Abstract
Intestinal adaptation is an important compensatory response to massive small bowel resection (SBR) and occurs because of a proliferative stimulus to crypt enterocytes by poorly understood mechanisms. Recent studies suggest the enteric nervous system (ENS) influences enterocyte proliferation. We, therefore, sought to determine whether ENS dysfunction alters resection-induced adaptation responses. Ret+/− mice with abnormal ENS function and wild-type (WT) littermates underwent sham surgery or 50% SBR. After 7 days, ileal morphology, enterocyte proliferation, apoptosis, and selected signaling proteins were characterized. Crypt depth and villus height were equivalent at baseline in WT and Ret+/− mice. In contrast after SBR, Ret+/− mice had longer villi (Ret+/− 426.7 ± 46.0 μm vs. WT 306.5 ± 7.7 μm, P < 0.001) and deeper crypts (Ret+/− 119 ± 3.4 μm vs. WT 82.4 ± 3.1 μm, P < 0.001) than WT. Crypt enterocyte proliferation was higher in Ret+/− (48.8 ± 1.3%) than WT (39.9 ± 2.1%; P < 0.001) after resection, but apoptosis rates were similar. Remnant bowel of Ret+/− mice also had higher levels of glucagon-like peptide 2 (6.2-fold, P = 0.005) and amphiregulin (4.6-fold, P < 0.001) mRNA after SBR, but serum glucagon-like peptide 2 protein levels were equal in WT and Ret+/− mice, and there was no evidence of increased c-Fos nuclear localization in submucosal neurons. Western blot confirmed higher crypt epidermal growth factor receptor (EGFR) protein levels (1.44-fold; P < 0.001) and more phosphorylated EGFR (2-fold; P = 0.003) in Ret+/− than WT mice after SBR. These data suggest that Ret heterozygosity enhances intestinal adaptation after massive SBR, likely via enhanced EGFR signaling. Reducing Ret activity or altering ENS function may provide a novel strategy to enhance adaptation attenuating morbidity in patients with short bowel syndrome.
Keywords: short bowel syndrome, enteric nervous system, epidermal growth factor receptor
intestinal adaptation following massive small bowel resection (SBR) is crucial to enhance absorptive and digestive surface area within the remnant bowel and prevent diarrhea, electrolyte abnormalities, and parenteral nutrition dependence. Adaptation is characterized by increased villus height and crypt depth as a consequence of mitogenic stimuli to intestinal crypt cells via incompletely understood mechanisms. Previous studies demonstrated important roles for many growth factors (9, 29, 33) and their receptors (44), as well as for smooth muscle (22, 27) and angiogenesis (28, 33, 35) in controlling epithelial proliferation and apoptosis. Enteric nervous system (ENS) function in intestinal adaptation has not been studied in detail.
Since the ENS controls most aspects of intestinal activity, we hypothesized that ENS defects might also alter adaptation. We became interested in this question because the receptor for glucagon-like peptide 2 (GLP2), a potent enterocyte proliferation growth factor (10), is expressed in enteric neurons (5). Furthermore, GLP2-induced c-Fos expression in crypt epithelium is blocked by tetrodotoxin (TTX), a voltage-gated sodium channel inhibitor, suggesting that the ENS is essential for normal intestinal epithelial turnover (5). Finally, our own studies demonstrated that intestinal epithelial gene expression, including epiregulin (Ereg) expression, is abnormal in neonatal mice with intestinal aganglionosis (43), suggesting the ENS could influence intestinotrophic growth factors expression.
To test the hypothesis that the ENS influences intestinal adaptation after SBR, we used Ret heterozygous (Ret+/−) mice. Ret is a transmembrane tyrosine kinase receptor activated by four ligands (glial cell line-derived neurotrophic factor, neurturin, artemin, and persephin) and essential for ENS development (2). Ret-deficient mice have essentially no ENS in the small bowel or colon (37), and Ret heterozygosity is a common risk factor for human Hirschsprung disease (1). In contrast to humans, Ret+/− mice have almost normal-appearing ENS anatomy, but reduced intestinal contractility and reduced release of some neurotransmitters (15). Nonetheless, Ret+/− mice appear healthy. We, therefore, hypothesized that, if the ENS is important for regulation of intestinal adaptation, functional defects in Ret+/− animals might be severe enough to alter adaptation after massive SBR. We now demonstrate that bowel adaptation is enhanced in Ret+/− mice compared with wild-type (WT) animals after SBR. Enhanced adaptation correlated with increased crypt cell proliferation, elevated intestinal amphiregulin (Areg) mRNA, increased epidermal growth factor receptor (EGFR) protein, and increased phosphorylated-EGFR (pEGFR) in crypt cells of the remnant bowel in Ret+/− mice. These observations suggest that drugs that modulate neuronal function and polymorphisms that reduce Ret expression could amplify intestinal adaptation in humans with short bowel syndrome.
MATERIALS AND METHODS
Animals
This protocol was approved by Washington University Institutional Animal Care and Use Committee. Ret+/− and WT mice were bred at least 10 generations into the C57BL/6 background (12).
Operative Procedure
Adult mice (7–11 wk old) were subjected to a 50% proximal SBR or sham surgery, as described (20). Food consumption after SBR was measured daily for many of the WT (n = 9) and Ret+/− (n = 5) mice. For SBR, bowel is resected starting 3 cm distal to the ligament of Treitz and extending to 12 cm proximal to the cecum, followed by end-to-end reanastomosis. For sham surgery, small bowel was transected 12 cm proximal to the cecum and reanastomosed. No antibiotics were given. Mice that die before harvest, show signs of illness (lethargy and unkempt fur), intestinal obstruction, or anastomotic leak (dilated proximal small intestine, absence of food in the cecum, and gastric distention) are excluded from analysis. In addition, mice that fail to show at least a 20% increase in villus height (i.e., the expected normal adaptation) are also excluded because they are presumed to be recovering poorly from surgery (41). Postoperative mortality and rates of nonadaptation were equal in WT and Ret+/− mice (P > 0.65). Overall, we evaluated 17 WT (sham n = 6, SBR n = 11) and 19 Ret+/− mice (sham n = 11, SBR n = 8).
