al. (35) and the present study. 2) Liver injury in the BDL model results from the toxicity of endogenous bile acids, whereas LCA-induced cholestasis results from exposure to an exogenous, more hydrophobic bile salt. 3) Cyp3a11 expression is increased in Nrf2−/− mice (Fig. 8), resulting most likely in the formation of more hydrophilic bile acids, as suggested by the increase in tetrahydroxylated bile acids in bile in the present study (Table 2). 4) The level of oxidative stress induced by LCA is probably more than develops after BDL. For example, activation of Nrf2 by LCA or chenodeoxycholic acid induces a battery of cytoprotective genes for GSH biosynthesis and GSH synthetic enzymes, resulting in increases in levels of GSH, a key player against oxidative stress (36). While activity of GSH synthetic enzymes also increases during BDL in mice, the hepatic expression of glutamate-cysteine ligase (Gcl) subunits and GSH synthase (Gs) increase transiently approximately twofold from 1 to 7 days after BDL but then fall to 50% of baseline after 2 wk (46). This correlates with a switch in nuclear ARE binding activity from Nrf2 to c-Maf/MafG ARE nuclear binding, which leads to decreased expression of GSH synthetic enzymes and GSH levels (45). Tan and co-workers (35, 36) showed that LCA treatment for 4 days significantly lowered total hepatic GSH and Trx, Gclc, and GST activities by 1.5- to 2-fold in Nrf2−/− compared with wild-type mice, while the activity of GSH synthetic enzymes during BDL was sustained in mice until day 7. Thus, overall, we speculate that the level of oxidative stress in the BDL mouse model might be less than that induced by LCA and contribute to the differences in cholestatic liver injury in Nrf2−/− mice between BDL and LCA.
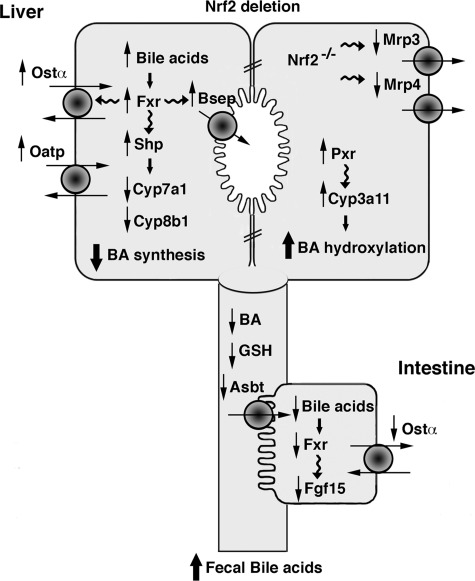
In summary, the present study shows for the first time that Nrf2 controls the expression of multiple genes involved in bile acid metabolism and bile acid homeostasis. Nrf2−/− mice develop a large number of adaptive compensatory changes involving nuclear transcription factors, including Fxr, Shp, and Pxr, efflux bile acid transporters, and altered GSH, as well as bile flow rates, that together resemble a cholestatic phenotype (Fig. 9). We postulate that together these multiple adaptations lessen the susceptibility of Nrf2−/− mice to liver injury induced by BDL as well as other forms of cholestatic liver injury. This study emphasizes the profound influence of Nrf2 on the regulation of bile acid homeostasis in the liver and intestine.
Fig. 9.
Nrf2−/− mice express a cholestatic phenotype, as illustrated in this model. In Nrf2−/− mice, accumulating hepatic bile acids (BA) activate Fxr and Shp, resulting in downregulation of Cyp7a1 and Cyp8b1 and bile acid synthesis and upregulation of Bsep, Ostα, and Oatp1a1. Pxr and Cyp3a11 are augmented, thus increasing bile acid hydroxylation. The Nrf2-dependent transporters Mrp3 and Mrp4 are downregulated. Asbt is downregulated, resulting in a reduction of ileal tissue bile acids and an increase in fecal bile acids.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R37 DK-25636 (to J. L. Boyer) and the Yale Liver Center P30 DK-34989.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.W. and J.L.B. are responsible for conception and design of the research; J.W., A.M., C.J.S., K.H., L.R.H., and T.W.K. performed the experiments; J.W. and C.J.S. analyzed the data; J.W. and J.L.B. interpreted the results of the experiments; J.W. prepared the figures; J.W. drafted the manuscript; J.W., C.J.S., L.R.H., T.W.K., and J.L.B. edited and revised the manuscript; J.W. and J.L.B. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dolan Patrick (Department of Environmental Health Sciences, Johns Hopkins University) for assistance with breeding and facilitating the export of Nrf2−/− mice.
