Abstract
More than 1,000 proteins of the nucleus, cytoplasm, and mitochondria are dynamically modified by O-linked β-N-acetylglucosamine (O-GlcNAc), an essential post-translational modification of metazoans. O-GlcNAc, which modifies Ser/Thr residues, is thought to regulate protein function in a manner analogous to protein phosphorylation and, on a subset of proteins, appears to have a reciprocal relationship with phosphorylation. Like phosphorylation, O-GlcNAc levels change dynamically in response to numerous signals including hyperglycemia and cellular injury. Recent data suggests that O-GlcNAc appears to be a key regulator of the cellular stress response, the augmentation of which is protective in models of acute vascular injury, trauma hemorrhage, and ischemia-reperfusion injury. In contrast to these studies, O-GlcNAc has also been implicated in the development of hypertension and type II diabetes, leading to vascular and cardiac dysfunction. Here we summarize the current understanding of the roles of O-GlcNAc in the heart and vasculature.
Keywords: cardioprotection, glycosylation, phosphorylation, signaling, survival
this review article is part of a collection on Post-translational Protein Modification in Metabolic Stress. Other articles appearing in this collection, as well as a full archive of all Review collections, can be found online at http://ajpheart.physiology.org/.
Introduction
The modification of intracellular proteins by monosaccharides of O-linked β-N-acetylglucosamine (O-GlcNAc) was first described in 1984 (162). Found on more than 1,000 proteins localized to the nucleus, cytoplasm, and mitochondria, O-GlcNAc is a common post-translational modification of metazoans (11, 51, 140, 159, 166, 170, 171, 174) and has recently been found on some prokaryotic proteins (148). O-GlcNAc modified proteins, or O-GlcNAcylated proteins, fall into diverse functional groups including: histones and other chromatin-associated proteins, transcription factors and RNA polymerase II, ribosomal proteins, proteasomal proteins, cytoskeletal proteins, signaling proteins such as protein kinases and phosphatases, metabolic enzymes, and viral proteins (11, 51, 140, 159, 166, 170, 171, 174).
The following observations support the notion that O-GlcNAc is a regulatory post-translational modification analogous to protein phosphorylation: 1) O-GlcNAc is not further extended into more complex oligo- or polysaccharides with the exception of plant nuclear pore proteins (62, 162); 2) the half-life of the O-GlcNAc modification is shorter that that of the protein it modifies (38, 86, 144); 3) O-GlcNAc levels respond dynamically to both extracellular (e.g., insulin) and intracellular (e.g., stress) stimuli (12, 13, 22, 38, 53, 74, 91, 118, 153, 188, 195); 4) sites of O-GlcNAc modification are similar to those used by protein kinases and are identical to those used by kinases on a subset of proteins (57); 5) O-GlcNAc is essential for life in mammals, arabidopsis and drosophila (45, 59, 131, 146, 149), and the deletion of the O-GlcNAc transferase (OGT) is lethal in mice [embryonic day (E) 4.5] cells and some tissues (131, 146); and 6) numerous studies have implicated O-GlcNAc in regulating enzyme activity, DNA binding, protein binding, localization, half-life, and regulating phosphorylation levels either by regulating protein kinases or by blocking amino acids that would otherwise be phosphorylated (58). For a subset of proteins, O-GlcNAc can block phosphorylation either by modifying the same Ser/Thr residue that would usually be modified (e.g., C-Myc, Thr 58) (23, 24), or sterically by modifying a nearby Ser/Thr residue (e.g., casein kinase 2, Ser347). Supporting a model in which a protein quickly cycles between O-GlcNAc modified and phosphorylated, OGT is found in a complex with protein phosphatase 1β and 1γ (177), and during cytokinesis OGT is found in a complex with OGT, O-GlcNAcase (the enzyme that removes O-GlcNAc), protein phosphatase 1, and aurora B kinase (152). Notably, on many proteins, such as α-B-crystalline, there is no relationship between phosphorylation and O-GlcNAcylation.
Recently, O-GlcNAc has been shown to modify many proteins that play key roles regulating the heart and vasculature (Table 1) and has been implicated in the etiology of hypertension and diabetes, as well as regulating the heart's ability to respond to both ischemia-reperfusion injury and trauma hemorrhage. Here we will focus on the role of O-GlcNAc in regulating physiological processes relevant to the heart and vasculature.
Table 1.
O-GlcNAc modified proteins in heart, erythrocytes, and vascular tissue*
| Protein and Site | Potential Role of O-GlcNAc | Tissue/Organism | Reference |
|---|---|---|---|
| α-Actin | None | Neonatal cardiomyocytes | (70) |
| Actin (Ser54, Ser157, Ser201, Ser234, Ser325, Ser370) | Unknown, although O-GlcNAc levels appear elevated in diabetic models (STZ treated rats and ob/ob mice). | Heart | (138) |
| Akt§ | O-GlcNAcylation is associated with reduced phosphorylation of Akt and is implicated in reducing angiogenesis. | Aorta | (95, 96, 106) |
| Ankyrin-1 (Ser288, Ser794, Ser960, Ser1162) | Unknown, but O-GlcNAc levels are elevated in patients with diabetes (Ser794 and Ser1162). | Erythrocytes | (169) |
| Aquaporin-1 (Ser236) | Unknown, but O-GlcNAc levels are elevated in patients with diabetes. | Erythrocytes | (169) |
| Band-3 anion transport protein (Ser162, Ser224, Ser745) | Unknown, but O-GlcNAc levels are reduced in patients with diabetes (Ser162 and Ser224). | Erythrocytes | (169) |
| Carbonic anhydrase (Ser130, Ser218) | Unknown, but O-GlcNAc levels are reduced in patients with diabetes (Ser30 and Ser218). | Erythrocytes | (169) |
| Catalase (Ser114, Ser254) | Unknown, but O-GlcNAc levels are elevated in patients with diabetes (Ser254). | Erythrocytes | (169) |
| Complex II, core 1† | Increased O-GlcNAcylation is linked to mitochondrial dysfunction in diabetes. | Neonatal cardiomyocytes | (64) |
| Complex III, core 2† | Increased O-GlcNAcylation is linked to mitochondrial dysfunction in diabetes. | Neonatal cardiomyocytes | (64) |
| Cox1† | Increased O-GlcNAcylation is linked to mitochondrial dysfunction in diabetes. | Neonatal cardiomyocytes | (64) |
| Desmin | Unknown | Neonatal cardiomyocytes | (70) |
| eNOS | Enhanced O-GlcNAc modification is associated with a reduction in phosphorylation at Ser1177 and a subsequent reduction in NO production. | Aorta | (95, 119) |
| Increased O-GlcNAcylation in DOCA-salt treated rats is associated with reduced phosphorylation at Ser1177. This may alter vascular relaxation contributing to hypertension. | |||
| Equilibrative nucleoside transporter 1 (Ser63) | Unknown. | Erythrocytes | (169) |
| Erythrocyte membrane protein band 4.2 (Ser82) | Unknown, but O-GlcNAc levels are reduced in patients with diabetes. | Erythrocytes | (169) |
| Fructose-bisphosphate aldolase | Unknown | Neonatal cardiomyocytes | (70) |
| Glut1 (Ser465) | Unknown | Erythrocytes | (169) |
| Glutathione S-transferase ω-1 (Ser13) | Unknown, but O-GlcNAc levels are elevated in patients with diabetes. | Erythrocytes | (169) |
| Glyceraldehyde-3-phosphate | Unknown | Neonatal cardiomyocytes | (70) |
| Hemoglobin subunit-α (Ser4, Ser36, Ser134) | Unknown, but O-GlcNAc levels are elevated in patients with diabetes (Ser 4, Ser 36). | Erythrocytes | (169) |
| Hemoglobin subunit-β (Ser50, Ser73, Thr85) | Unknown, but O-GlcNAc levels are elevated in patients with diabetes (Ser50 and Ser73). | Erythrocytes | (169) |
| HSP27 | Unknown | Neonatal cardiomyocytes | (70) |
| HSP60 | Unknown | Neonatal cardiomyocytes | (70) |
| Malate dehydrogenase | Unknown | Neonatal cardiomyocytes | (70) |
| Myosin heavy chain 6 (Ser172 Ser179, Ser196, Ser392, Ser622, Ser626, Ser644, Ser645, Ser749, Ser880, Ser1038, Ser1148, Ser1159, Thr1189, Ser1200, Ser1308, Ser1336, Ser1470, Ser1471, Ser1597, Thr1600, Thr1606, Ser1711, Ser1777, Ser19161) | Unknown | Heart | (138) |
| Myosin regulatory light chain 1 (Thr93, Thr164) | Unknown | Heart | (138) |
| Myosin regulatory light chain 2 (Ser15) | Unknown | Heart | (138) |
| NDUFA9∫† (Ser156) | Increased O-GlcNAcylation is linked to mitochondrial dysfunction in diabetes. | Neonatal cardiomyocytes | (64) |
| Peroxiredoxin-2 (Ser112, Thr18) | Unknown, but O-GlcNAc levels are reduced in patients with diabetes (Ser112 and Thr18). | Erythrocytes | (169) |
| Phospholamban† (Ser16, site identified by mutation of Ser residue to alanine) | O-GlcNAcylation is associated with reduced phosphorylation, and a subsequent increase in the association of phospholamban with SERCA2a. This association is correlated with decreased cardiac function in diabetic cardiomyopathy. | Neonatal cardiomyocytes | (189) |
| Proteasome subunit-α type-5 (Ser 198) | Unknown, but O-GlcNAc levels are elevated in patients with diabetes. | Erythrocytes | (169) |
| Protein 4.1 (Ser 491, Ser 792) | Unknown | Erythrocytes | (169) |
| Pyruvate kinase | Unknown | Neonatal cardiomyocytes | (70) |
| Sp1† | Increased O-GlcNAcylation is associated with reduced expression of SERCA2a in models of diabetes. | Neonatal cardiomyocytes | (26) |
| Spectrin-α chain (Ser844, Ser1250, Ser1737) | Unknown | Erythrocytes | (169) |
| Spectrin-β chain (Ser671, Ser767, Ser1297, Ser1652, Ser1936) | Unknown | Erythrocytes | (169) |
| Troponin I (Ser150). | Unknown | Heart | (138) |
| VDAC§†‡ | Decreased O-GlcNAcylation correlates with increased mitochondrial permeability. | Neonatal cardiomyocytes | (70, 127) |
| α-Synuclein (S87) | Unknown, but O-GlcNAc levels are elevated in patients with diabetes. | Erythrocytes | (169) |
As more than 1,000 proteins are now known to be O-linked β-N-acetylglucosamine (O-GlcNAc) modified, we refer readers to the following 2 sites for complete lists of O-GlcNAc modified proteins and amino acids: 1) http://cbsb.lombardi.georgetown.edu/hulab/OGAP.html and 2) http://www.phosphosite.org/homeAction.do;jsessionid=D89E4F582F1A5BE65C3034BC1883CDFF. ∫O-GlcNAc modification sites were mapped by BEMAD.
Identification by immunoprecipitation of the target protein was followed by Western blot with an O-GlcNAc specific antibody.
Identification by immunoprecipitation with an O-GlcNAc specific antibody or a GlcNAc-specific lectin was followed by detection with a protein specific antibody (this technique can produce false positives).
Spots that were reactive with an O-GlcNAc specific antibody on by two-dimensional PAGE were identified by mass spectrometry (this technique can produce false positives).STZ, streptozotocin; Cox1, cyclooxygenase 1; eNOS, endothelial nitric oxide (NO) synthase; DOCA, deoxycorticosterone acetate; HSP, heat shock protein; NDUFA9, NADH dehydrogenase [ubiquitin] 1α subcomplex subunit 9, mitochondrial; SERCA2a, cardiac sarcoplasmic reticulum Ca2+-ATPase; VDAC, voltage-dependent anion-selective channel.
STZ, streptozotocin; Cox1, cyclooxygenase 1; eNOS, endothelial nitric oxide (NO) synthase; DOCA, deoxycorticosterone acetate; HSP, heat shock protein; NDUFA9, NADH dehydrogenase [ubiquitin] 1α subcomplex subunit 9, mitochondrial; SERCA2a, cardiac sarcoplasmic reticulum Ca2+-ATPase; VDAC, voltage-dependent anion-selective channel.
