Abstract
The present study was conducted to determine the impact of suppressing trophoblast remodeling of the uterine spiral arteries by prematurely elevating estrogen levels in the first trimester of baboon pregnancy on uterine and umbilical blood flow dynamics. Uteroplacental blood flow was assessed by Doppler ultrasonography after acute administration of saline (basal state) and serotonin on days 60, 100, and 160 of gestation (term: 184 days) to baboons in which uterine spiral artery remodeling had been suppressed by the administration of estradiol on days 25–59 of gestation. Maternal blood pressure in the basal state was increased (P < 0.01), and uterine artery diastolic notching and the umbilical artery pulsatility index and systolic-to-diastolic ratio, reflecting downstream flow impedance, were increased (P < 0.01) after serotonin administration on day 160, but not earlier, in baboons treated with estradiol in early gestation. These changes in uteroplacental flow dynamics in serotonin-infused, estradiol-treated animals were accompanied by a decrease (P < 0.05) in uterine and umbilical artery volume flow and fetal bradycardia. The results of this study show that suppression of uterine artery remodeling by advancing the rise in estrogen from the second trimester to the first trimester disrupted uteroplacental blood flow dynamics and fetal homeostasis after vasochallenge late in primate pregnancy.
Keywords: placenta, fetus, hemodynamics, Doppler, ultrasound
during early human and nonhuman pregnancy, extravillous placental cytotrophoblasts migrate to, invade, and replace the endothelial and smooth muscle wall of the uterine spiral arteries (16, 21, 45, 46). As a result, the uterine spiral arteries are physiologically transformed from high-resistance, low-capacity to low-resistance, high-capacity vessels. Remodeling of the uterine arteries is thought, but has not been definitively established, to be important in promoting uteroplacental blood flow critical to fetal development. Indeed, defective uterine vessel remodeling appears to underpin clinical complications of human pregnancy, e.g., preeclampsia and fetal growth restriction (11, 12, 49, 57). In contrast, extensive restructuring of the uterine arteries conceivably would impair vasoregulatory processes, e.g., uterine artery vasoconstriction, within the endometrial basalis after delivery. Despite the fundamental importance of uterine vessel transformation, relatively little is known about the regulation of this process.
Using the baboon as a translational model, we have shown that advancing the physiological increase in estrogen from the second trimester to the first trimester markedly suppressed extravillous trophoblast invasion and remodeling of the uterine spiral arteries (4) and extravillous trophoblast mRNA levels of VEGF (9), which is thought to promote trophoblast migration and vessel invasion (6, 69). We have proposed, therefore, that the low level of estrogen in early gestation promotes trophoblast migration and vessel invasion, while the increase in estrogen of advancing pregnancy has a physiologically important role in controlling the extent to which the uterine arteries are remodeled during normal pregnancy. The impact of the estrogen-induced suppression of uterine artery transformation on uteroplacental blood flow and fetal development is unknown. However, the latter experimental paradigm in our nonhuman primate model provides a novel approach under controlled conditions to determine this important aspect of perinatal biology. It is hypothesized, therefore, that suppression of uterine spiral artery transformation by advancing the rise in estrogen from the second trimester to the first trimester of baboon pregnancy will disrupt uteroplacental blood flow dynamics and fetal homeostasis late in gestation. To test this hypothesis in the present study, uterine and umbilical blood flow dynamics were assessed by ultrasonography before and after vasochallenge with serotonin [5-hydroxytryptamine (5-HT)], a monamine vasoconstrictor that crosses the placenta (7, 56) and has been shown in other laboratory animals to be a potent constrictor of the uterine and umbilical arteries (29, 30, 66), at early, mid, and late gestation of baboons in which trophoblast invasion and remodeling of the uterine spiral arteries had been suppressed by prematurely elevating the levels of estrogen in early pregnancy.
MATERIALS AND METHODS
Animals.
Adult female baboons (Papio anubis) weighing 14–16 kg and obtained from the Southwest National Primate Research Center (San Antonio, TX) were housed individually in large primate cages in air-conditioned rooms with a 12:12-h light-dark lighting schedule. Baboons received standard primate chow (Teklad-Harlan, St. Louis, MO) and fresh fruit twice daily, vitamins daily, and water ad libitum. Females were paired with male baboons for 5 days at the anticipated time of ovulation, as estimated by menstrual cycle history and the daily pattern of perineal turgescence, and pregnancy was confirmed by palpation and ultrasonography. Day 1 of pregnancy was designated as the day preceding deturgescence and represented the day after ovulation. Animals were cared for and used strictly in accordance with United States Department of Agriculture regulations and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH, 1996). The experimental protocol used in this study was approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
Six pregnant baboons were treated daily on days 25–59 of gestation (term: 184 days) with estradiol benzoate [0.35 mg/day via a subcutaneous injection in 1.0 ml sesame oil; early estradiol (EE) treatment], and five baboons were untreated. A 2- to 3-ml blood sample was obtained from a maternal peripheral saphenous vein every 1–3 days between days 25 and 59 and on days 60, 100, and 160 of gestation after brief restraint and sedation with ketamine HCl (10 mg/kg body wt im) for the quantification of serum estradiol levels by RIA (3). At 10:00 hours on days 60, 100, and 160 of gestation, baboons were sedated with ketamine, endotracheally intubated, anesthetized with isoflurane, and sequentially administered via a catheter inserted into a maternal saphenous vein a constant infusion with a syringe pump (Harvard Apparatus, Dover, MA) of 0.9% normal saline (0.5 ml/min × 20 min, defined as the basal state) and then 5-HT (Sigma Life Sciences, St. Louis, MO) dissolved in saline (0.5 ml/min) at 4 μg·min−1·kg maternal body wt−1 × 20 min and then 8 μg·min−1·kg maternal body wt−1 × 20 min.
Throughout each saline and 5-HT 20-min infusion interval, multiple (i.e., n = 7) maternal blood pressure and heart rate measurements were made using a Dinamap Pro 400 V2 (GE Medical Systems, Milwaukee, WI), and the values were averaged. Mean arterial blood pressure was determined by the programmed algorithm from the calibrated Dinamap Pro 400 V2 blood pressure monitor. Uterine and fetal blood flow dynamics and fetal heart rate were assessed by ultrasonography as described below. On day 160 of gestation, immediately after Doppler analyses, fetuses were delivered by cesarean section, and blood samples (5 ml each) were obtained from a maternal saphenous vein and umbilical artery (i.e., fetal) to assess serum chemistry analytes (Antech Diagnostics, Lake Success, NY).
Doppler ultrasonography.
