Abstract
Changes in intestinal absorption of nutrients are important aspects of the aging process. To address this issue, we investigated the impact of accelerated mitochondrial DNA mutations on the stem/progenitor cells in the crypts of Lieberkühn in mice homozygous for a mitochondrial DNA polymerase gamma mutation, PolgD257A, that exhibit accelerated aging phenotype. As early as 3–7 mo of age, the small intestine was significantly enlarged in the PolgD257A mice. The crypts of the PolgD257A mice contained 20% more cells than those of their wild-type littermates and exhibited a 10-fold increase in cellular apoptosis primarily in the stem/progenitor cell zones. Actively dividing cells were proportionally increased, yet a significantly smaller proportion of cells was in the S phase of the cell cycle. Stem cell-derived organoids from PolgD257A mice failed to develop fully in culture and exhibited fewer crypt units, indicating an impact of the mutation on the intestinal epithelial stem/progenitor cell maintenance. In addition, epithelial cell migration along the crypt-villus axis was slowed and less organized, and the ATP content in the villi was significantly reduced. On a high-fat, high-carbohydrate diet, PolgD257A mice showed significantly restricted absorption of excess lipids accompanied by an increase in fecal steatocrits. We conclude that the PolgD257A mutation causes cell cycle dysregulation in the crypts leading to the age-associated changes in the morphology of the small intestine and contributes to the restricted absorption of dietary lipids.
Keywords: mitochondrial deoxyribonucleic acid mutations, gastrointestine, aging
intestinal dysfunction leading to mal-absorption is common in the elderly (12, 44). In humans, a compensatory increase in cell proliferation in the small intestines has been observed in the aging population correlating with increased size of the small intestine villi and crypt compartments (11, 50). Similarly, in aging rodents, increases in length and width of villi and crypt dimensions have been reported (18, 27). However, there have been no comprehensive studies demonstrating how these changes in morphology affect intestinal function. The epithelial cells of the intestinal mucosa are rapidly renewed throughout life. In the small intestine, epithelial stem cells reside near the base of crypts and give rise to progenitor cells. Epithelial progenitor cells are transit-amplifying cells and upon exit from the cell cycle at the junction of crypts and villi differentiate into absorptive enterocytes, goblet cells, and enteroendocrine cells. These differentiated epithelial cells reach the tip of villi in ∼4 days, undergo programmed cell death, and slough off into the intestinal lumen. Aging of the human small intestine is associated with increased proliferation and apoptosis of enterocytes (10). Similarly, cell proliferation in the small intestines of rats increases with age (26, 51); however, apoptosis decreases with age (51). These differences in cell proliferation and apoptosis between humans and rats have made it difficult to determine a molecular mechanism behind the aging small intestine in part due to a lack of an appropriate aging model that mimics what is seen in humans.
Aging is associated with mitochondrial dysfunction, which affects basic cellular function, initiates apoptosis, and accelerates the aging process (24). Insertions, deletions, and point mutations in the mitochondrial genome have their greatest effect in cells that require high energy production including neurons, hair cells of the inner ears, heart and skeletal myocytes, pancreatic β-cells, as well as gut and kidney epithelial cells (39, 40, 45, 49, 52). Cell division in a highly regenerative organ like the small intestine also requires high levels of energy production to synthesize lipid, protein, and nucleic acid. For example, mitochondrial DNA mutations causing respiratory chain defects in the colon of elderly subjects have been shown to attenuate cellular proliferation and increase apoptosis in the colonic crypts (29). Mitochondrial DNA polymerase gamma (Polg) is responsible for replicating the mitochondrial genome. Introducing an amino acid substitution, D257A, in the exonuclease domain II disrupts the proofreading ability of the enzyme without significantly affecting the replicating capacity of the polymerase (21, 46). Mice homozygous for the PolgD257A mutation have a reduced mean life span of 13 mo, and display a series of aging-associated phenotypes as early as 10 mo of age in conjunction with increased accumulations of mitochondrial (mt)DNA mutations (21, 46); these include graying and loss of hair, kyphosis, and decreased bone density. Hence, the homozygous PolgD257A mouse is an animal model of premature aging with many of the visible signs of human aging.
Previous work (21) utilizing the 10-mo-old PolgD257A mouse has shown two distinct changes in their small intestine: 1) increased villi epithelial cell apoptosis; and 2) elaborate branching and fusion of villi. In this study, we sought to investigate the role of the PolgD257A mutation in the proliferative cells in crypts of Lieberkühn of young adults 3–7 mo of age. The young PolgD257A mice are characterized by a striking cell cycle defect in the stem and progenitor cells that disrupts the epithelial cell migration pattern. While energy expenditure of the PolgD257A mice on a normal rodent chow (NC) diet were not altered, adaptation to a high-fat, high-carbohydrate Western-type (HFW) diet differed significantly between wild-type (WT) and PolgD257A mice, due in part to a significantly restricted absorption of excess dietary lipids.
MATERIALS AND METHODS
Mice.
Male mice homozygous for the PolgD257A mutation were derived from crosses between heterozygotes that had been extensively backcrossed (≥10 generations) onto a C57BL/6 background. Controls were male littermates that were WT at the Polg locus. Mice were fed ad libitum either NC diet [5.3% (wt/wt) fat and 0.019% (wt/wt) cholesterol; Prolab Isopro RMH 3000, 5P76; Agway, Syracuse, NY] or a HFW diet [21% (wt/wt) fat, 34% sucrose and 0.15% (wt/wt) cholesterol; TD88137; Teklad, Madison, WI]. All animal experiments were performed in accordance with the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill.
Histology.
Mice were euthanized with 2,2,2-tribromoethanol, and the small intestine was removed from the stomach to the cecum of the large intestine. Isolated intestines were placed in ice cold PBS, flushed with 10 ml of ice cold PBS, split into two sections, duodenum to jejunum and jejunum to ileum, and placed in 4% paraformaldehyde at 4°C for ≥24 h. Intestines were then cut length wise, rolled, and paraffin embedded. Sections (5 μm) were stained with hematoxylin and eosin, periodic acid-Schiff, or Masson's trichrome. Morphological parameters were measured on digitally scanned slides using ImageScope software. Cytochrome c oxidase (COX)/succinate dehydrogenase histochemistry was carried out as previously described (15, 33).
Apoptosis and S-phase detection.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) was used to detect DNA fragmentation caused by apoptosis signaling (ApopTag S7110; Chemicon). To determine cells in S phase, animals were injected with 50 μg of 5-ethynyl-2′-deoxyuridine (EdU) in 250 μl of PBS and euthanized 2 h after injection. EdU-labeled cells were detected as previously described (34) using a commercially available kit (Click-iT EdU C10084; Molecular Probes, Eugene, OR).
Cell migration.
For EdU pulse time course, mice were injected once with 50 μg of EdU in 250 μl of PBS. Mice were euthanized at 2, 24, and 48 h after injection. For EdU washout time course, mice were injected three times per day for 4 consecutive days with 75 μg of EdU in 250 μl of PBS. Mice were euthanized at 2, 24, 48, and 96 h after the last injection. EdU was detected as described previously (34).