The Biosynthesis of O-GlcNAc
The O-GlcNAc cycle is perpetuated by the differential regulation of just two enzymes, the OGT and O-GlcNAcase, as well as the levels of the substrate UDP-GlcNAc. How the cell regulates the dynamic modification of more than 1,000 proteins appropriately with just two enzymes has not yet been defined experimentally, though is thought to be regulated by protein-protein interactions of both OGT and O-GlcNAcase as well as alternative splicing of both enzymes (58).
The hexosamine biosynthetic pathway.
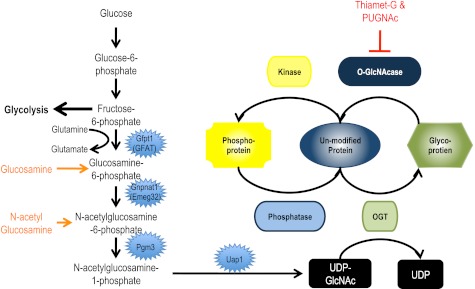
UDP-GlcNAc, the high-energy sugar donor used by OGT is synthesized by the hexosamine biosynthetic pathway (HBP). A small percentage of glucose imported into the cell is converted to UDP-GlcNAc through the HBP (Fig. 1), the rate-limiting step of which is catalyzed by glutamine:fructose-6 phosphatase amidotransferase (GFAT; Gfpt1). Notably, glucosamine, glutamine, and N-acetylglucosamine all feed into the HBP at different steps altering UDP-GlcNAc concentrations. These and other data have led to the hypothesis that many of the physiological effects of these compounds and hyperglycemia may be mediated and/or exacerbated by OGT and O-GlcNAcylation of proteins (56, 61, 143). Importantly, UDP-GlcNAc is also used for the biosynthesis of N-linked and O-linked glycans in the endoplasmic reticulum (ER)/Golgi, glycosylphosphatidylinositol anchors, and proteoglycans/glucosaminoglycans, and as such, changes to cell physiology attributed to altered UDP-GlcNAc concentrations may be independent of O-GlcNAc.
Fig. 1.
Metabolism of the O-linked β-N-acetylglucosamine (O-GlcNAc) modification. A small percentage of glucose imported into the cell is converted through the hexosamine biosynthetic pathway to UDP-GlcNAc. UDP-GlcNAc is the high-energy sugar donor for glycosylation by the O-GlcNAc transferase (OGT) and glycosylation in the endoplasmic reticulum and Golgi apparatus. Glucosamine and glutamine (orange) can be used for the synthesis of UDP-GlcNAc. Thiamet-G and O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc; red) are commercially available inhibitors of the O-GlcNAcase, the enzyme that removes O-GlcNAc. Notably, on a subset of proteins, the O-GlcNAc modification site is the same as that reported for phosphorylation. Thus, for some proteins, there is a reciprocal relationship between phosphorylation and the O-GlcNAc modification. In support of this model, OGT has been found in a complex with protein phosphatase 1α and γ, and during cytokinesis, OGT and O-GlcNAcase are found in a complex together and with protein phosphatase 1 and aurora kinase B. Gene names are as follows: Gfpt1, glutamine fructose-6-phosphate transaminase 1 (GFAT); Gnpnat1, glucosamine-phosphate N-acetyltransferase 1; Pgm3, phosphoglucomutase 3; Uap1, UDP-N-acetylglucosamine pyrophosphorylase 1.
Consistent with the OGT knockout, disrupting flux through the HBP and thus concentrations of UDP-GlcNAc has severe consequences (5, 49). Deletion of glucosamine-phosphate N-acetyltransferase 1 (EMeg32; Gnpnat1; Fig. 1), which reduces UDP-GlcNAc levels to 5% of normal, is embryonic lethal. However, embryonic fibroblasts isolated from these animals are viable although not adherent (5). More recently, the activity of phosphoglucomutase 3 (Pgm3), also known as phosphoacetylglucosamine mutase 1, which catalyzes the penultimate step of the HBP (Fig. 1), has been manipulated genetically (49). Similar to deleting OGT and glucosamine-phosphate N-acetyltransferase 1, deletion of Pgm3 results in embryonic lethality between E3.5 and E6.5, again highlighting the importance of O-GlcNAcylation and other glycoconjugates (49). Notably, the authors also generated a number of hypomorphic mutations in Pgm3, which allowed them to study the effects of incremental changes in UDP-GlcNAc levels (49). Male mice carrying the hypomorphic mutations are infertile, and structural changes are observed in the testis, kidney, pancreas, and salivary gland and in certain hematopoietic cell lineages (red blood cells, lymphocytes platelets) (49). The latter is not surprising, as Pgm3 is upregulated by erythropoietin. These mice should be useful tools for studying the role of flux though the HBP and subsequent O-GlcNAc addition.
The UDP-GlcNAc: polypeptide OGT (EC 2.4.1.94; GI:6006036) catalyzes the addition of O-GlcNAc.
OGT is a noncanonical glycosyltransferase expressed as a soluble protein in the nucleus, cytoplasm, and mitochondria (84, 104). OGT is characterized by two functional domains, the NH2-terminal tetratrichopeptide repeat domain (TPR domain) (84, 104) that is structurally similar to importin-α (69) and a COOH-terminal catalytic domain that belongs to the glycogen phosphorylase superfamily (179). Recently, the structures of a bacterial homolog of OGT (27, 116), a truncated human OGT (with only 4.5 of the 11.5 TPR domains) (90), and the TPR domain have been solved (69). Collectively, these data suggest that the TPR domain forms a long helical domain that is capped by the globular catalytic domain. Interestingly, the catalytic domain contains two Rossman-type folds separated by an intervening sequence (90, 179). The catalytic domain appears to have two conformations: an open conformation in which extended peptide substrates can access the narrow active site and a closed conformation (90). These and other data suggest that UDP-GlcNAc is bound first, before the peptide gains access to the active site (90).
While there is only one copy of OGT in the genome, on the X-chromosome, several splice variants exist of which three are well characterized (55, 84, 103, 104): 1) full-length OGT which is the predominant form in the nucleus and cytoplasm (ncOGT), 2) short OGT, and 3) mitochondrial OGT. These splice variants, as well as interacting proteins, and the concentrations of UDP-GlcNAc are thought to regulate the substrate specificity of OGT (19, 20). Data that supports these conclusions includes the following observations. First, ncOGT associates with p38 MAPK in response to glucose deprivation. While ncOGT and p38 MAPK do not modify each other, p38 MAPK appears to target ncOGT to its substrates (19). Second, short truncations of the TPR domain reduce the activity of ncOGT toward peptide and protein substrates (85, 105). Third, yeast two-hybrid and pull-down analyses demonstrate that OGT associates with numerous proteins (20, 21). Fourth, ncOGT changes its preferences for peptide substrates in vitro in the presence of different UDP-GlcNAc concentrations (85). These data suggest that at different UDP-GlcNAc concentrations in the cell, the enzyme would glycosylate different subsets of proteins and associate with different proteins providing differential targeting. Thus these changing specificities, as well as protein-protein interactions, may explain why there is no consensus motif for the addition of O-GlcNAc.
Walker and coworkers have isolated a number of OGT inhibitors that work well in vitro (50) and in some cells in vivo (10, 127). However, in our hands these inhibitors (5–100 μM, 4–18 h) have not had a significant effect on O-GlcNAc levels in U2OS, HeLa, MEF, and Cos-7 cells (N. E. Zachara, unpublished observations). Recently, Vocadlo and coworkers (48) have reported the use of a metabolic inhibitor that appears to work well in cell culture. Researchers interested in detecting the addition of O-GlcNAc to their proteins of interest are directed to recent reviews on the subject (196, 197). As both peptides and proteins modified by asparagine-N-GlcNAc (170, 171) and asparagine-N-GlcNAc2 (67) have been reported in the literature, particular care should be taken to perform appropriate controls sinces many techniques used to detect O-GlcNAc will also detect these modifications.
O-GlcNAcase catalyzes the removal of O-GlcNAc (EC 3.2.1.52, GI:1364613, mgea5).
O-GlcNAcase, sometimes known as hexosaminidase C, is a neutral β-N-acetylglucosaminidase that is expressed predominantly in the cytoplasm although it is also found in the mitochondria and nucleus (47, 64, 176). Unlike the lysosomal hexosaminidases, hexosaminidase A and hexosaminidase B, O-GlcNAcase is specific for β-N-acetylglucosamine and has a neutral pH optimum (pH 4.5–6) (32, 33, 47, 175). There are two major splice variants of O-GlcNAcase, a short form (∼75 kDa) and a longer form (∼130 kDa) (29, 47). Each splice variant contains the NH2-terminal hexosaminidase domain that is similar to hyaluronidases (32, 33, 47, 175). The COOH-terminal domain has similarity to acetyltransferases and has been suggested to have histone acetyltransferase activity, although the physiological significance of this activity is unknown (160). Supporting a role for O-GlcNAc in the development of type II diabetes, mutations and alternative splice variants of O-GlcNAcase are associated with the onset of type II diabetes (40, 92). Goto-Kakizaki rats express a 90-kDa isoform, Δ250–345, of O-GlcNAcase that appears to lack O-GlcNAcase activity (37).
Little is known about the regulation of O-GlcNAcase, although it purifies in a complex with other proteins (20, 47, 176), is both phosphorylated and O-GlcNAc modified (47, 175), and interestingly is cleaved by caspase-3 during apoptosis (9, 175). O-GlcNAcase is efficiently inhibited in vitro and in vivo by a number of compounds (25, 34, 35, 78–80, 89, 145, 147, 157, 191), although only two are commercially available (52, 191): O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc) and Thiamet-G.
Several investigators have shown that modulating O-GlcNAc levels pharmacologically or genetically does not result in the desired outcome, in part as the cell responds to this intervention by modulating the expression of the endogenous OGT and O-GlcNAcase (73, 154). For example, elevating O-GlcNAc levels is often accompanied by a suppression of the endogenous OGT and an induction of the endogenous O-GlcNAcase, presumably to restore basal levels of O-GlcNAc. Conversely, when O-GlcNAc levels are lowered, the expression of the O-GlcNAcase is lowered and OGT expression is elevated. The mechanism that regulates this internal O-GlcNAc rheostat is unknown; however, it appears to be regulated by the levels of O-GlcNAc rather than the association of OGT and O-GlcNAcase (20). Using an inducible OGT null cell line, Kazemi and coworkers (73) demonstrated that the OGT null is effectively an O-GlcNAcase null as well. Notably, if O-GlcNAc levels were elevated with an inhibitor of O-GlcNAcase at the induction of the knockout, O-GlcNAcase expression was stabilized in a dose-dependent manner (73). The mechanism that underlies these observations remains to be elucidated.
The Roles of O-GlcNAc in Regulating the Heart and Cardiovascular System
O-GlcNAc has been implicated in regulating many processes in the heart and vasculature, and these are discussed in detail below. Incongruously, O-GlcNAc has been implicated in regulating both the detrimental effects of diabetes as well as survival in models of trauma hemorrhage and ischemia-reperfusion injury. The molecular basis for these paradoxical observations has not yet been defined but may reflect changes in O-GlcNAc cycling, altered specificity of the enzymes, or the interaction of O-GlcNAc modified proteins with other signaling pathways that are differentially regulated during cellular stress/injury and diabetes.
O-GlcNAc as a regulator of cell survival.
O-GLcNAc, A NOVEL REGULATOR OF THE CELLULAR STRESS RESPONSE.
In 2004, O-GlcNAcylation was reported to increase on myriad proteins in response to multiple forms of cellular stress [heat shock, ER Stress (DTT), reductive stress, the hypoxia mimic CoCl2, oxidative stress, ethanolic stress, osmotic stress, genotoxic stress (UVB), arsenite stress, viral infection, translational inhibition (cycloheximide), and potentially ubiquitin stress (inhibition of the proteasome)] (195). Notably, the increase in O-GlcNAc levels was post-translational, found in numerous primary and transformed cell lines, and dose dependent (195). Moreover, elevating the levels of O-GlcNAc either genetically or pharmacologically promoted cell survival, whereas depressing the levels of O-GlcNAc suppressed cell survival (195). Together, these data suggested that O-GlcNAc is a novel post-translational modification used to sense and transmit cellular stress signals. In support of this hypothesis, O-GlcNAc appears to regulate numerous pathways (Table 2) in a manner consistent with stress tolerance in both in vivo and in vitro models of heat stress (156, 195), hypoxia (125, 127, 128), UV irradiation (102), oxidative stress (70), acute vascular injury (181), trauma hemorrhage (129, 130, 183, 198, 199), and ischemia-reperfusion injury (15, 16, 43, 44, 66, 88, 98–100, 126, 128).
