Abstract
Complex congenital heart disease (CHD) is often seen in conjunction with heterotaxy, the randomization of left-right visceral organ situs. However, the link between cardiovascular morphogenesis and left-right patterning is not well understood. To elucidate the role of left-right patterning in cardiovascular development, we examined situs anomalies and CHD in mice with a loss of function allele of Dnaic1, a dynein protein required for motile cilia function and left-right patterning. Dnaic1 mutants were found to have nodal cilia required for left-right patterning, but they were immotile. Half the mutants had concordant organ situs comprising situs solitus or mirror symmetric situs inversus. The remaining half had randomized organ situs or heterotaxy. Looping of the heart tube, the first anatomical lateralization, showed abnormal L-loop bias rather than the expected D-loop orientation in heterotaxy and nonheterotaxy mutants. Situs solitus/inversus mutants were viable with mild or no defects consisting of azygos continuation and/or ventricular septal defects, whereas all heterotaxy mutants had complex CHD. In heterotaxy mutants, but not situs solitus/inversus mutants, the morphological left ventricle was thin and often associated with a hypoplastic transverse aortic arch. Thus, in conclusion, Dnaic1 mutants can achieve situs solitus or inversus even with immotile nodal cilia. However, the finding of abnormal L-loop bias in heterotaxy and nonheterotaxy mutants would suggest motile cilia are required for normal heart looping. Based on these findings, we propose motile nodal cilia patterns heart looping but heart and visceral organ lateralization is driven by signaling not requiring nodal cilia motility.
Keywords: Dnaic1, heterotaxy, primary cilia dyskinesia, mouse disease model
some of the most complex congenital heart diseases (CHD) are found in patients with heterotaxy, a birth defect involving abnormal left-right patterning of heart and visceral organ situs. The heart is the single most left-right asymmetric organ in the body, and this asymmetry is critically important for efficient oxygenation of blood. Thus particularly devastating are structural heart defects in heterotaxy patients with ventriculoarterial and/or atrioventricular discordance, since these can cause inappropriate connections that violate the left-right separation of deoxygenated blood returning from the systemic circulation from oxygenated blood in the pulmonary circulation. Although heterotaxy affects only 1 in 10,000 individuals, such patients suffer an inordinate disease burden. Among CHD patients, those with heterotaxy exhibit more complicated postsurgical clinical course and suffer some of the highest morbidity and mortality (25, 26).
Recent human and animal model studies have provided important insights into the genetic and developmental etiology of heterotaxy. Human studies have yielded evidence that mutations in the transcription factor ZIC3 can cause heterotaxy (4, 30). Other genes identified as likely associated with human heterotaxy include nodal, EGF-CFC genes, LEFTY, PITX2, and other genes in the nodal signaling cascade (3, 24). Additional work from animal studies have expanded the repertoire to include genes encoding proteins required for ciliogenesis, such as intraflagellar transport protein IFT88 or centrosome protein MKS1 and others (4, 17). Mouse studies have shown motile cilia at the embryonic node are indispensible for left-right patterning (9). Cilia at the embryonic node exhibit a rotary motion that causes a leftward nodal flow. This is thought to generate a gradient that breaks symmetry and provide the left-sided activation of the nodal signaling cascade (9).
Clinically the link between motile cilia defects and abnormal left-right patterning is well documented in patients with primary ciliary dyskinesia (PCD). PCD patients have sinopulmonary disease due to mucociliary clearance defects caused by immotile or dyskinetic cilia in the airway epithelia (13, 18). Half of PCD patients exhibit situs inversus totalis, a condition known as Kartagener's syndrome in which all the visceral organs are mirror symmetric. Significantly, recent studies show 6.3% of PCD patients have heterotaxy, with nearly half of these associated with CHD (11). Because PCD and heterotaxy are both rare disorders, each affecting only 1 in 10,000 to 15,000 individuals (23), these findings would suggest a mechanistic link between these two rare disorders involving the disruption of motile cilia function. However, the inherent genetic diversity among patients is a confounding factor that makes drawing any definitive conclusion from such studies difficult. Fortunately, the availability of a mouse model with a mutation in Dnahc5, a motor dynein gene frequently mutated in patients with PCD, has provided confirming evidence that mutation in a PCD gene can cause not only situs inversus and situs solitus, but also heterotaxy and complex CHD (27). Although these observations showed the importance of motile cilia in cardiac morphogenesis, how left-right patterning is integrated with cardiac morphogenesis remains unclear. To investigate this question further, we examined left-right patterning and cardiac development in mutant mice with disruption of Dnaic1, a dynein gene also known to cause PCD.
Dnaic1 is an intermediate chain dynein that forms part of the outer dynein arm complex in motile cilia, and mutations in the human ortholog, DNAI1, are one of the most common causes for PCD. DNAI1 and DNAH5 mutations together account for 30–38% of all PCD cases (13, 31). Like Dnahc5, Dnaic1 mutant mice are a bona fide PCD model, exhibiting defects in airway ciliary motion and cilia ultrastructural defects consisting of missing outer dynein arms (19). Although Dnaic1 mutants can exhibit situs inversus totalis and situs solitus, the possible role of Dnaic1 in CHD and heterotaxy has not been systematically investigated. In this study, we characterized the cardiovascular anatomy and visceral organ situs of Dnaic1 mutant mice. Based on the results of our analyses of the Dnaic1 mutants, we propose two signaling pathways by which motile cilia at the node specifies heart and visceral organ situs—one signaling pathway requiring nodal cilia motility that specifies heart looping orientation and a second signaling pathway that requires nodal cilia but not cilia motility that patterns heart and visceral organ situs.
MATERIALS AND METHODS
Mouse breeding, genotyping, and embryo collection.
Dnaic1 mutant mice on the mixed C57Bl6;129Sv background were bred in accordance with a protocol approved by the Institutional Animal Care and Use Committee of the National Heart, Lung, and Blood Institute (Protocol No. H-0175). Mouse fetuses between E14 and E18 were harvested from adult Dnaic1+/− intercrosses. DNA extracted from tail snips was used for genotyping using primers and PCR conditions as described previously (19). Primers used included the following: IC78-CreF 5'-ATCTCTGGACACGAGGTGCATG-3′; IC78-CreR 5′-CAGCCTCACTACCTTACTGACCAGTG-3′; Dnaic1-WTF1 5′-TTCAAGGTGGGTTGCTGCTTACTG-3′; and Dnaic1-WTR1 5′-GCATCATAGGTGTCCAAGAACTGGC-3′. Wild-type product length was 402 bp, and mutant allele product length was 566 bp.
Episcopic fluorescence image capture.
