Abstract
The multifunctional CaMKII has been implicated in vascular smooth muscle cell (VSMC) migration, but little is known regarding its downstream targets that mediate migration. Here, we examined whether CaMKII regulates migration through modulation of matrix metalloproteinase 9 (MMP9). Using CaMKIIδ−/− mice as a model system, we evaluated migration and MMP9 regulation in vitro and in vivo. After ligation of the common carotid artery, CaMKII was activated in the neointima as determined by oxidation and autophosphorylation. We found that MMP9 was robustly expressed in the neointima and adventitia of carotid-ligated wild-type (WT) mice but was barely detectable in CaMKIIδ−/− mice. The perimeter of the external elastic lamina, a correlate of migration-related outward remodeling, was increased in WT but not in CaMKIIδ−/− mice. Migration induced by serum, platelet-derived growth factor, and tumor necrosis factor-α (TNF-α) was significantly decreased in CaMKIIδ−/− as compared with WT VSMCs, but migration was rescued with adenoviral overexpression of MMP9 in CaMKIIδ−/− VSMCs. Likewise, overexpression of CaMKIIδ in CaMKIIδ−/− VSMCs increased migration, whereas an oxidation-resistant mutant of CaMKIIδ did not. TNF-α strongly induced CaMKII oxidation and autophosphorylation as well as MMP9 activity, mRNA, and protein levels in WT, but not in CaMKIIδ−/− VSMC. Surprisingly, TNF-α strongly induced MMP9 promoter activity in WT and CaMKIIδ−/− VSMC. However, the MMP9 mRNA stability was significantly decreased in CaMKIIδ−/− VSMC. Our data demonstrate that CaMKII promotes VSMC migration through posttranscriptional regulation of MMP9 and suggest that CaMKII effects on MMP9 expression may be a therapeutic pathway in vascular injury.
Keywords: neointima
vascular smooth muscle cell (VSMC) migration is a central event in the response to vascular injury and leads to neointimal hyperplasia and remodeling. VSMC migration significantly contributes to the development of in-stent restenosis after balloon angioplasty (40) as well as intimal proliferation after vein grafts (32) or surgical endarterectomy (24). We and others have reported that the multifunctional CaMKII promotes neointimal formation after vascular injury (25, 35). Although the role of CaMKII in VSMC migration in vitro is now established (36, 39, 46, 59), the underlying mechanisms remain poorly understood. One of the few defined substrates of CaMKII in VSMC is the MAP kinase Erk1/2 (2, 11, 28), but CaMKII-mediated migration is likely independent of its effect on MAP kinase signaling (36, 39).
VSMC migration is a multistep process that involves actin filament and focal contact remodeling as well as extracellular matrix degradation and likely relies on multiple concerted signaling cascades (16), including those modulated by reactive oxygen species (ROS) (44, 53, 58). The proposed mechanisms for ROS-dependent VSMC migration include induction of monocyte chemotactic protein-1 and extracellular matrix breakdown through matrix metalloproteinase (MMP) activity. For example, deletion of functional MMP9 decreases VSMC migration in vitro and in vivo after vascular injury (10, 16). Recent studies in glia cells and cardiac myocytes and fibroblasts have implicated CaMKII as regulator of MMP9 expression (23, 57, 60), but it remains unclear whether this affects MMP9-dependent migration in the vasculature.
CaMKII is activated by binding calcium-bound calmodulin and subsequent autophosphorylation at Thr287 (26). Recently, an alternative pathway of CaMKII activation was described in which the oxidation of Met residues 281/282 in the CaMKII regulatory domain locks CaMKII into a Ca2+/calmodulin-autonomous conformation (15). In VSMC, CaMKII activation by autophosphorylation has been demonstrated (1). However, although several studies have reported an increase in CaMKII activity in response to oxidative stress (7, 37), it is unknown whether CaMKII is directly activated by oxidation in blood vessels or in VSMC. Using a model of CaMKII deficiency, we tested the hypothesis that VSMC-derived CaMKII is directly activated by ROS through oxidation and that CaMKII mediates VSMC migration and remodeling after injury by increasing the expression of MMP9.
METHODS
Reagents.
The following antibodies were used in this study: anti-α-smooth muscle actin, anti-GAPDH (Santa Cruz Biotechnology), anti-β-galactosidase (Chemicon), anti-MMP9, and anti-p-Thr286 CaMKII (Cell Signaling Technology). The generation of the anti-CaMKII (35) and anti-ox-Met 281/282 CaMKII (14) antibodies was described previously. Aphidicolin, a specific inhibitor of DNA polymerase A,D, was purchased from Sigma, the MMP9 inhibitor I from EMD Chemicals.
Mice.
Male and female CaMKIIδ−/− (5) and MEF2 reporter mice were kindly provided by Dr. Eric Olson, University of Texas Southwestern Medical Center. MEF2 reporter mice harbor a lacZ reporter gene under the control of tandem copies of the MEF2 consensus binding sites (42) and were crossbred with CaMKIIδ−/− mice. These mice and C57Bl/6 wild-type (WT) littermate controls were used according to the University of Iowa Institutional Animal Care and Use Committee guidelines, and all protocols were approved by the University of Iowa Institutional Animal Care and Use Committee. All procedures were in compliance with the standards for the care and use of laboratory animals of the Institute of Laboratory Animal Resource, National Academy of Science.
Carotid injury model.
WT C57Bl/6 mice and CaMKIIδ−/− mice (10 to 12 wk old) were anesthetized with ketamine and xylazine (2 and 0.3 mg ip, respectively). The left common carotid artery was ligated through a midline neck incision (31). Fourteen days after injury, all animals were anesthetized and perfused at physiological pressure with PBS followed by 4% paraformaldehyde for 3 min. The carotid arteries were excised, paraffin-embedded, and subjected to immunohistochemical analysis.
Immunohistochemistry.
For analyses of neointimal hyperplasia and remodeling, 5-μm sections were collected on Superfrost Plus slides. The sections were subjected to heat-mediated antigen retrieval using 0.01 M citrate buffer and were permeabilized in Triton X for 10 min. The sections were washed in PBS, and nonspecific binding was then blocked using a M.O.M. kit (Vector Labs) for 1 h followed by incubation in anti-α-smooth muscle actin antibody for 0.5 h at room temperature (1:200). After washes in PBS for 30 min at room temperature, the sections were preincubated in 5% goat serum for 30 min and then incubated with anti-MMP9 (1:100), anti-p-CaMKII (1:100), anti-ox-CaMKII (1:100), or anti-β-galactosidase (1:100) overnight at 4°C. The primary antibodies were detected with AlexaFluor 488- or 568-conjugated secondary antibodies (Invitrogen). Sections were counterstained with TO-PRO-3 (Invitrogen) or Vectashield containing DAPI (Vector Labs) to visualize nuclei. Images were captured with Zeiss LSM 510 META Laser confocal microscope. For in vitro studies, WT and CaMKIIδ−/− VSMC were fixed in 4% paraformaldehyde for 15 min, permeabilized, blocked in normal goat serum for 1 h, and incubated with primary antibody overnight at 4°C and with AlexaFluor 488-conjugated secondary antibody for 1 h. The slides were mounted with Vectashield after TO-PRO-3 nuclear counterstaining (Invitrogen). Digital images were taken with a Zeiss LSM 510 META Laser confocal microscope. Densitometry for different antigens was performed using National Institutes of Health (NIH) ImageJ.
For morphometric assessment of the external elastic lamina (EEL) circumference, the outermost elastic lamina was traced in five WT and CaMKIIδ-/- carotid arteries 14 days after ligation using NIH ImageJ (20 sections per mouse).
Cell culture.