Uterine and umbilical artery blood flow indexes were determined by pulsed-color Doppler ultrasonography/velocimetry using a 7-MHz sector-transducer and an Acuson Ultrasound (model 128 XP/10, Acuson, Mountain View, CA) with a high-pass filter at 100 Hz to separate vessel wall vibrations from flow velocity waveforms, as previously described (2). Right and left uterine arteries were identified with the transducer placed medial of the anterior iliac spines and directed slightly toward the pelvis and by visualization of the artificial crossing of the uterine and external iliac arteries (8, 10, 14). Because Doppler flow values were similar in right and left uterine arteries, the results were averaged. Doppler measurements of umbilical artery blood flow were obtained at the placental and fetal attachment sites, and respective values were averaged.
Color Doppler imaging was used to optimize placement of the pulsed wave gate at maximal color brightness by adjusting the velocity scale to identify the area and direction of maximum blood flow. The insonation angle was usually <30°, and the sample volume was adjusted to cover the entire vessel and thus allow for optimal color resolution, sensitivity, and frame rate. The pulsatility index (PI), calculated as peak systolic frequency − end-diastolic frequency ÷ mean, was measured as an index of downstream flow impedance (20, 25). Uterine and umbilical artery end-diastolic flow, the presence/absence of uterine artery diastolic notching, and the umbilical artery systolic flow-to-end-diastolic flow ratio (S/D ratio) were also assessed as indexes of downstream impedance to flow. Uterine and umbilical artery volume flow were quantified according to the following formula: QA (in ml/min) = V (in cm/s) × πr2 × 60 s/min, where QA is the arterial flow, V is the time-averaged mean velocity, and r is the radius of the vessel. Three Doppler flow and fetal heart rate measurements were obtained throughout each 20-min infusion interval during periods of fetal rest and apnea, and the results were averaged.
Statistical analysis.
Data on Doppler PIs, volume flows, S/D ratios, maternal and fetal blood pressures and heart rates, and serum estradiol levels were analyzed by mixed-model repeated-measures regression (32), with time-dependent covariates adjusted for within-animal correlation across days of gestation and treatment effects. Gestational age was entered as a continuous variable to test for linear trend. Post hoc analysis and inferences concerning the covariance parameters used χ2-statistic for comparisons (SAS 9.1, Cary, NC). The incidence of uterine artery diastolic notching, as assessed by Doppler, was analyzed by a χ2-test and post hoc Fisher's exact test. Fetal weights and serum chemistry analytes were analyzed by Student's t-test.
RESULTS
Serum estradiol.
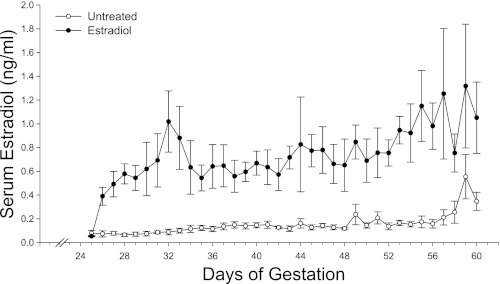
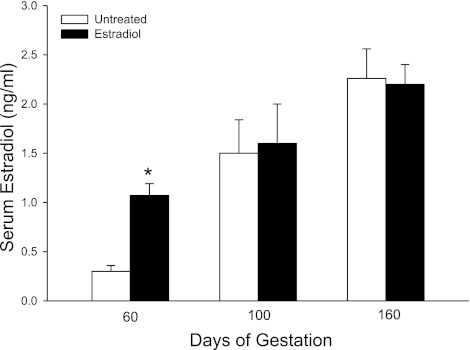
Maternal serum estradiol levels in untreated baboons were low (range of 0.10–0.15 ng/ml) between days 25 and 56 days of gestation and then increased slightly to ∼0.50 ng/ml on days 57–60 (i.e., at the end of the first trimester; Fig. 1). The daily administration of estradiol benzoate on days 25–59, i.e., EE treatment, increased maternal serum estradiol concentrations during this period to a mean ± SE value of 0.75 ± 0.03 ng/ml, a value fivefold greater (P < 0.001) than in untreated animals (0.14 ± 0.01 ng/ml; Fig. 1). In untreated baboons, serum estradiol levels exhibited a striking increase (P < 0.01) during the second trimester from 0.35 ± 0.07 ng/ml on day 60 to 1.50 ± 0.34 ng/ml on day 100 and to 2.26 ± 0.30 ng/ml on day 160 of gestation (Fig. 2). Serum estradiol on day 60 in EE-treated animals was elevated to a level (1.05 ± 0.30 ng/ml) similar to that normally achieved on day 100 in untreated animals (Fig. 2). However, estradiol concentrations on days 100 and 160 were similar in untreated and EE-treated baboons.
Fig. 1.
Maternal peripheral serum estradiol levels (means ± SE) in baboons untreated (n = 5) or treated daily on days 25–59 of gestation (term: 184 days) with estradiol benzoate (0.35 mg/day sc, n = 6). Serum estradiol levels on each of the days between days 27 and 60 were greater (P < 0.001) in estradiol-treated animals than in untreated animals.
Fig. 2.
Maternal serum estradiol levels on days 60, 100, and 160 in baboons untreated (n = 5) or treated daily on days 25–59 of gestation with estradiol (n = 6). *P < 0.01 vs. untreated animals on day 60.
Maternal blood pressure and maternal and fetal heart rate.
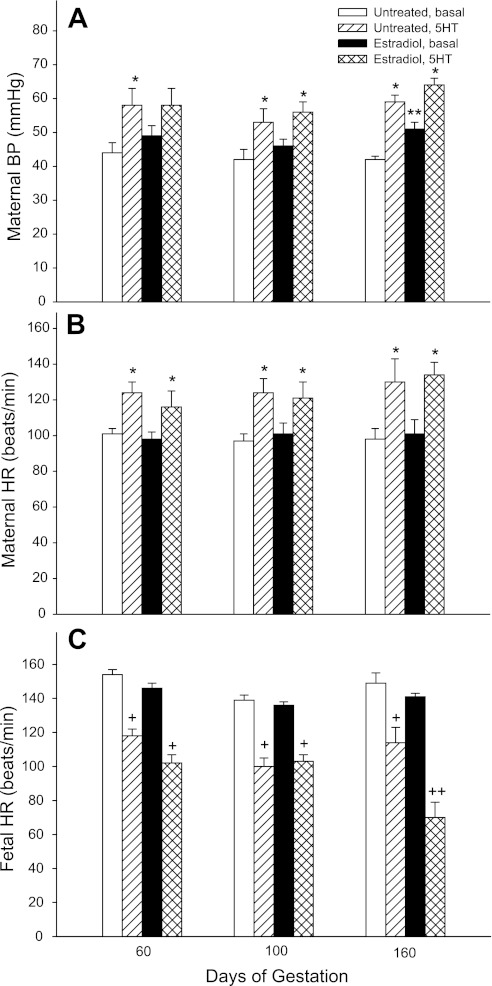
Maternal mean arterial blood pressure and heart rate after saline infusion (i.e., basal state) were similar on days 60, 100, and 160 of gestation in untreated baboons (Fig. 3). However, maternal blood pressure in the basal condition on day 160 was over 20% higher (P < 0.01) in EE-treated baboons (51.3 ± 1.9 mmHg) than in untreated baboons (42.3 ± 1.2 mmHg). Within 1–2 min of the onset of administration of 5-HT at both 4 (data not shown) and 8 μg·min−1·kg body wt−1 in untreated and EE-treated baboons at early, mid, and late gestation, maternal blood pressure (Fig. 3A) and heart rate (Fig. 3B) increased and plateaued to levels that were ∼25% greater (P < 0.05) than the respective values in the basal state.
