Abstract
Diet-induced obesity (DIO) attenuates the arterial cardiac baroreceptor reflex, but the mechanisms and sites of action are unknown. This study tested the hypothesis that DIO impairs central aortic baroreceptor pathways. Normal chow control (CON) and high-fat-chow obesity-resistant (OR) and obesity-prone (OP) rats were anesthetized (inactin, 120 mg/kg) and underwent sinoaortic denervation. The central end of the aortic depressor nerve (ADN) was electrically stimulated to generate frequency-dependent baroreflex curves (5–100 Hz) during selective activation of myelinated (A-fiber) or combined (A- and C-fiber) ADN baroreceptors. A mild stimulus (1 V) that activates only A-fiber ADN baroreceptors induced robust, frequency-dependent depressor and bradycardic responses in CON and OR rats, but these responses were completely abolished in OP rats. Maximal activation of A fibers (3 V) elicited frequency-dependent reflexes in all groups, but a dramatic deficit was still present in OP rats. Activation of all ADN baroreceptors (20 V) evoked even larger reflex responses. Depressor responses were nearly identical among groups, but OP rats still exhibited attenuated bradycardia. In separate groups of rats, the reduced heart rate (HR) response to maximal activation of ADN A fibers (3 V) persisted in OP rats following pharmacological blockade of β1-adrenergic or muscarinic receptors, suggesting deficits in both parasympathetic nervous system (PNS) and sympathetic nervous system (SNS) reflex pathways. However, the bradycardic responses to direct efferent vagal stimulation were similar among groups. Taken together, our data suggest that DIO severely impairs the central processing of myelinated aortic baroreceptor control of HR, including both PNS and SNS components.
Keywords: obesity, autonomic nervous system, arterial baroreflex
obesity is associated with multisystem morbidity, including endocrine, immune, and autonomic manifestations (3, 8, 28), such as impairment of the baroreceptor reflex pathways. The depression of baroreceptor reflex sensitivity during weight gain in humans (4, 21) is replicated in animal models of obesity (9, 24, 25, 39, 40). Furthermore, the restoration of baroreflex function with weight loss suggests a dynamic interrelationship between diet, metabolic status, and cardiovascular autonomic control (4, 21, 41, 42). Obesity not only increases sympathetic outflow (SNS) to muscle (1, 21) and kidneys (45), but it also decreases cardiac parasympathetic (PNS) drive (6, 36). Because a depressed cardiac PNS baroreflex is an important predictor of cardiac morbidity and mortality (29), independent of blood pressure changes, the mechanisms responsible for baroreflex modification during obesity are critical to understand.
Changes in arterial pressure are transmitted via the arterial baroreceptors to the central nervous system (CNS). Myelinated arterial baroreceptors are activated in the normotensive range of arterial pressures, but baroreceptors with C-fiber axons generally require supraphysiological pressures to reach threshold and fire action potentials (27). The CNS integrates this information to elicit reciprocal changes in PNS and SNS outflow, resulting in rapid reflex changes in heart rate (HR) and total peripheral resistance. A recent study using the obese Zucker rat, which is a genetic model of obesity, demonstrates that obesity impairs the CNS portions of baroreflex control of sympathetic nerve activity (SNA) without altering arterial baroreceptor afferent function (25). However, the mechanisms by which obesity impairs the PNS and SNS components of the cardiac baroreflex are unknown. Therefore, the present study examined how obesity alters the baroreceptor reflex control of PNS and SNS outflow during diet-induced obesity (DIO) in outbred rats, an obesity model that exhibits many characteristics of human obesity (15).
To study central portions of aortic baroreceptor pathways, we electrically stimulated the aortic depressor nerve (ADN) and measured blood pressure and HR responses. The rat ADN contains only arterial baroreceptor afferent axons and thus relays solely aortic baroreceptor information to the solitary tract nucleus (NTS) (19, 38). Direct electrical activation of ADN afferents offers key experimental advantages. First, shocks to ADN activate one-to-one action potential signals to the CNS, making quantification more reproducible (18–20). Second, gradations in electrical shock intensity precisely recruit two subclasses of baroreceptor axons based on myelination. This is a critical aspect, since myelinated (A) fibers compose ∼10% of ADN and are tonically active at resting blood pressure. In contrast, ∼90% of ADN axons are unmyelinated (C) fibers (5), which are often physiologically quiet at resting blood pressure, but have profound reflex effects when activated (16). Therefore, our studies contrasted the hemodynamic reflex responses to A-fiber selective baroreceptor activation relative to C-fiber ADN baroreceptor activation in rats with DIO.
Because obesity impairs baroreflex responses near resting arterial pressure, we hypothesized that DIO compromises central A-fiber baroreceptor pathways. To test this hypothesis, we selectively activated A-fiber ADN afferents and contrasted these reflex responses to those elicited by activating all ADN baroreceptors. Next, we blocked either β1-adrenergic receptors (atenolol) or muscarinic receptors (methscopolamine) to isolate the PNS or SNS cardiac efferent pathways, respectively. Finally, we tested whether the peripheral efferent vagus nerve contributed to the impairment in DIO rats. Our results indicate that DIO more strongly attenuates myelinated compared with unmyelinated, baroreceptor cardiovascular pathways.
METHODS
Animals
All experiments were conducted in male Sprague-Dawley rats (Charles River Laboratories). Animals were housed individually in cages in a temperature-controlled room (22 ± 2°C) with a 12:12-h light-dark cycle. Food and water were provided ad libitum. All experimental protocols conformed to the National Institutes of Health Guide for the Health and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Oregon Health & Science University.
DIO model.