Table 2.
Pathways implicated in protecting cells and tissues from cellular injury
| Pathway | Role of O-GlcNAc | Tissue | Reference |
|---|---|---|---|
| Regulation of reactive oxygen species | Reducing O-GlcNAc levels reduces catalase mRNA expression, whereas elevating O-GlcNAc levels increased catalase mRNA expression.† | Isolated neonatal cardiomyocytes | (128) |
| Reduced expression of proinflammatory cytokines | Reduced expression of circulating inflammatory cytokines (TNFα, IL-6, IL-10), probably through regulation of the NF-κB signaling pathway. | Cardiac tissue and isolated neonatal cardiomyocytes, kidney, liver | (129, 130, 183, 198, 199) |
| Regulation of protein stability | In proteins overexpressing OGT, proteins such as Sp1 appear more heat stable, and this enhances transcription of proteins such as HSPs. | HeLa | (94) |
| Expression of stress-related genes | OGT and O-GlcNAcase have been found on the promoters of numerous stress-related genes. Deletion of OGT renders Caenorhabditis elegans sensitive to UV irradiation. | C. elegans | (102) |
| Regulating the expression of HSPs | Regulation of GSK3β, Sp1, and HSF1 have been implicated in regulating the expression of HSPs. | Cos-7 Cells, mouse embryonic fibroblasts, neonatal cardiomyocytes, hepatocytes | (73, 94, 150, 195) |
| Regulation of GSK3β | O-GlcNAc appears to regulate pathways upstream of GSK3β, leading to inactivation by phosphorylation at Ser09. | MEFs, Cos-7 cells | (73, 87) |
| Mitochondrial membrane potential | Numerous studies have demonstrated the elevated levels of O-GlcNAc protect membrane potential and cellular ATP levels. | Isolated neonatal cardiomyocytes | (15, 70, 125, 127, 128) |
| Reduced calpain activation | May be regulated indirectly, though modulation of intracellular calcium levels | Isolated neonatal cardiomyocytes | (99) |
| Calcium homeostasis | The mechanisms by which O-GlcNAc mediates capacitive calcium entry and calcium overload are unknown, although recently O-GlcNAc was shown to modify and regulate the IP3 receptor. | Isolated neonatal cardiomyocytes | (122, 125, 127, 128, 132, 139) |
| Regulation of the p38 MAPK pathways | Glucosamine enhances signaling of p38 MAPK in response to agonist stimulation, leading to enhanced phosphorylation of HSP27 and αB-crystallin. | Isolated hearts | (43) |
| Formation of mPTP | O-GlcNAc reduces calcium overload and reactive oxygen species, two triggers known to stimulate pore opening. VDAC, which is thought by some to be a component of the mPTP, is O-GlcNAc modified.§ | Isolated neonatal cardiomyocytes | (125, 127, 128) |
The molecular mechanism by which this occurs has not been defined.
The importance of VDAC in forming the mitochondrial permeability transition pore (mPTP) is controversial. However, VDAC has been implicated in regulating cell death through other mechanisms including its association with hexokinase 2. OGT, O-GlcNAc transferase; GSK3β, glycogen synthase kinase 3β; HSF1, heat shock factor 1; IP3, inositol 1,4,5-trisphosphate.
While several pathways have been implicated in promoting cell survival in an O-GlcNAc-dependent manner (Table 2), only one of these has been attributed to O-GlcNAcylation of a specific protein. Recently, Ku and coworkers (87) demonstrated that O-GlcNAcylation of Keratin 18 (Ser30, Ser31, Ser49) was essential for signaling by Akt and that subsequent inactivation of the proapoptotic kinase GSK3β in a model of streptozotocin or FAS/PUGNAc induced apoptosis in the liver and pancreas (87). Another study has highlighted the role of O-GlcNAc in the inactivation of GSK3β, although here no defect in Akt signaling was observed (73). In search of additional mechanisms by which O-GlcNAc is protective, a number of studies have identified proteins whose O-GlcNAcylation status changes in response to stress [Table 3; (159, 194)]. These and other studies suggest that some proteins are modified in response to diverse forms of injuries, whereas other proteins are modified only in response to specific injuries.
Table 3.
Proteins whose O-GlcNAc modification state changes in response to stress or injury
| Protein and Site | Stress/Injury | Tissue/Organism | Reference |
|---|---|---|---|
| Carm1 | Heat stress | Cos-7 cells | (194) |
| Cytokeratin 8/18 | Heat stress | Cos-7 cells | (93) |
| DNA PK | Heat stress, ER Stress | Cos-7 cells | (194) |
| Histone H2B | Heat stress | HeLa cells | |
| IRS-1 | Urea (20 mM), probably working through increased ROS | 3T3-L1 adipocytes | (31) |
| Neurofilament H | Glucose deprivation | Neuro-2A | (19) |
| NF90 and NF110 | Heat stress | Cos-7 cells | (194) |
| Nup153 | Heat stress | Cos-7 cells | (194) |
| P125i (Associates with Sec23) | Heat stress | Cos-7 cells | (194) |
| Sec24b | Heat stress | Cos-7 cells | (194) |
| WNK1 | Heat stress | Cos-7 cells | (194) |
Recently 2 screens have focused on identifying proteins whose O-GlcNAcylation status responds to cellular injury (heat stress, hydrogen peroxide, hypoxia, urea, and trauma hemorrhage). Such proteins are listed here only if their O-GlcNAcylation status was confirmed either by immunoblot or the O-GlcNAc-peptide linkage was confirmed by mass spectrometry. Carm1, coactivator-associated arginine methyltransferase 1; DNA PK, DNA protein kinase; IRS-1, insulin receptor substrate 1; Nup153, nuclear pore protein 153; WNK1, with no lysine kinase 1.
The mechanisms that lead to enhanced levels of O-GlcNAc in response to different forms of cell stress appear varied, and it is unclear if this depends on the type of injury or the cell/tissue type. There is some evidence that glucose uptake and flux through the HBP are important in ischemia-reperfusion injury and trauma hemorrhage (16, 43, 156). This leads to the attractive hypothesis that one end point of “flight or fight response”-induced hyperglycemia is O-GlcNAc (17). Reinforcing this idea, it appears that various forms of stress can activate GFAT, which catalyzes the rate-limiting step in UDP-GlcNAc biosynthesis (36, 137). In response to a number of forms of stress the expression of OGT appears elevated, these include UV irradiation, osmotic stress, and ethanolic stress (195), whereas heat stress appears to increase the activity of OGT increases threefold (195). Finally, as discussed in section The UDP-GlcNAc: polypeptide OGT (EC 2.4.1.94; GI:6006036) catalyzes the addition of O-GlcNAc, in models of nutrient deprivation OGT appears to be targeted to substrates in the absence of increases in UDP-GlcNAc levels by p38 MAPK (19). Little is known about the regulation of O-GlcNAcase during stress; however, its expression and activity is not altered by heat stress (195).
O-GLcNAc, A NOVEL ENDOGENOUS CARDIOPROTECTIVE POST-TRANSLATIONAL MODIFICATION.
Ischemia-reperfusion injury in the heart is characterized by calcium overload, oxidative stress, ER stress, structural and functional changes to the mitochondria (17), and changes in the O-GlcNAcylation status of numerous proteins. In hearts subjected to either ischemia-reperfusion injury (in vivo) or simulated ischemia (ex vivo), O-GlcNAc levels appear to drop before increasing during reperfusion (15, 16, 43, 70). This can be mimicked in isolated neonatal cardiomyocytes subjected to hypoxia and reoxygenation, where lower levels of O-GlcNAc are observed during hypoxia and then increase above basal levels during reoxygenation (127). Notably, both the acute and delayed models of ischemic preconditioning lead to elevated levels of O-GlcNAc. As yet, it is unclear if increased O-GlcNAcylation is required for the protective effects of ischemic preconditioning (70).
Elevating O-GlcNAc levels before and after ischemia have been reported to reduce both cell/tissue death in isolated neonatal cardiomyocytes, isolated hearts, and in hearts in vivo by mitigating each of the characteristics of ischemia-reperfusion injury (15, 16, 43, 44, 66, 70, 88, 98–100, 122, 125–128, 132, 198) (Table 2). In addition, elevating O-GlcNAc levels prior to injury appears to reduce proinflammatory mediators (TNFα, IL-1, IL-6) in other models of tissue injury (18, 66, 181, 183, 198), suggesting additional pathways by which O-GlcNAc promotes survival of myocardial tissue. Glucose, glutamine, and glucosamine have all been shown to be cardioprotective, and several studies have attributed this to changes in O-GlcNAcylation (15, 16, 43, 98, 111, 121, 161). Interestingly, the cardioprotective effects of salidroside, a β-glucose analog, have also been attributed to O-GlcNAc by a mechanism that is still unclear (180).
O-GLcNAc, HYPERTROPHY, AND THE FAILING HEART.
In models of pressure overload-induced hypertrophy, the cellular concentrations of UDP-GlcNAc become elevated, and this is believed to be a consequence of increased expression of GFAT2 (190). Consistent with the idea that hypertrophy is associated with increased flux through the HBP, increased expression of GFAT and elevated levels of O-GlcNAcylation are observed in aged Brown-Norway rats, which also demonstrate age-dependent increases in cardiac hypertrophy (41). Moreover, in the failing heart, elevated levels of N-acyl-d-glucosamine 2-epimerase, which can contribute to HBP flux, have been reported (83). Together, these data suggest that flux through the HBP alters the physiology of hearts/cardiomyocytes in an O-GlcNAc-dependent manner. One consequence of this signaling appears to be a glucose-dependent change in gene expression. Young and coworkers (190) demonstrated in both ex vivo and in vivo models that glucose (25 mM) metabolism induced a change in the ratio of myosin heavy chain isoforms, increasing the expression of myosin heavy chain-β (fetal). Recently, reducing O-GlcNAc levels has been shown to restore hypertrophy signaling in a rodent model of diabetes (112). In contrast to these data, in exercise-induced hypertrophy, reduced levels of O-GlcNAc have been observed, suggesting that more research in this field is required before a clear model is delineated (4).
Recently, Watson and colleagues (172) demonstrated a role for O-GlcNAc in a model of infarct-induced heart failure (172). In addition to the typical phenotypes associated with heart failure (scaring, elevated heart weight, pulmonary edema, and increased atrial natriuretic peptide), elevated levels of O-GlcNAc were observed (172). Consistent with the models of hypertrophy discussed above, elevated expression of GFAT1 was observed, as well as increased expression of OGT and reduced expression of O-GlcNAcase. To study the role O-GlcNAc in heart failure further, OGT was deleted from hearts using Cre-lox technology and perhaps unexpectedly the significant reduction in O-GlcNAc did not result in cardiac dysfunction, hypertrophy, apoptosis, or fibrosis. However, deleting OGT appeared to exacerbate infarct-induced heart failure, resulting in reduced survival of mice, increasing the rate of apoptosis in the remote noninfarcted myocardium, and reducing ventricular function (172).
TRAUMA HEMORRHAGE.
Hypovolemia, a result of trauma hemorrhage, is a leading cause of death as a result of multiple organ failure. Enhanced glucose levels have been associated with improved survival in models of trauma hemorrhage, and recent studies have suggested that this is in part due to changes in O-GlcNAcylation (18, 129, 130, 183, 198, 199). In vivo trauma hemorrhage suppresses O-GlcNAc levels, and simply maintaining O-GlcNAc levels with glucosamine treatment improves the function of multiple organs and blood pressure and reduces the expression of the proinflammatory cytokines TNFα and IL-6 (129, 130, 183, 198, 199). Indicating that these affects are mediated by O-GlcNAc, these data could be recapitulated by treatment with PUGNAc or by altering the expression of OGT (199). More importantly, treating animals during resuscitation with glucosamine or PUGNAc maintained O-GlcNAc levels and elevated survival from 53 (control) to 85 and 86%, respectively (129).
INFLAMMATION.