Tissue Collection and Analysis
Remnant small intestine from the duodenojejunal to the ileocecal junction was harvested on postoperative day 7, a time of maximal sustained adaptation (19). The lumen was flushed with ice-cold phosphate-buffered saline. Bowel from anastomosis to ileocecal junction was measured after stretching with a 2-g weight. Samples distal to the anastomosis were analyzed. All analyses were blinded.
Histology.
The ileum was opened along the mesenteric border, fixed flat in 10% neutral buffered formalin, embedded in paraffin, and cut in 5-μm sections before staining with hematoxylin and eosin. Crypt depth and villus length were measured (Metamorph Software, UIC, Dowington, PA). At least 20 crypts and villi were counted per mouse. Only well-oriented villi with intact crypts, a continuous epithelial layer from the crypt to the villus tip, and a visible villus core were analyzed.
Enterocyte proliferation.
Ninety minutes before death, mice received subcutaneous 5-bromodeoxyuridine [BrdU; 30 μg/g body wt (3 mg/ml); Zymed Laboratories, San Francisco, CA]. Tissues were fixed (10% formalin), embedded in paraffin, sectioned, deparaffinized, and blocked (3% hydrogen peroxide, methanol 15 min, 25°C). Antigen retrieval was performed using the Diva Decloaking solution (Biocare Medical, Concord, CA) (120°C, 5 min and then 100°C, 1 min). Slides were blocked sequentially with avidin-pink and biotin-blue (Biocare Medical), treated with anti-BrdU antibody [1:500, (OBT0030), Accurate, Westbury, NY] in DaVinci Green (Biocare Medical) (1 h, 25°C), and visualized with biotinylated goat anti-rat IgG, followed by streptavidin-horseradish peroxidase and diaminobenzidine, and hematoxylin counterstaining. Twenty well-oriented crypts per mouse were analyzed.
Enterocyte apoptosis.
Dying cells were identified on hematoxylin and eosin-stained sections by pyknotic nuclei, condensed chromatin, and nuclear fragmentation. For activated caspase-3 staining, deparaffinized slides were blocked (0.5% hydrogen peroxide in methanol). Antigen retrieval was performed using Diva Decloaking solution (Biocare Medical) with pressurized heat. Slides were blocked using the Avidin/Biotin Kit (Biocare Medical), treated with primary anti-cleaved caspase-3 antibody [1:15,000, (9961L) Cell Signaling Technologies, Danvers, MA] overnight (4°C), and visualized with diaminobenzidine staining using Mach2 horseradish peroxidase-conjugated polymer probe (Biocare Medical, Concord, CA) and hematoxylin counterstaining. One hundred well-well oriented crypts per mouse were analyzed.
Enterocyte isolation.
Crypts and villi were separated from other cells in bowel wall using calcium chelation and mechanical disruption, as previously described with minor modifications (26). An 8- to 10-cm segment of distal small bowel was opened along the mesenteric border in phosphate-buffered saline with protease and phosphatase inhibitors (4°C), transferred to balanced salt solution (1.5 mM KCl, 96 mM NaCl, 27 mM sodium citrate, 8 mM KH2PO4, 5.6 mM Na2HPO4, 15 mM EDTA, and 1 mM dithiothreitol) with protease and phosphatase inhibitors (PhosphoSTOP and Complete, EDTA-free Protease Inhibitor Cocktail Tablets Roche Diagnostics, Indianapolis, IN) and then vigorously vortexed (4°C, 5 min) to remove intraluminal contents. Epithelia were detached by additional vortexing for 3 min and then again in a new tube to collect crypts and villi. Crypts were separated from villi using a 70-μm cell strainer (BD Biosciences), washed, and resuspended in 1 ml buffer A (150 mM NaCl, 1 mM EDTA, 50 mM Tris·HCl, pH 8.0) with the tablet protease and phosphatase inhibitors above. Crypt and villus fractions were sonicated and saved at −80°C for protein studies. Bowel remaining after crypt and villus removal was vortexed for 10 min in balanced salt solution to remove residual epithelia, homogenized in 1 ml buffer A, and centrifuged (1,000 g, 1 min, 4°C). The pellet was washed and resuspended in 1 ml of buffer A with protease and phosphatase inhibitors, sonicated, and stored at −80°C for protein analysis. Another 1- to 2-cm tissue segment was homogenized in 500 μl of lysis buffer (RNAqueous kit) and frozen at −80°C for RNA analysis.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
RNA prepared from <100-mg whole bowel 3–4 cm distal to the anastomosis following kit instructions (RNAqueous kit, Applied Biosystems) was stored at −80°C. RNA concentration was determined using a NanoDrop Spectrophotometer (ND-1000, NanoDrop Technologie, Wilmington, DE). Quality was evaluated using an Experion System with a RNA StdSens Chip and reagents (Bio-Rad Laboratories, Richmond, CA). Complementary DNA (cDNA) was prepared from high-quality RNA using an RT2 First Strand Kit (SABioscience; Fredrick, MD) and quantified using Quanti-iT OliGreen ssDNA Assay kit (Invitrogen, Carlsbad, CA). Equal amounts of cDNA were used in all reactions. mRNA levels were normalized to β-actin, and whole bowel cDNA was used as a plate-to-plate calibrator. Quantitative real-time PCR studies confirmed that β-actin levels are proportional to total mRNA in gut samples after both sham surgery and SBR (data not shown). Gene expression was examined using primers and reagents from SABioscience (Frederick, MD) and an Applied Biosystems 7500 Fast Real-Time PCR system (Foster City, CA).