Male Sprague-Dawley rats (57–59 days, 250–275 g) were placed on a high-fat diet (Purina, 571F, 33% kcal fat; LabDiet 5001 with 10% lard). After 2 wk on the high-fat diet, the weight distribution of rats was separated into a bottom and top tertile of weight gain. Based on this distribution, animals in the bottom tertile of weight gain were selected as obesity-resistant (OR), whereas animals in the top tertile of weight gain were selected as obesity-prone (OP) rats, as previously described (15, 31). The animals from the middle tertile were omitted from the study. An age-matched control (CON) group was concurrently placed on standard chow (13.5% kcal fat; LabDiet 5001) for the same period of time. Reflex experiments were conducted 4–6 wk after rats were placed on the high-fat diet (OR and OP rats) or the conventional chow (CON rats).
Surgery
Under ∼2% isoflurane (Novaplus) in 100% oxygen, a femoral arterial catheter (PE-50) and three venous catheters (PE-10 attached to PE-50) filled with heparinized saline were implanted for the measurement of mean arterial pressure (MAP) and HR, and for intravenous access, respectively. After the completion of the catheter surgery, an intravenous infusion of inactin (120 mg/ml; Sigma) was administered over 30 min as the isoflurane was slowly withdrawn. Under inactin, a ventral midline neck incision was made, and the trachea was cannulated (PE-250) to facilitate spontaneous breathing. Arterial oxygen was monitored by a pulse oximeter (Starr Life Sciences) to confirm adequate oxygenation. The left ADN was identified as it joined the superior laryngeal nerve. The ADN trunk was dissected caudally, and the peripheral end was cut. To remove potential counteracting responses from other arterial baroreceptor inputs, the right ADN was cut, and the carotid sinuses were bilaterally denervated by stripping the fibers and adventitia at the left and right carotid bifurcations and swabbing with 10% phenol in 95% ethanol (48). In successful sinoaortic baroreceptor denervations, blood pressure increases to intravenous injections of phenylephrine (5 μg/0.1 ml/30 s) failed to evoke reflex bradycardia. Experiments commenced following 60 min recovery. Rectal body temperature was maintained at 37 ± 1°C throughout using a heating pad. At the end of the experiment, rats were killed with an overdose of pentobarbital sodium (39 mg iv Euthosol).
Protocol 1: Baroreflex Responses to Graded Electrical Activation of the ADN
To elicit reflex MAP and HR responses, the central end of the ADN was placed on bipolar stainless steel electrodes and isolated in warm mineral oil. The electrodes were connected to a programmable stimulator (AMPI Master-8) through a stimulus isolation unit (AMPI ISO-Flex). Previous extensive studies directly measured the electroneurogram of the ADN (18) and found that low-intensity shocks (1–2 V, 0.1 ms) activated action potentials from myelinated fibers alone, whereas higher intensities always evoked mixed volleys of A- and C-fiber waves (18). Here, we used low-voltage shocks (1 V) to selectively activate A-fiber volleys, and moderate stimulation (3 V) to activate all A fibers plus a small contingent of C fibers. High-intensity shocks (20 V) were used to activate all A and C fibers of the ADN (18). At each test intensity, repeated shocks at 5, 10, 20, 50, and 100 Hz evoked reflex MAP and HR responses. Shocks were kept short (0.1-ms duration) to avoid the triggering of repetitive action potentials found with single shocks longer than 0.5 ms (Fan W and Andresen MC). Responses were measured as peak reflex changes in MAP and HR for 30-s stimulus ADN trains. Intensity and frequency combinations of the stimuli were applied in random order, with at least 2 min between stimulations.
Protocol 2: PNS Control of HR Mediated by Myelinated ADN Baroreceptors
Based on initial findings that DIO severely impaired myelinated baroreflex pathways, we tested if this impairment is due to a reduced responsiveness of PNS pathways in separate groups of rats (CON, OR, and OP). ADN frequency-response relationships were determined (5–100 Hz, see above) at the maximal A-fiber shock intensity of 3 V. To assess the PNS contribution to HR responses, the ADN frequency-response curve was tested before and 10 min following β1-adrenergic receptor blockade with atenolol (2 mg/kg iv; Sigma). Full block was verified by a lack of tachycardia to isoproterenol (1 μg/kg iv; Sigma). The contribution of the PNS was defined as the remaining HR response during ADN stimulation after β1-adrenergic receptor blockade.
Protocol 3: SNS Control of HR Mediated by Myelinated ADN Baroreceptors
To assess the contribution of the SNS to these responses, this protocol was repeated in separate, additional groups of CON, OR, and OP rats. Test procedures were comparable to PNS protocols and used a shock intensity of 3 V to maximally recruit A-fiber baroreceptors for this series. ADN stimulation was tested 10 min following muscarinic receptor blockade with methscopolamine (1 mg/kg iv; Sigma) that was verified by a lack of bradycardia to efferent vagal stimulation (10 V, 10 Hz). The contribution of the SNS was defined as the remaining HR response during ADN stimulation after muscarinic receptor blockade.
Protocol 4: Direct Efferent Vagal Stimulation
To test whether the PNS cardiac response impairment was due to peripheral nerve changes, we assessed the efferent limb of the cardiac PNS control in OP, OR, and CON rats. We measured the HR responses during electrical activation of the efferent vagal nerve trunk. The peripheral end of the cut vagus nerve was placed on the stimulating electrodes and isolated in warm mineral oil. Frequency-dependent HR responses were recorded to shocks (10 V, 0.1 ms duration) applied at 1, 3, 5, and 10 Hz for 30 s in random order.
Data Analysis
Data were sampled at 2,000 Hz using the Biopac MP100 data acquisition system and analyzed off-line (Acknowledge). MAP and HR responses were calculated as peak changes in MAP (ΔMAP) and HR (ΔHR) from baseline measurements. Baseline values were measured as averages over 10 s preceding each stimulation period, and the peak response was averaged over a stable period of at least 5 s. In addition, the change in MAP and HR during no stimulation (0 Hz) was also calculated. To determine this, MAP and HR were averaged during the first and last 10 s of a 30-s baseline measurement at the beginning of each series of shocks at a set voltage. The 0-Hz value was defined as the difference between the two 10-s averages. The time-to-peak value was determined by measuring the time duration of the fall in HR during each stimulation period. The slope of the response, (ΔHR)/(time to peak), was calculated by the Acknowledge software. All data are presented as means ± SE.
