Abstract
Sleep deprivation has been linked to hypertension, and recent evidence suggests that associations between short sleep duration and hypertension are stronger in women. In the present study we hypothesized that 24 h of total sleep deprivation (TSD) would elicit an augmented pressor and sympathetic neural response in women compared with men. Resting heart rate (HR), blood pressure (BP), and muscle sympathetic nerve activity (MSNA) were measured in 30 healthy subjects (age, 22 ± 1; 15 men and 15 women). Relations between spontaneous fluctuations of diastolic arterial pressure and MSNA were used to assess sympathetic baroreflex function. Subjects were studied twice, once after normal sleep and once after TSD (randomized, crossover design). TSD elicited similar increases in systolic, diastolic, and mean BP in men and women (time, P < 0.05; time × sex, P > 0.05). TSD reduced MSNA in men (25 ± 2 to 16 ± 3 bursts/100 heart beats; P = 0.02), but not women. TSD did not alter spontaneous sympathetic or cardiovagal baroreflex sensitivities in either sex. However, TSD shifted the spontaneous sympathetic baroreflex operating point downward and rightward in men only. TSD reduced testosterone in men, and these changes were correlated to changes in resting MSNA (r = 0.59; P = 0.04). Resting HR, respiratory rate, and estradiol were not altered by TSD in either sex. In conclusion, TSD-induced hypertension occurs in both sexes, but only men demonstrate altered resting MSNA. The sex differences in MSNA are associated with sex differences in sympathetic baroreflex function (i.e., operating point) and testosterone. These findings may help explain why associations between sleep deprivation and hypertension appear to be sex dependent.
Keywords: arterial blood pressure, microneurography, hypertension, autonomic activity
recent epidemiological studies demonstrate an association between sleep deprivation and hypertension (7, 12, 14). Moreover, a recent study examining the relations between sex, short sleep duration, and blood pressure reported that short durations of sleep were associated with hypertension in women, but not men (7). Therefore, evidence is accumulating to suggest that sleep deprivation contributes importantly to hypertension and that women may be at higher risk than men. Mechanisms underlying these potential sex differences remain unclear.
Increases in sympathetic neural activity have been suggested as a potential contributor to the increased blood pressure observed after sleep deprivation, but direct evidence is lacking. In fact, 24-h total sleep deprivation (TSD) has been reported to reduce resting muscle sympathetic nerve activity (MSNA) in humans (16). Kato et al. (16) reported an increase in blood pressure and decrease in MSNA after TSD in six men and two women. Ogawa et al. (19) followed up with similar findings in six men and attributed the increase in resting blood pressure to a resetting of the sympathetic arterial baroreflex. Thus our current knowledge of the relations between sleep deprivation, hypertension, and MSNA is limited to two studies of primarily men with no insight into potential sex differences. Given the reported associations between short sleep duration and hypertension in women (7), we hypothesized an augmented pressor response to TSD that would be associated with a potentiated MSNA response in women (i.e., an increase of MSNA compared with men). Because sleep deprivation has been shown to alter sex steroids (13), we also examined the potential interactions between TSD, sex steroids, and MSNA.
METHODS
Subjects
Thirty healthy subjects (15 men and 15 women) participated in the study. All subjects were nonsmokers and had no history of autonomic dysfunction, cardiovascular disease, asthma, or diabetes. All subjects were instructed to abstain from exercise, alcohol, and caffeine for 12 h before laboratory testing. All female subjects reported regular menstrual cycles (range, 26–30 days) and were tested during their early follicular phase to diminish potential confounding hormonal effects. Subjects could not participate if they were taking oral contraceptives or other hormonal supplementations. One female subject was excluded because her estradiol and progesterone levels indicated that she was not in her early follicular phase during one of the visits. Additionally, all subjects were screened for obstructive sleep apnea by a board certified sleep physician (J. DellaValla) using the at-home ApneaLink (ResMed, San Diego, CA). One male subject was excluded because his apnea-hypopnea index was ≥10 arbitrary units. Thus our final data set included 14 men and 14 women (Table 1). Testing procedures were explained to all subjects before obtaining written informed consent and were approved by the Michigan Technological University Institutional Review Board.
Table 1.
