Abstract
Inflammation contributes to liver injury in acetaminophen (APAP) hepatotoxicity in mice and is triggered by stimulation of immune cells. The purinergic receptor P2X7 is upstream of the nod-like receptor family, pryin domain containing-3 (NLRP3) inflammasome in immune cells and is activated by ATP and NAD that serve as damage-associated molecular patterns. APAP hepatotoxicity was assessed in mice genetically deficient in P2X7, the key inflammatory receptor for nucleotides (P2X7−/−), and in wild-type mice. P2X7−/− mice had significantly decreased APAP-induced liver necrosis. In addition, APAP-poisoned mice were treated with the specific P2X7 antagonist A438079 or etheno-NAD, a competitive antagonist of NAD. Pre- or posttreatment with A438079 significantly decreased APAP-induced necrosis and hemorrhage in APAP liver injury in wild-type but not P2X7−/− mice. Pretreatment with etheno-NAD also significantly decreased APAP-induced necrosis and hemorrhage in APAP liver injury. In addition, APAP toxicity in mice lacking the plasma membrane ecto-NTPDase CD39 (CD39−/−) that metabolizes ATP was examined in parallel with the use of soluble apyrase to deplete extracellular ATP in wild-type mice. CD39−/− mice had increased APAP-induced hemorrhage and mortality, whereas apyrase also decreased APAP-induced mortality. Kupffer cells were treated with extracellular ATP to assess P2X7-dependent inflammasome activation. P2X7 was required for ATP-stimulated IL-1β release. In conclusion, P2X7 and exposure to the ligands ATP and NAD are required for manifestations of APAP-induced hepatotoxicity.
Keywords: CD39; nod-like receptor family, pryin domain containing-3, caspase-1; inflammasome; damage-associated molecular pattern
acetaminophen (APAP) overdose is the most common cause of acute liver failure in the Unites States and Europe (15, 18, 24). Furthermore, cases of acute hepatic failure due to APAP continue to rise (24). Toxicity is initiated by metabolism of APAP via reductive pathways to reactive metabolites. An antidote exists for patients early in the course of poisoning during this metabolism phase. However, with increased delays in administration of therapy, the frequency with which patients develop hepatocellular injury worsens.
The initial, direct toxic injury induces an area of necrosis in the centrilobular regions. Liver injury is propagated by an inflammatory response after the drug has been metabolized and the initial centrilobular injury has occurred (23). The nature of this inflammatory response has been the subject of significant investigation in the past several years.
Prior research by our group indicates that inflammasome activation is a crucial initiating step in the propagation of APAP-induced hepatic injury (10). Proinflammatory cytokines IL-1β and IL-18 are thought to be crucial to the propagation of inflammation during APAP-induced hepatotoxicity (10). Production of these cytokines is dependent on cleavage by caspase-1, which is, in turn, dependent on the assembly of the inflammasome, a protein scaffold that assembles in response to a variety of danger signals or as damage-associated molecular patterns (DAMPs) (12). This results in the activation of caspase-1 and cleavage of pro-IL-1β and pro-IL-18 to IL-1β and IL-18.
It is unclear what signals the assembly of the inflammasome following APAP-induced hepatotoxicity. It is known that P2X7 receptor stimulation by extracellular ATP leads to inflammasome activation by its actions stimulating potassium efflux from cells (14). Recently, it was shown that the inflammatory response after thermal necrotic hepatocyte death was triggered by an ATP-driven P2X7 receptor-dependent pathway, and the same pathway has been shown to be important in bleomycin-induced pulmonary inflammation (25, 28). ATP is released from dying cells as well as inflammatory cells (3). In turn, extracellular ATP concentrations are tightly regulated by ubiquitous ectonucleotidases, principally CD39 (29).
The role of purinergic signaling mechanisms in APAP-induced hepatotoxicity is not known and has therapeutic implications as active targets for anti-inflammatory drug development that would include receptor agonists/antagonists or inducing or inhibiting the hydrolysis of ATP and other ligands. In an effort to investigate the role of purinergic mechanisms, we investigated the role of the P2X7 receptor in a murine model of APAP hepatotoxicity in mice genetically deficient in P2X7 or treated with a specific P2X7 antagonist. We investigated the effect of ATP and NAD using apyrase (soluble CD39) and by using an irreversible competitive NAD antagonist. Furthermore, mice genetically deficient in endogenous apyrase (CD39), with reduced ability to hydrolyze ATP and NAD, were also examined to study the effects of the master regulatory mechanism involved in purinergic signaling.