A three-way ANOVA with two-way repeated measures was used to determine between-group differences in the changes in MAP and HR during 1 V, 3 V, and 20 V ADN stimulation (group × amplitude × frequency) and for the effect of muscarinic or β1-adrenergic blockade (group × treatment × frequency). A two-way ANOVA with one-way repeated measures (group × frequency) was used to identify between-group differences in: 1) the PNS and SNS contributions to HR during 3 V ADN stimulation, 2) the time-to-peak and slope of the HR response, 3) the bradycardia during efferent vagal stimulation, and 4) the frequency threshold at which there was a significant fall in MAP and HR compared with MAP and HR with no stimulation (0 Hz). Within each protocol, a one-way ANOVA was used to compare body, weight, baseline MAP, and HR between groups. Specific comparisons were assessed using a Neuman-Keuls post hoc test. P < 0.05 indicated significance.
RESULTS
Baseline values.
As depicted in Tables 1–4, consumption of the high-fat diet for 4–6 wk accelerated weight gain only in OP rats. Despite exposure to the same high-fat diet, OR rats gained weight similarly to CON rats fed a conventional chow. Thus, at 4–6 wk, OP rats weighed more than either OR or CON rats (P < 0.05). Diet did not alter basal hemodynamic values across groups.
Table 1.
Baseline body weight, MAP, and HR for protocol 1
| Group | n | Body Wt, g | MAP, mmHg | HR, beats/min |
|---|---|---|---|---|
| CON | 5 | 423 ± 8 | 112 ± 5 | 395 ± 7 |
| OR | 5 | 446 ± 7 | 107 ± 6 | 403 ± 20 |
| OP | 6 | 516 ± 5*† | 110 ± 7 | 377 ± 22 |
Values are means ± SE; n, no. of animals. MAP, mean arterial pressure; HR, heart rate; CON, control; OR, obesity resistant; OP, obesity prone. Body weight was greater in OP vs. CON (*P < 0.05) and OR (†P < 0.05). There were no significant differences in baseline MAP and HR between groups.
Table 2.
Baseline body weight, MAP, and HR for protocol 2
| Pre-atenolol |
Post-atenolol |
|||||
|---|---|---|---|---|---|---|
| Group | n | Body Wt, g | MAP, mmHg | HR, beats/min | MAP, mmHg | HR, beats/min |
| CON | 6 | 440 ± 11 | 125 ± 7 | 360 ± 12 | 113 ± 8 | 315 ± 7# |
| OR | 5 | 448 ± 3 | 115 ± 6 | 367 ± 14 | 113 ± 6 | 338 ± 12# |
| OP | 5 | 538 ± 8*† | 112 ± 9 | 376 ± 8 | 104 ± 7 | 315 ± 13# |
Values are means ± SE; n, no. of animals. Body weight was greater in OP vs. CON (*P < 0.05) and OR (†P < 0.05). There were no significant differences in baseline MAP and HR between groups. Atenolol significantly decreased HR in all groups (#P < 0.05 vs. pre-atenolol).
Table 3.
Baseline body weight, MAP, and HR for protocol 3
| Pre-methscopolamine |
Post-methscopolamine |
|||||
|---|---|---|---|---|---|---|
| Group | n | Body Wt, g | MAP, mmHg | HR, beats/min | MAP, mmHg | HR, beats/min |
| CON | 6 | 441 ± 8 | 123 ± 6 | 380 ± 13 | 113 ± 8 | 389 ± 12 |
| OR | 8 | 449 ± 6 | 120 ± 4 | 372 ± 16 | 113 ± 6 | 383 ± 18 |
| OP | 6 | 530 ± 7*† | 110 ± 7 | 392 ± 10 | 104 ± 7 | 395 ± 5 |
Values are means ± SE; n, no. of animals. Body weight was greater in OP vs. CON (*P < 0.05) and OR (†P < 0.05). There were no significant differences in baseline MAP and HR between groups or treatment.
Table 4.
Baseline body weight, MAP, and HR for protocol 4
| Group | n | Body Wt, g | MAP, mmHg | HR, beats/min |
|---|---|---|---|---|
| CON | 7 | 430 ± 5 | 115 ± 4 | 393 ± 13 |
| OR | 6 | 439 ± 11 | 115 ± 6 | 398 ± 14 |
| OP | 7 | 517 ± 5*† | 113 ± 5 | 375 ± 16 |
Values are means ± SE; n, no. of animals. Body weight was greater in OP vs. CON (*P < 0.05) and OR (†P < 0.05). There were no significant differences in baseline MAP and HR between groups.
Obesity markedly attenuates myelinated ADN baroreflex responses.
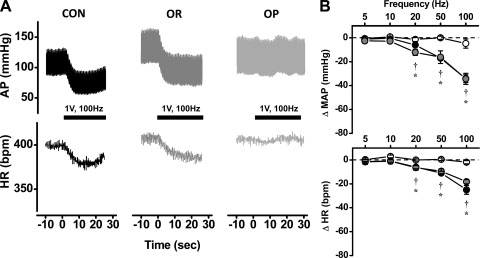
Mild shocks (1 V) selective for the activation of myelinated axons evoked robust depressor and bradycardic responses in CON rats (Fig. 1A). Such mild shocks required relatively high-frequency stimulation (≥50 Hz, P < 0.05) to trigger MAP and HR reflex responses (Fig. 1B). Similarly, OR rats on the high-fat diet also required high stimulation frequencies to evoke a robust fall in MAP (≥20 Hz, P < 0.05) and HR (≥50 Hz, P < 0.05). (Fig. 1, A and B). In stark contrast, identical mild ADN shocks failed to activate changes in MAP or HR in OP rats, even at the highest frequency of activation (100 Hz). Therefore, the baroreflex responses in OP rats were significantly impaired compared with those in CON and OR rats (P < 0.05, Fig. 1B).