Fig. 3.
Maternal mean arterial blood pressure (BP) and maternal and fetal heart rates (HR) during constant 20 min intravenous infusion of saline (i.e., basal state) and of serotonin [5-hydroxytryptamine (5-HT); 8 μg·min−1·kg body wt−1] to baboons untreated (n = 5) or treated with estradiol on days 25–59 of gestation (n = 6). *P < 0.05 vs. respective basal maternal BP and HR values; **P < 0.01 vs. the untreated basal maternal BP value; +P < 0.01 vs. the respective basal fetal HR value; ++P < 0.001 vs. untreated 5-HT-infused and estradiol-treated basal fetal HR values.
Fetal heart rate (in beats/min) was similar on days 60, 100, and 160 of gestation in untreated or EE-treated baboons (Fig. 3C). However, compared with the basal state, fetal heart rate was decreased (P < 0.01) within 2–3 min in a dose-dependent manner by the administration of 5-HT to untreated and EE-treated baboons at early, mid, and late gestation. Thus, on day 160 of gestation, fetal heart rate declined (P < 0.01) and plateaued throughout the 20-min infusion of 5-HT at the 0-μg dose (154 ± 4 and 141 ± 2 beats/min in untreated and EE-treated animals, respectively), 4-μg dose (125 ± 6 and 99 ± 6 beats/min in untreated and EE-treated animals, respectively), and 8-μg dose (120 ± 8 and 70 ± 9 beats/min in untreated and EE-treated animals, respectively). Statistical analysis showed an interaction between group and 5-HT treatment on day 160 but not on days 60 and 100. Thus, fetal heart rate in EE baboons on day 160 after 5-HT challenge at the 4- and 8-μg doses was 30% and 50% lower (P < 0.001), respectively, than before challenge and 20–30% lower (P < 0.03) than in untreated 5-HT-challenged animals (Fig. 3).
Uterine artery blood flow.
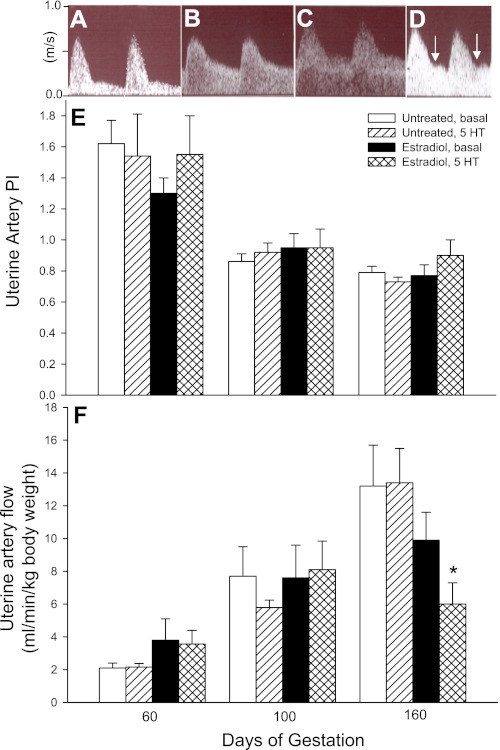
Figure 4 shows representative uterine artery waveforms as assessed by Doppler ultrasonography on day 60 (Fig. 4A) and day 160 (Fig. 4B) of gestation in untreated baboons after saline infusion (i.e., basal) and on day 160 in EE-treated baboons after a constant infusion of saline (i.e., basal; Fig. 4C) or 5-HT at 8 μg·min−1·kg body wt−1 (Fig. 4D). In untreated animals on day 160 of gestation (Fig. 4B), uterine artery waveforms showed high end-diastolic flow, indicative of low downstream flow impedance, compared with low end-diastolic flow/high impedance on day 60 (Fig. 4A). Although uterine artery waveforms on days 60, 100, and 160 appeared similar in untreated (Fig. 4B) and EE-treated (Fig. 4C) baboons in the basal state, the administration of 5-HT at both the 4 and 8 μg·min−1·kg body wt−1 doses at each stage of gestation caused uterine artery diastolic notching (Fig. 4D) in four of six EE-treated animals, reflecting flow impedance, compared (P < 0.05) with notching in only one of five untreated baboons. Uterine artery PI declined (P < 0.01) in untreated and EE-treated baboons in the basal and 5-HT-challenged states from elevated levels on day 60 (1.62 ± 0.15 in untreated, basal animals) to low levels on days 100 and 160 (Fig. 4E). The decline in uterine artery PI reflects a decline in downstream flow impedance and an increase in end-diastolic flow with advancing gestation. Uterine artery PI values were not significantly altered in untreated or EE-treated animals after challenge with either dose of 5-HT at early, mid, or late gestation. However, uterine artery PI in EE-treated baboons administered 5-HT at 8 μg·min−1·kg body wt−1 on day 160 (0.90 ± 0.10) was ∼25%, but was not significantly (P = 0.18) greater than that in vasochallenged untreated animals (0.73 ± 0.03).
Fig. 4.
A–D: representative uterine artery flow waveforms as assessed by Doppler ultrasonography on day 60 (A) and day 160 (B) during saline infusion (i.e., basal) in untreated baboons and on day 160 in animals treated with estradiol on days 25–59 and during infusion of saline (C) or 5-HT (8 μg·min−1·kg body wt−1; D). E and F: mean ± SE values of uterine artery pulsatility index (PI; E) and uterine artery volume flow (in ml·min−1·kg maternal body wt−1; F) as assessed on days 60, 100, and 160 of gestation in untreated (n = 5) and estradiol-treated (n = 6) baboons during saline (basal) or 5 HT (8 μg·min−1·kg body wt−1) infusion. Uterine artery PI values for untreated and estradiol-treated animals during the infusion of saline or 5-HT on days 100 and 160 were lower (P < 0.01) than the respective values on day 60. A progressive increase (P < 0.01) in volume flow occurred in untreated animals in the basal state between days 60, 100, and 160. An increase (P < 0.05) in volume flow in estradiol-treated animals in the basal state occurred between days 60 and 100 only. Arrows in D designate notching. *P < 0.01 vs. untreated 5-HT-infused animals and P < 0.05 vs. estradiol-treated animals in the basal state.