Finally, liver Kupffer cells were isolated and investigated for P2X7 dependence of inflammasome activation in response to ATP with priming by LPS. Collectively, our data show that ATP- and NAD-mediated activation of the P2X7 receptor is an integral part of the liver injury induced by APAP. This mechanism is modulated by the activity of CD39. Purinergic signaling, therefore, provides attractive targets for therapeutic intervention in APAP toxicity.
MATERIALS AND METHODS
Animals.
C57BL/6 male mice 5 to 8 wk of age were purchased from the National Cancer Institute or Taconic laboratories. P2X7−/−, and CD39−/− mice have been described (8, 32). All experiments and animal handling were performed under approved protocols at the Yale University and Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committees.
APAP-induced hepatotoxicity.
APAP (Sigma-Aldrich, St. Louis, MO) solution was prepared as described (10). APAP was dosed at 500 mg/kg and administered by intraperitoneal injection after 15 h of starvation. Animals were euthanized by isoflurane or ketamine/xylazine at 6 and 12 h for collection of serum, isolation of liver lymphocytes, or collection of liver tissue for histology, or they were observed every 4 h for 72 h until they became moribund.
Treatment with apyrase, etheno-NAD, and A438079.
Mice were treated by intraperitoneal injection with potato apyrase (Sigma-Aldrich) at 4 U/mouse 30 min prior and 6 h after APAP injection, etheno-NAD (Sigma-Aldrich) at 2 mg/mouse 1 h prior and 6 h after APAP injection, and A438079 (Tocris, Ellisville, MO) at 2 mg/mouse either 1 h prior or 2 h after APAP injection.
Liver histology scoring.
Liver histology was scored in a blinded manner in hematoxylin and eosin-stained, paraffin-embedded sections. Necrosis was scored from 0 to 3 when present in 0, 0–25%, 25–50%, or > 50% of the field, respectively. Hemorrhage was scored from 0 to 3 when present in 0, 0–5%, 5–20%, or > 20% of the field, respectively. At least five fields per section were examined under ×4 magnification and data are expressed as mean scores per experimental group.
Quantitation of liver-infiltrating neutrophils.
Neutrophil quantitation was performed in paraffin-embedded liver sections after immunolabeling with GR-1 monoclonal antibody (BD Biosciences, San Jose, CA) by scoring for positive cells in five high-power fields (×40). To confirm our results for neutrophil immunostaining, we immunolabeled liver sections for another neutrophil-specific epitope using Ly-6B.2 monoclonal antibody (AbD Serotec, Raleigh, NC). Imaging results represent Ly-6B.2 immunostained images.
Serum ALTs.
Serum was isolated from mice and alanine aminotransferase (ALT) levels were determined in the Yale New Haven Hospital clinical chemistry laboratory.
Quantitation of CYP 2E1 expression and APAP adducts.
Western blots of liver lysates were immunostained with rabbit anti-mouse IgG for CYP 2E1 (Abcam, Cambridge, MA), rabbit anti-APAP adduct IgG (gift of Lance Pohl, National Heart, Lung, and Blood Institute, Bethesda, MD), or rabbit anti-mouse IgG for b-actin (Abcam). The secondary antibody for immunodetection was goat anti-rabbit IgG horseradish peroxidase conjugate (Santa Cruz Biotechnology, Santa Cruz, CA). Immunodetection was performed with SuperSignal West Pico Chemiluminiscent Substrate (Thermo Scientific, Logan, UT). Densitometry of the predicted bands was determined using a Foto/Analyst Investigator digital imager (Fotodyne, Hartland, WI) and PC Imager software. The ratio of CYP2E1 and APAP adduct bands to β-actin bands was determined and normalized to the value of untreated wild-type animal liver run on the same Western blot analysis, which was set to one.
Caspase-1 activity assay.
Snap-frozen liver tissue stored in liquid nitrogen was homogenized with a rotor/stator homogenizer in cell lysis buffer, and 300 mg of liver protein was then incubated in a 96-well microtiter dish for 1 h at 37°C with the fluorescent caspase-1 substrate YVAD-AFC as per the supplier (Biovision, Mountain View, CA). Change in fluorescence at 505 nm after excitation at 400 nm was then determined with a Biotek Synergy fluorescent plate reader (Biotek, Winooski, VT). Values were normalized to blank samples containing assay buffer, YVAD-AFC substrate, and no liver protein, and expressed as fold change from untreated wild-type liver lysate run in the same experiment.
