Abstract
The aryl hydrocarbon receptor (AhR) mediates many toxic effects of environmental pollutants. AhR also interacts with multiple growth factor-driven signaling pathways. In the course of examining effects of growth factors on proliferation of human colon cancer cells, we identified cross talk between AhR and the epidermal growth factor receptor (EGFR). In the present work, we explored underlying signal transduction mechanisms and functional consequences of this interaction. With the use of two human colon cancer cell lines, H508 and SNU-C4, we examined the effects of AhR ligands including 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on cell proliferation and activation of EGFR, ERK1/2, and Src kinases. In colon cancer cells, 5-day incubation with TCDD stimulated a twofold dose-dependent increase in cell proliferation that was detectable with 1 nM and maximal with 30 nM TCDD. TCDD induced dose- and time-dependent phosphorylation of EGFR (Tyr845) and ERK1/2; maximal phosphorylation was observed 5 to 10 min after addition of 30 nM TCDD. Both TCDD-induced ERK1/2 phosphorylation and cell proliferation were abolished by AhR small interfering RNA, AhR-specific inhibitor CH223191, Src kinase inhibitor PP2, neutralizing antibodies against matrix metalloproteinase 7, heparin-binding-EGF-like growth factor and EGFR, EGFR inhibitors (AG1478 and PD168393), and MEK1 inhibitor PD98059. Coimmunoprecipitation experiments revealed that AhR forms a protein complex with Src and regulates Src activity by phosphorylating Src (Tyr416) and dephosphorylating Src (Tyr527). These data support novel observations that, in human colon cancer cells, Src-mediated cross talk between aryl hydrocarbon and EGFR results in ERK1/2 activation, thereby stimulating cell proliferation.
Keywords: colon cancer, Src kinase, matrix metalloproteinase
the aryl hydrocarbon receptor (AhR) is a cytosolic ligand-activated transcription factor belonging to the basic helix-loop-helix-Per/AhR nuclear translocator (ARNT)/Sim (PAS) family (17, 36, 43). In its inactive state, cytosolic AhR remains bound to several protein chaperones including heat-shock protein 90, p23, and the AhR-interacting protein. Upon ligand binding, AhR translocates into the cell nucleus, there forming a dimer with ARNT. Nuclear AhR-ARNT dimers activate gene transcription by binding to xenobiotic-responsive elements located within the target gene promoters. AhR target genes include phase I and II drug metabolizing enzymes such as the cytochrome P450 (CYP) enzymes CYP1A1 and CYP1B1, which play prominent roles in detoxifying environmental pollutants (27). AhR ligands are grouped into two major classes, synthetic and naturally occurring. Synthetic ligands include a large number of environmental pollutants; many are halogenated aromatic hydrocarbons, including 3-methylcholanthrene (MC) and the most potent ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Natural AhR ligands include indirubin and two dietary compounds, indole-3-carbinol (I3C) and 3,3′-diindolylmethane (DIM; Ref. 28).
In addition to its essential role in controlling xenobiotic metabolism, AhR plays an important role in regulating cell proliferation and apoptosis by interacting with several growth factor-mediated signaling pathways, including the epidermal growth factor receptor (EGFR), tumor necrosis factor-α, and transforming growth factor-β (16, 33). In rat liver and immune cells, AhR ligands including TCDD were shown to activate EGFR and mitogen-activated protein kinase (MAPK) extracellular signal-regulated kinases (ERK)1/2 (23, 32, 34). Depending on the cell type examined, these actions of AhR cause pleiotropic effects: increased cell proliferation in some cells and enhanced apoptosis in others. For example, in a human mammary epithelial cell line MCF10A, TCDD activates ERK1/2 via a Src-dependent mechanism to either stimulate cell proliferation (38) or antagonize insulin-induced cell growth (31). It was also reported in human T cells and macrophages that by activating ERK1/2 and p38 MAPK, TCDD induced apoptosis (32). To date, there are no reports regarding effects of AhR ligands on proliferation of intestinal epithelial cells.
The role of AhR in tumorigenesis remains controversial. Evidence supports both pro- and anticarcinogenic properties of AhR signaling. It has been known for decades that CYP1 enzymes catalyze conversion of polycyclic aromatic hydrocarbons including benzo[a]pyrene to active genotoxic metabolites, thereby initiating carcinogenesis. In addition, the AhR repressor was shown to act as a tumor suppressor in multiple human cancers, including colorectal cancer (46). Paradoxically, AhR was also reported to suppress intestinal and liver cancers (13, 21). In Apcmin/+ mice, Kawajiri et al. (21) showed that activation of AhR with natural ligands suppresses intestinal tumor formation. Using AhR-deficient mice, Fan et al. (13) showed that AhR functioned as a tumor suppressor for liver carcinogenesis.
Previously, we identified cross talk between the M3 muscarinic receptor, a G-protein-coupled receptor, and EGFR, a receptor tyrosine kinase (7, 9, 44). We demonstrated that M3 muscarinic receptor interacted with EGFR through activation of matrix metalloproteinase 7 (MMP7) and release of an EGFR ligand, heparin binding EGF-like growth factor. As a consequence of this interaction, muscarinic agonists stimulate colon cancer cell proliferation. These observations suggested to us that, in human intestinal epithelial cells, there might exist other examples of cross talk between EGFR and signaling pathways besides those with G-protein-coupled receptors. The objective of the present study was to seek evidence for AhR cross talk with EGFR and elucidate effects of this interaction. In particular, we asked whether activation of AhR results in pro-proliferative effects in human colon epithelial cells and what were the molecular mechanisms underlying these actions. Our results include the novel observation that TCDD-AhR interaction induces robust proliferation of human colon cancer cells through Src-mediated EGFR activation.
MATERIALS AND METHODS
Reagents.
TCDD, MC, indirubin, indole-3-carbinol (I3C), DIM, PP2, CH223191, and Tiron were purchased from Sigma-Aldrich; PD168393, AG1478, PD98059, U0126, LY294002, PP3, GM6001, and GM6001 negative control (NC) from Calbiochem; CellTiter 96 AQueous one solution cell proliferation assay (MTS) kit from Promega; RPMI 1640, DMEM, and McCoy's 5A growth media from Mediatech. TCDD was supplied in toluene solution. All other chemical inhibitors were dissolved in DMSO unless specified.