Western Blots and Immunohistochemistry
Isolated crypt, villus, or remaining bowel were lysed with sodium dodecyl sulfate (SDS) sample buffer (50 mM Tris·HCl, pH 6.8, 2% SDS, 10% glycerol, and 5% mercaptoethanol) and heated (5 min, 100°C), and protein concentration was determined by using the RC DC kit (Bio-Rad, Hercules, CA). Protein (25 and 55 μg) for EGFR and pEGFR, respectively, was separated on a 7% SDS-polyacrylamide gel (Novex gel, Invitrogen, Carlsbad, CA), transferred to Protran BA 83 (Whatman), and analyzed using EGFR (38), pEGFR [mix of 4 antibodies at 1:5,000 each: Tyr845 (2231), Tyr1068 (2234), Tyr1045 (2237), Tyr992 (2235), Cell Signaling Technologies, Danvers, MA], and β-actin [1:10,000, (4970) Cell Signaling Technologies] antibodies. c-Fos staining of submucosal ganglia employed an antibody from Santa Cruz Biotechnology (1:100) and followed the protocol outlined (21).
GLP2 Enzyme Linked Immunoassay
Serum was isolated from whole blood obtained 7 days after SBR by incubating at 4°C overnight, centrifuging for 20 min at 2,000 g, and taking the supernatant. A BioVendor (Candler, NC) mouse GLP2 enzyme linked immunoassay (ELISA) kit was used to determine serum GLP2 levels following the directions of the manufacturer.
Statistics
Analyses used SigmaStat software (SPSS 11.0, Chicago, IL). Two-way ANOVA was used for multiple comparisons. Student's t-test was used for single comparisons on normally distributed data and Mann-Whitney Rank Sum test on nonnormally distributed data. χ2 test was used for categorical variables. Results are presented as means ± SE. P values < 0.05 were considered significant.
RESULTS
Ret Heterozygous Mice Have Enhanced Adaptation After SBR
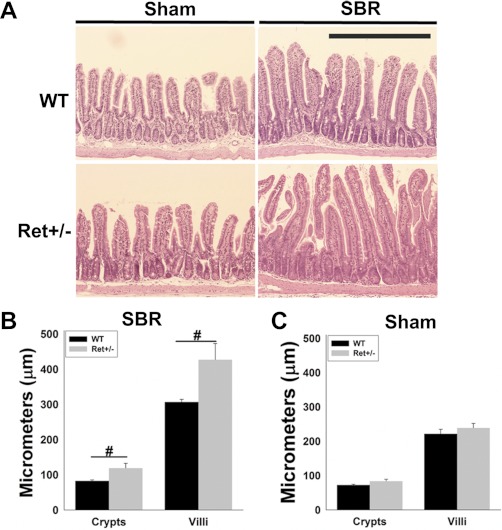
To test the hypothesis that ENS defects affect intestinal adaptation after bowel resection, we performed SBR or sham surgery on Ret+/− and WT mice and analyzed bowel morphology 7 days later. Remnant bowel length was equal in WT and Ret+/− mice after both sham surgery (WT 17.4 ± 0.7 cm, Ret+/− 16.0 ± 0.6 cm, P = 0.22) and SBR (WT 15.3 ± 0.7 cm, Ret+/− 15.6 ± 0.7 cm, P = 0.16). Bowel morphology 2 cm distal to the anastomosis was analyzed (Fig. 1A). After SBR, Ret+/− mice had significantly longer villi than WT mice (80 vs. 40% increase compared with sham, P < 0.001) (Fig. 1, B and C). Mean crypt depth was also greater in Ret+/− than WT mice after SBR (46 vs. 14% increase compared with sham, P < 0.001). In contrast, crypt depth and villus height after sham surgery or before surgery were equivalent in WT and Ret+/− mice (Fig. 1C and data not shown).
Fig. 1.
Small bowel morphology. A: representative hematoxylin and eosin (H&E) stained sections 7 days after surgery show longer villi and crypts in Ret+/− than wild-type (WT) mice after small bowel resection (SBR). Scale bar = 500 μm. B and C: quantitative analysis of villus height and crypt depth 7 days after SBR (B) or sham surgery (C). Values are means ± SE. #P < 0.001.
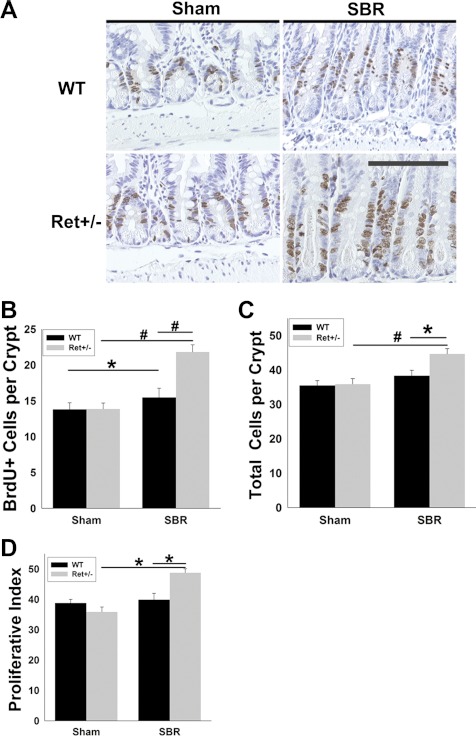
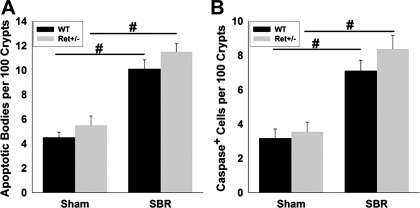
Consistent with increased villus length in Ret+/− mice, Ret heterozygotes had more BrdU+ epithelial cells per crypt (WT 15.5 ± 1.3, Ret+/− 21.8 ± 1.0, P < 0.001) and more total cells per crypt than WT mice after SBR (WT 38.3 ± 1.6, Ret+/− 44.7 ± 1.5; P = 0.006; Fig. 2, A–C). Ret+/− mice also had a greater percentage of BrdU+ crypt cells than WT animals after SBR (WT 39.9 ± 2.1%, Ret+/− 48.8 ± 1.3%, P = 0.003) (Fig. 2D). In contrast, these cell proliferation parameters were equivalent in WT and Ret+/− mice after sham surgery. Furthermore, although crypt apoptosis increased after SBR (Fig. 3), there was no difference in apoptosis rates between the WT and Ret+/− mice. Collectively, these data demonstrate that Ret+/− mice have an exaggerated proliferative response to SBR, but normal epithelial morphology in the absence of an adaptive stimulus. Because food intake can dramatically alter the adaptive response, we measured daily food intake in Ret+/− and WT mice after SBR, but found identical food intake in these groups (WT 10.6 ± 2.7 g/day, Ret+/− 10.7 ± 2.4 g/day, P = 0.87), suggesting that differences in food intake are unlikely to account for enhanced adaptation in Ret+/− mice.