Kupffer cell isolation and treatment.
Liver nonparenchymal cells were isolated as previously described with the following modifications (4). Mouse nonparenchymal cells were resuspended in 13% Optiprep (Axis Shield, Norton, MA) in HBSS. This was layered over 18% Optiprep in HBSS and then overlayed with HBSS. This was centrifuged at 1,400 g for 20 min at 4°C. The top layer and top interface were then recovered and plated on 24-well polystyrene dishes at 300,000 cells/well in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and gentamicin 50 μg/ml. One to two hours after plating, nonadherent cells were removed and adherent cells were assessed for cell surface expression of CD45 and F4/80 cell surface markers by fluorescence activated cell sorting using mouse anti-mouse CD45.2 phycoerythrin-conjugated antibody (BD Biosciences) and rat anti-mouse F4/80 allophycocyanin-conjugated antibody (eBiosciences, San Diego, CA). This data is shown in Fig. 5B. Cells were incubated overnight and then treated with LPS (Sigma-Aldrich) at 100 ng/ml for 6 h and then ATP (Sigma-Aldrich) at 5 mM for 20 min. Supernatant was collected, and cell lysate prepared in Cell Lysis Buffer (Cell Signaling, Danvers, MA) with Complete Protease Inhibitor (Roche, Mannheim, Germany).
Fig. 5.
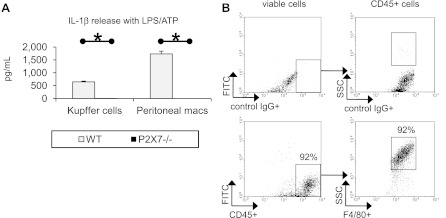
Kupffer cells require P2X7 to activate the nod-like receptor family, pryin domain containing-3 (NLRP3) inflammasome in response to extracellular ATP. Kupffer cells and thioglycollate-elicited peritoneal macrophages were isolated from WT and P2X7−/− mice and treated with LPS (100 ng/ml) and ATP (5 mM). Supernatant was assessed for IL-1β release (A). Adherent cells from the mouse liver nonparenchymal cell isolation were assessed for CD45.2 and F4/80 or control IgG positivity with representative results shown (n = 4) (B). *P < 0.05.
ELISA for IL-1β release.
High-binding, 96-well ELISA plates (BD Biosciences) were used per manufacturer instructions. The capture antibody was anti-mouse IL-1β (R&D Systems, Minneapolis, MN). 100 μl of Kupffer culture supernatant was added to each well and incubated overnight at 4°C. The detection antibody, polyclonal biotinylated anti-mouse IL-1β antibody (R&D Systems), was then added for 2 h at room temperature.
Statistics.
Kaplan-Meier plots and statistical analysis were performed using Microsoft Excel 2007 and MedCalc software version 9.2.0.1. Unpaired two-tailed Student's t-test was used to compare groups. A P value of < 0.05 was considered significant.
RESULTS
P2X7 is required for maximum liver injury in APAP hepatotoxicity.
Genetic deletion of P2X7 significantly reduces the liver necrosis score (1.3 ± 0.2 vs. 2.4 ± 0.3) and neutrophil count in the liver (9.9 ± 1.1 vs. 17.0 ± 1.0) relative to wild-type animals at 12 h post-APAP treatment (Fig. 1, A–B and D–E, respectively). Deletion of P2X7 also significantly decreases serum ALT values at 6 h post-APAP treatment (265 ± 53 vs. 3,526 ± 1,272) as shown in Fig. 1C.
Fig. 1.
Genetic deficiency of P2X7 decreases hepatic necrosis and inflammation in acetaminophen (APAP)-induced acute liver injury. Wild-type (WT; n = 6) and P2X7−/− mice (n = 7) were administered APAP at 500 mg/kg ip for 6 and 12 h. WT (n = 2) and P2X7−/− mice (n = 2) were also given PBS as control groups. Liver histology was assessed 12 h at posttreatment (A and B), serum ALTs at 6 h posttreatment (C), and neutrophils in the liver at 12 h posttreatment (D and E) . NS, not significant; *P < 0.05. Arrows point to neutrophils.