Cell culture.
H508 and SNU-C4 human colon cancer cells (ATCC) were grown in RPMI 1640 growth media supplemented with 10% FBS. Adherent cells were passaged weekly at subconfluence after trypsinization. Cultures were maintained in incubators at 37°C in an atmosphere of 5% CO2-95% air.
Cell proliferation assay.
Cells were seeded in 96-well plates at ∼10% confluence and allowed to attach for 24 h. After 18 h of serum starvation, 200 μl fresh serum-free growth media containing test agents were added. When chemical inhibitors and antibodies were used, they were added 30 min and 2 h, respectively, before test agents. All samples contained the same concentrations of solvents, i.e., either 0.1% toluene (TCDD and MC), 0.1% DMSO (CH223191, PP2, PP3, PD168393, AG1478, PD98059, U0126, LY294002, GM6001, GM6001NC, and indirubin), 0.1% ethanol (I3C and DIM), or combinations. Cells were incubated for 5 days at 37°C in an atmosphere of 5% CO2-95% air without further additions of test agents. After a 5-day incubation, cell proliferation was determined by addition 20 μl CellTiter 96 AQueous One solution (Promega) to each well. After a 1- to 2-h incubation at 37°C, absorbance was measured at 490 nm using a 96-well microtiter plate reader (SpectraMax384).
Antibodies and immunoblotting.
Rabbit polyclonal anti-ERK1/2 (p44/42) MAPK, mouse monoclonal anti-phospho-ERK1/2 MAPK, rabbit monoclonal anti-EGFR, rabbit polyclonal anti-phospho-EGFR (Tyr845), rabbit polyclonal anti-phospho-EGFR (Tyr992), rabbit polyclonal anti-phospho-EGFR (Tyr1173), rabbit polyclonal anti-phospho-Src (Tyr416), rabbit polyclonal anti-phospho-Src (Tyr527), and rabbit polyclonal anti-Src antibodies were from Cell Signaling Technology. Goat polyclonal anti-AhR and mouse monoclonal anti-AhR antibodies were from Santa Cruz Biotechnology and Sigma, respectively. Neutralizing anti-EGFR antibody (LA1) was from Millipore. Neutralizing anti-MMP7 and anti-heparin-binding-EGF-like growth factor (HBEGF) antibodies were from R&D Systems. Immunoblotting was performed as described previously (7). For chemical inhibitors (CH223191, PP2, PP3, AG1478, PD168393, PD98059, U0126, LY294002, wortmannin, GM6001, and GM6001NC) that are soluble only in DMSO, all samples and controls contained the same concentrations of DMSO (0.1%). Densitometry of immunoblots was performed using Adobe Photoshop and normalized to controls.
Immunoprecipitation.
Immunoprecipitation was performed according to New England Biolab protocols with minor modifications. Briefly, cells were lysed in RIPA buffer with proteinase and phosphatase inhibitors. Lysates were precleared with magnetic protein G agarose beads (New England Biolabs) and incubated with 2 μg goat anti-AhR antibody (Santa Cruz Biotech) overnight. Normal goat IgG (2 μg) was used as a negative control. Mouse monoclonal anti-AhR antibody (Sigma-Aldrich) was used to detect AhR. Immunoprecipitation was performed using actively growing cells with 10% FBS or cells that were serum starved overnight.
Quantitative real-time PCR.
Quantitative real-time PCR and quantification were performed as described previously (44). The following primers were used: human CYP1A1, forward, 5′-AGTGGCAGATCAACCATGAC and reverse, 5′-TTGTCGATAGCACCATCAGG; human CYP1B1, 5′-AGAACGTACCGGCCACTATC and reverse, 5′-ACTCGAGTCTGCACATCAGG; and human cyclooxygenase-2 (COX2), forward, 5′-TTAATGAGTACCGCAAACGC and reverse, 5′-ACCAGAAGGGCAGGATACAG. Specificity of amplifications was confirmed by melting-curve analysis. Relative levels of mRNA were calculated according to the standard ΔΔCt method. Individual expression values were normalized by comparison with glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
AhR small interfering RNA transfection.
H508 cells were seeded in 6- and 96-well plates at ∼10% confluence and incubated at 37°C for 24 h. Four pooled small interfering RNA (siRNA) duplex oligos targeting human AhR or nontargeting control oligos (control siRNA; Dharmacon) were transfected into H508 cells with DharmaFECT4 transfection agent according to the manufacturer's instructions. Two days following transfections, cells in 96-well plates were incubated for an additional 5 days in serum-free RPMI medium containing TCDD or various agents at 37°C in a CO2 incubator; cells in the sixwell plates were harvested for immunoblotting to confirm and quantify AhR siRNA knockdown.
Statistics.
All data are expressed as means ± SE of at least three independent experiments. Statistical calculations were performed using Student's unpaired t-test (SigmaPlot, Systat Software, San Jose, CA). P < 0.05 was considered significant.
RESULTS
AhR agonists induce proliferation of human colon cancer cells.
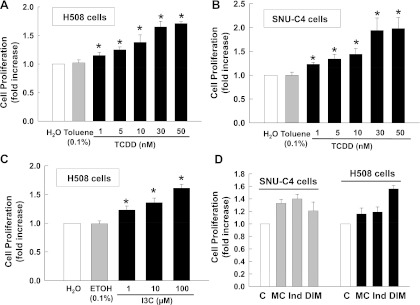
Because AhR activates EGFR in rat liver (34), we hypothesized that similar interactions occur in intestinal epithelial cells. We used two human colon cancer epithelial cell lines, H508 and SNU-C4, that express high levels of EGFR and exhibit EGFR-mediated interactions with other signaling mechanisms (2, 7) to test the effects of a variety of AhR agonists on colon cancer cell proliferation. AhR ligands included environmental contaminants (MC and TCDD) and natural ligands (indirubin, I3C, and DIM). As shown in Fig. 1, at the concentrations indicated, all AhR ligands stimulated proliferation of tested cell lines. TCDD induced dose-dependent cell proliferation in both H508 and SNU-C4 cells (Fig. 1, A and B). TCDD-induced proliferation was detectable at 1 nM and maximal at 30 nM. A 1.7- to 2.0-fold increase in cell proliferation was observed after 5 days, an incubation time selected based on our previous work with these cell lines (7, 44). Because toluene concentrations ≥0.2% were toxic to H508 and SNU-C4 cells (data not shown), the maximal TCDD concentration that could be used was 50 nM (0.1% toluene). As shown in Fig. 1C, I3C also induced dose-dependent proliferation of H508 cells. Likewise, as shown in Fig. 1D, a sampling of other AhR ligands revealed that MC, indirubin, and DIM also stimulated proliferation of both H508 and SNU-C4 cells. Because TCDD, one of the most potent AhR agonists caused the most robust increase in cell proliferation, we focused on TCDD for additional studies.