Cell cycle immunostaining.
Before immunostaining, the sections were treated for antigen retrieval using Reveal Decloaker (Biocare Medical, Concord, CA) per manufacturer's instructions. The sections were stained first for EdU as described above using Alexafluor 488 azide and then blocked with Dako blocking solution (X0909; Dako, Carpinteria, CA) for 5 min before antibody staining. All primary antibodies were diluted in Dako antibody diluent (S0809; Dako). To detect proliferating cells, sections were stained with Ki67 (1:500; no. M7249; Dako) and with anti-phospho histone H3 (1:500; Ser10, 06-570; Millipore, Temecula, CA) as a mitotic marker. Cells in G1 phase were visualized with anti-minichromosome maintenance 2 (MCM2; N-19, SC-9839; 1:200; Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies (Ki67: donkey anti-rat biotinylated, 712-067-003/streptavidin-405, 016-470-084; PH3: donkey anti-rabbit-Dylight 549, 711-505-152; MCM2: donkey anti-goat-Dylight 647, 705-496-147) conjugated to fluorophores that facilitated colocalization were diluted in DAKO diluent at 1:250 For nuclear staining, bis-benzimide (B2883; Sigma, St. Louis, MO) was diluted to 5 μg/ml in Tris-wash buffer and applied to tissue sections for 5 min.
Crypt isolation and culturing.
Crypts were isolated as previously described (14). The crypts were placed in crypt culturing medium [advanced DMEM/F12 (no. 12634; GIBCO) containing B27 supplements minus Vitamin A (no. 12587-010; Invitrogen), N2 supplements (no. 17502-048; Invitrogen), 10 mM HEPES (15630-106; GIBCO), and 100 μg/ml penicillin/streptomycin and 2 mM l-glutamine], and the numbers of crypts were counted. A volume containing 500 crypts was removed and pelleted at 500 g for 5 min at 4°C, and the crypt pellet was lightly and carefully resuspended in 5 μl of crypt culturing medium. Crypts were cultured under conditions previously described (14) with the exception that whole crypts were placed in 50 μl of Matrigel (BD Biosciences, San Jose, CA) containing R-spondin (1 μg/ml first day and 500 ng/ml thereafter; R&D Systems, Minneapolis, MN), Noggin (100 ng/ml; Peprotech, Rocky Hill, NJ), EGF (50 ng/ml; R&D Systems), Jagged (1 μM; AnaSpec, San Jose, CA), and Wnt-3a (2.5 ng/ml, first day only; R&D Systems). ATP was measured using the CellTiter-Glo luminescent cell viability assay per manufacturer's instructions (G7570; Promega, Madison, WI). ADP/ATP ratio was measured using the ApoSENSOR ADP/ATP ratio assay kit (K255-200; BioVision, Mountain View, CA).
Metabolic parameters and energy expenditure.
Mice were housed in metabolic cages for 48 h. Mice were allowed to acclimate to the new environment for 24 h before data and sample collection. Body weight, food intake, water intake, and urine output were measured during the subsequent 24 h. Urine and feces samples were collected over 24 h. Acid steatocrit was measured as described previously, and daily fat absorption was calculated as previously described (20, 42). For the analysis of energy expenditure, indirect calorimetry and physical activity were measured using the Labmaster system (TSE Systems, Bad Homburg, Germany) as previously described (22, 36). Mice were monitored for 96 h with the first 24 h discarded to allow for acclimation to the cages. During the first 48 h, animals were fed NC diet. After 48 h, the diet was switched to the HFW diet and monitoring was continued for an additional 48 h. The second 24-h period on either the NC or HFW diet was used for all comparisons.
Data analysis.
Values are reported as means ± SE. Statistical analyses were conducted with JMP 6.0.2 software (SAS Institute, Cary, NC) with ANOVA. Tukey-Kramer honestly significant difference test was used for post hoc analyses. P values <0.05 were considered significant.
RESULTS
Small intestine morphometrics.
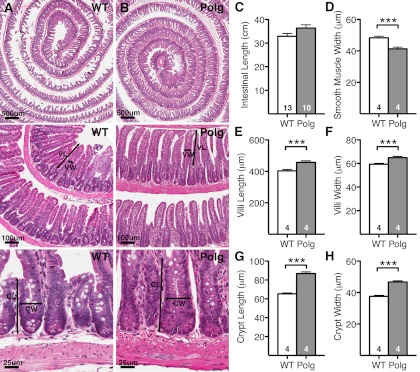
The structure of the villi and crypts of the small intestines appear normal in 7-mo-old PolgD257A mice (Fig. 1B) compared with 7-mo-old WT mice (Fig. 1A). The small intestine was slightly longer in PolgD257A mice compared with WT mice (Fig. 1C); however, the difference was not significant. The thickness of the smooth muscle, measured at 50 random locations from the proximal to distal end of the small intestine of each animal, was significantly smaller in Polg D257A mice compared with WT mice (Fig. 1D). In contrast, both the villi and crypt compartments of PolgD257A mice were enlarged as indicated by larger villus length (Fig. 1E), villus width (Fig. 1F), crypt length (Fig. 1G), and crypt width (Fig. 1H) than those of WT mice. These data show that the PolgD257A mutation affects the gross morphology of the small intestines mimicking the changes in dimension found in the small intestine in aged humans (11, 50).
Fig. 1.
Small intestine morphology of PolgD257A mice. Hematoxylin and eosin (H&E) histology of 7-mo-old (A) wild-type (WT) and (B) PolgD257A small intestines are represented as increasing magnifications. Black arrows indicate dimensions measured for villi length (VL), villi width (VW), crypt length (CL), and crypt width (CW). A comparison of small intestine morphology was made between WT and PolgD257A small intestine with regards to intestine length (C), smooth muscle width (D), villus length (E), villus width (F), crypt length (G), and crypt width (H). Approximately 50 villi and 50 crypts spanning the entire length of the intestine were measured per animal. Number of mice in each group is indicated within the bars. All data are represented as means ± SE. ***P < 0.001, between WT (n ≥ 4) and Polg (n ≥ 4).
Increased cell apoptosis in villi and crypts of PolgD257A mice.