Episcopic fluorescence image capture (EFIC) imaging was carried out as described previously (21). Briefly, embryos were embedded in paraffin (56.6 g; Thermo Scientific) with vybar (20 g; Baker Hughes), and Sudan IV dye (0.35 g; Sigma) dissolved in stearic acid (3.5 g; Sigma). Sectioning was carried out using a Leica SM2500 sledge microtome mounted with a Leica MZ16FA stereomicroscope equipped with green fluorescene protein fluorescent filters (excitation 470/40; emission 525/50), mercury lamp, and Orca-ER digital camera (C4742-95; Hamamatsu) (21). Microscope, camera, and microtome were all controlled using a custom written OpenLab automation (OpenLab 5.5.1; Improvision) running on an iMac (2.66 GHz Intel core 2 duo, 4 GB RAM; OS 10.5.8), which automatically sectioned and imaged through each paraffin embedded sample generating a registered two-dimensional image stack (21). Images collected of the block face were processed using OsiriX (v.3.6.1 64-bit) running on a MacPro (2.66 GHz quad-core; 6 GM RAM; OS 10.5.8) to assess anatomical structures in two-dimensional image stacks and three-dimensional rendered volumes.
Classifying structural heart defects.
The van Praagh terminology was used to track structural heart defects. This entails dividing the heart into atrial, ventricular, and arterial segments, with each segment assigned a left/right identity: the right versus left atrial chamber, the right versus left ventricular chamber, and the aortic versus pulmonary trunk (Table 1). Normal situs is classified as (S,D,S): solitus atrial appendages, dextral looping of the ventricles, and solitus positioning of the outflows. In addition, using EFIC data, we also assessed atrioventricular (AV) and ventriculoarterial connections, venous drainage, and septal defects.
Table 1.
Summary of cardiovascular, pulmonary, and abdominal situs phenotypes observed in Dnai1 mutant embryos
| Apex | Atrial Situs | Loop | AV Connection | VA Alignment | Ao-PA Relation | Segment Analysis | Septal Defects | Aortic Arch | Venous Return | Lung Situs | Abdomen |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Heterotaxy | |||||||||||
| Lev | SS,CA | D | AVC | DORV | Normal | (S,D,D) | ASD,VSD | LAA | 2 HVs | ND | R(St/Sp/P) |
| Lev | SI,CA | L(s-i) | AVC | DORV | Inverted | (I,L,L) | ASD,VSD | RAA | 2 HVs | ND | L(St/Sp/P) |
| Lev | SI,CA | L(s-i) | AVC | OA | P-Ao-Lev | (I,L,I) | ASD,VSD | RAA | AZY,2HVs | 1L:1R,LPI | R(St/Sp/P) |
| Lev | LAI,CA | D | AVC | OA | P-Ao-Lev | (A,D,D) | ASD,VSD | RAA | AZY,2HVs | 1L:1R,LPI | R(St/Sp/P) |
| Lev | SS | D | 2AVVs | CC | Normal | (S,D,S) | None | LAA | AZY | 1L:4R,SS | R(St/Sp/P) |
| Dex | SI,CA | L(s-i) | AVC | DORV | P-Ao-Lev | (I,L,L) | ASD,VSD | RAA | Normal | ND | L(St/Sp/P) |
| Dex | LAI | L | 2AVVs | CC | Inverted | (A,L,I) | ASD,VSD | RAA | AZY,2HVs | 2L:1R,SI | R(St/Sp/P) |
| Dex | SI | L | 2AVVs | CC | Inverted | (I,L,I) | None | RAA | Normal | ND | L(St/Sp/P) |
| Dex | SI | L | 2AVVs | CC | Inverted | (I,L,I) | None | RAA | AZY | 4L:1R,SI | L(St/Sp/P) |
| Dex | SI,CA | L(s-i) | AVC | DORV | P-Ao-Lev | (I,L,L) | ASD,VSD | RAA | AZY,2HVs | 1L:1R,LPI | R(St/Sp/P) |
| Dex | SI,CA | L(s-i) | AVC | DORV | P-Ao-Lev | (I,L,L) | ASD,VSD | IAA | AZY | 1L:1R,LPI | R(St/Sp/P) |
| Dex | SI,CA | L | 2AVVs | OA | Inverted | (I,L,L) | ASD,VSD | RAA | AZY | 1L:1R,LPI | R(St/Sp/P) |
| Mes | RAI | L | 1AVV(MV) | L-TGA | P-Ao-Lev | (A,L,D) | ASD,VSD | RAA | AZY,2HVs | 1L:1R,LPI | L(St/Sp/P) |
| Mes | SI,CA | D | 2AVVs | D-TGA | P-Ao-Dex | (S,D,D) | ASD,VSD | IAA | AZY,2HVs | 1L:1R,LPI | L(St/Sp/P) |
| Mes | CA | D | AVC | DORV | P-Ao-Dex | (S,D,D) | ASD,VSD | LAA | AZY,2HVs | 1L:1R,LPI | L(St/Sp/P) |
| Mes | LAI,CA | D | AVC | DORV | P-Ao-Dex | (A,D,D) | ASD,VSD | IAA | AZY | 1L:1R,LPI | R(St/Sp/P) |
| Situs solitus | |||||||||||
| Lev | SS | D | 2AVVs | Normal | Normal | (S,D,S) | None | LAA | Normal | 1L:4R,SS | L(St/Sp/P) |
| Lev | SS | D | 2AVVs | Normal | Normal | (S,D,S) | None | LAA | Normal | ND | L(St/Sp/P) |
| Lev | SS | D | 2AVVs | Normal | Normal | (S,D,S) | None | LAA | Normal | 1L:4R,SS | L(St/Sp/P) |
| Lev | SS | D | 2AVVs | Normal | Normal | (S,D,S) | None | LAA | Normal | 1L:4R,SS | L(St/Sp/P) |
| Lev | SS | D | 2AVVs | Normal | Normal | (S,D,S) | None | LAA | AZY | 1L:4R,SS | L(St/Sp/P) |
| Lev | SS | D | 2AVVs | Normal | Normal | (S,D,S) | VSD | LAA | AZY | 1L:4R,SS | L(St/Sp/P) |
| Situs Inversus | |||||||||||
| Dex | SI | L | 2AVVs | CC | Inverted | (I,L,I) | None | RAA | Normal | 4L:1R,SI | R(St/Sp/P) |
| Dex | SI | L | 2AVVs | CC | Inverted | (I,L,I) | None | RAA | Normal | 4L:1R,SI | R(St/Sp/P) |
| Dex | SI | L | 2AVVs | CC | Inverted | (I,L,I) | None | RAA | Normal | 4L:1R,SI | R(St/Sp/P) |
| Dex | SI | L | 2AVVs | CC | Inverted | (I,L,I) | None | RAA | Normal | 4L:1R,SI | R(St/Sp/P) |
| Dex | SI | L | 2AVVs | CC | Inverted | (I,L,I) | None | RAA | Normal | 4L:1R,SI | R(St/Sp/P) |
| Dex | SI | L | 2AVVs | CC | Inverted | (I,L,I) | None | RAA | AZY | 4L:1R,SI | R(St/Sp/P) |
| Dex | SI | L | 2AVVs | CC | Inverted | (I,L,I) | None | RAA | AZY | 4L:1R,SI | R(St/Sp/P) |
| Dex | SI | L | 2AVVs | CC | Inverted | (I,L,I) | VSD | RAA | AZY | 4L:1R,SI | R(St/Sp/P) |
| Dex | SI | L | 2AVVs | CC | Inverted | (I,L,I) | None | RAA | AZY | 4L:1R,SI | R(St/Sp/P) |
| Dex | SI | L | 2AVVs | CC | Inverted | (I,L,I) | VSD | RAA | Normal | 4L:1R,SI | R(St/Sp/P) |
| Dex | SI | L | 2AVVs | CC | Inverted | (I,L,I) | VSD | RAA | AZY | 4L:1R,SI | R(St/Sp/P) |
| Dex | SI | SI | 2AVVs | CC | Inverted | (I,L,I) | None | RAA | AZY | 4L:1R,SI | R(St/Sp/P) |
Lev: levocardia; Mes, mesocardia. SS: situs solitus; SI: situs inversus; RAI: right atrial isomerism; LAI: left atrial isomerism; CA: common atrium. D: D-loop; L: L-loop; s-i: superior-inferior positioning of the ventricles. AVV: atrioventricular valves; AVC: common atrioventricular canal; MV: mitral valve morphology. VA: Ventriculoarterial connections; CC: concordant; DORV: double outlet right ventricle; OA: overriding aorta. Ao, aorta; ant, anterior; dex, dextro; lev, levo; P, parallel i.e., Ao parallel and levo to PA. ASD: atrial septal defect; VSD: ventricular septal defect. LAA: left-sided aortic arch; RAA: right sided aortic arch; IAA: interrupted aortic arch. AZY: azygos continuation of interrupted inferior vena cava; HV: hepatic vein. L:R, number of left:right lung lobes. ND: not determined. L/R: left or right, St: stomach; Sp: spleen; P: pancreas.