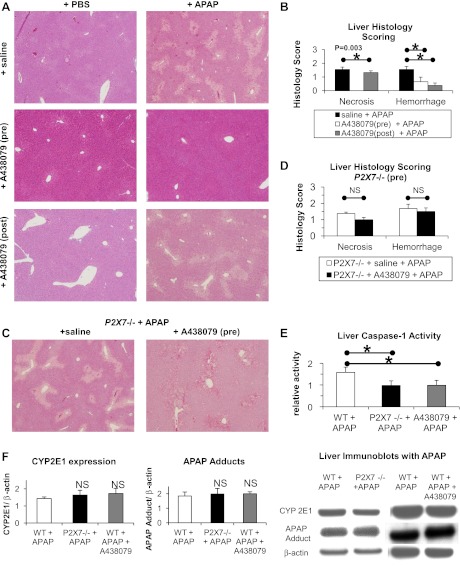
Pretreatment with a P2X7 antagonist A438079 before APAP prevented liver necrosis (0.0 ± 0.0 vs. 1.6 ± 0.2), significantly reduced hemorrhage (0.7 ± 0.3 vs. 1.6 ± 0.2), significantly reduced neutrophil count in the liver (9.9 ± 1.1 vs. 17.0 ± 1.0), and significantly reduced serum ALT values (310 ± 358 vs. 3,526 ± 1,272) as shown in Figs. 2, A–B and 3, D–E, respectively. Posttreatment with the P2X7 antagonist A438079 after APAP significantly reduced liver hemorrhage (0.4 ± 0.2 vs. 1.7 ± 0.4) as shown in Fig. 2, A and B but did not significantly alter liver necrosis (Fig. 2, A and B) or serum ALT values at 12 h posttreatment (6,200 ± 923 vs. 5,117 ± 1,117). A438079-dependent reductions in APAP liver injury required P2X7 as pretreatment with A438079 did not significantly change liver injury in P2X7−/− animals (Figs. 2, C and D).
Fig. 2.
Pre- and posttreatment with the antagonist A438079 prevents liver injury and inflammation in APAP-induced acute liver injury in a P2X7-dependent manner. WT mice were administered saline vehicle (n = 8) or A438079 at 300 mM/kg ip (n = 4) 1 h before or 2 h after (n = 5) administration of APAP at 500 mg/kg ip. P2X7−/− mice were administered saline vehicle (n = 5) or A438079 at 300 mM/kg ip (n = 5) 1 h before administration of APAP at 500 mg/kg ip. In control experiments, WT mice and P2X7−/− mice were also administered saline (n = 2 per group) or A438079 (n = 2 per group) 1 h prior to PBS vehicle. At 12 h post-APAP treatment, liver histology was assessed in WT mice (A and B) and P2X7−/− mice (C and D). Liver caspase-1 activity (E), liver CYP2E1 expression (F), and liver APAP adducts (F) were measured at 6 h post-APAP in WT mice (n = 5), P2X7−/− mice (n = 7), and WT pretreated with A438079 as above (n = 4). *P < 0.05.
Fig. 3.
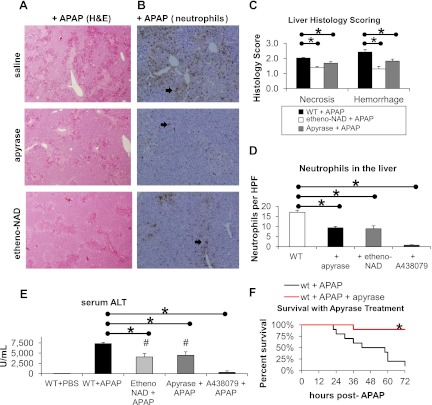
Treatment with apyrase or etheno-NAD decreases liver injury and inflammation in APAP-induced acute liver injury and improves survival. Saline vehicle (n = 5) or etheno-NAD at 2 mg per mouse (n = 6) was administered at 1 h before and 6 h after administration of APAP at 500 mg/kg ip. Apyrase at 4 units ip per mouse (n = 6) was administered 30 min before and 1 h after APAP. A438079 at 300 mM/kg ip (n = 4) was administered 1 h before APAP. At 12 h post-APAP treatment, liver histology (A and C), neutrophils in the liver (B and D), and serum ALT (E) were assessed. Survival was assessed to 72 h in WT mice administered apyrase (n = 10) or saline (n = 10) as above with APAP (F). H&E, hematoxylin and eosin; HPF, ×40 magnification high-powered field. *P < 0.05 between bracketed groups. #P < 0.05 compared with WT mice given APAP. Arrows point to neutrophils.