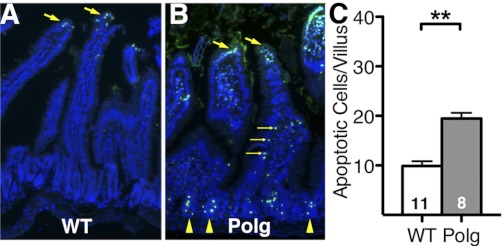
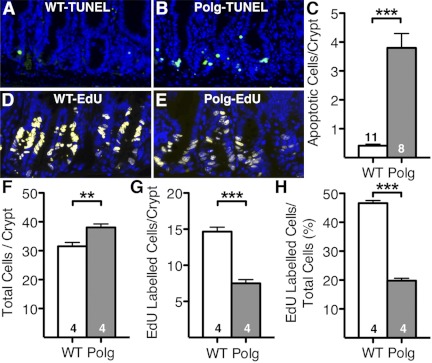
At 7 mo of age, both WT mice (Fig. 2A) and PolgD257A mice (Fig. 2B) had similar numbers of apoptotic epithelial cells in the tips of the villi. PolgD257A mice had significantly more apoptotic cells in the columnar epithelial cells compared with WT mice (Fig. 2B, small yellow arrows). These results confirm the previous report (21) of increased apoptosis in the columnar epithelial cells of the villi of PolgD257A mice. The number of apoptotic columnar epithelial cells of the villus indicates a twofold increase in cell death in PolgD257A mice (19.4 ± 1.2 cells/villus) compared with WT mice (9.9 ± 1.0 cells/villus; Fig. 2C). Strikingly, we also observed that PolgD257A intestine had a significant increase in apoptosis within the crypts of Lieberkühn (Fig. 2B, yellow arrowheads), where both intestinal stem cells and progenitor cells are present. High magnification (×40) of the crypts of Lieberkühn reveals very few apoptotic cells in the WT mice (Fig. 3A) while the TUNEL-positive cells were abundant in the PolgD257A mice (Fig. 3B). Quantification of the apoptotic crypt cells indicates a 10-fold increase in cell death in PolgD257A crypts (3.8 ± 0.5 cells/crypt) compared with WT crypts (0.4 ± 0.06 cells/crypt; Fig. 3C). Taken together, these data suggest that the increase in apoptotic cells in the PolgD257A crypts is not an age-related phenomenon but is already present in early adulthood.
Fig. 2.
Apoptosis in WT and PolgD257A small intestines. A: WT small intestine with apoptosis located at tip of each villus with large yellow arrows indicating apoptotic cells. Nuclei staining (DAPI), blue; terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), green. B: PolgD257A small intestines have similar levels of apoptosis at the tip of each villus indicated with large yellow arrows. Small yellow arrows indicate an increase in apoptotic cells in the columnar epithelial cells along the length of the villi. An increase in apoptosis was found at the base of the villi in the crypts of Lieberkühn. C: quantification of apoptosis found in the columnar epithelial cells along the length of the villi. Number of mice is indicated within the bars. All data are represented as means ± SE. **P < 0.01, between WT (n = 11) and Polg (n = 8).
Fig. 3.
Apoptosis and S-phase proliferation in WT and PolgD257A crypts of Lieberkühn. Apoptosis is located in the region of the transit amplifying cells of WT (A) and PolgD257A (B) crypts. WT crypts have few apoptotic cells while PolgD257A mice have numerous apoptotic cells. Quantification of apoptotic cells per crypt (C) comparing WT and PolgD257A mice. 5-Ethynyl-2′-deoxyuridine (EdU) incorporation into cells in the transit-amplifying region is found at 2 h after EdU administration in the crypts of WT (D) and PolgD257A (E) mice. WT mice have numerous and robust staining of S-phase cells in this region while PolgD257A mice have fewer and less intense staining of S-phase cells in this region. Quantification of total cells per crypt (F), EdU-labeled cells per crypt (G), and fraction of EdU-labeled cells of total cells (H) comparing WT and PolgD257A mice. Approximately 50 crypts were scored spanning the entire small intestines from each of 4 WT mice and 4 PolgD257A mice. Average number of cells/crypt section were obtained for each mouse and represented as means ± SE from the 4 animals in each group. Number of mice is indicated within the bars. Nuclei staining (DAPI), blue; apoptosis (TUNEL), green; and EdU cells, yellow. ***P < 0.001.
Decreased number of cells in S phase in the crypts of PolgD257A mice.
In normal tissues, a balance between apoptosis and proliferation is maintained. To examine this balance, we assessed cell proliferation in the crypts with EdU, which is incorporated into DNA during the S phase of the cell cycle and represents a marker of cell proliferation. In parallel with the increased size, the total number of cells per crypt sections (n = 50 from each animal) in PolgD257A intestine (38.4 ± 0.5) was 20% more than in WT intestine (31.5 ± 0.4; P < 0.0001; Fig. 3F). In a marked contrast to the pattern of apoptosis, visualization of EdU in the crypt region of PolgD257A mice (Fig. 3E) was less robust than the WT mice (Fig. 3D). In addition, the intensity was variable from one cell to the other, suggesting total reduction of EdU incorporation. The proportion of EdU-labeled cells in the crypts of Lieberkühn at 2 h after the EdU administration tended to be less in PolgD257A (7.9 ± 0.3 cells per crypt section) mice than in WT mice (14.6 ± 0.3; P = 0.0001; Fig. 3, G and H). These data suggest a reduction in DNA synthesis particularly in the crypts of Lieberkühn.
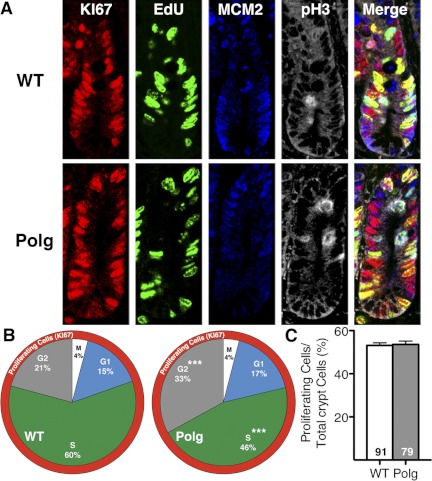
Alteration of cell cycle in intestinal crypts.
To examine whether the cell cycle dynamics of stem/progenitor cells differ between them, we next labeled epithelial cells in the crypt region of both PolgD257A and WT mice (Fig. 4A) with a proliferation marker (Ki67, red) and cell cycle markers for S phase (EdU, green), G1 phase (MCM2, blue), and M phase [phospho-histone H3 (pH3), white] and counted each cells in about 80 crypt sections per genotype. In parallel with the increased total number of cells per crypts in PolgD257A crypts, the numbers of proliferating cells detected by Ki67 were also more in PolgD257A crypts, but the fraction of proliferating cells per total crypt cells was similar between PolgD257A and WT mice (Fig. 4C). Among the proliferating cells, EdU labeling confirmed a decrease in DNA synthesis in crypt transit amplifying cells (progenitor cells) of PolgD257A mice compared with WT mice. The proportion of the S-phase cells, defined by EdU labeling but not with either M or G1 markers, was significantly reduced (P < 0.0001; Fig. 4B). Anti-MCM2 staining revealed a slightly higher percentage of crypt epithelial cells in G1 phase (growth arrest before DNA synthesis) in PolgD257A than in WT mice, although the difference did not reach significance. The proportions of the M-phase (mitosis) cells as measured by anti-pH3 staining were not different, both at 4%. In marked contrast, there were significantly higher percentages of crypt epithelial cells in late G2 (growth arrest before mitosis; 33.5 ± 1.4 vs. 20.7 ± 1.1%; P < 0.0001; Fig. 4B). The decrease in S-phase cells in PolgD257A mice and increase in G2 phase is even more dramatic considering that some of the G2 cells at this snapshot was likely counted as S phase since G2 cells were defined as Ki67 positive but negative for MCM2, pH3, or EdU since G2-specific markers were not used. We conclude that the PolgD257A mutation not only disrupts the synchronization of the cell cycle of the proliferating cells by decreasing cells in the S phase but also increases the percentage of cells in other phases of the cell cycle, suggesting a slowing of the total cell cycling time. The increase in the total number of Ki67-positive cells in the crypt region is likely to compensate for this cell cycle defect of the progenitor cells.
