Abstract
The enterohepatic circulation (EHC) of bile acids (BAs) plays a pivotal role in facilitating lipid absorption. Therefore, initiation of the EHC in newborns is of crucial importance for lipid absorption from milk. The purpose of this study was to determine at what age BA transporters in liver are expressed, and the mechanism for their initiation. Serum and liver samples were collected from C57BL/6 mice at 2 days before birth and various postnatal ages. Messenger RNA assays revealed a dramatic increase at birth in the expression of the BA transporters (Ntcp, Bsep, Mrp4, Ostβ), as well as the phospholipid floppase Mdr2 in mouse liver, with the highest expression at 1 day of age. The mRNA expression of the ileal BA transporters (Ostα and Ostβ) also markedly increased at birth. Meanwhile, taurine-conjugated cholic acid markedly increased in both serum and liver of newborns, correlated with upregulation of the classic pathway of BA biosynthesis in newborn liver. The mRNA levels of the major BA sensors, FXR and PXR, were increased at 1 day of age, and their prototypical target genes were upregulated in liver. The mRNA expression of transporters involved in the EHC of BAs was similar in wild-type and PXR-null mice. In contrast, in FXR-null mice, the “day 1 surge” pattern of Ntcp, Bsep, Ostβ, and Mdr2 was blocked in newborn mouse liver, and the induction of Ostα and Ostβ was also abolished in ileums of FXR-null mice. In conclusion, at birth, BAs from the classic pathway of synthesis trigger the induction of transporters involved in EHC of BAs in mice, through activation of the nuclear receptor FXR.
Keywords: postnatal development, lipid digestion
enterohepatic circulation (EHC) is the circulation of bile acids (BAs) and other bile constituents from liver to intestine and back to the liver. BAs are synthesized from cholesterol in the liver and excreted into the intestine. Once in the intestine, BAs facilitate the absorption of fat and fat-soluble vitamins by emulsifying the lipophilic substances in the diet. Most of the BAs in the intestine are recycled back to the liver, with only 5% of BAs eliminated into feces daily. Conservation of BAs ensures efficient nutrient and energy supply to the organism. During postnatal development, initiation of the EHC of BAs is crucial for nutrient and energy acquisition, to facilitate the rapid growth of developing tissues.
During the neonatal period in humans, there is a phase of physiological immaturity of the EHC of BAs, resulting in delayed hepatic clearance of BAs and bilirubin, as well as altered clearance of some exogenous chemicals. Immaturity of the EHC results in inefficient lipid digestion, and a period of cholestatic liver, that is termed “physiological cholestasis” (45). Clinically, newborns that have defects in BA transport systems develop deficiencies in fat-soluble vitamins (vitamins A, D, E, K), severe diarrhea (24), and progressive cholestasis (38), which can be lethal if untreated.
Transporters play essential roles in maintaining the EHC of BAs. The sodium taurocholate cotransporting polypeptide (Ntcp) is the major basolateral BA uptake transporter in liver (22), and the bile salt export pump (Bsep) is the rate-limiting canalicular transporter for BA excretion into bile (49). Although most BAs are sequestered in the EHC under physiological conditions, during cholestasis, BAs are also excreted into blood from the liver via the basolateral transporters, including the multidrug resistance-associated protein 4 (Mrp4) (31), as well as the organic solute transporters α and β (Ostα and Ostβ), which function as a heterodimer (5). In addition to BA transporters, the multidrug resistance protein 2 (Mdr2) in liver flops phospholipids from the interior to the exterior bileaflet of the canalicular membrane. Phospholipids enter the bile and form micelles with BAs, thereby protecting the biliary tree from injury (43), as well as facilitating nutrient absorption. In ileum, there is an active uptake system that brings BAs from the intestinal lumen back into the blood, and the transporters involved include the ileal apical sodium-dependent BA transporter (Asbt), as well as the basolateral transporters Ostα and Ostβ in enterocytes (5). Despite the crucial functions of the transporters in regulating the EHC, the ontogenic expression patterns of these transporters are poorly characterized.
Recently, it has been demonstrated that BAs are signaling molecules that activate certain nuclear receptors called “BA sensors” (14). Farnesoid X Receptor (FXR) is the major BA sensor, but the Pregnane X Receptor (PXR) may also regulate BA metabolism. Once activated by BAs, FXR in liver downregulates the major BA uptake transporter Ntcp (53), upregulates the main BA efflux transporter Bsep (36), and upregulates the basolateral BA efflux heterodimer transporter Ostα/β (54). FXR in the ileum induces the expression of Ostα and β on the basolateral membrane of the enterocytes to promote BA reabsorption (54). In addition, FXR in the ileum increases the expression of the intestinal signal fibroblast growth factor 15 (Fgf15) that travels to the liver and subsequently represses the expression of the major BA biosynthesizing enzyme, cytochrome P-450 (Cyp) 7a1(25). The nuclear receptor PXR increases the expression of some Cyp enzymes, conjugation enzymes, and transporters involved in the metabolism and elimination of potentially toxic chemicals, including BAs from the body (14). The physiological roles of FXR and PXR in regulating BA homeostasis during postnatal liver development are not well-characterized.
BA synthesis involves two pathways, namely the classic and the alternative pathway (15). The major enzymes involved in the classic pathway of BA synthesis include Cyp7a1, which is the rate-limiting enzyme for BA synthesis, as well as Cyp8b1, and these two enzymes catalyze the formation of cholic acid (CA). In the absence of Cyp8b1, chenodeoxycholic acid (CDCA) is the major BA (15). Therefore, CA and CDCA are the major primary BAs synthesized via the classic pathway. The enzymes in the alternative BA biosynthesis pathway include Cyp27a1, 7b1, as well as Cyp39a1 (relatively lowly expressed in liver), with CDCA as the primary product formed (37). The two primary BAs synthesized by humans are CA and CDCA. Mice further convert CDCA to α- and β-muricholic acid (MCA); therefore, the main primary BAs in mice are CA and MCA (20), which are then conjugated with taurine (major cosubstrate in mice) and excreted into bile. In enterocytes, BAs activate FXR, which results in the synthesis and secretion of Fgf15 into blood (the human analog of Fgf15 is FGF19). Fgf15 is then delivered to the liver where it activates the FgfR4 signaling pathway, which inhibits BA synthesis via downregulation of Cyp7a1 mRNA expression (25). It has been suggested, using a human hepatocyte culture system, that the mechanism of Cyp7a1 downregulation involves the FGF19-MAPK pathway (44). The alternative pathway is the major route of BA synthesis prenatally (50), whereas the classic pathway predominates in adults, except in certain diseases (17). However, when the switch from the alternative to the classic pathway of BA synthesis occurs during liver development remains unknown. This is partially due to the lack of a valid and simple analytical method for the quantification of individual BAs, as well as their taurine and glycine conjugates. Recently, a simple and sensitive ultra performance liquid chromatography (UPLC)-tandem mass spectrometry (MS/MS) method for the simultaneous quantification of BAs was developed in our laboratory (2), which was modified and used in the present study to determine the composition of BAs at various ages in mice (51).
Because of the importance of the EHC in mediating the absorption of fat-soluble nutrients, it is crucial to determine the mechanisms underlying the regulation of BA transport systems in the EHC during postnatal liver development. Therefore, the purpose of the present study was to determine at what age the BA transporters in the liver are expressed, and the mechanism of their initiation. Because BAs are substrates for many liver transporters involved in the EHC, the working hypothesis is that certain BAs initiate the expression of these transporters in liver after birth, via activating BA sensor-mediated pathways.
MATERIALS AND METHODS
Animals.
Eight-week-old C57BL/6 breeding pairs of mice were purchased from Charles River Laboratories (Wilmington, MA). They were housed according to the American Animal Association Laboratory Animal Care guidelines and were bred under standard conditions at the University of Kansas Medical Center. All studies were approved by the Institutional Animal Care and Use Committee. Breeding pairs were established at 4 PM and separated the following day at 8 AM. The body weight of females was recorded each day to monitor pregnancy status. Liver and ileum from offspring were collected at the following ages: day −2, 0 (right after birth and before the start of suckling), 1, 3, 5, 10, 15, 20, 30, and 45. Due to the small size of mice, less than 1-wk-old livers from male and female mice (same litter) were pooled at each age to achieve the minimum amount of tissue for subsequent analyses. After 10 days of age, males and females were separated. Due to potential variations caused by the estrous cycle in maturing adult female mice, only male livers were used after 10 days of age. Livers were frozen immediately in liquid nitrogen and stored at −80°C.
RNA isolation.
Total RNA was isolated using RNAzol Bee reagent (Tel-Test, Friendswood, TX) per the manufacturer's protocol. RNA concentrations were quantified using a NanoDrop Spectrophotometer (NanoDrop Technologies, Wilmington, DE) at a wavelength of 260 nm. Integrity of the total RNA samples was evaluated by formaldehyde-agarose gel electrophoresis and confirmed by visualization of 18S and 28S rRNA bands.
Branched DNA amplification technology (bDNA assay).
Mouse liver mRNA was quantified from total RNA. The mRNA of Ntcp, Bsep, Mdr2, Mrp4, Ostα, Ostβ, FXR, PXR, SHP, Cyp3a11, Cyp7a1, Cyp8b1, Cyp7b1, Cyp27a1, and Fgf15 was determined by a single-plex bDNA assay (QuantiGene 1.0 bDNA signal amplification kit, Affymetrix/Panomics, Fremont, CA) (23). Probe sets for Ntcp, Mrp4, Mdr2, Bsep, PXR, Cyp3a11, Ostα, and Ostβ were described previously (1, 6, 11–13, 30, 31). Multiple oligonucleotide probe sets for SHP, FXR, and Fgf15 (including capture, label, and blocker probes) were designed using ProbeDesigner Software v1.0 as described previously (13). The probe sets for SHP, FXR, and Fgf15 are shown in Table 1. Data are reported as relative light units per 10 μg total RNA. The ontogeny of various other uptake and efflux transporters (see Figs. 13–15) was determined by a multiplex bDNA array (QuantiGene 2.0 bDNA signal amplification kit, Affymetrix/Panomics), and data were normalized by glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA (probe sequences are available at http://www.panomics.com).
Table 1.