Uterine artery volume flow in untreated baboons in the basal state progressively increased (P < 0.01) from 2.1 ± 0.3 ml·min−1·kg maternal body wt−1 on day 60 to 7.7 ± 1.8 ml·min−1·kg maternal body wt−1 on day 100 and 13.2 ± 2.5 ml·min−1·kg maternal body wt−1 on day 160 (Fig. 4F). Although uterine artery blood flow in EE-treated baboons also exhibited an increase (P < 0.05) between early and midgestation, flow in the basal state on day 160 (9.9 ± 1.7 ml·min−1·kg maternal body wt−1) was not significantly different from the value on day 100. However, statistical analysis indicated an interaction between group and 5-HT treatment on day 160 but not earlier. Thus, on day 160, uterine artery volume flow after 5-HT challenge at the 8, but not 4, μg·min−1·kg body wt−1 dose of EE-treated animals (5.9 ± 1.3 ml·min−1·kg maternal body wt−1) was 55% lower (P < 0.01) than that in 5-HT-challenged untreated animals (13.5 ± 2.1 ml·min−1·kg maternal body wt−1) and 40% lower (P < 0.05) than in EE-treated animals in the basal state (9.9 ± 1.7 ml·min−1·kg maternal body wt−1). Uterine artery conductance, which was calculated as volume flow/arterial blood pressure and reflects vasoconstrictor tone, was also over threefold lower (P < 0.05) on day 160 in 5-HT-challenged EE-treated baboons (0.09 ± 0.01) than in untreated baboons (0.31 ± 0.05).
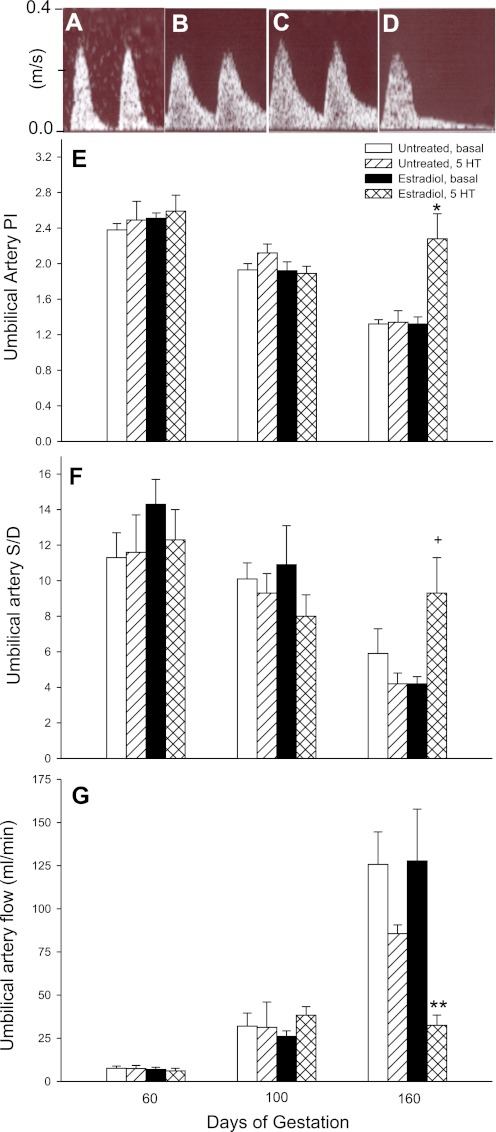
Umbilical artery blood flow.
Umbilical artery blood flow waveforms in untreated baboons exhibited low end-diastolic flow on day 60 (Fig. 5A) and high end-diastolic flow on day 160 (Fig. 5B). Consistent with this increase in end-diastolic flow, the umbilical artery Doppler PI (Fig. 5E) and S/D ratio (Fig. 5F) in untreated (basal) baboons decreased (P < 0.01) between day 60 (2.38 ± 0.02 and 11.3 ± 1.4, respectively) and day 160 (1.32 ± 0.05 and 5.9 ± 1.4, respectively) of gestation. The umbilical artery PI and S/D ratio in EE-treated baboons in the basal condition also declined (P < 0.01) between days 60 and 160 and showed high end-diastolic flow on day 160 (Fig. 5C). Umbilical artery PI and S/D ratio values in untreated baboons were not significantly altered at any stage of gestation by 5-HT treatment. However, there was a striking decline in end-diastolic flow after 5-HT challenge near term in EE-treated animals (Fig. 5D), and statistical analysis showed an interaction between group and 5-HT treatment. Thus, the umbilical artery PI and S/D ratio on day 160 in EE-treated baboons were approximately twofold greater after the administration of 5-HT at 8 (but not 4) μg/min (2.28 ± 0.28, P < 0.01, and 9.3 ± 2.0, P = 0.08, respectively) than in untreated baboons infused with 5-HT on day 160 (1.34 ± 0.13 and 4.2 ± 0.6, respectively) or saline-infused EE-treated baboons (1.32 ± 0.08 and 4.2 ± 0.4, respectively).
Fig. 5.
A–D: umbilical artery flow waveforms on day 60 (A) and day 160 (B) during saline infusion (basal) in untreated baboons and on day 160 in animals treated with estradiol on days 25–59 and during the infusion of saline (C) or 5-HT (8 μg·min−1·kg body wt−1; D). E–G: mean ± SE values of umbilical artery PI (E), systolic-to-end diastolic flow ratio (S/D ratio; F), and volume flow (in ml/min; G) in untreated (n = 5) and estradiol-treated (n = 6) animals during saline (basal) and 5-HT (8 μg·min−1·kg body wt−1) infusion. A decrease (P < 0.01) in the umbilical artery PI and S/D ratio occurred in untreated and estradiol-treated animals in the basal state on day 160 compared with the respective values on days 60 and 100. *P < 0.01 vs. untreated 5-HT-infused animals and vs. estradiol-treated basal animals for umbilical artery PI; +P = 0.08 vs. untreated 5-HT-infused animals and estradiol-treated basal animals for umbilical artery S/D ratio. A progressive increase (P < 0.01) in umbilical artery volume flow occurred in untreated and estradiol-treated animals in the basal state between days 60, 100, and 160. **P < 0.02 vs. estradiol-treated animals in the basal state and vs. untreated 5-HT-challenged animals for umbilical artery flow.