Nodal cilia video-microscopy.
To observe nodal cilia motility, E7.5-E7.75 embryos were harvested and imaged from intercrosses of Dnaic1+/− mice as described previously (5). The node containing distal visceral endoderm section of each embryo was dissected and transferred nodal side down onto a 35-mm glass bottomed culture dish (Willco Wells B.V, Netherlands) with a few drops of L-15 medium (+10% FBS) containing a small amount of 0.35 μm fluorescent polystyrene latex microspheres (Polysciences Cat. No. 17149). A round glass coverslip (FisherBrand) covered with a 0.3-mm thick silicone sheet (AAA Acme Rubber), with a small window cut out to make a thin walled chamber, was placed over the tissue section to secure it onto the 35-mm dish. Nodal cilia beat dynamics were subsequently captured at room temperature using a Leica inverted microscope (Leica DMIRE2) with a 100× oil-immersion objective and differential interference contrast optics. High-speed movies (200 fps) were collected using a Phantom v4.2 camera (Vision Research) and saved as audio video interleave format.
Statistics.
Graphs and linear regression lines were generated and analyzed using Prism 5 (GraphPad Software), with the F-test used to determine whether slopes were significantly different from zero.
RESULTS
Because mutations in DNAI1, the human homolog of Dnaic1, are known to cause airway ciliary dysmotility or immotility, we investigated whether nodal cilia in the Dnaic1 mutant mouse embryos are motile (Supplemental movie S1). Videomicroscopy of wildtype E7.5 embryos showed nodal cilia with normal clockwise rotational stoke, and tracking of fluorescent beads placed above the node showed the expected leftward flow across the node surface. In contrast, in Dnaic1 mutant littermate embryos the nodal cilia were completely immotile and fluorescent beads placed above the node displayed only random Brownian motion (Supplemental movie S1). Given the known importance of motile cilia at the node in left-right patterning, we expect the Dnaic1 mutant mice will likely exhibit a spectrum of laterality defects due to loss of motile cilia function at the node. To examine the situs and cardiac phenotypes of Dnaic1 mutants, we harvested 170 embryos from E14.5 to E18.5 from 22 pregnant dams. Harvesting preterm ensured stillborn pups were not lost due to cannibalization at birth. Genotyping yielded 34 homozygous mutants, with an overall Mendelian distribution observed consisting of 26.5% wildtype, 51.2% heterozygous, and 22.4% homozygous mutants. Necropsies of all 34 mutant fetuses were performed to determine visceral organ situs and examine for evidence of possible structural heart defects.
Dnaic1 mutants exhibit situs solitus, situs inversus, and heterotaxy.
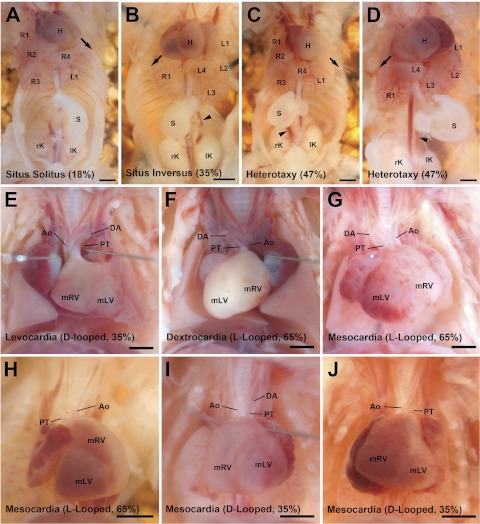
Necropsy showed amongst the 34 Dnaic1 homozygous mutants, 18% (6) had normal situs solitus (SS) (Fig. 1A), 35% (12) had situs inversus totalis (SIT) (Fig. 1B), and 47% (16) had randomized visceral organ situs or heterotaxy (Fig. 1, C and D). In SS the heart apex points to the left side of the chest cavity (levocardia) (Fig. 1E). The aortic arch is left sided, and there are four lung lobes on the right, one on the left. The stomach and spleen are both left sided (Fig. 1A). The normal mouse liver pattern comprises two liver lobes on the left and one large lobe on the right when the abdominal cavity is exposed without disturbing the visceral organs (6). With SIT, the visceral organ arrangement exhibits complete mirror symmetry (Fig. 1B and F), whereas heterotaxy refers to abnormal organ situs comprising any deviation from SS or SIT, whether associated with the cardiovascular, pulmonary, and/or abdominal organs (Fig. 1C, D, G–J).
Fig. 1.