Mouse aortic smooth muscle cells were isolated by enzymatic dispersion as described previously (48). Cells were cultured in DMEM supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 8 mM HEPES, and 2 mM l-glutamine at 37°C in a humidified 95% air-5% CO2 incubator. The purity of the mouse VSMC preparation in culture was confirmed by immunocytochemistry for α-smooth muscle actin. Mouse VSMC at passages 4–10 were used for experiments, with passage number for WT and CaMKIIδ−/− VSMCs matched with each experiment.
Immunoblotting.
Whole tissue extracts from mouse carotid arteries or mouse VSMC were prepared in ice-cold radioimmunoprecipitation assay lysis buffer and used for immunoblotting (34). Cell lysates or tissue homogenates were centrifuged at 4°C for 15 min at maximal speed, and the supernatant was stored at −80°C for further analysis. Protein concentrations were determined by modified Lowry assay (Bio-Rad DC protein assay). Samples were separated by SDS-PAGE (4–20%) and transferred to a polyvinylidene difluoride membrane. After membranes were blocked with 5% BSA, they were incubated with different primary antibodies for 1 h or overnight. The blots were then incubated with a horseradish peroxidase-conjugated secondary antibody and developed by a chemiluminescence detection system (Santa Cruz Biotechnology).
Detection of intracellular ROS.
WT and CaMKIIδ−/− VSMC were grown on chamber slides for 24 h in normal growth media at ∼70% confluence, then serum-deprived for 24 h before loading with 20 μM CM-H2DCFDA [5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate; Invitrogen] for 45 min (52). Cells were stimulated with 50 ng/ml TNF-α for 15 min, then rinsed with PBS before mounting the slides in Vectashield H-1000 mounting medium (Vector Laboratories). CM-H2DCFDA fluorescence intensity was visualized with Zeiss LSM 510 META laser confocal microscope.
Adenoviral transduction.
The point mutations CaMKIIδ2 M281,282V were generated with the QuikChange II Site-directed Mutagenesis kit (Stratagene). The cDNA for CaMKIIδ M281,282V was inserted into the multicloning site of the adenoviral shuttle vector pacAd5 CMV IRES enhanced green fluorescent protein pA using the BamH1 and Xho1 restriction sites. Adenovirus recombination and amplification was performed by the Gene Transfer Vector Core at the University of Iowa (3). Viral titers were determined with the Adeno-X rapid titer kit (Clontech). Control virus expressing enhanced green fluorescent protein was generated by recombination and amplification of the shuttle vector.
VSMC were incubated with adenoviruses expressing MMP9, CaMKIIδ M281,282V, or control adenovirus at a multiplicity of infection of 500 in serum-free media overnight. Subsequent experiments were conducted 72 h after adenoviral transduction as described previously (34).
Migration assays.
VSMC migration assays were performed using the modified Boyden chamber method in Transwell cell-culture chambers with a collagen polycarbonate membrane with 8-μm pores. WT or CaMKIIδ−/− VSMC (105 cells/well) were added to the upper chamber of the transwell and allowed 30 min to attach. The chambers contained DMEM with 0.1% FBS. Migration of VSMC was induced by the addition of 10 ng/ml PDGF, 50 ng/ml TNF-α, or DMEM with 10% FBS to the lower compartment. After 6 h, nonmigrated cells were removed from the upper chamber. VSMC migrating to the lower surface of the membrane were fluorescently stained with DAPI, imaged, and quantified using Cell Profiler software.
For scratch wound assays, the serum-starved VSMC monolayer was disrupted with a sterile cell scraper to create a cell-free zone. Cells were then washed with medium and treated in DMEM containing 10% FBS. Aphidicolin, a specific inhibitor of DNA polymerase A,D, was added at a concentration of 100 μM to prevent cell proliferation during the scratch wound assay. Images were taken 24 h after injury using a microscope equipped with a digital camera.
Some migration assays were performed in the presence of 10 nM MMP9 inhibitor I (PubChem Compound ID: 16760552). This inhibitor blocks MMP9 reversibly with an IC50 = 5 nM. MMP1 (IC50 = 1.05 μM) and MMP13 (IC50 = 113 nM) are only inhibited at much higher concentrations.
Where indicated, cells were infected with AdMMP9 or Adcontrol for 48 h, followed by serum starvation for an additional 24 h.
Quantitative RT-PCR.
Total RNA was isolated from VSMC per manufacturer's recommendation (Qiagen) followed by digestion with proteinase K and DNase I to eliminate genomic DNA contamination. cDNA was transcribed from 1 μg RNA using Superscript III enzyme (Invitrogen) and random nanomer primers. Message expression was quantified using an iQ Lightcycler (Bio-Rad) with SYBR green dye with MMP9 primers (sense: 5′-GAAGGCAAACCCTGTGTGTT-3′; antisense: 5′-AGAGTACTGCTTGCCCAGGA-3′) and normalized to acidic ribosomal phosphoprotein mRNA.
MMP9 gelatin zymography.
VSMC were serum-deprived for 24 h followed by incubation with TNF-α for 24 h. The culture medium was collected and concentrated by centrifugation at 7,500 g for 15 min at 4°C in Amicon Ultra-4 filter devices (Milipore). Protein concentrations were determined by modified Lowry assay. After electrophoresis (10% Zymogram gels; Bio-Rad), gels were soaked in zymogram renaturing buffer (Bio-Rad) for 1 h at room temperature and incubated in zymogram developing buffer (Bio-Rad) at 37°C for 48 h, to allow proteinase digestion of its substrate. Gels were then stained in a solution of 40% methanol, 10% acetic acid, and 0.25% brilliant blue R-250 for 1 h and destained in 10% acetic acid glacial and 40% ethyl alcohol. Gelatinolytic activity appeared as clear bands of digested gelatin against a dark blue background of stained gelatin. After gel staining, MMP2 and MMP9 were identified based on gelatin lysis at molecular masses of 62 and 84 kDa, respectively. Gel images were obtained using a digital imaging system (Eastman Kodak).
In vivo MEF2 reporter assay.
MEF2 X CaMKIIδ−/− and MEF2 reporter mice underwent carotid ligation as described above. Fourteen days after ligation, the mice were euthanized and MEF2-driven β-galactosidase expression was detected in paraffin-embedded section by immunostaining for β-galactosidase. Densitometry for β-galactosidase expression was performed in images using NIH ImageJ. For the purpose of analysis, the area between the external and internal elastic lamina was considered as media and the area luminal to the internal elastic lamina considered as neointima.
MMP9 promoter assays.
The murine MMP9 promoter (−1,261 to +1) was subcloned into pTAL-luc (Clontech) via blunt end ligation (22). The 5′-flanking sequence of the human MMP9 promoter (−1,284/+21) was inserted between KpnI and HindIII restriction sites in the pGL3 basic expression vector (41). The core MMP9 promoter luciferase construct included the 5′-flanking sequence up to −584 of the human MMP9 promoter without the putative MEF2 and a confirmed NF-κB consensus binding site. Cells were transfected using Lipofectamine LTX with Plus reagent (Invitrogen) according to the manufacturer's directions. To correct for transfection efficiency, cells were cotransfected with phL-TK vector encoding Renilla luciferase (Promega). Sixteen hours after transfection, media was replaced with fresh serum-containing media, and cells were allowed to recover for 48 h. TNF-α (50 ng/ml) was added for 20 h. Firefly and Renilla luciferase activities were determined in cell lysates using the Dual Luciferase reporter assay kit (Promega), according to the manufacturer's instructions, and were expressed in real light units.
MMP9 mRNA stability assays.
WT and CaMKIIδ−/− VSMC were grown to 70% confluence and then serum-starved for 24 h, before addition of 50 ng/ml TNF-α. After 24 h, cells were treated with 20 pg/ml DRB (5,6-dichlororibofuranosyl benzimidazole) or 5 pg/ml actinomycin D for varying lengths of time (0–16 h). Total RNA was isolated and quantitated by spectrophotometry. Quantitative RT-PCR was performed as described above. Relative MMP9/acidic ribosomal phosphoprotein mRNA ratios were calculated relative to the copy numbers at 0 h DRB/actinomycin. For determination of half-life, we assumed exponential decay. The indicated half-life was based on calculation for all four measured time points.