Fig. 4.
Cell cycle changes in PolgD257A crypt cells. A: representative crypt sections stained for cell proliferation by Ki67 (red), S-phase labeling by EdU (green), G1-phase labeling by minichromosome maintenance 2 (MCM2, blue), and M-phase labeling by pH3 (white) in WT and PolgD257A crypts. B: proportion of the proliferating cells in each phase of the cell cycle were determined from 2 WT and 2 PolgD257A mice, respectively. G2 cells are defined as being Ki67 positive but negative for all other markers. C: fraction of proliferating cells (Ki67 positive) out of total crypt cells in WT (91 crypts from 2 mice) and Polg (79 crypts from 2 mice). All data are represented as means ± SE. ***P < 0.001, between WT and Polg.
Culturing isolated crypts.
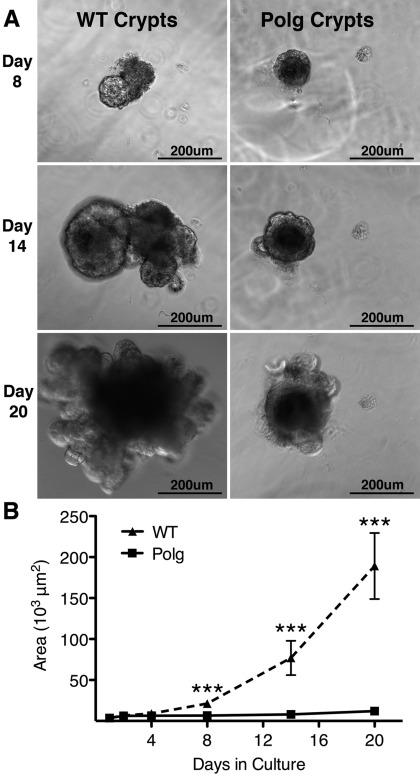
To confirm a defect in cell cycling, crypts were isolated from small intestines of 3-mo-old PolgD257A and WT mice to assess their ability to grow in culture. When in culture, isolated crypts lose the transit amplifying cells and form a ball of cells (organoids) that consist of the stem cells and Paneth cells. The Paneth cells provide many of the growth factors required by the stem cells, allowing effective growth of the stem cell population in vitro (35). During the first 4 days in culture, both WT and PolgD257A populations of cells appeared to multiply at similar rates and maintained similar sizes (Fig. 5). However, by 8 days, the WT organoid cultures began to multiply at a faster rate and became larger than the PolgD257A organoids (Fig. 5, A and B). Fourteen days after being plated, the size of the WT organoids significantly increased and began to show multiple crypt units, while crypt units of the PolgD257A organoids were rarely found (Fig. 5, A and B). Twenty days after plating, the WT organoids continued to increase in size and in formation of crypt units while growth was arrested in the PolgD257A organoids (Fig. 5, A and B). After 20 days in culture, seven of the nine independent WT organoids monitored continued to grow. In contrast, only 3 of the 13 independent PolgD257A organoids monitored grew to limited degrees. As previously described, crypt units contain stem, progenitor, and Paneth cells (14, 35). Similar results were seen in crypts isolated from 7-mo-old WT and PolgD257A mice in culture. Thus the in vitro culturing of intestinal crypts demonstrates that cellular expansion of intestinal crypt cells of the PolgD257A mice is dramatically decreased compared with that of WT mice. The decrease in cellular expansion in PolgD257A in culture is likely due to decreased proliferation of stem and progenitor cells, increased apoptosis, and/or the decreased ability of Paneth cells to support growth.
Fig. 5.
PolgD257A mutation impairs the culturing of isolated crypts. A: WT and PolgD257A cultures at days 8, 14, and 20. WT cultures have increased cellular mass with significant budding occurring at days 14 and 20. Polg have reduced cellular mass with very few buds developing. B: area of cell mass was determined on marked crypt cultures at days 1, 2, 4, 8, 14, and 20 after plating. WT cultures (▴; n = 9) grew at an exponential rate starting at day 4 in culture while Polg cultures (■; n = 13) only moderately increased in area through day 20. All data are represented as means ± SE. ***P < 0.001.
Intestinal cell migration.
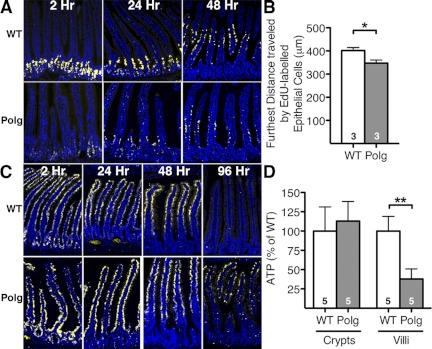
To test whether the defects in cell cycling of stem/progenitor cells alter epithelial cell migration along the crypt-villus axis in the intestine, we conducted an EdU pulse in both WT and PolgD257A mice and followed the labeled cells. EdU-labeled intestinal epithelial cells of WT mice were robustly labeled and displayed a highly coordinated migration pattern at 2, 24, and 48 h postinjection (Fig. 6A). In contrast, the EdU-labeled intestinal epithelial cells of PolgD257A mice were not only sparsely labeled at 2 h but displayed a random and asynchronous migration pattern at 24 and 48 h postinjection. The intensity of labeling and migration pattern of the EdU-labeled cells on one side of a single villus was often very different from that on the other side. We measured the distance from the base of the crypt to the EdU-labeled cell that migrated the farthest along the crypt-villi axis for each villus at 48 h to estimate the epithelial cell migration. The migration distance in PolgD257A villi was significantly less (median distance of 348 μm) than in WT villi (median distance of 402 μm; P < 0.001; Fig. 6B), indicating that the epithelial cells in PolgD257A mice are migrating more slowly than in WT mice.
Fig. 6.
Cell migration and ATP content in PolgD257A small intestines. A: migration of EdU-labeled cells at 2, 24, and 48 h postinjection in WT and PolgD257A mice. B: at 48 h postinjection of EdU, the lengths of migrating cells were measured from the base of the crypt to the farthest EdU-labeled cell in each villus. C: migration of EdU-labeled cells in WT and PolgD257A mice injected with EdU 3 times per day for 4 consecutive days at 2, 24, 48, and 96 h postinjection. D: intestinal crypts and villi were isolated from WT (n = 5) and PolgD257A (n = 5) mice. ATP in each sample was determined by normalizing to the amount of protein added to the assay and each experiment was normalized to WT mice, which was set at 100%. Number of mice is indicated within the bars. All data are represented as means ± SE. Nuclei staining (DAPI), blue; EdU cell, yellow. **P < 0.01 and *P < 0.05, between WT and Polg.