Umbilical artery volume blood flow in untreated baboons in the basal state progressively increased (P < 0.01) from 7.5 ± 1.3 ml/min on day 60 to 32.0 ± 7.6 ml/min on day 100 and to 125.7 ± 18.9 ml/min on day 160 (Fig. 5G). Umbilical artery volume flow in EE-treated animals also increased (P < 0.01) with advancing pregnancy and was similar to that in untreated animals. However, while the administration of 5-HT to untreated baboons on day 160 resulted in a nonsignificant 30% decrease in umbilical volume flow (85.7 ± 5.0 ml/min), in EE-treated animals challenged with 5-HT at a dosage of 8, but not 4, μg·min−1·kg body wt−1, umbilical artery flow was decreased (P < 0.02) to 32.5 ± 5.9 ml/min or by 75% compared with the respective basal state (127.8 ± 29.9 ml/min) and by >60% (P < 0.01) compared with untreated 5-HT-challenged animals.
Fetal weights and serum analytes.
Placental and fetal body and organ weights (Table 1) and serum chemistry analytes reflective of hepatic and renal function (Table 2) at the time of delivery after 5-HT infusion were similar in untreated and EE-treated baboons. Although each of the untreated baboon fetuses was delivered alive, one of the six fetuses from EE-treated baboons died at the end of the maternal infusion of 5-HT at the 8 μg·min−1·kg body wt−1 dose (Table 1).
Table 1.
Fetal baboon characteristics
| Body and Organ Weights |
|||||||
|---|---|---|---|---|---|---|---|
| Treatment | Number of Animals | Fetal Survival | Placenta, g | Body, g | Heart, g | Liver, g | Kidneys, g |
| Untreated | 5 | 5/5 | 180 ± 10 | 858 ± 19 | 4.6 ± 0.4 | 25.5 ± 1.6 | 5.4 ± 0.4 |
| Estradiol | 6 | 5/6 | 183 ± 9 | 859 ± 33 | 4.9 ± 0.5 | 23.2 ± 0.9 | 5.5 ± 0.3 |
Values are means ± SE. Parameters were measured after serotonin infusion (8 μg·min−1·kg body weight−1) on day 160 of gestation in baboons untreated or treated with estradiol benzoate (0.35 mg/day) on days 25–59 of gestation.
Table 2.
Serum chemistry analytes in fetal baboons
| Treatment | Number of Animals | Aspartate Aminotransferase, U/l | Alkaline phosphatase, U/l | Blood Urea Nitrogen/Creatinine | Albumin, g/dl | Na+, MEq/l | K+, MEq/l | Cl−, mEq/l |
|---|---|---|---|---|---|---|---|---|
| Untreated | 5 | 21.7 ± 3.5 | 670 ± 14 | 19.3 ± 2.4 | 3.5 ± 0.1 | 138 ± 1 | 6.3 ± 2 | 104 ± 2 |
| Estradiol | 6 | 19.3 ± 3.8 | 576 ± 38 | 12.8 ± 1.3 | 3.4 ± 0.1 | 141 ± 1 | 7.0 ± 2 | 104 ± 1 |
Values are means ± SE. Serum chemistry analytes were measured in the umbilical artery (i.e., fetal) after serotonin infusion (8 μg·min−1·μg body wt−1) on day 160 of gestation in baboons untreated or treated with estradiol benzoate on days 25–59 of gestation.
DISCUSSION
The present study shows that uterine and umbilical artery downstream flow impedance, as assessed by diastolic notching and a decrease in volume flow and conductance in the uterine artery as well as an increase in PI and decrease in end-diastolic flow in the umbilical artery, occurred during infusion of the vasoconstrictor 5-HT near term in baboons in which trophoblast uterine spiral artery remodeling was reduced by prematurely elevating estrogen levels in early pregnancy in contrast to animals with normal uterine vessel remodeling. Coinciding with the disruption in uteroplacental blood flow in EE-treated 5-HT-challenged baboons, fetal heart rate deceleration/bradycardia was elicited. Collectively, these results indicate that suppressing uterine artery remodeling by simply shifting the increase in estrogen from the second trimester to the first trimester of primate pregnancy disrupts uteroplacental blood flow dynamics and compromises fetal homeostasis after vasochallenge late in gestation.
Consistent with the decrease in uteroplacental flow impedance during advancing stages of normal human pregnancy (28, 42), the present study further shows that uterine and umbilical artery PI declined and end-diastolic flow increased with advancing gestation in both untreated and EE-treated baboons not challenged with 5-HT. It is well established that uterine and umbilical artery PI are directly proportional to downstream flow impedance on the maternal side and fetal side, respectively, of the placental circulation (27, 28). The decrease in uterine artery flow impedance with advancing gestation presumably reflects the remodeling of and change from high-resistance, low-capacity flow to low-resistance, high-capacity flow within the uterine arteries. The decline in vessel resistance and increase in vasodilation induced by vessel remodeling, coupled with enhanced maternal cardiac output and blood volume typical of advancing pregnancy, results in a progressive increase in uterine artery flow, as shown in baboons in the present study. The reduction in umbilical artery impedance with advancing pregnancy occurs primarily as a result of the progressive increase in vascular growth within the placental villous vessel bed (17, 60) exhibited during human (51, 54) and baboon (23) pregnancy. The decline in flow resistance, along with an increase in fetal cardiac output and blood volume characteristic of advancing gestation, leads to an increase in umbilical artery volume flow (47), as also observed in baboons in the present study.
Despite the reduction in uterine artery remodeling in baboons treated with estrogen in early pregnancy, uterine and umbilical artery flow dynamics appeared normal in the resting/basal state at early, mid, and late gestation. Transplacental O2 delivery to the conceptus appeared normal in transgenic mice with impaired spiral artery remodeling, challenging the physiological relevance of spiral artery modification (31). However, it seems unlikely that the normal uteroplacental flow observed in unchallenged estrogen-treated baboons with deficient vessel transformation means that uterine vessel transformation has no role in promoting uteroplacental blood flow. Rather, since isoflurance suppresses systemic vascular resistance in baboons (63), we suggest that the isoflurane anesthetization of baboons of the present study masked potential elevations in downstream flow impedance elicited by estrogen-induced impairment of uterine vessel transformation. However, it is also possible that compensatory mechanisms came into play to maintain normal flow in EE-treated baboons in the basal state. However, when challenged with 5-HT, the uteroplacental vessels of EE-treated but not untreated animals were highly responsive to this vasoconstrictor, suggesting that these vessels would also likely be hyperresponsive to other endogenously produced vasoconstrictor agents or autacoids.