Statistical analysis.
Data are shown as means ± SE unless noted otherwise. The SigmaPlot statistical package was used for the quantitative analyses of parameters such as EEL perimeter, staining intensity, and number of migrated cells (ANOVA and Student's t-test). A probability value <0.05 was considered significant.
RESULTS
CaMKII is activated by autophosphorylation and oxidation.
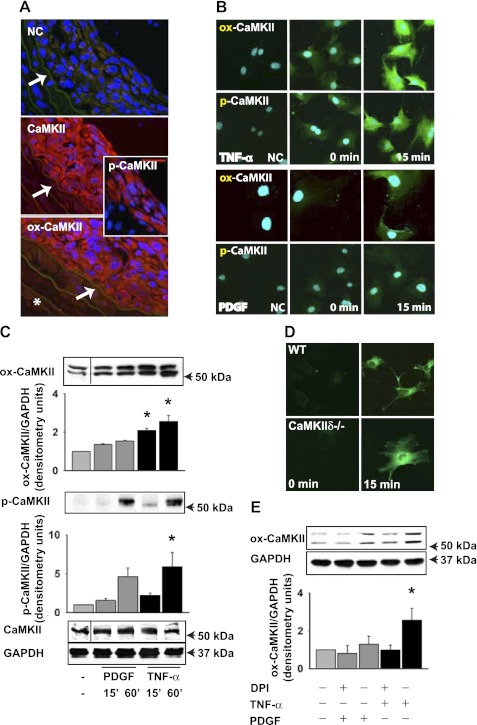
We recently reported that CaMKII is an important mediator of the response to vascular injury (35), and numerous studies have shown that this response is ROS dependent (44) . Given that oxidative stress results in CaMKII activation via oxidation of Met 281,282 in the regulatory domain of CaMKII (14), we examined CaMKII activation in the neointima in response to carotid injury. The neointima of WT mice had robust CaMKII expression following carotid ligation, consistent with our previous finding (35). We assessed CaMKII autophosphorylation (p-CaMKII) and oxidation (ox-CaMKII) by immunofluorescence. For ox-CaMKII detection, we used a validated antibody that does not recognize unoxidized or autophosphorylated CaMKII (15). We observed a strong signal for both ox- and p-CaMKII (Fig. 1A). Weak ox-CaMKII staining was seen in all layers of the carotid wall after injury, including the adventitia. In contrast, at baseline, before carotid ligation, no significant levels of p- or ox-CaMKII were detected (data not shown). Next, we isolated VSMC and analyzed how promigratory agonists that increase ROS production affect CaMKII oxidation and autophosphorylation. We detected low levels of ox-CaMKII and p-CaMKII at baseline in serum-starved VSMC (Fig. 1B). Exposure to TNF-α for 15 min resulted in an increase in ox- and p-CaMKII, whereas the response to PDGF was modest (Fig. 1B). Similarly, we detected significant increases in ox- and p-CaMKII after TNF-α treatment for 60 min by immunoblotting (Fig. 1C). A double band was consistently detected for ox- and p-CaMKII. These data demonstrate that ox-CaMKII is present in the neointima after vascular injury and that oxidative activation occurs in VSMC.
Fig. 1.
CaMKII oxidation is increased after carotid ligation and treatment with TNF-α. A: 14 days following carotid artery ligation, sections (1,000–2,000 μm proximal to the injury) were stained with antibodies that specifically detect oxidized Met281/282 CaMKII (ox-CaMKII), phosphorylated Thr286 CaMKII (p-CaMKII), or total CaMKII (red; blue DAPI). NC, negative control without primary antibody. The arrow points to the internal elastic lamina. The area luminal to the internal elastic lamina was considered as neointima. *Adventitia. B: serum-deprived wild-type (WT) vascular smooth muscle cells (VSMC) were treated with 10 ng/ml PDGF or 50 ng/ml TNF-α for 15 min, fixed, and stained for ox-CaMKII or p-CaMKII (green, blue TO-PRO-3). C: immunoblot for ox- and p-CaMKII in WT VSMC. Immunoblots are from a single experiment, and intervening lanes have been removed for ease of interpretation (indicated with a line). Representative results of 3 independent experiments are shown. *P < 0.05 compared with untreated. D: CM-H2DCFDA fluorescence was detected in WT and CaMKIIδ−/− VSMC after treatment with 50 ng/ml TNF-α for 15 min. E: WT VSMC were pretreated with 10 μM diphenyliodonium (DPI) for 30 min, followed by incubation with 10 ng/ml PDGF, or 50 ng/ml TNF-α for 60 min in the presence of DPI.
A recent report suggested that CaMKII may regulate the NADPH oxidase Nox 5 (43). Thus we assessed whether ROS production is impaired in CaMKIIδ−/− VSMC by CM-H2DCFDA fluorescence. After treatment with TNF-α for 15 min, ROS production was upregulated in both WT and CaMKIIδ−/− VSMC (Fig. 1D), suggesting that deficiency of CaMKII does not impair generation of ROS in VSMC and instead implies that CaMKII is a downstream mediator of ROS-dependent signaling. In accordance with this view, pretreatment of WT VSMC with NADPH oxidase inhibitor diphenyliodonium decreased ox-CaMKII levels in response to TNF-α (Fig. 1E). To our knowledge, these data provide the first evidence that CaMKII is oxidized and directly activated by ROS in VSMC.
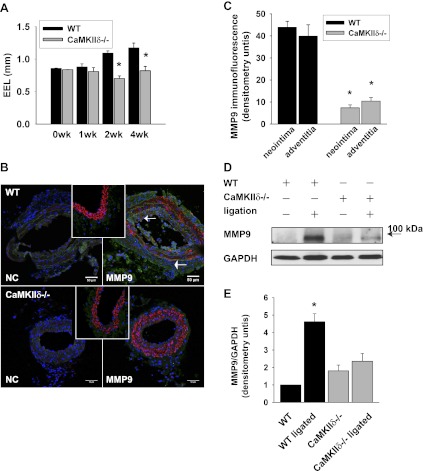
Outward remodeling and MMP9 expression are decreased in CaMKIIδ−/− carotid arteries after ligation.
We described previously that CaMKIIδ regulates neointimal hyperplasia by promoting VSMC proliferation (35). Because ROS-dependent migration is a key mechanism of vascular remodeling (44), we investigated whether remodeling is impacted by deletion of CaMKIIδ. We measured the perimeter of the EEL in hematoxylin-eosin-stained sections of WT and CaMKIIδ−/− carotid arteries 1, 2, and 4 wk after complete ligation of the common carotid artery. We detected an injury-induced increase in the EEL in WT mice but not in CaMKIIδ−/− mice (Fig. 2A). Because a similar phenotype has been described in MMP9−/− mice (17), we examined whether MMP9 expression is decreased under CaMKIIδ deletion. Whereas no MMP9 was detected at baseline in WT mice, 14 days after ligation the neointima and the adventitia of WT carotid arteries stained strongly for MMP9 (Fig. 2, B, top right, and C), suggesting active vascular remodeling. In contrast, no MMP9 was detected in the ligated CaMKIIδ−/− carotid arteries (Fig. 2, B, bottom right, and C), which may be attributed to reduced neointimal formation in these mice. Immunoblots for MMP9 also revealed a significant increase in MMP9 protein expression in ligated carotid arteries of WT but not of CaMKIIδ−/− mice (Fig. 2, D and E).
Fig. 2.
