Abstract
Irritable bowel syndrome (IBS) is characterized as functional because a pathobiological cause is not readily apparent. Considerable evidence, however, documents that sensitizing proinflammatory and lipotoxic lipids, mast cells and their products, tryptases, enteroendocrine cells, and mononuclear phagocytes and their receptors are increased in tissues of IBS patients with colorectal hypersensitivity. It is also clear from recordings in animals of the colorectal afferent innervation that afferents exhibit long-term changes in models of persistent colorectal hypersensitivity. Such changes in afferent excitability and responses to mechanical stimuli are consistent with relief of discomfort and pain in IBS patients, including relief of referred abdominal hypersensitivity, upon intra-rectal instillation of local anesthetic. In the aggregate, these experimental outcomes establish the importance of afferent drive in IBS, consistent with a larger literature with respect to other chronic conditions in which pain is a principal complaint (e.g., neuropathic pain, painful bladder syndrome, fibromyalgia). Accordingly, colorectal afferents and the environment in which these receptive endings reside constitute the focus of this review. That environment includes understudied and incompletely understood contributions from immune-competent cells resident in and recruited into the colorectum. We close this review by highlighting deficiencies in existing knowledge and identifying several areas for further investigation, resolution of which we anticipate would significantly advance our understanding of neural and neuro-immune contributions to IBS pain and hypersensitivity.
Keywords: colon, immune cells, neurogenic inflammation, silent afferent, visceral pain
many chronic abdominal and pelvic pain disorders, including irritable bowel syndrome (IBS), are characterized as functional because a well-defined pathobiological cause is not readily apparent. IBS is characterized by altered bowel habits, pain (typically the predominant complaint), and hypersensitivity. Accordingly, IBS patients typically exhibit significantly lower response thresholds to provocative stimuli (e.g., rectal distension), complain of increased sensitivity during normal organ function, and present increased tenderness in expanded areas of somatic (abdominal) referral (i.e., both visceral and somatic hypersensitivity). Hypersensitivity in IBS patients was first documented by Ritchie (93) and has since been extended to other functional visceral disorders (e.g., 22, 74, 92, 94). In contemporary studies, IBS patients exhibit generalized hypersensitivity, more prominent in the lower extremities and abdomen, consistent with the anatomical innervation of the distal bowel (87, 88, 109).
Functional visceral disorders are often advanced as resulting from altered central nervous system processing and/or dysregulated central modulation. However, in virtually all chronic diseases in which pain is a principal complaint, including IBS, the perceived sensation is initiated by activity in peripheral sensory (afferent) neurons. This is made most readily apparent by the simple expedient of blocking afferent input into the central nervous system. For example, infusion of local anesthetic into the rectum (87, 88, 109) rapidly relieves discomfort and pain in IBS patients, including relief of referred abdominal hypersensitivity (tenderness). Accordingly, colorectal afferents and the environment in which these receptive endings reside constitute the focus of this review.
The afferent endings of principal interest are nociceptors that are present in all tissues of the body and for which the adequate stimulus is typically potential or actual tissue damage. For hollow organs, the adequate stimulus is commonly distension, but also includes chemical mediators associated with visceral inflammation and immune-competent cells. Nociceptors are characterized by a variety of attributes (38), the most relevant of which for the present discussion is their ability to sensitize. Sensitization represents an increase in nociceptor excitability that is typically reflected in one or more of the following ways: a reduction in the threshold and an increase in the magnitude of response to a noxious intensity stimulus, acquisition of response to a previously ineffective stimulus, and/or development of spontaneous activity. Induction of sensitization requires more than an acute, noninjurious, noninflammatory stimulus; rather, longer-lasting nociceptor activation is necessary such as associated with burns and sprains, inflammation, and release or production of endogenous mediators from tissue and cells. For example, despite the absence of apparent colorectal pathobiology in IBS, nociceptor-sensitizing proinflammatory and lipotoxic lipids (50), mast cells and their products (4, 5), tryptases (104, 111), enteroendocrine cells (82) and mononuclear phagocytes and their receptors (68, 99, 113) are increased in tissues of IBS patients with colorectal hypersensitivity. This essay thus reviews 1) the classes of afferents innervating the colorectum, 2) intestinal immune cells and potential mediators of sensitization, and 3) the potential contribution of neurogenic inflammation in visceral hypersensitivity.
Afferent Innervation of the Colorectum
The distal colorectum is innervated by the hypogastric lumbar splanchnic nerve (LSN) and pelvic nerve (PN) with cell bodies in thoracolumbar and lumbosacral dorsal root ganglia (DRG), respectively. The afferent axons contained in these nerves are thinly myelinated Aδ- or unmyelinated C-fibers that subserve mechano-, chemo- and/or thermosensory functions. When studied, virtually all mechanosensitive afferents have been found also to be chemo- and/or thermosensitive (i.e., are multimodal). These nerves also contain a significant proportion of mechanically insensitive (or silent) afferents [∼25%; (32)], some of which acquire mechanosensitivity after exposure to inflammatory mediators (i.e., sensitize, see below) and others of which may be selectively chemo- or thermosensitive that remain to be studied systematically. Hollow organ distension in humans is painful and reproduces the range of sensations associated with pathological pain as well as the patterns of referred sensation (73). Accordingly, mechanical stimuli and mechanosensitive afferents have been the principal focus of experimental study. More recently, an in vitro colorectum-nerve preparation has been employed to more fully characterize receptive endings in the organ.
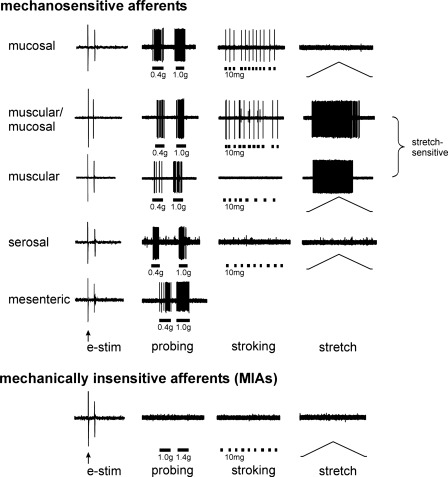
Earlier studies typically used mechanical search strategies and so found and studied only mechanosensitive endings. Recently, receptive endings in the colorectum have been localized using an unbiased electrical stimulation search strategy, thus revealing both mechanosensitive and mechanoinsensitive receptive endings (32). As displayed in Fig. 1, colorectal afferent endings localized in this way are classed based upon their responses or lack of response to three distinct mechanical stimuli: 1) punctuate probing of the receptive field using von Frey-like monofilaments, 2) circumferential stretch of the colorectum, and 3) fine stroking of the mucosal surface of the receptive field. Figure 2 illustrates differences in the topographical locations and proportions of classes of afferents in the LSN and PN innervations of the mouse colorectum; receptive fields of PN afferents are distributed throughout the distal colorectum, whereas those of LSN afferents extend further proximally and are concentrated near the mesenteric attachment. All mechanosensitive afferents respond to blunt probing of the receptive field; mucosal afferents respond also to stroking of the mucosal surface, muscular afferents also to circumferential stretch, and muscular-mucosal endings also to stroking and stretch. Afferent endings that respond only to probing and not to either stroking or stretch are classed as serosal. A fifth class of mechanosensitive ending that responds to probing is located on the mesenteric attachment (mesenteric afferents) and found only in the LSN innervation. Mechanically insensitive afferents (MIAs; or silent afferents) are present in both the LSN and PN pathways.
Fig. 1.
Functional classification of colorectal afferent endings. Afferent endings were located by electrical stimulation (e-stim; ↑, stimulus artifact). All mechanosensitive afferents respond to punctuate probing of their receptive field; muscular-mucosal afferents respond also to mucosal stroking (10 mg) and circumferential stretch (0–170 mN in 34 s), mucosal afferents respond also to stroking, and muscular afferents respond also to stretch. Serosal endings do not respond to either stroking or circumferential stretch. Mesenteric afferents have receptive fields on the mesenteric attachment and are typically not tested for responses to stroking or stretch. Muscular and muscular-mucosal afferents are often categorized together as stretch-sensitive afferents. In contrast, mechanically insensitive afferents (MIAs) do not respond to any mechanical stimuli.
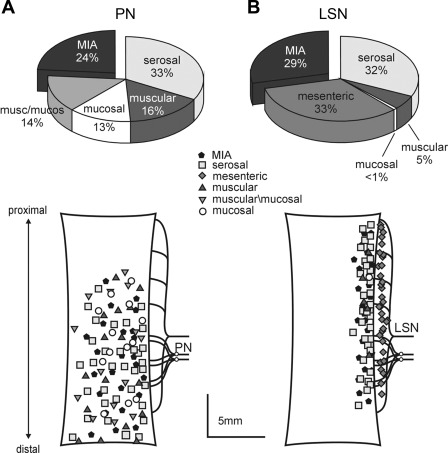
Fig. 2.
Proportions of colorectal afferent classes (top) and topographical distributions of their receptive fields (bottom) in the pelvic nerve (PN; A) and the lumbar splanchnic nerve (LSN) innervations of the mouse colorectum (B). Proportions in the PN were derived from ∼450 and in the LSN from ∼130 single fiber recordings (32, 33). For clarity, topographical distributions reflect locations of different afferent fiber classes of the same number of afferent endings (n = 100) between LS and PN innervations.
In addition to topographical differences and differences in proportions of classes of afferents between the two pathways, they differ in other ways as well. For example, mechanosensitive afferent classes in the PN have lower response thresholds, greater response magnitudes, and exhibit less adaptation compared with their LSN counterparts (14). Furthermore, PN MIAs are more likely to sensitize and acquire mechanosensitivity following brief exposure to inflammatory mediators than LSN MIAs (32). Accordingly, while LSN and PN afferents have overlapping functions, they importantly also have distinct functions. In support, a recent study demonstrated that visceromotor responses to noxious colorectal distension are unaffected after transection of the LSN pathway but absent after transection of the PN pathway (54).