Fig. 2.
Proliferation. A: representative 5-bromodeoxyuridine (BrdU) stained sections 7 days after surgery show longer crypts and more BrdU+ cells in Ret+/− than WT mice after SBR. Scale bar = 100 μm. B: BrdU+ cells per crypt were elevated after SBR in WT and Ret+/− mice compared with sham animals. Proliferation rates were higher in Ret+/− than WT after SBR. C: total cells per crypt were elevated in Ret+/− vs. WT mice after SBR. D: proliferative index (BrdU+ cells/total cells per crypt × 100) was higher in Ret+/− vs. WT mice after SBR. Values are means ± SE. #P < 0.001. *P < 0.006.
Fig. 3.
Apoptosis. Apoptotic bodies in H&E stained crypts (A) and activated caspase 3+ cells (B) were more abundant after SBR than sham surgery, but rates were not different in WT and Ret+/− mice. Values are means ± SE. #P < 0.001.
Ret+/− Mice Have Elevated Levels of Whole Bowel mRNA for GLP2 and the EGFR Ligand Areg After SBR
The morphological data suggest that Ret heterozygosity alters the resection-induced adaptive milieu in favor of enhanced epithelial proliferation. We hypothesized that this occurs because Ret heterozygosity induces expression of proteins already known to be important stimulants of small bowel adaptation. Although many molecules influence epithelial growth after SBR (29), we focused on GLP2 receptor (GLP2R) and EGFR signaling, since these proteins are considered to be important for intestinal adaptation (3, 17, 18, 23, 24, 32, 39–41, 44–46). Furthermore, GLP2R is expressed in the ENS (5), and levels of the EGFR ligand Ereg are elevated in neonatal Ret−/− mice (43).
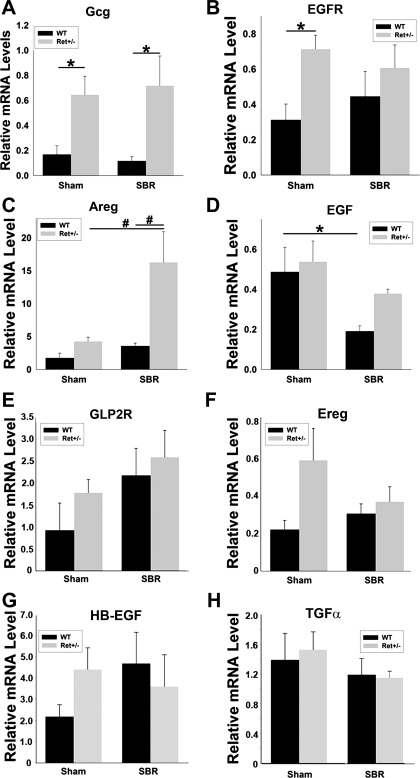
We found significantly higher levels of glucagon mRNA, the precursor for GLP2, after sham surgery (3.9-fold; P = 0.029) and SBR (6.2-fold; P = 0.005) in Ret+/− compared with WT mice (Fig. 4A). mRNA for the EGFR ligand Areg were also 4.6-fold higher in Ret+/− than WT mice after SBR (P < 0.001), but equivalent after sham surgery (P = 0.383; Fig. 4C). In contrast, EGFR mRNA was higher in the Ret+/− than WT mice after sham surgery, but equivalent in the SBR group (Fig. 4B). Interestingly, EGF mRNA levels were significantly lower in the WT SBR compared with WT sham mice (P = 0.019). Mean EGF mRNA level was also lower in Ret+/− mice after SBR than after sham surgery, but this difference was not statistically significant (P = 0.17). GLP2R, Ereg, heparin-binding-EGF, and transforming growth factor-α mRNA levels were also equivalent in Ret+/− and WT mouse small bowel after sham surgery or SBR (i.e., P > 0.05 for all comparisons; Fig. 4, E–H) Collectively, these data suggest that Ret heterozygous mice may have an increased adaptive response after SBR because of increased activation of GLP2R or EGFR signaling pathways compared with WT via elevated GLP2 or Areg production.
Fig. 4.
mRNA analysis. Whole bowel 7 days after SBR or sham surgery was analyzed by quantitative RT-PCR. A: glucagon (Gcg) mRNA [the precursor for glucagon-like peptide 2 (GLP2)] was more abundant in Ret+/− than WT mice after SBR and after sham surgery. B: epidermal growth factor (EGF) receptor (EGFR) mRNA was more abundant in Ret+/− than WT mice after sham surgery, but not after SBR. C: amphiregulin (Areg) mRNA was more abundant in Ret+/− than WT mice after SBR, but not after sham surgery. D: EGF mRNA levels were lower after SBR in WT mice than after sham surgery, but not statistically different in the SBR and sham groups of Ret+/− mice. E–H: mRNA levels for GLP2 receptor (GLP2R; E), epiregulin (Ereg; F), heparin-binding-EGF (HB-EGF; G), and transforming growth factor (TGF)-α (H) were equivalent in WT and Ret+/− after sham surgery or SBR (P > 0.05). Values are means ± SE. #P < 0.001. *P < 0.03.