Subject characteristics and sex steroids
| Men |
Women |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Variable | NS | TSD | NS | TSD | Condition | Sex | Condition × Sex |
| Age, years | 22 ± 1 | – | 22 ± 1 | – | – | – | – |
| Height, cm | 176 ± 2 | – | 165 ± 2 | – | – | < 0.001 | – |
| Weight, kg | 79 ± 4 | 79 ± 4 | 62 ± 4 | 63 ± 3 | 0.65 | < 0.01 | 0.27 |
| STAI, arbitrary units | |||||||
| Raw | 28 ± 2 | 35 ± 2 | 27 ± 2 | 32 ± 2 | < 0.01 | 0.43 | 0.36 |
| Standard | 42 ± 2 | 49 ± 2 | 40 ± 2 | 44 ± 2 | < 0.01 | 0.18 | 0.21 |
| Percentile STAI, % | 26 ± 6 | 48 ± 7 | 21 ± 6 | 33 ± 7 | < 0.01 | 0.24 | 0.18 |
| Estradiol, pg/ml | 25 ± 2 | 20 ± 1 | 36 ± 8 | 29 ± 3 | 0.13 | 0.07 | 0.79 |
| Progesterone, ng/ml | 2.2 ± 0.2 | 1.8 ± 0.2* | 2.1 ± 0.2 | 1.3 ± 0.1† | < 0.01 | 0.20 | < 0.05 |
| Testosterone, ng/dl | 589 ± 67 | 480 ± 54† | 46 ± 2 | 44 ± 4 | < 0.01 | < 0.01 | 0.01 |
Values are means ± SE; n = 14 men and n = 14 women unless otherwise noted. NS, normal sleep; TSD, total sleep deprivation; STAI, state-trait anxiety inventory.
P < 0.05, NS vs. corresponding TSD;
P < 0.01, NS vs. corresponding TSD. Estradiol/progesterone/testosterone, n = 27 (n = 14 men and n = 13 women).
Experimental Design
Subjects were tested twice: once after 24-h TSD in the laboratory and once after normal sleep (NS) at their homes. Trial order (TSD vs. NS) was randomized, and all subjects were tested ∼1 mo apart to ensure female subjects were tested during their early follicular phase for both visits. Wrist actigraphy (Actiwatch-64; Respironics, Bend, OR) was used to monitor limb movement for a minimum of three consecutive nights immediately preceding each trial. All wrist actigraphy data were analyzed by a board certified sleep physician (J. DellaValla). In the minority of nights (∼10%), actigraphy data were unavailable and self-reported sleep diary data were used. The actigraphy/sleep diary data from the three nights preceding each autonomic test demonstrate that participants were getting adequate and similar sleep before the NS (7.3 ± 0.2 h in men and 7.2 ± 0.2 h in women) and TSD (7.6 ± 0.3 h in men and 7.4 ± 0.3 h in women) trials.
During the TSD trial, subjects were contacted at 7:30 AM the morning before the sleep deprivation night. Subjects were instructed to remain awake (i.e., no naps) and report to the laboratory at 11:00 PM where two research assistants supervised the subject throughout the remainder of the night to ensure they remained awake (continuous visual observation and periodic auditory confirmations). Participants stopped eating a minimum of 8 h before the start of laboratory testing. Participants were provided a controlled light breakfast (i.e., water and granola bar) during each testing day (NS and TSD) after the resting seated blood pressure measurements and blood draw were completed.
On each day of testing (NS vs. TSD), three seated resting blood pressures were taken at 7:30 AM after 5 min of quiet rest. After the blood pressure recordings, state-anxiety was measured using the State-Trait Anxiety Inventory (STAI) questionnaire for adults (28), and fasting venous blood samples were then obtained to determine levels of estradiol, progesterone, and testosterone. State-anxiety is shown in Table 1 as raw scores, as well as percentile and standard scores based on age and sex. Subjects were then situated in the supine position on a laboratory table for hemodynamic and neural instrumentation. After instrumentation and at least 5 min of nonrecorded rest (to confirm hemodynamic and neural stability), a 10-min baseline was recorded. Subjects were continuously monitored by study investigators to ensure they did not fall asleep during the experiment.
Measurements
Microneurography.
Multifiber recordings of MSNA were made by inserting a tungsten microelectrode (Frederick Haer, Bowdoinham, ME) into the peroneal nerve of the right leg. A reference electrode was inserted subcutaneously 2 to 3 cm from the microneurography electrode. Both electrodes were connected to a differential preamplifier and then to an amplifier (total gain of 80,000) where the nerve signal was band-pass filtered (700–2,000 Hz) and integrated (time constant, 0.1 s) to obtain a mean voltage display of nerve activity. Quality recordings of MSNA were defined by spontaneous, pulse synchronous bursts that increased during end-expiratory apnea and remained unchanged during auditory stimulation or stroking of the skin.