Because the umbilical blood vessels are not innervated (59), the control of umbilical blood flow depends on vasoactive substances released locally and/or existing in the circulation, e.g., angiotensin II, histamine, and 5-HT (18, 26, 40, 52–55). 5-HT is transferred via a transporter across the placental syncytiotrophoblast (7, 62), where it has the capacity to induce umbilical artery contraction (35, 50, 56, 61). Many studies have shown that normal pregnancy suppresses the pressor responses of uterine and umbilical arteries to various vasoconstrictors, including 5-HT, angiotensin II, and epinephrine (19, 52, 53, 65, 66), and this process appears to be mediated by endothelium-dependent mechanisms, e.g., nitric oxide or prostacyclin (67). The refractory nature of the uteroplacental vessel bed to 5-HT in untreated baboons in the present study is consistent with the latter phenomenon. On the basis of the marked increase in PI and low end-diastolic flow within the umbilical artery after the administration of 5-HT to EE-treated baboons of the present study, it appears that the umbilical circulation in these baboons was particularly responsive to vasoconstrictor stimuli. The increase in umbilical artery flow impedance after 5-HT challenge of EE-treated animals did not result from improper placental villous vascularization, because we have shown that blood vessel density within the placental inner villous compartment was actually elevated by prematurely elevating estrogen in early baboon pregnancy (5, 48). Considering the latter finding and the fact that umbilical vessels lack autonomic innervation, EE treatment appears to have attenuated the refractoriness to vasoconstriction typical of normal pregnancy and enhanced responsivity of the umbilical-placental vessels to the action of 5-HT.
Importantly, based on the results of our recent and present studies, we propose that the low level of estrogen in the first trimester permits aggressive uterine artery remodeling and that the rise in estrogen thereafter suppresses and thus controls the extent to which the uterine arteries are remodeled. This would suggest that the temporal change in estrogen production/levels with advancing gestation is essential in regulating trophoblast uterine artery transformation. Once uterine vessel remodeling and uteroplacental perfusion have been established in the first half of gestation, however, they do not appear to be further impacted by estrogen. Thus, uterine, umbilical, and fetal blood flow parameters were not altered by suppressing estrogen production via the administration of an aromatase inhibitor throughout the second half of baboon pregnancy (2). The results of the present study take on heightened significance considering the potential deleterious effects that a premature increase in estrogen, or exposure to agents that enhance estrogen receptor signaling, would have on uterine vessel transformation and uteroplacental blood flow. For example, in vitro fertilization, which involves gonadotropin-induced ovarian hyperstimulation and thus elevated estrogen secretion, is associated with impaired endometrial vascularization, implantation, and embryonic development (33, 44, 58). Moreover, exposure to endocrine disruptors (15, 38) that enhance estrogen receptor signaling during early pregnancy would conceivably disrupt uterine vessel transformation and transplacental blood flow.
The decrease in uterine artery blood flow and increase in transplacental flow impedance exhibited near term in EE-treated baboons infused with 5-HT would be expected to impair the delivery of O2 and substrate to the fetus, particularly if vasochallenge was persistent. Fetal bradycardia (i.e., a heart rate of <80 beats/min) occurs in response to chronic hypoxia and vagal nerve innervation (36, 43) and is associated with redistribution of blood to the fetal brain and heart (68). Indeed, in the present study, vasochallenge of EE-treated baboons near term caused fetal bradycardia. Despite the disruption of uteroplacental blood flow observed in EE-treated baboons during 5-HT infusion, however, fetal growth was unaltered. Various experimental models of placental vascular flow impedance, e.g., uterine or umbilical artery ligation, placental embolism, and maternal hypoxia and hypothermia, have been used to induce fetal growth restriction. However, the extent to which fetal growth is compromised depends on whether the reduction in O2 and nutrient substrate supply to the fetus is acute or chronic and mild or severe (for a review, see Ref. 39). Preeclampsia in humans, which involves impaired uterine artery remodeling, is associated with only a 5–20% incidence of fetal growth restriction (13). The potential confounding effect of maternal stress on pregnancy outcome in the latter cases has not been evaluated; however, chronic maternal stress and anxiety lead to low fetal birth weight (41, 63). Other than instances of brief restraint and sedation for blood collection and treatment in early pregnancy, the animals of the present study were left undisturbed throughout most of gestation. Whether chronic vasochallenge of baboons after EE treatment, e.g., via frequent stress-related events, would inhibit fetal growth and impact development and physiological processes after birth in the offspring is unknown, but the present experimental paradigm provides an excellent approach to investigate this question.
A reduction in uteroplacental flow and fetal heart rate was observed in EE-treated animals after 5-HT vasochallenge in late gestation, but not in early or midgestation. Presumably, the metabolic demands imposed by the exponential growth of the fetus in the second half of gestation account for these observations. It is evident that the fetus exhibits resilience in combating disruptions of transplacental blood flow and only under conditions of pronounced vasoconstrictor challenge in late gestation, along with increased demands of the fetus, were significant fetal heart deceleration and in one case fetal demise observed.
In addition to impairment of uterine artery remodeling, disruption of uteroplacental blood flow dynamics after vasochallenge, and increase in maternal blood pressure, we (9) have also shown that prematurely elevating estrogen in early gestation decreased VEGF and increased soluble truncated Sflt-1 VEGF receptor expression by the extravillous placenta. Interestingly, comparable structural, physiological, and molecular derangements in uteroplacental development are hallmarks of preeclampsia in human pregnancy (1, 22, 24). Although numerous experimental animal models have been established in an attempt to replicate these hallmarks of human preeclampsia (37), defective uterine artery transformation as a key feature, which may be important in the etiology of preeclampsia and as shown in baboons in the present study, was not elicited in any of the other experimental animal models.
In summary, the present study shows that uterine and umbilical artery downstream flow impedance was increased, volume blood flow was decreased, and prolonged fetal heart deceleration was induced after vasochallenge with 5-HT near term in baboons in which trophoblast uterine spiral artery remodeling was suppressed by prematurely elevating estrogen levels in early pregnancy. These results indicate that suppressing uterine artery remodeling by advancing the rise in estrogen levels from the second trimester to the first trimester disrupts uteroplacental blood flow dynamics and fetal homeostasis during vasochallenge late in gestation.
GRANTS
This work was supported by National Institute of Child Health and Human Development Research Grant R01-HD-13294.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.W.A., G.J.P., and E.D.A. conception and design of research; G.W.A., T.W.B., and E.D.A. performed experiments; G.W.A., T.W.B., C.R.H., G.J.P., and E.D.A. analyzed data; G.W.A., T.W.B., C.R.H., G.J.P., and E.D.A. interpreted results of experiments; G.W.A., T.W.B., and E.D.A. prepared figures; G.W.A. and E.D.A. drafted manuscript; G.W.A., C.R.H., G.J.P., and E.D.A. edited and revised manuscript; G.W.A., T.W.B., C.R.H., G.J.P., and E.D.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The secretarial assistance of Wanda James with the computer graphics and preparation of the manuscript is greatly appreciated. The authors also gratefully acknowledge the scientific input from Dr. Kenneth Clark with respect to the application of serotonin to study uterine artery activity.