Two points merit emphasis here. First, the designation of mechanoreceptive afferent classes as mucosal, muscular, etc., is not histological, but rather functional based on response characteristics to applied mechanical stimuli. Interestingly, these classes are ordered by electrical stimulation thresholds for activation, consistent with their assumed anatomical location/depth from the mucosal surface (32). Nevertheless, it should be borne in mind that mucosal or serosal endings, for example, may not be located in or innervate the colorectal mucosa or serosa. Second, histological characterization of afferent endings in the rodent colorectum has resisted elaboration to date. Unlike the vagal innervation of the rodent gastrointestinal tract, in which Powley and colleagues have elegantly described mechanosensitive intraganglionic laminar endings (IGLEs; flattened plate-like structures located in myenteric ganglia) and intramuscular arrays (IMAs; branching varicose nerve fibers running parallel with muscle bundles) (84) and chemosensitive endings (86), LSN and PN receptive endings in the colorectum have resisted a similar analysis despite several creative efforts (see Ref. 116 for review).
Sensitization of Colorectal Afferents
As noted in the introduction, sensitization represents an increase in excitability and is characteristic of nociceptors in all tissues. Processes of nociceptor activation and sensitization are typically reversible (e.g., sunburn and sprains). Most chronic pain states are associated with tissue pathology (e.g., nerve injury, arthritis, cancers, etc.), persistent afferent drive, and consequently central sensitization. More puzzling are chronic pain states where sensitization is present in the absence of apparent tissue inflammation or pathology (e.g., fibromyalgia, migraine, IBS, etc.). Despite the absence of a pathobiological explanation, afferent drive has been documented as necessary and sufficient for persistence of many of these chronic pain states (see Ref. 38 for a recent review), including colorectal afferents in IBS (idem).
Either by recording directly from afferent fibers or their somata in nodose or DRG, afferents all along the gastrointestinal tract have been widely documented to increase excitability (i.e., sensitize) after organ ulceration, inflammation, ischemia or exposure to putative mediators of sensitization (see Ref. 6 for a recent overview). The classes of afferents sensitized were not able to be identified in most of these studies. However, use of in vitro colorectal-nerve attached preparations permits direct application of putative endogenous sensitizers [e.g., protons, potassium, ATP, bradykinin (BK), 5-HT, PGE2, histamine, bile salts, etc.] to functionally identified receptive endings upon which we focus here.
Mucosal afferents.
The vast majority of colorectal mucosal afferent endings are contained in the PN pathway with none/very few in the LSN (14, 32, 47) (Fig. 2). In addition to mechanosensitivity, a significant proportion of mucosal afferents also exhibit chemosensitivity to an array of chemicals and chemical mixtures [e.g., hyperosmolar solutions, acid, bile salts, 5-HT, ATP, capsaicin, and inflammatory soup (IS), a mixture of BK, 5-HT, PGE2, histamine and protons (17)], but sensitization is not commonly found. A recent study (48) reported that colorectal mucosal afferents are sensitized to mechanical stroking by mediators from peripheral blood mononuclear cells (PBMCs) of IBS patients, but sensitization was apparent only at the maximum stroking force tested (1,000 mg), an intensity of stimulation not likely limited only to the mucosa. Given their presumed location, one might expect mucosal afferents to exhibit sensitization to chemical stimuli such as would be present in the luminal chemical environment, but this has not been documented. Accordingly, a role for mucosal afferents in colorectal sensitization and contribution to enhanced peripheral input has not been established.
Muscular and muscular-mucosal (stretch-sensitive) afferents.
Muscular and muscular-mucosal afferents are stretch-sensitive, encode colorectal distension/stretch, and are present in both the PN and LSN innervations (14, 32, 47). Muscular and muscular-mucosal afferent endings predominate in the PN pathway, comprising ∼35–45% of mechanosensitive endings; muscular-mucosal afferents are absent in the LSN pathway and muscular afferents only contribute to 6–10% of LSN mechanosensitive afferent endings (Fig. 2). Consistent with this distribution, visceromotor responses to noxious colorectal distension are absent after axotomy of the PN pathway but unaffected after axotomy of the LSN pathway (54). Thus, studies on sensitization of stretch-sensitive colorectal afferents have generally focused on the PN pathway.
Most PN colorectal stretch-sensitive afferents have low response thresholds (<5 mmHg) to colon distension/stretch, but nevertheless encode the intensity of distension/stretch well into the noxious range; a smaller proportion (∼15%–25%) of stretch-sensitive afferents have high thresholds for response (20–40 mmHg depending on the species) and also encode distension intensity in the noxious range (32, 96). That both low- and high-threshold stretch-sensitive endings encode in the noxious range (and sensitize, see below) suggests that both can contribute to colorectal nociception. High-threshold stretch-sensitive endings in the mouse are more commonly located in the colon with fewer located closer to the anus (32). It has been reported that the guinea pig rectum, but not colon, is innervated by a specialized class of low-threshold stretch-sensitive mechanoreceptors with transduction sites corresponding to rectal IGLEs (117). They apparently function mainly as rectal mechanotransducers, do not seem to sensitize and thus may not contribute to colorectal nociception.
Stretch-sensitive colorectal endings are generally sensitized acutely and for 15–20 min after exposure to IS (an example is given in Fig. 3A). Importantly, both low- and high-threshold PN stretch-sensitive afferents exhibit increased response magnitudes and reduced response thresholds (high-threshold endings) to stretch (see example in Fig. 3A). More relevant to IBS, murine muscular and muscular-mucosal PN afferents were sensitized by brief exposure to mediators released from cultured PBMCs harvested from postinfectious diarrhea-predominant IBS (D-IBS) patients (48). Interestingly, mediators released from PBMCs harvested from healthy controls inhibited responses of muscular-mucosal afferents to mechanical stimulation, but had no effect on muscular afferents. While the foregoing is important with respect to identification and characterization of mechanosensitive colorectal afferent classes that can sensitize and thus potentially contribute increased afferent input to the central nervous system, the more relevant question is which class(es) of colorectal afferents contribute long-term to colorectal hypersensitivity? A priori, one would hypothesize that stretch-sensitive afferent endings, if contributory to colorectal hypersensitivity, would exhibit long-lasting sensitization in concert with functional colorectal hypersensitivity.
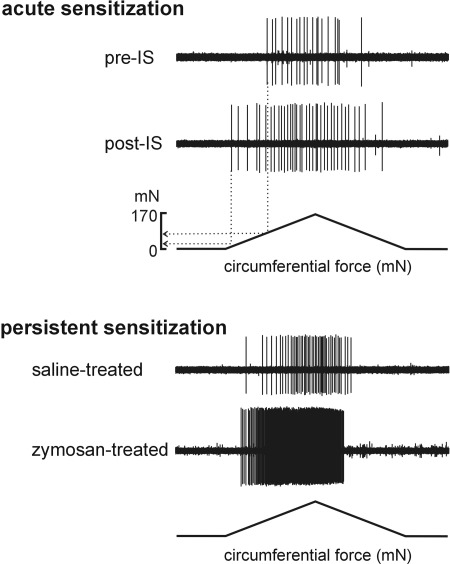
Fig. 3.
Representative single-fiber recordings illustrating acute sensitization by an inflammatory soup (IS) applied directly to the receptive ending of a stretch-sensitive muscular/muscular afferent (top) and persistent sensitization of a muscular-mucosal afferent ending 24 days after intracolonic zymosan instillation (bottom), a time at which colorectal hypersensitivity to distension was present, relative to the response of another muscular-mucosal afferent ending 24 days after intracolonic saline instillation (not hypersensitive). Note (top) that in addition to an increase in the response to stretch, response threshold was decreased from 91 mN to 31 mN after exposure to IS.
Studying long-term sensitization of colorectal afferents unavoidably relies on animal models that develop prolonged colorectal hypersensitivity. Intracolonic instillation of 2,4,6-trinitrobenzenesulfonic acid (TNBS) is commonly used to induce a transmural colon inflammation (colitis) in rodents, which, after resolution of the initial insult in rats, has been reported to be associated with weeks-long colorectal hypersensitivity (1). In a recent report (47), sensitization of LSN mesenteric and serosal afferents to mechanical probing was noted during both acute (7 days after TNBS treatment; mildly inflamed colon) and recovery (28 days; postinflammation) phases after intracolonic TNBS instillation. PN afferents in this study were generally unaffected during the acute, mild inflammatory phase but serosal afferent responses to probing were sensitized during the recovery phase after TNBS treatment. Similarly, responses of PN mucosal afferents to stroking (1,000 mg) and of muscular-mucosal afferents to stroking (1,000 mg), but not stretch, were significantly increased at day 28 relative to control and to afferent endings studied during the acute phase (day 7). Although the status of colorectal inflammation was evaluated in this study, colorectal hypersensitivity was not established and so linkage to the functional status of the colorectum cannot be made. In another study of long-lasting colorectal hypersensitivity (intracolonic zymosan instillation) in which both colorectal inflammation and hypersensitivity were evaluated, responses of PN muscular-mucosal endings to circumferential stretch were sensitized in colorectums taken from mice with established colorectal hypersensitivity (33). There was no evidence of sensitization of PN muscular endings to stretch, mucosal endings to stroking (10 mg), or serosal endings to probing (up to 1.0 g) in hypersensitive mice. It is difficult to conceive how responses to high intensity punctate probing of the colorectum (relative to stretch) translates to pain unless chemosensitivity of mucosal and/or serosal endings is the key contributor to increased excitability (see below). The foregoing suggests that stretch-sensitive, PN muscular-mucosal colorectal afferents contribute persistent afferent input that sustains, at least in part, colorectal hypersensitivity.