Blood pressure and heart rate.
Arterial blood pressures were obtained in both the seated position (described previously) and in the supine position immediately preceding the 10-min baseline. Both seated and supine resting arterial blood pressures were measured three consecutive times (separated by ∼1-min intervals) using an automated sphygmomanometer (Omron HEM-907XL; Omron Health Care). Beat-to-beat arterial blood pressure was recorded continuously throughout the 10-min baseline using the Finometer (Finapres Medical Systems, Amsterdam, The Netherlands) to allow spontaneous baroreflex assessment (described below). Three consecutive supine blood pressures were taken from the automated sphygmomanometer immediately preceding the 10-min baseline and were used to calibrate the Finometer to accurately depict absolute values. Arterial blood pressures are expressed as systolic arterial blood pressure (SAP), diastolic arterial blood pressure (DAP), and mean arterial blood pressure (MAP). Heart rate was recorded continuously via a three-lead electrocardiogram, and respiratory rate was continuously measured using a pneumobelt.
Data Analysis
MSNA.
Data were imported and analyzed in the WinCPRS software program (Absolute Aliens; Turku, Finland). R-waves were detected and marked in the time series. Muscle sympathetic nerve bursts were automatically detected on the basis of amplitude using a signal-to-noise ratio of 3:1, within a 0.5-s search window centered on a 1.3-s expected burst peak latency from the previous R-wave. Potential bursts were displayed and edited by one trained investigator. MSNA was expressed as burst frequency (in bursts/min) and burst incidence (in bursts/100 heart beats). Resting MSNA was successfully recorded during both NS and TSD conditions in 20 subjects (10 men and 10 women).
Spontaneous sympathetic baroreflex sensitivity analysis.
Sympathetic baroreflex function was determined using the spontaneous DAP-MSNA slope method, which examined the relations between spontaneous fluctuations in DAP and MSNA at rest (10, 29, 34). This analysis has been described in detail in previous studies (8, 17). Briefly, DAPs for each cardiac cycle were grouped into 3-mmHg intervals (bins) during baseline. Burst incidence for each DAP bin was calculated and plotted against the corresponding DAP. The slopes of these relationships were evaluated using linear regression analysis. All linear regression analyses were weighted for the number of cardiac cycles within each DAP bin. A minimum r value of 0.40 was set as the criteria for inclusion. As a result, one male and one female were excluded from the slope analysis (r ≤ 0.30). The mean coefficient values were ≥0.70 for the remaining nine men and nine women. Operating points for each regression line were determined as the mean value of burst incidence versus the mean DAP for the corresponding baseline period (n = 18 as in the spontaneous slope analysis).
Spontaneous cardiovagal baroreflex sensitivity analysis.
Spontaneous cardiovagal baroreflex sensitivity was determined from beat-to-beat changes in R-R interval and SAP (sequence method) as originally reported by Bertinieri et al. (5) and modified by Blaber et al. (6). Briefly, three or more beats relating to R-R intervals and progressive, spontaneous changes of SAP (lag 1) were identified as baroreflex sequences. We recorded both up-up sequences (progressive increases of SAP followed by a lengthening of the R-R interval) and down-down sequences (progressive decreases of SAP with a subsequent shortening of the R-R interval). Minimum criteria were set at 1 mmHg for SAP and 4-ms for R-R interval. Linear regression analysis was used to determine the slope of the linear relationship between the R-R intervals and SAP for each sequence. Up-up or down-down sequences within each 10-min baseline were averaged for each subject. Only sequences with linear r values >0.7 were accepted, resulting in 12 men and 10 women for up-up sequence analysis and 13 men and 12 women for down-down sequence analysis (Table 2).
Table 2.