Fig. 1.
A: representative arterial pressure (AP) and heart rate (HR) tracings show that the activation of myelinated aortic depressor nerve (ADN) baroreceptors (1 V, 100 Hz) evoked similarly robust reflex responses in a control (CON) and obesity-resistant (OR) rat, whereas these responses are completely absent in an obesity-prone (OP) rat. B: group data show that activation of myelinated ADN baroreceptors (1 V stimulus) evokes frequency-dependent depressor (top) and bradycardic (bottom) responses between 20 and 100 Hz in CON (n = 5) and OR (n = 5) rats, with no difference between groups. OP rats (n = 6) exhibited no significant responses at any stimulation. bpm, Beats/min. *P < 0.05 vs. CON; †P < 0.05 vs. OR.
Compared with the 1 V stimulation, moderate shocks (3 V) evoked brisk, much larger bradycardia and hypotension in both the CON and OR rats (Fig. 2). Significant hypotensive responses (P < 0.05) were activated at 10 Hz in CON and 20 Hz in OR rats; 50 Hz was the threshold for significant bradycardia (P < 0.05) in both groups. This moderate stimulus also evoked significant hypotension and bradycardia in OP rats at high frequencies (MAP: 100 Hz, HR: 50 Hz; P < 0.05 vs. no stimulation). In addition, the hypotensive and bradycardic responses to ADN activation were still weak in OP rats compared with CON and OR rats (Fig. 2B). Interestingly, the bradycardic responses in OP rats also developed more slowly compared with the swift HR changes in CON and OR rats (Figs. 2A and 3A). The time from the onset of stimulation to peak bradycardia was similar between groups (CON: 10.5 ± 1.1 s; OR: 10.5 ± 1.8 s; OP: 11.6 ± 1.1 s), but the rate of change for the fall in HR was slower (P < 0.05) in OP rats (−1.2 ± 0.4 beats·min−1·s−1) compared with CON (−4.3 ± 1.5 beats·min−1·s−1) and OR (−4.4 ± 0.8 beats·min−1·s−1) rats.
Fig. 2.
A: representative AP and HR tracings show that the activation of all myelinated and some unmyelinated ADN baroreceptors (3 V, 100 Hz) evoked similarly robust reflex responses in a CON and OR rat. Although these responses were also present in the OP rat, the magnitude is clearly impaired. B: group data show that activation of all myelinated baroreceptors (3 V) evokes frequency-dependent depressor (top) and bradycardic (bottom) responses between 10 and 100 Hz in CON (n = 5) and OR (n = 5) and OP (n = 6) rats, although responses were highly attenuated in OP rats compared with OR and CON rats. Again, there was no difference in reflex responses between CON and OR rats. *P < 0.05 vs. CON; †P < 0.05 vs. OR.
Fig. 3.
A: representative AP and HR tracings show that the activation of all myelinated and unmyelinated ADN baroreceptors (20 V, 100 Hz) evokes robust reflex responses in CON, OR, and OP rats. The depressor response was preserved in the OP rat, although the magnitude of the bradycardia was still clearly impaired. B: group data show that activation of all ADN baroreceptor afferents evoked frequency-dependent depressor (graph on top) and bradycardic (graph on bottom) responses between 5 and 100 Hz in CON (n = 5) and OR (n = 5) and OP (n = 6) rats. The magnitude of the depressor responses was similar between groups, but the fall in HR was still greatly attenuated in OP rats compared with OR and CON rats. There was no difference in reflex responses between CON and OR rats. *P < 0.05 vs. CON; †P < 0.05 vs. OR.
Obesity impairs bradycardic responses to maximal ADN activation.
Intense shocks that should activate all ADN axons evoked large and substantially similar MAP responses across all groups (Fig. 3). The depressor (CON: 10 Hz, OR and OP: 5 Hz; P < 0.05) and bradycardic (CON: 20 Hz, OR: 5 Hz, OP: 10 Hz; P < 0.05) thresholds were at low frequencies in all groups. Nevertheless, the degree of bradycardia was still impaired in OP rats compared with CON and OR (P < 0.05; Fig. 3B). Furthermore, the rate of change for the fall in HR was again slower (P < 0.05) in OP rats (−1.4 ± 0.2 beats·min−1·s−1) compared with CON (−5.6 ± 0.9 beats·min−1·s−1) and OR (−8.0 ± 2.7 beats·min−1·s−1) rats.
Obesity impairs cardiac parasympathetic and sympathetic reflex responses to myelinated ADN baroreceptor activation.
The response profiles to this point in our studies strongly suggested that obesity negatively affected the cardiac responses initiated by myelinated ADN afferents. Because PNS cardiac control is an intrinsically faster-acting bradycardic pathway compared with the SNS withdrawal (23), and the responses in OP rats were more slowly developing, we suspected that the attenuated overall bradycardia in OP rats might arise from an impairment of cardiac PNS pathways. To separately assess cardiac PNS and SNS contributions, we used cardiac selective autonomic blockers to remove either β1-adrenergic (protocol 2) or muscarinic (protocol 3) HR responses. In separate groups of rats, 3 V shocks were used to activate all myelinated ADN axons. With both muscarinic and β1-adrenergic receptors intact, baroreflex responses resembled those in the preceding series of experiments (Figs. 4 and 5). In all groups within both protocols, the 3 V ADN shocks evoked significant hypotension and bradycardia at frequencies no lower than 20 Hz (P < 0.05), with the exception of the OR group in protocol 2, which exhibited significant bradycardia at 10 Hz. Furthermore, OP rats exhibited consistently smaller depressor (P < 0.05, Figs. 4A and 5A) and bradycardic (P < 0.05, Figs. 4B and 5B) responses compared with CON and OR rats.