Oligonucleotide probes generated for analysis of mouse FXR and SHP mRNA by bDNA
| Gene | Function | Sequence |
|---|---|---|
| FXR | CE | ctctctgcacttccttagccgTTTTTctcttggaaagaaagt |
| CE | tggtctgccgtgagttccgTTTTTctcttggaaagaaagt | |
| CE | agattttatttgtgatttcctgaggTTTTTctcttggaaagaaagt | |
| CE | ctggaagcttttttgtgaattctaTTTTTctcttggaaagaaagt | |
| LE | gttgcccccgttcttacactTTTTTaggcataggacccgtgtct | |
| LE | gcatgtacatgtccatcacgcaTTTTTaggcataggacccgtgtct | |
| LE | cattcagccaacatccccatTTTTTaggcataggacccgtgtct | |
| LE | gatttacactggatttcagttaacaaaTTTTTaggcataggacccgtgtct | |
| LE | tcacatttttccttagccgtttaTTTTTaggcataggacccgtgtct | |
| LE | tgtctgatcagcgtgctgctTTTTTaggcataggacccgtgtct | |
| LE | ttcgctgtcgtcctcattcacTTTTTaggcataggacccgtgtct | |
| LE | ttgtcgcaagtcacgcccTTTTTaggcataggacccgtgtct | |
| LE | cattctctgtttgttgtacgaatccTTTTTaggcataggacccgtgtct | |
| LE | cgagaatctgtacatggctggtTTTTTaggcataggacccgtgtct | |
| BL | gcactcctggcacttcctgc | |
| BL | caaaacttggttgtggaggtcac | |
| BL | ataatataatccaggagggtctgc | |
| BL | tttcttctgcactaaattcttctttta | |
| BL | tgccatttctgttaatatgagaaaat | |
| SHP | CE | acggcaggttcctgaggaaTTTTTctcttggaaagaaagt |
| CE | gccccagcagcactctagcaTTTTTctcttggaaagaaagt | |
| CE | gctgctggcttcctctagcagTTTTTctcttggaaagaaagt | |
| CE | tgaactgcagccagtgagggTTTTTctcttggaaagaaagt | |
| LE | ccaaggcctccctgcaggTTTTTaggcataggacccgtgtct | |
| LE | gccgccgctgatcctcatTTTTTaggcataggacccgtgtct | |
| LE | aggtcacagcatcctgggcTTTTTaggcataggacccgtgtct | |
| LE | ggagcctcagccacctcgaTTTTTaggcataggacccgtgtct | |
| LE | ctgggcaccctgggtaccTTTTTaggcataggacccgtgtct | |
| LE | ttgtggccggtctgatggTTTTTaggcataggacccgtgtct | |
| LE | ggcagcgctgcagccacTTTTTaggcataggacccgtgtct | |
| BL | ggctactgtcttggctaggacat | |
| BL | ggggcaggtggcagaagg | |
| BL | caacccaagcaggaagagagg | |
| BL | gatcttcttaagtatactgggcacc | |
| BL | gatcttcttaagtatactgggcacc | |
| Fgf15 | CE | cttggcctggatgaagatgataQTTTQctcttggaaagaaagt |
| CE | aaggagcggtgaaacacggQTTTQctcttggaaagaaagt | |
| CE | ggctccgatggcagccaQTTTQctcttggaaagaaagt | |
| LE | ctgtactggttgtagcctaaacagtcQTTTQaggcataggacccgtgtct | |
| LE | ctggtccccggtttcaaagQTTTQaggcataggacccgtgtct | |
| LE | cagggagaacattttagacctcagQTTTQaggcataggacccgtgtct | |
| LE | tgatcaagtctagccactaacacaaQTTTQaggcataggacccgtgtct | |
| LE | ggcaggttgtcaacttaagttcaQTTTQaggcataggacccgtgtct | |
| LE | aactggagtaacttaggcacatatcaQTTTQaggcataggacccgtgtct | |
| BL | tggagatggtgcttcatggat | |
| BL | tcctggagctgttctctggg | |
| BL | ggataaagtttgagggtttctgg | |
| BL | atgctgtcactctccagggg | |
| BL | ccaccatcctgaacggatcc | |
| BL | actcttcactaggtggtctacatcct | |
| BL | ctgtcatttctggaagctggg | |
| BL | ttttctccatcctgtcggaatc | |
| BL | gttcacgggaccttgggg | |
| BL | tgtacagcttcctaagggggaa | |
| BL | cagggtccatgtgagacttagaata | |
| BL | gcagcctccaaagtcagtgg |
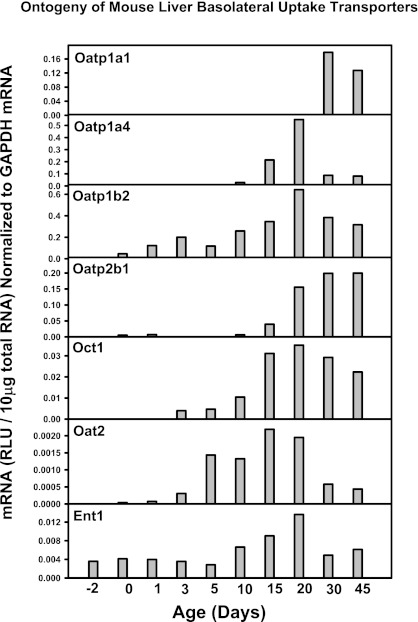
Fig. 13.
mRNA ontogeny of mouse liver basolateral uptake transporters.
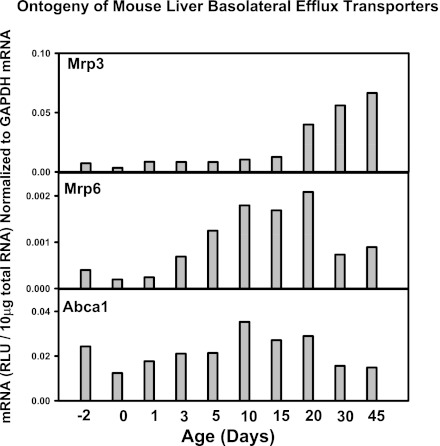
Fig. 14.
mRNA ontogeny of mouse liver basolateral efflux transporters.
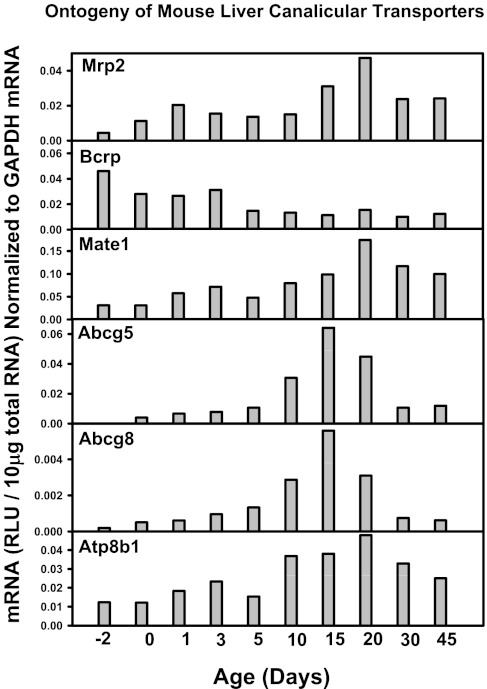
Fig. 15.
mRNA ontogeny of mouse liver canalicular transporters.
RT-PCR for Ibabp mRNA quantification during ileum development in mice.
The Ibabp mRNA was quantified by RT-PCR (n = 3 per age, male only). The total RNAs were reverse-transcribed into cDNAs using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The cDNA samples were used for isoform-specific RT-PCR using the Power SYBR Green PCR Master Mix and the 7900 HT Fast Real-Time PCR System (Applied Biosystems). The primer sequences for Ibabp mRNA are caccattggcaaagaatgtg (forward) and aacttgtcacccacgacctc (reverse). Data were normalized by Gapdh.
RNA-Seq.
The mRNAs of various FXR transcript isoforms, prolactin receptor, CEBPβ, HNF3β, FXR, BAL, and BAT, were quantified by RNA-Seq (from day −2 to 60 days of age, n = 3 per age, male livers only). The cDNA libraries from all total RNA samples were prepared using an Illumina TruSeq RNA sample prep kit (Illumina, San Diego, CA). The cDNA libraries were clustered onto a TruSeq paired-end flow cell and sequenced (2 × 100) using a TruSeq 200 cycle SBS kit (Illumina). For the initial run, a PhiX control as well as a universal human RNA reference were loaded on 2 of the 16 lanes of the Illumina HiSeq2000 sequencer (University of Kansas Medical Center Genome Sequencing Facility) and sequenced in parallel with other samples to ensure the data generated for each run are accurately calibrated during the image analysis and data analysis. In addition, the PhiX was spiked into each cDNA sample at ∼1% as an internal quality control. After the sequencing platform generated the sequencing images, the pixel-level raw data collection, image analysis, and base calling were performed by the real-time analysis software on a Dell PC attached to the HiSeq2000 sequencer. The data were streamed off to the analysis server as the run was progressing. The base calling (BCL) converter converted the BCL files to qseq files for downstream analysis. The Consensus Assessment of Sequence And VAriation (CASAVA) v. 1.7 was used to perform alignment of the sequencing run to the mouse reference genome (NCBI37/mm9) and the subsequent read counting. The output files were in FASTQ format. The RNA-Seq reads from the FASTQ files were mapped to the genome and the splice junctions were identified by TopHat. The output files in binary sequence alignment (BAM) format were analyzed by Cufflinks to estimate the transcript abundance and the presence of putative novel mRNA isoforms (Cuffcompare). The mRNA abundance was expressed in fragments per kilobase of exon per million reads mapped (FPKM).
Sample preparation for BA analysis.
To determine the concentration and composition of BAs during development, serum and liver samples were collected from pups at each age (n = 3 per age). Although BA analysis of bile from gallbladder is a better indication of BA composition, due to the small size of gallbladders in young mice, it is technically challenging to collect gallbladder bile. Therefore, only serum and liver BAs were quantified in this study. From 2 days before birth to 5 days of age, samples from the same litter were pooled (n = 3 litters) to achieve the desired amount of liver and serum for BA isolation. Internal standards (IS; 40 μg/ml d4-G-CDCA and 20 μg/ml d4-CDCA in MeOH), as well as serum and liver samples, were prepared for BA analysis with methods reported by Alnouti et al. (2) with some modifications (44). Briefly, for serum samples, simple protein precipitation by adding methanol (MeOH) was used. One milliliter of MeOH was added to 50 μl serum spiked with 5 μl IS, vortexed, and centrifuged at 12,000 g for 10 min. The supernatant was aspirated, evaporated under vacuum, and reconstituted in 50 μl of 50% MeOH. Liver samples were extracted by protein precipitation using ice-cold acetonitrile (ACN). Approximately 110 mg of liver were homogenized in 5 volumes of H2O. Liver homogenates (600 μl) were spiked with 10 μl IS, 3 ml of ice-cold ACN (5% NH4OH in ACN) were added, vortexed, shaked continuously for 1 h, and centrifuged at 12,000 g for 10 min. The supernatant was aspirated and precipitant was extracted with 1 ml of MeOH. Supernants from the two extraction steps were pooled, evaporated, and reconstituted in 100 μl of 50% MeOH.
UPLC-MS/MS of BA analysis.
A Waters ACQUITY ultra performance LC system (Waters, Milford, MA) and a Waters Quanttro Premier XE triple quadrupole instrument with an ESI source (Waters) were used. The entire liquid chromatography (LC)-MS/MS system was controlled by MassLynx 4.1 software. All chromatographic separations were performed with an ACQUITY UPLC C18 column (1.7 μm, 100 × 2.1 ID) equipped with an ACQUITY UPLC C18 guard column (Waters). Based on peak areas of BAs and internal standards, various conjugated and unconjugated BAs were quantified, namely the taurine-conjugated BAs including TCA, TαMCA, TβMCA, TωMCA, TMDCA, TUDCA, THDCA, TCDCA, TDCA, and TLCA; as well as the unconjugated BAs including CA, αMCA, βMCA, ωMCA, MDCA, UDCA, HDCA, CDCA, DCA, and LCA.
Immunofluorescence analysis.
Livers were embedded in optimal cutting temperature compound and rapidly frozen at −80°C. Six-micrometer sections were generated with a Leica CM3050 Cryostat (Meyer Instruments, Pittsburg, PA) and fixed with 4% paraformaldehyde in PBS. Briefly, slides were blocked with 5% goat serum in 0.1% Triton X-100 in PBS and incubated with primary antibody diluted 1:100 for Ntcp (K4 rabbit anti-rat) and Bsep (K44 rabbit anti-rat). There are no specific antibodies available for staining Mdr2. Staining of Mrp4 and Ostβ was not applicable due to low-protein expression levels in liver. After being washed, sections were incubated with a species-appropriate Alexa 488 IgG secondary antibody (1:200). Sections were mounted in Prolong Gold (Invitrogen, Carlsbad, CA). Fluorescent staining was visualized using an Olympus B×41 microscope (Olympus Optical, Tokyo, Japan). Images were captured using an Olympus DP70 camera (×40) and DP Controller software.