Hemodynamic and neural responses to sleep deprivation
| Men |
Women |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Variable | NS | TSD | NS | TSD | Condition | Sex | Condition × Sex |
| Seated arterial blood pressure, mmHg | |||||||
| Systolic | 109 ± 2 | 113 ± 2 | 99 ± 3 | 102 ± 2 | 0.02 | < 0.01 | 0.35 |
| Diastolic | 64 ± 2 | 68 ± 2 | 65 ± 2 | 67 ± 2 | 0.04 | 0.40 | 0.22 |
| Mean | 79 ± 2 | 83 ± 1 | 76 ± 2 | 78 ± 2 | 0.03 | < 0.05 | 0.25 |
| Supine arterial blood pressure, mmHg | |||||||
| Systolic | 109 ± 2 | 115 ± 2 | 95 ± 2 | 97 ± 2 | 0.02 | < 0.01 | 0.05 |
| Diastolic | 58 ± 2 | 61 ± 1 | 55 ± 1 | 59 ± 2 | < 0.01 | 0.12 | 0.20 |
| Mean | 75 ± 1 | 79 ± 1 | 68 ± 1 | 72 ± 2 | < 0.01 | < 0.01 | 0.35 |
| Heart rate, beats/min | 59 ± 2 | 60 ± 2 | 62 ± 2 | 60 ± 2 | 0.37 | 0.66 | 0.13 |
| MSNA burst frequency, bursts/min | 15 ± 2 | 10 ± 2* | 8 ± 1 | 10 ± 2 | 0.17 | 0.11 | 0.03 |
| MSNA burst incidence, bursts/100 heart beats | 25 ± 2 | 16 ± 3* | 14 ± 2 | 17 ± 3 | 0.16 | 0.11 | 0.02 |
| sympBRS, burst incidence/mmHg | −1.4 ± 0.2 | −1.3 ± 0.2 | −1.1 ± 0.2 | −1.4 ± 0.2 | 0.63 | 0.73 | 0.36 |
| cvBRS up-up, ms/mmHg | 23 ± 3 | 23 ± 2 | 25 ± 4 | 25 ± 3 | 0.92 | 0.58 | 0.97 |
| cvBRS d-d, ms/mmHg | 20 ± 2 | 22 ± 1 | 26 ± 4 | 24 ± 1 | 0.85 | 0.11 | 0.35 |
| Respiration, breaths/min | 15 ± 1 | 15 ± 1 | 16 ± 0 | 16 ± 1 | 0.25 | 0.37 | 0.23 |
Values are means ± SE; n = 14 men and n = 14 women unless otherwise noted. MSNA, muscle sympathetic nerve activity; sympBRS, spontaneous sympathetic baroreflex sensitivity; cvBRS up-up, spontaneous cardiovagal baroreflex sensitivity for up-up sequences; cvBRS d-d, spontaneous cardiovagal baroreflex sensitivity for down-down sequences.
P < 0.05, NS vs. corresponding TSD. MSNA, n = 20 (10 men and 10 women); sympBRS, n = 18 (9 men and 9 women); cvBRS up-up, n = 22 (12 men and 10 women); cvBRS d-d, n = 25 (13 men and 12 women).
Statistical Analysis
All data were analyzed statistically using commercial software (SPSS 18.0; SPSS, Chicago, IL). We used repeated-measures ANOVA with condition (NS vs. TSD) as the within-subjects factor and sex (men vs. women) as the between-subjects factor. Post hoc analysis was performed when significant condition × sex interactions were detected. Pearson correlations were used to examine the relations between MSNA and sex steroids (i.e., estradiol, progesterone, and testosterone). Results are expressed as means ± SE. Means were considered significantly different at P < 0.05.
RESULTS
Table 1 depicts subject characteristics and mean values for state-anxiety and sex steroids following NS and TSD. TSD elicited similar increases in state-anxiety in men and women (condition, P < 0.05; condition × sex, P ≥ 0.18). TSD significantly reduced testosterone in men, but not women (condition × sex, P = 0.01). Progesterone levels were reduced by TSD in both men and women, although decreases were more dramatic in women (condition × sex, P < 0.05). Estradiol was not significantly altered by TSD in either sex.