REFERENCES
- 1. Aboutwerat A, Pemberton PW, Smith A, Burrows PC, McMahon RF, Jain SK, Warnes TW. Oxidant stress is a significant feature of primary biliary cirrhosis. Biochim Biophys Acta 1637: 142–150, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Aleksunes LM, Slitt AL, Maher JM, Augustine LM, Goedken MJ, Chan JY, Cherrington NJ, Klaassen CD, Manautou JE. Induction of Mrp3 and Mrp4 transporters during acetaminophen hepatotoxicity is dependent on Nrf2. Toxicol Appl Pharmacol 226: 74–83, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aleksunes LM, Slitt AL, Maher JM, Dieter MZ, Knight TR, Goedken M, Cherrington NJ, Chan JY, Klaassen CD, Manautou JE. Nuclear factor-E2-related factor 2 expression in liver is critical for induction of NAD(P)H:quinone oxidoreductase 1 during cholestasis. Cell Stress Chaperones 11: 356–363, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, Madejczyk MS, Li N. OSTα-OSTβ: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology 42: 1270–1279, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Ballatori N, Truong AT, Ma AK, Boyer JL. Determinants of glutathione efflux and biliary GSH/GSSG ratio in perfused rat liver. Am J Physiol Gastrointest Liver Physiol 256: G482–G490, 1989 [DOI] [PubMed] [Google Scholar]
- 6. Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci USA 98: 4611–4616, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chiang JY. Bile acids: regulation of synthesis. J Lipid Res 50: 1955–1966, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chowdhry S, Nazmy MH, Meakin PJ, Dinkova-Kostova AT, Walsh SV, Tsujita T, Dillon JF, Ashford ML, Hayes JD. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radic Biol Med 48: 357–371, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Del Castillo-Olivares A, Gil G. Suppression of sterol 12α-hydroxylase transcription by the short heterodimer partner: insights into the repression mechanism. Nucleic Acids Res 29: 4035–4042, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O'Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci 59: 169–177, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol 45: 51–88, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Huang J, Tabbi-Anneni I, Gunda V, Wang L. Transcription factor Nrf2 regulates SHP and lipogenic gene expression in hepatic lipid metabolism. Am J Physiol Gastrointest Liver Physiol 299: G1211–G1221, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2: 217–225, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313–322, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47: 89–116, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res 48: 2664–2672, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol 244: 57–65, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal 7: 385–394, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Lamle J, Marhenke S, Borlak J, von Wasielewski R, Eriksson CJ, Geffers R, Manns MP, Yamamoto M, Vogel A. Nuclear factor-erythroid 2-related factor 2 prevents alcohol-induced fulminant liver injury. Gastroenterology 134: 1159–1168, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Li M, Wang W, Soroka CJ, Mennone A, Harry K, Weinman EJ, Boyer JL. NHERF-1 binds to Mrp2 and regulates hepatic Mrp2 expression and function. J Biol Chem 285: 19299–19307, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell 6: 507–515, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Maher JM, Dieter MZ, Aleksunes LM, Slitt AL, Guo G, Tanaka Y, Scheffer GL, Chan JY, Manautou JE, Chen Y, Dalton TP, Yamamoto M, Klaassen CD. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology 46: 1597–1610, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Mennone A, Soroka CJ, Cai SY, Harry K, Adachi M, Hagey L, Schuetz JD, Boyer JL. Mrp4−/− mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology 43: 1013–1021, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the β-globin locus control region. Proc Natl Acad Sci USA 91: 9926–9930, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moinova HR, Mulcahy RT. Up-regulation of the human γ-glutamylcysteine synthetase regulatory subunit gene involves binding of Nrf-2 to an electrophile responsive element. Biochem Biophys Res Commun 261: 661–668, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun 339: 79–88, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Osburn WO, Yates MS, Dolan PD, Chen S, Liby KT, Sporn MB, Taguchi K, Yamamoto M, Kensler TW. Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol Sci 104: 218–227, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: 45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prestera T, Talalay P, Alam J, Ahn YI, Lee PJ, Choi AM. Parallel induction of heme oxygenase-1 and chemoprotective phase 2 enzymes by electrophiles and antioxidants: regulation by upstream antioxidant-responsive elements (ARE). Mol Med 1: 827–837, 1995 [PMC free article] [PubMed] [Google Scholar]