Fig. 4.
Similar to protocol 1, OP rats exhibited impaired depressor and bradycardic responses compared with CON and OR (P < 0.05). During the activation of all myelinated ADN baroreceptors (3 V), blockade of β1-adrenergic receptors with atenolol had no effect on the depressor responses (A) in any group, but the bradycardic response (B) was reduced in CON and OR rats but not in OP rats. #P < 0.05 vs. pre-atenolol; *P < 0.05, OP vs. CON; †P < 0.05 OP vs. OR.
Fig. 5.
Similar to protocol 1, OP rats exhibited impaired depressor and bradycardic responses compared with CON and OR rats (P < 0.05). During the activation of all myelinated ADN baroreceptors (3 V), muscarinic blockade with methscopolamine had no effect on the depressor responses (A) in any group. The bradycardic response (B) was reduced in CON and OR rats but not in OP rats. #P < 0.05 vs. pre-methscopolamine; *P < 0.05 OP vs. CON; †P < 0.05 OP vs. OR.
Blockade of β1-adrenergic receptors with atenolol had no discernible effect on ADN-evoked MAP responses in any experimental group (Fig. 4A). Atenolol reduced the reflex bradycardia to ADN activation (Fig. 4B) in CON at 50 and 100 Hz (P < 0.05) and OR rats at 20, 50, and 100 Hz (P < 0.05). β1-Adrenergic block had no effect on the reflex bradycardia in OP rats.
To balance our experimental design, we blocked muscarinic receptors with methscopolamine. Methscopolamine had no significant impact on resting HR (Table 3), which may be related to substantial elimination of PNS drive by sinoaortic denervation (SAD) or the anesthetic. Similar to atenolol, methscopolamine had no effect on the MAP responses to any frequency of ADN activation (Fig. 5A). However, muscarinic blockade substantially reduced the bradycardia evoked by ADN activation in both CON (100 Hz, P < 0.05) or OR (50 and 100 Hz, P < 0.05) rats. Interestingly, muscarinic blockade failed to alter HR responses in OP rats (Fig. 5B).
To facilitate comparisons across protocols, we replotted these results together (Fig. 6). Cardiac responses measured following atenolol treatment represent PNS-mediated bradycardia (Fig. 6A), whereas responses after methscopolamine treatment were SNS-mediated (Fig. 6B). At higher frequencies (50 and 100 Hz), PNS responses in OP rats were less than OR and CON (P < 0.05), but, interestingly, the CON rats also expressed smaller responses compared with OR rats (P < 0.05). The OP rats also exhibited attenuated SNS responses (P < 0.05) compared with CON rats. However, the SNS responses were similar between OR and OP rats.
Fig. 6.
The contribution of the parasympathetic nervous system (PNS, A) and the sympathetic nervous system (SNS, B) to the bradycardic response during 3 V ADN stimulation during the activation of all myelinated ADN baroreceptors is varied between CON, OR, and OP rats. Although the total reflex bradycardia during activation of ADN baroreceptors is similar between CON and OR rats, CON rats are more reliant on SNS withdrawal to lower HR, whereas OR rats are more reliant on the activation of PNS pathways. When compared with both CON and OR rats at the same time, the overall attenuated reflex bradycardia in OP rats is clearly attributed to both attenuated contributions of PNS activation and SNS withdrawal. *P < 0.05 CON vs. OP; †P < 0.05 OR vs. OP, ‡P < 0.05 CON vs. OR.
Taken together, our findings suggest that the impairment of both PNS and SNS cardiac pathways contributes to the severely attenuated reflex bradycardia mediated by myelinated baroreceptors in OP rats. Despite the similar overall reflex bradycardia in CON and OR rats, the evidence also suggests that CON rats rely to a greater extent on SNS control, whereas OR rats depend more on PNS cardioinhibitory pathways.
Peripheral vagal efferent cardiac control is well preserved regardless of diet or selection group.
To assess whether diet or selection group might influence the final links of the PNS pathway, we directly activated the efferent end of the vagus nerve (protocol 4) with electrical shocks. Vagal activation resulted in a frequency-dependent bradycardia that was similar across all experimental groups (Fig. 7). This finding suggests that the observed alterations in PNS cardiac baroreflex responses occurred central to the vagal efferent axons and the heart.
Fig. 7.
The bradycardic response to efferent vagal stimulation was not different between CON, OR, and OP rats.
DISCUSSION
The present studies reveal that obesity triggers substantial CNS deficits, specifically in baroreceptor-linked autonomic reflex pathways controlling the cardiovascular system. By using graded stimulus shocks to directly activate ADN baroreceptor axons, a severe deficit in aortic myelinated baroreceptor pathways was distinguished. Furthermore, the use of selective cardiac postganglionic blockers revealed that obesity impairs both branches of the autonomic nervous system. We found that: 1) DIO depressed the myelinated ADN subset of aortic baroreflexes, which was attributed to deficits in PNS and SNS cardiac reflex pathways; 2) activation of ADN C-fiber baroreflex pathways masked the clear attenuation of ADN A-fiber baroreflexes in OP rats; 3) OR rats had preserved, if not sensitized, myelinated ADN PNS cardiac pathways; and 4) activation of the peripheral vagus nerve was equally effective in all animal groups.