One mechanism by which O-GlcNAc is thought to promote survival is by reducing the detrimental effects of prolonged or unregulated inflammation (66, 129, 181, 198), although O-GlcNAc has also been associated with hyperglycemia-induced inflammation (184). In numerous models, altering O-GlcNAc levels metabolically, pharmacologically, or genetically leads to a depression in the circulating levels of proinflammatory cytokines such as TNFα, IL-10, and IL-6 (66, 129, 181, 198). Moreover, the expression of intracellular adhesion molecule 1 (ICAM-1) is reduced, which should reduce neutrophil infiltration preventing further injury (198). Consistent with this observation, elevated levels of O-GlcNAc result in lower activity of cardiac myeloperoxidase in a model of trauma hemorrhage (198). O-GlcNAc also appears to regulate the NF-κB signaling pathway, resulting in these observations (198), both directly as NF-κB is O-GlcNAc modified (68) and indirectly as the modulation of O-GlcNAc levels appears to regulate the phosphorylation of IκB (198). This is probably a general response of cells, as this can be recapitulated in models in which cells are treated with LPS (198).
It is also possible that cells/tissues are less sensitive to inflammatory mediators. In endothelial cells, one outcome of TNFα activation is expression of monocyte chemoattractant protein-1 (MCP1) and ICAM-1 (71). Glucosamine, which was shown to elevate global O-GlcNAc levels, repressed the induction of MCP-1 and ICAM-1, suggesting that in human umbilical vein endothelial cells, glucosamine and O-GlcNAc are acting to suppress inflammatory signaling. Notably, TNFα acts through p38 MAPK to phosphorylate and activate the p65 subunit of NF-κB, and glucosamine appears to suppress this pathway (71).
Another model in which O-GlcNAc appears to reduce inflammation is a model of acute arterial injury, preventing neointima formation (181). Neointima, or thickening of the arterial intima, contributes to the pathology of atherosclerosis, in-stent restenosis, vein bypass graft failure, and transplant vasculopathy (120). Xing and coworkers (181) demonstrated that in response to acute injury of the carotid artery, the levels of O-GlcNAc dropped globally (181). Pretreating animals with glucosamine or PUGNAc increased O-GlcNAc on a subset of proteins and reduced the expression of a number of chemokines (cytokine-induced neutrophil chemoattractant-2β, MCP-1) and adhesion molecules (p-selectin, VCAM-1) associated with vascular remodeling (181). Consistent with this observation, elevating O-GlcNAc levels reduced infiltration of granulocytes and monocytes into the arterial wall and the appearance of neointima (181).
O-GlcNAc and diabetes.
The seminal work of Marshall and coworkers (114) demonstrated that the HBP (HBP; Fig. 1) plays a key role in the development and complications associated with type II diabetes. Here, high levels of glucose were unable to induce insulin resistance if the rate-limiting step of the HBP, GFAT, was inhibited pharmacologically with either 6-diazo-5-oxonorleucine or azaserine [Fig. 1; (114)]. Conversely, overexpression of GFAT exacerbated the effects of hyperglycemia (30, 61). Several laboratories have suggested that this is due to both changes in UDP-GlcNAc levels and thus the O-GlcNAc modification (1, 3, 8, 31, 39, 40, 46, 54, 60, 72, 82, 101, 109, 110, 133–136, 155, 167, 169, 185, 187), as well as other metabolites (115, 124) and possibly other forms of protein glycosylation (65, 173). Key data that support a role for O-GlcNAc in mediating the complications associated with type II diabetes includes 1) overexpression of O-GlcO-GlcOGT in the muscle and adipose of mice results in insulin resistance and hyperleptinemia, two hallmarks of type II diabetes (117); 2) deletion of either OGT or O-GlcNAcase in Caenorhabditis elegans leads to changes in glucose and trehalose metabolism, as well as dauer phenotypes characteristic of disrupted insulin signaling (40, 54); 3) PUGNAc treatment, an inhibitor of the O-GlcNAcase and lysosomal hexosaminidases, leads to insulin resistance in cell culture models and tissue explants (3, 134, 167); 4) in several models of diabetes O-GlcNAc levels are elevated on a subset of proteins (1, 2, 8, 42, 64, 113, 133, 138, 169); 5) numerous pathways are regulated by NAc, such as insulin signaling via Akt (3, 6, 40, 54, 119, 134–136, 167, 185); and 6) reducing the levels of O-GlcNAc in models of type II diabetes reverses the some of the complications associated with type II diabetes (26, 63, 64, 125). However, recent data suggests that elevating O-GlcNAc levels alone may not be sufficient to induce diabetes: 1) treating 3T3-L1 adipocytes with PUGNAc but not a more specific inhibitor of O-GlcNAcase (Thiamet-G) results in insulin resistance (107), 2) treating mice for long periods of time with Thiamet-G does not alter glucose metabolism (108), and 3) overexpressing O-GlcNAcase does not restore glucose metabolism in 3T3-L1 adipocytes (142). Collectively, these data suggest that elevating O-GlcNAc levels alone may not be sufficient for the induction of diabetes but must be associated with some other type of dysregulation [e.g., advanced glycation end-product formation, increased PKC activity, increased flux though the polyol pathway, and increased oxidative stress (7)] or that dysregulation of other pathways impacts the sites and dynamics of the O-GlcNAc modification contributing to diabetes. One mechanism by which diabetes may affect the sites of O-GlcNAcylation is increased UDP-GlcNAc levels, as discussed in section The UDP-GlcNAc: polypeptide OGT (EC 2.4.1.94; GI:6006036) catalyzes the addition of O-GlcNAc. An alternative mechanism may be altered targeting of the OGT, and recently Yao and coworkers (186) have demonstrated one example of this: one side effect of diabetes is increased modification of Arg/Lys residues with methylglyoxal, a highly reactive α-oxoaldehyde. Modification of the corepressor mSin3A with methylglyoxal at Arg925 and Lys938 resulted in recruitment of OGT and subsequent O-GlcNAcylation of the transcription factor specificity protein 3. Ultimately, O-GlcNAcylation of Sp3 transcription factor leads to an induction of angiopoitien-2, which sensitizes the microvasculature of the kidney to TNFα (186).
O-GLcNAc, DIABETES, AND CARDIOVASCULAR DYSFUNCTION.
Several laboratories have clearly demonstrated a role for O-GlcNAc in regulating proteins in a manner that would result in cardiac or vascular dysfunction, and these are discussed below. Several studies have determined the levels of O-GlcNAc in models of diabetes in cardiovascular tissues 1) on erythrocyte proteins in patients with type II diabetes (133, 169), 2) in hearts from Zucker diabetic fatty rats (42), 3) in hearts from db/db mice (112), 4) in rat hearts treated with streptozotocin (63), and 5) in hearts from ob/ob mice (138). In all cases diabetes results in elevated levels of O-GlcNAc on a subset of proteins.
One of the pathophysiologies associated with type II diabetes is the development of cardiomyopathy, in which many aspects of cardiac contractility are impaired. Underlying these observations are changes in cardiac calcium (Ca2+) handling thought to result from decreased sarcoplasmic reticulum (SR) Ca2+ uptake, reduced SR Ca2+ release, and reduced SR Ca2+ content. Like many of the studies discussed in O-GlcNAc and diabetes, glucose and glucosamine treatment of cardiomyocytes had been associated with impaired cardiac contractility and increased intracellular calcium. These data led to the hypothesis that O-GlcNAcylation of key proteins may lead to cardiac dysfunction in models of type II diabetes (26, 63, 125). Supporting this hypothesis, several studies have demonstrated that the diastolic decay phase of calcium transients is delayed in neonatal cardiomyocytes treated with high concentrations of extracellular glucose (25 mM), glucosamine (8 mM), or PUGNAc (50 μM) (26, 63, 125). Notably, they also demonstrated that overexpressing the O-GlcNAcase ablated the effect of high glucose on calcium handling, whereas overexpressing OGT exacerbated the effect (26, 63, 125). Together, these data suggest that nonphysiological elevations in O-GlcNAc levels alter calcium handling in neonatal cardiomyocytes but that this was dependent on high-glucose levels, since overexpressing either OGT or O-GlcNAcase had no effect on cells maintained in physiological concentrations of glucose (5.5 mM).
Several mechanisms have been reported that would alter calcium handling in the diabetic heart, and these center on cardiac sarcoplasmic reticulum Ca2+-ATPase (SERCA2a), the Ca2+ ATPase that transfers Ca2+ from the cytosol of the cell to the lumen of the SR. In one model, changes in the expression of SERCA2a appear to be regulated transcriptionally, and this is attributed to increased O-GlcNAcylation of Sp1 and decreased expression of myocytes enhancer factor 2A, two transcription factors known to regulate the expression of SERCA2a (26, 63, 125). O-GlcNAc has also been reported to regulate SERCA2a through its association with phospholamban. Yokoe and coworkers (189) showed that phospholamban was O-GlcNAcylated at Ser16 and that diabetes led to higher levels of O-GlcNAc at this site and lower phosphorylation. They suggest that this ultimately reduces SERCA2a activity through a direct association with phosphorylated-phospholamban (189). In an alternative model, diabetes has been shown to reduce expression of phospholamban, which would result in the same phenotype (63).
High glucose in neonatal cardiomyocytes also alters mitochondrial function, in part by direct modulation of mitochondrial proteins. Recently, Hu and coworkers (63) have shown that elevated levels of O-GlcNAc are associated with decreased function of the mitochondrial electron transport complexes—complex I, III, and IV. Importantly, reducing the levels of O-GlcNAc by overexpression of O-GlcNAcase reversed the high-glucose phenotype. Consistent with reduced mitochondrial function, lower levels of cellular ATP were observed in cells grown in high levels of glucose (63).
Hyperglycemia, and subsequent upregulation of O-GlcNAcylation, has also been linked to the development of atherosclerosis. Federici and coworkers (39) demonstrated that in human coronary artery endothelial cells high glucose and glucosamine elevated the levels of O-GlcNAc on a number of key proteins involved in insulin signaling: insulin receptor substrate 1, insulin receptor substrate 2, and the p85 subunit of phosphatidylinositol 3-kinase. Moreover, elevated O-GlcNAc levels were associated with decreased insulin signaling downstream of the insulin receptor, in particular through the phosphatidylinositol 3-kinase Akt/PKB signaling pathway. Increased levels of O-GlcNAc led to reduced phosphorylation of endothelial nitric oxide synthase (eNOS) at Ser1177; this inhibition of eNOS led to increased activity and expression of matrix metalloproteinase (MMP)-2 and MMP-9 (39). An altered balance between MMPs and inhibitors of MMPs has been implicated in the etiology of atherosclerosis. Consistent with increased MMP activity, plaques from patients with diabetes demonstrated higher levels of O-GlcNAcylation than controls (39).
O-GlcNAc and hypertension.
Hypertension is characterized by abnormal vascular reactivity, impaired endothelium-dependent relaxation, and enhanced sensitivity to vasoconstrictors. Hypertension is also a major risk factor for cardiovascular disease and is often associated with diabetes. Interestingly, the kinase “with no lysine kinase 1 (WNK1)” has been isolated as being O-GlcNAc modified in several studies (77, 159, 194), and O-GlcNAcylation increases in response to heat stress (194). While the function of WNK1 O-GlcNAcylation has not been studied, mutations in WNK1 and WNK4 lead to familial hypertension (178, 182).
Several studies have demonstrated that elevating O-GlcNAc levels induces many of the hallmarks of hypertension, such as increasing reactivity to constrictor stimuli (phenylephrine) and impaired endothelium-dependent vasodilatation (95–97). The mechanisms that underlie these observations appear to be regulated in part by decreased phosphorylation of Akt (Ser473) and eNOS (Ser1177) (95–97). Previously, eNOS was shown to be hyper-O-GlcNAcylated in a model of diabetes-induced erectile dysfunction. Notably, O-GlcNAcylation of eNOS prevented phosphorylation by Akt at this site and was associated with reduced nitric oxide production (119).
Lima and coworkers (96) demonstrated that hypertensive rats, induced by deoxycorticosterone acetate and salt (DOCA-salt), had elevated levels of O-GlcNAc on numerous proteins in both aorta and mesenteric arteries. The effect of hypertension, impaired endothelium-dependent relaxation, and enhanced sensitivity of vasoconstrictors could be recapitulated simply by elevating O-GlcNAc levels with an inhibitor of the O-GlcNAcase (PUGNAc, Fig. 1). However, PUGNAc and DOCA-salt did not have an additive effect, suggesting that DOCA-salt may be working through O-GlcNAc. Again, the effects of hypertension were linked to a reduction in phosphorylation of key signaling molecules eNOS and Akt. Notably, the reduction in eNOS phosphorylation may result from direct competition with O-GlcNAc, as Lima and coworkers (96) demonstrated that DOCA induced eNOS O-GlcNAcylation.