Spectrum of visceral organ situs phenotypes in Dnai1 mutant mice. A–D: examples of ventral cavity organ situs. A: Eighteen percent (6 of 34) Dnaic1 mutant mice displayed normal situs solitus [left pointed heart, 1 left (L1) and 4 right lung lobes (R1–R4), left-sided stomach]. B: Thirty-five percent (12 of 34) displayed complete mirror image reversal of organ arrangement or situs inversus [right pointed heart, 1 right (R1) and 4 left lung lobes (L1–L4), right-sided stomach]. C: example of 1 of the 16 of 34 (47%) Dnaic1 mutant mice displaying left-right randomization of organ arrangement or heterotaxy [left pointed heart, 1 left and 4 right lung lobes, right-sided stomach]. D: another heterotaxy example [right pointed heart, 1 right and 4 left lung lobes, left-sided stomach]. E–J: examples of heart and great artery looping situs. E: example of a Dnaic1 mutant mouse with normal D-looped heart and right looped great arteries. F: example of a Dnaic1 mutant mouse with reversed L-looped heart and left looped great arteries. G–I: examples of abnormal hearts found in Dnaic1 mutant mice with heterotaxy. G: reversed L-looped heart with left looped great arteries; this heart was also found to have tricuspid atresia, multiple VSDs, atrioventricular septation defects, and aortic stenosis. H: reversed L-looped heart displaying a superior-inferior arranged ventricles and left looped great arteries. I: D-looped heart with interrupted aortic arch. J: D-looped heart with interrupted aortic arch. Arrow: heart apex direction; Arrowhead: azygos/hemiazygos continuations. All scale bars = 1 mm. PT, pulmonary trunk; DA, ductus arteriosus; Ao, aorta.
In the Dnaic1 mutants with heterotaxy, the majority of animals displayed left pulmonary isomerism consisting of a single lung lobe on each side (Table 1). Heterotaxy mutants also showed a spectrum of abnormal liver lobation, the most common being bilaterally symmetric liver lobation reminiscent of midline liver seen in patients with heterotaxy. Heterotaxy mutants also showed randomized stomach situs (Table 1), with ∼50% exhibiting right-sided stomach. No spleen abnormalities were observed, and in all cases, stomach and spleen situs were concordant. In the 16 SS/SIT Dnaic1 mutant mice, cardiac morphogenesis on a gross level appears to have proceeded normally (Table 1).
Parallel examination of over 30 wildtype littermate controls in conjunction with the Dnaic1 homozygous mutants showed no heart defects or anomalies in either heart or visceral organ situs.
Analysis for structural heart defects.
To assess for structural heart defects, the intracardiac anatomy in each of the 34 Dnaic1 mutants was analyzed using EFIC. EFIC is a histological imaging technique that provides two-dimensional serial image stacks that can be examined with digital resectioning in any imaging plane. In addition, high resolution 3D reconstructions can be readily generated to visualize 3D anatomy (21). To facilitate tracking and tabulating the structural heart defects relative to left-right situs, we used van Praagh's method for segmental analysis and these are tabulated in Table 1 (28).
With SS, cardiovascular morphogenesis proceeded normally with levocardia and normal outflow tract septation with positioning of the aorta (Ao) to the right and posterior of the pulmonary artery (PA; Fig. 1E). The superior vena cavas (SVCs) return to the right atrium - the left SVC via the coronary sinus and the inferior vena cava (IVC) to the right atrium. Similarly, mutants with SI showed normal cardiovascular anatomy, except all structures were positioned in a mirror symmetric pattern. Thus the heart apex points rightward (dextroardia) and the aortic arch is right sided. The Ao remains posterior but is left of the PA (Fig. 1F), and the super vena cava (SVC) and IVC show inverted positioning. Overall, SS/SI mutants showed no major CHD, although EFIC imaging revealed some minor defects as noted below.
In contrast with the normal cardiovascular anatomy of the SS and SI mutants, all of the heterotaxy mutants exhibited structural heart defects. These are systematically presented below; of the 16 heterotaxy mutants, 5 had levocardia, 4 mesocardia, and 7 dextrocardia (Table 1). There was a wide spectrum of complex structural heart defects that included malpositioning of the outflow tract, as well as discordance in ventriculoarterial and atrioventricular connections arising from the disturbance in left-right patterning.
Abnormalities in heart looping and ventricular chamber situs.
One of the first anatomic lateralization of the embryo is looping of the heart tube. During normal heart development, the heart tube undergoes dextral looping (D-loop), but in the Dnaic1 mutants, L-looped hearts (22 of 34; 64.7%) outnumbered D-looped hearts (12 of 34; 35.3%) by 2:1. A similar L-loop bias was observed in the heterotaxy (10 vs. 6) and nonheterotaxy (SS/SI) mutants (12 vs. 6). Left-right positioning of the aortic arch was linked to the direction of heart looping, since mutants exhibiting D-looped hearts invariably had left-sided aortic arch, whereas L-looped hearts had right-sided aortic arch. Although looping of the heart tube showed abnormal L-loop bias in the Dnaic1 mutants, the subsequent development of CHD was not linked to the direction of heart tube looping. Thus CHD was found in 16 mutants—half of the D-looped (6/12) and half of the of L-looped (10/22) hearts (Table 1). These were in fact the 16 mutants that exhibited heterotaxy. In six of these heterotaxy mutants, rather than left-right positioning of the ventricles, we observed a superior-inferior arrangement of the ventricles, with the morphologic right ventricle (mRV) situated superiorly (Fig. 1H). The mRV can be easily identified as the ventricle in which there is papillary muscle attached to the septum. In three of the mutants, the inferior ventricle was displaced leftward and hence designated D-superior-inferior, whereas in another two, the left ventricle was positioned rightward, and thus designated L-superior-inferior (2/34) (Fig. 1H, Table 1).
Atrial situs anomaly in heterotaxy mutants.
To determine atria situs in the Dnaic1 mutants, we examined the connection of the SVCs to the atrial chambers. The morphologic right atrium is the chamber in which the ipsilateral SVC of an atrium returns directly to it, while for the morphological left atrium, the ipsilateral SVC returns via a coronary sinus to the contralateral atrium. In all mutants with situs solitus and situs inversus, atria situs were normal, being solitus in the solitus mutants and inverted in the situs inversus mutants (Table 1). In contrast, almost all of the heterotaxy mutants showed defects in atrial septation. They exhibited common atria, with nearly half showing discordance in atrial situs. Thus inverted atria situs was observed in two mutants with levocardia and L-looped hearts, and one mutant with mesocardia and a D-looped heart. Three heterotaxy mutants had left atrial isomerism, which is indicated by a common atrium to which both SVCs return. This was seen in two mutants with D-looped hearts and one with an L-lopped heart (Table 1). We observed only one mutant with right atrial isomerism, seen as each SVC returning directly to its ipsilateral atrium and with an inferior venous connection to each atrium. Right atrial isomerism was found in an L-looped heart that was the only mutant found to have L-transposition of the great arteries (L-TGA; Table 1).
Malpositioning of the great arteries.