Serum prolactin quantification.
Serum prolactin concentrations from male pups at various ages as well as from the mothers were quantified by a radioimmunoassay (RIA; I125; n = 5).
Quantification of FXR-DNA binding.
Chromatin immunoprecipitation for sequencing was performed by Genpathway (San Diego, CA) using a ChIP-grade FXR antibody (H-130x, Santa Cruz Biotechnology, Santa Cruz, CA) as described previously (46).
Statistics.
Differences between multiple age groups were analyzed by SPSS (1-way ANOVA followed by post hoc Duncan test). Differences between two groups at the same age were determined by Student's t-test. Statistical significance was considered at P < 0.05.
RESULTS
Ontogeny of major transporters in mice involved in EHC of BAs.
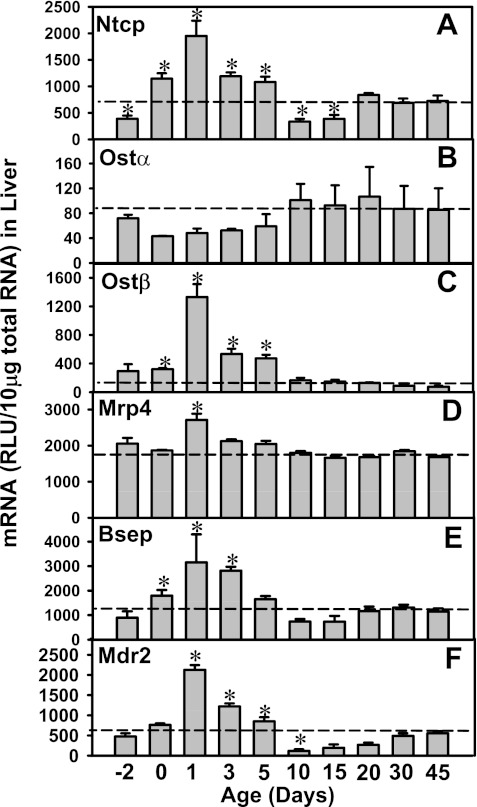
The mRNA expression of major hepatic and ileal transporters involved in the EHC of BAs was determined 2 days before birth to 45 days of age in mice as shown in Figs. 1 and 2. Among the basolateral transporters in liver, Ntcp is the major BA uptake transporter. Ntcp mRNA was low at 2 days before birth (Fig. 1A). At birth, the Ntcp mRNA increased about twofold and then reached a peak at 1 day of age (5-fold prenatal levels), followed by a decrease at 3 and 5 days of age, and returned to prenatal levels at 10 and 15 days of age. After 20 days of age, Ntcp mRNA gradually approached adult levels (∼2-fold higher than prenatal levels) till 45 days of age. The basolateral BA efflux transporters, Ostα and β, function as a dimer. Interestingly, although Ostα mRNA is lowly expressed in liver throughout development (Fig. 1B), Ostβ mRNA markedly increased in newborn liver that reached a peak at 1 day of age (4.5-fold of prenatal levels; Fig. 1C). Between 5 and 10 days of age, Ostβ mRNA decreased to adult levels. The mRNA of hepatic Mrp4 was consistent from 2 days before birth to 45 days of age, except for a moderate increase at 1 day of age (Fig. 1D).
Fig. 1.
Ontogeny of liver transporters: sodium taurocholate cotransporting polypeptide (Ntcp; basolateral uptake), organic solute transporter (Ost)α, Ostβ, multidrug resistance-associated protein 4 (Mrp4; basolateral efflux), bile salt export pump (Bsep), and multidrug resistance protein 2 (Mdr2; canalicular efflux) in wild-type mice from 2 days before birth to 45 days of age. Total RNA was isolated from liver at each age and analyzed by the bDNA assay as described in materials and methods. Data are presented as mean relative light units (RLU) ± SE (n = 5 animals). *Significant differences (P < 0.05) compared with values at 45 days of age.
Fig. 2.
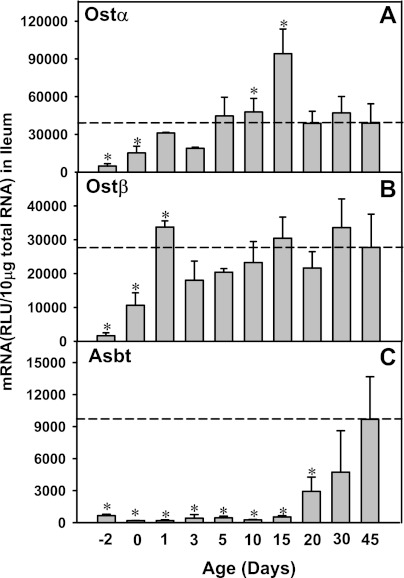
Ontogeny of ileal transporters: Ostα and Ostβ (basolateral efflux) and Asbt (apical uptake) in wild-type mice from 2 days before birth to 45 days of age. Total RNA was isolated from ileum at each age and analyzed by the bDNA assay as described in materials and methods. Data are presented as mean RLU ± SE (n = 5 animals). *Significant differences (P < 0.05) compared with values at 45 days of age.
Among the canalicular transporters, Bsep is the major BA efflux transporter, whereas Mdr2 is the major canalicular phospholipid floppase in liver. Similar to the ontogeny of Ntcp, Ostβ, and Mrp4, the mRNA of both Bsep and Mdr2 was also increased markedly in mouse liver after birth, which was about three- to fourfold higher at 1 day of age than before birth. The expression of Bsep and Mdr2 then returned to before birth levels at 10 and 15 days of age and then gradually increased to adult levels by ∼30 days of age (Fig. 1, E and F).
In summary, the liver transporters Ntcp, Bsep, Mrp4, Mdr2, and Ostβ, which all play important roles in the EHC, share a common day 1 surge pattern in their mRNA expression during development.
In ileum, a marked increase in mRNA of the basolateral BA transporters, Ostα and β, was observed shortly after birth (Fig. 2). Ostα mRNA in ileum at 1 day of age was 6.4-fold higher than before birth, which reached a peak at 15 days of age (19.4-fold; Fig. 2A). The ileal Ostβ mRNA reached a peak at 1 day of age (20.9-fold of prenatal levels) and remained relatively similar thereafter (Fig. 2B). Therefore, the neonatal increase of the major BA efflux transporters Ostα and Ostβ in ileum correlates with the upregulation of BA uptake and efflux transporters in liver; however, the ileal apical BA uptake transporter Asbt was low both before birth and the first 15 days of age and then gradually increased during development and reached a peak at 45 days of age (14.8-fold prenatal levels; Fig. 2C).
Because the peak mRNAs of most transporters involved in the EHC of BAs were observed at 1 day of age, the present phenomenon was termed the day 1 surge pattern. To determine whether the protein expression of some of these transporters is also upregulated shortly after birth, and whether the transporter proteins are properly inserted into the plasma membranes to exert their functions, immunofluorescence staining was performed in the following experiments.
Immunofluorescence staining of Ntcp and Bsep in livers of mice during development.
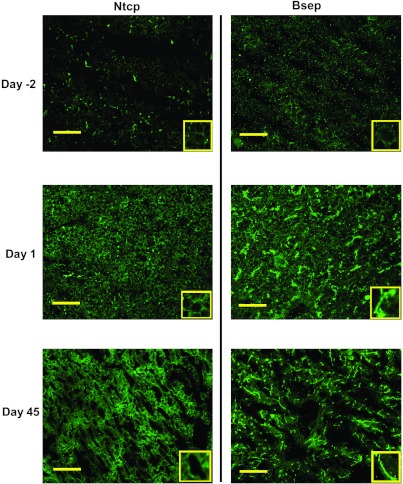
To determine the expression and localization of Ntcp and Bsep, immunofluorescence was performed on frozen liver sections of mice at 2 days before birth and 1 and 45 days of age for these two proteins (Fig. 3). Ntcp protein was low but detectable at the basolateral membrane of hepatocytes at 2 days before birth. At 1 day of age, enhanced basolateral Ntcp staining (green) throughout the liver lobule was observed, and the fluorescence intensity is comparable to that at 45 days of age (Fig. 3, left column). Bsep protein was barely detectable at 2 days before birth, but at 1 day of age, the staining of Bsep protein (green) was markedly enhanced and was localized to the canalicular junctions between adjacent hepatocytes, and the fluorescence intensity at 1 day of age was similar to that at 45 days of age. Mrp4 and Ostβ immunofluorescence staining was not detectable, probably due to low-protein expression levels in liver (data not shown). In summary, the day 1 surge pattern in the mRNA expression of hepatic transporters, Ntcp and Bsep, was detected at both the mRNA and protein levels in liver at 1 day of age, and it appeared to be inserted into the plasma membranes of hepatocytes, indicating the BA transport system in liver is functioning in livers of newborn mice.
Fig. 3.
Immunofluorescence of Ntcp and Bsep protein in livers from 2 days before birth, 1 day, and 45 days of age. Immunofluorescence against basolateral Ntcp as well as canalicular Bsep (green) was conducted on liver cryosections as described in materials and methods. Portions of images were enlarged and provided as insets. Representative images are shown. Bar = 50 μm.
Because BAs are substrates for many hepatic transporters that share the day 1 surge pattern, we hypothesized that an elevation in BAs, due to their increased de novo biosynthesis in newborns, initiates the mRNA increase of these transporters during development.
Ontogeny of BA biosynthesizing enzymes during liver development.
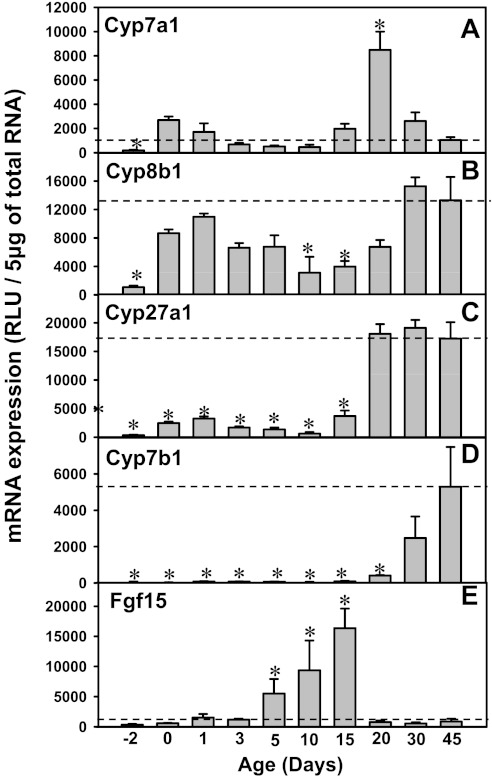
To determine whether BA biosynthesizing enzymes are increased in liver during development, the mRNA expression of major enzymes from both the classic and alternative pathways of BA biosynthesis was quantified (Fig. 4). For the classic pathway of BA synthesis, both Cyp7a1 and 8b1 mRNAs were low before birth, but increased markedly at birth (14.4- and 8.1-fold of prenatal levels, respectively; Fig. 4, A and B). After 1 day of age, both Cyp7a1 and 8b1 mRNAs decreased, but increased again after 10 days of age. Cyp7a1 mRNA reached a peak at 20 days of age and decreased thereafter, whereas Cyp8b1 mRNA reached a peak at 30 days of age and remained stable thereafter. In contrast, for the alternative pathways of BA synthesis, Cyp27a1 mRNA increased markedly after 20 days of age, which remained stable until 45 days of age (between days 20 and 45: ∼50-fold higher than that before birth). Cyp7b1 mRNA was also consistently low before 20 days of age and then increased markedly to adult values levels (46-fold higher at 30 days of age, and 99-fold higher at 45 days of age than 2 days before birth; Fig. 4, C and D).