Matrix metalloproteinase (MMP)9 expression is decreased in CaMKIIδ−/− carotid arteries after complete carotid ligation. A: structural outward remodeling was assessed by measuring the circumference of the external elastic lamina (EEL) in hematoxylin-eosin-stained sections of in WT and CaMKIIδ−/− carotid arteries 14 days after ligation (n = 20 sections of 5 mice per group; *P < 0.05 vs. WT). B: immunofluorescent staining for MMP9 (green) in 5-μm paraffin-embedded sections (1,000 μm proximal to the injury) of WT control and CaMKIIδ−/− carotid arteries 14 days after ligation (α-smooth muscle-actin red, DAPI blue). B, left: negative control. B, insets: MMP9 immunofluorescence at baseline. C: MMP9 densitometry (n = 5 carotid arteries per group). D: immunoblots for MMP9 from protein extracts of WT and CaMKIIδ−/− carotid arteries collected at baseline or 14 days after carotid ligation. GAPDH immunoblots served as a loading control. E: densitometry of 4 independent MMP9 immunoblots. The MMP9 protein levels were adjusted for GAPDH expression.
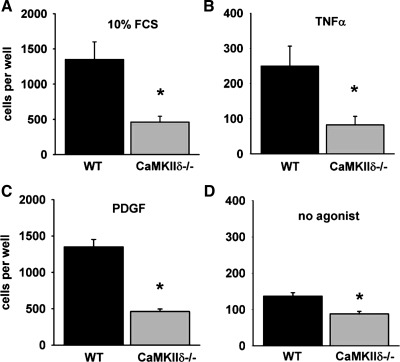
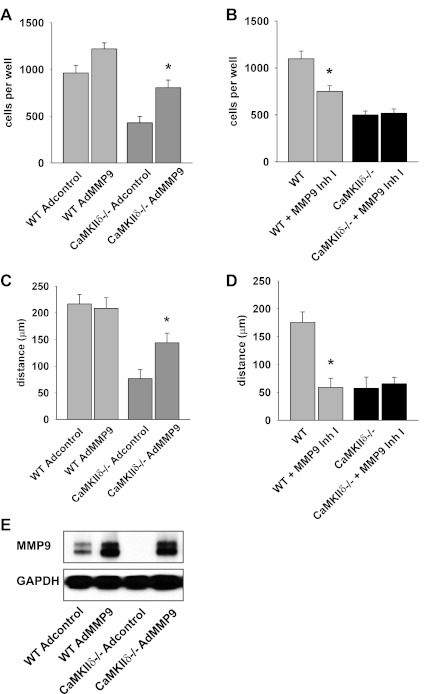
Impaired CaMKIIδ−/− VSMC migration is rescued by MMP9 overexpression.
We found that serum, PDGF, and TNF-α increased WT VSMC migration but that migration responses to PDGF and TNF-α were significantly reduced in CaMKIIδ−/− VSMCs (Fig. 3, A–D). Adenoviral overexpression of MMP9 restored migration to serum in CaMKIIδ−/− VSMC (Fig. 4, A and C). The levels of MMP9 protein expression were equal in WT and CaMKIIδ−/− VSMC and exceeded the baseline MMP9 expression in WT cells. Conversely, when WT and CaMKIIδ−/− VSMC were treated with the MMP9 inhibitor I at a concentration that selectively inhibits MMP9, migration was only affected in WT cells. These data support a concept that MMP9 is required for CaMKII-mediated promotion of VSMC migration.
Fig. 3.
Migration is decreased in CaMKIIδ−/− VSMC. A–D: cell migration was assessed using the modified Boyden chamber assay. Quiescent, serum-deprived WT or CaMKIIδ−/− aortic VSMC were treated with 10% FCS (A), 50 ng/ml TNF-α (B), 10 ng/ml PDGF (C), or serum-free DMEM (D) for 5 h. (*P < 0.05 vs. WT; n = 3 to 4 independent experiments).
Fig. 4.
Migration is rescued by MMP9 expression in CaMKIIδ−/− aortic VSMC. A and C: WT and CaMKIIδ−/− VSMC were transduced with an adenovirus containing MMP9 (AdMMP9) or no transgene [Adcontrol; multiplicity of infection (MOI) 100 for both] for 72 h. Cell migration was examined by Boyden chamber assay in response to 10% FCS after 5 h (A) or in wound scratch assay 24 h after the VSMC monolayer was disrupted (C; *P < 0.05 vs. Adcontrol; n = 3 independent experiments). B and D: 10 nM MMP9 inhibitor I (Inh I) were added to WT and CaMKIIδ−/− VSMC during Boyden chamber assays with 10% FCS (B) or wound scratch assays (D; *P < 0.05 vs. untreated; n = 3 independent experiments). E: expression of MMP9 after adenoviral transduction with Adcontrol or AdMMP9 in CaMKIIδ−/− VSMC was examined by immunoblotting.
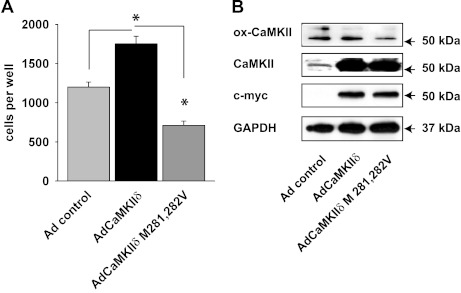
Furthermore, we tested whether migration could be enhanced in CaMKIIδ−/− VSMC by CaMKII reconstitution. As expected, adenoviral overexpression of WT CaMKIIδ2 increased migration. We also overexpressed the CaMKII mutant Met 281,282 Val that is missing the two methionine residues required for the oxidative activation of CaMKII (15). In stark contrast, VSMC migration further decreased below the level of control CaMKIIδ−/− VSMC, indicating that the oxidation-deficient mutant exerted a dominant negative effect (Fig. 5A). Proliferating VSMC are known to produce only low concentrations of ROS. This may explain why the levels of ox-CaMKII were not increased despite WT CaMKII overexpression (Fig. 5B); however, a lower level of ox-CaMKII with CaMKIIδ M281,282V was noted. The residual ox-CaMKII with CaMKIIδ M281,282V overexpression band is likely explained by the presence of the CaMKII isoform γ in CaMKIIδ−/− VSMC.
Fig. 5.
Migration is rescued by WT CaMKIIδ but not by CaMKIIδ M281,282V expression in CaMKIIδ−/− aortic VSMC. A: CaMKIIδ−/− VSMC were transduced with an adenovirus containing WT CaMKIIδ (Ad CaMKIIδ), the oxidation-resistant mutant CaMKIIδ M281,282V (Ad CaMKIIδ M281,282V), or no transgene (Adcontrol; MOI 100 for all) for 72 h. Cell migration was assessed using the modified Boyden chamber assay. Quiescent, serum-deprived VSMC were treated with 10% FCS for 5 h. (*P < 0.05 vs. WT, n = 4 independent experiments). B: immunoblots for c-myc, GAPDH, and ox- and total CaMKII in proliferating CaMKIIδ−/− VSMC that are transduced as described in A. Representative Western blot of 5 independent experiments is shown.
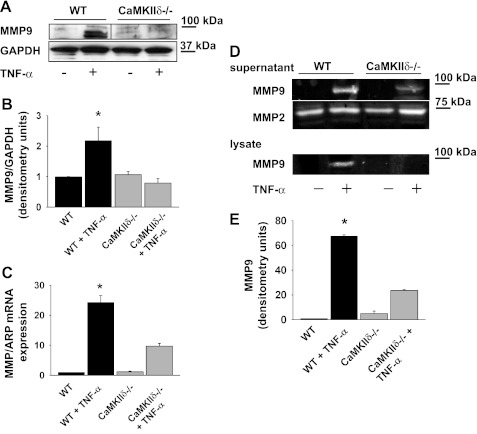
MMP9 expression and activity are decreased in CaMKIIδ−/− VSMC.