Genetic deficiency of P2X7 or pretreatment of wild-type animals with the P2X7 antagonist A438079 significantly decreased liver caspase-1 activity relative to wild-type animals given APAP (1.0 ± 0.2 and 1.0 ± 0.2 vs. 1.6 ± 0.2) as shown in Fig. 2E. Genetic deficiency of P2X7 or pretreatment of wild-type animals with the P2X7 antagonist A438079 did not significantly change liver CYP2E1 expression or APAP adduct formation 6 h post-APAP treatment compared with wild-type animals given APAP (Fig. 2F).
Extracellular ATP and NAD promote liver injury in APAP hepatotoxicity.
Treatment with apyrase or etheno-NAD significantly reduced liver necrosis (1.7 ± 0.1 and 1.4 ± 0.1 vs. 2.0 ± 0.0) and liver hemorrhage (1.8 ± 0.1 and 1.3 ± 0.2 vs. 2.4 ± 0.2) in APAP liver injury relative to saline vehicle-treated animals shown in Fig. 3, A and C. Treatment with apyrase or etheno-NAD significantly reduced neutrophil count in the liver (8.9 ± 1.4 and 9.3 ± 0.7 vs. 17.0 ± 1.0) as shown in Fig. 3D. Serum ALTs were significantly reduced with pretreatment with etheno-NAD or apyrase (4,083 ± 830 and 4,480 ± 851 vs. 7,320 ± 313) shown in Fig. 3E. Treatment with apyrase also significantly decreased mortality at 72 h post-APAP treatment relative to saline vehicle (1/10 vs. 9/10 expired animals) shown in Fig. 3F.
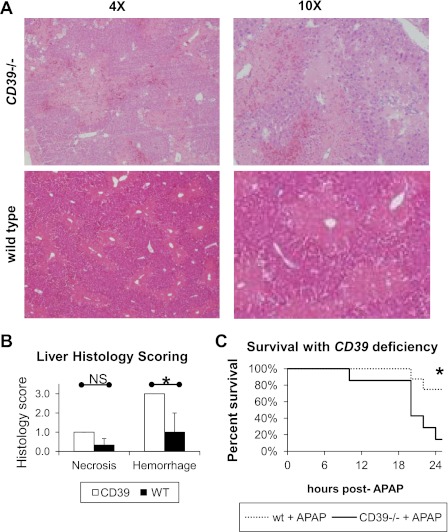
Genetic deletion of CD39 is expected to increase local concentrations of extracellular ATP and NAD at sites of injury and resulted in significantly increased liver hemorrhage (1.0 ± 1.0 and 3.0 ± 0.0) at 12 h post-APAP treatment shown in Fig. 4, A and B. Genetic deletion of CD39 also significantly increased mortality at 24 h post-APAP treatment relative to saline vehicle (6/8 vs. 1/7 expired animals) as shown in Fig. 4C.
Fig. 4.
Genetic deficiency of CD39 accelerates hemorrhage and increases mortality in APAP hepatotoxicity. WT (n = 6) and CD39−/− mice (n = 6) were administered APAP at 200 mg/kg ip. Liver histology was assessed at 12 h post-APAP treatment (A and B). Survival was assessed to 72 h in WT (n = 8) and CD39−/− mice (n = 8) administered APAP at 500 mg/kg ip (C). *P < 0.05.
P2X7 is required for IL-1β release in Kupffer cells.
Liver Kupffer cells were isolated from wild-type and P2X7−/− mice. Treatment with LPS and ATP-induced caspase-1 proteolytic activation and IL-1β release in wild-type but not P2X7−/− Kupffer cells (Fig. 5). In parallel experiments, thioglycollate-elicited peritoneal macrophages obtained from wild-type but P2X7−/− mice could be induced to release IL-1β in response to LPS and ATP. The Kupffer cell isolation was >92% positive for the macrophage marker F4/80 as determined by flow cytometric analysis.
DISCUSSION
Liver injury following APAP overdose is initiated by the metabolism of excess amounts of APAP. A portion of ingested APAP undergoes reductive metabolism, primarily via CYP2E1 to produce the electrophilic reactive metabolite, n-amino para-benzoquinone imine, which binds intracellular proteins resulting in mitochondrial failure (36). However, APAP metabolites are only found in the centrilobular areas of the liver following acute poisoning. As the time course of liver injury following APAP demonstrates, worsening liver injury and panlobular necrosis 8–16 h after the completion of APAP metabolism in mice and humans, this supports a second mechanism in propagating liver injury (16).