Other models of persistent colorectal hypersensitivity would appear to merit study to evaluate possible contributions of classes of afferents to hypersensitivity. For example, colorectal mechanical (distension) or chemical (e.g., mustard oil, acetic acid) insult in rodents early in life (postnatal days 8–21) results in colorectal hypersensitivity in adulthood (3). Increased afferent input in adult rats after neonatal insult is suggested from L6–S1 spinal dorsal root multiunit recordings of afferent activity (61), but which class(es) of colorectal afferents are involved has yet to be investigated in this model.
Serosal and mesenteric afferents.
Colorectal serosal afferents were initially defined as responsive only to punctuate probing of the receptive field and not to mucosal stroking or to stretch (14), and have been alternatively termed colonic high-threshold afferents (43). Serosal afferents in PN and LSN innervations exhibit distinct mechano- and chemosensitive characteristics, suggesting phenotypic and perhaps even structural differences (12, 14). Morphological studies of the LSN innervation of the distal colorectum failed to reveal the presence of afferent fibers in the serosa and thus the designation of serosal may be a misnomer (see Ref. 116). Instead, LSN serosal afferents were reported to have varicose branching endings on intramural blood vessels, particularly in the submucosa, a finding analogous to LSN mesenteric afferents, which have varicose endings on mesenteric blood vessels. In support, both LSN serosal and mesenteric afferents have receptive endings on or near the vasculature and can be activated by forceful compression of the vessel (14). It has been suggested that these receptive endings may sense ischemia of the colorectum as they reportedly respond to occlusion of the mesenteric arteries (44). Most LSN serosal afferents also exhibit chemosensitivity to both endogenously released (e.g., BK and ATP) as well as exogenously applied chemicals: icilin (a transient receptor potential channel melastatin 1 agoinst), capsaicin [a transient receptor potential vanilloid 1 (TRPV1) agonist], allyl isothiocyanate and trans-cinnamaldehyde (TRPA1 agonists; 12, 15, 43). LSN serosal afferents are reportedly sensitized to mechanical probing in vitro by BK (15) and desensitized by capsaicin (12).
In contrast, PN serosal afferents respond to lower intensity mechanical probing, give greater responses to probing, and exhibit less adaptation than their LSN counterparts (14). In addition, a significantly smaller proportion of PN than LSN serosal afferents respond to BK (15) or ATP (12); PN afferents showed no sensitization to probing after exposure to BK or capsaicin (12). PN serosal afferent responses to probing have been reported to be sensitized by mediators released from PBMCs harvested from IBS patients and during the recovery phase of TNBS-induced colorectal inflammation (47, 48).
MIAs.
On average, ∼25% of LSN and PN colorectal afferents are mechanically insensitive (32, 33). MIAs are characterized by their ability to acquire mechanosensitivity in inflammatory conditions (95), thus contributing significant new peripheral input to the development of hyperalgesia. Accordingly, awakening of silent nociceptors in the colorectum could contribute to persistent hypersensitivity. In support, MIAs acquire mechanosensitivity (sensitize) following exposure in vitro to IS, but, as above, MIAs in the LSN and PN pathways are different. In the colorectal PN innervation, 71% of MIAs acquired mechanosensitivity after exposure to IS, whereas only 23% of MIAs in the LSN innervation did so (32). In this in vitro experimental preparation, sensitization of MIAs was short lasting (∼20 min) and reversible, but reproducible. In a recent study of long-lasting visceral hypersensitivity, a key finding was that the proportion of PN MIAs was significantly decreased by 50% in colorectums from hypersensitive mice (24 days after intracolonic zymosan treatment) (33), consistent with the switch of some MIAs from a mechanically insensitive to sensitive phenotype. Interestingly, it was the serosal and not either of the stretch-sensitive afferent classes (muscular or muscular-mucosal) that correspondingly increased in proportion. Population studies such as these can be difficult to interpret, but because there was no increase in the proportions of afferents in colorectums from hypersensitive mice that were stretch-sensitive, the role of MIAs in persistent colorectal hypersensitivity is uncertain. We did not find sensitization of PN serosal endings to probing in this model, but did not examine possible sensitization to chemical stimuli.
Intestinal Immune Cells in IBS
Absent in the preceding discussion is consideration of potential underlying mechanisms of sensitization and hypersensitivity in IBS, which is considered a functional disorder without an underlying organic abnormality. It has been argued, however, that a low-grade inflammation and altered activity of the immune system contribute to the pain and hypersensitivity in IBS, and thus to persistent afferent drive from the colorectum. As an alimentary tract constantly exposed to foreign dietary materials and commensal microorganisms, the gut is equipped with abundant lymphoid tissues and immune cells for host defense. The gut immune system is unique in that it is designed to respond to harmful pathogens but not to food antigens or commensal bacteria. In IBS patients, however, many studies have documented changes or dysregulation of the gut immune system that could directly or indirectly influence afferent excitability.
Mast cells.
Intestinal mast cells (MC) are perhaps the most extensively studied immune cells in relation to IBS and are the cell type most consistently identified as increased in IBS. MC granules contain substances such as histamine and proteases which, when MCs degranulate, can trigger inflammatory reactions. In IBS patients, the majority of studies report an increase in the number of MCs in terminal ileum, ileocecal junction, cecum, colon, and rectum (for a review, see Ref. 79). In D-IBS patients, Park et al. (81) found more MCs degranulated and in close proximity (<2 μm) to enteric nerves in both the cecum and rectum. Barbara et al. (4) reported similar findings in descending colon biopsies from both D-IBS and constipation-predominant IBS (C-IBS) patients. In addition, they detected higher contents of mucosal histamine and tryptase in IBS patients than in healthy subjects, and a significant correlation between MCs in close proximity to nerves and the severity/frequency of abdominal pain/discomfort. In patients with ulcerative colitis in remission, who frequently report IBS-like symptoms, a similarly higher percentage of degranulated MCs were found close to nerve endings (108). MC hyperplasia or increased MC degranulation has also been noted in animal models that developed colorectal hypersensitivity after stress (41), inflammation (57), or maternal separation (107). In these animal models, the MC stabilizer doxantrazole reduced colorectal hypersensitivity to distension without affecting responses in control animals. Likewise, the MC stabilizer ketotifen increased the mechanical threshold for discomfort in hypersensitive IBS patients, but not in normosensitive patients (52).
How might MCs sensitize visceral afferents and contribute to hypersensitivity? Barbara et al. (5) collected colonic mucosal mediators by incubating biopsies from IBS patients, where the number of MCs and contents of MC mediators histamine, tryptase, and PGE2 were higher than in controls. When applied to mesenteric afferent endings or to isolated rat DRG neurons, the mediators enhanced action potential firing and increased intracellular calcium concentration, respectively. These excitatory effects of mediators on sensory neurons were inhibited by an H1 histamine receptor antagonist or a serine protease inhibitor, but not by a 5-HT3 receptor antagonist. In contrast, Cremon et al. (23) found a 10-fold increase (relative to controls) in 5-HT release in the supernatant of incubated colon biopsies from IBS patients that was correlated with MC counts. The supernatant increased action potential discharge in rat mesenteric afferents, and the response was significantly inhibited by a 5-HT3 receptor antagonist. In a behavioral assessment, visceromotor responses in rats that developed colorectal hypersensitivity after receiving the MC degranulating drug BrX-537A were attenuated by a 5-HT1A receptor antagonist but neither by a 5-HT3 receptor antagonist nor by H1-, H2-, or H3-histamine receptor antagonists (20). MC typtase is a serine protease that activates the protease-activated receptor-2, a G protein-coupled receptor expressed in sensory neurons in rodents (100). Activation of protease-activated receptor-2 releases neuropeptides such as substance P (SP) and calcitonin gene-related peptide (CGRP) from sensory nerve terminals to initiate neurogenic inflammation (see following section), and potentiates TRPV1 and TRPV4 channels, both of which have been implicated in colorectal mechanosensation and/or hypersensitivity (8).
Intestinal macrophages.
In addition to MCs, the gut harbors abundant macrophages (Mϕ) for innate immune activity. Unlike monocytes in blood and Mϕ in other tissues, intestinal macrophages (iMϕ) do not function as typical antigen-presenting cells and lack cellular machinery for production of proinflammatory cytokines (such as TNF-α, IL-1β, IL-6, IL-8, and IL-12) and induction of potent adaptive immune responses. Nevertheless, the phagocytic activity of iMϕ is very potent. In the healthy gut, iMϕ constitutively produce the anti-inflammatory cytokine IL-10 and maintain the differentiation/survival of regulatory T cells that produce another anti-inflammatory cytokine, TGF-β, which together with IL-10, keep the intestinal iMϕ in the state of anergy. When there is pathogenic invasion, however, a large number of inflammatory Mϕ are recruited to the site of invasion and produce proinflammatory mediators (for review, see Ref. 72). In inflammatory bowel diseases, such as ulcerative colitis and Crohn's disease, iMϕ are known to play a pivotal role in the inappropriate inflammatory responses against harmless commensal microorganisms. Transgenic mice deficient in IL-10 develop chronic enterocolitis if not raised under specific pathogen-free conditions (53), and this chronic colitis is attenuated by elimination of local iMϕ (112).