We next examined how CaMKII deficiency affects expression and activity of MMP9 in cultured VSMC. MMP9 protein expression increased about 2.2-fold in WT VSMC after incubation with TNF-α for 24 h, but no significant increase was observed in the CaMKIIδ−/− VSMC (Fig. 6, A and B). Accordingly, MMP9 mRNA levels were increased 24.3-fold in WT VSMC but only 9.7-fold in CaMKIIδ−/− cells (P < 0.05; Fig. 6C). Next, MMP9 activity was measured by gelatin zymography in the supernatant and lysate of VSMC that were incubated with TNF-α for 24 h. At baseline, minimal MMP9 activity was seen in WT and CaMKIIδ−/− VSMC (Fig. 6D). After TNF-α treatment, deficiency in CaMKII resulted in a 65% decrease in MMP9 activity in the supernatant compared with WT. MMP9 was induced in WT but not CaMKIIδ−/− VSMC in response to TNF-α, but no differences in MMP2 activity at baseline or with TNF-α were observed in VSMC lysates (Fig. 6E). Collectively, these results implicate CaMKII in the expression and activation of MMP9 in VSMC.
Fig. 6.
MMP9 protein and mRNA expression is significantly impaired in CaMKIIδ−/− VSMC. A: immunoblot of WT and CaMKIIδ−/− VSMC for MMP9 after treatment with 50 ng/ml TNF-α for 24 h. Representative Western blot of 5 independent experiments is shown. Immunoblots are from a single experiment, and intervening lanes have been removed for ease of interpretation (indicated with a line). B: densitometry of MMP9 expression normalized to GAPDH expression in 5 independent experiments. C: MMP9 mRNA levels in WT and CaMKIIδ−/− VSMC after 24-h treatment with 50 ng/ml TNF-α (n = 7 independent experiments; *P < 0.05). D: MMP2 and -9 activities were determined by gelatin zymography in the supernatant of WT and CaMKIIδ−/− VSMC after 24 h treatment with 50 ng/ml TNF-α. MMP9 activity was also assessed in lysates. E: quantification of MMP9 activity in the supernatant of WT and CaMKIIδ−/− VSMC, adjusted for cell number at the time of supernatant collection (n = 4 independent experiments; *P < 0.05 vs. untreated WT). ARP, acidic ribosomal phosphoprotein.
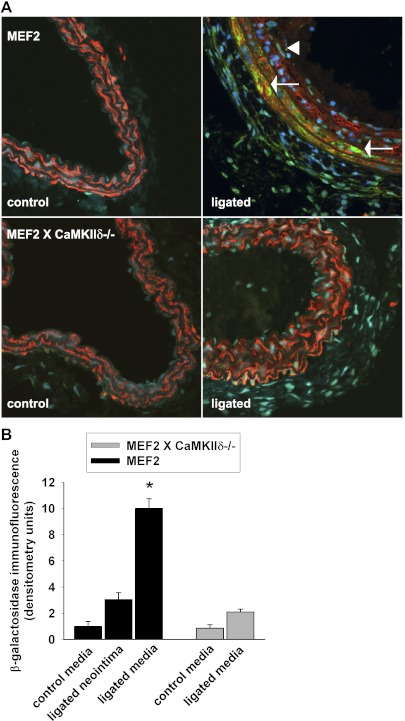
MEF2 transcription is activated after carotid ligation.
The transcription factor MEF2 is regulated in VSMC by CaMKII through derepression of HDAC4 (34). A recent report identified a putative MEF2 binding site in the MMP9 promoter and suggested that CaMKII regulates MMP9 transcription in myocardial cells. To test whether MEF2 may regulate MMP9 expression in vivo after carotid ligation, we cross-bred CaMKIIδ−/− mice with MEF2 reporter mice that express lacZ once MEF2 transcriptional activity is induced (42). As expected, no MEF2 transcriptional activity was detected at baseline (Fig. 7, A, left, and B). However, after carotid ligation, MEF2 was strongly activated in the media of WT mice (Fig. 7A, top right). In the neointima and adventitia where MMP9 is robustly expressed after carotid ligation (Fig. 2B), only weak MEF2 activity was detected. In MEF2 reporter X CaMKIIδ-/- mice, weak MEF2 transcriptional activity was noted after carotid ligation (Fig. 7A, bottom right). The distinct localization of MEF2 transcriptional activity and MMP9 in different layers of the ligated WT carotid artery suggests that MEF2-dependent gene transcription, while activated after vascular injury, does not control MMP9 expression. Under CaMKII deletion, both MMP9 expression and MEF2-controlled transcription are abolished, but the two processes are unlikely to be related.
Fig. 7.
MEF2 transcription after carotid ligation. MEF2 reporter mice that harbor a lacZ reporter gene under the control of tandem copies of the MEF2 DNA-binding sites were cross-bred with CaMKIIδ−/− mice. A: immunofluorescence for β-galactosidase (green) was performed in carotid arteries (1,000 μm proximal to the injury) of MEF2- and MEF2 X CaMKIIδ−/− mice that were collected at baseline or 14 days after ligation (green, red smooth muscle-actin, blue TO-PRO-3). MEF2 transcriptional activity was detected in the carotid media only in ligated MEF2 reporter mice (arrow), whereas the MEF2 activation in the neointima (arrow head) and adventitia was weak. B: densitometric quantitation of β-galactosidase in the neointima and media of the ligated MEF2 reporter and in the media of the ligated MEF2XCaMKIIδ−/− carotid arteries and unligated controls, adjusted for area and background. Because no measurable neointima developed after ligation in MEF2XCaMKIIδ−/− carotid arteries, no densitometry of the MEF2XCaMKIIδ−/− neointima is provided. Three to four sections of 3 mice per group were analyzed (*P < 0.05 vs. untreated WT).
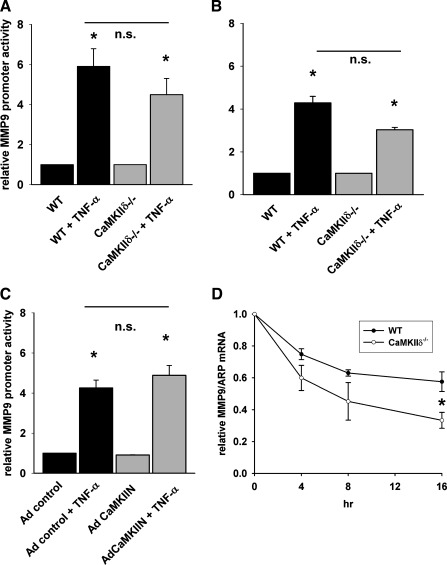
MMP9 promoter activity is not dependent on MEF2 transcription in vitro.
To further dissect the regulation of MMP9 by CaMKII, we first investigated whether deficiency in CaMKII decreases MMP9 transcription in response to TNF-α. For this purpose, we transfected a construct containing the human MMP9 promoter (−1,284 to +21) with a luciferase reporter into WT and CaMKIIδ−/− VSMC (Fig. 8A) (41). After treatment with TNF-α for 20 h, we detected a 7.3-fold increase in MMP9 promoter activity in WT VSMC (Fig. 8A). In contrast with our working hypothesis that CaMKII regulates MMP9 mRNA transcription, we did not detect a decrease in MMP9 promoter activity in CaMKIIδ−/− VSMC after TNF-α treatment. Very similar results were obtained with a murine MMP9 promoter (−1,180 to +30) construct (data not shown). We also used a truncated human MMP9 luciferase promoter construct (−584 to +21) that lacks the putative MEF2 consensus binding site at −678 and an established NF-κB consensus binding site at −598 (Fig. 8B) (41). In WT VSMC, the MMP9 promoter activity rose 4.3-fold, yet the MMP9 promoter activity was nonsignificantly higher in CaMKIIδ−/− VSMC compared with WT control, suggesting that MMP9 mRNA transcription is independent of CaMKII.
Fig. 8.