Fig. 1.
Aryl hydrocarbon receptor (AhR) agonists stimulate proliferation of colon cancer cells. A: dose-response of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced proliferation of H508 human colon cancer cells. B: dose-response of TCDD-induced proliferation of SNU-C4 human colon cancer cells. C: actions of indole-3-carbinol (I3C) on proliferation of H508 cells. D: actions of 3-methylcholanthrene (MC; 1 μM), indirubin (100 nM), and 3,3′-diindolylmethane (DIM; 1 μM) on proliferation of H508 and SNU-C4 cells. Cells were incubated for 5 days at 37°C with AhR ligands at the indicated concentrations. Cell proliferation was measured as described in materials and methods. Values represent means ± SE from ≥3 separate experiments. *P < 0.05 vs. controls (C; Student's t-test).
TCDD-induced cell proliferation is mediated by AhR.
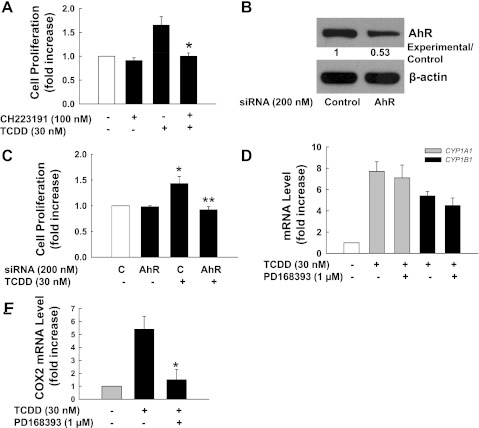
To demonstrate that TCDD-stimulated cell proliferation required AhR, we examined the actions of an AhR-specific chemical inhibitor, CH223191, and AhR siRNA. As shown in Fig. 2A, CH223191 completely blocked TCDD-induced cell proliferation in H508 cells. When expression of AhR was reduced with siRNA (Fig. 2B), TCDD-induced proliferation of H508 cells was strongly attenuated (Fig. 2C).
Fig. 2.
TCDD-induced proliferation of H508 cells is AhR dependent. A: H508 cells were incubated for 5 days at 37°C with 30 nM TCDD in the presence or absence of AhR inhibitor CH22319. Cell proliferation was measured as described in materials and methods. *P < 0.05 vs. TCDD-stimulated cells in the absence of CH223191. B: H508 cells in 6-well plates were transfected with either AhR small interfering (si)RNA or control siRNA as described in materials and methods. Numbers between immunoblots represent densitometry. Experimental/control ratios were calculated after normalizing each test band to the respective β-actin band. After 48 h, AhR protein levels were determined by immunoblotting with anti-AhR antibody. the β-actin levels were used as loading controls. C: 2 days after transfection, AhR siRNA-transfected cells or control siRNA-transfected cells in 96-well plates were incubated for an additional 5 days in serum-free medium containing 30 nM TCDD or 0.1% toluene as a control. *P < 0.05 vs. control siRNA-transfected cells in the absence of TCDD. **P < 0.05 vs. control siRNA-transfected cells in the presence of TCDD. D: TCDD stimulates CYP1A1 and CYP1B1 mRNA expression. E: TCDD stimulates cyclooxygenase-2 (COX2) mRNA expression. Quantitative PCR was performed using cDNAs synthesized from total RNAs prepared from H508 cells that were stimulated for 4 h with 30 nM TCDD or 0.1% toluene in the presence or absence of epidermal growth factor receptor (EGFR) inhibitor PD168393 (1 μM). *P < 0.05 vs. TCDD-stimulated cells in the absence of PD168393. Values represent means ± SE from ≥3 separate experiments.
TCDD stimulates expression of pro-proliferative genes through EGFR.
To confirm that TCDD activated AhR in H508 cells, we examined the effect of TCDD on expression of two AhR target genes, CYP1A1 and CYP1B1. As shown in Fig. 2D, at 4 h in H508 cells, 30 nM TCDD increased mRNA levels of CYP1A1 and CYP1B1 by ∼6.7- and 4.4- fold, respectively. To determine whether TCDD-AhR interaction also upregulated expression of pro-proliferative genes, we performed quantitative PCR using primers for COX2, a proproliferative gene the expression of which is often increased in neoplastic intestinal cells (15). As shown in Fig. 2E, TCDD stimulated a 5.4-fold increase in COX2 mRNA levels. TCDD-induced upregulation of COX2 transcription was abolished by the EGFR inhibitor PD168393. In contrast, as shown in Fig. 2D, TCDD-induced upregulation of CYP1A1 and CYP1B1 transcription was not altered by preincubation with the EGFR inhibitor PD168393. These results indicate that TCDD-AhR interaction results in activation of gene transcription through two distinct pathways; AhR directly activates transcription of xenobiotic enzymes but indirectly activates transcription of proproliferative genes through EGFR.
TCDD stimulates EGFR phosphorylation through Src kinase.