To evaluate the concept that IBS is due to a low-grade inflammation in the gut, Mϕ and IL-10 contents in blood or intestinal biopsies have been studied. O'Sullivan et al. (77) reported normal levels of colonic iMϕ in IBS patients in which all three subgroups of IBS (D-IBS, C-IBS, and alternating IBS) were pooled. In contrast, Spiller et al. (98) reported a 50% reduction in the number of resident mucosal iMϕ with an apparent increase in calprotectin-positive, presumably recruited iMϕ in postinfectious IBS (PI-IBS) patients, suggesting a shift toward dominance of proinflammatory Mϕ in the intestinal mucosa in this IBS group. Similarly, the change in IL-10 content in blood or intestine in IBS patients is inconsistent across studies. In cultured PBMCs harvested from IBS patients, O'Mahony et al. (76) reported a decrease in basal content of IL-10 with an increase in IL-12 content, resulting in a significantly lower IL-10:IL-12 ratio in IBS patients than in healthy subjects; the ratio was normalized by ingestion of Bifidobacterium infantis but not by Lactobacillus salivarius. Macsharry et al. (64) reported decreases in IL-10 mRNA amount in colonic and rectal mucosal biopsies from IBS patients, but no increase in IL-12 mRNA. In other studies, basal content of IL-10 did not differ between IBS patients and healthy subjects (for a review, see Ref. 80).
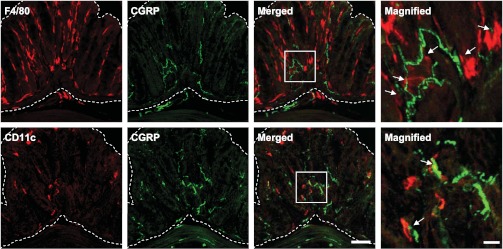
It is noteworthy that resident iMϕ are also in close proximity to CGRP-positive nerve fibers in murine colon (Fig. 4). In endometriosis, a chronic inflammatory condition related to pelvic pain and infertility in women, an increase in Mϕ is associated with a higher nerve fiber density in endometriotic regions (105) where the nerve fibers consist of autonomic and sensory Aδ- and C-fibers, suggesting that these nerve fibers can potentially be stimulated by inflammatory mediators secreted by Mϕ. This potentially pronociceptive interaction between iMϕ and nerve fibers remains to be established in IBS.
Fig. 4.
Immunolocalization of mucosal immune-competent cells and calcitonin gene-related peptide (CGRP) in the murine distal colon. F4/80-immunoreactive (IR) iMϕand CD11c-IR intestinal dendritic cells (red) were identified in close proximity to CGRP-IR nerve fibers (green) in colonic mucosa (area bordered by dashed lines). Boxed fields in the merged micrographs were magnified in the far right column. Arrows indicate mucosal immune-competent cells and CGRP-IR nerve fibers in contact or juxtaposition. Bar indicates 10 μm in the magnified micrographs and 50 μm in the others.
Cytokines and lymphocytes.
Various cytokines together with lymphocytes also have been examined in IBS patients as a measure of activities of innate and adaptive immune responses. Unlike inflammatory bowel disease patients who exhibit markedly elevated content of proinflammatory cytokines and chemokines in colon biopsies, IBS patients show no changes in the content of proinflammatory cytokines or chemokines (IL-1β, TNF-α, IL-6, CXCL-10) or even a decrease (IL-8, CCL-2, CXCL-9), suggesting a persistent deficit in the production of cytokines and chemokines important for mucosal defense (64). In contrast, the basal content of proinflammatory cytokines IL-6 and IL-8 was increased in plasma of IBS patients (24), and isolated PBMCs from IBS patients produced more proinflammatory cytokines (IL-1β, TNF-α, and IL-6) in vitro than PBMCs from healthy controls (60). Kindt et al. (51) found that stimulated lymphocyte expression of IL-5 and IL-13 was enhanced, whereas stimulated monocytic IL-12 and lymphocytic IL-10 expression was reduced in IBS, postulating a shift toward a helper T cell type 2 (Th2) cytokine profile in IBS without changes in the number of circulating immune cells. Accordingly, the content of total lymphocytes {T-lymphocytes, CD4+ T [helper T (Th)] lymphocytes and CD8+ T (cytotoxic T) lymphocytes} in the peripheral blood of IBS patients were all found to be normal (for a review, see Ref. 79).
In intestinal mucosa, intraepithelial lymphocytes were found to be increased in the colon or jejunum of D-IBS patients (19, 42) and in the rectum of PI-IBS patients (98), but were unchanged in the rectum of both PI- and non PI-IBS patients (25) or duodenum of D-IBS patients (90). Similarly, no changes in the number of lymphocytes or leukocytes were noted in cecum or colon of IBS patients (77), whereas a high number of T lymphocytes was observed in the rectal lamina propria of IBS patients (25).
It is of note that other immune-competent cells such as B lymphocytes and natural killer (NK) cells have been rarely studied in relation to IBS. Circulating B lymphocytes in IBS patients were found to express surface markers for enhanced capacity of antigen presentation with no apparent changes in their number (78). In women with IBS, activated NK cells, together with T lymphocytes, were significantly fewer than in control (71) and their postprandial decrease in circulation was attenuated (27). Significant negative correlation was observed between the number of NK cells and depression, anxiety, overall distress (71), and the postprandial level of norepinephrine (27), suggesting disturbances in psychological and neuroendocrinological interactions with the immune system in these patients.
The variability in reported content of cytokines and the numbers of lymphocytes in IBS patients should not be taken to deemphasize the modulatory effects of cytokines on sensory neurons. IL-1β sensitizes splanchnic afferents to mesenteric ischemia and histamine (34), induces firing of action potentials in pulmonary afferents (115), and increases the excitability of cultured rat DRG neurons to mechanical/thermal stimulation by activating p38 MAP kinase and relieving resting slow inactivation of tetrodotoxin-resistent Na+ channels (7). Sensitizing effects of IL-6 and IL-2 on primary afferents were also reported in knee joint (11) and hairy skin (67) of rats, respectively. Whereas the contributions of immune-competent cell products to processes of nociceptor sensitization have been established in nonvisceral tissues, that the gut harbors an enormous immune system underscores the likely importance and contribution of neural-immune interactions to persistent pain and hypersensitivity in IBS. The foregoing has focused on localized neural-immune interactions. Other literature suggests that top-down neuroendocrine contributions from the hypothalamic-pituitary-adrenal axis and/or sympathetic nervous system have the ability to modulate afferent signaling from the gut, but that is beyond the scope of this discussion (see Ref. 26 for an overview).
Neurogenic Inflammation and IBS
Inflammation is neurogenic when inflammatory neuropeptides are released from the peripheral terminals of afferent (sensory) neurons. That afferent neurons have efferent functions has been long appreciated and neurogenic inflammation is important to the pathogenesis of a number of diseases. SP and CGRP are the principal peptides released from Aδ- and C-fiber axons associated with small-diameter nodose and DRG somata (see Ref. 91 for a review). Colorectal sensory neurons include intrinsic primary afferent neurons (IPANs), whose cell bodies are located in the myenteric and/or submucosal plexi, and extrinsic primary afferent neurons (EPANs) with somata in DRG. It should be appreciated that neurogenic inflammation does not exclude a neurogenic contribution from the intrinsic nervous system of the gut (e.g., see Ref. 37), and many studies do not clearly distinguish between release of peptides from intrinsic and extrinsic innervations.
Although a previous episode(s) of or on-going, low-grade colonic inflammation has been associated with IBS, the potential contribution of neurogenic inflammation to IBS has not been systemically studied. Thus, review of the efferent functions of sensory neurons and how that might contribute to development and/or maintenance of IBS is relevant here.
SP and CGRP.
Neurogenic inflammation in the gut is characterized by arteriolar vasodilation and extravasation of plasma proteins and neutrophils. Immunohistochemical studies have documented SP- and CGRP-immunoreactivity in nerves throughout the wall of the GI tract (35). The content of SP is increased in the inflamed colon of patients with ulcerative colitis (39) and in animal models of intestinal inflammation (18, 101). SP and CGRP are frequently coexpressed in peptidergic sensory neurons and presumably coreleased from the same terminals, where SP and CGRP can exert distinct effects. For example, it is well-documented in skin that the vasodilatory action of CGRP is attenuated by SP through a mechanism thought to involve the release of proteases from MCs (10), whereas CGRP appears to prolong the action of SP by preventing its degradation (58). There are, however, relatively few studies that have examined the roles of SP and CGRP in intestinal inflammation, mostly in rodent models of colitis, and fewer still have addressed IBS. In a rat model of chronic colorectal hypersensitivity, a CGRP receptor antagonist reduced the hypersensitivity, suggesting that CGRP receptors can modulate colorectal hypersensitivity and may provide a promising target for treatment of IBS (9). Correspondingly, Wang et al. (110) reported that the expression of SP in the enteric nervous system in a rat model of IBS is abnormal, suggesting that local changes in SP may be involved in the pathogenesis of IBS and may play an important role in the regulation of gastrointestinal function. In a rat model of colitis induced by TNBS, both SP- and CGRP-immunoreactivities were significantly reduced in colorectal mucosal and muscular layers during the first week after intracolonic treatment, which recovered over the next week, suggesting reinnervation of the muscle layers (70). Furthermore, there is a dense CGRP innervation of blood vessels associated with adherent mesentery and in damaged areas of the colon. The studies mentioned above and other authors (e.g., Ref. 30) suggest that the mechanism underlying the reduction in SP and CGRP may involve cytokines that have been shown to be upregulated in colitis. In addition, colorectal inflammation is associated with a decreased coexpression of SP and CGRP in both colorectal thoracolumbar and lumbosacral DRGs, suggesting increased release of these peptides at peripheral and central endings (e.g., Ref. 106).