Propagation of hepatocellular injury occurs through activation of the innate immune system and induction of inflammatory injury, although the contribution of this pathway is debated (5, 10, 11, 13, 20–22, 34). Necrotic cells or cells undergoing apoptosis release intracellular contents, which have been demonstrated to induce sterile inflammation in a variety of models of tissue injury (27). A key example is DNA from apoptotic cells, which can bind TOLL-like receptor 9 (TLR9) (7, 17). In the setting of APAP-induced injury, Imaeda et al. (10) identified TLR9 activation by assaying IL-1b in mice deficient in Tlr9, finding that APAP as well as DNA from apoptotic mammalian cells, increased pro-IL-1b transcript in the wild-type animals in a TLR9-dependent manner, and more importantly demonstrating that Tlr9 null animals were protected from hepatotoxicity. They further studied animals deficient in the components of the NRLP3 inflammasome required to activate caspase-1 and cleave pro-IL-1b to form IL-1b. They found that deficiency of nod-like receptor family, pryin domain containing-3 (Nlrp3) and caspase-1 reduced liver injury and mortality without effecting pro-IL-1b transcript levels, demonstrating that posttranscriptional processing of procytokines, is also a key regulated determinant of innate immune injury in APAP.
Intrahepatic release of DAMPs has been demonstrated after APAP toxicity, and apoptotic and secondary necrotic cells following the initial APAP-induced injury release DNA, which activates TLR9 to transcribe pro-IL-1b and pro-IL-18 (23). These procytokines are cleaved by caspase-1 activated by the NLRP3 inflammasome. In turn, mature IL-1b and IL-18 upregulate innate immune responses in local immune cells (23, 33). Specifically, Kupffer cells (the resident macrophages of the liver) can propagate the hepatic innate inflammatory response through the production of proinflammatory TNF-α (19, 20). Subsequently there is a migration of neutrophils into the liver, and the ensuing cytotoxic actions are thought to cause further cause hepatocellular damage (21).
The link between the initial necrosis in APAP injury and upregulation of inflammasome activity has not, to date, been elucidated. Activation of the NLRP3 inflammasome can be triggered by extracellular ATP, a ligand for the purinergic receptor P2X7. As such, we investigated purinergic mechanisms, specifically ATP and NAD activation of the P2X7 receptor, in the setting of necrotic cell death induced by APAP. There is increasing evidence that P2X7 mediates sterile inflammation in many organs, including the lung (28) and the pancreas (9). Despite this accepted role of the P2X7 receptor in the sterile inflammatory response, there are important organ-specific differences. In the lung, inhibition of P2X7 receptor function results in a very significant reduction in inflammation and injury, but this occurs to a lesser degree in the pancreas. Of note, the contribution of P2X7 activation to sterile inflammation in the liver was previously unknown.
Using genetically deficient animals, receptor antagonists, and enzymatic depletion, we have demonstrated an important role for purinergic signaling methods in APAP liver injury and inflammation. P2X7 was required for full injury in APAP hepatotoxicity (Figs. 1B and 2B). This P2X7-dependent effect did not appear to be through regulation of CYP2E1 expression or alteration of APAP metabolism and APAP adduct formation (Fig. 2F). P2X7 was required for innate immune response to APAP hepatotoxicity, specifically hepatic caspase-1 activation (Fig. 2E) and neutrophil migration into the liver (Figs. 1E and 3D). In addition to this central finding, our work supports the use of P2X7 specific antagonists in liver injury after APAP, and may reduce the incidence of APAP-induced acute liver failure (Figs. 2B and 3E).
It is of interest that the P2X7 receptor antagonist A438079 reduced APAP liver injury to a greater degree than seen in the P2X7-deficient mouse (Figs. 1 and 2). To confirm that this finding was not related to off-target effects of the antagonist, we demonstrated that A438079 did not significantly alter APAP liver injury in P2X7-deficient mice (Fig. 2, C and D). The reasons for these differences are therefore likely attributable to developmental adaptations of the immune system in the P2X7 receptor deficient mice, with upregulation of non-P2X7 receptor-dependent pathways.