MMP9 promoter activity and mRNA stability in WT and CaMKIIδ−/− VSMC. A: full-length (−1,284/+21) and a core (B) MMP9 promoter luciferase construct that includes the 5′-flanking sequence up to −584 of the human MMP-9 promoter were transfected into WT and CaMKIIδ−/− VSMC with TK Renilla luciferase as control. Promoter activity was induced with 50 ng/ml TNF-α for 24 h. MMP9 promoter activity was normalized for TK Renilla luciferase activity. C: CaMKII peptide inhibitor CaMKIIN or Adcontrol was expressed in WT VSMC by adenoviral transduction (MOI 500). Forty-eight hours after transduction, the full-length MMP9 promoter luciferase and the TK Renilla luciferase constructs were transfected and promoter activity induced with TNF-α as in A and B. D: MMP9 mRNA stability was assessed by quantitative RT-PCR in DRB- and actinomycin B-treated WT and CaMKIIδ−/− VSMC. MMP9 mRNA transcription was first induced by TNF-α treatment for 24 h, followed by arrest with DRB and actinomycin. MMP9 mRNA levels were normalized to the expression of the housekeeping gene ARP and expressed as ratio in comparison with the time point 0 h when DRB and actinomycin were added. n.s., not significant. *P < 0.05 vs. untreated WT or untreated Adcontrol.
CaMKIIδ−/− VSMC are proliferation deficient (35). Consequently, although equal cell numbers were plated, fewer CaMKIIδ−/− VSMC may have been present during the transfection, and the ratio of promoter construct to cells may have influenced the promoter assay. We therefore tested whether acute CaMKII inhibition by adenoviral overexpression of the CaMKII peptide inhibitor CaMKIIN affects MMP9 transcription. However, MMP9 promoter activity (−1,284 to +21) was similar in cells that were transduced with adenovirus expressing the inhibitor peptide CaMKIIN compared with cells infected with a control virus (Fig. 8C).
Because MMP9 mRNA and protein levels and activity were lower in CaMKIIδ−/− VSMC (Fig. 6), we hypothesized that CaMKII regulates MMP9 mRNA stability. Baseline MMP9 mRNA levels were low; thus cells were treated with TNF-α for 24 h to induce MMP9 transcription, followed by treatment with DRB and actinomycin to arrest transcription. MMP9 mRNA was quantified at different time points after arrest. The MMP9 mRNA half-life was calculated as 20 h in WT VSMC (Fig. 8D). In contrast, the MMP9 mRNA turnover in CaMKIIδ−/− VSMC was significantly decreased to about 10 h. These findings imply that MMP9 expression in VSMC in response to TNF-α is regulated by CaMKII at the posttranscriptional level.
DISCUSSION
VSMC migration following injury is well-established to be ROS dependent. CaMKII has also been linked to migration, although the mechanism was unclear. Here we reveal that CaMKII is activated following injury and induces MMP9 expression to thereby regulate vascular remodeling. We found that CaMKII deficiency strongly decreased MMP9 expression after complete carotid ligation. VSMC migration is diminished in CaMKIIδ−/− VSMC and recovered with MMP9 overexpression. MMP9 mRNA and protein levels and activity are significantly lower after TNF-α treatment in CaMKIIδ−/− VSMC. We did not detect a significant difference in MMP9 promoter activity with CaMKIIδ deficiency. However, our data support that CaMKII influences MMP9 mRNA stability. In addition, this study demonstrates that CaMKII is directly activated by oxidation of methionine 281,282 in vivo after ligation and in response to a growth factor that induces ROS and ROS-dependent migration. Collectively, our data implicate CaMKII regulation of MMP9 expression and activity as a key step neointimal formation.
Direct activation of CaMKII has been reported to occur by oxidation of methionine 281,282 adjacent to the CaMKII autophosphorylation site and mediates cardiac pathology after myocardial infarction and in sinus node dysfunction (14, 15, 54). Oxidant stress is a common mechanism of injury in vascular tissue (20), and excessive ROS is implicated in the pathogenesis of hypertension, atherosclerosis, and stroke (21). Given the important role of ROS in smooth muscle migration (53, 56, 58) and proliferation (38, 45) and the role of CaMKII in these VSMC phenotypes, we speculated that CaMKII is oxidized and thereby activated in the vasculature, contributing to the development of vascular disease. A role for CaMKII as mediator of ROS-dependent signaling in the vasculature has been postulated (58) based on previous studies reporting an increase in autophosphorylated CaMKII in response to oxidative stress in vascular smooth muscle (17, 37) . The inhibition of CaMKII blocks activation of ROS-dependent signaling pathways (7, 37). The current study demonstrates for the first time that CaMKII is directly activated by methionine oxidation in the neointima after ligation and after treatment with TNF-α. These data strengthen the evidence for CaMKII as mediator of oxidative signaling in VSMC. In addition to oxidized CaMKII, we also observed an increase in phosphorylated CaMKII with injury and in response to agonists. Although the signaling networks activated by p-CaMKII have been well-studied, it is unclear whether the two activation mechanisms occur simultaneously in one CaMKII subunit or in different subunits of the holoenzyme. Our data with an oxidation-resistant mutant of CaMKIIδ (Fig. 5A) show that oxidative CaMKII activation is necessary for migration. This finding suggests that the two activation pathways are not redundant. Whether this is true for other VSMC phenotypes is currently unknown.
Several previous studies have reported that CaMKII mediates VSMC migration (36, 39, 46, 59), but the downstream signaling targets are not well defined. Others have detected active p-CaMKII at the wound edge of migrating VSMC, and CaMKIIδ2 downregulation by short hairpin RNA decreased the activation of the Rho GTPase Rac and migration in response to PDGF (39). However, VSMC migration is a multistep process that is likely regulated at multiple levels. In this study, we provide evidence that CaMKII controls expression of MMP9, a master regulator of extracellular matrix turnover and a well-established mediator of VSMC migration (30). Importantly, we found that migration could be restored in CaMKIIδ−/− VSMC with re-expression of MMP9, thus revealing one apparent mechanism by which CaMKII mediates VSMC migration.
The role of ROS in VSMC migration is established, including several lines of evidence demonstrating that MMP9 activity and expression are regulated by ROS (22, 41, 47, 50). What remains unclear, however, are the precise pathways by which ROS activate MMP9. Others have shown that regulation of MMP9 expression occurs at the transcriptional and posttranscriptional levels (6, 13, 16, 29, 49). The transcription factors NF-κB (27, 37, 51) and AP-1 (18) can be regulated by CaMKII and have been shown to promote MMP9 transcription (9, 12, 33). A recent study reported on CaMKII regulating MMP9 transcription through the transcription factor MEF2 in cardiomyocytes (23). Our data do not support this pathway in VSMCs, because the known binding sites for these transcription factors are included in the full-length MMP9 promoter construct used in our study (Fig. 8). In contrast, we found that the MMP9 mRNA stability was decreased in CaMKIIδ−/− VSMC. Regulation of mRNA half-life occurs primarily through conserved AU-rich elements (AREs) that are present in multiple, nontandem copies within the 3′-untranslated region (3′-UTR) of mRNAs(8). AREs interact with specific RNA-binding proteins that control the rate of mRNA decay. The 3′-UTR of the mouse and rat MMP9 transcripts each contain several functional ARE consensus sequences (13, 19, 55). Future studies should determine whether CaMKII promotes MMP9 mRNA stability by regulating the functions of specific RNA-binding proteins that bind to these AREs.
A potential candidate is the cytoplasmic polyadenylation element binding protein, which binds to the 3′-UTR of cytoplasmic mRNAs and, when phosphorylated, initiates mRNA polyadenylation and translation. Furthermore, cytoplasmic polyadenylation element binding protein has been shown to be phosphorylated by CaMKII in neurons (4).