The lack of robust EdU labeling in the PolgD257A mice could be related to cellular uptake of EdU and/or triphosphate formation. To bypass these issues and to confirm a delayed cell migration of PolgD257A intestinal epithelial cells, both PolgD257A and WT mice were injected with EdU three times per day for 4 consecutive days before tissue was harvested at the indicated time points (Fig. 6B). At 2 h after the final injection, all of the intestinal epithelial cells examined in WT mice were completely labeled by this method. In contrast, labeling of the epithelial cells of PolgD257A mice was only found in the lower two-thirds of the crypt-villus axis while the cells at the tips of the villi were sparsely labeled. At 24 h postinjection, labeled WT epithelial cells had migrated completely out of the crypts and started to migrate up the villi, whereas in the PolgD257A mice the border between labeled and unlabeled epithelial cells could be seen to migrate out of the crypts and just into the lower portions of the villi at this time point. At 48 h postinjection, labeled WT epithelial cells had migrated to the upper half of the villi, whereas the PolgD257A epithelial cells migrated completely out of the crypts and started to migrate up the villi. By 96 h postinjection, labeled WT epithelial cells had completely migrated out of the villi and were no longer visible. In contrast, labeled PolgD257A epithelial cells still remained near the distal end of the villi at this time point, with some cells randomly dispersed throughout the villi. Taken together, these data show that the epithelial cell migration in the small intestines of PolgD257A mice is significantly slower compared with WT mice with an ∼24-h delay in migration thereby extending the lifespan of the epithelial cells.
Mitochondrial dysfunction in isolated intestinal crypts and villi.
To assess whether mitochondrial dysfunction contributes to the cell cycle changes in PolgD257A mice, production of ATP was measured in crypts and villi isolated from 3-mo-old mice. In the crypt population, the content of ATP in PolgD257A and WT mice was similar (Fig. 6D). CD44-positive, CD31-negative, and CD45-negative stem and progenitor cells sorted from the isolated crypts of PolgD257A mice also showed ATP levels (18.9 ± 0.3 fM ATP/cell) similar to those in WT cells (19.5 ± 0.6 fM ATP/cell). In the isolated villi, the content of ATP in PolgD257A (6.2 ± 0.2 fM ATP/cell) tissue was decreased to ∼38% of WT levels (Fig. 6D). In the crypt population, the ratio of ADP to ATP in cells was also similar (0.39 ± 0.08 in PolgD257A; n = 6, and 0.43 ± 0.08 in WT; n = 6). In the isolated villi, the ratio of ADP to ATP in PolgD257A cells (0.41 ± 0.1; n = 6) was slightly higher than WT cells (0.19 ± 0.1; n = 6), but the difference was not significant. These data indicate that levels of ATP cannot explain the cell cycle changes in Polg D257A crypts. However, decreased ATP content and the higher ADP/ATP ratio in PolgD257A villi could indicate a reduced functionality of the enterocytes.
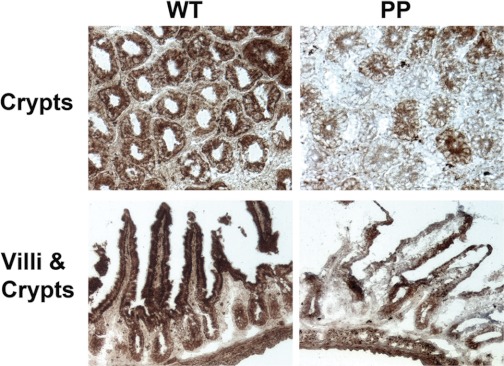
We next assessed mitochondrial function by visualizing COX enzyme activity in frozen tissue sections from upper portions of WT and PolgD257A ileum. Epithelial cells from WT sections displayed a strong and uniform COX enzyme activity in both crypts and villi (brown stain, Fig. 7). In contrast, PolgD257A crypts and villi showed general reduction as well as less uniform labeling of COX enzyme activity than WT ileum sections (Fig. 7). These data confirm that the small intestines of PolgD257A mice have a respiratory chain defect consistent with impaired mitochondrial function compared with WT small intestines.
Fig. 7.
Respiratory chain defect in PolgD257A (PP) small intestines. Segments of upper ileum were freshly isolated, frozen, and sectioned (transverse and sagittal) for cytochrome c oxidase (COX) enzymatic activity by COX/SDH histochemistry. Top: transverse sectioning of the small intestines through the crypts of WT and PolgD257A mice, respectively. Bottom: sagittal sectioning of the small intestines through the villi and crypts of WT and PolgD257A mice, respectively. Brown staining indicates a positive activity of COX. Segments isolated from 2 WT and 2 PolgD257A mice showed very similar staining patterns.
Response of PolgD257A mice to a HFW diet.
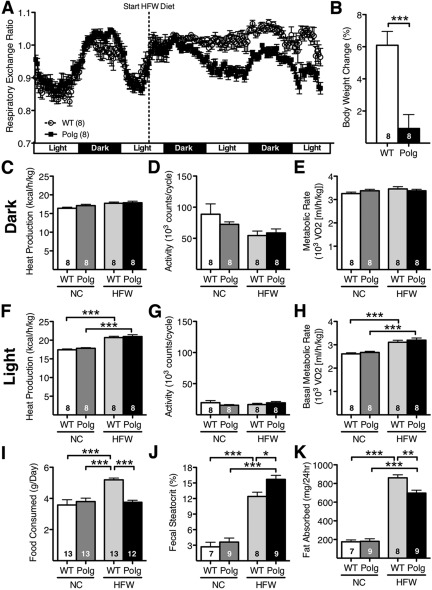
To determine the physiological consequence of the observed changes in intestinal morphology, slower renewal rate of the epithelial cells in the villi, and reduced mitochondrial function, we measured indexes of overall energy metabolism. When mice were maintained on NC diet, which is low in fat and high in fiber, the body weight, food consumption, and plasma levels of cholesterol, triglycerides, glucose, and insulin were indistinguishable between PolgD257A and WT mice at both 3 mo and 7 mo of age (not shown). Whole body metabolic activity was similar in PolgD257A males compared with WT males as measured by respiratory exchange ratio (RER), heat production, physical activity, and metabolic rate (Fig. 8). Regardless of age, the WT and PolgD257A mice responded very differently to a switch to a HFW diet. Upon diet replacement, RER in WT mice dramatically increased to >1.0 accompanied by a loss of diurnal rhythm, indicating the use of carbohydrate as a main energy source. In contrast, the response in the PolgD257A mice was blunted and they maintained diurnal rhythm of RER similar to when they were on NC diet (Fig. 8A). Overall heat production, activity, and metabolic rate during the second 24-h period after administration of the HFW diet did not differ between the two genotype groups, although the HFW diet significantly increased heat production (Fig. 8F) and basal metabolic rate (Fig. 8E) during the light cycle.