Increased GLP2R Signaling Is Unlikely to Mediate Enhanced Adaptation in Ret+/− Mice After SBR
GLP2R is expressed within enteric neurons; however, these cells are challenging to isolate from adult mice and difficult to study biochemically. Because GLP2 peptide is released into serum from L cells after complex posttranslational processing (34) and serum levels increase after SBR (6, 25, 30), we hypothesized that higher glucagon mRNA levels in Ret+/− might lead to higher serum levels of GLP2 peptide. To test this hypothesis, we directly measured serum GLP2 peptide levels by ELISA, but found identical serum levels in WT and Ret+/− mice 7 days after SBR (WT 1.38 ± 0.14 ng/ml; Ret+/− 1.38 ± 0.08 ng/ml, P = 1.0). The possibility remained that local GLP2 release could activate enteric neurons. Because exogenous GLP2 increases the number of submucosal neurons with nuclear c-Fos localization in rats (21), we evaluated c-Fos protein nuclear localization in submucosal neurons 7 days after SBR by immunohistochemistry. Unlike the results reported in rat, we found very few submucosal neurons with nuclear c-Fos localization in either WT or Ret+/− mice. Collectively, these data suggest that increased GLP2R activation is unlikely to account for the enhanced adaptive response in Ret+/− mice after SBR.
Ret Heterozygosity Increases EGFR Activity in Small Bowel Crypt Cells
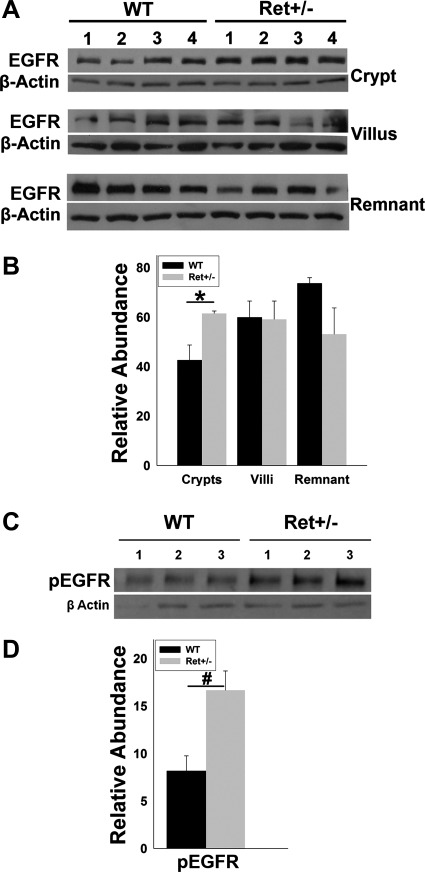
We next investigated the possibility that EGFR activation was increased in Ret+/− compared with WT mice after SBR. EGFR is expressed in gut epithelial cells that can be readily isolated in large numbers from mouse bowel. Crypt epithelial cells are ideal for investigating EGFR activation, since proliferation rates are elevated in these cells in Ret+/− mice after SBR compared with WT animals. If elevated mRNA levels for Areg found in Ret+/− mice after SBR reflect protein abundance, then Ret+/− mice should have increased EGFR activation compared with WT animals after SBR. To test this hypothesis, we separated crypt epithelial cells from villi and remaining bowel 7 days after SBR. Western blot demonstrated higher levels of EGFR protein in crypt epithelial cells of Ret+/− than WT mice (1.44-fold higher, P = 0.03), but comparable levels of EGFR protein in villi (P = 0.94) and remnant bowel (P = 0.11) (Fig. 5, A and B). Interestingly, pEGFR (i.e., activated receptor) in crypt cells was dramatically higher (2-fold, P = 0.003) in Ret+/− than in WT mice 7 days after SBR (Fig. 5, C and D). These data are consistent with the hypothesis that Ret heterozygosity increases levels of the EGFR ligand Areg after SBR, and that EGFR activation contributes to the increased adaptive response in Ret+/− mice.
Fig. 5.
EGFR activation after SBR. A: protein immunoblot for EGFR and β-actin in crypt, villus, and bowel remnant fractions obtained 7 days after SBR from WT and Ret+/− mice. B: quantitative analysis demonstrated elevated EGFR in crypt epithelial cells of Ret+/− mice compared with WT after SBR (normalized to β-actin), but equivalent levels in villus and bowel remnant fractions. C: protein immunoblot of crypt epithelial cells 7 days after SBR shows elevated phosphorylated EGFR (pEGFR) in Ret+/− compared with WT mice. D: quantitative analysis of pEGFR protein levels after SBR. Values are means ± SE. #P = 0.003. *P = 0.03.
DISCUSSION
Small bowel has the remarkable ability to adapt after resection by increasing villus and crypt length to enhance absorptive surface area. This process requires that bowel remaining after resection “senses” intestinal loss and responds by sustained increases in stem cell proliferation in the intestinal crypt. Our investigations demonstrate that Ret+/− mice adapt more vigorously than WT after SBR. Specifically, after 50% SBR, Ret+/− mice have longer villi, more crypt epithelial cells, and greater rates of crypt epithelial cell proliferation than WT. Ret+/− animals also have higher levels of GLP2 and Areg mRNA in remnant bowel and increased EGFR phosphorylation. Interestingly, however, we did not detect elevated levels of GLP2 peptide in serum 7 days after SBR or increased submucosal neuron c-Fos accumulation in Ret+/− vs. WT mice, suggesting that the mechanism of enhanced adaptation in Ret+/− animals may be GLP2 independent. These observations imply that a novel genetic mechanism may impact the adaptive response in short bowel syndrome. Furthermore, these data indicate that one or more Ret-responsive cell populations normally limit adaptation after SBR, and that reduced Ret signaling may enhance adaptation.