In subsequent work, Lima and coworkers (97) have demonstrated the endothelin-1 (ET-1) and PUGNAc augment vascular contraction to phenylephrine in vascular smooth muscle cells. Interestingly, ET-1 appears to enhance O-GlcNAc levels, suggesting that like DOCA-salt, ET-1 may work via O-GlcNAc. Consistent with the hypothesis that ET-1 regulates the RhoA/Rho kinase pathways in an O-GlcNAc-dependent manner, ET-1-dependent phosphorylation of protein phosphatase 1 regulatory protein, myosin light chain, and myosin light chain phosphatase target protein 1 could be reversed with an OGT inhibitor or small interfering RNA of OGT (97). Moreover, ET-1 induction of RHO activity was again blocked by small interfering RNA of OGT (97). Together,these data may suggest that O-GlcNAc plays a role in regulating vascular reactivity.
Conclusions
O-GlcNAc is a novel post-translational modification that appears to be a key regulator of cell function that is crucial for heart and vascular function. Our understanding of the molecular mechanisms by which O-GlcNAc regulates key proteins and signaling events and how this is altered by diseases such as aging, hypertension, and diabetes is in its infancy. One challenge to advancing the field lies in the modification itself, which is challenging to detect and resists genetic/pharmacological manipulation. There have been a number of recent advances in methodologies for detecting O-GlcNAc modified proteins (28, 75, 76, 81, 123, 141, 151, 158, 159, 163–166, 170, 171, 192, 193), mapping the sites of addition of O-GlcNAc (11, 14, 81, 141, 151, 170), probing the dynamics of O-GlcNAcylation (12, 13, 77, 165, 168, 194), and genetic models in which O-GlcNAc levels can be manipulated (5, 49, 73, 131, 146, 172). Together, this suite of technologies should allow researchers to answer some of the key remaining questions: 1) How is the specificity of OGT and O-GlcNAcase regulated, and how is this altered during aging or in disease models? 2) What is the molecular basis of the O-GlcNAc paradox, and why is O-GlcNAc protective in acute models of injury and destructive in models of sustained injury? and, finally, 3) What is the function of O-GlcNAc on proteins, and how does this network of O-GlcNAc modified proteins act to regulate heart and vascular function?
GRANTS
This work was funded by the American Heart Association and by National Heart, Lung, and Blood Institute Grants R21-HL-108003 and P01-HL-107153 (to N. E. Zachara).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.E.Z. prepared figures, drafted manuscript, edited and revised manuscript, and approved final version of manuscript.
ACKNOWLEDGMENTS
We apologize to our colleagues whose work was not cited in this paper because of theme or space restrictions. We acknowledge the constructive comments of Dr. Chad Slawson (University of Kansas Medical Center).
REFERENCES
- 1.Akimoto Y, Kreppel LK, Hirano H, Hart GW. Hyperglycemia and the O-GlcNAc transferase in rat aortic smooth muscle cells: elevated expression and altered patterns of O-GlcNAcylation. Arch Biochem Biophys 389: 166–175, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Akimoto Y, Kreppel LK, Hirano H, Hart GW. Increased O-GlcNAc transferase in pancreas of rats with streptozotocin-induced diabetes. Diabetologia 43: 1239–1247, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Arias EB, Kim J, Cartee GD. Prolonged incubation in PUGNAc results in increased protein O-Linked glycosylation and insulin resistance in rat skeletal muscle. Diabetes 53: 921–930, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Belke DD. Swim-exercised mice show a decreased level of protein O-GlcNAcylation and expression of O-GlcNAc transferase in heart. J Appl Physiol 111: 157–162, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Boehmelt G, Wakeham A, Elia A, Sasaki T, Plyte S, Potter J, Yang Y, Tsang E, Ruland J, Iscove NN, Dennis JW, Mak TW. Decreased UDP-GlcNAc levels abrogate proliferation control in EMeg32-deficient cells. EMBO J 19: 5092–5104, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosch RR, Janssen SW, Span PN, Olthaar A, van Emst-de Vries SE, Willems PH, Martens JM, Hermus AR, Sweep CC. Exploring levels of hexosamine biosynthesis pathway intermediates and protein kinase C isoforms in muscle and fat tissue of Zucker diabetic fatty rats. Endocrine 20: 247–252, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Buse MG, Robinson KA, Marshall BA, Hresko RC, Mueckler MM. Enhanced O-GlcNAc protein modification is associated with insulin resistance in GLUT1-overexpressing muscles. Am J Physiol Endocrinol Metab 283: E241–E250, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Butkinaree C, Cheung WD, Park S, Park K, Barber M, Hart GW. Characterization of beta-N-acetylglucosaminidase cleavage by caspase-3 during apoptosis. J Biol Chem 283: 23557–23566, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, Vosseller K, Reginato MJ. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene 29: 2831–2842, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Carapito C, Klemm C, Aebersold R, Domon B. Systematic LC-MS analysis of labile post-translational modifications in complex mixtures. J Proteome Res 8: 2608–2614, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Carrillo LD, Froemming JA, Mahal LK. Targeted in vivo O-GlcNAc sensors reveal discrete compartment-specific dynamics during signal transduction. J Biol Chem 286: 6650–6658, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrillo LD, Krishnamoorthy L, Mahal LK. A cellular FRET-based sensor for beta-O-GlcNAc, a dynamic carbohydrate modification involved in signaling. J Am Chem Soc 128: 14768–14769, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Chalkley RJ, Thalhammer A, Schoepfer R, Burlingame AL. Identification of protein O-GlcNAcylation sites using electron transfer dissociation mass spectrometry on native peptides. Proc Natl Acad Sci USA 106: 8894–8899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am J Physiol Cell Physiol 294: C1509–C1520, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol 292: C178–C187, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Chatham JC, Marchase RB. The role of protein O-linked beta-N-acetylglucosamine in mediating cardiac stress responses. Biochim Biophys Acta 1800: 57–66, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatham JC, Not LG, Fulop N, Marchase RB. Hexosamine biosynthesis and protein O-glycosylation: the first line of defense against stress, ischemia, and trauma. Shock 29: 431–440, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Cheung WD, Hart GW. AMP-activated protein kinase and p38 MAPK activate O-GlcNAcylation of neuronal proteins during glucose deprivation. J Biol Chem 283: 13009–13020, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung WD, Sakabe K, Housley MP, Dias WB, Hart GW. O-linked beta-N-acetylglucosaminyltransferase substrate specificity is regulated by myosin phosphatase targeting and other interacting proteins. J Biol Chem 283: 33935–33941, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chikanishi T, Fujiki R, Hashiba W, Sekine H, Yokoyama A, Kato S. Glucose-induced expression of MIP-1 genes requires O-GlcNAc transferase in monocytes. Biochem Biophys Res Commun 394: 865–870, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Chou CF, Omary MB. Mitotic arrest with anti-microtubule agents or okadaic acid is associated with increased glycoprotein terminal GlcNAc's. J Cell Sci 107: 1833–1843, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Chou TY, Dang CV, Hart GW. Glycosylation of the c-Myc transactivation domain. Proc Natl Acad Sci USA 92: 4417–4421, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou TY, Hart GW, Dang CV. c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J Biol Chem 270: 18961–18965, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Choubdar N, Bhat RG, Stubbs KA, Yuzwa S, Pinto BM. Synthesis of 2-amido, 2-amino, and 2-azido derivatives of the nitrogen analogue of the naturally occurring glycosidase inhibitor salacinol and their inhibitory activities against O-GlcNAcase and NagZ enzymes. Carbohydr Res 343: 1766–1777, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem 2003 [DOI] [PubMed] [Google Scholar]
- 27.Clarke AJ, Hurtado-Guerrero R, Pathak S, Schuttelkopf AW, Borodkin V, Shepherd SM, Ibrahim AF, van Aalten DM. Structural insights into mechanism and specificity of O-GlcNAc transferase. EMBO J 27: 2780–2788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comer FI, Vosseller K, Wells L, Accavitti MA, Hart GW. Characterization of a mouse monoclonal antibody specific for O-linked N-acetylglucosamine. Anal Biochem 293: 169–177, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Comtesse N, Maldener E, Meese E. Identification of a nuclear variant of MGEA5, a cytoplasmic hyaluronidase and a beta-N-acetylglucosaminidase. Biochem Biophys Res Commun 283: 634–640, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Crook ED, Daniels MC, Smith TM, McClain DA. Regulation of insulin-stimulated glycogen synthase activity by overexpression of glutamine: fructose-6-phosphate amidotransferase in rat-1 fibroblasts. Diabetes 42: 1289–1296, 1993 [DOI] [PubMed] [Google Scholar]
- 31.D'Apolito M, Du X, Zong H, Catucci A, Maiuri L, Trivisano T, Pettoello-Mantovani M, Campanozzi A, Raia V, Pessin JE, Brownlee M, Giardino I. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J Clin Invest 120: 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dennis JW, Pawling J, Cheung P, Partridge E, Demetriou M. UDP-N-acetylglucosamine:alpha-6-d-mannoside beta1, 6 N-acetylglucosaminylt ransferase V (Mgat5) deficient mice. Biochim Biophys Acta 1573: 414–422, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Dong DL, Hart GW. Purification and characterization of an O-GlcNAc selective N-acetyl-beta-d-glucosaminidase from rat spleen cytosol. J Biol Chem 269: 19321–19330, 1994 [PubMed] [Google Scholar]
- 34.Dorfmueller HC, Borodkin VS, Schimpl M, Shepherd SM, Shpiro NA, van Aalten DM. GlcNAcstatin: a picomolar, selective O-GlcNAcase inhibitor that modulates intracellular O-glcNAcylation levels. J Am Chem Soc 128: 16484–16485, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorfmueller HC, van Aalten DM. Screening-based discovery of drug-like O-GlcNAcase inhibitor scaffolds. FEBS Lett 584: 694–700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA 97: 12222–12226, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farook VS, Bogardus C, Prochazka M. Analysis of MGEA5 on 10q24.1-q243 encoding the beta-O-linked N-acetylglucosaminidase as a candidate gene for type 2 diabetes mellitus in Pima Indians. Mol Genet Metab 77: 189–193, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Favreau C, Worman HJ, Wozniak RW, Frappier T, Courvalin JC. Cell cycle-dependent phosphorylation of nucleoporins and nuclear pore membrane protein Gp210. Biochemistry 35: 8035–8044, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 106: 466–472, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Forsythe ME, Love DC, Lazarus BD, Kim EJ, Prinz WA, Ashwell G, Krause MW, Hanover JA. Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 (O-GlcNAcase) knockout impacts O-GlcNAc cycling, metabolism, and dauer. Proc Natl Acad Sci USA 103: 11952–11957, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fulop N, Feng W, Xing D, He K, Not LG, Brocks CA, Marchase RB, Miller AP, Chatham JC. Aging leads to increased levels of protein O-linked N-acetylglucosamine in heart, aorta, brain and skeletal muscle in Brown-Norway rats. Biogerontology 9: 139–151, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fulop N, Mason MM, Dutta K, Wang P, Davidoff AJ, Marchase RB, Chatham JC. Impact of Type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart. Am J Physiol Cell Physiol 292: C1370–C1378, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Fulop N, Zhang Z, Marchase RB, Chatham JC. Glucosamine cardioprotection in perfused rat heart associated with increased O-linked N-acetylglucosamine protein modification and altered p38 activation. Am J Physiol Heart Circ Physiol 292: H2227–H2236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fulop N, Zhang Z, Marchase RB, Chatham JC. Glucosamine cardioprotection in perfused rat hearts associated with increased O-linked N-acetylglucosamine protein modification and altered p38 activation. Am J Physiol Heart Circ Physiol 292: H2227–H2236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gambetta MC, Oktaba K, Muller J. Essential role of the glycosyltransferase sxc/Ogt in polycomb repression. Science 325: 93–96, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Gao Y, Miyazaki J, Hart GW. The transcription factor PDX-1 is post-translationally modified by O-linked N-acetylglucosamine and this modification is correlated with its DNA binding activity and insulin secretion in min6 beta-cells. Arch Biochem Biophys 415: 155–163, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Gao Y, Wells L, Comer FI, Parker GJ, Hart GW. Dynamic O-glycosylation of nuclear and cytosolic proteins - Cloning and characterization of a neutral, cytosolic beta-N-acetylglucosam inidase from human brain. J Biol Chem 276: 9838–9845, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Gloster TM, Zandberg WF, Heinonen JE, Shen DL, Deng L, Vocadlo DJ. Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nat Chem Biol 7: 174–181, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greig KT, Antonchuk J, Metcalf D, Morgan PO, Krebs DL, Zhang JG, Hacking DF, Bode L, Robb L, Kranz C, de Graaf C, Bahlo M, Nicola NA, Nutt SL, Freeze HH, Alexander WS, Hilton DJ, Kile BT. Agm1/Pgm3-mediated sugar nucleotide synthesis is essential for hematopoiesis and development. Mol Cell Biol 27: 5849–5859, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gross BJ, Kraybill BC, Walker S. Discovery of O-GlcNAc transferase inhibitors. J Am Chem Soc 127: 14588–14589, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Gurcel C, Vercoutter-Edouart AS, Fonbonne C, Mortuaire M, Salvador A, Michalski JC, Lemoine J. Identification of new O-GlcNAc modified proteins using a click-chemistry-based tagging. Anal Bioanal Chem 390: 2089–2097, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Haltiwanger RS, Grove K, Philipsberg GA. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-beta-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate. J Biol Chem 273: 3611–3617, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Haltiwanger RS, Philipsberg GA. Mitotic arrest with nocodazole induces selective changes in the level of O-linked N-acetylglucosamine and accumulation of incompletely processed N-glycans on proteins from HT29 cells. J Biol Chem 272: 8752–8758, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Hanover JA, Forsythe ME, Hennessey PT, Brodigan TM, Love DC, Ashwell G, Krause M. A Caenorhabditis elegans model of insulin resistance: altered macronutrient storage and dauer formation in an OGT-1 knockout. Proc Natl Acad Sci USA 102: 11266–11271, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanover JA, Yu S, Lubas WB, Shin SH, Ragano-Caracciola M, Kochran J, Love DC. Mitochondrial and nucleocytoplasmic isoforms of O-linked GlcNAc transferase encoded by a single mammalian gene. Arch Biochem Biophys 409: 287–297, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Hart GW. Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu Rev Biochem 66: 315–335, 1997 [DOI] [PubMed] [Google Scholar]
- 57.Hart GW, Greis KD, Dong LYD, Blomberg MA, Chou TY, Jiang MS, Roquemore EP, Snow DM, Kreppel LK, Cole RN, Comer FI, Arnold CS, Hayes BK. O-linked N-acetylglucosamine: The “yin-yang” of Ser/Thr phosphorylation? Nuclear and cytoplasmic glycosylation. In: Glycoimmunology, edited by Alavi A, Axford JS. New York: Plenum Press, 1995, p. 115–123 [PubMed] [Google Scholar]
- 58.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem 80: 825–858, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hartweck LM, Scott CL, Olszewski NE. Two O-linked N-acetylglucosamine transferase genes of Arabidopsis thaliana L. Heynh have overlapping functions necessary for gamete and seed development. Genetics 161: 1279–1291, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heart E, Choi WS, Sung CK. Glucosamine-induced insulin resistance in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab 278: E103–E112, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Hebert LF, Jr, Daniels MC, Zhou JX, Crook ED, Turner RL, Simmons ST, Neidigh JL, Zhu JS, Baron AD, McClain DA. Overexpression of glutamine:fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J Clin Invest 98: 930–936, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heese-Peck A, Cole RN, Borkhsenious ON, Hart GW, Raikhel NV. Plant nuclear pore complex proteins are modified by novel oligosaccharides with terminal N-acetylglucosamine. Plant Cell 7: 1459–1471, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, Dillmann WH. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res 96: 1006–1013, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Hu Y, Suarez J, Fricovsky E, Wang H, Scott BT, Trauger SA, Han W, Oyeleye MO, Dillmann WH. Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J Biol Chem 284: 547–555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang JB, Hernandez J, Leduc R, Frost SC. Alternative glycosylation of the insulin receptor prevents oligomerization and acquisition of insulin-dependent tyrosine kinase activity. Biochim Biophys Acta 1499: 74–84, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Hwang SY, Shin JH, Hwang JS, Kim SY, Shin JA, Oh ES, Oh S, Kim JB, Lee JK, Han IO. Glucosamine exerts a neuroprotective effect via suppression of inflammation in rat brain ischemia/reperfusion injury. Glia 58: 1881–1892, 2010 [DOI] [PubMed] [Google Scholar]
- 67.Isono T. O-GlcNAc-specific antibody CTD110.6 cross-reacts with N-GlcNAc2-modified proteins induced under glucose deprivation. PLoS One 6: e18959, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.James LR, Tang D, Ingram A, Ly H, Thai K, Cai L, Scholey JW. Flux through the hexosamine pathway is a determinant of nuclear factor kappaB-dependent promoter activation. Diabetes 51: 1146–1156, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Jinek M, Rehwinkel J, Lazarus BD, Izaurralde E, Hanover JA, Conti E. The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin alpha. Nat Struct Mol Biol 11: 1001–1007, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marban E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 117: 1172–1182, 2008 [DOI] [PubMed] [Google Scholar]
- 71.Ju Y, Hua J, Sakamoto K, Ogawa H, Nagaoka I. Modulation of TNF-alpha-induced endothelial cell activation by glucosamine, a naturally occurring amino monosaccharide. Int J Mol Med 22: 809–815, 2008 [PubMed] [Google Scholar]
- 72.Kaneto H, Xu G, Song KH, Suzuma K, Bonner-Weir S, Sharma A, Weir GC. Activation of the hexosamine pathway leads to deterioration of pancreatic beta-cell function through the induction of oxidative stress. J Biol Chem 276: 31099–31104, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Kazemi Z, Chang H, Haserodt S, McKen C, Zachara NE. O-linked [beta]-N-acetylglucosamine (O-GlcNAc) regulates stress-induced heat shock protein expression in a GSK-3[beta] dependent manner. J Biol Chem 285: 39096–39107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kearse KP, Hart GW. Lymphocyte activation induces rapid changes in nuclear and cytoplasmic glycoproteins. Proc Natl Acad Sci USA 88: 1701–1705, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khidekel N, Arndt S, Lamarre-Vincent N, Lippert A, Poulin-Kerstien KG, Ramakrishnan B, Qasba PK, Hsieh-Wilson LC. A chemoenzymatic approach toward the rapid and sensitive detection of O-GlcNAc posttranslational modifications. J Am Chem Soc 125: 16162–16163, 2003 [DOI] [PubMed] [Google Scholar]
- 76.Khidekel N, Ficarro SB, Clark PM, Bryan MC, Swaney DL, Rexach JE, Sun YE, Coon JJ, Peters EC, Hsieh-Wilson LC. Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics. Nat Chem Biol 3: 339–348, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Khidekel N, Ficarro SB, Peters EC, Hsieh-Wilson LC. Exploring the O-GlcNAc proteome: direct identification of O-GlcNAc-modified proteins from the brain. Proc Natl Acad Sci USA 101: 13132–13137, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim EJ, Amorelli B, Abdo M, Thomas CJ, Love DC, Knapp S, Hanover JA. Distinctive inhibition of O-GlcNAcase isoforms by an alpha-GlcNAc thiolsulfonate. J Am Chem Soc 129: 14854–14855, 2007 [DOI] [PubMed] [Google Scholar]
- 79.Kim EJ, Love DC, Darout E, Abdo M, Rempel B, Withers SG, Rablen PR, Hanover JA, Knapp S. OGA inhibition by GlcNAc-selenazoline. Bioorg Med Chem 18: 7058–7064, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim EJ, Perreira M, Thomas CJ, Hanover JA. An O-GlcNAcase-specific inhibitor and substrate engineered by the extension of the N-acetyl moiety. J Am Chem Soc 128: 4234–4235, 2006 [DOI] [PubMed] [Google Scholar]
- 81.Klement E, Lipinszki Z, Kupihar Z, Udvardy A, Medzihradszky KF. Enrichment of O-GlcNAc modified proteins by the periodate oxidation-hydrazide resin capture approach. J Proteome Res 9: 2200–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Konrad RJ, Mikolaenko I, Tolar JF, Liu K, Kudlow JE. The potential mechanism of the diabetogenic action of streptozotocin: inhibition of pancreatic beta-cell O-GlcNAc-selective N-acetyl-beta-d-glucosaminidase. Biochem J 356: 31–41, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koya D, Dennis JW, Warren CE, Takahara N, Schoen FJ, Nishio Y, Nakajima T, Lipes MA, King GL. Overexpression of core 2 N-acetylglycosaminyltransferase enhances cytokine actions and induces hypertrophic myocardium in transgenic mice. FASEB J 13: 2329–2337, 1999 [DOI] [PubMed] [Google Scholar]
- 84.Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem 272: 9308–9315, 1997 [DOI] [PubMed] [Google Scholar]
- 85.Kreppel LK, Hart GW. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem 274: 32015–32022, 1999 [DOI] [PubMed] [Google Scholar]
- 86.Ku NO, Omary MB. Expression, glycosylation, and phosphorylation of human keratins 8 and 18 in insect cells. Exp Cell Res 211: 24–35, 1994 [DOI] [PubMed] [Google Scholar]
- 87.Ku NO, Toivola DM, Strnad P, Omary MB. Cytoskeletal keratin glycosylation protects epithelial tissue from injury. Nat Cell Biol 12: 876–885, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laczy B, Marsh SA, Brocks CA, Wittmann I, Chatham JC. Inhibition of O-GlcNAcase in perfused rat hearts by NAG-thiazolines at the time of reperfusion is cardioprotective in an O-GlcNAc-dependent manner. Am J Physiol Heart Circ Physiol 299: H1715–H1727, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lameira J, Alves CN, Moliner V, Marti S, Kanaan N, Tunon I. A quantum mechanics/molecular mechanics study of the protein-ligand interaction of two potent inhibitors of human O-GlcNAcase: PUGNAc and NAG-thiazoline. J Phys Chem B 112: 14260–14266, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Lazarus MB, Nam Y, Jiang J, Sliz P, Walker S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature 469: 564–567, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lefebvre T, Baert F, Bodart JF, Flament S, Michalski JC, Vilain JP. Modulation of O-GlcNAc glycosylation during Xenopus oocyte maturation. J Cell Biochem 93: 999–1010, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Lehman DM, Fu DJ, Freeman AB, Hunt KJ, Leach RJ, Johnson-Pais T, Hamlington J, Dyer TD, Arya R, Abboud H, Goring HH, Duggirala R, Blangero J, Konrad RJ, Stern MP. A single nucleotide polymorphism in MGEA5 encoding O-GlcNAc-selective N-acetyl-beta-D glucosaminidase is associated with type 2 diabetes in Mexican Americans. Diabetes 54: 1214–1221, 2005 [DOI] [PubMed] [Google Scholar]