Many studies of neurogenic inflammation have addressed the interaction of SP with its preferred receptor, neurokinin 1 (NK1). The hypothesis that SP is a mediator of neurogenic inflammation was strengthened when it was shown that SP caused vasodilatation and plasma extravasation, which could be abolished by SP receptor (i.e., NK1) antagonists and antibodies to SP (62). The site of SP action is not restricted to the vasculature, but also includes MCs, from which histamine and other soluble mediators are released, contributing further to the local inflammatory response (46). NK1 receptors are significantly upregulated in surgical specimens from patients with inflammatory bowel diseases (65); in support, pharmacological antagonism or genetic deletion of the NK1 receptor attenuated intestinal inflammation in an animal model of enteritis (85). There is strong evidence implicating SP in inflammatory bowel disease: SP tissue content and immunoreactivity are both elevated (66), receptors for SP and its mRNA are upregulated (40), and altered NK receptor-mediated contraction occurs in inflammatory bowel disease-affected colon (69). Similarly, neutralization of SP or NK-1 receptors reduced the severity of inflammation in mouse models of experimental colitis (2, 97), whereas impaired degradation of SP in a knockout model worsened intestinal inflammation (101).
Afferents that release SP and CGRP also express the TRPV1 ion channel, which can modulate peptide release (46). Neuropeptide release may be responsible for the ascending inflammation that is associated with colitis. Increased activity in sensory neurons innervating the distal colon may facilitate an initial neuronally triggered inflammatory response that may spread to the more proximal parts of the colon through sensitization of TRPV1 in adjacent sensory neurons, which will cause an increase in CGRP and SP tissue content that maintain the inflammatory process (28). Recently, Tan et al. (103) showed in mice that 59% of TRPV1-immunoreactive DRG neurons projecting to the colorectum colocalize with CGRP and conversely that 70% of CGRP-positive neurons express TRPV1. CGRP released from these TRPV1-expressing afferents is suggested to contribute to mucosal protection and play a protective role in colonic inflammation because sensory denervation using capsaicin (TRPV1 agonist) or pretreatment with a CGRP receptor antagonist promoted acute and chronic inflammation (29, 89). Therefore, it is important to note that although studies have indicated a pivotal role for sensory neurons in proinflammatory processes of the colon, controversial data exist about the role of neurogenic inflammation in the gut.
Neurogenic inflammation and neuro-immune interactions.
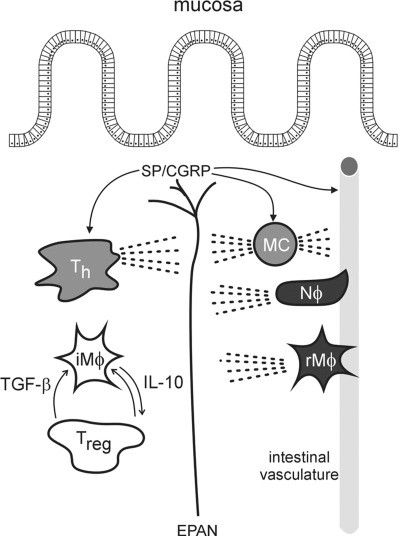
The extensive network of colorectal afferents and their proximity to intestinal immune cells reinforces the likelihood of extensive physiological crosstalk between the two. Thus, excitation of afferents can trigger inflammation by their efferent function (neurogenic inflammation), which establishes a positive feedback between afferents and the local immune system. In addition to direct effects on the vasculature and likely afferent endings, neuropeptides released near immune cells, such as dendritic cells, MCs, T cells, and B cells, reportedly contribute to inflammatory diseases (45, 83), further linking neural and immune interactions in the gut. For example, CGRP stimulates T-cell migration (102) and both CGRP and SP induce secretion of proinflammatory cytokines IFN-γ and IL-2 from Th cells. Additionally, SP exerts direct proinflammatory responses in human colonic epithelial cell lines by inducing the secretion of IL-8 (59, 118) and CGRP induces release of TNF-α from MCs (75). In further support of direct actions of neuropeptides on immune cells, Gad et al. (36) reported that either blockade of NK1 receptors or capsaicin-induced ablation of both IPANs and EPANs protected mice with severe combined immunodeficiency in an immunogenic model of chronic colitis. Figure 5 attempts to capture the interplay between the extrinsic innervation of the gut and immune-competent cells and their products.
Fig. 5.
Illustration of the relationships and interplay between the extrinsic primary afferent neuron (EPAN) innervation of the gut and immune-competent cells and their products. Neuropeptides such as substance P and CGRP are released from primary afferent endings and initiate neurogenic inflammation (arrows indicate some of the peptides' sites of action). Immune-competent cells [e.g., neutrophils (Nϕ) and recruited macrophages (rMϕ)] are recruited to the site of inflammation. Unlike anergic resident immune-competent cells [e.g., intestinal Mϕ and regulatory T cells (Treg)], the recruited cells, together with mast cells (MCs) and T helper cells (Th), secrete inflammatory mediators that act on/regulate receptors (e.g., neurokinin 1), ligand-gated ion channels (e.g., transient receptor potential channel vanilloid 1) and voltage-gated ion channels (which underly changes in neuron excitability and thus sensitization). The dashed lines are intended to indicate release of endogenous substances that can influence vascular permeability and modulate neuron excitability.
Summary
The foregoing documents potential neural and neuro-immune mechanisms of colorectal hypersensitivity. From human psychophysical evidence gathered over the past 70–80 years, distension/stretch of muscle layers constitutes the adequate stimulus for activation of colorectal nociceptors. Not inappropriately, this stimulus has been widely applied in the study of visceral nociceptive mechanisms, most profitably in in vitro organ-nerve attached preparations. The finding that hollow organs are innervated by stretch-sensitive receptive endings having low or high thresholds for response is not surprising, but that both low- and high-threshold endings encode stimulus intensity well into the noxious range (established in behavioral experiments) and, moreover, sensitize were unexpected findings. Accordingly, stretch-sensitive receptive endings innervating the colorectum have the potential to contribute to pain and hypersensitivity irrespective of response threshold. A role for mucosal, serosal, or mesenteric afferent endings remains to be established. Generally, afferent endings in these classes are unaffected by sensitizing mediators or may show a decrease in response to mechanical stimulation after exposure (see Table 1). Importantly, study of the two nerves innervating the colorectum reveal clear topological and functional differences among classes of afferent endings, suggesting that stretch sensitivity, and thus pain and hypersensitivity, are important functional features relegated to the pelvic pathway.
Table 1.
Summary of colorectal afferent activation and sensitization in mouse and rat in vitro organ-nerve preparations (afferent class)
| Mucosal | Muscular | Muscular-Mucosal | Serosal | Mesenteric | MIAs | Ref. No. | |
|---|---|---|---|---|---|---|---|
| Agonists/Mediators | |||||||
| 48/80 | U (r-LSN) | U (r-LSN) | (21) | ||||
| 5-HT | A (r-LSN) | A (r-LSN) | 21) | ||||
| AHS | ↓(m-PN) | ↓(m-PN) | A (m-PN) | AS (m-PN) | (55,56) | ||
| ATP | A (m-LSN, PN) | A (m-LSN) | (12) | ||||
| U (m-PN) | |||||||
| Bile | A (r-LSN) | U (r-LSN) | A (r-LSN) | (63) | |||
| U (m-LSN, PN) | (32) | ||||||
| BK | AS (m-LSN) | A (m-LSN) | (15) | ||||
| A (m-PN) | U (m-PN) | ||||||
| HCl | A (r-LSN) | U (r-LSN) | A (r-LSN) | (63) | |||
| IS | A (m-PN) | AS (m-PN) | AS (m-PN) | A (m-PN) | (17,49) | ||
| AS (m-LSN,PN) | (32) | ||||||
| NaCl (308 mM) | A (r-LSN) | U (r-LSN) | A (r-LSN) | (63) | |||
| PBMCs | S (m-PN) | S (m-PN) | S (m-PN) | S (m-PN) | (48) | ||
| SLIGRL | AU (m-LSN) | (13) | |||||
| TRPV1 | U (r-LSN) | U (r-LSN) | A (r-LSN) | (63) | |||
| A↓(m-LSN) | A (m-LSN) | (12) | |||||
| AU (m-PN) | U (m-PN) | ||||||
| A (m-PN) | A (m-PN) | A (m-PN) | A (m-PN) | (49) | |||
| AU (m-LSN, PN) | (32) | ||||||
| TRPA1 | S (m-PN) | S (m-LSN, PN) | (13) | ||||
| TRPV4 | S (m-PN) | S (m-LSN) | (16) | ||||
| TRPM8 | A↓(m-LSN) | A↓(m-LSN) | (43) | ||||
| IBS/IBD models | |||||||
| DSS | U (r-LSN) | U (r-LSN) | (21) | ||||
| TNBS | S (m-PN) | U (m-PN) | U (m-PN) | S (m-LSN, PN) | S (m-LSN) | (47) | |
| S (m-PN) | ↓(m-PN) | U (m-PN) | S (m-PN) | (31) | |||
| Zymosan | U (m-PN) | U (m-PN) | S (m-PN) | U (m-PN) | S (m-PN) | (33) | |
A, activated; S, sensitized; ↓ mechanical response reduced; U, unaffected; AU, activated but not sensitized; A↓, activated, response decreased; r, rat; m, mouse; PN, pelvic nerve; LSN, lumbar splanchnic nerve; 48/80, compound 48/80 that promotes mast cell degranulation; AHS, acidified hypertonic solution; BK, bradykinin; IS, inflammatory soup; TRP, transient receptor potential cation channel; TRPV1, TRP, subfamily V, member 1; TRPV4, member 4; TRPA1, subfamily A, member 1; TRPM8, subfamily M, member 8; DSS, dextran sodium sulfate; TNBS, 2,4,6-trinitrobenzenesulfonic acid; SLIGRL, protease- activated receptor-2 activating peptide; PBMCs, mediators from peripheral blood mononuclear cells.