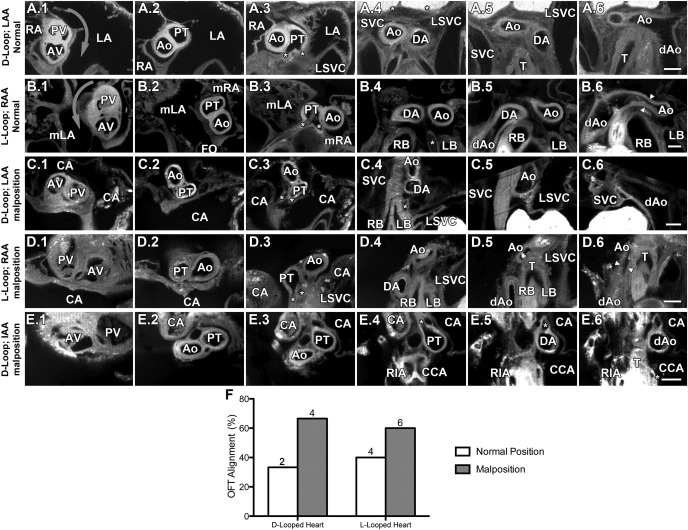
We carefully examined the relative positioning of the great arteries in the Dnaic1 mutants. In the normal situs solitus animal, the semilunar valve of the pulmonary artery is positioned anterior of the aortic valve and exhibits a characteristic clockwise spiraling relative to the aorta (29). This normal arrangement of the aortic and pulmonary outflows was observed in Dnaic1 mutants with situs solitus (Fig. 2, A1–A6). In contrast, with situs inversus totalis, a mirror image arrangement of the pulmonary trunk results in reversed spiraling of the pulmonary trunk around the ascending aorta. Nevertheless, the aortic valve remains posterior of the pulmonary valve (Fig. 2, B1–B6). Analysis of the heterotaxy mutants showed nine had malpositioning of the great arteries and abnormal semilunar valve arrangement (Fig. 2, C–E). In most instances, this was characterized by a shift to a parallel or side-by-side arrangement of the aortic-pulmonary trunks (Fig. 2, C1–E1). This was associated with a disruption of the normal spiraling of the outflows (Fig. 2, C1–C6, D1–D6, E1–E6). Malpositioning of the great arteries and abnormal semilunar valve arrangement was seen only with heterotaxy and was found in four of six D-looped hearts and six of 10 L-looped among the 16 mutants with heterotaxy (Fig. 2F).
Fig. 2.
Malposition of the great vessels in Dnaic1 mutant mice. Serial transverse sections start at the aortic valve (AV) and pulmonary valve (PV) (column 1) and continue in the cranial direction until reaching the dorsal aorta (column 6). A1–A6: D-looped heart, left-sided aortic arch (LAA), and normal great vessel rotation. B1–B6: L-looped heart, right-sided aortic arch (RAA), and completely reversed great vessel rotation. Arrowheads: highlight aortic stenosis. C1–C6: D-looped heart, left-sided aortic arch, and malrotated great vessels. D1–D6: L-looped heart, right-sided aortic arch, and malrotated great vessels. E1–E6: D-looped heart with interrupted aortic arch [IAA; aorta drains into the RIA and CCA, whereas the DA drains into the descending aorta (dAo)]. F: outflow tract rotation phenotypes in heterotaxic Dnaic1 mutant hearts grouped according to heart looping. *Highlights pulmonary arteries; arrows indicate rotation direction of great vessels. Scale bars = 0.2 mm. LA, left atrium; RA, right atrium; SVC, superior vena cava; LSVC, left SVC; mLA, morphological left atrium; FO, foramen ovale; CA, common atrium; mRA, morphological right atrium; RIA, innominate artery; CCA, common carotid artery; RB, right bronchus; LB, left bronchus; T, trachea.
Ventriculoarterial connection, atrioventricular septation, and venous return.
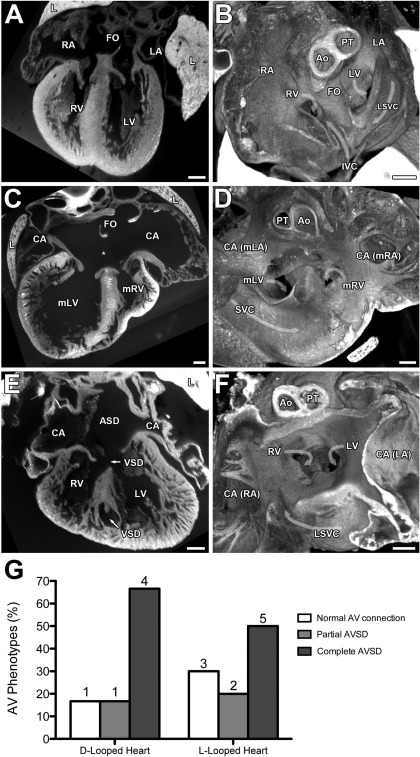
Abnormal ventriculoarterial connections were observed in 13 of the 16 heterotaxy mutants (Table 1). This included overriding aorta or double outlet right ventricle. In double outlet right ventricle, the two outflows emerge from the mRV, which can be positioned either on the right or left. In two mutants we observed either an L or D-transposition of the great arteries. Almost all of the heterotaxy mutants also displayed septation defects. This included not only common atria associated with defects in atrial septation, but often this is seen in conjunction with defects in ventricular septation that resulted in atrioventricular septation defects (AVSD) (Fig. 3, C and D) or partial AVSD (Fig. 3, E and F). There were no pulmonary vein defects observed, either by location of drainage or alteration in the size of the vessel. Analysis of the venous return revealed nearly all of the heterotaxy mutants had azygos or hemiazygos continuations associated with interruption of the IVC (Table 1).
Fig. 3.
Ventricular and atrioventricular septal defects (AVSD) in Dnaic1 mutant hearts. Coronal 2-dimensional sections (A, C, E) and transverse 3-dimensionsional reconstructions looking from the atrium down into the AV junction (B, D, F) are shown. A and B: example of an L-looped heart with normal atrioventricular septum and heart anatomy. C and D: D-looped heart with partial AVSD as demonstrated by the presence of a large inter-atrial communication (*) below an intact FO and above 2 discrete AV valves connected to an intact VS. E and F: D-looped heart with perimembranous VSD forming part of a complete AVSD, muscular VSD within the ventricular septum, and noncompaction of the ventricular myocardium. G: AVSD relative to D- and L-loop hearts in Dnaic1 mutant hearts with heterotaxy. Scale bars = 0.2 mm. IVC, inferior vena cava.
Situs solitus/situs inversus mutants with minor isolated defects.
A parallel analysis of all of the 18 SS/SI mutants showed none of them had the complex structural heart defects seen in the heterotaxy mutants noted above. This is consistent with the fact that SS and SI mutants are postnatal viable. However, EFIC imaging revealed some of the SS/SI mutants had small VSDS. This was observed in one of the mutants with levocardia and three of the mutants with dextrocardia. Three of the four SS/SI mutants with VSD also exhibited azygos continuation. In addition, five other SS/SI mutants exhibited azygos or hemiazygos continuation as an isolated defect (Table 1).