- 93.Liao J, Lowthert LA, Omary MB. Heat stress or rotavirus infection of human epithelial cells generates a distinct hyperphosphorylated form of keratin 8. Exp Cell Res 219: 348–357, 1995 [DOI] [PubMed] [Google Scholar]
- 94.Lim KH, Chang HI. O-linked N-acetylglucosamine suppresses thermal aggregation of Sp1. FEBS Lett 580: 4645–4652, 2006 [DOI] [PubMed] [Google Scholar]
- 95.Lima VV, Giachini FR, Carneiro FS, Carneiro ZN, Fortes ZB, Carvalho MH, Webb RC, Tostes RC. Increased vascular O-GlcNAcylation augments reactivity to constrictor stimuli—Vasoactive Peptide Symposium. J Am Soc Hypertens 2: 410–417, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lima VV, Giachini FR, Choi H, Carneiro FS, Carneiro ZN, Fortes ZB, Carvalho MH, Webb RC, Tostes RC. Impaired vasodilator activity in deoxycorticosterone acetate-salt hypertension is associated with increased protein O-GlcNAcylation. Hypertension 53: 166–174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lima VV, Giachini FR, Hardy DM, Webb RC, Tostes RC. O-GlcNAcylation: a novel pathway contributing to the effects of endothelin in the vasculature. Am J Physiol Regul Integr Comp Physiol 300: R236–R250, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol 42: 177–185, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu J, Marchase RB, Chatham JC. Increased O-GlcNAc levels during reperfusion lead to improved functional recovery and reduced calpain proteolysis. Am J Physiol Heart Circ Physiol 293: H1391–H1399, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol 40: 303–312, 2006 [DOI] [PubMed] [Google Scholar]
- 101.Liu K, Paterson AJ, Chin E, Kudlow JE. Glucose stimulates protein modification by O-linked GlcNAc in pancreatic beta cells: linkage of O-linked GlcNAc to beta cell death. Proc Natl Acad Sci USA 97: 2820–2825, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Love DC, Ghosh S, Mondoux MA, Fukushige T, Wang P, Wilson MA, Iser WB, Wolkow CA, Krause MW, Hanover JA. Dynamic O-GlcNAc cycling at promoters of Caenorhabditis elegans genes regulating longevity, stress, and immunity. Proc Natl Acad Sci USA 107: 7413–7418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Love DC, Kochran J, Cathey RL, Shin SH, Hanover JA. Mitochondrial and nucleocytoplasmic targeting of O-linked GlcNAc transferase. J Cell Sci 116: 647–654, 2003 [DOI] [PubMed] [Google Scholar]
- 104.Lubas WA, Frank DW, Krause M, Hanover JA. O-Linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J Biol Chem 272: 9316–9324, 1997 [DOI] [PubMed] [Google Scholar]
- 105.Lubas WA, Hanover JA. Functional expression of O-linked GlcNAc transferase - Domain structure and substrate specificity. J Biol Chem 275: 10983–10988, 2000 [DOI] [PubMed] [Google Scholar]
- 106.Luo B, Soesanto Y, McClain DA. Protein modification by O-linked GlcNAc reduces angiogenesis by inhibiting Akt activity in endothelial cells. Arterioscler Thromb Vasc Biol 28: 651–657, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Macauley MS, Bubb AK, Martinez-Fleites C, Davies GJ, Vocadlo DJ. Elevation of global O-GlcNAc levels in 3T3-L1 adipocytes by selective inhibition of O-GlcNAcase does not induce insulin resistance. J Biol Chem 283: 34687–34695, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Macauley MS, Shan X, Yuzwa SA, Gloster TM, Vocadlo DJ. Elevation of Global O-GlcNAc in rodents using a selective O-GlcNAcase inhibitor does not cause insulin resistance or perturb glucohomeostasis. Chem Biol 17: 949–958, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Majumdar G, Harmon A, Candelaria R, Martinez-Hernandez A, Raghow R, Solomon SS. O-glycosylation of Sp1 and transcriptional regulation of the calmodulin gene by insulin and glucagon. Am J Physiol Endocrinol Metab 285: E584–E591, 2003 [DOI] [PubMed] [Google Scholar]
- 110.Majumdar G, Harrington A, Hungerford J, Martinez-Hernandez A, Gerling IC, Raghow R, Solomon S. Insulin dynamically regulates calmodulin gene expression by sequential o-glycosylation and phosphorylation of sp1 and its subcellular compartmentalization in liver cells. J Biol Chem 281: 3642–3650, 2006 [DOI] [PubMed] [Google Scholar]
- 111.Malhotra R, Brosius FC., 3rd Glucose uptake and glycolysis reduce hypoxia-induced apoptosis in cultured neonatal rat cardiac myocytes. J Biol Chem 274: 12567–12575, 1999 [DOI] [PubMed] [Google Scholar]
- 112.Marsh SA, Dell'Italia LJ, Chatham JC. Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids 40: 819–828, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marsh SA, Dell'italia LJ, Chatham JC. Interaction of diet and diabetes on cardiovascular function in rats. Am J Physiol Heart Circ Physiol 296: H282–H292, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem 266: 4706–4712, 1991 [PubMed] [Google Scholar]
- 115.Marshall S, Nadeau O, Yamasaki K. Glucosamine-induced activation of glycogen biosynthesis in isolated adipocytes. Evidence for a rapid allosteric control mechanism within the hexosamine biosynthesis pathway. J Biol Chem 280: 11018–11024, 2005 [DOI] [PubMed] [Google Scholar]
- 116.Martinez-Fleites C, Macauley MS, He Y, Shen DL, Vocadlo DJ, Davies GJ. Structure of an O-GlcNAc transferase homolog provides insight into intracellular glycosylation. Nat Struct Mol Biol 15: 764–765, 2008 [DOI] [PubMed] [Google Scholar]
- 117.McClain DA, Lubas WA, Cooksey RC, Hazel M, Parker GJ, Love DC, Hanover JA. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc Natl Acad Sci USA 99: 10695–10699, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miller MW, Caracciolo MR, Berlin WK, Hanover JA. Phosphorylation and glycosylation of nucleoporins. Arch Biochem Biophys 367: 51–60, 1999 [DOI] [PubMed] [Google Scholar]
- 119.Musicki B, Kramer MF, Becker RE, Burnett AL. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc Natl Acad Sci USA 102: 11870–11875, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Muto A, Model L, Ziegler K, Eghbalieh SD, Dardik A. Mechanisms of vein graft adaptation to the arterial circulation: insights into the neointimal algorithm and management strategies. Circ J 74: 1501–1512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nagy T, Champattanachai V, Marchase RB, Chatham JC. Glucosamine inhibits angiotensin II-induced cytoplasmic Ca2+ elevation in neonatal cardiomyocytes via protein-associated O-linked N-acetylglucosamine. Am J Physiol Cell Physiol 290: C57–C65, 2006 [DOI] [PubMed] [Google Scholar]
- 123.Nandi A, Sprung R, Barma DK, Zhao Y, Kim SC, Falck JR, Zhao Y. Global identification of O-GlcNAc-modified proteins. Anal Chem 78: 452–458, 2006 [DOI] [PubMed] [Google Scholar]
- 124.Nelson BA, Robinson KA, Buse MG. Defective Akt activation is associated with glucose- but not glucosamine-induced insulin resistance. Am J Physiol Endocrinol Metab 282: E497–E506, 2002 [DOI] [PubMed] [Google Scholar]
- 125.Ngoh GA, Facundo HT, Hamid T, Dillmann W, Zachara NE, Jones SP. Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circ Res 104: 41–49, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ngoh GA, Hamid T, Prabhu SD, Jones SP. O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death. Am J Physiol Heart Circ Physiol 297: H1711–H1719, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ngoh GA, Watson LJ, Facundo HT, Dillmann W, Jones SP. Non-canonical glycosyltransferase modulates post-hypoxic cardiac myocyte death and mitochondrial permeability transition. J Mol Cell Cardiol 45: 313–325, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ngoh GA, Watson LJ, Facundo HT, Jones SP. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids 40: 895–911, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Not LG, Brocks CA, Vamhidy L, Marchase RB, Chatham JC. Increased O-linked beta-N-acetylglucosamine levels on proteins improves survival, reduces inflammation and organ damage 24 hours after trauma-hemorrhage in rats. Crit Care Med 38: 562–571, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Not LG, Marchase RB, Fulop N, Brocks CA, Chatham JC. Glucosamine Administration Improves Survival Rate after Severe Hemorrhagic Shock Combined with Trauma in Rats. Shock 2007 [DOI] [PubMed] [Google Scholar]
- 131.O'Donnell N, Zachara NE, Hart GW, Marth JD. Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol 24: 1680–1690, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pang Y, Hunton DL, Bounelis P, Marchase RB. Hyperglycemia inhibits capacitative calcium entry and hypertrophy in neonatal cardiomyocytes. Diabetes 51: 3461–3467, 2002 [DOI] [PubMed] [Google Scholar]
- 133.Park K, Saudek CD, Hart GW. Increased expression of beta-N-acetylglucosaminidase in erythrocytes from individuals with pre-diabetes and diabetes. Diabetes 59: 1845–1850, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Park SY, Ryu J, Lee W. O-GlcNAc modification on IRS-1 and Akt2 by PUGNAc inhibits their phosphorylation and induces insulin resistance in rat primary adipocytes. Exp Mol Med 37: 220–229, 2005 [DOI] [PubMed] [Google Scholar]
- 135.Parker G, Taylor R, Jones D, McClain D. Hyperglycemia and inhibition of glycogen synthase in streptozotocin-treated mice: role of O-linked N-acetylglucosamine. J Biol Chem 279: 20636–20642, 2004 [DOI] [PubMed] [Google Scholar]
- 136.Parker GJ, Lund KC, Taylor RP, McClain DA. Insulin resistance of glycogen synthase mediated by o-linked N-acetylglucosamine. J Biol Chem 278: 10022–10027, 2003 [DOI] [PubMed] [Google Scholar]
- 137.Ram AF, Arentshorst M, Damveld RA, vanKuyk PA, Klis FM, van den Hondel CA. The cell wall stress response in Aspergillus niger involves increased expression of the glutamine: fructose-6-phosphate amidotransferase-encoding gene (gfaA) and increased deposition of chitin in the cell wall. Microbiology 150: 3315–3326, 2004 [DOI] [PubMed] [Google Scholar]
- 138.Ramirez-Correa GA, Jin W, Wang Z, Zhong X, Gao WD, Dias WB, Vecoli C, Hart GW, Murphy AM. O-linked GlcNAc modification of cardiac myofilament proteins: a novel regulator of myocardial contractile function. Circ Res 103: 1354–1358, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rengifo J, Gibson CJ, Winkler E, Collin T, Ehrlich BE. Regulation of the inositol 1,4,5-trisphosphate receptor type I by O-GlcNAc glycosylation. J Neurosci 27: 13813–13821, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rexach JE, Clark PM, Hsieh-Wilson LC. Chemical approaches to understanding O-GlcNAc glycosylation in the brain. Nat Chem Biol 4: 97–106, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rexach JE, Rogers CJ, Yu SH, Tao J, Sun YE, Hsieh-Wilson LC. Quantification of O-glycosylation stoichiometry and dynamics using resolvable mass tags. Nat Chem Biol 6: 645–651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Robinson KA, Ball LE, Buse MG. Reduction of O-GlcNAc protein modification does not prevent insulin resistance in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab 292: E884–E890, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Roos MD, Han IO, Paterson AJ, Kudlow JE. Role of glucosamine synthesis in the stimulation of TGF-α gene transcription by glucose and EGF. Am J Physiol Cell Physiol 270: C803–C811, 1996 [DOI] [PubMed] [Google Scholar]
- 144.Roquemore EP, Chevrier MR, Cotter RJ, Hart GW. Dynamic O-GlcNAcylation of the small heat shock protein alpha B-crystallin. Biochemistry 35: 3578–3586, 1996 [DOI] [PubMed] [Google Scholar]
- 145.Scaffidi A, Stubbs KA, Dennis RJ, Taylor EJ, Davies GJ, Vocadlo DJ, Stick RV. A 1-acetamido derivative of 6-epi-valienamine: an inhibitor of a diverse group of beta-N-acetylglucosaminidases. Org Biomol Chem 5: 3013–3019, 2007 [DOI] [PubMed] [Google Scholar]
- 146.Shafi R, Lyer SPN, Ellies LG, O'Donnell N, Marek KW, Chui D, Hart GW, Marth JD. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA 97: 5735–5739, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shanmugasundaram B, Debowski AW, Dennis RJ, Davies GJ, Vocadlo DJ, Vasella A. Inhibition of O-GlcNAcase by a gluco-configured nagstatin and a PUGNAc-imidazole hybrid inhibitor. Chem Commun (Camb) 4372–4374, 2006 [DOI] [PubMed] [Google Scholar]