Local release or synthesis of sensitizing mediators from immune-competent and other cells in tissue has long been advanced as contributing to functional gastrointestinal disorders and particularly IBS. Both clinical and basic science studies have moved this notion from speculative to significantly contributory. That supernatants from IBS biopsy samples sensitize colorectal afferent endings supports contributions of afferent drive to IBS symptoms. Not surprisingly, the amine, cytokine, chemokine, and other players are many and the pathways complex, but are now more tractable than previously, providing targets for improved management of IBS symptoms. Less well studied, but likely also important, are efferent contributions of primary afferents innervating the colorectum, both extrinsic and intrinsic. That NK or CGRP receptor antagonists are reported effective in some models does not address the source of these (or other) peptides, but nevertheless implicates these peptides in local inflammatory processes and thus sensitization. Neurogenic inflammatory contributions to IBS symptoms have not been widely studied and merit further examination.
Concluding Comments
The current discussion about the generator(s) of IBS pain and hypersensitivity has unfortunately become polarizing, peripherists citing evidence such as reviewed above and centralists emphasizing top-down models. From a sensory neurobiological perspective, afferent drive is necessary, though not always sufficient, for generation of the pain and persistent hypersensitivity that characterizes functional gastrointestinal disorders. Without question, affective, cognitive, experiential, neuroendocrinological, etc. influences are potent modulators of all peripheral inputs, probably more so with respect to nociceptive, and particularly enteroceptive input into the central nervous system. Accordingly, we discussed here evidence of neural and neuro-immune interactions that plausibly give rise to the necessary peripheral input contributing to IBS pain and hypersensitivity. This activated, sensitized, and/or amplified afferent input is extensively modulated and interpreted in the mind/brain with widespread somatic consequences (114).
Although many questions remain to be resolved, the evidence that colorectal afferent sensitization/input is necessary for generation of pain and hypersensitivity in IBS is wholly consistent with many other chronic pain-associated conditions (see Ref. 38). Similarly, mounting evidence strengthens the argument that IBS is a consequence of a low-grade colorectal inflammation and/or dysregulated colonic immune system.
Despite significant advances in our understanding of the functional characteristics of the afferent innervation of the colorectum, there remain many unresolved issues and questions. Key among them relates to the histological localization of functionally determined classes of colorectal afferent endings and whether they are morphologically similar or dissimilar. Stretch-sensitive afferents exhibit the property of sensitization, which in most experimental models is reversible within a short time frame. Recent studies reveal longer-lasting sensitization of colorectal afferents. Hypothetically, IBS could be a driven by sensitized afferents which, if correct, suggests that some colorectal afferents undergo a long-term, if not irreversible, change in phenotype. The foregoing discloses our ignorance about which class(es) of mechano-sensitive and/or -insensitive ending provides key afferent input with respect to IBS pain and hypersensitivity. It has long been assumed that stretch-sensitive afferents are those that provide the key input, and this is supported by the most recent literature. While MIA endings are present in the colorectal innervation and can acquire mechanosensitivity when acutely sensitized, MIAs appear not to acquire stretch sensitivity in models of persistent colorectal hypersensitivity, suggesting no or only a limited role in IBS pain and hypersensitivity. A largely unexplored, but potentially important contribution to IBS pain and hypersensitivity could arise from chemosensitive colorectal afferent endings. In conjunction with the likely contributions of products of immune-competent cells in the colorectum, the chemosensitivity of colorectal afferents should be more systematically investigated. Knowledge about the histological localization of endings, their relationship with immune-competent cells, and the anatomical and functional interaction(s) between the intrinsic and extrinsic innervations of the colorectum are essentially unknown and should be a focus of future studies. For example, studies addressing sensitization of IPANs and possible crosstalk with EPANs are virtually nonexistent. Do gila or other cells associated with IPANs elaborate mediators that sensitize EPANs or alter bowel motility in IBS? We know very little about how changes in the innate/adaptive immune components of the colorectum might reveal underlying mechanisms for prolonged sensitization of colorectal afferents. Similarly, the neurogenic inflammatory contribution to colorectal afferent sensitization and hypersensitivity remains largely unexplored. Long-term sensitization of colorectal afferents could result either from infection/inflammation in the gut or from sensitized afferent activity, which induces a localized inflammation.
GRANTS
The authors are supported by National Institutes of Health awards R01-NS-035790, R01-DK-093525, and UL1-RR-024153, and NIH Roadmap for Medical Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.F., J.H.L., E.S.S., and G.F.G. analyzed data; B.F., J.H.L., E.S.S., and G.F.G. interpreted results of experiments; B.F., J.H.L., E.S.S., and G.F.G. prepared figures; B.F., J.H.L., E.S.S., and G.F.G. drafted manuscript; B.F., J.H.L., E.S.S., and G.F.G. edited and revised manuscript; B.F., J.H.L., E.S.S., and G.F.G. approved final version of manuscript; B.F., J.H.L., E.S.S. and G.F.G. conception and design of research.
ACKNOWLEDGMENTS
We thank Michael Burcham for preparation of the figures and Tim McMurray for immunohistochemical and technical support and regret that limitations of space prevented us from citing many important contributions of others.
REFERENCES
- 1.Adam B, Liebregts T, Gschossmann JM, Krippner C, Scholl F, Ruwe M, Holtmann G. Severity of mucosal inflammation as a predictor for alterations of visceral sensory function in a rat model. Pain 123: 179–186, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Agro A, Stanisz AM. Inhibition of murine intestinal inflammation by anti-substance P antibody. Reg Immunol 5: 120–126, 1993 [PubMed] [Google Scholar]
- 3.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology 119: 1276–1285, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126: 693–702, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Barbara G, Wang B, Stanghellini V, De Giorgio R, Cremon C, Di NG, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 132: 26–37, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Beyak MJ. Visceral afferents–determinants and modulation of excitability. Auton Neurosci 153: 69–78, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1β sensors. J Neurosci 28: 14062–14073, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boesmans W, Owsianik G, Tack J, Voets T, Van den BP. TRP channels in neurogastroenterology: opportunities for therapeutic intervention. Br J Pharmacol 162: 18–37, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourdu S, Dapoigny M, Chapuy E, Artigue F, Vasson MP, De chelotte P, Bommelaer G, Eschalier A, Ardid D. Rectal instillation of butyrate provides a novel clinically relevant model of noninflammatory colonic hypersensitivity in rats. Gastroenterology 128: 1996–2008, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Brain SD, Williams TJ. Substance P regulates the vasodilator activity of calcitonin gene-related peptide. Nature 335: 73–75, 1988 [DOI] [PubMed] [Google Scholar]
- 11.Brenn D, Richter F, Schaible HG. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum 56: 351–359, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Brierley SM, Carter R, Jones W, III, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol 567: 267–281, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O'Donnell TA, Cooper NJ, Harrington AM, Adam B, Liebregts T, Holtmann G, Corey DP, Rychkov GY, Blackshaw LA. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology 137: 2084–2095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brierley SM, Jones RC, III, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127: 166–178, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Brierley SM, Jones RC, III, Xu L, Gebhart GF, Blackshaw LA. Activation of splanchnic and pelvic colonic afferents by bradykinin in mice. Neurogastroenterol Motil 17: 854–862, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, Holtmann G, Liedtke W, Blackshaw LA. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 134: 2059–2069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brumovsky PR, Feng B, Xu L, McCarthy CJ, Gebhart GF. Cystitis increases colorectal afferent sensitivity in the mouse. Am J Physiol Gastrointest Liver Physiol 297: G1250–G1258, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castagliuolo I, Keates AC, Qiu B, Kelly CP, Nikulasson S, Leeman SE, Pothoulakis C. Increased substance P responses in dorsal root ganglia and intestinal macrophages during Clostridium difficile toxin A enteritis in rats. Proc Natl Acad Sci USA 94: 4788–4793, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 122: 1778–1783, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Coelho AM, Fioramonti J, Bueno L. Mast cell degranulation induces delayed rectal allodynia in rats: role of histamine and 5-HT. Dig Dis Sci 43: 727–737, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Coldwell JR, Phillis BD, Sutherland K, Howarth GS, Blackshaw LA. Increased responsiveness of rat colonic splanchnic afferents to 5-HT after inflammation and recovery. J Physiol 579: 203–213, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Constantini M, Sturniolo CG, Zaninotto G. Altered esophageal pain threshold in irritable bowel syndrome. Dig Dis Sci 38: 206–212, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Cremon C, Carini G, Wang B, Vasina V, Cogliandro RF, De Giorgio R, Stanghellini V, Grundy D, Tonini M, De PF, Corinaldesi R, Barbara G. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol 106: 1290–1298, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Dinan TG, Clarke G, Quigley EM, Scott LV, Shanahan F, Cryan J, Cooney J, Keeling PW. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol 103: 2570–2576, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol 98: 1578–1583, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun 25: 386–394, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Elsenbruch S, Holtmann G, Oezcan D, Lysson A, Janssen O, Goebel MU, Schedlowski M. Are there alterations of neuroendocrine and cellular immune responses to nutrients in women with irritable bowel syndrome? Am J Gastroenterol 99: 703–710, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Engel MA, Khalil M, Mueller-Tribbensee SM, Becker C, Neuhuber WL, Neurath MF, Reeh PW. The proximodistal aggravation of colitis depends on substance P released from TRPV1-expressing sensory neurons. J Gastroenterol 2011 [DOI] [PubMed] [Google Scholar]