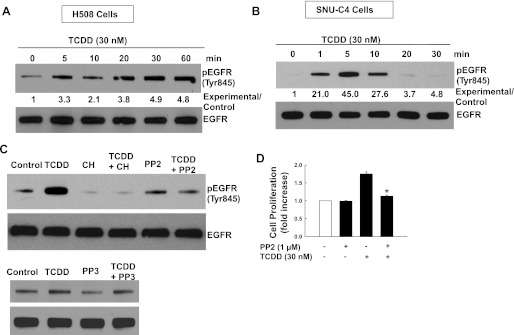
EGFR plays a central role in regulating cell proliferation in many cancer cells (45). In human mammary epithelial cells, TCDD transactivates EGFR through Src kinase (31). To identify AhR cross talk with EGFR in human colon cancer cells, we examined the actions of TCDD on EGFR (Tyr845) phosphorylation, which is primarily mediated by Src kinase (4, 37). As shown in Fig. 3, A and B, TCDD induced robust time-dependent phosphorylation of EGFR (Tyr845) in both H508 and SNU-C4 cells. In H508 cells, TCDD-induced EGFR (Tyr845) phosphorylation was detected at 5 min and persisted for ≥60 min. In SNU-C4 cells, TCDD-induced EGFR Tyr845 phosphorylation was more robust but transient: it was detected at 1 min, peaked at 5 min, and returned to baseline by 20 min. As anticipated, TCDD-induced EGFR (Tyr845) phosphorylation in H508 cells was blocked by the AhR inhibitor CH223191 (Fig. 3C). In addition, TCDD-induced EGFR Tyr845 phosphorylation was attenuated by a Src kinase inhibitor PP2 but not by PP3, an inactive PP2 analog (Fig. 3C). In both cell lines, TCDD did not alter phosphorylation of EGFR (Tyr992) and (Tyr1173); sites that are not Src kinase substrates (data not shown). Furthermore, as shown in Fig. 3D, TCDD-induced H508 cell proliferation was abolished with a Src inhibitor PP2. Collectively, these data support the conclusion that TCCD stimulates cell proliferation by activating EGFR (Tyr845) by a Src kinase-dependent mechanism.
Fig. 3.
Src kinase mediates TCDD-induced phosphorylation of EGFR (Tyr845). A: time course for TCDD-induced EGFR (Tyr 845) phosphorylation in H508 human colon cancer cells. B: time course for TCDD-induced EGFR (Tyr 845) phosphorylation in SNU-C4 human colon cancer cells. Immunoblotting for total EGFR was used as a loading control. H508 or SNU-C4 cells were treated with 30 nM TCDD for the indicted time at 37°C. Numbers between immunoblots represent densitometry. Experimental/control ratios were calculated after normalizing each test band to the respective EGFR band. Total EGFR was used as a loading control. C: H508 cells were incubated for 5 min at 37°C, with 30 nM TCDD in the presence or absence of AhR inhibitor CH223191 (1 μM), Src inhibitor PP2 (10 μM), or PP3 (10 μM). Phosphorylation of EGFR (Tyr845) was detected by immunoblotting. Immunoblots are representative of ≥3 separate experiments. D: H508 cells were incubated for 5 days at 37°C with 30 nM TCDD in the presence or absence of the Src inhibitor PP2. Values represent means ± SE from ≥3 separate experiments *P < 0.05 vs. TCDD alone.
TCDD activates ERK1/2 through AhR, Src, and EGFR.
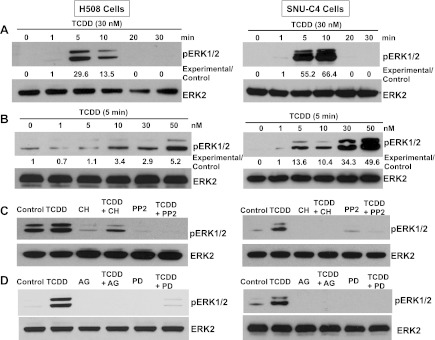
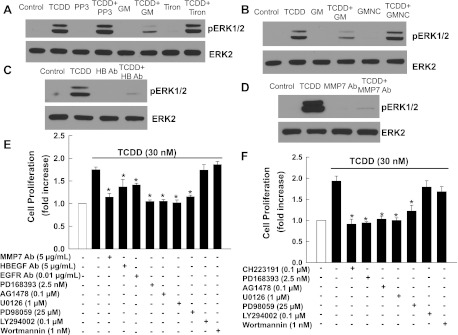
To determine whether TCDD-induced transactivation of EGFR activates downstream EGFR signaling, we examined the actions of TCDD on the phosphorylation status of ERK1/2. As shown in Fig. 4A, TCDD induced time- and dose-dependent activation of ERK1/2 in both cell lines that was transient and robust, peaking in 5–10 min. As shown in Fig. 4B, in both cell lines, ERK1/2 phosphorylation was detectable with 5–10 nM TCDD and maximal with 50 nM TCDD, the highest concentration tested. In addition, as shown in Fig. 4, C and D, in both H508 and SNU-C4 cells, TCDD-induced phosphorylation of ERK1/2 was abolished with the AhR inhibitor CH223191, Src inhibitor PP2, and EGFR inhibitors AG1478 and PD168393. PP3, an inert PP2 analog, did not alter TCDD-induced ERK1/2 phosphorylation (Fig. 5A). These results confirmed that TCDD-AhR interaction stimulates Src-dependent transactivation of EGFR and downstream ERK1/2 phosphorylation.
Fig. 4.
TCDD-induced time- and dose-dependent ERK1/2 phosphorylation is mediated by AhR, Src kinase, and EGFR. A: time-course for TCDD-induced ERK1/2 phosphorylation in H508 and SNU-C4 cells. Cells were treated with 30 nM TCDD for the indicated time at 37°C, and phosphorylated ERK1/2 was detected by immunoblotting with anti-phospho-ERK1/2 antibody. B: dose-response for TCDD-induced ERK1/2 phosphorylation in H508 and SNU-C4 cells. Cells were treated with the indicated concentrations of TCDD for 5 min at 37°C. ERK1/2 phosphorylation was detected by immunoblotting with anti-phospho-ERK1/2 antibody. Numbers below immunoblots represent densitometry. Experimental/control ratios were calculated after normalizing each test band to the respective ERK2 band. C: H508 or SNU-C4 cells were treated with 30 nM TCDD for 5 min at 37°C with or without a 30-min preincubation with AhR inhibitor CH223191 (CH; 1 μM) and Src kinase inhibitor PP2 (10 μM). D: H508 or SNU-C4 cells were treated with 30 nM TCDD for 5 min at 37°C with or without a 30-min preincubation with EGFR inhibitors AG1478 (AG; 0.1 μM) and PD168393 (PD; 1 μM). Phosphorylation of ERK1/2 was detected by immunoblotting with anti-phospho-ERK1/2 antibody. Immunoblotting for total ERK2 was used as a loading control. Immunoblots are representative of at ≥3 separate experiments.