Fig. 4.
A–D: messenger RNA of bile acid (BA) synthesizing enzymes (Cyp7a1, 8b1, 27a1, and 7b1) in livers of wild-type mice during development. E: ontogeny of the mRNA expression of ileal fibroblast growth factor 15 (Fgf15) and in wild-type mice. Total RNA was isolated from liver at each age and analyzed as described in materials and methods. Individual samples (n = 5) were analyzed by bDNA assay. Data are presented as mean RLU normalized to 5 μg total RNA.
In addition to liver enzymes contributing to BA biosynthesis, Fgf15 is secreted from the ileum and thought to be transported by the blood to the liver, where it binds to the FgfR4 receptor, and subsequently decreases the transcription of the BA synthesizing enzyme Cyp7a1. To determine the contribution of Fgf15 signaling on BA synthesis in development, the ontogeny of FgfR4 mRNA in liver as well as that of Fgf15 mRNA in ileum was determined. FgfR4 mRNA was consistently low throughout liver development (data not shown). The Fgf15 mRNA in ileum was low 2 days before birth to 3 days of age, and it increased after 5 days of age, which reached a peak at 15 days of age (49-fold prenatal levels; Fig. 4E), and markedly decreased by 20 days of age. The low perinatal expression of the Fgf15 pathway provides a permissive environment for the first induction of Cyp7a1 mRNA in newborns. In contrast, the downregulation of Fgf15 after day 15 of age likely disinhibits the Cyp7a1 mRNA expression, which may contribute to the second peak of Cyp7a1 mRNA after 15 days of age.
In summary, the neonatal induction of BA synthesizing enzymes, Cyp7a1 and 8b1, together with low expression of the suppression signal Fgf15, promotes an increase in BA biosynthesis in newborns. Because the major BA synthesized by Cyp7a1 and 8b1 in the classic pathway is CA, and the major BA synthesized by the alternative pathway enzymes in mice is muricholic acid and CA, we hypothesized that CA is the predominant BA in newborns that contributes to the day 1 surge pattern of liver transporters.
BA concentrations in serum and liver during development.
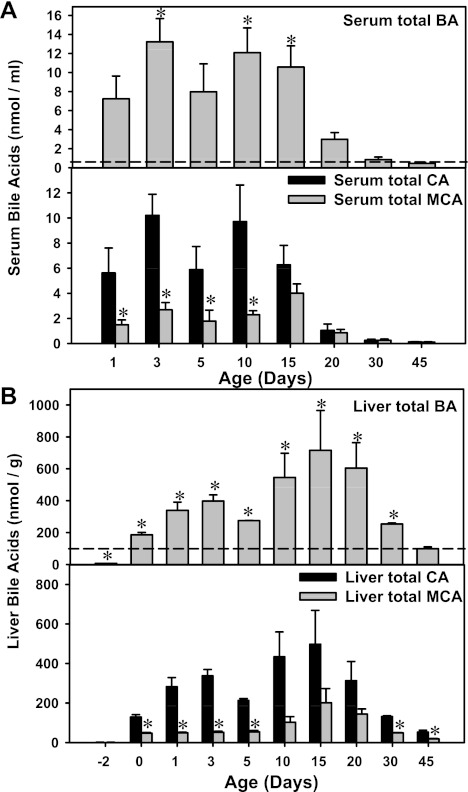
As a first approach to determine the association between BA concentrations and the expression of major liver transporters involved in the EHC, BAs in serum and liver were quantified during development as described in materials and methods (Fig. 5). Total BA concentrations in serum were highest in newborns (day 1), which were 15.8-fold higher than adult levels. After 15 days of age, the serum BAs markedly decreased (Fig. 5A, top). In liver, the total BA concentrations were lowest before birth (7.5% of day 45 levels), followed by a marked increase at birth (1.88-fold higher than day 45 levels), which continued to increase until 3 days of age. At 5 days of age, the total liver BA concentrations decreased moderately to 2.8-fold higher than adult levels, but increased again after 10 days of age, which reached a peak at 15 days of age (7.2-fold higher than day 45 levels) and decreased thereafter (Fig. 5B, top).
Fig. 5.
A: serum total BA concentrations (top) as well as concentrations of total cholic acid (CA; taurine-conjugated CA and unconjugated CA) and total muricholic acid (TαMCA, TβMCA, αMCA, and βMCA; bottom) during development (n = 3 per age). The ωMCA was excluded because it is a secondary BA. B: liver total BA concentrations (top) as well as concentrations of total CA (taurine-conjugated CA and unconjugated CA) and total primary muricholic acid (TαMCA, TβMCA, αMCA, and βMCA) during development (n = 3 per age). BAs from serum and liver were quantified by LC-MS/MS as described in materials and methods. For serum, data are expressed as nmol of BAs per ml. For liver, data are expressed as nmol/g. A and B, top: *signfificant differernces (P < 0.05) compared with levels at 45 days of age. A and B, bottom: *significant differences (P < 0.05) between total CA and MCA concentrations at the same age.
To determine whether BAs synthesized from the classic pathway are responsible for the day 1 surge of transporters, serum and liver BA concentrations were quantified by LC/MS-MS (Fig. 5). In this study, the taurine-conjugated CA (TCA) and unconjugated CA were defined as total CA, which represents the primary product from the classic pathway of BA biosynthesis. The taurine-conjugated αMCA and βMCA (TαMCA and TβMCA) as well as their unconjugated forms (αMCA and βMCA) were defined as total primary MCA. In serum, both total CA and MCA concentrations were markedly higher before 15 days of age than their concentrations thereafter (Fig. 5A, bottom). As shown in Fig. 5A, bottom, total CA was significantly higher than total MCA (which can be synthesized by both pathways) in serum from 1 to 10 days of age. After 15 days of age, both CA and MCA concentrations decreased markedly till 45 days of age.
In liver, both total CA and MCA concentrations were low before birth, followed by a marked increase right after birth (Fig. 5B, bottom). The total CA concentrations in liver continued to increase at 1 and 3 days of age, which moderately decreased at 5 days of age. After 5 days of age, the total CA concentrations increased again, which reached a peak at 15 days of age, and decreased thereafter. For total MCA, the concentrations remained constant from 0 to 5 days of age, which moderately increased after 10 days of age, reached a peak at 15 days of age, and decreased thereafter. Interestingly, similar to serum BA concentrations, total CA, which is the specific product from the classic pathway of BA biosynthesis, was higher than total MCA during the neonatal period. In addition, from 10 to 20 days of age, total CA concentrations in liver tended to be higher than MCA, although statistical significance was not achieved. In livers of adult mice, the total CA concentrations were also higher than total MCA.
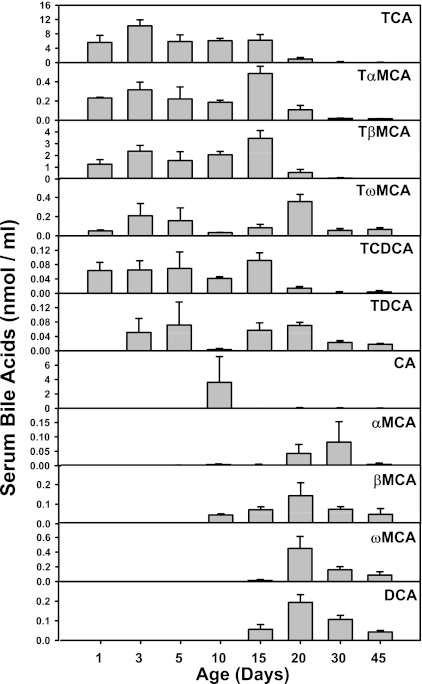
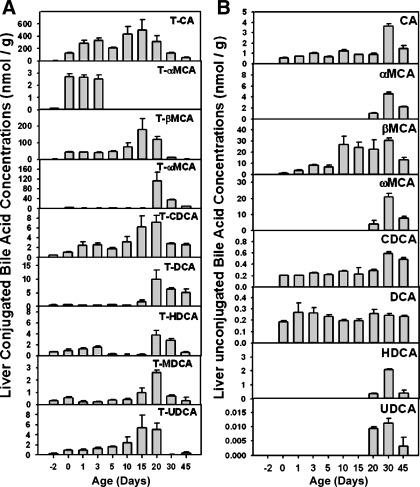
The concentrations of individual BAs in serum and liver are shown in Figs. 6 and 7. In serum, LC/MS-MS detected 11 BAs, including TCA, TαMCA, TβMCA, TωMCA, TCDCA, TDCA, as well as unconjugated BAs, including CA, αMCA, βMCA, ωMCA, and DCA (Fig. 6). In general, serum taurine-conjugated BAs were high from 1 to 20 days of age and decreased after 30 days of age. However, the unconjugated BAs in liver were low from 1 to 5 days of age, but increased after 10 days of age, and then decreased in adults. In liver, LC/MS-MS detected 17 BAs, including 9 taurine-conjugated BAs (TCA, TαMCA, TβMCA, TωMCA, TCDCA, TDCA, THDCA, TMDCA, and TUDCA; Fig. 7A) and 9 unconjugated BAs (CA, αMCA, βMCA, ωMCA, CDCA, DCA, HDCA, and UDCA; Fig. 7B). For conjugated BAs, TCA and TCDCA tended to be high in livers up to 20 days of age; TαMCA was only present in the neonatal period (day 0 to day 3), whereas the other BAs were high in the adolescent period (10 to 20 days of age). For unconjugated BAs, αMCA, ωMCA, HDCA, and UDCA were present in livers only after 20 days of age. DCA was detected in livers immediately after birth and remained constant thereafter. CA, βMCA, and CDCA were detected right after birth, and gradually increased, which reached a peak at 30 days of age, and decreased moderately by 45 days of age.
Fig. 6.
Serum unconjugated BA concentrations during development (n = 3 per age). BAs from serum were quantified by LC-MS/MS as described in materials and methods. Data are expressed as nmol of BAs per ml.
Fig. 7.
Liver conjugated (A) and unconjugated (B) BA concentrations during development (n = 3 per age). BAs from liver were quantified by LC-MS/MS as described in materials and methods. Data are expressed as nmol/g.
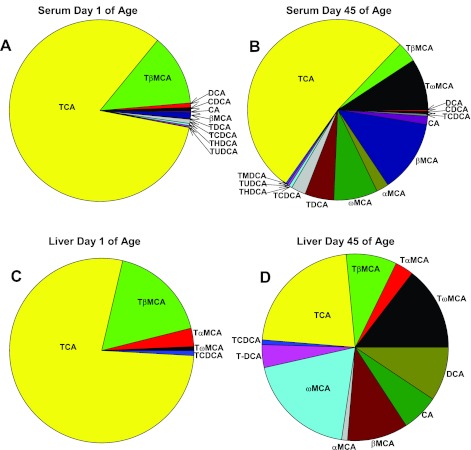
To further consolidate the hypothesis that the classic pathway of BA biosynthesis predominates in the neonatal period, percentages of BAs in serum and liver were calculated at 1 day of age compared with 45 days of age (Fig. 8). In both serum and liver, TCA was the predominant BA at 1 day of age (77.8% of total BAs in serum, and 82.9% of total BAs in liver). In contrast, at 45 days of age, although TCA was still a major BA, it decreased to 22% in serum and 52.3% in liver. In addition, there was an increase in both the variety and percentages of other BAs increased in adults.