- 29.Eysselein VE, Reinshagen M, Patel A, Davis W, Nast C, Sternini C. Calcitonin gene-related peptide in inflammatory bowel disease and experimentally induced colitis. Ann NY Acad Sci 657: 319–327, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Eyssleein V, Sternini C, Cominelli F, Nast C. Putative mediators in inflammatory bowel disease: substance P and calcitonin gene-related peptide. In: Effects of Immune Cells and Inflammation on Smooth Muscle and Enteric Nerves, edited by Snape W, Collins S. Boca Raton, FL: CRC, 1991, p. 281–293 [Google Scholar]
- 31.Feng B, La JH, Tanaka T, Gebhart GF. Silent afferents and colorectal hypersensitivity. Program No 682 19/VV3 2010 Neuroscience Meeting Planner San Diego, CA: Society for Neuroscience, 2010 [Google Scholar]
- 32.Feng B, Gebhart GF. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am J Physiol Gastrointest Liver Physiol 300: G170–G180, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng B, La JH, Schwartz ES, Tanaka T, McMurray TP, Gebhart GF. Long-term sensitization of mechano-sensitive and -insensitive afferents in mice with persistent colorectal hypersensitivity. Am J Physiol Gastrointest Liver Physiol 302: G676–G683, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu LW, Longhurst JC. Interleukin-1β sensitizes abdominal visceral afferents of cats to ischaemia and histamine. J Physiol 521: 249–260, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furness J, Costa M. The Enteric Nervous System. Edinburgh, UK: Churchill-Livingston, 1987 [Google Scholar]
- 36.Gad M, Pedersen AE, Kristensen NN, Fernandez CF, Claesson MH. Blockage of the neurokinin 1 receptor and capsaicin-induced ablation of the enteric afferent nerves protect SCID mice against T-cell-induced chronic colitis. Inflamm Bowel Dis 15: 1174–1182, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Genton L, Kudsk KA. Interactions between the enteric nervous system and the immune system: role of neuropeptides and nutrition. Am J Surg 186: 253–258, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med 16: 1248–1257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldin E, Karmeli F, Selinger Z, Rachmilewitz D. Colonic substance P levels are increased in ulcerative colitis and decreased in chronic severe constipation. Dig Dis Sci 34: 754–757, 1989 [DOI] [PubMed] [Google Scholar]
- 40.Goode T, O'Connell J, Anton P, Wong H, Reeve J, O'Sullivan GC, Collins JK, Shanahan F. Neurokinin-1 receptor expression in inflammatory bowel disease: molecular quantitation and localisation. Gut 47: 387–396, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gue M, DelRio-Lacheze C, Eutamene H, Theodorou V, Fioramonti J, Bueno L. Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil 9: 271–279, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Guilarte M, Santos J, deTorres I, Alonso C, Vicario M, Ramos L, Martinez C, Casellas F, Saperas E, Malagelada JR. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut 56: 203–209, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrington AM, Hughes PA, Martin CM, Yang J, Castro J, Isaacs NJ, Blackshaw LA, Brierley SM. A novel role for TRPM8 in visceral afferent function. Pain 152: 1459–1468, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Haupt P, Janig W, Kohler W. Response pattern of visceral afferent fibres, supplying the colon, upon chemical and mechanical stimuli. Pflügers Arch 398: 41–47, 1983 [DOI] [PubMed] [Google Scholar]
- 45.Herbert MK, Holzer P. Neurogenic inflammation. II. Pathophysiology and clinical implications. Anasthesiol Intensivmed Notfallmed Schmerzther 37: 386–394, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience 24: 739–768, 1988 [DOI] [PubMed] [Google Scholar]
- 47.Hughes PA, Brierley SM, Martin CM, Brookes SJ, Linden DR, Blackshaw LA. Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut 58: 1333–1341, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Hughes PA, Brierley SM, Martin CM, Liebregts T, Persson J, Adam B, Holtmann G, Blackshaw LA. TRPV1-expressing sensory fibres and IBS: links with immune function. Gut 58: 465–466, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Jones RC, III, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci 25: 10981–10989, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kajander K, Myllyluoma E, Kyronpalo S, Rasmussen M, Sipponen P, Mattila I, Seppanen-Laakso T, Vapaatalo H, Oresic M, Korpela R. Elevated pro-inflammatory and lipotoxic mucosal lipids characterise irritable bowel syndrome. World J Gastroenterol 15: 6068–6074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kindt S, Van OL, Broekaert D, Kasran A, Ceuppens JL, Bossuyt X, Fischler B, Tack J. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil 21: 389–398, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der HS, Schemann M, Bischoff SC, van den Wijngaard RM, Boeckxstaens GE. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 59: 1213–1221, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75: 263–274, 1993 [DOI] [PubMed] [Google Scholar]
- 54.Kyloh M, Nicholas S, Zagorodnyuk VP, Brookes SJ, Spencer NJ. Identification of the visceral pain pathway activated by noxious colorectal distension in mice. Front Neurosci 5: 16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.La JH, Feng B, Brumovsky PR, Tanaka T, Gebhart GF. Intraluminal hypertonicity with mild acidity induces colorectal hypersensitivity in mice. Society for Neuroscience, 682 14/UU6 2010, San Diego, CA, 2010 [Google Scholar]
- 56.La JH, Feng B, Schwartz ES, Gebhart GF. Intracolonic hypertonicity with mild acidity sensitizes mechanically-insensitive visceral afferents and induces persistent colorectal hypersensitivity in the mouse. Society for Neuroscience, 494 07 2011, Washington, DC, 2011 [Google Scholar]
- 57.La JH, Kim TW, Sung TS, Kim HJ, Kim JY, Yang IS. Role of mucosal mast cells in visceral hypersensitivity in a rat model of irritable bowel syndrome. J Vet Sci 5: 319–324, 2004 [PubMed] [Google Scholar]
- 58.Le GP, Nyberg F, Terenius L, Hokfelt T. Calcitonin gene-related peptide is a potent inhibitor of substance P degradation. Eur J Pharmacol 115: 309–311, 1985 [DOI] [PubMed] [Google Scholar]
- 59.Levite M. Neurotransmitters activate T-cells and elicit crucial functions via neurotransmitter receptors. Curr Opin Pharmacol 8: 460–471, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Liebregts T, Adam B, Bredack C, Roth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ, Holtmann G. Immune activation in patients with irritable bowel syndrome. Gastroenterology 132: 913–920, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res 971: 73–82, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Lundberg JM, Saria A, Rosell S, Folkers K. A substance P antagonist inhibits heat-induced oedema in the rat skin. Acta Physiol Scand 120: 145–146, 1984 [DOI] [PubMed] [Google Scholar]
- 63.Lynn PA, Blackshaw LA. In vitro recordings of afferent fibres with receptive fields in the serosa, muscle and mucosa of rat colon. J Physiol 518: 271–282, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Macsharry J, O'Mahony L, Fanning A, Bairead E, Sherlock G, Tiesman J, Fulmer A, Kiely B, Dinan TG, Shanahan F, Quigley EM. Mucosal cytokine imbalance in irritable bowel syndrome. Scand J Gastroenterol 43: 1467–1476, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Mantyh CR, Gates TS, Zimmerman RP, Welton ML, Passaro EP, Jr, Vigna SR, Maggio JE, Kruger L, Mantyh PW. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc Natl Acad Sci USA 85: 3235–3239, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mantyh CR, Vigna SR, Bollinger RR, Mantyh PW, Maggio JE, Pappas TN. Differential expression of substance P receptors in patients with Crohn's disease and ulcerative colitis. Gastroenterology 109: 850–860, 1995 [DOI] [PubMed] [Google Scholar]
- 67.Martin HA, Murphy PR. Interleukin-2 activates a sub-population of cutaneous C-fibre polymodal nociceptors in the rat hairy skin. Arch Physiol Biochem 103: 136–148, 1995 [DOI] [PubMed] [Google Scholar]
- 68.McKernan D, Gaszner G, Quiqley E, Cryan J, Dinan T. Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment Pharmacol Ther 33: 1045–1052, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Menzies JR, McKee R, Corbett AD. Differential alterations in tachykinin NK2 receptors in isolated colonic circular smooth muscle in inflammatory bowel disease and idiopathic chronic constipation. Regul Pept 99: 151–156, 2001 [DOI] [PubMed] [Google Scholar]