When we initiated these studies, we had the simplistic idea that mutations that reduce ENS function would impair intestinal adaptation after SBR. This hypothesis was based on the observation that the ENS was necessary for GLP2 activity, since the GLP2R is expressed in enteric neurons and TTX blocks GLP2-induced c-Fos activation in crypt cells (5, 16). We had hypothesized that Ret+/− mice might provide insight into the relative importance of GLP2R in the ENS vs. GLP2R in myofibroblasts and enteroendocrine cells, since Ret heterozygosity impairs ENS function, but Ret is not expressed in myofibroblasts or enteroendocrine cells. The observation that Ret+/− mice have a greater adaptive response to SBR than WT demonstrates that our original hypothesis was wrong. However, our results are interesting when considered in the context of prior studies.
In contrast to Ret+/− mice after SBR, we found normal villus and crypt morphology in Ret+/− animals after sham surgery. This suggests that one or more trophic factors are differentially regulated in Ret heterozygotes after SBR compared with baseline conditions. One intriguing observation that could provide a plausible explanation for this dichotomy in intestinal architecture is that Areg mRNA is dramatically elevated after SBR, but not after sham surgery in Ret+/− compared with WT mice. Areg is an EGFR ligand, and assuming that increased mRNA leads to elevated protein at the EGFR, elevated Areg would be anticipated to cause the morphological changes and increases in crypt EGFR phosphorylation that we observed.
Why Areg levels are elevated in the bowel of Ret+/− mice after SBR is unknown. Ret+/− mice are known to have reduced release of some neurotransmitters in response to electrical stimulation and to have reduced intestinal motility in vitro (15). It is, therefore, possible that Areg expression is controlled by neuronal activity. In this context, several prior observations suggest that components of the ENS may have an inhibitory effect on intestinal adaptation. For example, destruction of the ENS with benzalkonium chloride causes enhanced adaptation after SBR that closely mimics our results (8, 14). Benzalkonium chloride-induced bowel denervation, however, increases crypt depth and villus height in rats, even without SBR (8), whereas Ret+/− have similar morphology to WT animals in the absence of SBR. This difference could occur because benzalkonium chloride causes a 75–85% loss of enteric neurons, a defect much more severe than Ret heterozygosity. Nonetheless, the chemical injury model adds credence to the idea that Ret+/−-induced reductions in ENS function enhance bowel adaptation after SBR. Prior coculture studies of human ENS and epithelial components also demonstrated that epithelial proliferation increased when ENS activity was blocked with TTX or by vasoactive intestinal peptide (VIP) receptor antagonist. Furthermore, VIP administration even in the presence of TTX reduced epithelial cell proliferation (42). Because VIP release from electrically stimulated Ret+/− bowel (15) is reduced compared with WT mice, it is possible that lower VIP levels might cause the enhanced proliferative response after SBR in Ret+/− mice. Linking specific cellular and molecular changes within the ENS to effects of Ret heterozygosity on small bowel adaptation will, however, require considerable additional investigation.
From a mechanistic standpoint, Ret also has essential roles in the sympathetic and parasympathetic nervous system, selected sensory neurons, and kidney development (12, 13, 37). Since the autonomic nervous system regulates many organs, it is conceivable that imbalances between sympathetic and parasympathetic innervation could alter growth factor or hormonal signaling to enhance adaptation after SBR in Ret+/− mice, independent of ENS function. The effect of Ret heterozygosity might then only become evident in the setting of stressors that activate the autonomic nervous system. One intriguing possibility is that enhanced adaptation in Ret+/− mice after SBR results in part from altered autonomic innervation of salivary gland, an essential source of EGF after SBR (19). Salivary gland receives both sympathetic and parasympathetic innervation that is Ret dependent, and EGF synthesis and release depend on the balance of sympathetic and parasympathetic signaling. Moreover, because EGF induces the expression of other EGFR ligands (4, 7), the increased Areg mRNA in Ret+/− mouse bowel might be a secondary effect of altered salivary EGF secretion. Autonomic innervation to other organs could also contribute to the observed phenotype by altering systemic hormone levels that impact the adaptive response after SBR.
These observations imply that Ret signaling may have physiological relevance after SBR in human populations, since a common human Ret intronic polymorphism reduces Ret protein levels and increases the risk of Hirschsprung disease by severalfold (11). Specifically, the +9.7T allele (SNP rs245357) that is present in 25% of Caucasians and 50% of Asians is part of an enhancer element that reduces the expression of Ret reporter constructs six- to eightfold (11). The +9.7T polymorphism alone does not, however, cause distal bowel aganglionosis, since it has a “mild” effect on Ret expression compared with haploinsufficiency, which entails a 50% risk of human Hirschsprung disease. Interestingly, Ret+/− mice have full colonization of the bowel by ENS precursors, but, like the +9.7T allele, Ret heterozygosity sensitizes animals to other mutations that, in combination, cause Hirschsprung-like disease (31). It is, therefore, tempting to speculate that Ret+/− mice are a closer mimic for the human +9.7T allele than for human Ret heterozygosity. If this is true, then common Ret enhancer polymorphisms may be a major determinant of the adaptive response of the small bowel to intestinal resection in humans. This is a potentially testable hypothesis that will be pursued by linking small bowel length at the time of resection, duration of TPN dependence, and human genetics.
Summary
Our data indicate that a Ret-responsive program limits intestinal adaptation after SBR. Deciphering which Ret+/− mouse defects enhance bowel adaptation after SBR will require the type of analysis we just completed using a series of tissue or cell type specific Ret mutant animals that do not currently exist. Nonetheless, these findings provide hope that modulation of neuronal function could enhance bowel adaptation in individuals with short bowel syndrome. Furthermore, since polymorphisms that alter Ret expression are common in humans, variable Ret expression may underlie in part the variable clinical adaptation observed after SBR and influence the likelihood of long-term total parenteral nutrition dependence. These studies, therefore, open new avenues of investigation and provide a stronger impetus to investigate neural mechanisms regulating bowel adaptation. They also suggest that low levels of Ret kinase inhibitors (36) currently entering clinical trials for cancer might be beneficial in the individuals with short bowel syndrome to enhance intestinal adaptation.