- 148.Shen A, Kamp HD, Grundling A, Higgins DE. A bifunctional O-GlcNAc transferase governs flagellar motility through anti-repression. Genes Dev 20: 3283–3295, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sinclair DA, Syrzycka M, Macauley MS, Rastgardani T, Komljenovic I, Vocadlo DJ, Brock HW, Honda BM. Drosophila O-GlcNAc transferase (OGT) is encoded by the Polycomb group (PcG) gene, super sex combs (sxc). Proc Natl Acad Sci USA 106: 13427–13432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Singleton KD, Wischmeyer PE. Glutamine induces heat shock protein expression via O-glycosylation and phosphorylation of HSF-1 and Sp1. JPEN J Parenter Enteral Nutr 32: 371–376, 2008 [DOI] [PubMed] [Google Scholar]
- 151.Skorobogatko YV, Deuso J, Adolf-Bergfoyle J, Nowak MG, Gong Y, Lippa CF, Vosseller K. Human Alzheimer's disease synaptic O-GlcNAc site mapping and iTRAQ expression proteomics with ion trap mass spectrometry. Amino Acids 2011 [DOI] [PubMed] [Google Scholar]
- 152.Slawson C, Lakshmanan T, Knapp S, Hart GW. A mitotic GlcNAcylation/phosphorylation signaling complex alters the posttranslational state of the cytoskeletal protein vimentin. Mol Biol Cell 19: 4130–4140, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Slawson C, Shafii S, Amburgey J, Potter R. Characterization of the O-GlcNAc protein modification in Xenopus laevis oocyte during oogenesis and progesterone-stimulated maturation. Biochim Biophys Acta 1573: 121–129, 2002 [DOI] [PubMed] [Google Scholar]
- 154.Slawson C, Zachara NE, Vosseller K, Cheung WD, Lane MD, Hart GW. Perturbations in O-linked beta-N-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem 280: 32944–32956, 2005 [DOI] [PubMed] [Google Scholar]
- 155.Soesanto YA, Luo B, Jones D, Taylor R, Gabrielsen JS, Parker G, McClain DA. Regulation of Akt signaling by O-GlcNAc in euglycemia. Am J Physiol Endocrinol Metab 295: E974–E980, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sohn KC, Lee KY, Park JE, Do SI. OGT functions as a catalytic chaperone under heat stress response: a unique defense role of OGT in hyperthermia. Biochem Biophys Res Commun 322: 1045–1051, 2004 [DOI] [PubMed] [Google Scholar]
- 157.Stubbs KA, Zhang N, Vocadlo DJ. A divergent synthesis of 2-acyl derivatives of PUGNAc yields selective inhibitors of O-GlcNAcase. Org Biomol Chem 4: 839–845, 2006 [DOI] [PubMed] [Google Scholar]
- 158.Tai HC, Khidekel N, Ficarro SB, Peters EC, Hsieh-Wilson LC. Parallel identification of O-GlcNAc-modified proteins from cell lysates. J Am Chem Soc 126: 10500–10501, 2004 [DOI] [PubMed] [Google Scholar]
- 159.Teo CF, Ingale S, Wolfert MA, Elsayed GA, Not LG, Chatham JC, Wells L, Boons GJ. Glycopeptide-specific monoclonal antibodies suggest new roles for O-GlcNAc. Nat Chem Biol 6: 338–343, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Toleman C, Paterson AJ, Whisenhunt TR, Kudlow JE. Characterization of the histone acetyltransferase (HAT) domain of a bifunctional protein with activable O-GlcNAcase and HAT activities. J Biol Chem 279: 53665–53673, 2004 [DOI] [PubMed] [Google Scholar]
- 161.Tong H, Chen W, London RE, Murphy E, Steenbergen C. Preconditioning enhanced glucose uptake is mediated by p38 MAP kinase not by phosphatidylinositol 3-kinase. J Biol Chem 275: 11981–11986, 2000 [DOI] [PubMed] [Google Scholar]
- 162.Torres CR, Hart GW. Topography and Polypeptide Distribution of Terminal N-Acetylglucosamine Residues on the Surfaces of Intact Lymphocytes. J Biol Chem 259: 3308–3317, 1984 [PubMed] [Google Scholar]
- 163.Viner RI, Zhang T, Second T, Zabrouskov V. Quantification of post-translationally modified peptides of bovine alpha-crystallin using tandem mass tags and electron transfer dissociation. J Proteomics 72: 874–885, 2009 [DOI] [PubMed] [Google Scholar]
- 164.Vocadlo DJ, Hang HC, Kim EJ, Hanover JA, Bertozzi CR. A chemical approach for identifying O-GlcNAc-modified proteins in cells. Proc Natl Acad Sci USA 100: 9116–9121, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vosseller K, Hansen KC, Chalkley RJ, Trinidad JC, Wells L, Hart GW, Burlingame AL. Quantitative analysis of both protein expression and serine/threonine post-translational modifications through stable isotope labeling with dithiothreitol. Proteomics 5: 388–398, 2005 [DOI] [PubMed] [Google Scholar]
- 166.Vosseller K, Trinidad JC, Chalkley RJ, Specht CG, Thalhammer A, Lynn AJ, Snedecor JO, Guan S, Medzihradszky KF, Maltby DA, Schoepfer R, Burlingame AL. O-linked N-acetylglucosamine proteomics of postsynaptic density preparations using lectin weak affinity chromatography and mass spectrometry. Mol Cell Proteomics 5: 923–934, 2006 [DOI] [PubMed] [Google Scholar]
- 167.Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci USA 99: 5313–5318, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang Z, Pandey A, Hart GW. Dynamic interplay between O-linked N-acetylglucosaminylation and glycogen synthase kinase-3-dependent phosphorylation. Mol Cell Proteomics 6: 1365–1379, 2007 [DOI] [PubMed] [Google Scholar]
- 169.Wang Z, Park K, Comer F, Hsieh-Wilson LC, Saudek CD, Hart GW. Site-specific GlcNAcylation of human erythrocyte proteins: potential biomarker(s) for diabetes mellitus. Diabetes 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang Z, Udeshi ND, O'Malley M, Shabanowitz J, Hunt DF, Hart GW. Enrichment and site mapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photochemical cleavage, and electron transfer dissociation mass spectrometry. Mol Cell Proteomics 9: 153–160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang Z, Udeshi ND, Slawson C, Compton PD, Sakabe K, Cheung WD, Shabanowitz J, Hunt DF, Hart GW. Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Sci Signal 3: ra2, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, Long BW, Prabhu SD, Xuan YT, Jones SP. O-linked beta-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci USA 107: 17797–17802, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wellen KE, Lu C, Mancuso A, Lemons JM, Ryczko M, Dennis JW, Rabinowitz JD, Coller HA, Thompson CB. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev 24: 2784–2799, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wells L. Mapping sites of O-GlcNAc modification using affinity tags for serine and threonine post-translational modifications. Mol Cell Proteomics 1: 791–804, 2002 [DOI] [PubMed] [Google Scholar]
- 175.Wells L, Gao Y, Mahoney JA, Vosseller K, Chen C, Rosen A, Hart GW. Dynamic O-glycosylation of nuclear and cytosolic proteins-Further characterization of the nucleocytoplasmic beta-N-acetylglucosamini dase, O-GlcNAcase. J Biol Chem 277: 1755–1761, 2002 [DOI] [PubMed] [Google Scholar]
- 176.Wells L, Gao Y, Mahoney JA, Vosseller K, Chen C, Rosen A, Hart GW. Dynamic O-glycosylation of nuclear and cytosolic proteins: further characterization of the nucleocytoplasmic beta-N-acetylglucosaminidase, O-GlcNAcase. J Biol Chem 277: 1755–1761, 2002 [DOI] [PubMed] [Google Scholar]
- 177.Wells L, Kreppel LK, Comer FI, Wadzinski BE, Hart GW. O-GlcNAc transferase is in a functional complex with protein phosphatase 1 catalytic subunits. J Biol Chem 279: 38466–38470, 2004 [DOI] [PubMed] [Google Scholar]
- 178.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 179.Wrabl JO, Grishin NV. Homology between O-linked GlcNAc transferases and proteins of the glycogen phosphorylase superfamily. J Mol Biol 314: 365–374, 2001 [DOI] [PubMed] [Google Scholar]
- 180.Wu T, Zhou H, Jin Z, Bi S, Yang X, Yi D, Liu W. Cardioprotection of salidroside from ischemia/reperfusion injury by increasing N-acetylglucosamine linkage to cellular proteins. Eur J Pharmacol 613: 93–99, 2009 [DOI] [PubMed] [Google Scholar]
- 181.Xing D, Feng W, Not LG, Miller AP, Zhang Y, Chen YF, Majid-Hassan E, Chatham JC, Oparil S. Increased protein O-GlcNAc modification inhibits inflammatory and neointimal responses to acute endoluminal arterial injury. Am J Physiol Heart Circ Physiol 295: H335–H342, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xu BE, Lee BH, Min X, Lenertz L, Heise CJ, Stippec S, Goldsmith EJ, Cobb MH. WNK1: analysis of protein kinase structure, downstream targets, and potential roles in hypertension. Cell Res 15: 6–10, 2005 [DOI] [PubMed] [Google Scholar]
- 183.Yang S, Zou LY, Bounelis P, Chaudry I, Chatham JC, Marchase RB. Glucosamine administration during resuscitation improves organ function after trauma hemorrhage. Shock 25: 600–607, 2006 [DOI] [PubMed] [Google Scholar]
- 184.Yang WH, Park SY, Nam HW, Kim do H, Kang JG, Kang ES, Kim YS, Lee HC, Kim KS, Cho JW. NFkappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci USA 105: 17345–17350, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature 451: 964–969, 2008 [DOI] [PubMed] [Google Scholar]
- 186.Yao D, Taguchi T, Matsumura T, Pestell R, Edelstein D, Giardino I, Suske G, Rabbani N, Thornalley PJ, Sarthy VP, Hammes HP, Brownlee M. High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. J Biol Chem 282: 31038–31045, 2007 [DOI] [PubMed] [Google Scholar]
- 187.Yki-Jarvinen H, Virkamaki A, Daniels MC, McClain D, Gottschalk WK. Insulin and glucosamine infusions increase O-linked N-acetyl-glucosamine in skeletal muscle proteins in vivo. Metabolism 47: 449–455, 1998 [DOI] [PubMed] [Google Scholar]
- 188.Yki-Jarvinen H, Virkamaki A, Daniels MC, McClain D, Gottschalk WK. Insulin and glucosamine infusions increase O-linked N-acetyl-glucosamine in skeletal muscle proteins in vivo. Metabolism 47: 449–455, 1998 [DOI] [PubMed] [Google Scholar]
- 189.Yokoe S, Asahi M, Takeda T, Otsu K, Taniguchi N, Miyoshi E, Suzuki K. Inhibition of phospholamban phosphorylation by O-GlcNAcylation: implications for diabetic cardiomyopathy. Glycobiology 20: 1217–1226, 2010 [DOI] [PubMed] [Google Scholar]
- 190.Young ME, Yan J, Razeghi P, Cooksey RC, Guthrie PH, Stepkowski SM, McClain DA, Tian R, Taegtmeyer H. Proposed regulation of gene expression by glucose in rodent heart. Gene Regul Syst Bio 1: 251–262, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, Whitworth GE, Stubbs KA, McEachern EJ, Davies GJ, Vocadlo DJ. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol 4: 483–490, 2008 [DOI] [PubMed] [Google Scholar]
- 192.Zachara NE. Detecting the “O-GlcNAc-ome”; detection, purification, and analysis of O-GlcNAc modified proteins. Methods Mol Biol 534: 251–279, 2009 [DOI] [PubMed] [Google Scholar]
- 193.Zachara NE. Detection and analysis of (O-linked beta-N-acetylglucosamine)-modified proteins. Methods Mol Biol 464: 227–254, 2009 [DOI] [PubMed] [Google Scholar]
- 194.Zachara NE, Molina H, Wong KY, Pandey A, Hart GW. The dynamic stress-induced “O-GlcNAc-ome” highlights functions for O-GlcNAc in regulating DNA damage/repair and other cellular pathways. Amino Acids 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zachara NE, O'Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem 279: 30133–30142, 2004 [DOI] [PubMed] [Google Scholar]
- 196.Zachara NE, Vosseller K, Hart GW. Detection and analysis of proteins modified by O-linked N-acetylglucosamine. Curr Protoc Protein Sci 12: 12.8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zachara NE, Vosseller K, Hart GW. Detection and analysis of proteins modified by O-linked N-acetylglucosamine. Curr Protoc Mol Biol 17: 17.6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zou L, Yang S, Champattanachai V, Hu S, Chaudry IH, Marchase RB, Chatham JC. Glucosamine improves cardiac function following trauma-hemorrhage by increased protein O-GlcNAcylation and attenuation of NF-κB signaling. Am J Physiol Heart Circ Physiol 296: H515–H523, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zou L, Yang S, Hu S, Chaudry IH, Marchase RB, Chatham JC. The protective effects of PUGNAc on cardiac function after trauma-hemorrhage are mediated via increased protein O-GlcNAc levels. Shock 27: 402–408, 2007 [DOI] [PubMed] [Google Scholar]