Obesity impairs the arterial baroreflex with broad detrimental consequences, including increased risk for cardiac arrhythmias, sudden cardiac death, and end-organ damage (17, 29, 46). However, few mechanistic details are understood. Because only a small fraction of human obesity is associated with a currently recognized genetic origin (11), we chose to study a DIO model, which utilizes outbred, genetically diverse animals to mimic the polygenic clinical profile of human obesity. DIO models have been extensively studied in the context of energy balance (30, 31), but less is known about blood pressure regulation. We started with pubescent animals that gained weight steeply and studied these animals early in adulthood to minimize the potential confounding effects of age and weight gain (9, 10). Like obese humans, DIO impairs baroreflex control of HR (49). However, the site of impairment within the baroreflex loop is unknown. Therefore, we assessed whether obesity impairs central processing of BP inputs beyond the level of the baroreceptors by testing the baroreflex responses elicited by stimulation of ADN afferents.
Low-intensity electrical ADN stimulation selectively activates myelinated baroreceptors to evoke reflex responses with a highly characteristic frequency dependence (16, 18). With this knowledge, we designed specific protocols to compare baroreflex curves mediated by myelinated aortic baroreceptors with that of mixed A- and C-fiber ADN baroreceptors. As a result, our study revealed that obesity severely impaired the central processing of myelinated ADN baroreceptors (Figs. 1 and 2). It is important to appreciate that A-fiber baroreceptors normally dominate baroreflex responses near resting blood pressure and below, such as in conditions of postural hypotension (44).
Most commonly, baroreflex studies electrically activate all ADN baroreceptors to examine the central control of the baroreflex in several pathological states, such as obesity (2, 9, 25), hypertension (37), and high sodium intake (14). Such high-intensity shocks may well mask the contributions of the myelinated baroreceptors and reflect primarily the powerful C-fiber baroreflex pathways. Previous studies show robust reflex responses at low activation frequencies (≤10 Hz) in the obese state (10, 25), a clear indication of unmyelinated baroreceptor reflexes (18, 20). Indeed, our study confirms this. While our findings suggest an A-fiber pathway deficit during DIO, the OP rats still exhibited strong, predominating C-fiber ADN responses. This interpretation is supported by the emergence of substantial low-frequency MAP and HR responses (5 Hz) with high-intensity shocks in OP rats (P < 0.05, Fig. 3B). These responses are associated with only ADN C-fiber activation (16), although future studies using anodal blockade to isolate C-fiber responses (20) are needed to determine whether obesity directly affects unmyelinated baroreflex pathways. Furthermore, these data highlight the importance of selective activation of myelinated vs. unmyelinated ADN baroreceptors when assessing baroreflex dysfunction.
Previous work suggested that obesity decreases cardiac vagal tone and suppresses the contribution of the PNS to the baroreflex (6, 36). In support of this, our studies demonstrated that: 1) the myelinated baroreceptor responses of cardiac pathways were more slowly developing in OP compared with CON and OR rats, 2) methscopolamine had no effect on reflex bradycardia in OP rats, and 3) the remaining PNS response after atenolol was highly attenuated compared with OR and CON rats. A new finding is that the bradycardia during efferent vagal stimulation was normal in OP rats, indicating a central deficit of the PNS pathway. In addition, the remaining SNS response after methscopolamine was also attenuated in OP rats compared with CON rats (Fig. 6B), indicating that obesity simultaneously impairs the PNS and SNS components of the cardiac baroreflex beyond the level of the baroreceptor afferents.
However, it is curious as to why a significant reflex bradycardia remained in the OP rats following either SNS or PNS blockade. For example, if PNS blockade with methscopolamine had no effect, this would indicate that the fall in HR is solely mediated by SNS withdrawal. Therefore, it would be expected that the blockade of the SNS pathway with atenolol would completely abolish the response. From a mechanistic perspective, the inability of PNS or SNS blockade to alter the HR response in OP rats may be indicative of functional antagonism between cardiac muscarinic and β1-adrenergic signaling. The activation of muscarinic receptors suppresses β-adrenergic sensitivity (34) and vice versa (35). Therefore, blocking either muscarinic or β1-adrenergic receptors may have disinhibited the remaining SNS or PNS responses, respectively.
Another major finding was that the depressor responses during ADN A-fiber stimulation were also clearly attenuated in OP rats. Similar observations in the obese Zucker rat were attributed to central deficits in reflex control of sympathetic activity (25). Alternatively, the attenuated response in OP rats may be due to impaired vascular noradrenergic responsiveness, as seen in other models of obesity (7, 33). Whether DIO differentially impacts sympathoinhibition during the activation of only myelinated baroreceptors compared with simultaneous activation of A and C ADN fibers, or detrimentally affects vascular function, each requires future investigation.
An intriguing result was the dominance of PNS pathways in OR rats to more strongly regulate HR in baroreflex responses than even CON rats. However, the mechanisms are unclear. In other aspects, OR rats are comparable to CON rats. They are not insulin (49) or leptin (31) resistant and have normal baroreflex sensitivity (49). Indeed, our study showed that there is no impairment of the hemodynamic reflexes mediated by myelinated baroreceptors since the overall MAP and HR responses were almost identical to CON rats. Despite these general response similarities, more subtle but potentially critical differences may be important between OR and CON rats. For example, OR rats have even higher insulin sensitivity than CON rats, despite being on a high-fat diet (49). Because impaired vagal control of the baroreflex is associated with insulin resistance (32), the concurrent elevation in insulin sensitivity and enhanced PNS control of the cardiac baroreflex in OR rats may be functionally linked. Alternatively, because the OR and OP animals are only identified after being placed on the high-fat diet, the moderate PNS responsiveness of CON rats may be the result of a blending of genetically disposed OR and OP rats.