Fig. 5.
Actions of various inhibitors on TCDD-induced ERK1/2 phosphorylation and cell proliferation. A: H508 cells were treated with 30 nM TCDD for 5 min at 37°C with or without a 30-min preincubation with PP3 (10 μM), an inert PP2 analog, and a reactive oxygen species (ROS) scavenger Tiron (200 μM). B: H508 cells were treated with 30 nM TCDD for 5 min at 37°C with or without 30-min preincubation with the matrix metalloproteinase (MMP) inhibitor GM6001 (20 μM) and inactive analog GM 6001NC (20 μM). C: H508 cells were treated with 30 nM TCDD for 5 min at 37°C with or without 30-min preincubation with neutralizing anti-heparin-binding-EGF-like growth factor (HBEGF) antibody (5 μg/ml). D: H508 cells were treated with 30 nM TCDD for 5 min at 37°C with or without 30-min preincubation with neutralizing anti-MMP7 antibody (5 μg/ml). E: H508 cells were incubated for 5 days at 37°C with 30 nM TCDD in the presence or absence of neutralizing antibodies against MMP7, HBEGF, and EGFR; EGFR inhibitors PD168393 and AG1478; MEK inhibitors PD98059 and U0126; and phosphatidylinositol 3-kinase inhibitors LY294002 and wortmannin. F: SNU-C4 cells were incubated for 5 days at 37°C with 30 nM TCDD in the presence or absence of CH223191, PD168393, AG1478, U0126, PD98059, LY294002, and wortmannin. Values represent means ± SE from ≥3 separate experiments *P < 0.05 vs. TCDD alone.
TCDD activation of EGFR involves MMP7 and HBEGF.
Because transactivation of EGFR often involves an MMP (18), we examined the action of a nonselective MMP inhibitor, GM6001. As shown in Fig. 5B, GM6001, but not GM6001NC, an inert GM6001 analog, strongly inhibited TCDD-induced ERK1/2 phosphorylation. Previously, we (7, 9) showed in colon cancer cell lines that MMP7-catalyzed release of an endogenous EGFR ligand, HBEGF, mediates transactivation of EGFR. Hence, we examined the actions of neutralizing antibodies against MMP7 and HBEGF on TCDD-induced ERK1/2 phosphorylation. As shown in Fig. 5, C and D, both anti-MMP7 and anti-HBEGF antibodies abolished TCDD-induced ERK1/2 phosphorylation. Concentrations of GM6001, GM6001NC, anti-MMP7, and anti-HBEGF used in these experiments were those previously shown to inhibit transactivation of EGFR in colon cancer cell lines (7, 9, 35, 44). In addition, because generation of reactive oxygen species (ROS) may be required for transactivation of EGFR (35), we examined the actions of Tiron, a ROS scavenger. TCDD-induced ERK1/2 phosphorylation was not affected by adding Tiron (Fig. 5A). Collectively, these data indicate that in colon epithelial cells cross talk between AhR and EGFR requires participation by Src, MMP7, and HBEGF.
TCDD-induced cell proliferation is mediated by transactivation of EGFR and post-EGFR ERK1/2 signaling.
To determine whether transactivation of EGFR and post-EGFR ERK1/2 signaling plays a key role in mediating the actions of TCDD on cell proliferation, we examined the actions of various inhibitors. As shown in Fig. 5, E and F, TCDD-induced cell proliferation was significantly attenuated or abolished by preincubation with neutralizing antibodies against MMP7, HBEGF, and EGFR; EGFR inhibitors PD168393 and AG1478; and inhibitors of MEK, the kinase immediately upstream of ERK1/2 (U0126 and PD98059). In contrast, preincubation with phosphatidylinositol 3-kinase inhibitors LY294002 and wortmannin did not alter TCDD-induced cell proliferation. All inhibitors alone at the concentrations used did not alter cell proliferation (data not shown; Refs. 7–9). These results provide additional support for the conclusion that, in human colon cancer cells, TCDD stimulates cell proliferation via MMP7- and HBEGF-mediated transactivation of EGFR.
TCDD stimulates Src kinase phosphorylation.
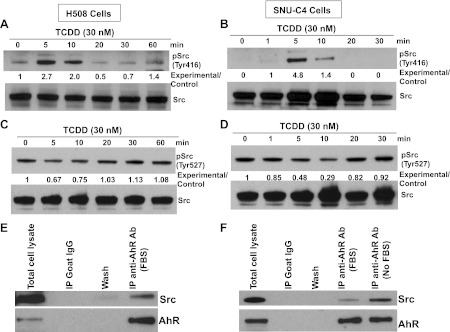
To investigate further the mechanisms underlying TCDD-induced Src activation, we examined the effects of TCDD on the phosphorylation status of Src kinase at Tyr416 and Tyr527. Phosphorylation of Src (Tyr416) upregulates enzyme activity, whereas phosphorylation of Src (Tyr527) reduces enzyme activity (20). As shown in Fig. 6, A–D, in both cell lines, TCDD induced phosphorylation of Src (Tyr416) and dephosphorylation of Src (Tyr527) in a time-dependent manner. In both H508 and SNU-C4 cells, maximal phosphorylation of Src (Try416) and dephosphorylation of Src (Tyr527) occurred at 5–10 min after addition of TCDD. These time courses are consistent with those for TCD-stimulated phosphorylation of EGFR and ERK1/2 (Figs. 3 and 4).
Fig. 6.