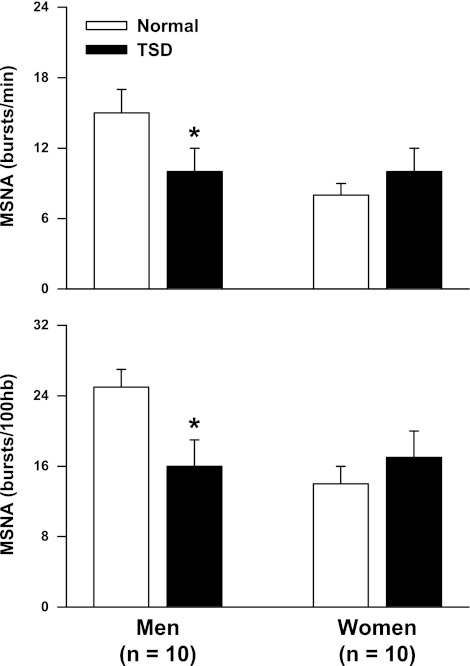
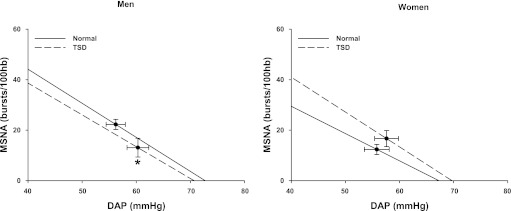
Table 2 compares the hemodynamic and neural responses to TSD in men and women. TSD elicited similar increases in SAP, DAP, and MAP in men and women (condition, P < 0.05 for all). This hypertensive response was observed regardless of body position (i.e., seated vs. supine). TSD did not alter resting heart rate in either sex. Figure 1 demonstrates that TSD decreased MSNA in men, but not women, when expressed as both burst frequency and burst incidence. TSD did not alter spontaneous sympathetic baroreflex sensitivity as determined by the spontaneous DAP-MSNA linear regression analysis in either sex (Table 2). However, TSD significantly reduced the operating point in men, but not women (condition × sex, P < 0.05). This downward, rightward shift of the spontaneous sympathetic baroreflex operating point in men is illustrated in Fig. 2. Importantly, blood pressure responses to TSD in the subjects with complete MSNA recordings (n = 20) revealed similar results to the blood pressure responses in the 28 subjects reported in Table 2. Specifically, TSD increased supine DAP in the men (56 ± 2 to 60 ± 2 mmHg) and women (55 ± 2 to 59 ± 2 mmHg), and these increases were not different between sexes (condition, P = 0.001; condition × time, P = 0.827). Table 2 also demonstrates that TSD did not alter spontaneous cardiovagal baroreflex sensitivities in either sex.
Fig. 1.
Muscle sympathetic nerve activity (MSNA) after a normal night of sleep (NS) and 24 h of total sleep deprivation (TSD) in men and women. TSD decreased MSNA burst frequency and burst incidence in men, but did not alter MSNA in women (condition × sex interactions; P < 0.05). *P < 0.05 vs. corresponding NS.
Fig. 2.
Mean linear regression lines and operating points (·) for MSNA burst incidence and diastolic arterial blood pressure. TSD altered the operating point in men (downward, rightward shift), but not women. TSD did not alter the burst incidence slopes, an index of sympathetic baroreflex sensitivity, in either sex. Burst incidence slopes and respective operating points were determined from 9 men and 9 women. *P < 0.05 vs. corresponding operating point.
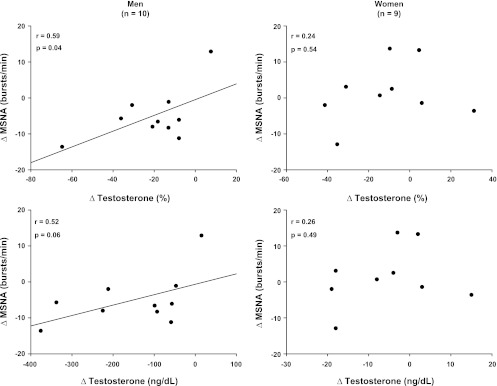
Figure 3 depicts the relations between changes in testosterone and MSNA in response to TSD in men and women. Changes in testosterone were correlated to changes in MSNA in men, but not women. Changes in progesterone and estradiol were not correlated to changes in MSNA in either sex.
Fig. 3.
Correlations of MSNA and testosterone in men and women. Changes in MSNA were correlated with changes in testosterone in men, but not women.
DISCUSSION
The present study examined the influence of TSD on neural cardiovascular control in men and women. Our results support previous findings that TSD increases resting blood pressure (16, 19) and state-anxiety (2, 26, 33), and we extend these previous studies by demonstrating that the TSD pressor responses subsist in both men and women. More important, the present study introduces three new and novel findings. First, MSNA responses to TSD are sex dependent. Specifically, TSD decreased MSNA in men, but did not alter MSNA in women. Second, TSD did not alter spontaneous sympathetic and cardiovagal baroreflex sensitivities in either sex. However, TSD elicited a rightward, downward shift of the sympathetic baroreflex operating point in men, but not women. Third, TSD reduced testosterone in men, and these changes were associated with changes in resting MSNA. Collectively, our findings suggest that mechanisms underlying the acute hypertensive response to TSD differ in men and women and that both neural (i.e., spontaneous sympathetic baroreflex) and non-neural (i.e., testosterone) mechanisms may be underlying these sex differences.