REFERENCES
- 1.Aardena MW, Oosterhof H, Timmer A, van Rooy I, Aarnouodse JG. Uterine artery Doppler flow and uteroplacental vascular pathology in normal pregnancies and pregnancies complicated by preeclampsia and small for gestational age fetuses. Placenta 22: 405–411, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Aberdeen GW, Baschat AA, Harman CR, Weiner CP, Langenberg PW, Pepe GJ, Albrecht ED. Uterine and fetal blood flow indexes and fetal growth assessment after chronic estrogen suppression in the second half of baboon pregnancy. Am J Physiol Heart Circ Physiol 298: H881–H889, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht ED, Aberdeen GW, Pepe GJ. The role of estrogen in the maintenance of primate pregnancy. Am J Obstet Gynecol 182: 432–438, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Albrecht ED, Bonagura TW, Burleigh DW, Enders AC, Aberdeen GW, Pepe GJ. Suppression of extravillous trophoblast invasion of uterine spiral arteries by estrogen during early baboon pregnancy. Placenta 27: 483–490, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Albrecht ED, Robb VA, Pepe GJ. Regulation of placental vascular endothelial growth/permeability factor expression and angiogenesis by estrogen during early baboon pregnancy. J Clin Endocrinol Metab 89: 5803–5809, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Aplin JD, Haigh T, Lacey H, Chen CP, Jones CJ. Tissue interactions in the control of trophoblast invasion. J Reprod Fertil Suppl 55: 57–64, 2000 [PubMed] [Google Scholar]
- 7.Balkovetz DF, Tiruppathi C, Leibach FH, Mahesh VB, Ganapathy V. Evidence for an imipramine-sensitive serotonin transporter in human placental brush-border membranes. J Biol Chem 264: 2195–2198, 1989 [PubMed] [Google Scholar]
- 8.Bilardo CM, Nicolaides KH, Campbell S. Doppler measurements of fetal and uteroplacental circulations: relationship with umbilical venous blood gases measured at cordocentesis. Am J Obstet Gynecol 162: 115–120, 1990 [DOI] [PubMed] [Google Scholar]
- 9.Bonagura TW, Pepe GJ, Enders AC, Albrecht ED. Suppression of extravillous trophoblast vascular endothelial growth factor expression and uterine spiral artery invasion by estrogen during early baboon pregnancy. Endocrinology 149: 5078–5087, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bower S, Schuchter K, Campbell S. Doppler ultrasound screening as part of routine antenatal scanning: prediction of pre-eclampsia and intrauterine growth retardation. Br J Obstet Gynaecol 100: 989–994, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu 1: 177–191, 1972 [PubMed] [Google Scholar]
- 12.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol 187: 1416–1423, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Gilstrap L, Wenstrom KD. Hypertension disorders in pregnancy. In: Williams Obstetrics (22nd ed.). New York: McGraw-Hill, 2005, p. 761–808 [Google Scholar]
- 14.Detti L, Akiyama M, Mari G. Doppler blood flow in obstetrics. Curr Opin Obstet Gynecol 14: 587–593, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 30: 293–342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enders AC, Blankenship TN. Modification of endometrial arteries during invasion by cytotrophoblast cells in the pregnant macaque. Acta Anat 159: 169–193, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Giles WB, Trudinger BJ, Baird PJ. Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. Br J Obstet Gynaecol 92: 31–38, 1985 [DOI] [PubMed] [Google Scholar]
- 18.Glance DG, Elder MG, Bloxam DL, Myatt L. The effect of the components of the renin-angiotensin system on the isolated perfused human placental cotyledon. Am J Obstet Gynecol 149: 450–454, 1984 [DOI] [PubMed] [Google Scholar]
- 19.Gokina NI, Kuzina OY, Fuller R, Osol G. Local uteroplacental influences are responsible for the induction of uterine artery myogenic tone during rat pregnancy. Reprod Sci 16: 1072, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gosling RG, King DH. Ultrasound angiology. In: Arteries and Veins, edited by Marcus AW, Adamson L. Edinburgh, UK: Churchill Livingstone, 1975, p. 61–98 [Google Scholar]
- 21.Hamilton WJ, Boyd JD. Development of the human placenta in the first three months of gestation. J Anat 94: 297–328, 1960 [PMC free article] [PubMed] [Google Scholar]
- 22.Harman CR, Baschat AA. Arterial and venous Dopplers in IUGR. Clin Obstet Gynecol 46: 931–946, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Hildebrandt VA, Babischkin JS, Koos RD, Pepe GJ, Albrecht ED. Developmental regulation of vascular endothelial growth/permeability factor messenger ribonucleic acid levels in and vascularization of the villous placenta during baboon pregnancy. Endocrinology 142: 2050–2057, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Ilekis JV, Reedy UM, Roberts JM. Preeclampsia–a pressing problem: an executive summary of a National Institute of Child Health and Human Development workshop. Reprod Sci 14: 508–523, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Joern H, Funk A, Goetz M, Kuehlwein H, Klein A, Fendel H. Development of quantitative Doppler indices for uteroplacental and fetal blood flow during the third trimester. Ultrasound Med Biol 22: 823–835, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Kaiser JR, Cox BE, Roy TA, Rosenfeld CR. Differential development of umbilical and systemic arteries. I. ANG II receptor subtype expression. Am J Physiol Regul Integr Comp Physiol 274: R797–R807, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol 175: 1534–1542, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Kurjak A, Chervenak FA. Donald School Textbook of Ultrasound in Obstetrics and Gynecology. New York: Parthenon Publishing Group, 2003, p. 395–421 [Google Scholar]
- 29.Lang U, Baker RS, Braems G, Zygmunt M, Künzel W, Clark KE. Uterine blood flow-a determinant of fetal growth. Eur J Obstet Gynecol Reprod Biol 110, Suppl 1: S55–S61, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Lang U, Prada J, Clark KE. Systemic and uterine vascular response to serotonin in third trimester pregnant ewes. Eur J Obstet Gynecol Reprod Biol 51: 131–138, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Leno-Duran E, Hatta K, Bianco J, Yamada AT, Ruiz-Ruiz C, Olivares EG, Croy BA. Fetal-placental hypoxia does not result from failure of spiral arterial modification in mice. Placenta 31: 731–737, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Lindsey JK. Models for Repeated Measurements. Oxford, UK: Oxford Univ. Press, 1999 [Google Scholar]