Fig. 8.
Short-term administration of high-fat, high-carbohydrate Western-type diet (HFW) in PolgD257A mice. A: combined Respiratory exchange ratio in WT and PolgD257A mice given a normal diet for 48 h followed by high fat diet for 48 h. Data from four 3-mo-old and four 7-mo-old mice were indistinguishable and were pooled. B: body weight change in WT and PolgD257A mice measured over the course of the experiment heat production (C and F), activity (D and G), and metabolic rate (E and H) were measured using indirect calorimetry during the last 24 h on each diet and averaged based on the dark and light cycles in WT and PolgD257A mice. Food consumption (I), fecal steatocrit (J), and fat absorbed (K) and before and after the HFW diet was administered to WT and PolgD257A mice. NC, normal chow diet. Number of mice is indicated within the bars. All data are represented as means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001, by Tukey-Kramer honestly significant difference test.
Upon the switch to a HFW diet, food intake of 3- to 7-mo-old WT mice significantly increased (5.2 ± 0.1 g/day) compared with PolgD257A mice (3.8 ± 0.1 g/day; P < 0.001; Fig. 8I). In contrast, food intake remained the same in PolgD257A mice compared with mice given a NC diet. Fecal excretion of fat also increased dramatically within 2 days after switching to the HFW diet, as the fecal steatocrit was significantly higher in the PolgD257A mice (15.7 ± 0.6%; n = 9) than in the WT mice (12.4 ± 0.4%; n = 8; P < 0.002). Daily fat absorption based on food consumption and fecal fat loss was significantly greater in the WT mice (860 ± 33 mg/day) than the PolgD257A mice (697 ± 31 mg/day; P < 0.003) resulting in a gain in weight only in the WT mice.
Taken together, these data demonstrate that mice with the PolgD257A mutation control the excess fat in the diet in part through a restricted absorption by the intestine and in part by restricting the food consumption.
DISCUSSION
The central finding of the present study is that the editing defect of mitochondrial DNA polymerase gamma, PolgD257A, increases cell apoptosis and decreases proliferating cells in S phase in the small intestine crypts of Lieberkühn. The cell cycle disruption in the stem/progenitor cell population results in slower cell migration of the epithelial cell up into the villi and affects the absorption of excess dietary fat. Our study underscores the central role of mitochondria in the regulation of the stem/progenitor cell cycle in the small intestine of the prematurely aging PolgD257A mice.
Although whether accumulations of mitochondrial DNA mutations per se play a definitive causal role in human aging has yet to be proven, increases in mitochondrial DNA mutations have been found in the elderly (9), and mice with the PolgD257A mutation exhibit an accelerated aging phenotype (21, 46). In addition, mitochondrial DNA mutations have been demonstrated as the underlying factor in several metabolic diseases (43, 48) and have their greatest effect in cells that are metabolically active or highly proliferative (39, 40, 45, 49, 52). Although the fusion and branching of the villi were previously observed in the small intestine of PolgD257A mice at 10 mo of age (21), they were not prominent features in our 7-mo-old PolgD257A mice. However, the increased length and width of the villi in these mice are consistent with similar observations made in aged humans (11, 50) and rodents (18, 27). Increased villus surface area could be a compensatory response for the reduction of absorptive capacity of enterocytes stemming from an aging-associated hyperproliferation of precursor cells (17). The increased number of total as well as proliferating Ki67-positive cells in the crypts of PolgD257A mice indicates hyperplasia consistent with hyperproliferation of epithelial cells commonly seen during aging.
Apoptosis is a common event at the tips of the villi in the small intestine due to the high rate of epithelial cell turnover, which is 3.3 days in C57BL/6 mice (8, 38). Consistent with increased apoptosis of enterocytes described in aged humans and animals (10, 23), we found increased TUNEL-positive cells in the villus columnar epithelial cells of the PolgD257A mice at 7 mo of age. Additionally, we found significantly increased apoptosis in the transit amplifying cells (progenitors) of the PolgD257A crypts. This latter is a novel observation that has not been reported as a typical aging phenotype. The crypt region houses both stem and progenitor cells, which are required to replenish epithelial cells lost during shedding and apoptosis. Under normal circumstances, there is only a low level of spontaneous apoptosis in the crypt region that is thought to maintain the stem cell population by removing excess or damaged stem cells (30). The increased apoptosis in the crypts of the PolgD257A mice suggests that stem and progenitor cells in the crypt are particularly susceptible to the damage triggered by the loss of editing function of Polg.
In conjunction with the increase in apoptosis, we also found that crypts of the PolgD257A intestine showed a reduced EdU incorporation into cells in S phase during the 2-h pulse time. In addition, EdU labeling was not as robust as in WT crypts, although the weak staining could be slower DNA synthesis and/or defects in uptake and triphophorylation of EdU. In contrast, staining with Ki67 (a marker of cell proliferation in all phases of the cell cycle) was higher in PolgD257A crypts compared with WT crypts. Thus the decreased S-phase labeling indicates that the PolgD257A mutation directly affects the cell cycle progression of the rapidly dividing transit-amplifying cells.
Mitochondria play an instrumental role in apoptosis (16, 19, 37, 41); the same can be said about cell division. During the Gap1 (G1) phase, all proteins required during DNA replication are synthesized, while DNA synthesis occurs during the S phase of the cell cycle. Both processes demand a high concentration of ATP. After the completion of S phase, the cell enters the Gap2 (G2) phase where ATP is synthesized for mitosis (6). Cells exit G2 and enter the mitosis (M) phase, during which the physical separation of chromosomes occurs through ATP-mediated movement of kinesin along microtubules and cell division takes place (32). Hence, ATP produced by the mitochondria is an essential requirement to drive the cell cycle. Inhibition of mitochondrial protein synthesis leads to G1 arrest and attenuates DNA replication (13, 47), whereas increasing mitochondrial DNA copy number increases the transition from G1 to S and G2 to M, thereby accelerating the progression through the cell cycle (3). Our analysis of markers for cell cycle phase demonstrated that significantly more cells in the crypts of PolgD257A mice are in G2 phase, while fewer cells are in S phase compared with WT mice.
In addition to the defects seen in the PolgD257A progenitor cells, in vitro culturing of isolated crypts demonstrated that proliferation of PolgD257A stem cells is also severely affected. In this culture system, the stem cells are capable of both symmetric and asymmetric division, which lead to progenitor cells and new stem cells, respectively (2, 14, 35). Stem cell maintenance and growth are supported by the necessary factors provided by the Paneth cells (35). Both growth and formation of new stem cells were severely compromised in isolated PolgD257A crypts. Our results, thus, confirm that dysfunction at the stem cell level is a contributing factor involved in passing deleterious mutations on to progenitor cells leading to progenitor cell apoptosis and the decreased cell migration found in the PolgD257A small intestine.