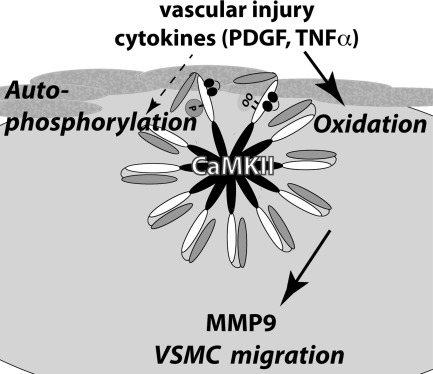
In summary, this study provides in vivo and in vitro evidence that CaMKIIδ controls VSMC migration through regulation of MMP9 (Fig. 9). Our data suggest that CaMKII regulates MMP9 expression posttranscriptionally in VSMC. Moreover, we demonstrated that active oxidized CaMKII is present in vivo after vascular injury and in vitro after treatment with agonists that are well known to promote ROS production and VSMC migration. These data provide novel insights into the mechanisms involved in VSMC migration and raise questions regarding whether CaMKII inhibition in clinical settings could decrease VSMC neointimal formation after angioplasty, arteriosclerosis, and transplant-associated arteriopathy.
Fig. 9.
Model of mechanism for CaMKIIδ function in migration. CaMKIIδ can be activated by autophosphorylation and oxidation in response to carotid ligation or promigratory agonists. Oxidative CaMKII activation is required for VSMC migration. CaMKII controls MMP9 expression likely through posttranscriptional mechanisms.
GRANTS
This work was supported in part by funding from the following grants: an American Heart Association Scientist Development Grant, a VA Merit grant by the Office of Research and Development, Department of Veterans Affairs (I. M. Grumbach), National Heart, Lung, and Blood Institute Grants HL-079031 and R01 HL-70250 (to M. E. Anderson); NIH grants ES-015981 and ES-014871 (to A. B. Carter); and the University of Iowa Cardiovascular Center Interdisciplinary Research Fellowship (to W. Li).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.A.S., L.X., H.L., W.L., J.B.H., P.N.S., and I.M.G. performed experiments; J.A.S., L.X., H.L., W.L., and I.M.G. analyzed data; J.A.S. and I.M.G. interpreted results of experiments; J.A.S., L.X., H.L., W.L., and I.M.G. prepared figures; J.A.S., A.B.C., M.E.A., and I.M.G. edited and revised manuscript; J.A.S., L.X., H.L., W.L., J.B.H., A.B.C., J.B., M.E.A., and I.M.G. approved final version of manuscript; J.B.H., A.B.C., J.B., M.E.A., and I.M.G. conception and design of research; I.M.G. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Chantal Allamargot (Central Microscopy Research Facilities, University of Iowa) for expert technical support and Dr. Kristina W. Thiel for assistance in the preparation of the manuscript.
REFERENCES
- 1. Abraham ST, Benscoter H, Schworer CM, Singer HA. In situ Ca2+ dependence for activation of Ca2+/calmodulin-dependent protein kinase II in vascular smooth muscle cells. J Biol Chem 271: 2506–2513, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Abraham ST, Benscoter HA, Schworer CM, Singer HA. A role for Ca2+/calmodulin-dependent protein kinase II in the mitogen-activated protein kinase signaling cascade of cultured rat aortic vascular smooth muscle cells. Circ Res 81: 575–584, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Anderson RD, Haskell RE, Xia H, Roessler BJ, Davidson BL. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther 7: 1034–1038, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Atkins CM, Nozaki N, Shigeri Y, Soderling TR. Cytoplasmic polyadenylation element binding protein-dependent protein synthesis is regulated by calcium/calmodulin-dependent protein kinase II. J Neurosci 24: 5193–5201, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA, Katus HA, Bassel-Duby R, Maier LS, Olson EN. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci USA 106: 2342–2347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bond M, Chase AJ, Baker AH, Newby AC. Inhibition of transcription factor NF-kappaB reduces matrix metalloproteinase-1, -3 and -9 production by vascular smooth muscle cells. Cardiovasc Res 50: 556–565, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Bouallegue A, Pandey NR, Srivastava AK. CaMKII knockdown attenuates H2O2-induced phosphorylation of ERK1/2, PKB/Akt, and IGF-1R in vascular smooth muscle cells. Free Radic Biol Med 47: 858–866, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci 58: 266–277, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng G, Wei L, Xiurong W, Xiangzhen L, Shiguang Z, Songbin F. IL-17 stimulates migration of carotid artery vascular smooth muscle cells in an MMP-9 dependent manner via p38 MAPK and ERK1/2-dependent NF-kappaB and AP-1 activation. Cell Mol Neurobiol 29: 1161–1168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cho A, Reidy MA. Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ Res 91: 845–851, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Cipolletta E, Monaco S, Maione AS, Vitiello L, Campiglia P, Pastore L, Franchini C, Novellino E, Limongelli V, Bayer KU, Means AR, Rossi G, Trimarco B, Iaccarino G, Illario M. Calmodulin-dependent kinase II mediates vascular smooth muscle cell proliferation and is potentiated by extracellular signal regulated kinase. Endocrinology 151: 2747–2759, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doyle GA, Pierce RA, Parks WC. Transcriptional induction of collagenase-1 in differentiated monocyte-like (U937) cells is regulated by AP-1 and an upstream C/EBP-beta site. J Biol Chem 272: 11840–11849, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Eberhardt W, Akool e Rebhan J, Frank S, Beck KF, Franzen R, Hamada FM, Pfeilschifter J. Inhibition of cytokine-induced matrix metalloproteinase 9 expression by peroxisome proliferator-activated receptor alpha agonists is indirect and due to a NO-mediated reduction of mRNA stability. J Biol Chem 277: 33518–33528, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Erickson JR, He BJ, Grumbach IM, Anderson ME. CaMKII in the cardiovascular system: sensing redox states. Physiol Rev 91: 889–915, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O′Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133: 462–474, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fahling M, Steege A, Perlewitz A, Nafz B, Mrowka R, Persson PB, Thiele BJ. Role of nucleolin in posttranscriptional control of MMP-9 expression. Biochim Biophys Acta 1731: 32–40, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Galis ZS, Johnson C, Godin D, Magid R, Shipley JM, Senior RM, Ivan E. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ Res 91: 852–859, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Gordon JW, Pagiatakis C, Salma J, Du M, Andreucci JJ, Zhao J, Hou G, Perry RL, Dan Q, Courtman D, Bendeck MP, McDermott JC. Protein kinase A-regulated assembly of a MEF2(middle dot)HDAC4 repressor complex controls c-Jun expression in vascular smooth muscle cells. J Biol Chem 284: 19027–19042, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Graubert T, Johnston J, Berliner N. Cloning and expression of the cDNA encoding mouse neutrophil gelatinase: demonstration of coordinate secondary granule protein gene expression during terminal neutrophil maturation. Blood 82: 3192–3197, 1993 [PubMed] [Google Scholar]
- 20. Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation 108: 1912–1916, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: animal and human studies. Circulation 108: 2034–2040, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Gurjar MV, Sharma RV, Bhalla RC. eNOS gene transfer inhibits smooth muscle cell migration and MMP-2 and MMP-9 activity. Arterioscler Thromb Vasc Biol 19: 2871–2877, 1999 [DOI] [PubMed] [Google Scholar]
- 23. He BJ, Joiner ML, Singh MV, Luczak ED, Swaminathan PD, Koval OM, Kutschke W, Allamargot C, Yang J, Guan X, Zimmerman K, Grumbach IM, Weiss RM, Spitz DR, Sigmund CD, Blankesteijn WM, Heymans S, Mohler PJ, Anderson ME. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med 17: 1610–1618, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Healy DA, Zierler RE, Nicholls SC, Clowes AW, Primozich JF, Bergelin RO, Strandness DE., Jr Long-term follow-up and clinical outcome of carotid restenosis. J Vasc Surg 10: 662–668, 1989 [PubMed] [Google Scholar]