Fig. 8.
Percentage of various components of BAs in serum (A and B) and liver (C and D) at day 1 (A and C) and 45 days of age (B and D). BAs were quantified by LC-MS/MS as described in materials and methods.
In summary, both serum and liver BAs increased right after birth, which associates with the high mRNA expression of liver transporters involved in the EHC of BAs. The major BAs in both serum and liver are taurine conjugates, and TCA is the predominant BA in newborns, providing strong evidence that the classic pathway of BA biosynthesis predominates during the neonatal period (from birth to 5 days of age).
Neonatal upregulation of FXR and PXR pathways in liver.
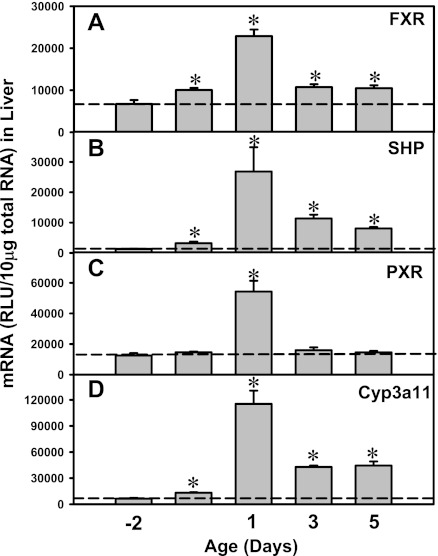
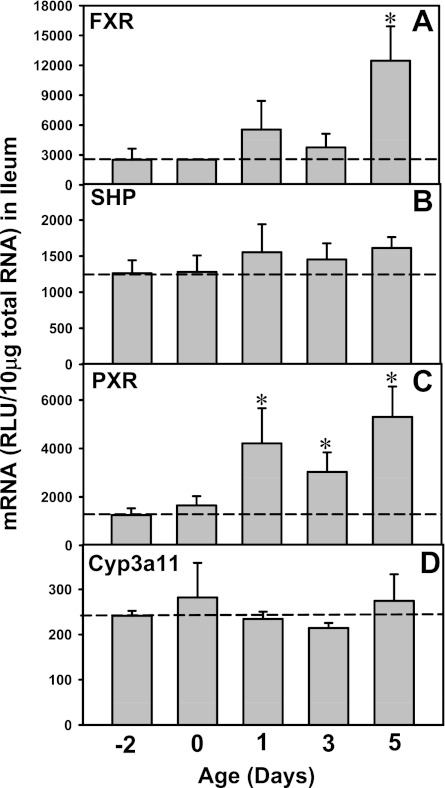
FXR and PXR are major BA sensors in liver. To determine whether FXR and PXR are expressed in newborns, and whether FXR- and PXR-mediated pathways are activated by high concentrations of BAs in newborns, the mRNA expression of both FXR and PXR as well as their prototypical target genes were quantified from 2 days before birth to 5 days of age (Fig. 9). FXR mRNA was low before birth, followed by an increase at birth (1.5-fold higher than prenatal levels), which then reached a peak at 1 day of age (3.4-fold), which gradually decreased at 3 (1.6-fold) and 5 days (1.6-fold) of age (Fig. 9A). Correlated with the day 1 surge pattern of FXR-mRNA, the mRNA of the prototypical FXR target gene, SHP, also increased in newborns and reached a peak at 1 day of age (20-times prenatal levels; Fig. 9B). Similar to the expression pattern of FXR, PXR mRNA also increased after birth, which reached a peak at 1 day of age (4.3-fold prenatal levels; Fig. 9C). The prototypical PXR target gene, Cyp3a11, also reached a peak at 1 day of age (18-fold prenatal levels; Fig. 9D). In summary, the day 1 surge pattern was observed in both FXR- and PXR-mRNA in liver, as well as in their prototypical target genes, indicating not only the major BA sensors are highly expressed in newborn liver, but also the FXR- and PXR-mediated pathways are functionally activated. In contrast, although the ileal FXR- and PXR-mRNAs were increased in newborns, their target genes were not readily activated in ileums of newborns (Fig. 10), indicating a delayed maturation of FXR and PXR pathways in newborn ileum compared with fully functioning FXR and PXR pathways in newborn liver.
Fig. 9.
Upregulation of the mRNA expression of Farnesoid X Receptor (FXR), SHP (prototypical target gene of FXR), Pregnane X Receptor (PXR), and Cyp3a11 (prototypical target gene of PXR) in neonatal wild-type mouse livers. Total RNA was isolated from liver at each age and analyzed by the bDNA assay as described in materials and methods. Data are presented as mean RLU ± SE (n = 5 animals). *Significant differences (P < 0.05) compared with values at day −2 of age.
Fig. 10.
Messenger RNA expression of FXR, SHP (prototypical target gene of FXR), PXR, and Cyp3a11 (prototypical target gene of PXR) in neonatal wild-type mouse ileum. Total RNA was isolated from ileum at each age and analyzed by the bDNA assay as described in materials and methods. Data are presented as mean RLU ± SE (n = 5 animals). *Significant differences (P < 0.05) compared with values at day −2 of age.
Expression of liver transporters in FXR- and PXR-null mice at day 1 of age.
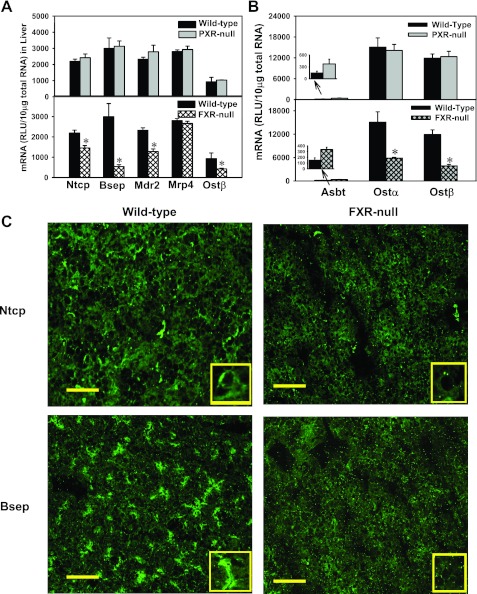
To determine whether FXR and PXR contribute to the day 1 surge pattern of hepatic transporters (Ntcp, Bsep, Mdr2, Mrp4, and Ostβ), the mRNA of these transporters was quantified in livers from FXR- and PXR-null mice at 1 day of age (Fig. 11A). In PXR-null mice, the day 1 surge pattern of all five hepatic transporters was still maintained. In contrast, in FXR-null mice, except for Mrp4, which remained unchanged, the day 1 surge pattern was attenuated as indicated by the mRNA expression of Ntcp, Bsep, Mdr2, and Ostβ in liver, which were 66, 18, 56, and 45% of wild-type mice, respectively (Fig. 11A). At 3 and 5 days of age, the expression of Bsep and Ostβ in FXR-null mouse liver remained lower than that in wild-type mice, whereas the expression of Ntcp and Bsep was similar between FXR-null and wild-type mice (data not shown). To determine whether FXR and PXR contribute to the neonatal upregulation of the ileal BA transporters, Ostα and β, the mRNA of these transporters was quantified in livers from FXR- and PXR-null mice at 1 day of age (Fig. 11B). In PXR-null mice, the day 1 surge pattern of Ostα and β transporters was maintained. In contrast, in FXR-null mice, the expression of both Ostα and β was decreased at 1 day of age, which were 39 and 32% of wild-type mice, respectively. In summary, the day 1 surge pattern of the liver transporters Ntcp, Bsep, Mdr2, and Ostβ, as well as the ileal transporters, Ostα and β, was attenuated in FXR-null mice, but not in PXR-null mice.
Fig. 11.
A: mRNA expression of liver transporters Ntcp, Bsep, Mdr2, Mrp4, and Ostβ in wild-type, PXR-null (top), and FXR-null mice (bottom) at 1 day of age. B: mRNA expression of ileal transporters Asbt, Ostα, and Ostβ in wild-type, PXR-null (top), and FXR-null mice (bottom) at 1 day of age. C: immunofluorescence of Ntcp and Bsep protein in livers from wild-type and FXR-null mice at 1 day of age. Immunofluorescence against basolateral Ntcp protein as well as canalicular Bsep (green) was conducted on liver cryosections as described in materials and methods. Portions of images were enlarged and provided as insets. Representative images are shown. Bar = 50 μm.
Immunofluorescence of Ntcp and Bsep in livers of wild-type and FXR-null mice at 1 day of age.
To determine whether the protein expression and membrane localization of transporters were also impaired in FXR-null mice in newborns, immunofluorescence of Ntcp and Bsep was performed on frozen liver sections of wild-type and FXR-null mice at 1 day of age (Fig. 11C). The basolateral staining of Ntcp protein was reduced, but still detectable in livers of FXR-null mice, indicating FXR at least partially contributes to the day 1 surge pattern of Ntcp in liver. The canalicular staining of Bsep protein was decreased in livers of FXR-null mice, suggesting FXR is important in maintaining the neonatal surge of Bsep expression.
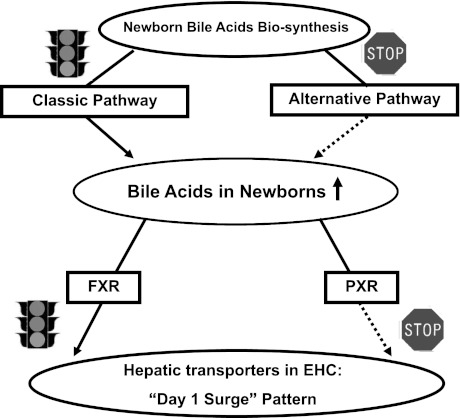
Collectively, increased BAs via the classic pathway of biosynthesis trigger the day 1 surge pattern of major transporters involved in the EHC of BAs during development, through activating the FXR-mediated pathway. Our working model is that during development, increased BA biosynthesis occurs in liver during the neonatal period primarily by the classic pathway, which contributes to the increased BAs in newborns. In turn, high concentrations of BAs activate both the FXR and PXR receptors, but FXR is responsible for the day 1 surge pattern of hepatic transporters for the EHC of BAs (Fig. 12).
Fig. 12.
Schematic of the working hypothesis for the molecular mechanisms underlying the neonatal induction of hepatic transporters involved in enterohepatic circulation (EHC).
Other liver transporters that are not thought to have a major role in the EHC of BAs, such as the basolateral uptake transporters for xenobiotics (the Oatps, Oct1, Oat2, and Ent1), the basolateral efflux transporters (Mrp3 and 6, Abca1), and many canalicular transporters for xenobiotics and other chemicals (Mrp2, Bcrp, Mate1, Abcg5, g5, and Atp8b1) do not have a day 1 surge pattern in their mRNA expression, and the majority of these transporters increased between 15 to 45 days of age, except for Bcrp, which is enriched prenatally (Figs. 13, 14, and 15). In addition, the housekeeping gene Gapdh, used for normalization of some mRNA assays in the present study, is consistently expressed throughout liver development (data not shown). Therefore, the day 1 surge pattern appears to be a specific phenomenon for the expression of transporters involved in the EHC of BAs.