We note that both increased apoptosis and cell cycle dysregulation of the crypt cells were present in young PolgD257A mice equally at 3 and 7 mo of age. This observation could be explained if we assume that the random mitochondrial DNA mutations were generated during development and that the respiratory defects in a small population of the stem cells caused their daughter cells to stall at multiple stages during the cell cycle. Further evidence for a global defect in the stem-progenitor cells is the impaired growth and budding of PolgD257A crypt cells while in culture. In addition, no significant difference in ATP content was detected in the isolated intestinal stem and progenitor cells or the isolated intact crypts of PolgD257A and WT mice, although COX activity was significantly compromised in PolgD257A crypts, confirming impaired mitochondrial function. These observations suggest an alternative possibility that the PolgD257A mutation is directly affecting the cell cycle progression. For example, a previously observed pausing during the mitochondrial DNA replication by the PolgD257A protein (1) could be an additional contributing factor for the perturbed cell cycle regulation of the rapidly replicating cells. While mitochondria clearly participate in cellular proliferation, current knowledge regarding the fundamental biology of mitochondria, such as the timing and exact mode of their replication through the progression of cell cycle, is still not complete. It is not even clear whether all the mitochondrial DNA molecules replicate once in each cell division or a subset of them are amplified (4). Radsak and Schutz (31) showed that peak activity of polymerase gamma occurs in the G2 phase concurrent to a period of enhanced mitochondrial DNA synthesis in synchronized mouse cells in culture, which is consistent with our observation of increased cells in G2 phase and possible reduction in the rate of mitochondrial DNA synthesis. However, others (4, 25) have shown that the replication of mitochondrial DNA proceeds continuously during the cell cycle, and mitochondria undergo dynamic morphologic changes in size and shape controlled by the fission and fusion events (5). A recent study by Mitra et al. (28) indicated that, during the cell cycle, mitochondria convert from isolated, fragmented elements into a single, giant network at the G1-S transition. Whether this hyperfusion takes place in rapidly proliferating cells in the intestine and whether the PolgD257A mutant protein and/or aging would be involved in this process have yet to be explored. It is interesting to note that genetic loss of mitofusin1, a gene important for mitochondrial fusion, dramatically reduces viability and further impairs mitochondrial function when combined with the PolgD257A background (7). This highlights the role that mitochondrial fusion may play in dampening the deleterious effects of mitochondrial DNA mutations.
Any reduction in the actively dividing transit amplifying cells could contribute to a slower cell migration, reduced shedding of epithelial cells, and a prolonged presence of the epithelial cells in the villi. Two separate experiments showed that the migrations of epithelial cells from the crypt to the tip of the villi are severely compromised in the mice with the PolgD257A mutation. Particularly noteworthy is the loss of synchrony in the intestinal epithelial cell migration pattern along with the prolonged time for their replacement that can increase exposure to a toxic environment and hamper cell function. Because energy demands of the absorptive epithelial cells are high, reduced ATP production as we have demonstrated in isolated villi would likely affect the physiological function of the small intestine of the PolgD257A mice. While no alteration in basal metabolism in the mice fed the NC diet was observed at either 3 or 7 mo of age, the PolgD257A and WT mice responded very differently to a HFW diet. WT mice fed the HFW diet overwhelming used carbohydrates, converted unused carbohydrates to fat for storage, and stored excess fat in the adipose tissue, while PolgD257A mice fed the HFW diet used more fat than carbohydrates. Thus the PolgD257A mutation plays a pivot role in food intake, intestinal fat absorption, and adipose storage of fat. Further studies are necessary to evaluate how these mice respond to the long-term feeding of the HFW diet and how this phenotype relates to aging.
In conclusion, the PolgD257A mutation causes changes in the morphology of the small intestine similar to the age-associated changes in small intestinal morphology of aging humans and mice. The changes in morphology are the result of increased apoptosis and a slower cell cycle in the proliferating progenitor and stem cells located in the crypts of Lieberkühn. The most striking evidence for intestinal dysfunction in PolgD257A mice is the increase in fecal fat excretion as a consequence of limited fat absorption. Therefore, the premature aging PolgD257A mouse is a cost effective and physiologically relevant model for studying in vivo aging of the small intestine.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-42630 and HL-087946 (toN. Maeda) and T32-HL-69768 (to R. Fox).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.G.F. and N.M. conception and design of research; R.G.F. and S.T.M. performed experiments; R.G.F., S.T.M., and N.M. analyzed data; R.G.F., S.T.M., and N.M. interpreted results of experiments; R.G.F. prepared figures; R.G.F. drafted manuscript; R.G.F., S.T.M., G.C.K., T.A.P., and N.M. edited and revised manuscript; R.G.F., S.T.M., G.C.K., T.A.P., and N.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Marcus McNair, Xianwen Yi, and Victoria Newton for skillful assistance.