Regarding the endogenous ligands for P2X7, NAD and ATP were first identified as DAMPs released by injured erythrocytes (30). ATP was later identified as a DAMP-type ligand for P2X7 released from extracellular mitochondria of necrotic cell origin (12). Extracellular NAD is a much more potent ligand for P2X7 receptor activation, presumably due to membrane localization through NAD-ribosylation (31). We provide evidence that endogenous ATP and NAD likely released from injured hepatocytes in APAP toxicity are required for hepatocyte necrosis and sinusoidal injury with intrahepatic hemorrhage. Specifically, depletion of extracellular ATP with apyrase and competitive antagonism of NAD-ribosylation sites with etheno-NAD both resulted in decreased liver necrosis and hemorrhage (Fig. 3, C and E). Additionally, apyrase and etheno-NAD also reduced neutrophil migration into the liver to a significant but lesser degree than use of a P2X7 antagonist (Fig. 3D). Finally, apyrase decreased mortality from APAP-induced acute liver failure (Fig. 3F). To further confirm that endogenous ATP and NAD are key determinants of sterile liver injury and mortality in APAP hepatotoxicity, we demonstrated that genetic deletion of CD39, the predominant ecto-NTPDase in the metabolism of extracellular ATP and NAD, enhanced liver hemorrhage and increased mortality in this model (Fig. 4, B and C). Several cell types in the liver are known to express P2X7 and CD39. Liver sinusoidal endothelial cells and Kupffer cell subpopulations express P2X7, but function was not confirmed in these studies (1, 2, 35). Liver sinusoidal endothelial cells and Kupffer cell also express CD39 (1, 2). The role of Kupffer cells in promoting liver injury in APAP hepatotoxicity was demonstrated in macrophage depletion experiments employing gadolinium chloride (26). Additionally, selective deletion of macrophages employing diphtheria toxin and the CD11b-DTR transgene implicated macrophages in the progression of toxin-mediated liver injury (6). We now show that Kupffer cells respond to extracellular ATP in a P2X7-dependent manner and through activation of the NLRP3 inflammasome with IL-1β release (Fig. 5). It is possible that functional P2X7 on hepatocytes or sinusoidal endothelial cells may also mediate APAP hepatotoxicity through recognition of ATP and NAD and P2X7-mediated cell permeabilization and cell death.
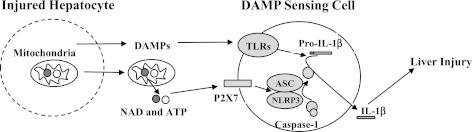
Inflammation promotes liver injury in APAP hepatotoxicity, mediated at least in part through DAMP-mediated innate immune signaling. DAMP receptors previously implicated in APAP hepatotoxicity include TLR4 and TLR9, which recognize the DAMPs high mobility group box-1 and self DNA, respectively (1, 12, 39). Through activation of the NLRP3 inflammasome, DAMP-mediated activation of P2X7 provides a link between TOLL-like receptor mediated transcriptional induction of proinflammatory cytokines, specifically pro-IL-1b and pro-IL-18, and cytokine maturation and secretion (Fig. 6).
Fig. 6.
Injured hepatocytes release ATP and NAD, which signal NLRP3 inflammasome activation through P2X7. DAMP, damage-associated molecular patterns; TLR, TOLL-like receptor.
In summary, we have identified P2X7 and extracellular ATP and NAD as significant contributors to liver injury, inflammation, and mortality, in APAP hepatotoxicity. We have also shown that Kupffer cells have functional P2X7 and may thereby mediate these effects through activation of the NLRP3 inflammasome consistent with the paradigm of DAMP mediated sterile inflammation.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-076674-01A2 and a Veterans Affairs Merit Grant (to W. A. Mehal), National Institute of Diabetes and Digestive and Kidney Diseases Grants T32-DK-7356 and K08DK092281-01 (to R. Hoque), National Heart, Lung, and Blood Institute Grants PO1-HL-076540 and RO1-HL-094400 (to S. C. Robson), a Miles and Eleanor Shores Award, Harvard Medical School, and the Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, MA (to S. Salhanick). This work was also supported by a National Institutes of Health Grant R37 DK25636 to the Yale Liver Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.H., A.M.S., S.S., S.C.R., and W.Z.M. conception and design of research; R.H., A.M.S., S.S., A.F.M., and A.G. performed experiments; R.H., A.M.S., S.S., A.F.M., and A.G. analyzed data; R.H., A.M.S., S.S., A.F.M., and S.C.R. interpreted results of experiments; R.H., A.M.S., S.S., A.F.M., and A.G. prepared figures; R.H., S.C.R., and W.Z.M. drafted manuscript; R.H., S.C.R., and W.Z.M. edited and revised manuscript; R.H., A.M.S., S.C.R., and W.Z.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Ahmad Farooq for assistance with preparation of mouse liver tissue for Western blot analysis and preparation of mouse serum for ALTs.