GRANTS
This work was supported by National Institutes of Health (NIH) 5 T32 DK077653-18, the Digestive Diseases Research Core Center Morphology Core Grant no. P30 DK52574, RO1DK087715, DK57038, The Children's Discovery Institute CH-II-2008-123, St. Louis Children's Hospital Foundation—Children's Surgical Sciences Institute, and Burroughs Wellcome Fund 1008525 and RO1-DK 53234.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.C.H., J.A.L., D.W., J.G., C.R.E., K.J.R., E.C.M., R.O.H., and B.W.W. conception and design of research; M.C.H., J.A.L., D.W., J.G., C.R.E., K.J.R., and E.C.M. performed experiments; M.C.H., J.A.L., D.W., J.G., C.R.E., K.J.R., E.C.M., R.O.H., and B.W.W. analyzed data; M.C.H., J.A.L., D.W., J.G., C.R.E., K.J.R., E.C.M., R.O.H., and B.W.W. interpreted results of experiments; M.C.H., R.O.H., and B.W.W. prepared figures; M.C.H., R.O.H., and B.W.W. drafted manuscript; M.C.H., J.A.L., D.W., J.G., C.R.E., K.J.R., E.C.M., R.O.H., and B.W.W. edited and revised manuscript; M.C.H., J.A.L., D.W., J.G., C.R.E., K.J.R., E.C.M., R.O.H., and B.W.W. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of M. C. Hitch: University of Alabama at Birmingham, Dept. of Pediatrics, Divisions of Gastroenterology and Nutrition Science, 618 Ambulatory Care Center, 1600 7th Ave. South, Birmingham, AL 35233-1711 (e-mail: mhitch@peds.uab.edu).
REFERENCES
- 1. Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, Pelet A, Arnold S, Miao X, Griseri P, Brooks AS, Antinolo G, de Pontual L, Clement-Ziza M, Munnich A, Kashuk C, West K, Wong KK, Lyonnet S, Chakravarti A, Tam PK, Ceccherini I, Hofstra RM, Fernandez R. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet 45: 1–14, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev 16: 441–467, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Bahrami J, Yusta B, Drucker DJ. ErbB activity links the glucagon-like peptide-2 receptor to refeeding-induced adaptation in the murine small bowel. Gastroenterology 138: 2447–2456, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Barnard JA, Graves-Deal R, Pittelkow MR, DuBois R, Cook P, Ramsey GW, Bishop PR, Damstrup L, Coffey RJ. Auto- and cross-induction within the mammalian epidermal growth factor-related peptide family. J Biol Chem 269: 22817–22822, 1994 [PubMed] [Google Scholar]
- 5. Bjerknes M, Cheng H. Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc Natl Acad Sci U S A 98: 12497–12502, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dahly EM, Gillingham MB, Guo Z, Murali SG, Nelson DW, Holst JJ, Ney DM. Role of luminal nutrients and endogenous GLP-2 in intestinal adaptation to mid-small bowel resection. Am J Physiol Gastrointest Liver Physiol 284: G670–G682, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Damstrup L, Kuwada SK, Dempsey PJ, Brown CL, Hawkey CJ, Poulsen HS, Wiley HS, Coffey RJ., Jr Amphiregulin acts as an autocrine growth factor in two human polarizing colon cancer lines that exhibit domain selective EGF receptor mitogenesis. Br J Cancer 80: 1012–1019, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deniz M, Kilinc M, Hatipoglu ES. Morphological alterations in small intestine of rats with myenteric plexus denervation. Eur Surg Res 36: 152–158, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Drozdowski L, Thomson AB. Intestinal hormones and growth factors: effects on the small intestine. World J Gastroenterol 15: 385–406, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A 93: 7911–7916, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Emison ES, McCallion AS, Kashuk CS, Bush RT, Grice E, Lin S, Portnoy ME, Cutler DJ, Green ED, Chakravarti A. A common sex-dependent mutation in a RET enhancer underlies Hirschsprung disease risk. Nature 434: 857–863, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Enomoto H, Crawford PA, Gorodinsky A, Heuckeroth RO, Johnson EM, Jr, Milbrandt J. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development 128: 3963–3974, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Enomoto H, Heuckeroth RO, Golden JP, Johnson EM, Jr, Milbrandt J. Development of cranial parasympathetic ganglia requires sequential actions of GDNF and neurturin. Development 127: 4877–4889, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Garcia SB, Kawasaky MC, Silva JC, Garcia-Rodrigues AC, Borelli-Bovo TJ, Iglesias AC, Zucoloto S. Intrinsic myenteric denervation: a new model to increase the intestinal absorptive surface in short-bowel syndrome. J Surg Res 85: 200–203, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Gianino S, Grider JR, Cresswell J, Enomoto H, Heuckeroth RO. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development 130: 2187–2198, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell D, Nichols BL, Burrin DG. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology 130: 150–164, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Helmrath MA, Erwin CR, Warner BW. A defective EGF-receptor in waved-2 mice attenuates intestinal adaptation. J Surg Res 69: 76–80, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Helmrath MA, Shin CE, Erwin CR, Warner BW. The EGF\EGF-receptor axis modulates enterocyte apoptosis during intestinal adaptation. J Surg Res 77: 17–22, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Helmrath MA, Shin CE, Fox JW, Erwin CR, Warner BW. Adaptation after small bowel resection is attenuated by sialoadenectomy: the role for endogenous epidermal growth factor. Surgery 124: 848–854, 1998 [PubMed] [Google Scholar]
- 20. Helmrath MA, VanderKolk WE, Can G, Erwin CR, Warner BW. Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg 183: 441–449, 1996 [PubMed] [Google Scholar]