REFERENCES
- 1. Bailey LJ, Cluett TJ, Reyes A, Prolla TA, Poulton J, Leeuwenburgh C, Holt IJ. Mice expressing an error-prone DNA polymerase in mitochondria display elevated replication pausing and chromosomal breakage at fragile sites of mitochondrial DNA. Nucleic Acids Res 37: 2327–2335, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Blank HM, Li C, Mueller JE, Bogomolnaya LM, Bryk M, Polymenis M. An increase in mitochondrial DNA promotes nuclear DNA replication in yeast. PLoS Genet 4: e1000047, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bogenhagen D, Clayton DA. Mouse L cell mitochondrial DNA molecules are selected randomly for replication throughout the cell cycle. Cell 11: 719–727, 1977 [DOI] [PubMed] [Google Scholar]
- 5. Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22: 79–99, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Chapman JD, Webb RG, Borsa J. ATP pool levels in synchronously growing Chinese hamster cells. J Cell Biol 49: 229–233, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141: 280–289, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat 141: 461–479, 1974 [DOI] [PubMed] [Google Scholar]
- 9. Chomyn A, Attardi G. MtDNA mutations in aging and apoptosis. Biochem Biophys Res Commun 304: 519–529, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Ciccocioppo R, Di Sabatino A, Luinetti O, Rossi M, Cifone MG, Corazza GR. Small bowel enterocyte apoptosis and proliferation are increased in the elderly. Gerontology 48: 204–208, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Corazza GR, Ginaldi L, Quaglione G, Ponzielli F, Vecchio L, Biagi F, Quaglino D. Proliferating cell nuclear antigen expression is increased in small bowel epithelium in the elderly. Mech Ageing Dev 104: 1–9, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Drozdowski L, Thomson AB. Aging and the intestine. World J Gastroenterol 12: 7578–7584, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gattermann N, Dadak M, Hofhaus G, Wulfert M, Berneburg M, Loeffler ML, Simmonds HA. Severe impairment of nucleotide synthesis through inhibition of mitochondrial respiration. Nucleosides Nucleotides Nucleic Acids 23: 1275–1279, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Gracz AD, Ramalingam S, Magness ST. Sox9 expression marks a subset of CD24-expressing small intestine epithelial stem cells that form organoids in vitro. Am J Physiol Gastrointest Liver Physiol 298: G590–G600, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greaves LC, Barron MJ, Plusa S, Kirkwood TB, Mathers JC, Taylor RW, Turnbull DM. Defects in multiple complexes of the respiratory chain are present in ageing human colonic crypts. Exp Gerontol 45: 573–579, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris MH, Thompson CB. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ 7: 1182–1191, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Holt PR, Luk GD. Aging and intestinal polyamine metabolism in the rat. Exp Gerontol 25: 173–181, 1990 [DOI] [PubMed] [Google Scholar]
- 18. Holt PR, Pascal RR, Kotler DP. Effect of aging upon small intestinal structure in the Fischer rat. J Gerontol 39: 642–647, 1984 [DOI] [PubMed] [Google Scholar]
- 19. Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zuniga-Pflucker JC, Kroemer G, Penninger JM. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410: 549–554, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Kalivianakis M, Minich DM, Havinga R, Kuipers F, Stellaard F, Vonk RJ, Verkade HJ. Detection of impaired intestinal absorption of long-chain fatty acids: validation studies of a novel test in a rat model of fat malabsorption. Am J Clin Nutr 72: 174–180, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309: 481–484, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Kuperman Y, Issler O, Regev L, Musseri I, Navon I, Neufeld-Cohen A, Gil S, Chen A. Perifornical Urocortin-3 mediates the link between stress-induced anxiety and energy homeostasis. Proc Natl Acad Sci USA 107: 8393–8398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee EK, Jung KJ, Choi J, Kim HJ, Han YK, Jeong KS, Ji AR, Park JK, Yu BP, Chung HY. Molecular basis for age-related changes in ileum: involvement of Bax/caspase-dependent mitochondrial apoptotic signaling. Exp Gerontol 45: 970–976, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Lee HC, Wei YH. Mitochondrial role in life and death of the cell. J Biomed Sci 7: 2–15, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Magnusson J, Orth M, Lestienne P, Taanman JW. Replication of mitochondrial DNA occurs throughout the mitochondria of cultured human cells. Exp Cell Res 289: 133–142, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Mandir N, FitzGerald AJ, Goodlad RA. Differences in the effects of age on intestinal proliferation, crypt fission and apoptosis on the small intestine and the colon of the rat. Int J Exp Pathol 86: 125–130, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin K, Kirkwood TB, Potten CS. Age changes in stem cells of murine small intestinal crypts. Exp Cell Res 241: 316–323, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci USA 106: 11960–11965, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nooteboom M, Johnson R, Taylor RW, Wright NA, Lightowlers RN, Kirkwood TB, Mathers JC, Turnbull DM, Greaves LC. Age-associated mitochondrial DNA mutations lead to small but significant changes in cell proliferation and apoptosis in human colonic crypts. Aging Cell 9: 96–99, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Potten CS. The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer Metastasis Rev 11: 179–195, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Radsak K, Schutz E. Changes of mitochondrial DNA polymerase-gamma activity in synchronized mouse cell cultures. Eur J Biochem 89: 3–9, 1978 [DOI] [PubMed] [Google Scholar]
- 32. Rice S, Lin AW, Safer D, Hart CL, Naber N, Carragher BO, Cain SM, Pechatnikova E, Wilson-Kubalek EM, Whittaker M, Pate E, Cooke R, Taylor EW, Milligan RA, Vale RD. A structural change in the kinesin motor protein that drives motility. Nature 402: 778–784, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Ross JM. Visualization of mitochondrial respiratory function using cytochrome c oxidase/succinate dehydrogenase (COX/SDH) double-labeling histochemistry. J Vis Exp 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA 105: 2415–2420, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schoiswohl G, Schweiger M, Schreiber R, Gorkiewicz G, Preiss-Landl K, Taschler U, Zierler KA, Radner FP, Eichmann TO, Kienesberger PC, Eder S, Lass A, Haemmerle G, Alsted TJ, Kiens B, Hoefler G, Zechner R, Zimmermann R. Adipose triglyceride lipase plays a key role in the supply of the working muscle with fatty acids. J Lipid Res 51: 490–499, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shi Y. A structural view of mitochondria-mediated apoptosis. Nat Struct Biol 8: 394–401, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Shibahara T, Sato N, Waguri S, Iwanaga T, Nakahara A, Fukutomi H, Uchiyama Y. The fate of effete epithelial cells at the villus tips of the human small intestine. Arch Histol Cytol 58: 205–219, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Simmons RA, Suponitsky-Kroyter I, Selak MA. Progressive accumulation of mitochondrial DNA mutations and decline in mitochondrial function lead to beta-cell failure. J Biol Chem 280: 28785–28791, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Someya S, Yamasoba T, Kujoth GC, Pugh TD, Weindruch R, Tanokura M, Prolla TA. The role of mtDNA mutations in the pathogenesis of age-related hearing loss in mice carrying a mutator DNA polymerase gamma. Neurobiol Aging 29: 1080–1092, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y, Alnemri ES. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 410: 112–116, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Takahashi N, Li F, Hua K, Deng J, Wang CH, Bowers RR, Bartness TJ, Kim HS, Harp JB. Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell Metab 6: 506–512, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet 6: 389–402, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thomson AB. Small intestinal disorders in the elderly. Best Pract Res Clin Gastroenterol 23: 861–874, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Trifunovic A, Larsson NG. Mitochondrial dysfunction as a cause of ageing. J Intern Med 263: 167–178, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly- YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417–423, 2004 [DOI] [PubMed] [Google Scholar]
- 47. van den Bogert C, van Kernebeek G, de Leij L, Kroon AM. Inhibition of mitochondrial protein synthesis leads to proliferation arrest in the G1-phase of the cell cycle. Cancer Lett 32: 41–51, 1986 [DOI] [PubMed] [Google Scholar]
- 48. Wallace DC. Mitochondrial DNA mutations in disease and aging. Environ Mol Mutagen 51: 440–450, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. J Neurochem 96: 825–832, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Webster SG, Leeming JT. The appearance of the small bowel mucosa in old age. Age Ageing 4: 168–174, 1975 [DOI] [PubMed] [Google Scholar]
- 51. Xiao ZQ, Moragoda L, Jaszewski R, Hatfield JA, Fligiel SE, Majumdar AP. Aging is associated with increased proliferation and decreased apoptosis in the colonic mucosa. Mech Ageing Dev 122: 1849–1864, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Zhang D, Mott JL, Farrar P, Ryerse JS, Chang SW, Stevens M, Denniger G, Zassenhaus HP. Mitochondrial DNA mutations activate the mitochondrial apoptotic pathway and cause dilated cardiomyopathy. Cardiovasc Res 57: 147–157, 2003 [DOI] [PubMed] [Google Scholar]