Abstract
The complete nucleotide sequence of the 16S RNA gene from the rrnB cistron of Escherichia coli has been determined by using three rapid DNA sequencing methods. Nearly all of the structure has been confirmed by two to six independent sequence determinations on both DNA strands. The length of the 16S rRNA chain inferred from the DNA sequence is 1541 nucleotides, in close agreement with previous estimates. We note discrepancies between this sequence and the most recent version of it reported from direct RNA sequencing [Ehresmann, C., Stiegler, P., Carbon, P. & Ebel, J.P. (1977) FEBS Lett. 84, 337-341]. A few of these may be explained by heterogeneity among 16S rRNA sequences from different cistrons. No nucleotide sequences were found in the 16S rRNA gene that cannot be reconciled with RNase digestion products of mature 16S rRNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chapman N. M., Noller H. F. Protection of specific sites in 16 S RNA from chemical modification by association of 30 S and 50 S ribosomes. J Mol Biol. 1977 Jan 5;109(1):131–149. doi: 10.1016/s0022-2836(77)80049-1. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Carbon P., Ebel J. P. Recent progress in the determination of the primary sequence of the 16 S RNA of Escherichia coli. FEBS Lett. 1977 Dec 15;84(2):337–341. doi: 10.1016/0014-5793(77)80720-5. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Ebel J. P. Sequence analysis of the 3'-T1 oligonucleotide of 16S ribosomal RNA from Escherichia coli. FEBS Lett. 1974 Dec 1;49(1):47–48. doi: 10.1016/0014-5793(74)80628-9. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Fellner P., Ebel J. P. The determination of the primary structure of the 16S ribosomal RNA of Escherichia coli. 2. Nucleotide sequences of products from partial enzymatic hydrolysis. Biochimie. 1972;54(7):901–967. doi: 10.1016/s0300-9084(72)80007-5. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Fellner P., Ebel J. P. The determination of the primary structure of the 16S ribosomal RNA of Escherichia coli. III. Further studies. Biochimie. 1975;57(6-7):711–748. doi: 10.1016/s0300-9084(75)80047-2. [DOI] [PubMed] [Google Scholar]
- Fellner P. Nucleotide sequences from specific areas of the 16S and 23S ribosomal RNAs of E. coli. Eur J Biochem. 1969 Nov;11(1):12–27. doi: 10.1111/j.1432-1033.1969.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan J. J., Noller H. F. Altered topography of 16S RNA in the inactive form of Escherichia coli 30S ribosomal subunits. Biochemistry. 1978 Feb 21;17(4):587–593. doi: 10.1021/bi00597a005. [DOI] [PubMed] [Google Scholar]