TCDD-induced cell proliferation is dependent on Src kinase that interacts with AhR. A: time course for TCDD-induced phosphorylation of Src (Tyr416) in H508 cells. B: time course for TCDD-induced phosphorylation of Src (Tyr416) in SNU-C4 cells. C: time course for TCDD-induced phosphorylation of Src (Tyr527) in H508 cells. D: time course for TCDD-induced phosphorylation of Src (Tyr527) in SNU-C4 cells. Cells were treated with 30 nM TCDD for the indicted time at 37°C. Phosphorylation of Src (Tyr416) or (Tyr527) was detected by immunoblotting with the corresponding antibodies. Numbers between the immnunoblots represent densitometry. Experimental/control ratios were calculated after normalizing each test band to the respective Src band. Immnoblotting for total Src was used as a loading control. E: total H508 cell lysates (positive control), H508 cell proteins immunoprecipitated (IP) with normal goat IgG (negative control), wash solution from IgG immunoprecipitation, and H508 cell proteins immunoprecipitated with goat polyclonal anti-AhR antibody were probed with rabbit polyclonal anti-Src and mouse monoclonal anti-AhR antibodies. F: total SNU-C4 cell lysates (positive control), SNU-C4 cell proteins immunoprecipitated with normal goat IgG (negative control), wash solution from IgG immunoprecipitation, and SNU-C4 cell proteins immunoprecipitated with goat polyclonal anti-AhR antibody were probed with rabbit polyclonal anti-Src and mouse monoclonal anti-AhR antibodies. Immunoprecipitation was performed as described in materials and methods using cell lysates from actively growing cells in the presence of 10% FBS (FBS) or serum-starved cells (No FBS). Normal goat IgG was used as a negative control. All cell lysates used for immunoprecipitation were precleared with protein G magnetic beads. Immunoblots are representative of ≥3 separate experiments.
AhR interacts with Src.
In rodent adipose and liver tissues, it was reported that Src may be associated with components of the cytosolic AhR complex (5, 12). To determine whether AhR and Src are present in the same protein complex in colon epithelial cells, we performed coimmunoprecipitation experiments. As shown in Fig. 6, E and F, anti-AhR antibodies successfully immunoprecipitated AhR. Moreover, we detected Src in AhR immunoprecipitation complexes in both cell lines but not in control goat IgG immunoprecipitates or washes. These results demonstrate that in both colon cancer cell lines AhR and Src coexist in the same protein complex. Nonetheless, at present, it is unclear whether AhR and Src interact directly or indirectly through other protein intermediaries.
DISCUSSION
Cancer cell proliferation is regulated by a variety of growth factors and receptors. EGFR plays a key role in promoting growth of many human cancers, including colorectal cancer (25, 42). As a consequence, EGFR-targeted biological therapies are Food and Drug Administration approved for treatment of EGFR-positive advanced colorectal cancer (29, 40). However, to maximize their growth potential, cancer cells may develop new mechanisms to activate EGFR indirectly through cross talk with other signaling pathways. Such cross talk may limit the benefits of currently approved EGFR-targeted biological (3, 19, 39). For example, in mouse models of colon cancer and several human colon cancer cell lines including H508 cells used in this study, we showed that cholinergic muscarinic receptors cross talk with EGFR to stimulate cell proliferation, tumor growth, and invasion (2, 7, 9, 44).
Findings described in the present study reveal a novel mechanism whereby colon cancer cells can gain growth advantages through EGFR signaling. AhR ligands including TCDD, MC, I3C, and DIM robustly stimulated proliferation of two colon epithelial cell lines (Fig. 1); TCDD-AhR interaction results in time- and dose-dependent phosphorylation of Src (Tyr416), EGFR (Tyr 845), and ERK1/2 (Figs. 3, A and B, and 4, A and B); TCDD-induced ERK1/2 phosphorylation and cell proliferation are either abolished or strongly inhibited by inhibitors of AhR, Src, MMP7, HBEGF, and EGFR (Figs. 3–5); and AhR and Src coimmunoprecipitate (Fig. 6, E and F), thereby identifying a physical interaction. Src is capable of activating EGFR either directly through phosphorylating EGFR (Tyr845) in the cytoplasm (4, 37) or indirectly through MMP and HBEGF (24, 30). We showed in the current study that, in colon intestinal epithelial cells, TCDD-induced transactivation of EGFR was mediated by Src kinase through MMP7-catalyzed release of HBEGF, an endogenous EGFR ligand. Collectively, these data support the novel observation that AhR cross talk with EGFR via Src, MMP7, and HBEGF stimulates proliferation of human colon epithelial cells.
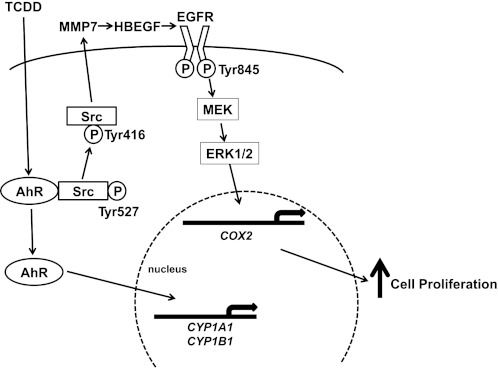
On the basis of the present findings, in Fig. 7 we illustrate a proposed model delineating key steps from TCDD interaction with AhR to increased cell proliferation in human colon cancer cells. In unstimulated cells, Src and AhR coexist in a protein complex that also likely contains heat-shock protein 90, AhR-interacting protein, and P23 (6). Binding of TCDD to AhR activates Src (Tyr416 phosphorylation), which in turn activates EGFR (Tyr845 phosphorylation) by a mechanism involving activation of MMP7 and release of HBEGF. Downstream ERK1/2 signaling and pro-proliferative gene (e.g., COX2) transcription stimulates cell proliferation. Activated AhR can also translocate into the nucleus and act as a transcriptional factor to directly regulate levels of genes involved in xenobiotic metabolism such as CYP1A1 and CYP1B1. The EGFR family consists of four structurally related members, EGFR (ERBB1), ERBB2, ERBB3, and ERBB4. We (2) previously showed that H508 and SNU-C4 cells primarily express EGFR and ERBB2. Neutralizing anti-EGFR antibodies attenuated TCDD-induced cell proliferation (Fig. 5A). Hence, we believe that EGFR plays a dominant role in mediating the actions of TCDD in colon cancer cells used in this study (Fig. 7).
Fig. 7.
Model depicting proposed mechanisms underlying TCDD-induced, Src-mediated activation of EGFR signaling in colon epithelial cells. Binding of AhR by a ligand, e.g., TCDD, results in phosphorylation of Src kinase at Tyr416 and de-phosphorylation of Src kinase at Tyr527. Phosphorylated Src (Tyr416) activates EGFR indirectly through activation of MMP7 and release of an EGFR ligand, HBEGF. Post-EGFR downstream ERK1/2 signaling stimulates transcription of pro-proliferative genes, e.g., COX2, and cell proliferation. Binding of AhR by TCDD also causes translocation of AhR from the cytosol to the nucleus to act as transcription factor for CYP1A1 and CYP1B1.