- 21. Kaji T, Tanaka H, Redstone H, Wallace LE, Holst JJ, Sigalet DL. Temporal changes in the intestinal growth promoting effects of glucagon-like peptide 2 following intestinal resection. J Surg Res 152: 271–280, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Knott AW, Juno RJ, Jarboe MD, Profitt SA, Erwin CR, Smith EP, Fagin JA, Warner BW. Smooth muscle overexpression of IGF-I induces a novel adaptive response to small bowel resection. Am J Physiol Gastrointest Liver Physiol 287: G562–G570, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Knott AW, Juno RJ, Jarboe MD, Zhang Y, Profitt SA, Thoerner JC, Erwin CR, Warner BW. EGF receptor signaling affects bcl-2 family gene expression and apoptosis after massive small bowel resection. J Pediatr Surg 38: 875–880, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Kumagai K, Horikawa T, Gotoh A, Yamane S, Yamada H, Kobayashi H, Hamada Y, Suzuki S, Suzuki R. Up-regulation of EGF receptor and its ligands, AREG, EREG, and HB-EGF in oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 110: 748–754, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Ljungmann K, Hartmann B, Kissmeyer-Nielsen P, Flyvbjerg A, Holst JJ, Laurberg S. Time-dependent intestinal adaptation and GLP-2 alterations after small bowel resection in rats. Am J Physiol Gastrointest Liver Physiol 281: G779–G785, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Longshore SW, Nair R, Perrone EE, Erwin CR, Guo J, Warner BW. p21(waf1/cip1) deficiency does not perturb the intestinal crypt stem cell population after massive small bowel resection. J Pediatr Surg 44: 1065–1071; discussion 1071, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin CA, Bernabe KQ, Taylor JA, Nair R, Paul RJ, Guo J, Erwin CR, Warner BW. Resection-induced intestinal adaptation and the role of enteric smooth muscle. J Pediatr Surg 43: 1011–1017, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Martin CA, Perrone EE, Longshore SW, Toste P, Bitter K, Nair R, Guo J, Erwin CR, Warner BW. Intestinal resection induces angiogenesis within adapting intestinal villi. J Pediatr Surg 44: 1077–1082; discussion 1083, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin GR, Beck PL, Sigalet DL. Gut hormones, and short bowel syndrome: the enigmatic role of glucagon-like peptide-2 in the regulation of intestinal adaptation. World J Gastroenterol 12: 4117–4129, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin GR, Wallace LE, Hartmann B, Holst JJ, Demchyshyn L, Toney K, Sigalet DL. Nutrient-stimulated GLP-2 release and crypt cell proliferation in experimental short bowel syndrome. Am J Physiol Gastrointest Liver Physiol 288: G431–G438, 2005 [DOI] [PubMed] [Google Scholar]
- 31. McCallion AS, Stames E, Conlon RA, Chakravarti A. Phenotype variation in two-locus mouse models of Hirschsprung disease: tissue-specific interaction between Ret and Ednrb. Proc Natl Acad Sci U S A 100: 1826–1831, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McMellen ME, Wakeman D, Erwin CR, Guo J, Warner BW. Epidermal growth factor receptor signaling modulates chemokine (CXC) ligand 5 expression and is associated with villus angiogenesis after small bowel resection. Surgery 148: 364–370, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McMellen ME, Wakeman D, Longshore SW, McDuffie LA, Warner BW. Growth factors: possible roles for clinical management of the short bowel syndrome. Semin Pediatr Surg 19: 35–43, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Orskov C, Holst JJ, Knuhtsen S, Baldissera FG, Poulsen SS, Nielsen OV. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology 119: 1467–1475, 1986 [DOI] [PubMed] [Google Scholar]
- 35. Parvadia JK, Keswani SG, Vaikunth S, Maldonado AR, Marwan A, Stehr W, Erwin C, Uzvolgyi E, Warner BW, Yamano S, Taichman N, Crombleholme TM. Role of VEGF in small bowel adaptation after resection: the adaptive response is angiogenesis dependent. Am J Physiol Gastrointest Liver Physiol 293: G591–G598, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Phay JE, Shah MH. Targeting RET receptor tyrosine kinase activation in cancer. Clin Cancer Res 16: 5936–5941, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Schuchardt A, D'Agati V, Larsson-Blomberg L, Constantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367: 380–383, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Sheng G, Bernabe KQ, Guo J, Warner BW. Epidermal growth factor receptor-mediated proliferation of enterocytes requires p21waf1/cip1 expression. Gastroenterology 131: 153–164, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Sinclair EM, Yusta B, Streutker C, Baggio LL, Koehler J, Charron MJ, Drucker DJ. Glucagon receptor signaling is essential for control of murine hepatocyte survival. Gastroenterology 135: 2096–2106, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Taylor JA, Bernabe KQ, Guo J, Warner BW. Epidermal growth factor receptor-directed enterocyte proliferation does not induce Wnt pathway transcription. J Pediatr Surg 42: 981–986, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Taylor JA, Martin CA, Nair R, Guo J, Erwin CR, Warner BW. Lessons learned: optimization of a murine small bowel resection model. J Pediatr Surg 43: 1018–1024, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toumi F, Neunlist M, Cassagnau E, Parois S, Laboisse CL, Galmiche JP, Jarry A. Human submucosal neurones regulate intestinal epithelial cell proliferation: evidence from a novel co-culture model. Neurogastroenterol Motil 15: 239–242, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Vohra BP, Tsuji K, Nagashimada M, Uesaka T, Wind D, Fu M, Armon J, Enomoto H, Heuckeroth RO. Differential gene expression and functional analysis implicate novel mechanisms in enteric nervous system precursor migration and neuritogenesis. Dev Biol 298: 259–271, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Warner BW, Erwin CR. Critical roles for EGF receptor signaling during resection-induced intestinal adaptation. J Pediatr Gastroenterol Nutr 43, Suppl 1: S68–S73, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Warner BW, VanderKolk WE, Can G, Helmrath MA, Shin CE, Erwin CR. Epidermal growth factor receptor expression following small bowel resection. J Surg Res 70: 171–177, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Yusta B, Holland D, Koehler JA, Maziarz M, Estall JL, Higgins R, Drucker DJ. ErbB signaling is required for the proliferative actions of GLP-2 in the murine gut. Gastroenterology 137: 986–996, 2009 [DOI] [PubMed] [Google Scholar]