- Kirschbaum J. B., Konrad E. B. Isolation of a specialized lambda transducing bacteriophage carrying the beta subunit gene for Escherichia coli ribonucleic acid polymerase. J Bacteriol. 1973 Nov;116(2):517–526. doi: 10.1128/jb.116.2.517-526.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
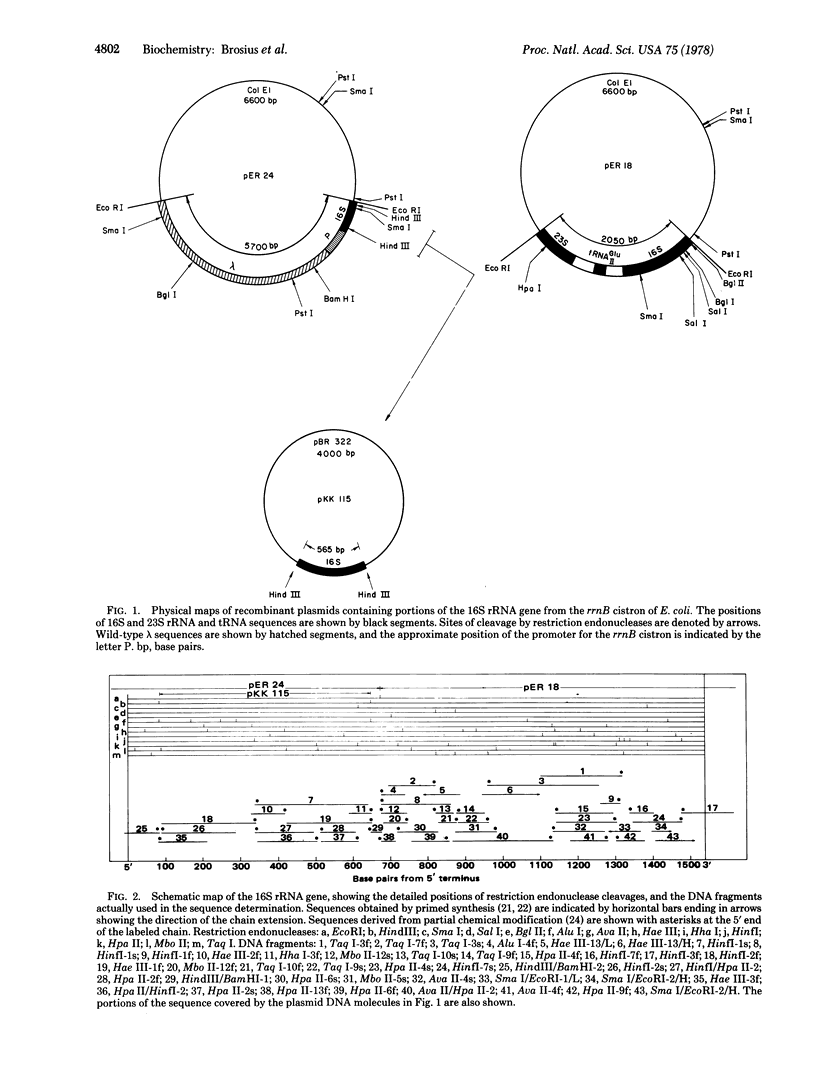
- Lindahl L., Jaskunas S. R., Dennis P. P., Nomura M. Cluster of genes in Escherichia coli for ribosomal proteins, ribosomal RNA, and RNA polymerase subunits. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2743–2747. doi: 10.1073/pnas.72.7.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt M. Structural studies on transfer ribonucleic acid. I. Labeling of exposed guanine sites in yeast phenylalanine transfer ribonucleic acid with kethoxal. Biochemistry. 1969 Aug;8(8):3249–3253. doi: 10.1021/bi00836a017. [DOI] [PubMed] [Google Scholar]
- Magrum L., Zablen L., Stahl D., Woese C. Corrections in the catalogue of oliogonucleotides produced by digestion of Escherichia coli 16S rRNA with T1 RNase. Nature. 1975 Oct 2;257(5525):423–426. doi: 10.1038/257423a0. [DOI] [PubMed] [Google Scholar]
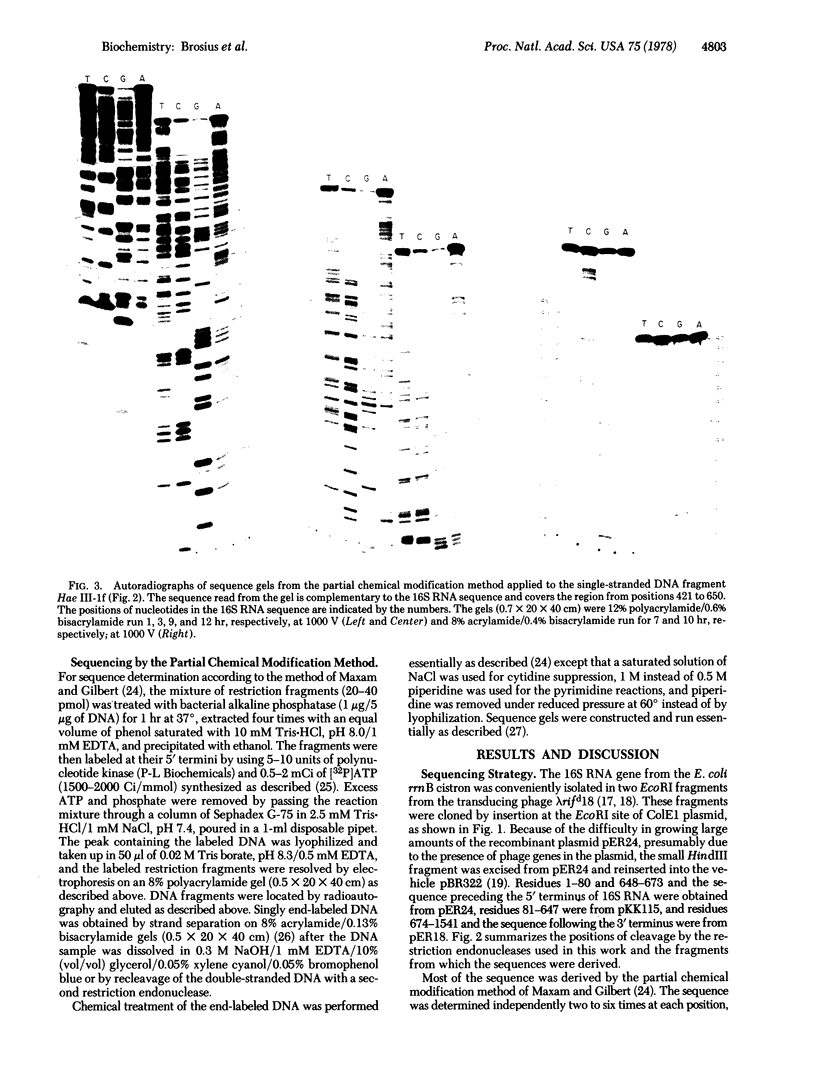
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley J. E. The estimation of polynucleotide chain length by a chemical method. Biochim Biophys Acta. 1965 Nov 8;108(3):340–347. doi: 10.1016/0005-2787(65)90026-2. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Chaires J. B. Functional modification of 16S ribosomal RNA by kethoxal. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3115–3118. doi: 10.1073/pnas.69.11.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F., Herr W. Nucleotide sequence of the 3' terminus of E. coli 16S ribosomal RNA. Mol Biol Rep. 1974 Dec;1(8):437–439. doi: 10.1007/BF00360668. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Topography of 16S RNA in 30S ribosomal subunits. Nucleotide sequences and location of sites of reaction with kethoxal. Biochemistry. 1974 Nov 5;13(23):4694–4703. doi: 10.1021/bi00720a003. [DOI] [PubMed] [Google Scholar]
- Ortega J. P., Hill W. E. A molecular weight determination of the 16S ribosomal ribonucleic acid from Escherichia coli. Biochemistry. 1973 Aug 14;12(17):3241–3243. doi: 10.1021/bi00741a015. [DOI] [PubMed] [Google Scholar]