- 25. House SJ, Singer HA. CaMKII-delta isoform regulation of neointima formation after vascular injury. Arterioscler Thromb Vasc Biol 28: 441–447, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Hudmon A, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem 71: 473–510, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Hughes K, Edin S, Antonsson A, Grundstrom T. Calmodulin-dependent kinase II mediates T cell receptor/CD3- and phorbol ester-induced activation of IkappaB kinase. J Biol Chem 276: 36008–36013, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Illario M, Cavallo AL, Bayer KU, Di Matola T, Fenzi G, Rossi G, Vitale M. Calcium/calmodulin-dependent protein kinase II binds to Raf-1 and modulates integrin-stimulated ERK activation. J Biol Chem 278: 45101–45108, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Jiang Y, Muschel RJ. Regulation of matrix metalloproteinase-9 (MMP-9) by translational efficiency in murine prostate carcinoma cells. Cancer Res 62: 1910–1914, 2002 [PubMed] [Google Scholar]
- 30. Johnson C, Galis ZS. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler Thromb Vasc Biol 24: 54–60, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol 17: 2238–2244, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Lawrie GM, Lie JT, Morris GC, Jr, Beazley HL. Vein graft patency and intimal proliferation after aortocoronary bypass: early and long-term angiopathologic correlations. Am J Cardiol 38: 856–862, 1976 [DOI] [PubMed] [Google Scholar]
- 33. Lee SJ, Park SS, Lee US, Kim WJ, Moon SK. Signaling pathway for TNF-alpha-induced MMP-9 expression: mediation through p38 MAP kinase, and inhibition by anti-cancer molecule magnolol in human urinary bladder cancer 5637 cells. Int Immunopharmacol 8: 1821–1826, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Li H, Li W, Gupta AK, Mohler PJ, Anderson ME, Grumbach IM. Calmodulin kinase II is required for angiotensin II-mediated vascular smooth muscle hypertrophy. Am J Physiol Heart Circ Physiol 298: H688–H698, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li W, Li H, Sanders PN, Mohler PJ, Backs J, Olson EN, Anderson ME, Grumbach IM. The multifunctional Ca2+/calmodulin-dependent kinase II (delta) (CaMKIIdelta) controls neointima formation after carotid ligation and vascular smooth muscle cell proliferation through cell cycle regulation by p21. J Biol Chem 286: 7990–7999, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lundberg MS, Curto KA, Bilato C, Monticone RE, Crow MT. Regulation of vascular smooth muscle migration by mitogen-activated protein kinase and calcium/calmodulin-dependent protein kinase II signaling pathways. J Mol Cell Cardiol 30: 11: 2377–2389, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Luo SF, Chang CC, Lee IT, Lee CW, Lin WN, Lin CC, Yang CM. Activation of ROS/NF-kappaB and Ca2+/CaM kinase II are necessary for VCAM-1 induction in IL-1beta-treated human tracheal smooth muscle cells. Toxicol Appl Pharmacol 237: 8–21, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Menshikov M, Plekhanova O, Cai H, Chalupsky K, Parfyonova Y, Bashtrikov P, Tkachuk V, Berk BC. Urokinase plasminogen activator stimulates vascular smooth muscle cell proliferation via redox-dependent pathways. Arterioscler Thromb Vasc Biol 26: 801–807, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Mercure MZ, Ginnan R, Singer HA. CaM kinase II δ2-dependent regulation of vascular smooth muscle cell polarization and migration. Am J Physiol Cell Physiol 294: C1465–C1475, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE, Investigators SIRIUS Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 349: 1315–1323, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Murthy S, Ryan A, He C, Mallampalli RK, Carter AB. Rac1-mediated mitochondrial H2O2 generation regulates MMP-9 gene expression in macrophages via inhibition of SP-1 and AP-1. J Biol Chem 285: 25062–25073, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Naya FJ, Wu C, Richardson JA, Overbeek P, Olson EN. Transcriptional activity of MEF2 during mouse embryogenesis monitored with a MEF2-dependent transgene. Development 126: 2045–2052, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Pandey D, Gratton JP, Rafikov R, Black SM, Fulton D. Calcium/calmodulin-dependent kinase II mediates the phosphorylation and activation of NADPH oxidase 5. Mol Pharmacol 80: 407–415, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Papaharalambus CA, Griendling KK. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc Med 17: 48–54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Patterson C, Ruef J, Madamanchi NR, Barry-Lane P, Hu Z, Horaist C, Ballinger CA, Brasier AR, Bode C, Runge MS. Stimulation of a vascular smooth muscle cell NAD(P)H oxidase by thrombin. Evidence that p47(phox) may participate in forming this oxidase in vitro and in vivo. J Biol Chem 274: 19814–19822, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Pfleiderer PJ, Lu KK, Crow MT, Keller RS, Singer HA. Modulation of vascular smooth muscle cell migration by calcium/calmodulin-dependent protein kinase II-δ2. Am J Physiol Cell Physiol 286: C1238–C1245, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Poitevin S, Garnotel R, Antonicelli F, Gillery P, Nguyen P. Type I collagen induces tissue factor expression and matrix metalloproteinase 9 production in human primary monocytes through a redox-sensitive pathway. J Thromb Haemost 6: 1586–1594, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Ray JL, Leach R, Herbert JM, Benson M. Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci 23: 185–188, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Sehgal I, Thompson TC. Novel regulation of type IV collagenase (matrix metalloproteinase-9 and -2) activities by transforming growth factor-beta1 in human prostate cancer cell lines. Mol Biol Cell 10: 407–416, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shin MH, Moon YJ, Seo JE, Lee Y, Kim KH, Chung JH. Reactive oxygen species produced by NADPH oxidase, xanthine oxidase, and mitochondrial electron transport system mediate heat shock-induced MMP-1 and MMP-9 expression. Free Radic Biol Med 44: 635–645, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Singh MV, Kapoun A, Higgins L, Kutschke W, Thurman JM, Zhang R, Singh M, Yang J, Guan X, Lowe JS, Weiss RM, Zimmermann K, Yull FE, Blackwell TS, Mohler PJ, Anderson ME. Ca2+/calmodulin-dependent kinase II triggers cell membrane injury by inducing complement factor B gene expression in the mouse heart. J Clin Invest 119: 986–996, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stanic B, Katsuyama M, Miller FJ., Jr An oxidized extracellular oxidation-reduction state increases Nox1 expression and proliferation in vascular smooth muscle cells via epidermal growth factor receptor activation. Arterioscler Thromb Vasc Biol 30: 2234–2241, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270: 296–299, 1995 [DOI] [PubMed] [Google Scholar]
- 54. Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, Gao Z, He BJ, Luczak ED, Joiner ML, Kutschke W, Yang J, Donahue JK, Weiss RM, Grumbach IM, Ogawa M, Chen PS, Efimov I, Dobrev D, Mohler PJ, Hund TJ, Anderson ME. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest 121: 3277–3288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tanaka H, Hojo K, Yoshida H, Yoshioka T, Sugita K. Molecular cloning and expression of the mouse 105-kDa gelatinase cDNA. Biochem Biophys Res Commun 190: 732–740, 1993 [DOI] [PubMed] [Google Scholar]
- 56. ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, Rosenkranz S. Novel nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res 71: 331–341, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Wang HH, Hsieh HL, Yang CM. Calmodulin kinase II-dependent transactivation of PDGF receptors mediates astrocytic MMP-9 expression and cell motility induced by lipoteichoic acid. J Neuroinflammation 7: 84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, Griendling KK. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res 94: 1219–1226, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Yang M, Kahn AM. Insulin-inhibited and stimulated cultured vascular smooth muscle cell migration are related to divergent effects on protein phosphatase-2A and autonomous calcium/calmodulin-dependent protein kinase II. Atherosclerosis 196: 227–233, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Zhang W, Chen DQ, Qi F, Wang J, Xiao WY, Zhu WZ. Inhibition of calcium-calmodulin-dependent kinase II suppresses cardiac fibroblast proliferation and extracellular matrix secretion. J Cardiovasc Pharmacol 55: 96–105, 2010 [DOI] [PubMed] [Google Scholar]