The overall role of AhR in carcinogenesis remains somewhat controversial. Seemingly strong evidence supports a key role for AhR in tumor initiation; AhR-regulated phase I enzymes such as CYP1A1 and CYP1B1 convert inert pollutant compounds to active genotoxic carcinogens (10, 27). AhR-deficient mice are resistant to carcinogenic effects of most polycyclic aromatic hydrocarbons (14), and AhR-overexpressing transgenic mice spontaneously developed hepatic and gastrointestinal tumors (1, 22, 26). Zudaire et al. (46) showed that a repressor of AhR suppressed multiple human cancers including colon tumors. In contrast, other work (14) indicated that AhR suppressed intestinal tumor initiation. AhR-deficient mice spontaneously developed cecal tumors, and in Apcmin/+ mice, natural AhR ligands suppress intestinal tumor formation by promoting ligand-activated degradation of endogenous β-catenin.
Recent evidence (11) suggests that AhR plays an important role in tumor promotion; growth of preexisting neoplasia. In animal models, TCDD is one of the most potent promoters of liver and gastrointestinal tumors (1, 26, 41). In contrast to polycyclic aromatic hydrocarbons such as benzo[a]pyrene, TCDD is metabolically inert and does not generate genotoxic metabolites. Therefore, the canonical AhR pathway of regulating xenobiotic metabolism fails to explain the tumor-promoting effects of TCDD. A hallmark of tumor promotion is uncontrolled cell proliferation. In the present study, we demonstrate that interaction of AhR and EGFR robustly stimulates colon epithelial cell proliferation. Hence, Src-mediated cross talk between AhR and EGFR signaling pathways provides an important link between AhR canonical function and TCDD-mediated tumor promotion. In addition to TCDD, our study revealed that other AhR ligands, including MC, indirubin, I3C, and DIM, stimulated cell proliferation, likely also through EGFR activation.
Results of our work may have important clinical implications. Colorectal cancer (CRC) is one of the leading causes of cancer death worldwide. The presence of abundant synthetic and natural AhR ligands in the environment and from dietary sources may contribute to colon carcinogenesis through the actions of CYP1 enzymes and AhR interaction with EGFR. Our study is the first to demonstrate pro-proliferative effects of AhR ligands in intestinal epithelial cells. Based on these findings, we propose that this novel pro-proliferative interaction of AhR and EGFR can be targeted for CRC treatment. Based on their observation that natural AhR ligands suppress intestinal carcinogenesis, Kawajiri et al. (21) proposed to use naturally occurring and chemically designed AhR agonists as chemo-preventive agents for CRC. Although natural AhR ligands may inhibit initiation of intestinal tumors in mice through the APC-β-catenin pathway, our data indicate that they can strongly promote proliferation of established human colon cancer cells. It is evident that more studies are needed to fully understand the role of xenobiotics and AhR in intestinal carcinogenesis.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants K08-DK-080843 (to G. Xie) and T32-DK-067872 (to J.-P. Raufman) and National Cancer Institute Grants R01-CA-107345 and R01-CA-120407 (to J. P. Raufman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.X. conception and design of research; G.X. and Z.P. performed experiments; G.X. and Z.P. analyzed data; G.X. and Z.P. interpreted results of experiments; G.X. and Z.P. prepared figures; G.X. drafted manuscript; G.X. and J.-P.R. edited and revised manuscript; G.X., Z.P., and J.-P.R. approved final version of manuscript.
REFERENCES
- 1. Andersson P, McGuire J, Rubio C, Gradin K, Whitelaw ML, Pettersson S, Hanberg A, Poellinger L. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc Natl Acad Sci USA 99: 9990–9995, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belo A, Cheng K, Chahdi A, Shant J, Xie G, Khurana S, Raufman JP. Muscarinic receptor agonists stimulate human colon cancer cell migration and invasion. Am J Physiol Gastrointest Liver Physiol 300: G749–G760, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhola NE, Grandis JR. Crosstalk between G-protein-coupled receptors and epidermal growth factor receptor in cancer. Front Biosci 13: 1857–1865, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem 274: 8335–8343, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Blankenship A, Matsumura F. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced activation of a protein tyrosine kinase, pp60src, in murine hepatic cytosol using a cell-free system. Mol Pharmacol 52: 667–675, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Bock KW, Kohle C. Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions. Biochem Pharmacol 72: 393–404, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Cheng K, Zimniak P, Raufman JP. Transactivation of the epidermal growth factor receptor mediates cholinergic agonist-induced proliferation of H508 human colon cancer cells. Cancer Res 63: 6744–6750, 2003 [PubMed] [Google Scholar]
- 8. Cheng K, Raufman JP. Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem Pharmacol 70: 1035–1047, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Cheng K, Xie G, Raufman JP. Matrix metalloproteinase-7-catalyzed release of HB-EGF mediates deoxycholyltaurine-induced proliferation of a human colon cancer cell line. Biochem Pharmacol 73: 1001–1012, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol 43: 309–334, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Dietrich C, Kaina B. The aryl hydrocarbon receptor (AhR) in the regulation of cell-cell contact and tumor growth. Carcinogenesis 31: 1319–1328, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Enan E, Matsumura F. Identification of c-Src as the integral component of the cytosolic Ah receptor complex, transducing the signal of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) through the protein phosphorylation pathway. Biochem Pharmacol 52: 1599–1612, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Fan Y, Boivin GP, Knudsen ES, Nebert DW, Xia Y, Puga A. The aryl hydrocarbon receptor functions as a tumor suppressor of liver carcinogenesis. Cancer Res 70: 212–220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol 34: 605–614, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Fournier DB, Gordon GB. COX-2 and colon cancer: potential targets for chemoprevention. J Cell Biochem Suppl 34: 97–102, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Haarmann-Stemmann T, Bothe H, Abel J. Growth factors, cytokines and their receptors as downstream targets of arylhydrocarbon receptor (AhR) signaling pathways. Biochem Pharmacol 77: 508–520, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol 35: 307–340, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Higashiyama S. Metalloproteinase-mediated shedding of heparin-binding EGF-like growth factor and its pathophysiological roles. Protein Pept Lett 11: 443–450, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Hu T, Li C. Convergence between Wnt-beta-catenin and EGFR signaling in cancer. Mol Cancer 9: 236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hunter T. A tail of two src's: mutatis mutandis. Cell 49: 1–4, 1987 [DOI] [PubMed] [Google Scholar]