- 70.Miampamba M, Sharkey KA. Distribution of calcitonin gene-related peptide, somatostatin, substance P and vasoactive intestinal polypeptide in experimental colitis in rats. Neurogastroenterol Motil 10: 315–329, 1998 [DOI] [PubMed] [Google Scholar]
- 71.Motzer SA, Jarrett M, Heitkemper MM, Tsuji J. Natural killer cell function and psychological distress in women with and without irritable bowel syndrome. Biol Res Nurs 4: 31–42, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Mowat AM, Bain CC. Mucosal macrophages in intestinal homeostasis and inflammation. J Innate Immun 3: 550–564, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain 41: 167–234, 1990 [DOI] [PubMed] [Google Scholar]
- 74.Ness TJ, Powell-Boone T, Cannon R, Lloyd LK, Fillingim RB. Psychophysical evidence of hypersensitivity in subjects with interstitial cystitis. J Urol 173: 1983–1987, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Niizeki H, Alard P, Streilein JW. Calcitonin gene-related peptide is necessary for ultraviolet B-impaired induction of contact hypersensitivity. J Immunol 159: 5183–5186, 1997 [PubMed] [Google Scholar]
- 76.O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O'Sullivan GC, Kiely B, Collins JK, Shanahan F, Quigley EM. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 128: 541–551, 2005 [DOI] [PubMed] [Google Scholar]
- 77.O'Sullivan M, Clayton N, Breslin NP, Harman I, Bountra C, McLaren A, O'Morain CA. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil 12: 449–457, 2000 [DOI] [PubMed] [Google Scholar]
- 78.Ohman L, Lindmark AC, Isaksson S, Posserud I, Strid H, Sjovall H, Simren M. B-cell activation in patients with irritable bowel syndrome (IBS). Neurogastroenterol Motil 21: 644–50, e27, 2009 [DOI] [PubMed] [Google Scholar]
- 79.Ortiz-Lucas M, Saz-Peiro P, Sebastian-Domingo JJ. Irritable bowel syndrome immune hypothesis. Part one: the role of lymphocytes and mast cells. Rev Esp Enferm Dig 102: 637–647, 2010 [DOI] [PubMed] [Google Scholar]
- 80.Ortiz-Lucas M, Saz-Peiro P, Sebastian-Domingo JJ. Irritable bowel syndrome immune hypothesis. Part two: the role of cytokines. Rev Esp Enferm Dig 102: 711–717, 2010 [DOI] [PubMed] [Google Scholar]
- 81.Park CH, Joo YE, Choi SK, Rew JS, Kim SJ, Lee MC. Activated mast cells infiltrate in close proximity to enteric nerves in diarrhea-predominant irritable bowel syndrome. J Korean Med Sci 18: 204–210, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park JH, Rhee PL, Kim YH, Kim JJ, Rhee JC, Song SY. Enteroendocrine cell counts correlate with visceral hypersensitivity in patients with diarrhoea-predominant irritable bowel syndrome. Neurogastroenterol Motil 18: 539–546, 2006 [DOI] [PubMed] [Google Scholar]
- 83.Peters JH, Ritter RC, Simasko SM. Leptin and CCK modulate complementary background conductances to depolarize cultured nodose neurons. Am J Physiol Cell Physiol 290: C427–C432, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Rev 34: 1–26, 2000 [DOI] [PubMed] [Google Scholar]
- 85.Pothoulakis C, Castagliuolo I, LaMont JT, Jaffer A, O'Keane JC, Snider RM, Leeman SE. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci USA 91: 947–951, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Powley TL, Spaulding RA, Haglof SA. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J Comp Neurol 519: 644–660, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Price DD, Craggs JG, Zhou O, Verne GN, Peristein WM, Robinson ME. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: evidence from human physchophysics, animal models, and neuroimaging. Neuroimage 47: 995–1001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Price DD, Zhou O, Moshiree B, Robinson ME, Verne GN. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain 7: 529–535, 2006 [DOI] [PubMed] [Google Scholar]
- 89.Reinshagen M, Flamig G, Ernst S, Geerling I, Wong H, Walsh JH, Eysselein VE, Adler G. Calcitonin gene-related peptide mediates the protective effect of sensory nerves in a model of colonic injury. J Pharmacol Exp Ther 286: 657–661, 1998 [PubMed] [Google Scholar]
- 90.Remes-Troche JM, Adames K, Castillo-Rodal AI, Ramirez T, Barreto-Zuniga R, Lopez-Vidal Y, Uscanga LF. Intraepithelial gammadelta + lymphocytes: a comparative study between celiac disease, small intestinal bacterial overgrowth, and irritable bowel syndrome. J Clin Gastroenterol 41: 671–676, 2007 [DOI] [PubMed] [Google Scholar]
- 91.Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther 302: 839–845, 2002 [DOI] [PubMed] [Google Scholar]
- 92.Richter JE, Barish CF, Castell DO. Abnormal sensory perception in patients with esophageal chest pain. Gastroenterology 91: 845–852, 1986 [DOI] [PubMed] [Google Scholar]
- 93.Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut 14: 125–132, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salet GA, Samsom M, Roelofs JM, van BergeHenegouwen GP, Smout AJ, Akkermans LM. Responses to gastric distension in functional dyspepsia. Gut 42: 823–829, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schaible HG, Schmidt RF. Time course of mechanosensitivity changes in articular afferents during a developing experimental arthritis. J Neurophysiol 60: 2180–2195, 1988 [DOI] [PubMed] [Google Scholar]
- 96.Sengupta JN, Gebhart GF. Gastrointestinal afferent fibers and sensation. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR, Alpers DH, Christensen J, Jacobson ED, Walsh JH. New York: Raven, 1994, vol. 1 [Google Scholar]
- 97.Sonea IM, Palmer MV, Akili D, Harp JA. Treatment with neurokinin-1 receptor antagonist reduces severity of inflammatory bowel disease induced by Cryptosporidium parvum. Clin Diagn Lab Immunol 9: 333–340, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in postdysenteric irritable bowel syndrome. Gut 47: 804–811, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology 136: 1979–1988, 2009 [DOI] [PubMed] [Google Scholar]
- 100.Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med 6: 151–158, 2000 [DOI] [PubMed] [Google Scholar]
- 101.Sturiale S, Barbara G, Qiu B, Figini M, Geppetti P, Gerard N, Gerard C, Grady EF, Bunnett NW, Collins SM. Neutral endopeptidase (EC 3.42411) terminates colitis by degrading substance P. Proc Natl Acad Sci USA 96: 11653–11658, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Talme T, Liu Z, Sundqvist KG. The neuropeptide calcitonin gene-related peptide (CGRP) stimulates T cell migration into collagen matrices. J Neuroimmunol 196: 60–66, 2008 [DOI] [PubMed] [Google Scholar]
- 103.Tan LL, Bornstein JC, Anderson CR. Distinct chemical classes of medium-sized transient receptor potential channel vanilloid 1-immunoreactive dorsal root ganglion neurons innervate the adult mouse jejunum and colon. Neuroscience 156: 334–343, 2008 [DOI] [PubMed] [Google Scholar]
- 104.Theoharides TC, Kempuraj D, Sant GR. Massive extracellular tryptase from activated bladder mast cells in interstitial cystitis. Urology 58: 605–606, 2001 [DOI] [PubMed] [Google Scholar]
- 105.Tran LV, Tokushige N, Berbic M, Markham R, Fraser IS. Macrophages and nerve fibres in peritoneal endometriosis. Hum Reprod 24: 835–841, 2009 [DOI] [PubMed] [Google Scholar]
- 106.Traub RJ, Hutchcroft K, Gebhart GF. The peptide content of colonic afferents decreases following colonic inflammation. Peptides 20: 267–273, 1999 [DOI] [PubMed] [Google Scholar]
- 107.van den Wijngaard RM, Klooker TK, Welting O, Stanisor OI, Wouters MM, van der CD, Bulmer DC, Peeters PJ, Aerssens J, de HR, Lee K, de Jonge WJ, Boeckxstaens GE. Essential role for TRPV1 in stress-induced (mast cell-dependent) colonic hypersensitivity in maternally separated rats. Neurogastroenterol Motil 21: 1107–1e94, 2009 [DOI] [PubMed] [Google Scholar]
- 108.van Hoboken EA, Thijssen AY, Verhaaren R, van d V, Prins FA, Verspaget HW, Masclee AA. Symptoms in patients with ulcerative colitis in remission are associated with visceral hypersensitivity and mast cell activity. Scand J Gastroenterol 46: 981–987, 2011 [DOI] [PubMed] [Google Scholar]
- 109.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyper-algesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain 105: 223–230, 2003 [DOI] [PubMed] [Google Scholar]
- 110.Wang WF, Yang YS, Peng LH, Sun G. Alternation of substance P-containing neural pathways in a rat model of irritable bowel syndrome with rectal distension. Chin J Dig Dis 7: 211–218, 2006 [DOI] [PubMed] [Google Scholar]
- 111.Wang ZY, Wang P, Bjorling DE. Role of mast cells and protease-activated receptor-2 in cyclooxygenase-2 expression in urothelial cells. Am J Physiol Regul Integr Comp Physiol 297: R1127–R1135, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Watanabe N, Ikuta K, Okazaki K, Nakase H, Tabata Y, Matsuura M, Tamaki H, Kawanami C, Honjo T, Chiba T. Elimination of local macrophages in intestine prevents chronic colitis in interleukin-10-deficient mice. Dig Dis Sci 48: 408–414, 2003 [DOI] [PubMed] [Google Scholar]
- 113.Weber B, Saurer L, Mueller C. Intestinal macrophages: differentiaton and involvement in intestinal immunopathologies. Semin Immunopathol 31: 171–184, 2009 [DOI] [PubMed] [Google Scholar]
- 114.Wilder-Smith CH. The balancing act: endogenous modulation of pain in functional gastrointestinal disorders. Gut 60: 1589–1599, 2011 [DOI] [PubMed] [Google Scholar]
- 115.Yu J, Lin S, Zhang J, Otmishi P, Guardiola JJ. Airway nociceptors activated by pro-inflammatory cytokines. Respir Physiol Neurobiol 156: 116–119, 2007 [DOI] [PubMed] [Google Scholar]
- 116.Zagorodnyuk VP, Brookes SJ, Spencer NJ. Structure-function relationship of sensory endings in the gut and bladder. Auton Neurosci 153: 3–11, 2010 [DOI] [PubMed] [Google Scholar]
- 117.Zagorodnyuk VP, Lynn P, Costa M, Brookes SJ. Mechanisms of mechanotransduction by specialized low-threshold mechanoreceptors in the guinea pig rectum. Am J Physiol Gastrointest Liver Physiol 289: G397–G406, 2005 [DOI] [PubMed] [Google Scholar]
- 118.Zhao D, Kuhnt-Moore S, Zeng H, Pan A, Wu JS, Simeonidis S, Moyer MP, Pothoulakis C. Substance P-stimulated interleukin-8 expression in human colonic epithelial cells involves Rho family small GTPases. Biochem J 368: 665–672, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]