- 33.Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci USA 100: 2963–2968, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magness RR, Rosenfeld CR. Systemic and uterine responses to alpha-adrenergic stimulation in pregnant and nonpregnant ewes. Am J Obstet Gynecol 155: 897–904, 1986 [DOI] [PubMed] [Google Scholar]
- 35.Mak KK, Gude NM, Walters WA, Boura AL. Effects of vasoactive autacoids on the human umbilical-fetal placental vasculature. Br J Obstet Gynaecol 91: 99–106, 1984 [DOI] [PubMed] [Google Scholar]
- 36.Martin CB, Jr, de Haan J, van der Wildt B, Jongsma HW, Dieleman A, Arts TH. Mechanisms of late decelerations in the fetal heart rate. A study with autonomic blocking agents in fetal lambs. Eur J Obstet Gynecol Reprod Biol 9: 361–373, 1979 [DOI] [PubMed] [Google Scholar]
- 37.McCarthy FP, Kingdom JC, Kenny LC, Walsh SK. Animal models of preeclampsia; uses and limitations. Placenta 32: 413–419, 2011 [DOI] [PubMed] [Google Scholar]
- 38.McLachlan JA. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocr Rev 22: 319–341, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol 35: 730–743, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Myatt L. Control of vascular resistance in the human placenta. Placenta 13: 329–341, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Paarlberg KM, Vingerhoets AJ, Passchier J, Dekker GA, Heinen AG, van Geijn HP. Psychosocial predictors of low birthweight: a prospective study. Br J Obstet Gynaecol 106: 834–841, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Papageorghiou AT, Yu CK, Cicero S, Bower S, Nicolaides KH. Second trimester uterine artery Doppler screening in unselected populations: a review. J Matern Fetal Neonatal Med 12: 78–88, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Parer J. Fetal heart rate. In: Maternal-Fetal Medicine (4th ed.), edited by Creasy RK, Resnik R. Philadelphia, PA: Saunders, 1999, p. 270–299 [Google Scholar]
- 44.Paulson RJ, Sauer MV, Lobo RA. Embryo implantation after human in vitro fertilization: importance of endometrial receptivity. Fertil Steril 53: 870–874, 1990 [DOI] [PubMed] [Google Scholar]
- 45.Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 4: 397–413, 1983 [DOI] [PubMed] [Google Scholar]
- 46.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 27: 939–958, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Poston L. The control of blood flow to the placenta. Exp Physiol 82: 377–387, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Robb VA, Pepe GJ, Albrecht ED. Acute temporal regulation of placental vascular endothelial growth/permeability factor expression in baboons by estrogen. Biol Reprod 71: 1694–1698, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol 16: 5–15, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Robson JM, Sullivan FM. Analysis of action of 5-hydroxytryptamine in pregnancy. J Physiol 184: 717–732, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenfeld CR. Changes in uterine blood flow during pregnancy. In: The Uterine Circulation, edited by Rosenfeld CR. Ithaca, NY: Perinatology, 1989, p. 135–156 [Google Scholar]
- 52.Rosenfeld CR. Consideration of the uteroplacental circulation in intrauterine growth. Semin Perinatol 8: 42–51, 1984 [PubMed] [Google Scholar]
- 53.Rosenfeld CR. Mechanisms regulating angiotensin II responsiveness by the uteroplacental circulation. Am J Physiol Regul Integr Comp Physiol 281: R1025–R1040, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Rosenfeld CR. Regulation of the placental circulation. In: Fetal and Neonatal Physiology (3rd ed.), edited by Polin RA, Fox WW, Abman SH. Philadelphia, PA: Saunders, 2004, p. 97–103 [Google Scholar]
- 55.Rosenfeld CR, Gresores A, Roy TA, Magness RR. Comparison of ANG II in fetal and pregnant sheep: metabolic clearance and vascular sensitivity. Am J Physiol Endocrinol Metab 268: E237–E247, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Schneider TJ, Struijk PC, Lotgering FK, Wallenburg HC. Placental transfer and maternal and fetal hemodynamic effects of ketanserin in the pregnant ewe. Eur J Obstet Gynecol Reprod Biol 68: 179–184, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Sheppard BL, Bonnar J. The ultrastructure of the arterial supply of the human placenta in pregnancy complicated by fetal growth retardation. Br J Obstet Gynaecol 83: 948–959, 1976 [DOI] [PubMed] [Google Scholar]
- 58.Simon C, Dominguez F, Valbuena D, Pellicer A. The role of estrogen in uterine receptivity and blastocyst implantation. Trends Endocrinol Metab 14: 197–199, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Spivac M. On the presence or absence of nerves in the umbilical vessels of man and guinea pig. Anat Rec 85: 85–109, 1943 [Google Scholar]
- 60.Todros T, Piccoli E, Rolfo A, Cardaropoli S, Guiot C, Gaglioti P, Oberto M, Vasario E, Caniggia I. Review: feto-placental vascularization: a multifaceted approach. Placenta 32: S165–S169, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Tulenko TN. Regional sensitivity to vasoactive polypeptides in the human umbilicoplacental vasculature. Am J Obstet Gynecol 135: 629–636, 1979 [DOI] [PubMed] [Google Scholar]
- 62.Vähäkangas K, Myllynen P. Drug transporters in the human blood-placental barrier. Br J Pharmacol 158: 665–678, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Aken H, Fitch W, Graham DI, Brüssel T, Themann H. Cardiovascular and cerebrovascular effects of isoflurane-induced hypotension in the baboon. Anesth Analg 65: 565–574, 1986 [PubMed] [Google Scholar]
- 64.Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. Am J Obstet Gynecol 169: 858–865, 1993 [DOI] [PubMed] [Google Scholar]
- 65.Weiner C, Hdez M, Chestnut D, Wang JP, Herrig J. The interaction between serotonin and angiotensin II in the chronically instrumented guinea pig and its alteration by indomethacin. Am J Obstet Gynecol 156: 869–875, 1987 [DOI] [PubMed] [Google Scholar]
- 66.Weiner CP, Thompson LP, Liu KZ, Herrig JE. Pregnancy reduces serotonin-induced contraction of guinea pig uterine and carotid arteries. Am J Physiol Heart Circ Physiol 263: H1764–H1769, 1992 [DOI] [PubMed] [Google Scholar]
- 67.Weiner CP, Thompson LP, Liu KZ, Herrig JE. Endothelium-derived relaxing factor and indomethacin-sensitive contracting factor alter arterial contractile responses to thromboxane during pregnancy. Am J Obstet Gynecol 166: 1171–1178, 1992 [DOI] [PubMed] [Google Scholar]
- 68.Wood CE. Local and endocrine factors in the control of the circulation. In: Fetus and Neonate: Physiology and Clinical Implications, edited by Hanson MA, Spencer JAD, Hanson CH. Cambridge, UK: Cambridge Univ. Press, 1983, p. 110–115 [Google Scholar]
- 69.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol 160: 1405–1423, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]