- 21. Kawajiri K, Kobayashi Y, Ohtake F, Ikuta T, Matsushima Y, Mimura J, Pettersson S, Pollenz RS, Sakaki T, Hirokawa T, Akiyama T, Kurosumi M, Poellinger L, Kato S, Fujii-Kuriyama Y. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc Natl Acad Sci USA 106: 13481–13486, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koliopanos A, Kleeff J, Xiao Y, Safe S, Zimmermann A, Buchler MW, Friess H. Increased arylhydrocarbon receptor expression offers a potential therapeutic target for pancreatic cancer. Oncogene 21: 6059–6070, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Kwon MJ, Jeong KS, Choi EJ, Lee BH. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-induced activation of mitogen-activated protein kinase signaling pathway in Jurkat T cells. Pharmacol Toxicol 93: 186–190, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Luttrell DK, Luttrell LM. Not so strange bedfellows: G-protein-coupled receptors and Src family kinases. Oncogene 23: 7969–7978, 2004 [DOI] [PubMed] [Google Scholar]
- 25. McKay JA, Murray LJ, Curran S, Ross VG, Clark C, Murray GI, Cassidy J, McLeod HL. Evaluation of the epidermal growth factor receptor (EGFR) in colorectal tumours and lymph node metastases. Eur J Cancer 38: 2258–2264, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Moennikes O, Loeppen S, Buchmann A, Andersson P, Ittrich C, Poellinger L, Schwarz M. A constitutively active dioxin/aryl hydrocarbon receptor promotes hepatocarcinogenesis in mice. Cancer Res 64: 4707–4710, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer 6: 947–960, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol 21: 102–116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Overman MJ, Hoff PM. EGFR-targeted therapies in colorectal cancer. Dis Colon Rectum 50: 1259–1270, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med 8: 289–293, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Park S, Mazina O, Kitagawa A, Wong P, Matsumura F. TCDD causes suppression of growth and differentiation of MCF10A, human mammary epithelial cells by interfering with their insulin receptor signaling through c-Src kinase and ERK activation. J Biochem Mol Toxicol 18: 322–331, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Park SJ, Yoon WK, Kim HJ, Son HY, Cho SW, Jeong KS, Kim TH, Kim SH, Kim SR, Ryu SY. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activates ERK and p38 mitogen-activated protein kinases in RAW 264.7 cells. Anticancer Res 25: 2831–2836, 2005 [PubMed] [Google Scholar]
- 33. Puga A, Ma C, Marlowe JL. The aryl hydrocarbon receptor cross talks with multiple signal transduction pathways. Biochem Pharmacol 77: 713–722, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Randi AS, Sanchez MS, Alvarez L, Cardozo J, Pontillo C, Kleiman de Pisarev DL. Hexachlorobenzene triggers AhR translocation to the nucleus, c-Src activation and EGFR transactivation in rat liver. Toxicol Lett 177: 116–122, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Riemann A, Schneider B, Ihling A, Nowak M, Sauvant C, Thews O, Gekle M. Acidic environment leads to ROS-induced MAPK signaling in cancer cells. PLos One 6: e22445, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rowlands JC, Gustafsson JA. Aryl hydrocarbon receptor-mediated signal transduction. Crit Rev Toxicol 27: 109–134, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Sato K, Sato A, Aoto M, Fukami Y. c-Src phosphorylates epidermal growth factor receptor on tyrosine 845. Biochem Biophys Res Commun 215: 1078–1087, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Tannheimer SL, Ethier SP, Caldwell KK, Burchiel SW. Benzo[a]pyrene- and TCDD-induced alterations in tyrosine phosphorylation and insulin-like growth factor signaling pathways in the MCF-10A human mammary epithelial cell line. Carcinogenesis 19: 1291–1297, 1998 [DOI] [PubMed] [Google Scholar]
- 39. van der Veeken J, Oliveira S, Schiffelers RM, Storm G, van Bergen En Henegouwen PM, Roovers RC. Crosstalk between epidermal growth factor receptor- and insulin-like growth factor-1 receptor signaling: implications for cancer therapy. Curr Cancer Drug Targets 9: 748–760, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Venook AP. Epidermal growth factor receptor-targeted treatment for advanced colorectal carcinoma. Cancer 103: 2435–2446, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Viluksela M, Bager Y, Tuomisto JT, Scheu G, Unkila M, Pohjanvirta R, Flodstrom S, Kosma VM, Maki-Paakkanen J, Vartiainen T, Klimm C, Schramm KW, Warngard L, Tuomisto J. Liver tumor-promoting activity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in TCDD-sensitive and TCDD-resistant rat strains. Cancer Res 60: 6911–6920, 2000 [PubMed] [Google Scholar]
- 42. Wan CW, McKnight MK, Brattain DE, Brattain MG, Yeoman LC. Different epidermal growth factor growth responses and receptor levels in human colon carcinoma cell lines. Cancer Lett 43: 139–143, 1988 [DOI] [PubMed] [Google Scholar]
- 43. Whitlock JP., Jr Mechanistic aspects of dioxin action. Chem Res Toxicol 6: 754–763, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Xie G, Cheng K, Shant J, Raufman JP. Acetylcholine-induced activation of M3 muscarinic receptors stimulates robust matrix metalloproteinase gene expression in human colon cancer cells. Am J Physiol Gastrointest Liver Physiol 296: G755–G763, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yarden Y. The EGFR family and its ligands in human cancer signalling mechanisms and therapeutic opportunities. Eur J Cancer 37, Suppl 4: S3–S8, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Zudaire E, Cuesta N, Murty V, Woodson K, Adams L, Gonzalez N, Martinez A, Narayan G, Kirsch I, Franklin W, Hirsch F, Birrer M, Cuttitta F. The aryl hydrocarbon receptor repressor is a putative tumor suppressor gene in multiple human cancers. J Clin Invest 118: 640–650, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]