- Ross A., Brimacombe R. Application of a rapid gel method to the sequencing of fragments of 16S ribosomal RNA from Escherichia coli. Nucleic Acids Res. 1978 Jan;5(1):241–256. doi: 10.1093/nar/5.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer M., Shane S. Area of 16S ribonucleic acid at or near the interface between 30S and 50S ribosomes of Escherichia coli. J Bacteriol. 1977 May;130(2):900–910. doi: 10.1128/jb.130.2.900-910.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz I., Ofengand J. Photo-affinity labeling of tRNA binding sites in macromolecules. I. Linking of the phenacyl-p-azide of 4-thiouridine in (Escherichia coli) valyl-tRNA to 16S RNA at the ribosomal P site. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3951–3955. doi: 10.1073/pnas.71.10.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague K. U., Steitz J. A. The 3' terminal oligonucleotide of E. coli 16S ribosomal RNA: the sequence in both wild-type and RNase iii- cells is complementary to the polypurine tracts common to mRNA initiator regions. Nucleic Acids Res. 1975 Jun;2(6):787–798. doi: 10.1093/nar/2.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley W. M., Jr, Bock R. M. Isolation and physical properties of the ribosomal ribonucleic acid of Escherichia coli. Biochemistry. 1965 Jul;4(7):1302–1311. doi: 10.1021/bi00883a014. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalay A. A., Grohmann K., Sinsheimer R. L. Separation of the complementary strands of DNA fragments on polyacrylamide gels. Nucleic Acids Res. 1977;4(5):1569–1578. doi: 10.1093/nar/4.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Bonen L., Schaup H. W., Lewis B. J., Zablen L., Woese C. The use of ribonuclease U2 in RNA sequence determination. Some corrections in the catalog of oligomers produced by ribonuclease T1 digestion of Escherichia coli 16S ribosomal RNA. J Mol Evol. 1974 Feb 28;3(1):63–77. doi: 10.1007/BF01795977. [DOI] [PubMed] [Google Scholar]
- Valenzuela P., Bell G. I., Venegas A., Sewell E. T., Masiarz F. R., DeGennaro L. J., Weinberg F., Rutter W. J. Ribosomal RNA genes of Saccharomyces cerevisiae. II. Physical map and nucleotide sequence of the 5 S ribosomal RNA gene and adjacent intergenic regions. J Biol Chem. 1977 Nov 25;252(22):8126–8135. [PubMed] [Google Scholar]
- Woese C. R., Fox G. E., Zablen L., Uchida T., Bonen L., Pechman K., Lewis B. J., Stahl D. Conservation of primary structure in 16S ribosomal RNA. Nature. 1975 Mar 6;254(5495):83–86. doi: 10.1038/254083a0. [DOI] [PubMed] [Google Scholar]
- Young R. A., Steitz J. A. Complementary sequences 1700 nucleotides apart form a ribonuclease III cleavage site in Escherichia coli ribosomal precursor RNA. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3593–3597. doi: 10.1073/pnas.75.8.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]