Aortic stenosis and thinning of the LV chamber wall.
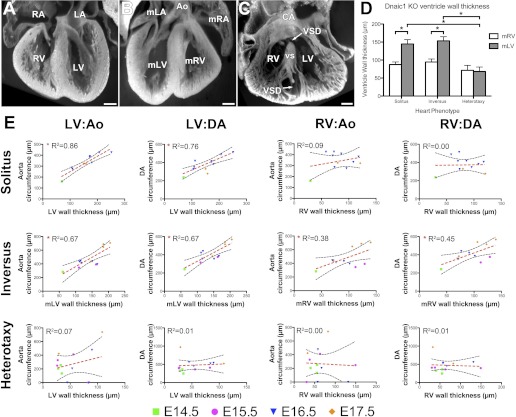
We observed narrowing of the aortic arch (i.e., hypoplastic transverse arch) in 12 of the 16 heterotaxy Dnaic1 mutants (Fig. 4). Using EFIC three-dimensional reconstructions, we measured the Ao circumference just proximal to where the Ao joins the ductus arteriosus (DA) and the DA circumference just distal to this junction (Fig. 4B). This analysis showed a significant narrowing of the Ao in heterotaxy versus SS/SI mutants, but no significant difference was seen for the DA when compared with similar measurements of the SS/SI mutants (Fig. 5B). In three heterotaxy mutants where we found a complete interruption of the aortic arch (Table 1; Fig. 4A), a ligamentous structure was observed between the Ao-left carotid and the DA-Ao junctions, likely representing the closed aorta remnant (arrowhead in Fig. 4, E and H).
Fig. 4.
Summary of heart looping and great artery phenotypes observed in Dnaic1 mutant embryos. A: great artery phenotypes grouped according to great artery looping. B: quantification of Ao and DA circumferences grouped according to heart situs. Aortic and DA circumferences were measured just proximal to the DA-Ao junction. *P < 0.05. C–H: sagittal (C–E) and transverse (F–H) sections of great arteries. C, F: L-looped heart with left looped aortic arch of similar circumference to the DA. D, G: L-looped heart displaying a hypoplastic transverse arch of the left looped aortic arch when compared with the DA. Arrows highlight hypoplastic transverse arch just proximal to the DA-Ao junction. E, H: L-looped heart displaying an interrupted aortic arch. Arrowhead highlights a ligamentous like structure that is probably the completely closed Ao remnant between the Ao-left common carotid artery (ALCC) and DA-Ao junctions. Scale bars = 0.2 mm.
Fig. 5.
Ventricular wall thickness is correlated with aorta and ductal vessel size in normal but not diseased Dnaic1 mutant hearts. A–C: coronal 3-dimensional section volumes of solitus heart with LV thicker than RV (A), inversus heart with thicker right-sided ventricle (i.e., mLV; B), and heterotaxic heart with thin walled RV and LV (C). D: quantification of ventricular wall thicknesses grouped according to situs; only measurements from E16 to E17 hearts are presented, error bars are ± SE. *P < 0.05. E: correlation graphs mapping the relationship between ventricular wall thicknesses (x-axis) and great artery circumferences (y-axis). Morphological left ventricle (mLV) wall thickness in solitus and inversus hearts was significantly correlated with both aorta and ductus arteriosus circumferences; morphological right ventricles (mRV) were not significantly correlated in solitus hearts but showed a weak correlation in to both aorta and ductus arteriosus circumferences in inversus hearts. No correlation was seen for ventricular wall thicknesses in heterotaxy hearts (both mRV and mLV) vs. either aorta or ductus arteriosus circumferences. Scale bars = 0.2 mm. CHD, congenital heart disease.
Examination of the EFIC three-dimensional reconstructions also suggested the ventricles in the heterotaxy mutants were thin. This was confirmed with measurement of the ventricular wall thickness using the EFIC three-dimensional reconstructions (Fig. 5A). In the normal SS hearts, the LV chamber wall was significantly thicker than that of the RV (Fig. 5A). Similarly, in SI mutants, the mLV, which is the right-sided ventricle, was thicker than the mRV (Fig. 5B). In contrast, in heterotaxy mutants, the mLV were significantly thinner than that of the SS/SI mutants (Fig. 5D). The mLV chamber wall thickness increased in parallel with the Ao/DA circumferences as development progressed between E14.5 and E17.5 in the SS/SI but not heterotaxy mutants (Fig. 5E). Although a similar trend was observed for the RV in the SS/SI mutants, this was not found for the heterotaxy mutants (Fig. 5E). We hypothesize that the thinning of the mLV wall in mutant embryos with heterotaxy includes a spectrum of severity where noncompaction of the ventricular myocardium represents the worst case scenario (as highlighted in Fig. 3E and Fig. 5C).
DISCUSSION
The Dnaic1 mutant analysis presented here provides the first evidence that Dnaic1 function is required for nodal cilia motility. It confirms that defects in nodal cilia motility caused by Dnaic1 deficiency can result in heterotaxy and complex CHD. Although a clinical connection between motile cilia dysfunction and heterotaxy is suggested by recent studies of a large PCD cohort (11), a causal role for ciliary dysfunction in heterotaxy cannot be concluded based on the clinical studies alone given the inherent human genetic heterogeneity. Analysis based on the use of inbred mutant mice in the present study confirms that mutation in a single gene, Dnaic1, can cause ciliary dysfunction, with half of the Dnaic1 mutant mice exhibiting heterotaxy and the other half with either SS or SI. Significantly, complex CHD was only observed in the heterotaxy mutants, but never in the mutants exhibiting either SS or SI.
Situs inversus and situs solitus mutants have no defects or mild defects.
SI or SS mutants had no structural heart defects or only mild defects such as small VSDs, or interruption of the IVC with azygos continuation. Clinically, small VSDs as well as azygos continuation are not incompatible with postnatal viability, and in fact, these defects can go undetected clinically. Consistent with this, the Dnaic1 mutants with SI/SS are generally postnatal viable. The finding of VSDs is interesting, since VSDs are among some of the most common CHDs (1, 15). These findings suggest processes involved in the specification of laterality may also affect developmental processes that impact cardiac chamber septation. Because azygos continuation was observed in nearly half of the SI/SS mutants, this may be useful as a biomarker for potential genetic risk of mutations causing heterotaxy, especially in individuals with a family history of CHD and situs anomalies.