REFERENCES
- 1. Beldi G, Enjyoji K, Wu Y, Miller L, Banz Y, Sun X, Robson SC. The role of purinergic signaling in the liver and in transplantation: effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Front Biosci 13: 2588–2603, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beldi G, Wu Y, Sun X, Imai M, Enjyoji K, Csizmadia E, Candinas D, Erb L, Robson SC. Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration. Gastroenterology 135: 1751–1760, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol 22: 364–373, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Crispe IN. Isolation of mouse intrahepatic lymphocytes. Curr Protoc Immunol Chapter 3, Unit 3 21, 2001 [doi: 10.1002/0471142735.im0321s22] [DOI] [PubMed] [Google Scholar]
- 5. Dear JW, Simpson KJ, Nicolai MP, Catterson JH, Street J, Huizinga T, Craig DG, Dhaliwal K, Webb S, Bateman DN, Webb DJ. Cyclophilin A is a damage-associated molecular pattern molecule that mediates acetaminophen-induced liver injury. J Immunol 187: 3347–3352, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 115: 56–65, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391: 43–50, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, 2nd, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med 5: 1010–1017, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Hoque R, Sohail M, Malik A, Sarwar S, Luo Y, Shah A, Barrat F, Flavell R, Gorelick F, Husain S, Mehal W. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology 141: 358–369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest 119: 305–314, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishida Y, Kondo T, Kimura A, Tsuneyama K, Takayasu T, Mukaida N. Opposite roles of neutrophils and macrophages in the pathogenesis of acetaminophen-induced acute liver injury. Eur J Immunol 36: 1028–1038, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci USA 106: 20388–20393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jaeschke H. Role of inflammation in the mechanism of acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol 1: 389–397, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol 286: C1100–C1108, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Kaplowitz N. Acetaminophen hepatoxicity: what do we know, what don't we know, and what do we do next? Hepatology 40: 23–26, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Kim YC, Lee SJ. Temporal variation in hepatotoxicity and metabolism of acetaminophen in mice. Toxicology 128: 53–61, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Lamphier MS, Sirois CM, Verma A, Golenbock DT, Latz E. TLR9 and the recognition of self and non-self nucleic acids. Ann NY Acad Sci 1082: 31–43, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, Lee WM. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 42: 1364–1372, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Laskin DL, Laskin JD. Role of macrophages and inflammatory mediators in chemically induced toxicity. Toxicology 160: 111–118, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Liu ZX, Govindarajan S, Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology 127: 1760–1774, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology 43: 1220–1230, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol 2: 493–503, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Martin-Murphy BV, Holt MP, Ju C. The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol Lett 192: 387–394, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marx J. Toxicology. Protecting liver from painkiller's lethal dose. Science 298: 341–342, 2002 [DOI] [PubMed] [Google Scholar]
- 25. McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330: 362–366, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Michael SL, Pumford NR, Mayeux PR, Niesman MR, Hinson JA. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology 30: 186–195, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Pedra JH, Cassel SL, Sutterwala FS. Sensing pathogens and danger signals by the inflammasome. Curr Opin Immunol 21: 10–16, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, Ryffel B, Couillin I. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med 182: 774–783, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2: 409–430, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scheuplein F, Schwarz N, Adriouch S, Krebs C, Bannas P, Rissiek B, Seman M, Haag F, Koch-Nolte F. NAD+ and ATP released from injured cells induce P2X7-dependent shedding of CD62L and externalization of phosphatidylserine by murine T cells. J Immunol 182: 2898–2908, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Seman M, Adriouch S, Scheuplein F, Krebs C, Freese D, Glowacki G, Deterre P, Haag F, Koch-Nolte F. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity 19: 571–582, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem 276: 125–132, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Tsutsui H, Adachi K, Seki E, Nakanishi K. Cytokine-induced inflammatory liver injuries. Curr Mol Med 3: 545–559, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Williams CD, Antoine DJ, Shaw PJ, Benson C, Farhood A, Williams DP, Kanneganti TD, Park BK, Jaeschke H. Role of the Nalp3 inflammasome in acetaminophen-induced sterile inflammation and liver injury. Toxicol Appl Pharmacol 252: 289–297, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xiang Z, Lv J, Jiang P, Chen C, Jiang B, Burnstock G. Expression of P2X receptors on immune cells in the rat liver during postnatal development. Histochem Cell Biol 126: 453–463, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Zaher H, Buters JT, Ward JM, Bruno MK, Lucas AM, Stern ST, Cohen SD, Gonzalez FJ. Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol Appl Pharmacol 152: 193–199, 1998 [DOI] [PubMed] [Google Scholar]