- 30. Reisman SA, Csanaky IL, Yeager RL, Klaassen CD. Nrf2 activation enhances biliary excretion of sulfobromophthalein by inducing glutathione-S-transferase activity. Toxicol Sci 109: 24–30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soroka CJ, Lee JM, Azzaroli F, Boyer JL. Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology 33: 783–791, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Soroka CJ, Mennone A, Hagey LR, Ballatori N, Boyer JL. Mouse organic solute transporter-α deficiency enhances renal excretion of bile acids and attenuates cholestasis. Hepatology 51: 181–190, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA 98: 3369–3374, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sugimoto H, Okada K, Shoda J, Warabi E, Ishige K, Ueda T, Taguchi K, Yanagawa T, Nakahara A, Hyodo I, Ishii T, Yamamoto M. Deletion of nuclear factor-E2-related factor-2 leads to rapid onset and progression of nutritional steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol 298: G283–G294, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Tan KP, Wood GA, Yang M, Ito S. Participation of nuclear factor (erythroid 2-related) factor 2 in ameliorating lithocholic acid-induced cholestatic liver injury in mice. Br J Pharmacol 161: 1111–1121, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tan KP, Yang M, Ito S. Activation of nuclear factor (erythroid-2 like) factor 2 by toxic bile acids provokes adaptive defense responses to enhance cell survival at the emergence of oxidative stress. Mol Pharmacol 72: 1380–1390, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Tanaka Y, Aleksunes LM, Cui YJ, Klaassen CD. ANIT-induced intrahepatic cholestasis alters hepatobiliary transporter expression via Nrf2-dependent and independent signaling. Toxicol Sci 108: 247–257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, Zatloukal K, Guo GL, Schuetz JD, Gonzalez FJ, Marschall HU, Denk H, Trauner M. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology 125: 825–838, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, Zatloukal K, Denk H, Trauner M. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology 42: 420–430, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Wagner M, Zollner G, Trauner M. Nuclear receptor regulation of the adaptive response of bile acid transporters in cholestasis. Semin Liver Dis 30: 160–177, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Wang W, Soroka CJ, Mennone A, Rahner C, Harry K, Pypaert M, Boyer JL. Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology 131: 878–884, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weerachayaphorn J, Cai SY, Soroka CJ, Boyer JL. Nuclear factor erythroid 2-related factor 2 is a positive regulator of human bile salt export pump expression. Hepatology 50: 1588–1596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weerachayaphorn J, Luo Y, Mennone A, Soroka CJ, Harry K, Boyer JL. Oltipraz, an Nrf2 activator, aggravates liver injury in a mouse model of extrahepatic cholestasis (Abstract). Hepatology Suppl 52: 665A, 2010 [Google Scholar]
- 44. Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, Evans RM. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA 98: 3375–3380, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang H, Ko K, Xia M, Li TW, Oh P, Li J, Lu SC. Induction of avian musculoaponeurotic fibrosarcoma proteins by toxic bile acid inhibits expression of glutathione synthetic enzymes and contributes to cholestatic liver injury in mice. Hepatology 51: 1291–1301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang H, Ramani K, Xia M, Ko KS, Li TW, Oh P, Li J, Lu SC. Dysregulation of glutathione synthesis during cholestasis in mice: molecular mechanisms and therapeutic implications. Hepatology 49: 1982–1991, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang M, Chiang JY. Transcriptional regulation of the human sterol 12α-hydroxylase gene (CYP8B1): roles of heaptocyte nuclear factor 4α in mediating bile acid repression. J Biol Chem 276: 41690–41699, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Zhang Y, Klaassen CD. Effects of feeding bile acids and a bile acid sequestrant on hepatic bile acid composition in mice. J Lipid Res 51: 3230–3242, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang YK, Guo GL, Klaassen CD. Diurnal variations of mouse plasma and hepatic bile acid concentrations as well as expression of biosynthetic enzymes and transporters. PLos One 6: e16683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]