The effects of TSD on arterial blood pressure and catecholamines are inconsistent. Several studies report increases in arterial blood pressure following TSD (16, 19, 25, 27), whereas others have demonstrated no change (1, 22, 32, 37). Likewise, multiple studies report an increase in plasma or urine catecholamines after TSD (4, 18, 27, 31), whereas others report no change (1, 11, 16, 19, 23). Studies have also measured heart rate variability as an index of autonomic function in sleep-deprived subjects and reported an increase in sympathetic cardiac modulation (27, 37). An underlying theme of these prior studies is the lack of female participants and/or emphasis on sex differences. Moreover, the use of catecholamine levels and heart rate variability as indices of autonomic function are not as direct as post-ganglionic sympathetic nerve activity (i.e., MSNA).
To date, only two studies have examined resting MSNA following TSD in humans (16, 19). Kato et al. (16) reported that TSD increased blood pressure and decreased MSNA in six men and two women. Similarly, Ogawa et al. (19) demonstrated an increase in blood pressure and decrease of MSNA following TSD in six men. Thus to date our understanding of the effects of sleep deprivation on MSNA is limited to two studies with a total of 12 men and two women. The aim of the present study was to comprehensively examine neural cardiovascular responses to TSD in men and women. Our findings support the existing concept that TSD reduces MSNA in men. However, TSD reductions of MSNA were not observed in women, despite a similar pressor response to that of the men.
Ogawa et al. (19) attributed the TSD reduction of MSNA in men to a resetting of the sympathetic baroreflex. The authors support this claim by reporting a significantly elevated set point as determined by the x-intercept of the spontaneous DAP-MSNA linear regression line (19). However, the x-intercept is strongly influenced by slope; thus we used the DAP-MSNA linear regression slope in conjunction with resting DAP (via the automated sphygmomanometer) and resting MSNA to determine the operating point of the spontaneous sympathetic baroreflex (20). We demonstrate that TSD did not alter spontaneous sympathetic baroreflex sensitivity in men or women, but did significantly alter the spontaneous sympathetic baroreflex operating point in men. Specifically, the operating point was shifted rightward and downward in men, but not women. We believe the response exhibited by the men was an appropriate response given the rise in arterial blood pressure during TSD. In other words, the baroreflex detected increases in arterial pressure and consequently reduced MSNA. Women, on the other hand, demonstrated a significant increase in arterial blood pressure similar to the men, but the acute hypertensive response was not accompanied by a concurrent decrease of MSNA. We conclude that TSD was associated with some degree of sympathetic baroreflex dysfunction in women compared with men. We recognize that this assumes that the baroreflex is primarily driving MSNA responses, as opposed to MSNA driving arterial blood pressure. In reality, a dynamic interaction exists between arterial blood pressure, the arterial baroreflex, and MSNA, in which MSNA can either drive or respond to changes in arterial blood pressure. Moreover, nonbaroreflex factors are known to contribute importantly to the control of resting MSNA (21). Regardless, the novelty of the present study is that men and women respond differently regarding MSNA responses to TSD, as well as DAP-MSNA relations. Moreover, these findings are consistent with recent evidence that men and women have different strategies for regulating arterial blood pressure and MSNA (15). Such findings might help explain why women appear to be more susceptible to developing hypertension as a result of short sleep duration (7).
TSD has repeatedly been shown to significantly decrease testosterone levels in male rats and humans (3, 13, 36). The decreased testosterone levels in our study were correlated to reductions of MSNA in men (Fig. 3). To our knowledge, only one microneurographic study has examined the relations between MSNA and testosterone. Specifically, women with polycystic ovary syndrome demonstrated increased levels of MSNA that were positively correlated to testosterone (30). The authors suggest that testosterone modulated MSNA in women with polycystic ovary syndrome and is an important contributor to the sex difference in sympathetic outflow observed in the normal population. Our results reveal for the first time that the positive relation between changes of MSNA and testosterone observed in young women may also be important in young men. Thus, in addition to the role of the spontaneous arterial baroreflex in MSNA control during TSD discussed previously, we conclude that reductions of testosterone may also play a key role in reducing resting MSNA after TSD in men.