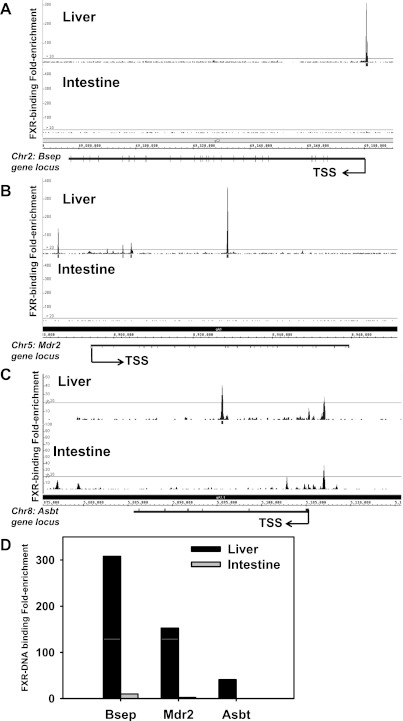
In vivo FXR-DNA binding has been shown for BA transporters in mouse liver and intestine (46). For example, Ostβ has two in vivo FXR-binding sites (one at the promoter and one at the 3′-end) in mouse liver and three FXR-binding sites (two at the promoter and one at the 3′-end) in intestine; Ostα has no in vivo FXR-binding sites in liver, but it has three binding sites in intestine [one within −1245 to −1145 bp upstream of the transcription start site (TSS), one within 100 bp upstream of the TSS, and one within intron 2] (46). For Ntcp, there are two in vivo FXR-binding sites in liver (one distal and one proximal), but there are no FXR-binding sites in intestine (46). In addition, the in vivo FXR-DNA binding activities for Bsep, Mdr2, and Asbt were examined in adult mouse liver and intestine (Fig. 16). FXR binds to none of these three transporters in intestine (Fig. 16, A–C, bottom). In contrast, in mouse liver there is one FXR-binding site around the Bsep gene locus (−191 bp upstream of TSS; Fig. 16A), four FXR-binding sites around the Mdr2 gene locus (−7717 upstream, 8603 downstream, 10,715 bp downstream, and 34,971 bp downstream of TSS; Fig. 16B), and one FXR-binding site around the Asbt gene locus (9648 bp downstream of TSS; Fig. 16C). The FXR-binding fold-enrichment to these three gene loci is shown in Fig. 16D, which indicates that FXR binding to all these three genes appears to be liver-specific, and the fold-enrichment for FXR binding was highest for Bsep, followed by Mdr2 and Asbt.
Fig. 16.
Location and fold enrichment of FXR binding to the gene loci of Bsep (A), Mdr2 (B), and Asbt (C) in adult mouse liver and intestine. Threshold for positive FXR enrichment: average peak value >20-fold based on calculation of false discovery rate (FDR). Images were generated by integrated genome browser. D: overall FXR DNA binding fold enrichment in livers and intestines of adult mice for these 3 genes.
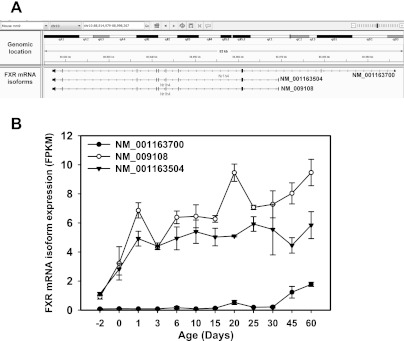
There are three known transcript variants of FXR in mice, namely NM_00163700 (variant 1, the longest isoform), NM_001163504 (variant 2, a shorter isoform due to alternative 5′-UTR and alternative start codon), and NM_009108 (variant 3, another shorter isoform compared with variant 1 due to an alternate in-frame splice site in the coding region; Fig. 17A). All three transcript variants of FXR have been quantified by RNA-Seq as shown in Fig. 17B. Among the three FXR mRNA isoforms, the longest FXR transcript (variant 1) was minimally expressed in mouse liver before 30 days of age, and only moderately increased from 30 to 60 days of age. In contrast, variant 2 and 3 were significantly expressed in mouse liver; variant 2 was expressed at a lower level than variant 3 at most postnatal ages. Both variant 2 and 3 increased markedly from day −2 to 1 day of age. After 1 day of age, variant 2 was expressed at a relatively stable level. In contrast, variant 3 reached a peak at 1 day of age, followed by a second peak at 20 days, and a third peak at 60 days of age. Therefore, the day 1 surge appears to correlate with FXR transcript variant 3, which encodes the FXR protein isoform 2.
Fig. 17.
A: genomic location of the 3 known FXR transcript variants in mice. B: ontogeny of the 3 known FXR transcript variants during liver development in mice.
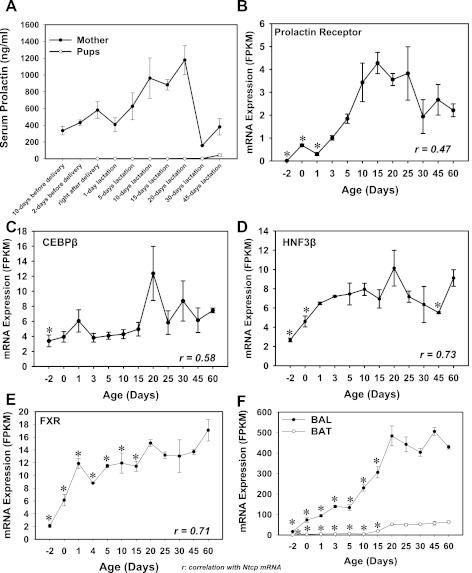
Regarding the regulation of Ntcp by prolactin, it has been shown that prolactin induces Ntcp expression in rat liver through Stat5 (8, 9, 21). Therefore, an initial hypothesis was that prolactin is a contributing factor in inducting Ntcp mRNA in neonatal mouse livers. To test this hypothesis, serum prolactin concentrations in pups at various ages were quantified by RIA (I125), and the serum prolactin from the mothers was used as a positive control. The mRNA of the prolactin receptor was also quantified. The data indicate that although the maternal serum prolaction increased markedly during lactation period, the serum prolactin in the suckling pups remained low (Fig. 18A). In addition, the mRNA of the prolaction receptor in livers of pups was very low throughout development (Fig. 18B). A linear regression between the ontogeny of Ntcp mRNA and the mRNA of the prolactin receptor showed that there is not a positive association (r = 0.47; Fig. 18B). Therefore, although it has been shown in maternal lactating rats that Ntcp is regulated by prolactin, prolactin does not appear to be a major contributor for the day 1 surge of Ntcp mRNA in neonatal mice.
Fig. 18.
A: serum prolactin concentrations in mother and the male pups (n = 5) at various time points from before birth to postweaning. Prolactin was quantified using a radioimmunoassay (RIA; I125). B–F: mRNA ontogeny of prolactin receptor (B), CEBPβ (C), HNF3β (D), FXR (E), as well as BAL and BAT (F) during liver development in mice (n = 5 per age, male only). Data are represented as means ± SE. *Statistically significant differences compared with 60 days of age.
It has been shown that CEBPβ trans-activates the mouse Ntcp promoter; in contrast, HNF3β decreases Ntcp promoter activity (26). Regarding CEBPβ, very recent findings indicate that in HepG2 cells, activation of FXR by CDCA induces detoxifying enzymes through AMPK and ERK1/2-mediated phosphorylation of CEBPβ (32), suggesting that there is crosstalk between FXR and CEBPβ signaling pathways. Therefore, the mRNAs of CEBPβ and HNF3β were quantified in the present study during mouse liver development (Fig. 18, C and D). The mRNAs of both CEBPβ and HNF3β increased after birth and reached a peak at 20 days of age. Regression analyses indicate that there is not a positive association between CEBPβ mRNA and Ntcp mRNA (r = 0.58; Fig. 18C), and there is not an apparent negative association between HNF3β mRNA and Ntcp mRNA (r = 0.73; Fig. 18D). Because CEBPβ is very lowly expressed in neonatal livers, and it poorly correlates with the Ntcp mRNA ontogeny, the protein phosphorylation of CEBPβ was not determined. In summary, although it has been shown with in vitro systems that CEBPβ and HNF3β regulate Ntcp transcription, they do not appear to play major roles in the neonatal increase of Ntcp mRNA in mouse liver. In contrast, the mRNA of FXR was low before birth, but it increased right after birth and reached a peak at 1 day of age in mouse liver (6-fold of prenatal levels; Fig. 18E). FXR mRNA in liver decreased after 1 day of age, but it increased again to adult levels after 20 days of age. Linear regression analysis indicates a positive association for the ontogeny of FXR mRNA and Ntcp mRNA (r = 0.71). In adult mouse livers, direct FXR-binding sites have been identified in the Ntcp gene loci (46). In the neonatal period, BA concentrations increased markedly in liver (Fig. 5). Therefore, the evidence suggests that FXR is important for the increased Ntcp mRNA in the neonatal period.
The ratio between unconjugated and amidated BAs shifts toward unconjugated BAs during the later time points of maturation. It has been shown that FXR regulates the key enzymes of amidation, namely the BA-CoA ligase (BAL) and the BA CoA: amino acid N-acetytransferase (BAT) (35). The mRNA ontogeny of both BAL and BAT was quantified by RNA-Seq (Fig. 18F). Both BAL and BAT mRNAs gradually increased and reached their peak during the adult period (45 days of age for BAL, and 60 days of age for BAT; Fig. 18F). The ontogeny of these two genes does not appear to correlate with the shift from conjugated to unconjugated BAs in liver during development. It is possible that the shift between the two types of BAs during liver development is due to transporters, which promote the biliary excretion of conjugated BAs to reduce their hepatic concentrations.
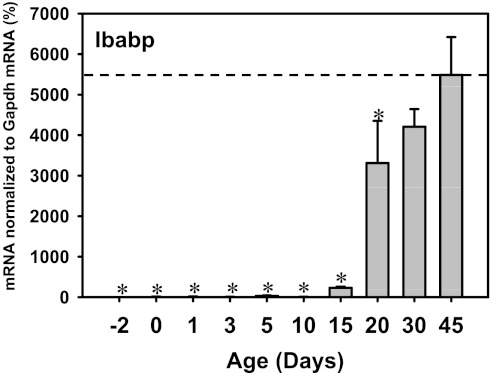
The ontogeny of Ibabp, another prototypical FXR target gene in ileum of mice, was also determined (Fig. 19). The Ibabp mRNA was low before 10 days of age, but it increased markedly thereafter, and reached peak values at 45 days of age (Fig. 19). This ontogeny pattern of Ibabp mRNA is different from Ostβ mRNA ontogeny. In addition, Fgf15 mRNA was low in neonatal and adult periods, but it was high between 5 and 15 days of age. Therefore, there appear to be regulators other than FXR that determine the various ontogenic patterns of Ibabp, Ostβ, and Fgf15. Nevertheless, among the known FXR target genes in intestine, namely Ostα, Ostβ, Fgf15, and Ibabp, all except Ostβ were low at 1 day of age, suggesting that FXR activation in the intestine is delayed compared with liver during development in mice.
Fig. 19.
Ontogeny of Ibabp mRNA in ilea of mice (n = 3 per age, male only). Data are represented as means ± SE. *Statistically significant differences compared with 45 days of age.
DISCUSSION
In conclusion, the present study revealed a day 1 surge pattern in the expression of major transporters involved in the EHC of BAs in newborns and demonstrated that it is the BAs from the classic pathway of synthesis that trigger the induction of these transporters in newborns, through activating FXR-mediated pathways.