Although the hearts of mice with SI are considered anatomically normal given all of the intracardiac connections are concordant, it is worth noting that the L-looped hearts from these mice with SI may not be functionally equivalent to D-looped hearts in mice with SS. L-looped hearts in mice (or patients) with SI do not exhibit ventricular torsion in mirror symmetry, but, instead, retain the same counterclockwise ventricular rotation as D-looped hearts despite their mirror symmetric cardiac anatomy. This suggests that the normal spiral arrangement of the myocardial fibers is independent of early heart looping direction (2, 7, 8). Whether the ventricular torsion discordance in L-looped hearts may cause hemodynamic perturbation in the Dnaic1 mutant mice with SI will require further investigations in the future.
Heterotaxy mutants exhibit complex CHD.
In contrast with SS/SI mutants, which had no CHD, all heterotaxy mutants exhibited complex CHD that included a spectrum of defects involving discordance in the left-right identity of the cardiac chambers and the atrioventricular and/or arterioventricular connections. The CHD phenotypes seen in the Dnaic1 heterotaxy mutants overlap with those seen previously in the Dnahc5 mutants (27). Clinically, mutations in DNAI1 and DNAH5 account for 30–38% of PCD (13, 31). PCD patients can exhibit heterotaxy (11), albeit the incidence observed clinically is only 6% vs. 50–60% in the Dnahc5/Dnaic1 mutant mouse models. The lower incidence of heterotaxy in PCD patients may reflect attrition from preterm loss due to complex CHD associated with heterotaxy; this is frequently observed in the mouse Dnaic1 and Dnahc5 mutants. Consistent with this, we note heterotaxy patients exhibit some of the most complex structural heart defects, and these patients have some of the highest morbidity/mortality among CHD patients (24).
Requirement for nodal cilia in heart looping and heart/visceral organ lateralization.
We found heart looping in the Dnaic1 mutants had an L-loop bias. Looping of the heart tube represents the first anatomic lateralization of the embryo and is a distinct morphogenetic event that specifies the first left-right asymmetry in cardiac morphogenesis (14). Because L-loop bias was observed in both heterotaxy and nonheterotaxy Dnaic1 mutants, this would suggest heart-looping orientation is specified before downstream events determining heart and visceral organ situs. In fact, half of the Dnaic1 mutant mice exhibited either normal or mirror symmetric heart and visceral organ situs. This is despite the fact that the Dnaic1 mutant embryos have immotile cilia at the node and with no nodal fluid flow. This same result was observed for the Dnahc5 mutant mice, which also showed a high incidence of SS and SI despite having paralyzed nodal cilia (27). In contrast, mouse mutants with complete loss of cilia at the node, such as in the Mks1del64−323 Meckel Gruber syndrome mutant mouse model. Mks1del64–323 mutants exhibit heterotaxy with randomization of heart looping orientation and visceral organ situs, and in all cases, this is accompanied by complex CHD (5). These observations suggest paralyzed nodal cilia in the Dnaic1 mutants can nevertheless subserve function required for left-right patterning of heart and visceral organ situs.
Different signals modulating heart and visceral organ lateralization.
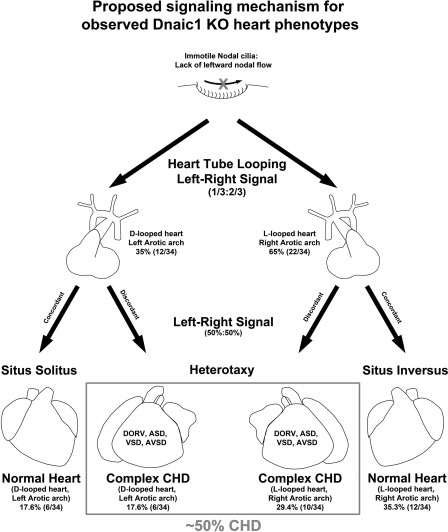
In light of these findings, we propose two different signals controlling heart and visceral organ situs specification. In this model, one signal controls heart tube looping and requires motile nodal cilia function (Fig. 6). Consistent with this model, Dnaic1 mutants with paralyzed nodal cilia show abnormal heart L-loop bias. A second signal specifies visceral organ lateralization that requires cilia at the node but does not require cilia motility. This visceral organ lateralization signal is preserved in the Dnaic1 and Dnahc5 mutants with paralyzed nodal cilia (27), but not in mutants such as Mks1 with no nodal cilia (5). Although the potential function of paralyzed cilia in the node is unknown, motile cilia in the respiratory epithelia have been shown to mediate chemosensory function that can sense and respond to environmental stimuli, not unlike characteristics of nonmotile primary cilia (22).
Fig. 6.
Proposed signaling mechanisms for observed Dnaic1 mutant heart and visceral organ phenotypes.
Transverse aortic arch hypoplasia and cardiac dysfunction in heterotaxy mutants.
We observed a high incidence of hypoplastic transverse arch among the Dnaic1 heterotaxy mutants, but hypoplastic transverse arch was not observed among mutants with SS or SI. The hypoplastic transverse arch showed a range of severity with interrupted aortic arch being the most severe form. In comparison, clinically only a few patients with heterotaxy have been reported to exhibit aortic stenosis (10, 12). Because all of the heterotaxy mutants die either prenatally or neonatally, this suggests the possibility that deleterious hemodynamic changes brought on by the hypoplastic transverse arch may have caused the fetal demise. Consistent with this, we observed a marked thinning of the LV chamber wall in the heterotaxy mutants that would suggest poor contractility. It should be noted that although atrioventricular block is quite often present in clinical cases of heterotaxy syndromes and almost always due to left atrial isomerism (20), this is not likely to be a major cause of fetal demise in the Dnaic1 mutants, since only four out of the 34 mutant fetuses analyzed were found to have atrial isomerism (Table 1). It is interesting to note that the hypoplastic transverse arch and interrupted aortic arch phenotypes seen in the Dnaic1 mutants closely matched the phenotype of the Tgfβ2 KO mice (16). TGFβ signaling via Nodal is well described to play an essential role in left-right patterning (9). Thus it is appealing to consider whether disruption in aortic arch patterning in the Dnaic1 mutants may involve defective TGFβ signaling.
Conclusion
Our studies show left-right patterning of cardiac and visceral organ situs requires motile cilia at the node, but this may involve both motile and nonmotile cilia function. We propose that two signaling pathways exist in which the initial specification of heart tube looping orientation requires motile cilia function, whereas lateralization of the heart and visceral organ situs involves signaling function that can be transduced by even paralyzed nodal cilia. In the context of this model, it is interesting to consider whether looping of the heart tube itself may play a role in driving downstream signals that pattern organ lateralization, since heterotaxy is observed to be tightly coupled with complex CHD. The finding of hypoplastic transverse arch and thinning of the LV chamber walls in the heterotaxy mutants would suggest there may be significant hemodynamic perturbation in the heterotaxy mutants. This could cause prenatal/neonatal lethality and perhaps account for the lower incidence of heterotaxy among PCD patients.