If increases in MSNA are not driving the TSD-induced hypertension, what is? Perry et al. (24) recently reported that paradoxical sleep deprivation in Wistar-Hannover male rats resulted in selective alterations of sympathetic nerve activity. Specifically, both 24-h and 96-h paradoxical sleep deprivation increased renal sympathetic nerve activity but did not alter splanchnic sympathetic nerve activity (24). This preferential sympathoexcitation to the kidney was also associated with reduced plasma angiotensin II concentrations (24). Moreover, Charloux et al. (9) has demonstrated that TSD modifies the 24-h profile of aldosterone. The microneurography technique used in the present study was specific to the muscular bed of the lower leg. Wallin and colleagues (35) have reported a strong positive correlation between MSNA and renal norepinephrine spillover at rest, but it is unclear whether this strong relationship persists during interventions such as TSD. Based on the recent findings of Perry et al. (24), it may be warranted to examine the influence of TSD on renal norepinephrine spillover in humans.
Numerous studies have noted an association between anxiety and abnormal sleeping patterns; however, relatively few have examined how TSD influences state-anxiety (2, 26, 33). These previous studies all report that TSD increases state-anxiety, but no studies have focused on potential sex differences. The present study supports previous conclusions that TSD significantly increases state-anxiety, and our results extend these findings by demonstrating that TSD increases state-anxiety to a similar extent in men and women. Despite sex differences in both neural and hormonal responses to TSD, our findings suggest that self-perceived state-anxiety responses to TSD are not sex dependent.
In conclusion, TSD increases resting arterial blood pressure and state-anxiety to a similar extent in both men and women. In contrast, MSNA decreased in men following TSD, but not women. The divergent MSNA responses appear to be related, in part, to alterations in spontaneous sympathetic baroreflex function and/or testosterone responses to TSD. Such findings may help to explain why relations between sleep deprivation and hypertension are stronger in women (7) and may provide important mechanistic insight to better understand sex-specific risks for hypertension.
GRANTS
This project was supported by the National Heart, Lung, and Blood Institute Grant HL-098676.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.R.C. and J.P.D. conception and design of research; J.R.C., J.J.D., R.A.L., and H.Y. performed experiments; J.R.C., J.J.D., R.A.L., J.P.D., and H.Y. analyzed data; J.R.C., J.J.D., R.A.L., J.P.D., and H.Y. interpreted results of experiments; J.R.C. and J.J.D. drafted manuscript; J.R.C., J.J.D., R.A.L., J.P.D., and H.Y. edited and revised manuscript; J.R.C., J.J.D., R.A.L., J.P.D., and H.Y. approved final version of manuscript; J.J.D., R.A.L., and H.Y. prepared figures.
ACKNOWLEDGMENTS
We thank Thomas Drummer, Christopher Schwartz, Michelle King, and Kristen Reed for technical assistance. We also thank all the subjects for participation.
REFERENCES
- 1.Acheson A, Richards JB, de Wit H. Effects of sleep deprivation on impulsive behaviors in men and women. Physiol Behav 91: 579–587, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Babson KA, Trainor CD, Feldner MT, Blumenthal H. A test of the effects of acute sleep deprivation on general and specific self-reported anxiety and depressive symptoms: an experimental extension. J Behav Ther Exp Psychiatry 41: 297–303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgartner A, Graf KJ, Kurten I, Meinhold H, Scholz P. Neuroendocrinological investigations during sleep deprivation in depression. I. Early morning levels of thyrotropin, TH, cortisol, prolactin, LH, FSH, estradiol, and testosterone. Biol Psychiatry 28: 556–568, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Benedict C, Hallschmid M, Lassen A, Mahnke C, Schultes B, Schioth HB, Born J, Lange T. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr 93: 1229–1236, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol Heart Circ Physiol 254: H377–H383, 1988 [DOI] [PubMed] [Google Scholar]
- 6.Blaber AP, Yamamoto Y, Hughson RL. Methodology of spontaneous baroreflex relationship assessed by surrogate data analysis. Am J Physiol Heart Circ Physiol 268: H1682–H1687, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, Ferrie JE, Shipley MJ, Brunner EJ, Marmot MG. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension 50: 693–700, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter JR, Lawrence JE, Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J Physiol Endocrinol Metab 297: E85–E91, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charloux A, Gronfier C, Chapotot F, Ehrhart J, Piquard F, Brandenberger G. Sleep deprivation blunts the night time increase in aldosterone release in humans. J Sleep Res 10: 27–33, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 84: 65–81, 1972 [DOI] [PubMed] [Google Scholar]