Transporters are essential in maintaining the EHC of BAs, as evidenced by numerous clinical cases in pediatrics and animal studies using knock-out mice. For example, Bsep is the major BA efflux transporter in liver that determines bile flow, and inborn errors in human BSEP result in severe progressive familial intrahepatic cholestasis (PFIC type II) (48), as well as hepatocellular carcinoma in young children (27). In addition, although the phospholipid floppase Mdr2 in liver does not transport BAs, the defect in its human analog MDR3 results in PFIC type III (19). Mdr2-null mice develop hepatocyte degeneration and focal necrosis, as well as abnormalities in bile composition (43), indicating the importance of Mdr2/MDR3 in maintaining BA circulation and liver protection. On the basolateral membrane of hepatocytes, Ntcp is the major BA uptake transporter that returns serum BAs back into the hepatocytes, evidenced by a 95% blockade of sodium-dependent BA uptake following a Ntcp-specific antisense oligonucleotide administration (22). By transporting BAs back into liver, Ntcp keeps BAs in the EHC for the next cycle of nutrient absorption and prevents toxicity to extrahepatic organs.
In comparison to the high expression of the liver-specific transporters, Ntcp, Bsep, and Mdr2 (16, 18), the expression of the basolateral BA efflux transporters, Mrp4, Ostα, and Ostβ, is very lowly expressed in the liver (30), indicating the liver protects the extrahepatic organs from high concentrations of BAs by minimizing the excretion of BAs back into the systemic circulation. These basolateral BA efflux transporters are induced by cholestasis, which is necessary for hepatic protection. For example, Mrp4-null mice have more severe liver injury following bile duct ligation (31). In ileum, both Ostα and β are highly expressed during the postnatal period, which favors the retrieval of BAs from the lumen into the EHC to facilitate lipophilic nutrient absorption. Although numerous studies have been reported on the regulation of liver transporters in various adult disease models, less attention has been paid to the regulation of these transporters during liver development under physiological conditions. The present study filled this knowledge gap by identifying a day 1 surge pattern in the ontogeny of these transporters, as well as the FXR-signaling pathway as a regulatory mechanism for the activation of these genes at birth.
The general dogma for the regulation of Ntcp states that high levels of BAs decrease Ntcp mRNA expression through activating the FXR-SHP signaling pathway in liver, as observed in various models of cholestasis (28, 41) and BA feeding studies (53) performed in adult mice or rats. Teleologically this may be protective, because unrestricted hepatic influx of BAs from serum might lead to accumulation of toxic BAs in hepatocytes. However, the present study challenges the existing paradigm by demonstrating that both Ntcp mRNA and protein immunofluorescence staining are high in newborn livers, despite approximately twofold higher BA concentrations in liver at birth than at 45 days of age, and upregulated hepatic FXR-SHP pathway in newborns. In addition, the present study also demonstrates that the day 1 surge pattern of Ntcp is dependent on the presence of the FXR-signaling pathway in newborns. Evidence from clinical studies demonstrates that children with severe hypercholanemia had normal expression of NTCP, even though the serum concentrations of BAs were extremely high (39). Thus, the regulation of Ntcp by BAs is not as simple as anticipated. It can be speculated that the FXR-SHP signaling pathway is not the major pathway in regulating the expression of Ntcp in newborns, and FXR may be involved in certain neonatal-predominant signaling pathways that promote the day 1 surge pattern of Ntcp in newborns. Very little is known regarding how FXR induces Ntcp in mouse liver, but it seems that the role of FXR is age- and context-specific. Recently, a large body of evidence suggested that distinct chromatin epigenetic signatures are very important for cell development and are essential in predefining transcription-DNA binding patterns. It is possible that a distinct chromatin epigenetic environment favors the recruitment of FXR directly to the response element of Ntcp and promotes gene expression in cooperation with other cofactors. Supporting evidence for this hypothesis includes: 1) a positive association for the ontogeny of FXR mRNA and Ntcp mRNA (r = 0.71) in that FXR mRNA also markedly increased and reached a peak at 1 day of age (6-fold of prenatal levels), 2) in adult mouse liver, direct FXR-binding sites have been identified in the Ntcp gene loci (46), suggesting that direct FXR binding to Ntcp occurs, and 3) BAs as FXR ligands markedly increase in neonatal mouse livers. Further investigations will determine age-specific interactions between FXR and genes encoding BA transporters in the liver.
It is well-known that before birth, liver is mainly a hematopoietic organ, whereas after birth, it becomes the major organ for chemical metabolism and detoxification. Therefore, functionally speaking, during the neonatal period when BAs are high in serum and liver due to the immaturity in EHC, it is necessary to upregulate Ntcp in liver to reduce BA concentrations in the systemic circulation, even at the cost of stressing the hepatocytes, so as to protect extrahepatic organs that are critical for survival, including the brain where the blood-brain barrier is not completely functioning, as well as other developing organs that are vulnerable to BA-induced toxicity.
Previous studies determined the ontogenic expression of Ntcp and Bsep in rat livers. It has been shown that Ntcp mRNA was detectable by Northern blot as early as gestation day 18 in rat liver when sodium-dependent BA transport already occurs (7). The present study shows that in mice, Ntcp protein was already localized to the basolateral membrane at day −2 of age (although the fluorescence staining was relatively weak), suggesting that low levels of Ntcp-mediated BA transport may also occur in prenatal mouse livers. However, more BA uptake likely occurs in newborns, when both the mRNA expression and the protein immunofluorescence staining of Ntcp reached a peak in neonatal livers. The expression of Bsep was undetectable in prenatal rat livers, but a marked upregulation of Bsep protein occurs in newborn rat livers (52), which is consistent with the present study in mice. The present study further demonstrates that the induction of Bsep in newborn mouse liver is due to activation of FXR, likely by direct activation, because it has been demonstrated that Bsep is a FXR target gene (36).
A large body of evidence indicates that FXR is important in regulating BA homeostasis, lipid metabolism, and glucose metabolism in adults (15). FXR-null mice have elevated serum BA concentrations in adults (40), indicating the important role of FXR in BA synthesis and/or disposition. Less is known of the role of FXR during development. Clinically, decreased FXR expression in ileum was observed in children with PFIC type I (10), and functional variants of FXR in liver have been identified in intrahepatic cholestasis of pregnancy (47), indicating FXR is important for both perinatal and maternal BA homeostasis. The present study demonstrates that FXR is essential in triggering the day 1 surge of hepatic and ileal transporters to promote the EHC of BAs, so as to facilitate the absorption of fat-soluble nutrients in newborns. Mrp4 is the only transporter whose neonatal surge is independent of FXR. It has been shown that the constitutive androstane receptor (CAR) can induce Mrp4 expression in mouse liver (4, 29, 42), and the expression of CAR mRNA is upregulated right after birth (J. Y. Cui and C. D. Klaassen, unpublished data). Therefore, it can be speculated that CAR contributes to the moderate induction of hepatic Mrp4 at 1 day of age.
It has been suggested that “physiological cholestasis” in newborns is due to the immaturity of the EHC and delayed hepatic clearance of BAs (45). However, how the neonatal BA concentrations are elevated remains poorly understood. It has been demonstrated that maternal cholestasis in rats does not affect the expression of Ntcp in pups (3), indicating the high levels of BAs in newborns are not likely from the mother through placental transfer. The present study demonstrates that the increase in BA concentrations in newborns is likely due to upregulation of the classic pathway of BA synthesis, as evidenced by the marked increase in Cyp7a1 and 8b1 mRNAs, as well as increase in taurine-conjugated CA in newborns. It is well-known that the liver X receptor (LXR) transcriptionally upregulates Cyp7a1 in mice, and oxysterols, which are metabolized from cholesterol, serve as ligands for LXR (15). Therefore, it is possible that the upregulation of the classic pathway of BA synthesis is triggered by cholesterol-enriched milk from the mother. In addition, the low expression of the suppression signal Fgf15 from the intestine also provides a permissive environment to allow the induction of Cyp7a1. Although Cyp39a1 from the alternative pathway was also induced in newborns, the significance of this 24-hydroxysterol 7-hydroxylase in producing BAs in liver is not well-understood.
Different BAs have various affinities for FXR. CDCA has been shown to be the most potent FXR agonist in cell cultures (33). In addition, 6α-ethyl-CDCA (6-ECDCA) is a potent and selective FXR agonist endowed with anti-cholestatic activity when given to an in vivo rat model of cholestasis (34). However, most CDCA synthesized in mice is converted to MCA, whereas 6-ECDCA is a synthetic BA and is not produced in mice. Another study showed that the FXR target gene Bsep in mice can be induced by CA feeding in an FXR-dependent manner, indicating that CA might also be a ligand for FXR (53). Therefore, during the neonatal period, the increased concentrations of CA are likely the mechanism for the FXR-mediated pathway.
At later ages (from day 20 to day 30), serum BA concentrations decreased in mice, whereas liver BA concentrations remained high and correlated with high mRNA levels of the BA-synthesizing enzymes (Cyp7a1, 8b1, and 27a1). The decrease in serum BA concentrations probably reflects maturation of other BA uptake transport systems in liver at later ages, for example, the basolateral uptake transporters Oatp1a1, 1a4, and 1b2 increase gradually and reach adult levels around 30 days of age (12), which may act in concert with Ntcp to further reduce serum BAs and thus protect other organs from BA toxicity.
In the neonatal period, the expression profiles of a gene can be different in liver and ileum. For example, the prototypical target genes of FXR and PXR (SHP and Cyp3a11) were only upregulated in liver but not in ileum, even though FXR and PXR mRNAs were increased in both tissues. Ostα, a well-known FXR target gene (54), was only induced in newborn ileum but not in liver. In addition, the intestinal Fgf15, which is a target of FXR, was not induced at 1 day of age even though FXR mRNA is induced at this age. The mechanism for the tissue-specific regulation of these genes is not clearly understood, but it can be speculated that certain epigenetic mechanisms, like microRNAs, might regulate the expression of certain genes in the intestine, like FXR and PXR, which either inhibit the formation of a functional protein or decrease mRNA stability. The posttranscriptional modifications of these genes in liver and ileum will be determined in future studies.
Although there appears to be an increase in the mRNAs of Ostα and Ostβ in the neonatal mouse intestine, Asbt mRNA remains low until 20 days of age. Because Asbt is the major apical transporter for BA reabsorption in the intestine, the low levels of Asbt mRNA in the neonatal period indicate that reabsorption of BAs from the intestinal lumen is low. In addition, the mRNA of the prototypical FXR target gene, Ibabp, was also low during the neonatal period. Therefore, induction of BA transporters in liver right after birth precedes the intestinal transporters, whereas the BA transport system in the intestine appears to be only functioning at a low level. The present study provides some insights in understanding the mechanisms for the maturation of the EHC of BAs in the neonatal period, and it demonstrates that FXR might be a therapeutic target in regulating BAs and nutrient homeostasis in pediatric patients.
GRANTS
This work was supported by National Institutes of Health Grants ES-009649, ES-019487, DK-081461, and ES-07079 to Dr. C. D. Klaassen.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.Y.C., L.M.A., Y.T., G.L.G., H.L., X.-b.Z., and C.D.K. conception and design of research; J.Y.C., Z.D.F., Y.G., and G.L.G. performed experiments; J.Y.C. and Z.D.F. analyzed data; J.Y.C., L.M.A., Y.T., and Z.D.F. interpreted results of experiments; J.Y.C. and Z.D.F. prepared figures; J.Y.C. drafted manuscript; J.Y.C., L.M.A., Y.T., Z.D.F., Y.G., G.L.G., H.L., X.-b.Z., and C.D.K. edited and revised manuscript; J.Y.C., L.M.A., Y.T., Z.D.F., Y.G., G.L.G., H.L., X.-b.Z., and C.D.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Youcai Zhang for technical assistance in serum bile acid quantification with LC-MS/MS, and Dr. Jay Patrick for designing the probe sets for the bDNA assay of mouse FXR mRNA.