A major aim of the present study was to determine if DIO impairs baroreflex function central to the peripheral nerve endings of aortic baroreceptors. Our data support this hypothesis, since the bradycardic and depressor responses to ADN stimulation were severely depressed in OP rats. However, our experimental design has two limitations. First, all animals were subjected to both anesthesia and SAD, which may affect the central integration of the baroreflex compared with a conscious, intact animal. Nevertheless, all groups were treated similarly, but myelinated baroreceptor pathways were only attenuated in OP animals. These findings suggest that the deficit is independent of the anesthetic or SAD. Second, we did not assess whether baroreceptor afferent sensitivity to changes in arterial pressure might contribute during DIO. Studies of single A- and C-fiber baroreceptors could address this question, but were beyond the scope of the present work.
The mechanisms responsible for these central baroreflex impairments, or even precisely which brain sites are affected, were not discerned. However, we offer the following possibilities. Central impairment of the baroreceptor reflex during obesity may be attributed to insulin resistance. Baroreflex gain is impaired in several insulin resistance states, including pregnancy (13) and DIO (49), suggesting a functional link between the two. Alternatively, this central impairment may be attributed to a chronic low-grade inflammation during the obese state (47). Inflammatory mediators, such as interleukin-6, act directly in NTS to impair cardiac baroreflex gain (43). Nevertheless, our results indicate that obesity differentially impairs the baroreflex beyond the level of the baroreceptors, with a more severe loss of myelinated- than C-fiber-initiated aortic baroreflex pathways, and impairment of SNS and central PNS portions of autonomic baroreflex pathways. Identifying the sites of dysfunction along these pathways and determining the impact of insulin resistance and/or inflammation on myelinated baroreceptor reflexes remain potentially interesting aspects for future investigation.
GRANTS
This work was supported by National Institutes of Health Grants NS-045553 (B. H. McCully), HL-088552 (V. L. Brooks), and HL-41119 (M. C. Andresen) and by American Heart Association Grants 7500041 (B. H. McCully) and 2060630 (V. L. Brooks).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: B.H.M., V.L.B., and M.C.A. conception and design of research; B.H.M. performed experiments; B.H.M. analyzed data; B.H.M., V.L.B., and M.C.A. interpreted results of experiments; B.H.M. prepared figures; B.H.M. drafted manuscript; B.H.M., V.L.B., and M.C.A. edited and revised manuscript; B.H.M., V.L.B., and M.C.A. approved final version of manuscript.
REFERENCES
- 1. Agapitov AV, Correia ML, Sinkey CA, Haynes WG. Dissociation between sympathetic nerve traffic and sympathetically mediated vascular tone in normotensive human obesity. Hypertension 52: 687– 695, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ai J, Liang F, Zhou H, Zhao J, Wang N, Zhu S, Yang B. Mechanism of impaired baroreflex sensitivity in Wistar rats fed a high-fat and -carbohydrate diet. Br J Nutr 104: 291– 297, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 23: 469– 480, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation 106: 2533– 2536, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Andresen MC, Krauhs JM, Brown AM. Relationship of aortic wall and baroreceptor properties during development in normotensive and spontaneously hypertensive rats. Circ Res 43: 728– 738, 1978 [DOI] [PubMed] [Google Scholar]
- 6. Arone LJ, Mackintosh R, Rosenbaum M, Leibel RL, Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol Regul Integr Comp Physiol 269: R222– R225, 1995 [DOI] [PubMed] [Google Scholar]
- 7. Belin de Chantemele EJ, Mintz JD, Rainey WE, Stepp DW. Impact of leptin-mediated sympatho-activation on cardiovascular function in obese mice. Hypertension 58: 271– 279, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benarroch EE. Autonomic-mediated immunomodulation and potential clinical relevance. Neurology 73: 236– 242, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Bunag RD, Eriksson L, Krizsan D. Baroreceptor reflex impairment and mild hypertension in rats with dietary-induced obesity. Hypertension 15: 397– 406, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Bunag RD, Krizsan D, Itoh H. Diminished cardiovascular responsiveness to vagal stimulation in obese rats. Am J Physiol Regul Integr Comp Physiol 259: R842– R848, 1990 [DOI] [PubMed] [Google Scholar]
- 11. Choquet H, Meyre D. Genetics of Obesity: What have we Learned? Curr Genomics 12: 169– 179, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Czaja K, Lakomy M, Kaleczyc J, Barb CR, Rampacek GB, Kraeling RR. Leptin receptors, NPY, and tyrosine hydroxylase in autonomic neurons supplying fat depots in a pig. Biochem Biophys Res Commun 293: 1138– 1144, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Daubert DL, Chung MY, Brooks VL. Insulin resistance and impaired baroreflex gain during pregnancy. Am J Physiol Regul Integr Comp Physiol 292: R2188– R2195, 2007 [DOI] [PubMed] [Google Scholar]
- 14. DiBona GF, Jones SY. Endogenous angiotensin affects responses to stimulation of baroreceptor afferent nerves. J Hypertens 21: 1539– 1546, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Dobrian AD, Davies MJ, Prewitt RL, Lauterio TJ. Development of hypertension in a rat model of diet-induced obesity. Hypertension 35: 1009– 1015, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Douglas WW, Ritchie JM, Schaumann W. A study of the effect of the pattern of electrical stimulation of the aortic nerve on the reflex depressor responses. J Physiol 133: 232– 242, 1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duflou J, Virmani R, Rabin I, Burke A, Farb A, Smialek J. Sudden death as a result of heart disease in morbid obesity. Am Heart J 130: 306– 313, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Fan W, Andresen MC. Differential frequency-dependent reflex integration of myelinated and nonmyelinated rat aortic baroreceptors. Am J Physiol Heart Circ Physiol 275: H632– H640, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Fan W, Reynolds PJ, Andresen MC. Baroreflex frequency-response characteristics