- 11.Fiorica V, Higgins EA, Iampietro PF, Lategola MT, Davis AW. Physiological responses of men during sleep deprivation. J Appl Physiol 24: 167–176, 1968 [DOI] [PubMed] [Google Scholar]
- 12.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension 47: 833–839, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Santos MR, Gaja-Rodriguez OV, Alonso-Uriarte R, Sojo-Aranda I, Cortes-Gallegos V. Sleep deprivation and adaptive hormonal responses of healthy men. Arch Androl 22: 203–207, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep 29: 1009–1014, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Joyner MJ, Charkoudian N, Wallin BG. Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension 56: 10–16, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension 35: 1173–1175, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muslce sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol 573: 445–451, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am J Hypertens 12: 63–68, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Ogawa Y, Kanbayashi T, Saito Y, Takahashi Y, Kitajima T, Takahashi K, Hishikawa Y, Shimizu T. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique. Sleep 26: 986–989, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Ogoh S, Fisher JP, Raven PB, Fadel PJ. Arterial baroreflex control of muscle sympathetic nerve activity in the transition from rest to steady-state dynamic exercise in humans. Am J Physiol Heart Circ Physiol 293: H2202–H2209, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Osborn JW, Jacob F, Guzman P. A neural set point for the long-term control of arterial pressure: beyond the arterial baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 288: R846–R855, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Pagani M, Pizzinelli P, Traon AP, Ferreri C, Beltrami S, Bareille MP, Costes-Salon MC, Beroud S, Blin O, Lucini D, Philip P. Hemodynamic, autonomic and baroreflex changes after one night sleep deprivation in healthy volunteers. Auton Neurosci 145: 76–80, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Palmblad J, Akerstedt T, Froberg J, Melander A, von Schenck H. Thyroid and adrenomedullary reactions during sleep deprivation. Acta Endocrinol (Copenh) 90: 233–239, 1979 [DOI] [PubMed] [Google Scholar]
- 24.Perry JC, Bergamaschi CT, Campos RR, Andersen ML, Montano N, Casarini DE, Tufik S. Sympathetic and angiotensinergic responses mediated by paradoxical sleep loss in rats. J Renin Angiotensin Aldosterone Syst 12: 146–152, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Robillard R, Lanfranchi PA, Prince F, Filipini D, Carrier J. Sleep deprivation increases blood pressure in healthy normotensive elderly and attenuates the blood pressure response to orthostatic challenge. Sleep 34: 335–339, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sagaspe P, Sanchez-Ortuno M, Charles A, Taillard J, Valtat C, Bioulac B, Philip P. Effects of sleep deprivation on color-word, emotional, and specific stroop interference and on self-reported anxiety. Brain Cogn 60: 76–87, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Sauvet F, Leftheriotis G, Gomez-Merino D, Langrume C, Drogou C, Van Beers P, Bourrilhon C, Florence G, Chennaoui M. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol 108: 68–75, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, 1983 [Google Scholar]
- 29.Sundlof G, Wallin B. Human muscle nerve sympathetic activity at rest. J Physiol 274: 621–637, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sverrisdottir YB, Mogren T, Kataoka J, Janson PO, Stener-Victorin E. Is polycystic ovary syndrome associated with high sympathetic nerve activity and size at birth? Am J Physiol Endocrinol Metab 294: E576–E581, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Tochikubo O, Ikeda A, Miyajima E, Ishii M. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension 27: 1318–1324, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Vaara J, Kyrolainen H, Koivu M, Tulppo M, Finni T. The effect of 60-h sleep deprivation on cardiovascular regulation and body temperature. Eur J Appl Physiol 105: 439–444, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Vardar SA, Ozturk L, Kurt C, Bulut E, Sut N, Vardar E. Sleep deprivation induced anxiety and anaerobic performance. J Sport Sci Med 6: 532–537, 2007 [PMC free article] [PubMed] [Google Scholar]
- 34.Wallin BG, Eckberg DL. Sympathetic transients caused by abrupt alterations of carotid baroreceptor activity in humans. Am J Physiol Heart Circ Physiol 242: H185–H190, 1982 [DOI] [PubMed] [Google Scholar]
- 35.Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol 491: 881–887, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu JL, Wu RS, Yang JG, Huang CC, Chen KB, Fang KH, Tsai HD. Effects of sleep deprivation on serum testosterone concentrations in the rat. Neurosci Lett 494: 124–129, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Zhong X, Hilton HJ, Gates GJ, Jelic S, Stern Y, Bartels MN, Demeersman RE, Basner RC. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol 98: 2024–2032, 2005 [DOI] [PubMed] [Google Scholar]