Portions of this work were presented at the national meeting of the America Association for the Study of Liver Diseases (AASLD) for which J. Y. Cui received the Presidential Award of Distinction, October 31-November 4, 2008.
REFERENCES
- 1.Aleksunes LM, Slitt AM, Cherrington NJ, Thibodeau MS, Klaassen CD, Manautou JE. Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol Sci 83: 44–52, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Alnouti Y, Csanaky IL, Klaassen CD. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 873: 209–217, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrese M, Trauner M, Ananthanarayanan M, Boyer JL, Suchy FJ. Maternal cholestasis does not affect the ontogenic pattern of expression of the Na+/taurocholate cotransporting polypeptide (ntcp) in the fetal and neonatal rat liver. Hepatology 28: 789–795, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, Zelcer N, Adachi M, Strom S, Evans RM, Moore DD, Borst P, Schuetz JD. Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem 279: 22250–22257, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, Madejczyk MS, Li N. OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology 42: 1270–1279, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Beilke LD, Aleksunes LM, Holland RD, Besselsen DG, Beger RD, Klaassen CD, Cherrington NJ. Constitutive androstane receptor-mediated changes in bile acid composition contributes to hepatoprotection from lithocholic acid-induced liver injury in mice. Drug Metab Dispos 37: 1035–1045, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyer JL, Hagenbuch B, Ananthanarayanan M, Suchy F, Stieger B, Meier PJ. Phylogenic and ontogenic expression of hepatocellular bile acid transport. Proc Natl Acad Sci USA 90: 435–438, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao J, Huang L, Liu Y, Hoffman T, Stieger B, Meier PJ, Vore M. Differential regulation of hepatic bile salt and organic anion transporters in pregnant and postpartum rats and the role of prolactin. Hepatology 33: 140–147, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Cao J, Wood M, Liu Y, Hoffman T, Hyde J, Park-Sarge OK, Vore M. Estradiol represses prolactin-induced expression of Na+/taurocholate cotransporting polypeptide in liver cells through estrogen receptor-alpha and signal transducers and activators of transcription 5a. Endocrinology 145: 1739–1749, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Chen F, Ananthanarayanan M, Emre S, Neimark E, Bull LN, Knisely AS, Strautnieks SS, Thompson RJ, Magid MS, Gordon R, Balasubramanian N, Suchy FJ, Shneider BL. Progressive familial intrahepatic cholestasis, type 1, is associated with decreased farnesoid × receptor activity. Gastroenterology 126: 756–764, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Cheng X, Klaassen CD. Regulation of mRNA expression of xenobiotic transporters by the pregnane × receptor in mouse liver, kidney, and intestine. Drug Metab Dispos 34: 1863–1867, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Cheng X, Maher J, Chen C, Klaassen CD. Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (Oatps). Drug Metab Dispos 33: 1062–1073, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Cheng X, Maher J, Dieter MZ, Klaassen CD. Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos 33: 1276–1282, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Chiang JY. Bile acid regulation of gene expression: roles of nuclear hormone receptors. Endocr Rev 23: 443–463, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Chiang JY. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol 40: 539–551, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Choudhuri S, Cherrington NJ, Li N, Klaassen CD. Constitutive expression of various xenobiotic and endobiotic transporter mRNAs in the choroid plexus of rats. Drug Metab Dispos 31: 1337–1345, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Crosignani A, Del Puppo M, Longo M, De Fabiani E, Caruso D, Zuin M, Podda M, Javitt NB, Kienle MG. Changes in classic and alternative pathways of bile acid synthesis in chronic liver disease. Clin Chim Acta 382: 82–88, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Cui Y, Cheng X, Miao Weaver Y, Klaassen CD. Tissue distribution, gender-divergent expression, ontogeny and chemical induction of multidrug resistance transporter genes (Mdr1a, Mdr1b, Mdr2) in mice. Drug Metab Dispos 37: 203–210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, Deleuze JF, Desrochers M, Burdelski M, Bernard O, Oude Elferink RP, Hadchouel M. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci USA 95: 282–287, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott WH, Hyde PM. Metabolic pathways of bile acid synthesis. Am J Med 51: 568–579, 1971 [DOI] [PubMed] [Google Scholar]
- 21.Ganguly TC, O'Brien ML, Karpen SJ, Hyde JF, Suchy FJ, Vore M. Regulation of the rat liver sodium-dependent bile acid cotransporter gene by prolactin. Mediation of transcriptional activation by Stat5. J Clin Invest 99: 2906–2914, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagenbuch B, Scharschmidt BF, Meier PJ. Effect of antisense oligonucleotides on the expression of hepatocellular bile acid and organic anion uptake systems in Xenopus laevis oocytes. Biochem J 316: 901–904, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartley DP, Klaassen CD. Detection of chemical-induced differential expression of rat hepatic cytochrome P450 mRNA transcripts using branched DNA signal amplification technology. Drug Metab Dispos 28: 608–616, 2000 [PubMed] [Google Scholar]
- 24.Heubi JE, Balistreri WF, Partin JC, Schubert WK, McGraw CA. Refractory infantile diarrhea due to primary bile acid malabsorption. J Pediatr 94: 546–551, 1979 [DOI] [PubMed] [Google Scholar]
- 25.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2: 217–225, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Jung D, Hagenbuch B, Fried M, Meier PJ, Kullak-Ublick GA. Role of liver-enriched transcription factors and nuclear receptors in regulating the human, mouse, and rat NTCP gene. Am J Physiol Gastrointest Liver Physiol 286: G752–G761, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, Bull LN, Pawlikowska L, Bilezikci B, Ozcay F, Laszlo A, Tiszlavicz L, Moore L, Raftos J, Arnell H, Fischler B, Nemeth A, Papadogiannakis N, Cielecka-Kuszyk J, Jankowska I, Pawlowska J, Melin-Aldana H, Emerick KM, Whitington PF, Mieli-Vergani G, Thompson RJ. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 44: 478–486, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, MacKenzie KI, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest 112: 1678–1687, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD. Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metab Dispos 33: 956–962, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Maher JM, Slitt AL, Cherrington NJ, Cheng X, Klaassen CD. Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab Dispos 33: 947–955, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Mennone A, Soroka CJ, Cai SY, Harry K, Adachi M, Hagey L, Schuetz JD, Boyer JL. Mrp4−/− mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology 43: 1013–1021, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Noh K, Kim YM, Kim YW, Kim SG. Farnesoid X receptor activation by chenodeoxycholic acid induces detoxifying enzymes through AMP-activated protein kinase and extracellular signal-regulated kinase 1/2-mediated phosphorylation of CCAAT/enhancer binding protein beta. Drug metabolism and disposition: the biological fate of chemicals. Drug metab Dispos 39: 1451–1459, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Pellicciari R, Costantino G, Fiorucci S. Farnesoid X receptor: from structure to potential clinical applications. J Med Chem 48: 5383–5403, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, Morelli A, Parks DJ, Willson TM. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem 45: 3569–3572, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Pircher PC, Kitto JL, Petrowski ML, Tangirala RK, Bischoff ED, Schulman IG, Westin SK. Farnesoid X receptor regulates bile acid-amino acid conjugation. J Biol Chem 278: 27703–27711, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Plass JR, Mol O, Heegsma J, Geuken M, Faber KN, Jansen PL, Muller M. Farnesoid X receptor and bile salts are involved in transcriptional regulation of the gene encoding the human bile salt export pump. Hepatology 35: 589–596, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 72: 137–174, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Scheimann AO, Strautnieks SS, Knisely AS, Byrne JA, Thompson RJ, Finegold MJ. Mutations in bile salt export pump (ABCB11) in two children with progressive familial intrahepatic cholestasis and cholangiocarcinoma. J Pediatr 150: 556–559, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Shneider BL, Fox VL, Schwarz KB, Watson CL, Ananthanarayanan M, Thevananther S, Christie DM, Hardikar W, Setchell KD, Mieli-Vergani G, Suchy FJ, Mowat AP. Hepatic basolateral sodium-dependent bile acid transporter expression in two unusual cases of hypercholanemia and in extrahepatic biliary atresia. Hepatology 25: 1176–1183, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102: 731–744, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Slitt AL, Allen K, Morrone J, Aleksunes LM, Chen C, Maher JM, Manautou JE, Cherrington NJ, Klaassen CD. Regulation of transporter expression in mouse liver, kidney, and intestine during extrahepatic cholestasis. Biochim Biophys Acta 1768: 637–647, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Slitt AL, Cherrington NJ, Dieter MZ, Aleksunes LM, Scheffer GL, Huang W, Moore DD, Klaassen CD. trans-Stilbene oxide induces expression of genes involved in metabolism and transport in mouse liver via CAR and Nrf2 transcription factors. Mol Pharmacol 69: 1554–1563, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA, van der Valk MA, Offerhaus GJA, Berns AJM, Borst P. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 75: 451–462, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology 49: 297–305, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suchy FJ, Balistreri WF, Heubi JE, Searcy JE, Levin RS. Physiologic cholestasis: elevation of the primary serum bile acid concentrations in normal infants. Gastroenterology 80: 1037–1041, 1981 [PubMed] [Google Scholar]
- 46.Thomas AM, Hart SN, Kong B, Fang J, Zhong XB, Guo GL. Genome-wide tissue-specific farnesoid X receptor binding in mouse liver and intestine. Hepatology 51: 1410–1419, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Mil SW, Milona A, Dixon PH, Mullenbach R, Geenes VL, Chambers J, Shevchuk V, Moore GE, Lammert F, Glantz AG, Mattsson LA, Whittaker J, Parker MG, White R, Williamson C. Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology 133: 507–516, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Soroka CJ, Boyer JL. The role of bile salt export pump mutations in progressive familial intrahepatic cholestasis type II. J Clin Invest 110: 965–972, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang R, Lam P, Liu L, Forrest D, Yousef IM, Mignault D, Phillips MJ, Ling V. Severe cholestasis induced by cholic acid feeding in knockout mice of sister of P-glycoprotein. Hepatology 38: 1489–1499, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Yamato Y, Kimura A, Inoue T, Kurosawa T, Kato H. Fetal bile acid metabolism: analysis of urinary 3beta-monohydroxy-delta(5) bile acid in preterm infants. Biol Neonate 80: 19–25, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Klaassen CD. Effects of feeding bile acids and a bile acid sequestrant on hepatic bile acid composition in mice. J Lipid Res 51: 3230–3242, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zinchuk VS, Okada T, Akimaru K, Seguchi H. Asynchronous expression and colocalization of Bsep and Mrp2 during development of rat liver. Am J Physiol Gastrointest Liver Physiol 282: G540–G548, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Zollner G, Wagner M, Fickert P, Geier A, Fuchsbichler A, Silbert D, Gumhold J, Zatloukal K, Kaser A, Tilg H, Denk H, Trauner M. Role of nuclear receptors and hepatocyte-enriched transcription factors for Ntcp repression in biliary obstruction in mouse liver. Am J Physiol Gastrointest Liver Physiol 289: G798–G805, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU, Trauner M. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-α/β in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol 290: G923–G932, 2006 [DOI] [PubMed] [Google Scholar]