GRANTS
This work was supported by grants from National Heartl, Lung, and Blood Institute Grant ZO1-HL-005701 (to C. Lo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.J.B.F. and C.L. conception and design of research; R.J.B.F., A.C., and L.E.O. performed experiments; R.J.B.F., A.C., and W.A.D. analyzed data; R.J.B.F., W.A.D., and C.L. interpreted results of experiments; R.J.B.F. prepared figures; R.J.B.F. drafted manuscript; R.J.B.F., L.E.O., and C.L. edited and revised manuscript; C.L. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1. Bedard E, Shore DF, Gatzoulis MA. Adult congenital heart disease: a 2008 overview. Br Med Bull 85: 151– 180, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bonnafe E, Touka M, AitLounis A, Baas D, Barras E, Ucla C, Moreau A, Flamant F, Dubruille R, Couble P, Collignon J, Durand B, Reith W. The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification. Mol Cell Biol 24: 4417– 4427, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen CM, Norris D, Bhattacharya S. Transcriptional control of left-right patterning in cardiac development. Pediatr Cardiol 31: 371– 377, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Chung B, Shaffer LG, Keating S, Johnson J, Casey B, Chitayat D. From VACTERL-H to heterotaxy: variable expressivity of ZIC3-related disorders. Am J Med Genet A 155A: 1123– 1128, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Cui C, Chatterjee B, Francis D, Yu Q, SanAgustin JT, Francis R, Tansey T, Henry C, Wang B, Lemley B, Pazour GJ, Lo CW. Disruption of Mks1 localization to the mother centriole causes cilia defects and developmental malformations in Meckel-Gruber syndrome. Dis Model Mech 4: 43– 56, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danforth CH, Center E. Development and genetics of a sex-influenced trait in the livers of mice. Proc Natl Acad Sci USA 39: 811– 817, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Delhaas T, Kroon W, Bovendeerd P, Arts T. Left ventricular apical torsion and architecture are not inverted in situs inversus totalis. Prog Biophys Mol Biol 97: 513– 519, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Frank LH, Yu Q, Francis R, Tian X, Samtani R, Sahn DJ, Leatherbury L, Lo CW. Ventricular rotation is independent of cardiac looping: a study in mice with situs inversus totalis using speckle-tracking echocardiography. J Am Soc Echocardiogr 23: 315– 323, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell 125: 33– 45, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Kapa S, Gleeson FC, Vege SS. Dorsal pancreas agenesis and polysplenia/heterotaxy syndrome: a novel association with aortic coarctation and a review of the literature. JOP 8: 433– 437, 2007 [PubMed] [Google Scholar]
- 11. Kennedy MP, Omran H, Leigh MW, Dell S, Morgan L, Molina PL, Robinson BV, Minnix SL, Olbrich H, Severin T, Ahrens P, Lange L, Morillas HN, Noone PG, Zariwala MA, Knowles MR. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation 115: 2814– 2821, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Kondrachuk O, Yalynska T, Yemets I. Heterotaxy syndrome (mirror-image dextrocardia, coarctation of the aorta, polysplenia): a previously unreported association. Pediatr Cardiol 30: 864– 865, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Leigh MW, Zariwala MA, Knowles MR. Primary ciliary dyskinesia: improving the diagnostic approach. Curr Opin Pediatr 21: 320– 325, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manner J. The anatomy of cardiac looping: a step towards the understanding of the morphogenesis of several forms of congenital cardiac malformations. Clin Anat 22: 21– 35, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Mitchell ME, Sander TL, Klinkner DB, Tomita-Mitchell A. The molecular basis of congenital heart disease. Semin Thorac Cardiovasc Surg 19: 228– 237, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Molin DG, DeRuiter MC, Wisse LJ, Azhar M, Doetschman T, Poelmann RE, Gittenberger-de Groot AC. Altered apoptosis pattern during pharyngeal arch artery remodelling is associated with aortic arch malformations in Tgfbeta2 knock-out mice. Cardiovasc Res 56: 312– 322, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development 127: 2347– 2355, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Noone PG, Leigh MW, Sannuti A, Minnix SL, Carson JL, Hazucha M, Zariwala MA, Knowles MR. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med 169: 459– 467, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Ostrowski LE, Yin W, Rogers TD, Busalacchi KB, Chua M, O′Neal WK, Grubb BR. Conditional deletion of Dnaic1 in a murine model of primary ciliary dyskinesia causes chronic rhinosinusitis. Am J Respir Cell Mol Biol 43: 55– 63, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pepes S, Zidere V, Allan LD. Prenatal diagnosis of left atrial isomerism. Heart 95: 1974– 1977, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Rosenthal J, Mangal V, Walker D, Bennett M, Mohun TJ, Lo CW. Rapid high resolution three dimensional reconstruction of embryos with episcopic fluorescence image capture. Birth Defects Res C Embryo Today 72: 213– 223, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science 325: 1131– 1134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Storm van's Gravesande K, Omran H. Primary ciliary dyskinesia: clinical presentation, diagnosis and genetics. Ann Med 37: 439– 449, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Sutherland MJ, Ware SM. Disorders of left-right asymmetry: heterotaxy and situs inversus. Am J Med Genet C Semin Med Genet 151C: 307– 317, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Swisher M, Jonas R, Tian X, Lee ES, Lo CW, Leatherbury L. Increased postoperative and respiratory complications in patients with congenital heart disease associated with heterotaxy. J Thorac Cardiovasc Surg 141: 637– 644, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takeuchi K, McGowan FX, Jr, Bacha EA, Mayer JE, Jr, Zurakowski D, Otaki M, del Nido PJ. Analysis of surgical outcome in complex double-outlet right ventricle with heterotaxy syndrome or complete atrioventricular canal defect. Ann Thorac Surg 82: 146– 152, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Tan SY, Rosenthal J, Zhao XQ, Francis RJ, Chatterjee B, Sabol SL, Linask KL, Bracero L, Connelly PS, Daniels MP, Yu Q, Omran H, Leatherbury L, Lo CW. Heterotaxy and complex structural heart defects in a mutant mouse model of primary ciliary dyskinesia. J Clin Invest 117: 3742– 3752, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Praagh R. Terminology of congenital heart disease. Glossary and commentary. Circulation 56: 139– 143, 1977 [DOI] [PubMed] [Google Scholar]
- 29. Webb S, Qayyum SR, Anderson RH, Lamers WH, Richardson MK. Septation and separation within the outflow tract of the developing heart. J Anat 202: 327– 342, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wessels MW, Kuchinka B, Heydanus R, Smit BJ, Dooijes D, de Krijger RR, Lequin MH, de Jong EM, Husen M, Willems PJ, Casey B. Polyalanine expansion in the ZIC3 gene leading to X-linked heterotaxy with VACTERL association: a new polyalanine disorder? J Med Genet 47: 351– 355, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Zariwala MA, Knowles MR, Omran H. Genetic defects in ciliary structure and function. Annu Rev Physiol 69: 423– 450, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.