to aortic depressor and carotid sinus nerve stimulation in rats. Am J Physiol Heart Circ Physiol 271: H2218– H2227, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Fan W, Schild JH, Andresen MC. Graded and dynamic reflex summation of myelinated and unmyelinated rat aortic baroreceptors. Am J Physiol Regul Integr Comp Physiol 277: R748– R756, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, Mancia G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation 97: 2037– 2042, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Habecker BA, Sachs HH, Rohrer H, Zigmond RE. The dependence on gp130 cytokines of axotomy induced neuropeptide expression in adult sympathetic neurons. Dev Neurobiol 69: 392– 400, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Head GA, McCarty R. Vagal and sympathetic components of the heart rate range and gain of the baroreceptor-heart rate reflex in conscious rats. J Auton Nerv Syst 21: 203– 213, 1987 [DOI] [PubMed] [Google Scholar]
- 24. Hilzendeger AM, Goncalves AC, Plehm R, Diedrich A, Gross V, Pesquero JB, Bader M. Autonomic dysregulation in ob/ob mice is improved by inhibition of angiotensin-converting enzyme. J Mol Med (Berl) 88: 383– 390, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Huber DA, Schreihofer AM. Attenuated baroreflex control of sympathetic nerve activity in obese Zucker rats by central mechanisms. J Physiol 588: 1515– 1525, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hyatt Sachs H, Rohrer H, Zigmond RE. The conditioning lesion effect on sympathetic neurite outgrowth is dependent on gp130 cytokines. Exp Neurol 223: 516– 522, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jones JV, Thoren PN. Characteristics of aortic baroreceptors with non-medullated afferents arising from the aortic arch of rabbits with chronic renovascular hypertension. Acta Physiol Scand 101: 286– 293, 1977 [DOI] [PubMed] [Google Scholar]
- 28. Katagiri H, Yamada T, Oka Y. Adiposity and cardiovascular disorders: disturbance of the regulatory system consisting of humoral and neuronal signals. Circ Res 101: 27– 39, 2007 [DOI] [PubMed] [Google Scholar]
- 29. La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT, Jr, Camm AJ, Schwartz PJ. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation 103: 2072– 2077, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Levin BE. Factors promoting and ameliorating the development of obesity. Physiol Behav 86: 633– 639, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725– R730, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Miller AW, Sims JJ, Canavan A, Hsu T, Ujhelyi MR. Impaired vagal reflex activity in insulin-resistant rats. J Cardiovasc Pharmacol 33: 698– 702, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Mingorance C, Alvarez dS, Jimenez-Palacios FJ, Callejon MM, Casto C, Marhuenda E, Herrera MD. Effects of chronic treatment with the CB1 antagonist, rimonabant on the blood pressure, and vascular reactivity of obese Zucker rats. Obesity (Silver Spring) 17: 1340– 1347, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Muscholl E. Peripheral muscarinic control of norepinephrine release in the cardiovascular system. Am J Physiol Heart Circ Physiol 239: H713– H720, 1980 [DOI] [PubMed] [Google Scholar]
- 35. Richardson RM, Kim C, Benovic JL, Hosey MM. Phosphorylation and desensitization of human m2 muscarinic cholinergic receptors by two isoforms of the beta-adrenergic receptor kinase. J Biol Chem 268: 13650– 13656, 1993 [PubMed] [Google Scholar]
- 36. Rissanen P, Franssila-Kallunki A, Rissanen A. Cardiac parasympathetic activity is increased by weight loss in healthy obese women. Obes Res 9: 637– 643, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Salgado HC, Barale AR, Castania JA, Machado BH, Chapleau MW, Fazan R., Jr Baroreflex responses to electrical stimulation of aortic depressor nerve in conscious SHR. Am J Physiol Heart Circ Physiol 292: H593– H600, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Sapru HN, Gonzalez E, Krieger AJ. Aortic nerve stimulation in the rat: cardiovascular and respiratory responses. Brain Res Bull 6: 393– 398, 1981 [DOI] [PubMed] [Google Scholar]
- 39. Schreihofer AM, Mandel DA, Mobley SC, Stepp DW. Impairment of sympathetic baroreceptor reflexes in obese Zucker rats. Am J Physiol Heart Circ Physiol 293: H2543– H2549, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Silvani A, Bastianini S, Berteotti C, Franzini C, Lenzi P, Lo MV, Zoccoli G. Dysregulation of heart rhythm during sleep in leptin-deficient obese mice. Sleep 33: 355– 361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Skrapari I, Tentolouris N, Perrea D, Bakoyiannis C, Papazafiropoulou A, Katsilambros N. Baroreflex sensitivity in obesity: relationship with cardiac autonomic nervous system activity. Obesity (Silver Spring) 15: 1685– 1693, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Straznicky NE, Lambert GW, Lambert EA. Neuroadrenergic dysfunction in obesity: an overview of the effects of weight loss. Curr Opin Lipidol 21: 21– 30, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Takagishi M, Waki H, Bhuiyan ME, Gouraud SS, Kohsaka A, Cui H, Yamazaki T, Paton JF, Maeda M. IL-6 microinjected in the nucleus tractus solitarii attenuates cardiac baroreceptor reflex function in rats. Am J Physiol Regul Integr Comp Physiol 298: R183– R190, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Thoren P, Saum WR, Brown AM. Characteristics of rat aortic baroreceptors with nonmedullated afferent nerve fibers. Circ Res 40: 231– 237, 1977 [DOI] [PubMed] [Google Scholar]
- 45. Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation 96: 3423– 3429, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Wang TJ, Parise H, Levy D, D'Agostino RB, Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. J Am Med Assoc 292: 2471– 2477, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 15: 2792– 2800, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Yoshimoto M, Nagata K, Miki K. Differential control of renal and lumbar sympathetic nerve activity during freezing behavior in conscious rats. Am J Physiol Regul Integr Comp Physiol 299: R1114– R1120, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Zhao D, Azar AS, Brooks VL. Diet-induced obesity in rats decreases insulin sensitivity and baroreflex gain (Abstract). FASEB J 23: 785 4, 2009 [Google Scholar]